Abstract
Porridge (koozh) is one of the traditional foods made from Eleusine coracana L. grains (Finger millet). It is a soft food prepared from processed (germinated & fermented) finger millet flour (FMF). However, in the modern world of fast food, koozh is usually prepared from non-processed (non-germinated & non-fermented) FMF. Hence, present study was undertaken to evaluate the macro and micro nutrient contents in koozh prepared from germinated (fermented & non-fermented) and non-germinated (fermented & non-fermented) FMF. Highest protein, carbohydrate and glycoprotein contents were found in koozh prepared from germinated & non-fermented FMF. The free amino acid contents are higher in germinated & fermented condition when compare to other preparations. No significant change was observed in the calorific value of all preparations. There is no statistical difference in macro-nutrients & micro-nutrients minerals such as calcium, iron, magnesium, manganese, phosphorous and zinc among all the preparations. However, copper content is higher in non-germinated condition, whereas selenium, silicon and sulphur are higher in germinated FMF when compared to others. Significant level of total phenol, total flavonoid and free radical scavenging activity was observed in all preparations, which increased further during fermentation. The present observations, lead us to conclude that koozh prepared from germinated & non-fermented FMF contains higher level of carbohydrate, protein and glycoprotein, however germinated & fermented koozh has increased aminoacids, phytochemicals and free radical scavenging activity. Hence it is suggested that the consumption of koozh made from germinated & fermented FMF may provide easily digestible and energetic nutrients for healthier life.
Keywords: Finger millet, Koozh, Nutrients, Germination, Fermentation
Introduction
In modern lifestyle, consumption of rapidly processed fast food leads to various diseases (Kearney 2010). Human epidemiological studies brought out that almost 90 % of diseases occur due to intake of food with high calorific value. Earlier studies showed that intake of fatty food leads to chronic metabolic diseases like diabetes, cardiovascular diseases and ageing (Everitt et al. 2006). Hence, disease prevention through dietary modulation plays a vital role in the present world of fast food. In this scenario, cereals are preferred as foremost choice for low calorie diet. Recently, preparations of low calorie food from cereals have become the major focus of health care industries in which either traditionally processed (germinated & fermented) or non- processed (non-germinated & non-fermented) cereals are used. Hence present study was conducted to explore the nutrient values of koozh (porridge) made from one such familiar cereal, finger millet as an example of low calorie food.
Finger millet (Ragi, Eleusine coracana L.) is a prominent drought resistant crop and used as a staple prime food in India as well as African countries (Devi et al. 2014). Finger millet serves as a good source of carbohydrate, protein, dietary fiber, amino acids and phytochemicals. It is nutritionally rich in minerals such as calcium, magnesium, phosphorous and manganese which are essential for the normal growth of body tissue and energy metabolism. It’s phytochemicals play a vital role as hypoglycemic agent to control blood glucose level in diabetic patients (Kang et al. 2008; Chethan et al. 2008) and to reduce the cancer risk (Chandrasekara and Shahidi 2011). Further, finger millet koozh serves as an ideal low calorie diet for all age groups especially growing infants and pregnant women.
Typically finger millet is used for preparations of various traditional foods such as roti, mudde, ambali and koozh (Shobana et al. 2013). These preparations from finger millet powder were made after either traditional processing (germinated & fermented) or non-processing (non-germinated & non-fermented). This processed finger millet products have received great importance among the consumers because of its functional constituents (Saleh et al. 2013). Many traditional processes including milling, germination, fermentation, popping and decortication for preparing finger millet koozh are common throughout the world. Among these traditional processes germination and fermentation are more widely used in rural households (Rao and Muralikrishna 2001). In recent days, traditionally processed foods have poor scientific consideration and its commercialization has been restricted.
In general, germination has been proved to improve nutritional value of grains in numerous food preparations. Previous studies shown that carbohydrate, protein, minerals such as phosphorous and calcium and essential amino acids contents were increased due to germination (Mamiro et al. 2001). Saleh et al. (2013) had shown that germinated finger millet augments digestibility of food and improves its flavor. Most of the consumed forms of traditional finger millet foods are prepared through natural fermentation process. During fermentation, flour will undergo major biochemical reactions like starch hydrolysis, sugar transformation and softening. So the conventional process of fermentation is believed to increase the nutritive value of grains. The germinated and fermented finger millet flour is largely used in preparation of weaning food, instant mixes, beverages and pharmaceutical products (Rao and Muralikrishna 2001).
Finger millet koozh is one of the popular traditional food made in South India. It is an excellent hydrating drink and is favorable for easy digestion and health maintenance. It is commonly consumed by rural population for breakfast and lunch. But, the detailed information on the effect of different processing methods on the nutritional value of finger millet based food is scarce.
Due to its significant nutritive value, processed finger millet-based food preparations should be analyzed for their microbiological, enzymological and biochemical properties for their certification as quality food for consumers. Hence, in view of this deliberation the present study was undertaken to find out the nutritive value of germinated and non-germinated (with or without fermentation) koozh samples.
Materials and methods
Grain collection
Grains of Eleusine coracana L. (Finger millet) were procured from local market in Pondicherry, cleaned, washed with water and then dried in shade.
Germination
Grains were soaked in water for 3 h, after water was drained; grains were allowed to germinate on moist cotton cloth at room temperature (25–27 °C) for 48 h. The germinated grains were dried for 48 h in shade and finally powdered using blender.
Fermentation
50 g of germinated or non-germinated finger millet powder was mixed in 100 mL of deionised water (1:2) in a 250 mL glass beaker and concealed with aluminium foil and left over for 24 h at room temperature to undergo fermentation. Thick slurry was formed at the end of the fermentation process.
Koozh preparation
Slurry was prepared by using 50 g of germinated and non-germinated finger millet flour (with or without fermentation). The slurry was mixed individually with 120 mL of boiled deionised water and then cooked with constant stirring without any lumps for about 15–20 min when it started thickening, it was left to reach room temperature and stored in air tight container at 4 °C.
Sample preparation
To 5 g of each koozh sample 10 mL of deionised water was added and mixed thoroughly. It was then sonicated (Branson Digital Sonifier) and filtered by using Whatman filter paper no.1 and filtrate was used as sample for following assays.
Determination of pH and acidity
pH was determined using pH meter. Acidity in samples was determined as explained by Antony and Chandra (1997). 10 mL of koozh filtrate sample was taken in a titration flask and 2–3 drops of phenolphthalein was added to it. The sample containing indicator was titrated against 0.1 N NaOH until pale pink end point appeared. Volume of 0.1 N NaOH used was measured to determine acidity of sample by using following formula:
Biochemical analysis
Protein content in samples was estimated by Bradford (1976) method using BSA as a standard. Total carbohydrate was estimated by phenol sulphuric acid method (Dubois et al. 1956). Glycoprotein of sample was quantified by Schiff’s reagent microtiter plate by the method of Kilcoynea et al. (2011). N-acetylglucosamine (GlcNAc) was used as a standard. Presence of free amino acids in sample was quantified calorimetrically by ninhydrin method (Chen et al. 2009). Tryptophan was used as a standard. Minerals present in samples were analyzed by X-ray Fluorescence Instrument (Bruker S4 Pioneer) (Nielson et al. 1991).
The proteins in sample were fractionated using SDS-PAGE electrophoresis (Laemmli 1970). 30 μg protein sample was run on 12 % reduced SDS-PAGE. After the run, gel was fixed in staining solution for 30 min at room temperature. Subsequently the gel was destained for 45 min and then photographed.
Staining for glycoprotein was performed according to Schiff’s method. 30 μg of protein sample was loaded on 12 % reduced SDS-PAGE. Gel was fixed in 50 % methanol for 30 min and washed with 3 % acetic acid (in RT) for 15 min (3× for 5 min). Then gel was treated with oxidation reagent containing 1 % periodic acid for 15 min in dark at room temperature, and again washed with 3 % acetic acid (3× for 10 min). The washed gel was stained with Schiff's reagent at 4 °C in dark for 15 min. The stained gel was treated with reducing reagent (0.5 % Potassium metabisulphate) for 5 min until bands become visible.
Total calorific value of the sample was estimated by using Bomb calorimeter (IKA C 5000 control/ duo control calorimeter system).
Antioxidant property
Total flavonoid content was estimated by calorimetric method of Zhishen et al. (1999). Phenol content was measured spectrophotometrically by the method of Psomiadou and Tsimidou (2002). DPPH scavenging assay was carried out according to method of Shimada et al. (1992).
Statistical analysis
All biochemical assays were performed in triplicate and the data were analyzed by using SPSS 16 software. All the values were expressed as mean ± S.D.
Results and discussion
In South India most of the people in rural areas have been consuming koozh as a healthy food. Koozh has been prepared predominantly by subjecting finger millet grains to some traditional processes. Earlier reports recorded the nutrient profile of the processed finger millet alone (Shobana et al. 2013). However, nutritional values of processed and non-processed finger millet-based foods are very meager. In recent days koozh is consumed by all the people and further it has been gaining popularity as one of the leading nutrient rich food. In this context, koozh has received much attention of consumers because of the awareness about the probiotic values of nutrient constituents.
pH and acidity of koozh
pH and acidity of koozh is depicted in Table 1. Low pH and higher acidity were observed in fermented koozh of both germinated and non-germinated finger millet grains when compared to that of non-fermented one. This may be due to the increased concentration of H+ ion generated during fermentation process. Further Antony et al. (1996) reported that the predominant organic acids such as lactic acid and acetic acid are produced during the fermentation of finger millet. So the acidity of koozh may be attributed to the production of these acids. Further, Karovicova (2007) reported that fermentation gives an appetizing flavor due to increased acidity and low pH. Similar appetizing excellent flavor has been observed in fermented koozh when compared to non-fermented one in the present study.
Table 1.
pH and acidity of koozh prepared from finger millet grains
| Particular | pH | Acidity (%) |
|---|---|---|
| Germinated & fermented koozh | 4.55 ± 0.02 | 2.0 ± 0.08 |
| Germinated & non-fermented koozh | 5.26 ± 0.4 | 1.3 ± 0.05 |
| Non-germinated & fermented koozh | 4.8 ± 0.05 | 2.4 ± 0.05 |
| Non-germinated & non-fermented koozh | 5.87 ± 0.2 | 1 ± 0.2 |
Macro and micro nutrients of koozh
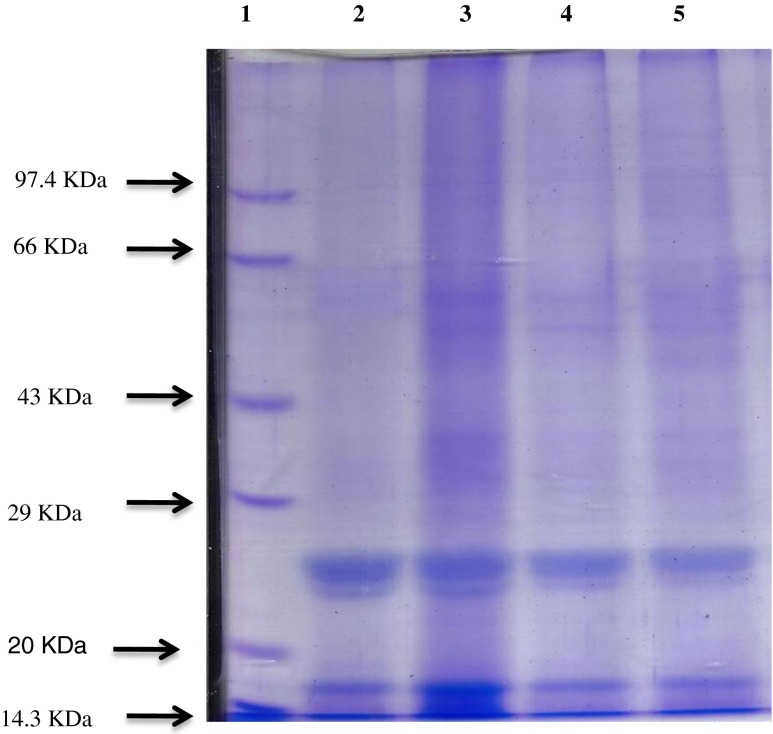
Germinated & non-fermented finger millet koozh was found to exhibit significant (P ≤ 0.05) increase in the protein content when compared to that of other preparations (Table 2). The augmentation in protein content of germinated and non-fermented finger millet koozh may be due to quantitative reduction in anti-nutritional factors such as tannin, phytic acid, polyphenols which are known to interact with protein to form complexes (Hassan et al. 2006). Further, this observation was confirmed by SDS-PAGE analysis (Fig. 1). Prominent protein expressions were observed at 42–66 kDa and 14–29 kDa of germinated and non-germinated koozh samples in non-fermented condition respectively, those bands are more intensive than fermented koozh samples. But electrophoretic pattern of fermented koozh indicates that the protein bands above 43 kDa were diminished in non-germinated condition while these bands were completely absent in germinated koozh samples. But some of the prominent bands in fermented samples were observed in the molecular range of 14–29 kDa. This observation clearly shows that germination and fermentation processes slightly reduce the total protein content of the samples. Decreased protein content due to fermentation may be due to the enzymatic breakdown of protein by microbial protease activity and decreased pH during fermentation (Saleh et al. 2013). This substantiates the work of El Hag et al. (2002) who reported that during fermentation proteolytic activity was enhanced by fermenting microbes and it was associated with protein digestibility which increased amino nitrogen by partial breakdown of protein.
Table 2.
Nutrient contents of koozh prepared from finger millet grains
| Varieties | Protein (mg/g of flour) | Carbohydrate (mg/g of flour) | Glycoprotein (mg/g of flour) | Free amino acid (mg/g of flour) | Calories (kcal/g of flour) |
|---|---|---|---|---|---|
| Germinated & fermented koozh | 29.81 ± 2.17#@ | 93.33 ± 11.17#@$ | 23.8 ± 2.7 | 14.8 ± 1.25#$ | 3.801 ± 0.03 |
| Germinated & non-fermented koozh | 38.57 ± 3.29*@$ | 126.67 ± 10.3*@$ | 25.7 ± 4.4@ | 10.47 ± 0.8* | 3.816 ± 0.04 |
| Non-germinated & fermented koozh | 17.02 ± 3.39*#$ | 61.52 ± 5.5*#$ | 17.4 ± 2.4# | 12.46 ± 1.48$ | 3.695 ± 0.06 |
| Non-germinated & non-fermented koozh | 27.3 ± 4.27 # @ | 83.6 ± 0.3*#@ | 21.8 ± 2.6 | 9.6 ± 0.5*@ | 3.740 ± 0.11 |
*represents significant difference at P ≤ 0.05 Germinated & fermented koozh
#represents significant difference at P ≤ 0.05 Germinated & non-fermented koozh
@represents significant difference at P ≤ 0.05 Non-germinated & fermented koozh
$represents significant difference at P ≤ 0.05 Non-germinated & non-fermented koozh
Fig. 1.
Protein profile by SDS-PAGE. (Lane 1: Protein marker; Lane 2: Germinated & fermented koozh; Lane 3: Germinated & non-fermented koozh; Lane 4: Non-germinated & fermented koozh; Lane 5: Non-germinated & non-fermented koozh)
The germinated & non-fermented koozh showed high carbohydrate content (P ≤ 0.05) when compared to that of other preparations (Table 2). It was proved that during germination starch is broken down by the starch hydrolyzing enzymes such as amylases and diastases (Antony et al. 1996). Malleshi and Desikachar (1986) found higher amylase activity during finger millet germination than other millets. Higher amylase activity leads to an elevated carbohydrate level in germinated samples when compared to non-germinated samples. Hence, our results strongly agree with the previous report (Malleshi and Desikachar 1986). Further increased carbohydrate in germinated finger millet was reported by Saleh et al. (2013). On the other hand fermented koozh samples showed low carbohydrate content. This observation confirms the utilization of the carbohydrate by the fermenting microbes. Carbohydrates are a major carbon source for fermenting microbes (Adams 1990). This observation is in conformity with previous reports that the carbohydrate content is decreased during fermentation in pearl millet (Khetarpaul and Chauhan 1990) and foxtail millet (Antony et al. 1996).
The results on glycoprotein in koozh are depicted in Table 2. Significant level of glycoprotein content was observed in all the koozh samples; lowest glycoprotein content was observed in koozh prepared from (P ≤ 0.05) non-germinated & fermented finger millet powder when compared to other preparations. When comparison was made between fermentation and non-fermentation processes the glycoprotein content of koozh was found to be more in non-fermented sample. Furthermore, this finding was confirmed by glycoprotein staining gel-method. The prominent glycoprotein bands were observed in the molecular range of 14.3-29 kDa (Fig. 2) in all koozh samples. The germinated samples showed more intensified bands when compared to those of non-germinated samples. However, analysis of the samples of fermented and non-fermented koozh showed prominent glycoprotein bands when compared to those of fermented koozh. The difference in glycoprotein in fermented samples may be perhaps due to the activity of fermenting microbial enzyme on glycoprotein in the sample.
Fig. 2.
Staining of processed and non processed koozh for glycoprotein through SDS-PAGE (Lane 1: Protein marker; Lane 2: Germinated & fermented koozh; Lane 3: Germinated & non-fermented koozh; Lane 4: Non-germinated & fermented koozh; Lane 5: Non-Germinated & non-fermented koozh)
The amino acid content was found to be increased (P ≤ 0.05) in fermented koozh of germinated and non-germinated grains (Table 2) when compared to that of non-fermented ones. This result suggests that during fermentation microbes degrade proteins into peptides and free amino acids due to increased amounts of proteolytic enzymes. Thus there is a marked increase in free amino acid content in fermented samples when compared to the non-fermented samples. Furthermore, Au and Fields (1981) reported that fermentation produces better essential amino acid composition as a result of de novo production of important amino acids. Similarly Saleh et al. (2013) brought out that fermentation increased the amino nitrogen level in samples through breakdown of proteins. Free amino acid content of our samples closely agrees with the earlier report on finger millet (Mbithi-Mwikya et al. 2000). All koozh samples showed significant level of calorific values. However, no statistical significance was observed between the samples (Table 2) (Saleh et al. 2013).
Mineral contents of koozh samples were tabulated in Table 3. The results showed that minerals such as calcium, potassium, phosphorous and sulphur exhibited high level in all preparations of koozh when compared to other minerals (chlorine, iron, manganese, magnesium, zinc, copper and silicon). Increased content of selenium (P ≤ 0.05) was noticed in germinated & non-fermented koozh whereas higher (P ≤ 0.05) concentration of silicon and sulphur in germinated & fermented koozh when compared to those of remaining methods. Similarly, iron content (P ≤ 0.05) increased significantly in germinated and fermented koozh as compared to that of non-germinated and non-fermented sample. This observation strongly agrees with previous reports (Saleh et al. 2013; Mamiro et al. 2001). Further, it was found that the germination of finger millet increased the mineral contents. Moreover, copper level is higher (P ≤ 0.05) in non-germinated koozh than that of germinated one. Similarly potassium also showed similar trend in different processed samples. However, other minerals such as calcium, chlorine, magnesium, manganese, phosphorous and zinc content did not show any significant variation among all the koozh samples.
Table 3.
Mineral contents of koozh prepared from different processed finger millet grains
| Minerals (mg/g of flour) | Germinated & fermented koozh | Germinated & non-fermented koozh | Non-germinated & fermented koozh | Non-germinated & non-fermented koozh |
|---|---|---|---|---|
| Calcium | 43.15 ± 2.3 | 43.91 ± 3.53 | 38.2 ± 3.27 | 40.45 ± 4.65 |
| Chlorine | 2.46 ± 3.16 | 2.23 ± 2.20 | 3.51 ± 3.15 | 3.96 ± 2.11 |
| Iron | 2 ± 0.3 | 1.44 ± 0.28 | 1.48 ± 0.16 | 1.29 ± 0.24 |
| Potassium | 28.54 ± 3.5$ | 30.66 ± 4.7$ | 36.46 ± 4.3 | 42.5 ± 4.2*# |
| Magnesium | 3.75 ± 0.51 | 3.65 ± 0.41 | 2.81 ± 0.22 | 3.7 ± 0.42 |
| Manganese | 1.48 ± 0.21 | 1.74 ± 0.21 | 1.6 ± 0.16 | 1.67 ± 0.13 |
| Phosphorous | 19.1 ± 2.1 | 21.6 ± 3.5 | 17.4 ± 2.3 | 20.4 ± 2.1 |
| Zinc | 0.66 ± 0.11 | 0.64 ± 0.12 | 0.72 ± 0.12 | 0.62 ± 0.11 |
| Copper | 0.74 ± 0.01@$ | 0.64 ± 0.04@$ | 7.62 ± 1.13*# | 6.28 ± 2.25*# |
| Selenium | 12.82 ± 1.4#@$ | 23.68 ± 0.42*@$ | 4.5 ± 0.41*# | 3.9 ± 4.1*# |
| Silicon | 6 ± 0.26#@$ | 5.1 ± 0.81*@ | 2.47 ± 0.43*# | 1.88 ± 0.1* |
| Sulphur | 19.38 ± 2.5#@$ | 12.32 ± 2.5* | 9.98 ± 2.3* | 11.9 ± 2.14* |
*represents significant difference at P ≤ 0.05 Germinated & fermented koozh
#represents significant difference at P ≤ 0.05 Germinated & non-fermented koozh
@represents significant difference at P ≤ 0.05 Non-germinated & fermented koozh
$represents significant difference at P ≤ 0.05 Non-germinated & non-fermented koozh
Antioxidant properties
Table 4 shows the total phenolic and flavonoid content of processed and non-processed finger millet koozh. No significant variation in total phenol and flavonoid contents has been observed in all the koozh samples; however, fermented samples showed increased values in comparison to non-fermented samples. Similarly, significant level of free radical scavenging activity was noticed in all the samples (Table 4). The fermented koozh showed an increase in free radical scavenging activity when compared to that of non-fermented samples. Koozh made from germinated or non-germinated grains with fermentation showed enhanced total phenol, flavonoid and free radical scavenging activity (P ≤ 0.05) when compared to that of non-fermented koozh. It is evident from the enhanced free radical scavenging activity that bound polyphenolic and flavonoid contents were liberated by the structural break down of cereal cell wall fibrils such as cellulose and hemicelluloses by fermenting microbes (Banu et al. 2011; Dordevic et al. 2010).
Table 4.
Antioxidant activity of koozh prepared from finger millet grains
| Particulars | Total phenol (mg/g of flour) | Total flavonoid (mg/g of flour) | Free radical scavenging activity (%) |
|---|---|---|---|
| Germinated & fermented koozh | 0.57 ± 0.5 | 0.13 ± 0.08 | 20.85 ± 2.2$ |
| Germinated & non-fermented koozh | 0.43 ± 0.02 | 0.11 ± 0.02 | 18.6 ± 1.1@ |
| Non-germinated & fermented koozh | 0.46 ± 0.03 | 0.26 ± 0.3 | 24.6 ± 1.2#$ |
| Non-germinated & non-fermented koozh | 0.22 ± 0.01 | 0.14 ± 0.03 | 15.65 ± 1.3*@ |
*represents significant difference at P ≤ 0.05 Germinated & fermented koozh
#represents significant difference at P ≤ 0.05 Germinated & non-fermented koozh
@represents significant difference at P ≤ 0.05 Non-germinated & fermented koozh
$represents significant difference at P ≤ 0.05 Non-germinated & non-fermented koozh
Food processing methods such as germination and fermentation have been practiced over the centuries and maintained to produce the final food products with more attractive flavor, and taste and further these methods are known to increase the qualitative value of nutrient of the food. Germination enhances the endogenous phytase activity in cereals through activation of intrinsic phytase, or de novo synthesis. Increased α-amylase activity in germination also increases hydrolysis of amylose and amylopectin to dextrose and maltose it enhances the nutrient values and energy levels in cereals. Fermentation process involves the use of microorganisms and their enzymes for the production of foods with distinctive quality features that are quite peculiar from the agricultural raw cereals. Fermentation may lead to significant enhancement in the nutrients of foods by increasing the protein digestibility through hydrolysis of proteins to free amino acids, increasing bio-availability of minerals and polyphenols through hydrolysis of complex agents such as phytate and oxalate, and elevating nutrient B-vitamins levels through microbial synthesis. Therefore traditional food processing and preparation methods can improve nutrients level of foods significantly.
Conclusion
In the present work, nutrient contents were investigated in the differently processed “koozh”. Germinated and non-fermented koozh has more macro-nutrients when compared to those of other preparations; on the other hand free amino acid, phytochemicals, micro-nurtients such as selenium and radical scavenging activity are higher in fermented koozh sample. In mineral analysis, major minerals such as calcium, potassium and phosphorous were higher in all preparations of koozh when compared to those of other mineral contents. But calorific value of koozh samples did not show any considerable difference among different samples. Our current study elucidates that all preparative methods of koozh showed significant level of macro and micro nutrients; however, these nutrients are higher in koozh prepared from germinated and fermented grains. It is evident in the koozh prepared from germinated grains brings out desirable nutritional changes due to break down of complex compounds into a simple form, where as fermentation intensifies the flavor and further simplify the protein and carbohydrate. Hence it may be concluded the koozh made from germinated and fermented grains is considered to be easily digestible and energetic drink.
Acknowledgments
The authors duly acknowledge the funding support from Indian Council of Medical Research (ICMR Ref: 52/13/2007) and Department of Science and Technology (NO.SR/FT/LS-63/2011 & DST-FIST), New Delhi, India. Central Instrumentation Facility, Pondicherry University and the first author (A. Subastri) also acknowledge the UGC, India for the financial assistance in the form UGC –BSR Fellowship. We thank Prof. B. Kannabiran (Retired), Department of Biochemistry and Molecular Biology, Pondicherry University, for his invaluable support in language editing.
References
- Adams MR. Topical aspects of fermented foods. Trends Food Sci Tech. 1990;1:141–144. doi: 10.1016/0924-2244(90)90111-B. [DOI] [Google Scholar]
- Antony U, Sripriya G, Chandra TS. Effect of fermentation on the primary nutrients in finger millet (Eleusine coracana) J Agric Food Chem. 1996;44:2616–2618. doi: 10.1021/jf950787q. [DOI] [Google Scholar]
- Antony U, Chandra TS. Microbial population and biochemical changes in fermenting finger millet (Eleusine coracana) World J Microb Biot. 1997;13:533–537. doi: 10.1023/A:1018561224777. [DOI] [Google Scholar]
- Au PM, Fields ML. Nutritive quality of fermented sorghum. J Food Sci. 1981;46:652–654. doi: 10.1111/j.1365-2621.1981.tb04937.x. [DOI] [Google Scholar]
- Banu I, Vasilean I, Aprodu I. Effect of Select Parameters of the Sourdough Rye fermentation on the activity of some mixed starter cultures. Food Biotech. 2011;25:275–291. doi: 10.1080/08905436.2011.617251. [DOI] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Devi PB, Vijayabharathi R, Sathyabama S, Malleshi NG, Priyadarisini VB. Health benefits of finger millet (Eleusine coracana L.) polyphenols and dietary fiber: A review. J Food Sci Technol. 2014;51:1021–1040. doi: 10.1007/s13197-011-0584-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekara A, Shahidi F. Antiproliferative potential and DNA scission inhibitory activity of phenolics from whole millet grains. J Funct Food. 2011;3:159–170. doi: 10.1016/j.jff.2011.03.008. [DOI] [Google Scholar]
- Chen L, Chen Q, Zhang Z, Wan X. A novel colorimetric determination of free amino acids content in tea infusions with 2, 4-dinitrofluorobenzene. J Food Comp Anal. 2009;22:137–141. doi: 10.1016/j.jfca.2008.08.007. [DOI] [Google Scholar]
- Chethan S, Sreerama YN, Malleshi NG. Mode of inhibition of finger millet malt amylase by the millet phenolics. Food Chem. 2008;111:187–191. doi: 10.1016/j.foodchem.2008.03.063. [DOI] [Google Scholar]
- Dordevic TM, Siler-Marinkovic SS, Dimitrijevic-Brankovic SI. Effect of fermentation on antioxidant properties of some cereals and pseudo cereals. Food Chem. 2010;119:957–963. doi: 10.1016/j.foodchem.2009.07.049. [DOI] [Google Scholar]
- Dubois MK, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28:350–356. doi: 10.1021/ac60111a017. [DOI] [Google Scholar]
- El Hag ME, Abdullahi HE, Nabila EY. Effect of fermentation and dehulling on starch, total polyphenols, phytic acid content and in vitro protein digestibility of pearl millet. Food Chem. 2002;77:193–196. doi: 10.1016/S0308-8146(01)00336-3. [DOI] [Google Scholar]
- Everitt AV, Hilmer SN, Brand-Miller JC, Jamieson HA, Truswell AS, Sharma AP, Mason RS, Morris BJ, Le Couteur DG. Dietary approaches that delay age-related diseases. Clin Inter Aging. 2006;1:11–31. doi: 10.2147/ciia.2006.1.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan AB, Ahmed IAM, Osman NM, Eltayeb MM, Osman GA, Babiker EE. Effect of processing treatments followed by fermentation on protein content and digestibility of Pearl millet (Pennisetum typhoideum) cultivars. Pakistan J Nutr. 2006;5:86–89. doi: 10.3923/pjn.2006.86.89. [DOI] [Google Scholar]
- Kang RK, Jain R, Mridula D. Impact of indigenous fiber rich premix supplementation on blood glucose levels in diabetics. Am J Food Technol. 2008;3:50–55. doi: 10.3923/ajft.2008.50.55. [DOI] [Google Scholar]
- Karovicova ZKJ. Fermentation of cereals for specific purpose. J Food Nutr Res. 2007;46:51–57. [Google Scholar]
- Kearney J. Food consumption trends and drivers. Phil Trans R Soc B. 2010;365:2793–2807. doi: 10.1098/rstb.2010.0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khetarpaul N, Chauhan BM. Improvement in HCl extractability of minerals from pearl millet by natural fermentation. Food Chem. 1990;37:69–75. doi: 10.1016/0308-8146(90)90046-7. [DOI] [Google Scholar]
- Kilcoynea M, Gerlacha JQ, Farrella MP, Bhavan VP, Joshi L. Periodic acid–Schiff’s reagent assay for carbohydrates in a microtiter plate format. Anal Biochem. 2011;416:18–26. doi: 10.1016/j.ab.2011.05.006. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Malleshi NG, Desikachar HSR. Influence of malting conditions on quality of finger millet. J Inst Brew. 1986;92:81–86. doi: 10.1002/j.2050-0416.1986.tb04377.x. [DOI] [Google Scholar]
- Mamiro PRS, Vancamp J, Mwiky SM, Huyghrbaert A. In vitro extractability of calcium, iron and zinc in finger millet and kidney beans during processing. J Food Sci. 2001;66:1271–1275. doi: 10.1111/j.1365-2621.2001.tb15200.x. [DOI] [Google Scholar]
- Mbithi-Mwikya S, Ooghe W, Van Camp J, Nagundi D, Huyghebaert A. Amino acid profile after sprouting, autoclaving and lactic acid fermentation of finger millet (Elusine coracana) and kidney beans (Phaseolus vulgaris L.) J Agric Food Chem. 2000;48:3081–3085. doi: 10.1021/jf0002140. [DOI] [PubMed] [Google Scholar]
- Nielson KK, Mahoney AW, Williams LS, Rogers BC. Mineral concentrations and variations in fast food samples analyzed by X-Ray Fluorescence. J Agric Food Chem. 1991;39:887–892. doi: 10.1021/jf00005a016. [DOI] [Google Scholar]
- Psomiadou E, Tsimidou M. Stability of Virgin Olive Oil. 1. Autoxidation Studies. J Agric Food Chem. 2002;50:716–721. doi: 10.1021/jf0108462. [DOI] [PubMed] [Google Scholar]
- Rao SMVSST, Muralikrishna G. Non-starch polysaccharides and bound phenolic acids from native and malted finger millet (Ragi, Eleusine coracana, Indaf-15) Food Chem. 2001;72:187–192. doi: 10.1016/S0308-8146(00)00217-X. [DOI] [Google Scholar]
- Saleh ASM, Zhang Q, Chen J, Shen Q. Millet grains: Nutritional quality, processing and potential health benefits. Compr Rev Food Sci F. 2013;12:281–295. doi: 10.1111/1541-4337.12012. [DOI] [Google Scholar]
- Shimada K, Fujikawa K, Yahara K, Nakamura T. Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J Agric Food Chem. 1992;40:945–48. doi: 10.1021/jf00018a005. [DOI] [Google Scholar]
- Shobana S, Krishnaswamy K, Sudha V, Malleshi NG, Anjana RM, Palaniappan L, Mohan V. Finger Millet (Ragi, Eleusine coracana L.): A review of its nutritional properties, processing, and plausible health benefits. Adv Food Nutr Res. 2013;69:1043–4526. doi: 10.1016/B978-0-12-410540-9.00001-6. [DOI] [PubMed] [Google Scholar]
- Zhishen J, Mengcheng T, Jianming W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999;64:555–559. doi: 10.1016/S0308-8146(98)00102-2. [DOI] [Google Scholar]