Abstract
The aim of this study was to design and produce biologically active edible hydrosols, which, when applied to the surface of food products, will protect them from oxidative changes, spoilage and growth of microorganisms. Verification of testing hypothesis and the degree of aim realization were performed by assessing a DPPH radical scavenging activity and microbial reduction of experimental hydrosols on the basis of hydroxypropylmethylcellulose (HPMC), chitosan (CH), lysozyme (L) and nanocolloidal silver (NAg). Antimicrobial activity of different concentrations of CH, L and NAg hydrosols against Gram (+) bacteria: Bacilllus cereus and Micrococcus flavus and Gram (−) bacteria: Escherichia coli and Pseudomonas fluorescens, which exist more often in food, were analyzed using serial dilution test. Total number of microorganisms was determined on meat sample covered by tested sols. Hydrosols containing chitosan and other bioactive substances caused death of each tested microorganism. Lack of chitosan in hydrosols is reflected in a slight inhibition of M. flavus, E. coli and P. fluorescens. Simultaneous influence of CH, L and NAg addition and storage time on total number of bacteria in meat samples with hydrosols was showed. The addition of lysozyme to sols composition significantly increases antioxidant activity.
Keywords: Hydrosols, Chitosan, Lysozyme, Nanosilver, Antimicrobial activity
Introduction
“Hurdles Technology” or barriers is commonly used as a system of combined preservation of food products that can exploit the synergistic action of many factors, resulting in inhibition of microbial growth, each acting alone is not very effective. Barriers are the parameters that inactivate microorganisms, such as water activity (aw), pH and redox potential (Eh), or bioactive substances that can damage some, or at least one of the homeostatic mechanisms of microorganisms (Leistner and Gould 2002).
Chitosan is the N-acetylated chitin derivative β (1–4) linked of 2-amino-2-deoxy-β-D-glucose (GlcN) and N-acetylglucosamine (GlcNAc)—occasionally occurring in the chain. This polymer is given a GRAS status (Generally Recognized As Safe), which is a guarantee of safety in use as a natural food component (Shepherd et al. 1997; Terbojevich and Muzzarelli 2000). Moreover, it is a biorenewable, biodegradable and non-toxic, environmentally friendly product, which can be used as a preventive substance in food system (Rosca et al. 2005). It was also successfully used as component of preparation in wound healing (Dai et al. 2011; Ribeiro et al. 2013). Chitosan is dissolved in most organic acids such as formic acid, acetic acid, lactic acid, and oxalic acids. Most commonly used solvent is 0.1 M solution of acetic acid. Antimicrobial activity of chitosan is largely dependent on the molecular weight, degree of deacetylation, the concentration in solution and the pH of the medium (Lim and Hudson 2003). Previous studies indicate that the chitosan is dissolved in an aqueous salt solution to allow protonation of the amine, resulting in activity against some strains of fungi, yeasts and bacteria (Sagoo et al. 2002; Coma 2010). However, the strongest activity has against bacteria (Tsai et al. 2002), more against Gram (+) than Gram (−) (Jeon et al. 2001). It was observed that the polymer acts more quickly to fungi than bacteria and algae (Cuero 1999). There are also reports in the literature confirming the theory of hurdles and synergism of the applied chitosan in combination with several preservatives (Chen et al. 1996; Roller et al. 2002), and the antimicrobial factors (Lee et al. 2003; Song et al. 2002).
Lysozyme is muramidase (N-acetyl-muramyl-hydrolase) known bacteriostatic agent, discovered by Alexander Fleming in 1922. It decomposes β (1–4) glycosidic linkage between N-acetylmuramic acid and N-acetylglucosamine in the cell wall of polysaccharides, including bacteria (Leśnierowski and Kijowski 2007). Lysozyme is attached to the rest of the amino sugar polysaccharide bacterial cell wall, causing the deformation and unsealing, which results in a perforation of the cell wall (Proctor and Cunningham 1988). Because of its lytic properties, lysozyme has been applied as an antiseptic substance. Its function is mainly sensitive against Gram (+). Bacteriostatic effect of lysozyme can also be extended to the influence on the growth inhibition of Gram (−) by the application of permeable membrane for lysozyme with other substances or to connect other substances to lysozyme (Masschalck and Michiels 2003). The use of lysozyme in chitosan’s protective coatings extends the range of its effects on the bacteria Escherichia coli and Streptococcus faecalis (Park et al. 2004). Also the mixture of enzyme and chito-oligomers exhibits a strong inhibiting effect of the growth of such bacteria as Escherichia coli, Pseudomonas fluorescens, Bacillus cereus, and Staphylococcus aureus in minced meat stored in refrigerator (Rao et al. 2008). Bacteriostatic efficiency of lysozyme is potentiated as a result of: a complementary action in the combination with hydrogen peroxide and ascorbic acid (Miller 1959), EDTA (Padgett et al. 1998), coffee acid, cinnamic acid (Valenta et al. 1998), alginate, carrageenan (Cha and Chinnan 2004) and chitosan (Duan et al. 2008). It is also possible to inactivate the growth of Listeria monocytogenes, aerobic bacteria, yeast and fungi in smoked salmon coated by a film produced with whey protein and lysozyme (Min et al. 2005).
Silver colloid is a kind of antibiotic with a broad spectrum of activity (Galeano et al. 2003; Tankhiwale and Bajpai 2009; Marambio-Jones and Hoek 2010). It reacts with the thiol group of proteins and enzymes of bacteria and causes their inactivation (Lok et al. 2006). Silver, silver ions and silver compounds were analyzed for antibacterial and antiviral effects (Sondi and Salopek-Sondi 2004; Pal et al. 2007; Duran et al. 2007). In the literature, there are known methods for producing coatings with nanosilver dispensed within the structure of chitosan and silver in filter paper. Both food packagings have strong bacteriostatic effect against Escherichia coli (Tankhiwale and Bajpai 2009, 2010).
Using bioactive substances such as: chitosan, lysozyme (Moreira et al. 2011; Lian et al. 2012) and nanosilver (Llorens et al. 2012) might extend spectrum of their activity to the other food species of bacteria. The aim of this study was to design and produce biologically active edible hydrosols, which, when applied to the surface of the food product, will protect them from oxidative changes, spoilage and growth of microorganisms.
Materials and methods
Materials
The solid base was hydroxypropylmethylcellulose—Walocel preparate, HM 100 PA 2208 FG from Dow Wolff Cellulosics, Poland. Low molecular weight chitosan (DD = 75–85 %) and DL lactic acid (85 % syrup) were obtained from Sigma Aldrich, Poland. Lysozyme from white egg hen with 2000 U/mg activity was purchased from Ovopol, Poland. Nonionic form of colloidal silver with particles’ sizes in the range of 10–60 nm and a concentration of 50 ppm was obtained from Nano-Tech, Poland.
Preparation of sols biocomposite
Hydroxypropylmethyl cellulose (HPMC), as a based component, was dissolved in distilled water and heated with stirring in a water bath at 70 ° C. After 12 h, evaporated water was supplemented and inserted into the refrigerator at a temperature of 4 °C. At the same time, chitosan (CH) solution was prepared in 2 % aqueous solution of lactic acid by stirring with 400 rpm for 12 h. Then, water stock solution of lysozyme was prepared. Glycerol was used as plasticizer in amount of 25 % of polymers dry mass. Thus prepared solutions of sols, preparate of nanocolloidal silver (NAg) and glycerol were mixed in suitable proportions to obtain final concentrations of the components shown in Table 1.
Table 1.
Experimental design of hydrosols variants
| Variant | Variation factors | Constant factors | |||
|---|---|---|---|---|---|
| Chitosan [%] | Lysozyme [%] | Ag nanocolloid [ppm] | HPMC [%] | Glycerol [%] | |
| 1. | 0.0 | 0.0 | 0.0 | 0.5 | 25 (based on dry mass of polymers) |
| 2. | 0.5 | 2.0 | |||
| 3. | 1.0 | 1.0 | |||
| 4. | 1.0 | 0.0 | 2.0 | ||
| 5. | 0.5 | 1.0 | |||
| 6. | 1.0 | 0.0 | |||
| 7. | 2.0 | 0.0 | 1.0 | ||
| 8. | 0.5 | 0.0 | |||
| 9. | 1.0 | 2.0 | |||
Antioxidant activity as a free radical scavenging ability of DPPH
Scavenging capacity was measured using a methanolic solution of radicals of 1,1-diphenyl-2-pikrylohydrazylu (DPPH) and was described by Chen et al. (2007). Sols were mixed with 0.3 mM DPPH solution of alcohol in a 1:1 ratio. After 30-min incubation at room temperature the reduction of the DPPH free radical was measured by reading the absorbance at 517 nm. The antioxidant activity of experimental hydrosols was expressed as micromole of Trolox per milliliter of film solution (μg Trolox/ml).
Antimicrobial activity
The method was described by Song et al. (2002). The following microorganisms were used in the test: Bacillus cereus B3P, Micrococcus flavus, Escherichia coli, Pseudomonas fluorescens. The bacterial cultures were grown on the nutrient agar slant and kept at 4 °C. Microorganisms were taken from the slant and transferred to a suitable medium for propagation: B. cereus to Tryptic Soy Broth (Sigma Aldrich) for 24 h at 37 °C, M. flavus to enriched broth (BTL) for 24 h at 37 °C, E. coli to broth with sheep blood, P. fluorescens—broth with sheep blood for 24 h at 25 °C. The bacterial suspension was standardized by transferring 1 ml of culture to fresh medium to a density of 1 McFarland scale. The absorbance was measured at a wavelength of λ = 550 to determine cell density and obtained 106 cfu/ml of bacterial cultures concentration. 0.1 ml of the bacterial cell suspension was introduced into 1 ml sols sample (to a final concentration of bacterial cells of 105 cfu/ml) and incubated at 37 °C for 30 min. Then, the samples were applied in an amount of 0.1 ml of the suspension on a plate of solidified agar. The plates were incubated at 37 °C for 24 h (B. subtilis, M. flavus, E. coli) and 25 °C for 48 h (P. fluorescens). After this time the colonies grown were counted. A control sample was inoculated without the addition of experimental sol. The results are presented as log cfu/ml.
Total number of microorganism of meat sample covered by hydrosol
The total number of microorganisms was determined by plate count agar at 30 °C, for 72 h, according to PN-EN ISO4833 (“Meat and meat products. Microbiological examination. Determination of total number of microorganism”—Polish Standard). Refrigerated bovine tenderloin were obtained within 48 h of slaughtering from a local meat packer and ground through a 20 ± 0,3 mm thick slices (0 week of storage). Meat slices were sprayed out with biocomposites hydrosols, vacuum packed on the insert adsorbent in foil bags and stored in 4 °C for 1, 2, 3, 4 weeks. Meat slices were minced using sterilized grinder, homogenized in sterile bags with the Stomacher homogenizer and serial diluted. An agar plate with peptone-tryptone, yeast extract and glucose broth was flooded with a culture. Grown colonies were counted after incubation time. The results are presented in log cfu/g.
Statistical analysis
Data were analyzed by analysis of variance (Anova) using Statistic 9. The differences between means were established with Duncan Test with 5 % significance. All experiments were performed in triplicate.
Results and discussion
Statistical analysis of DPPH radical scavenging ability of hydrosols is presented in Table 2. Proportional relationship between the increase of L and NAg concentration and increase amount of Trolox, which is needed to neutralize 0,3 mM free radicals of DPPH were shown. Increasing concentration of CH in sols reflects reduction of bonded Trolox. The amount of bonded Trolox was at the same level, regardless of CH concentration. It was noted that hydrosols with the highest concentration of L were presented with the best efficiency of free radical scavenging. Samples without enzyme addition were characterized by the weakest antioxidant capacity (Fig. 1). The ability of hydrosols to free radical scavenging was increased with increasing concentration of the enzyme, what may be related with a higher content of hydrolysis products of used polysaccharides. Antioxidant properties of chito-oligomers and their derivatives are dependent on the degradation of chitosan (Sun et al. 2008). Furthermore, with the increase in the degree of substitution, decreases the ability to bind free radicals. This is due to the fact that chito-oligomers contain smaller amount of active amino groups, which can react with DPPH and create a colorful and reduced form of DPPH-H (Huang et al. 2006). The molecular weight of chito-oligomers in hydrolysates also affects their antioxidant properties. The increase of molecular weight of 60 kDa greatly decreases possibility of chito-oligomers to scavenge free radicals (Kim and Thomas 2007). However, Youn et al. (2001) obtained a slightly different result and suggested that chitosan with higher molecular weight and in higher concentration may present stronger antioxidant effect. Other reports indicate higher capacity chito-oligomers for radical scavenging of DPPH, hydroxide-oxide anion and superoxide anion to higher deacetylation degree, around 90 % (Je et al. 2004; Jarmila and Vavrikova 2011).
Table 2.
Antioxidant activity of DPPH for biocomposite hydrosols (main effects)
| Parameter | Main effects | |||||
|---|---|---|---|---|---|---|
| Chitosan [%] | Lysozyme [%] | Ag nanocolloid [ppm] | ||||
| μg Trolox/ml | 0.0 | 17.202a | 0.0 | 4.394a | 0.0 | 10.648a |
| 1.0 | 13.223b | 0.5 | 14.109b | 1.0 | 14,980b | |
| 2.0 | 12.239b | 1.0 | 24.161c | 2.0 | 17.037c | |
| Free radical scavenging [%] | 0.0 | 51.370a | 0.0 | 7.287a | 0.0 | 28.811a |
| 1.0 | 37.674b | 0.5 | 40.724b | 1.0 | 43.721b | |
| 2.0 | 34.289b | 1.0 | 75.323c | 2.0 | 50.801c | |
a–cStatistically significant differences for the columns within the factors at p ≤ 0.05
n = 3
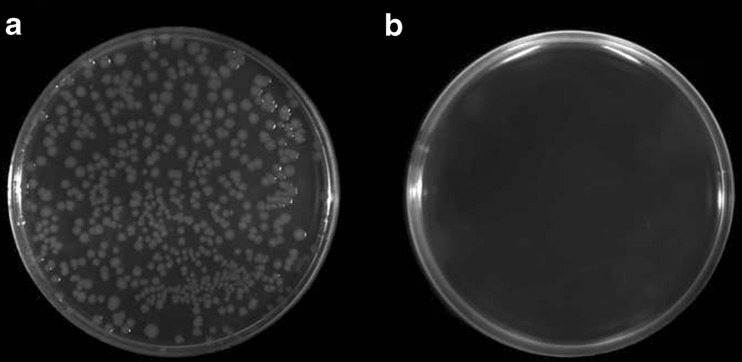
Fig. 1.
a Growth of E. coli of 105 cfu/ml; b Inhibition of E. coli growth of 105 cfu/ml by experimental hydrosol, 9 variant
Determination of antimicrobial activity was performed by modified method of Song et al. (2002) and allowed the quantification of the inhibition degree of microbial growth by the bioactive components from the experimental sols. It was found that 104 log cfu/ml of B. cereus was completely inactivated as a result of simultaneous effect of every bioactive components (Table 3). The amount of B. cereus at the control plate was 6,64 log cfu/ml. The growth of M. flavus was completely inhibited by sols with chitosan, lysozyme and nanosilver. It was also noted that lysozyme present positive impact against this bacteria. The addition of the enzyme to the sol composition caused reduction of Micrococcus growth by about 2 log cfu/ml. The variant 5 shows less M. flavus reduction comparing with 4 variant, what is related with higher concentration of nanosilver in second one. According to Rhim et al., the bacteriostatic effect of chitosan film could be improved with the addition of nanosilver (Rhim et al. 2006). Also the difference between those two samples is in lysozyme dose; the 0.5 % in variant 5 could be not enough to inhibit growth if this bacteria. Bacteriostatic effect against Gram (−) of the experimental hydrosols was also demonstrated. Complete inhibition of growth of E. coli (Fig. 1a and b) and P. fluorescens was observed just for samples containing chitosan (Table 3). It was noted that the greatest antimicrobial activity to create biocomposites among all used components has chitosan. Our results indicate also higher bacteriostatic effect of chitosan and chitosan oligomers against B. cereus, E. coli and P. fluorescens, and are in accordance with other reports (No et al. 2002; Cho et al. 1998). These authors noted an increase in chitosan activity against Bacillus sp. and E. coli with a decreasing viscosity of 1000 to 10 cP (Cho et al. 1998). Chitosan oligomers obtained after lysozyme degradation exhibit stronger antilisterial activity than not hydrolyzed chitosan (Zimoch-Korzycka et al. 2014). According to Wu, the increasing concentration of chitosan in membrane with the addition of cellulose also greatly reduces the growth of E. coli (Wu et al. 2004). Reducing the number of E. coli and B. cereus by 5–6 logarithmic orders and P. fluorescens about 3 orders by chitosan and abut 1–2 orders by chitooligosaccharides obtained by irradiation were achieved by Rao et al. (2008). The mechanism of microbial growth inhibition by the chitosan molecules is still not clear but it is associated with the polycationic nature of the polysaccharide, which interrupts the cell membrane (Helander et al. 2001) or a film-forming ability around the bacterial cell (Zheng and Zhu 2003). Lysozyme (which is a component of hydrosol protective layer) can be effective against Gram (−), when the outer membrane of bacteria is interrupted or is not covered with chitosan (Park et al. 2004). It is well known that the Gram (−) are resistant to the native form of lysozyme, which is why so many scientists undertake the study to improve the bacteriostatic properties of the enzyme, through the creation of mixtures interactively affecting the microorganisms (Nakamura et al. 1992; Song et al. 2002). Davidson and Branen’s research suggests the possibility of strengthening the activity of lysozyme against Gram (−) by the addition of EDTA, which changes permeability of the outer membrane (Branen and Davidson 2004). Antimicrobial activity of lysozyme has been successfully used against Gram (+), what is related to the hydrolysis of the glycoside bond of bacterial cell walls, especially the β (1–4) bond between the C-1 of N-acetylmuramic and C-4 of N-acetyl - D -glucosamine (Masschalck and Michiels 2003). However, Gram (−) bacteria are more sensitive to the effects of silver ions introduced into chitosan film than Gram (+), which is related to changes in the membrane permeability (Rhim et al. 2006). Silver has an antimicrobial effect against susceptible strains of Escherichia coli, Pseudomonas sp. and yeast alcohol (Clement and Jarrett 1994).
Table 3.
Effect of antimicrobial activity against Gram (+) and Gram (−) bacteria of biocomposite hydrosols
| Variant | Growth of microorganisms [log CFU/mL] | |||
|---|---|---|---|---|
| Gram (+) | Gram (−) | |||
| Bacillus cereus | Micrococcus flavus | Escherichia coli | Pseudomonas fluorescens | |
| 1 | 0.00 ± 0.00* | 6.82 ± 0.01 | 2.34 ± 0.01 | 4.78 ± 0.43 |
| 2 | 0.00 ± 0.00 | 4.63 ± 0.21 | 2.39 ± 0.01 | 4.77 ± 0.10 |
| 3 | 0.00 ± 0.00 | 4.15 ± 0.21 | 2.41 ± 0.00 | 5.02 ± 0.03 |
| 4 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| 5 | 0.00 ± 0.00 | 4.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| 6 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| 7 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| 8 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| 9 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Control | 6.64 ± 0.02 | 7.00 ± 0.00 | 2.51 ± 0.02 | 5.16 ± 0.02 |
* p ≤ 0.05
n = 3
The antimicrobial activity of experimental films on meat samples with increasing concentrations of chitosan was observed (Table 4). The control sample after a week of storage contained less than 1 order more colony forming units in 1 g of meat. While after 4 weeks, there was an increase of 2 orders compared to the fresh meat. Meat samples covered by every tested variant of hydrosols after first week of storage was an increase in the total number of microorganisms, which were higher than the number of colony forming units found in a control sample. Total number of bacteria in reference sample of meat after 4 weeks of storage was 8 log, but the same sample sprayed with sol of 9 variant resulted in inhibition of microorganisms about of 2,5 log. Storage time of refrigerated meat with tested films was influenced on inhibition of microbial growth. These results confirm the report of Sagoo et al., who showed a reduction in the total number of microorganisms in minced meat dipped in the chitosan solution of 0.3 and 0.6 %. They have found that the inhibition of microorganisms growth at the level of 1–3 log depends on the storage time of the products. The same researchers have also noted that the number of microorganisms in each of the test samples was always higher than the reference sample (Sagoo et al. 2002), what was in agreement with our results. According Darmadji and Izumimoto, who added 1 % of chitosan to beef patties stored at 4 °C for 10 days, have caused a reduction in the number of bacteria by 1–2 orders of logarithmic. However, change in temperature, or lowering the concentration of chitosan, was resulted in no inhibition of microbial growth (Darmadji and Izumimoto 1994). But Garcia et al., were noted that the addition of chitosan to model sausage batter did not result in the reduction of microorganisms, their growing was occurred within 26 days of storage (Garcia et al. 2010). It suggests possible simultaneous effect of many factors on reduction of microbial growth.
Table 4.
Antibacterial activity of structured biocomposite hydrosols on meat sample
| Variant | Growth of total number of bacteria [log CFU/g] | ||||
|---|---|---|---|---|---|
| Time [weeks] | |||||
| 0 | 1 | 2 | 3 | 4 | |
| 1 | 4.28 ± 0.02* | 5.00 ± 0.04 | 5.77 ± 0.05 | 6.64 ± 0.05 | 7.98 ± 0.01 |
| 2 | 5.32 ± 0.05 | 5.69 ± 0.10 | 6.28 ± 0.03 | 7.91 ± 0.02 | |
| 3 | 5.30 ± 0.05 | 5.54 ± 0.03 | 6.04 ± 0.03 | 6.69 ± 0.02 | |
| 4 | 4.66 ± 0.04 | 4.80 ± 0.12 | 5.64 ± 0.03 | 5.90 ± 0.04 | |
| 5 | 4.59 ± 0.08 | 5.64 ± 0.03 | 5.71 ± 0.03 | 5.85 ± 0.05 | |
| 6 | 4.76 ± 0.01 | 5.60 ± 0.04 | 5.72 ± 0.03 | 5.75 ± 0.04 | |
| 7 | 4.64 ± 0.06 | 4.99 ± 0.02 | 5.65 ± 0.02 | 5.72 ± 0.05 | |
| 8 | 4.58 ± 0.04 | 5.18 ± 0.01 | 5.36 ± 0.04 | 5.93 ± 0.03 | |
| 9 | 4.49 ± 0.01 | 4.80 ± 0.03 | 4.98 ± 0.01 | 5.57 ± 0.05 | |
| Control | 5.54 ± 0.05 | 5.98 ± 0.01 | 6.67 ± 0.02 | 8.04 ± 0.02 | |
*p ≤ 0.05
n = 3
Conclusions
It was demonstrated that experimental sols exhibit antimicrobial activity against all strains of bacteria chosen to test but is varied depending on the concentration and relative proportions of biologically active ingredients, what was confirmed in experiment with meat sample. Antioxidant properties were improved by addition of lysozyme to the composition of sols, which suggest higher reducing power of smaller chains of used polysaccharides (HPMC and chitosan). Many papers have reported water-soluble chitosan that presents higher physiological activity, such as anti-tumor or lifting immunity (Jeon and Kim 2002; Qin et al. 2002; Suzuki 1996). However, antimicrobial component characterized with strong antioxidant properties presents low antimicrobial properties because the antioxidant activity may play protective function against bacteria (Rao et al. 2008).
References
- Branen JK, Davidson PM. Enhancement of nisin, lysozyme, and monolaurin antimicrobial activities by ethylenediaminetetraacetic acid and lactoferrin. Int J Food Microbiol. 2004;90:63–74. doi: 10.1016/S0168-1605(03)00172-7. [DOI] [PubMed] [Google Scholar]
- Cha DS, Chinnan MS. Biopolymer-based antimicrobial packaging: review. Crit Rev Food Sci Nutr. 2004;44:223–237. doi: 10.1080/10408690490464276. [DOI] [PubMed] [Google Scholar]
- Chen JC, Yeh JY, Chen PC, Hsu CK. Phenolic Content and DPPH Radical Scavenging Activity of Yam-containing Surimi Gels Influenced by Salt and Heating. Asian J Health Inf Sci. 2007;2:1–11. [Google Scholar]
- Chen M, Yeh GH, Chiang B. Antimicrobial and physicochemical properties of methylcellulose and chitosan films containing a preservative. J Food Process Preserv. 1996;20:379–390. doi: 10.1111/j.1745-4549.1996.tb00754.x. [DOI] [Google Scholar]
- Cho HR, Chang DS, Lee WD, Jeong ET, Lee EW. Utilization of chitosan hydrolysate as a natural food preservative for fish meat paste products. Korean J Food Sci Technol. 1998;30:817–822. [Google Scholar]
- Clement JL, Jarrett PS. Antibacterial silver. Metal-Based Drugs. 1994;5–6:467–482. doi: 10.1155/MBD.1994.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coma V. Polysaccharide-based biomaterials with antimicrobial and antioxidant properties. Polimeros. 2010;20:1–12. [Google Scholar]
- Cuero RG. Antimicrobial action of exogenous chitosan. EXS. 1999;87:315–33. doi: 10.1007/978-3-0348-8757-1_23. [DOI] [PubMed] [Google Scholar]
- Dai T, Tanaka M, Huang YY, Hamblin MR. Chitosan preparations for wounds and burns: antimicrobial and wound-healing effects. Expert Rev Anti-Infect Ther. 2011;9:857–879. doi: 10.1586/eri.11.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darmadji P, Izumimoto M. Effect of chitosan in meat preservation. Meat Sci. 1994;38:243–254. doi: 10.1016/0309-1740(94)90114-7. [DOI] [PubMed] [Google Scholar]
- Duan J, Kim K, Daeschel MA, Zhao Y. Storability of antimicrobial chitosan-lysozyme composite coating and film-forming solutions. J Food Sci. 2008;73:321–329. doi: 10.1111/j.1750-3841.2008.00849.x. [DOI] [PubMed] [Google Scholar]
- Duran N, Marcarto PD, De Souza GIH, Alves OL, Esposito E. Antibacterial effect of silver nanoparticles produced by fungal process on textile fabrics and their effluent treatment. J Biomed Nanotechnol. 2007;3:203–208. doi: 10.1166/jbn.2007.022. [DOI] [Google Scholar]
- Galeano B, Korff E, Nicholson WL. Inactivation of vegetative cells, but not spores, of Bacillus anthracis, B. cereus, and B. subtilis on stainless steel surfaces coated with an antimicrobial silver- and zinc- containing zeolite formulation. Appl Environ Microbiol. 2003;69:4329–4331. doi: 10.1128/AEM.69.7.4329-4331.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia M, Diaz R, Puerta F, Beldarrain T, Gonzales J, Gonzales I. Influence of chitosan addition on quality properties of vacuum-packaged pork sausages. Cienc Tecnol Aliment. 2010;30:560–564. doi: 10.1590/S0101-20612010000200041. [DOI] [Google Scholar]
- Helander IM, Nurmiaho-Lassila EL, Ahvenainen R, Rhoades J, Roller S. Chitosan disrupts the barrier properties of the outer membrane of Gram-negative bacteria. Int J Food Microbiol. 2001;71:235–244. doi: 10.1016/S0168-1605(01)00609-2. [DOI] [PubMed] [Google Scholar]
- Huang RH, Rajapakse N, Kim SK. Structural factors affecting radical scavenging activity of chitooligosaccharides (COS) and its derivatives. Carbohydr Polym. 2006;63:122–129. doi: 10.1016/j.carbpol.2005.08.022. [DOI] [Google Scholar]
- Jarmila V, Vavrikova E. Chitosan Derivatives with Antimicrobial, Antitumour and Antioxidant Activities—a Review. Curr Pharm Des. 2011;17:3596–3607. doi: 10.2174/138161211798194468. [DOI] [PubMed] [Google Scholar]
- Je JY, Park PJ, Kim SK. Free radical scavenging properties of hetero-chitooligosaccharides using an ESR spectroscopy. Food Chem Toxicol. 2004;42:381–387. doi: 10.1016/j.fct.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Jeon YJ, Kim SK. Antitumor activity of chitosan oligosaccharides produced in ultrafiltration membrane reactor system. J Microbiol Biotechnol. 2002;12:503–507. [Google Scholar]
- Jeon YJ, Park PJ, Kim SK. Antimicrobial effect of chitooligosaccharides produced by bioreactor. Carbohydr Polym. 2001;44:71–76. doi: 10.1016/S0144-8617(00)00200-9. [DOI] [Google Scholar]
- Kim KW, Thomas RL. Antioxidative activity of chitosans with varying molecular weights. Food Chem. 2007;101:308–313. doi: 10.1016/j.foodchem.2006.01.038. [DOI] [Google Scholar]
- Lee CH, An DS, Park HF, Lee DS. Wide-spectrum antimicrobial packaging materials incorporating nisin and chitosan in the coating. Packag Technol Sci. 2003;16:99–106. doi: 10.1002/pts.617. [DOI] [Google Scholar]
- Leistner L, Gould GW. Hurdle Technologies Combination Treatments for Food Stability, Safety and Quality. New York: Springer; 2002. [Google Scholar]
- Leśnierowski G, Kijowski J. Lysozyme. In: Huopalhti R, Lopez-Fandino R, Anton M, Schade R, editors. Bioactive egg compounds. Berlin: Springer; 2007. [Google Scholar]
- Lian ZX, Ma ZS, Wei J, Liu H. Preparation and characterization of immobilized lysozyme and evaluation of its application in edible coatings. Process Biochem. 2012;47:201–208. doi: 10.1016/j.procbio.2011.10.031. [DOI] [Google Scholar]
- Lim S, Hudson SM. Review of chitosan and its derivatives as antimicrobial agents and their uses as textile chemicals. J Macromol Sci. 2003;43:223–269. doi: 10.1081/MC-120020161. [DOI] [Google Scholar]
- Llorens A, Lloret E, Picouet PA, Trbojevich R, Fernandez A. Metallic-based micro and nanocomposites in food contact materials and active food packaging. Trends Food Sci Technol. 2012;24:19–29. doi: 10.1016/j.tifs.2011.10.001. [DOI] [Google Scholar]
- Lok CN, Ho CM, Chen R, He QY, Yu WY, Sun H, Tam PK, Chiu JF, Chen CM. Proteomic analysis of the mode of antibacterial action of silver nanoparticles. J Proteome Res. 2006;5:916–924. doi: 10.1021/pr0504079. [DOI] [PubMed] [Google Scholar]
- Marambio-Jones C, Hoek EMV. A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment. J Nanoparticle Res. 2010;12:1531–1551. doi: 10.1007/s11051-010-9900-y. [DOI] [Google Scholar]
- Masschalck B, Michiels CW. Antimicrobial properties of lysozyme in relation to foodborne vegetative bacteria. Crit Rev Microbiol. 2003;29:191–214. doi: 10.1080/713610448. [DOI] [PubMed] [Google Scholar]
- Miller GL. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem. 1959;31:426–428. doi: 10.1021/ac60147a030. [DOI] [Google Scholar]
- Min S, Harris LJ, Han JH, Krochta JM. Listeria monocytogenes inhibition by whey protein films and coatings incorporating lysozyme. J Food Prot. 2005;68:2317–2325. doi: 10.4315/0362-028x-68.11.2317. [DOI] [PubMed] [Google Scholar]
- Moreira MR, Roura SI, Ponce A. Effectiveness of chitosan edible coatings to improve microbiological and sensory quality of fresh cut broccoli. LWT Food Sci Technol. 2011;44:2335–2341. doi: 10.1016/j.lwt.2011.04.009. [DOI] [PubMed] [Google Scholar]
- Nakamura S, Kato A, Kobayashi K. Bifunctional lysozyme-galactomannan conjugate having excellent emulsifying properties and bactericidal effect. J Agric Food Chem. 1992;40:735–739. doi: 10.1021/jf00017a005. [DOI] [Google Scholar]
- No HK, Na YP, Lee SH, Meyers SP. Antibacterial activity of chitosans and chitosan oligomers with different molecular weights. Int J Food Microbiol. 2002;74:65–72. doi: 10.1016/S0168-1605(01)00717-6. [DOI] [PubMed] [Google Scholar]
- Padgett T, Han IY, Dawson PL. Incorporation of food-grade antimicrobial compounds into biodegradable packaging films. J Food Prot. 1998;61:1330–1335. doi: 10.4315/0362-028x-61.10.1330. [DOI] [PubMed] [Google Scholar]
- Pal S, Tak YK, Song JM. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the Gram-negative bacterium Escherichia coli. Appl Environ Microbiol. 2007;73:1712–1720. doi: 10.1128/AEM.02218-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SI, Daeschel MA, Zhao Y. Functional properties of antimicrobial lysozyme-chitosan composite films. J Food Sci. 2004;8:215–221. doi: 10.1111/j.1365-2621.2004.tb09890.x. [DOI] [Google Scholar]
- Proctor VA, Cunningham FE. The chemistry of lysozyme and its use as a food preservative and a pharmaceutical. CRC Crit Rev Food Sci Nutr. 1988;26:359–395. doi: 10.1080/10408398809527473. [DOI] [PubMed] [Google Scholar]
- Qin CQ, Du YM, Xiao L, Zhan L, Gao XH. Enzymic preparation of water-soluble chitosan and their antitumor activity. Int J Biol Macromol. 2002;31:111–117. doi: 10.1016/S0141-8130(02)00064-8. [DOI] [PubMed] [Google Scholar]
- Rao MS, Chander R, Sharma A. Synergistic effect of chitooligosaccharides and lysozyme for meat preservation. Food Sci Technol. 2008;41:1995–2001. [Google Scholar]
- Rhim JW, Hong SI, Park HM, Ng PKW. Preparation and characterization of chitosan-based nanocomposite films with antimicrobial activity. J Agric Food Chem. 2006;54:5814–5822. doi: 10.1021/jf060658h. [DOI] [PubMed] [Google Scholar]
- Ribeiro MP, Morgado PI, Moguel SP, Coutinho P, Correia IJ. Dextran-based hydrogel containing chitosan microparticles loaded with growth factors to be used in wound healing. Mater Sci Eng C. 2013;33:2958–2966. doi: 10.1016/j.msec.2013.03.025. [DOI] [PubMed] [Google Scholar]
- Roller S, Sagoo S, Board R, O’Mahony T, Caplice E, Fitzgerald G, Fogden M, Owen M, Fletcher H. Novel combination of chitosan, carnocin and sulphite for the preservation of chilled pork sausages. Meat Sci. 2002;62:165–77. doi: 10.1016/S0309-1740(01)00243-1. [DOI] [PubMed] [Google Scholar]
- Rosca C, Chitanu GC, Popa MI. Interaction of chitosan with natural or synthetic anionic polyelectrolytes. The chitosan-carcoxymethylcellulose complex. Carbohydr Polym. 2005;62:34–41. doi: 10.1016/j.carbpol.2005.07.004. [DOI] [Google Scholar]
- Sagoo S, Board R, Roller S. Chitosan inhibits growth of spoilage microorganisms in chilled pork products. Food Microbiol. 2002;19:175–182. doi: 10.1006/fmic.2001.0474. [DOI] [Google Scholar]
- Shepherd R, Reader S, Falshaw A. Chitosan functional properties. Glycoconj J. 1997;14:535–542. doi: 10.1023/A:1018524207224. [DOI] [PubMed] [Google Scholar]
- Sondi I, Salopek-Sondi B. Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteria. J Colloid Interface Sci. 2004;275:177–182. doi: 10.1016/j.jcis.2004.02.012. [DOI] [PubMed] [Google Scholar]
- Song Y, Babiker EE, Usui M, Saito A, Kato A. Emulsifying properties and bactericidal action of chitosan-lysozyme conjugates. Food Res Int. 2002;35:459–466. doi: 10.1016/S0963-9969(01)00144-2. [DOI] [Google Scholar]
- Sun T, Yao Q, Zhou D, Mao F. Antioxidant activity of N-carboxymethyl chitosan oligosaccharides. Bioorg Med Chem Lett. 2008;18:5774–5776. doi: 10.1016/j.bmcl.2008.09.072. [DOI] [PubMed] [Google Scholar]
- Suzuki S. Studies on biological effects of water soluble lower homologous oligosaccharides of chitin and chitosan. Fragrance J. 1996;15:61–68. [Google Scholar]
- Tankhiwale R, Bajpai SK. Graft copolymerization onto cellulose-based filter paper and its further development as silver nanoparticles loaded antibacterial food-packaging material. Colloids Surf B. 2009;69:164–168. doi: 10.1016/j.colsurfb.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Tankhiwale R, Bajpai SK. Silver-nanoparticle-loaded chitosan lactate films with fair antibacterial properties. J Appl Polym Sci. 2010;115:1894–1900. doi: 10.1002/app.31168. [DOI] [Google Scholar]
- Terbojevich M, Muzzarelli RAA. Chitosan. In: Phillips GO, Williams PA, editors. Handbook of Hydrocolloids. Cambridge: CRC Press; 2000. pp. 367–376. [Google Scholar]
- Tsai GJ, Su WH, Chen HC, Pan CL. Antimicrobial activity of shrimp chitin and chitosan from different treatments and applications of fish preservation. Fish Sci. 2002;68:170–177. doi: 10.1046/j.1444-2906.2002.00404.x. [DOI] [Google Scholar]
- Valenta C, Schwarz EG, Bernkop-Schnürch A. Lysozyme caffeic acid conjugates: possible novel preservatives for dermatics. Int J Pharm. 1998;174:125–132. doi: 10.1016/S0378-5173(98)00252-X. [DOI] [Google Scholar]
- Wu YB, Yu SH, Mi FL, Wu CW, Shyu SS, Peng CK, Chao AC. Preparation and characterization on mechanical and antibacterial properties of chitsosan/cellulose blends. Carbohydr Polym. 2004;57:435–440. doi: 10.1016/j.carbpol.2004.05.013. [DOI] [Google Scholar]
- Youn SK, Kim YJ, Ahn DH. Antioxidative effects of chitosan in meat sausage. J Korean Soc Food Sci Nutr. 2001;30:477–481. [Google Scholar]
- Zheng LY, Zhu JF. Study on antimicrobial activity of chitosan with different molecular weights. Carbohydr Polym. 2003;54:527–530. doi: 10.1016/j.carbpol.2003.07.009. [DOI] [Google Scholar]
- Zimoch-Korzycka A, Gardrat C, Castellan A, Jarmoluk A, Coma V (2014) The use of lysozyme to prepare biologically active chito-oligomers. Polimeros (approved August 19, 2014)