Abstract
The kinetics of cysteine and divalent ion modulation viz. Ca2+, Cu2+, Hg2+ of fruit bromelain (EC 3.4.22.33) have been investigated in the present study. Kinetic studies revealed that at pH 4.5, cysteine induced V-type activation of bromelain catalyzed gelatin hydrolysis. At pH 3.5, Ca2+ inhibited the enzyme noncompetitively, whereas, both K-and V-type activations of bromelain were observed in the presence of 0.5 mM Ca2+ at pH 4.5 and 7.5. Bromelain was inhibited competitively at 0.6 mM Cu2+ ions at pH 3.5, which changed to an uncompetitive inhibition at pH 4.5 and 7.5. An un-competitive inhibition of bromelain catalyzed gelatin hydrolysis was observed in the presence of 0.6 mM Hg2+ at pH 3.5 and 4.5. These findings suggest that divalent ions modulation of fruit bromelain is pH dependent.
Keyword: Ananas Comosus, Bromelain kinetics, Competitive inhibition, K-type activation, V-type activation, Uncompetitive inhibition
Introduction
Bromelain is a sulfhydryl enzyme (predominance of cysteine) obtained from stem and fruit of pineapple [Ananas comosus (L.) Merr.] (Hale et al. 2005; Hebbar et al. 2008; Dubey et al. 2013). Stem bromelain (EC 3.4.22.32) is a glycoprotein (mannose, fucose, xylose and N-acetylglucosamine), basic in nature with isoelectric pH 9.5 (Ota et al. 1964; Gortner and Singleton 1965; Yasuda et al. 1970) and exhibits a broad specificity for protein cleavage but prefers Z-Arg-Arg-|-NHMec amongst the small molecule substrates (Harrach et al. 1998; Hatano et al. 2002; Gaspani et al. 2002; Khan et al. 2003; Haq et al. 2005). Fruit Bromelain (E.C. 3.4.22.33) lacks carbohydrate moiety and is acidic with isoelectric pH 4.6 (Ota et al. 1964; Gortner and Singleton 1965). It hydrolyzes proteins with a broad specificity for peptide bonds and possesses specificity towards Bz-Phe-Val-Arg-|-NHMec with no action on Z-Arg- Arg-|-NHMec (Maurer 2001). The carbohydrate moiety of stem bromelain may contribute towards its functional stability at alkaline pH (Khan et al. 2003).
Bromelain is widely used in food, cosmetic, chemical and textile industries. It has been extensively used in food industry for baking, meat tenderization and to avoid browning of apple (Esnault 1995; Godfrey and West 1996). It is also used to improve dyeing properties of protein fibers in textile industry (Koh et al. 2006). In leather industry, bromelain is employed for skin pre-tanning, softening and bating (Walsh 2002). There are numerous therapeutic applications of the thiol protease in inflammation, arthritis, indigestion, hay fever, ulcers and pulmonary edema (Brien et al. 2004; Beuth 2008; Fitzhugh et al. 2008). Furthermore, it increases the absorption of antibiotics used for the treatment of bacterial infections (Bala et al. 2013).
Stem bromelain is activated by cysteine, hydrogen sulphide and sodium cyanide (Maurer 2001; Padma et al. 2012) and is inhibited by heavy metals i.e. mercury, silver, copper, cobalt, zinc and trans-epoxysu- ccinyl-L-leucylamido {4-guanidino} butane, etc. (McGarvey and Christoffersen 1992; Shukor et al. 2008; Masdor and Said 2011; Padma et al. 2012; Bala et al. 2013). In 2012, Marshall and Golden characterized bromelain from Morinda citrifolia (Noni) and observed the non-competitive inhibition of bromalein by HgCl2. Most of the kinetics studies have been performed on the enzyme extracted from the stem of Ananas comosus, and since it is known that kinetics depend on the source of enzyme and conditions such as pH or temperature or ionic environment, we had an interest to analyze the kinetic behavior of fruit bromelain in the presence of cysteine and divalent ions i.e. Ca2+, Cu2+ and Hg2+ in the present study.
Materials and methods
Extraction
Extraction of bromealin from fruit of Ananas comosus was carried out following the method as described by Gautam et al. (2010). Purely ripe pineapple fruits were taken, cleaned and cut into small slices and were homogenized and filtered. Sodium benzoate (0.6 g) was added to 300 ml of filtrate to obtain crude extract, which was used as the source of bromelain.
Protein estimation
Protein was determined following the method of Lowry et al. (1951) using bovine serum albumin as a standard.
Bromelain activity assay
Bromelain assay was performed by the method described by Moodie (2001). Gelatin was chosen as the substrate for the analysis of activity of bromelain. Bromelain was assayed by measuring digestion action on gelatin and was expressed as GDU.
Ammonium sulfate precipitation and dialysis
The crude extract was first subjected to ammonium sulfate precipitation and then dialysis was performed following the method of Gautam et al. (2010). Ammonium sulfate salt (6.6 g) was added to the extract (pinch by pinch), with constant stirring for 45 min and was incubated overnight at 4 °C. The precipitated enzymes were centrifuged at 10,000 rpm for 10 min at 4 °C. The pellet was separated and dissolved in 10 ml of 10 mM Tris HCl buffer and was dialysed against 100 mM phosphate buffer NaCl overnight at 4 °C with several changes of buffer.
Ion exchange chromatography
Bromelain was purified following modified method of Gautam et al. (2010). About 5 g of DEAE cellulose was suspended in 12 ml 0.5 M sodium phosphate buffer (pH 8.0). Poured thick slurry of DEAE cellulose along the sides of column and equilibrated the column with 0.5 M sodium phosphate buffer (pH 8.0), followed by elution with buffer A i.e. 25 mM Tris–HCl and 100 mM NaCl. The dialysed sample was then loaded and enzyme was eluted with elution buffer A. Eluate was collected in test tube and elution was done at a flow rate of 1 ml/min. The ion exchange eluates of fruit bromelain were then analyzed for the enzyme activity with GDU assay (Moodie 2001).
Kinetics studies
Effect of substrate concentration
Kinetic experiments were performed in triplicates by using 5–6 concentrations of gelatin covering from about 0.3 to 3 times the expected Km (i.e. 9.6, 19.2, 48.0, 67.2 and 86.4 mM) as was recommended by Cleland (1967) and kinetic parameters (Km and Vmax) were determined from Lineweaver Burk Plot.
Effect of cysteine and divalent ions (Ca2+, Hg2+ and Cu2+)
Bromelain activity was assayed in the absence and presence of cysteine (1, 2, 5, 7 and 9 mM), CaCl2 (0.1, 0.2, 0.3, 0.4 and 0.5 mM), CuSO4 (0.1 mM, 0.3 mM, 0.5 mM, 0.7 mM, 0.9 mM), and HgSO4 (0.3 mM, 0.6 mM, 1.0 mM, 1.3 mM, 1.6 mM). The gelatin hydrolysis by bromelain was also investigated at different pH (i.e. 3.5, 4.5 and 7.5) by assaying enzyme activity in the presence and absence of CaCl2 (0.5 mM), CuSO4 (0.6 mM) and HgSO4 (0.6 mM), and Km and Vmax were calculated using Lineweaver Burk plot.
Results and discussion
The crude extract of fruit bromelain was subjected to salt precipitation, dialysis and ion exchange chromatography using DEAE as anion exchanger resin. The concentration of protein and enzyme activity was analyzed in sample preparations after each purification step (Table 1). Specific activity was increased by 2.97 folds after dialysis, and 7.23 folds after ion exchange chromatography, this implies enhanced separation of bromelain from crude extract.
Table 1.
Enzyme activity of Bromelain in Ananas Comosus extract at different levels of purification
| Sample | Protein (mg/ml) | Enzyme activity (GDU/g) | Specific activity | Purification fold |
|---|---|---|---|---|
| Crude extract | 2.43 ± 0.41 | 844 ± 3.46 | 347.32 | 1.0 |
| Extract after salt precipitation and dialysis | 0.83 ± 0.056 | 857 ± 3.46 * | 1032.53 | 2.97 |
| Extract after ion exchange Chromatography | 0.45 ± 0.19 | 1130.6 ± 122.4* | 2512.44 | 7.23 |
Mean ± S.D (n = 3), * p < 0.05 vs crude extract
The enzyme activity of gelatin hydrolysis was 1000 GDU/g at the concentration 10–50 M. However, fruit bromelain activity was enhanced markedly (66.6 %) as the concentration of substrate was increased to 80 M (data not shown). The enzyme activity as a function of pH was also observed. Optimum pH for fruit bromelain for gelatin hydrolysis was pH 4–7, which corroborated the earlier observations that fruit bromelain has an effective pH of 3.0–8.0 (Ketnawa et al. 2011; Bala et al. 2012). At pH 5–8, the enzyme has hydrogen bond between thiol and imidazole functional group (i.e. Cys-His) and is critical for catalytic activity (Valle et al. 2008). Furthermore, the observed fruit bromelain-pH profile was consistent with the thiol proteases of papain family, which display a wide profile of maximum activity around neutral pH (Rowan et al. 1990).
Bromelain is a sulfhydryl enzyme (predominance of cysteine) and a free cysteine contributes to its maximum activity (Hale et al. 2005; Hebbar et al. 2008; Dubey 2013). There are reports available which suggest that bromelain is activated by cysteine predominately, while hydrogen sulphide and sodium cyanide are less effective in thiol protease activation (Maurer 2001; Padma et al. 2012). In the present study, fruit bromelain was incubated with cysteine and observed that cysteine enhanced the gelatin hydrolysis by bromelain in a concentration dependant manner (Fig. 1a). These results are consistent with the earlier reports (Jiang et al. 2007), which suggested that cysteine could act as both chelating and reducing agent, which could recover the activity of cysteine proteases either by removing metal ions or reducing their catalytic sites. The Michaelis-Menten constant (Km) and maximum reaction velocity (Vmax) were calculated by plotting the activity data obtained in the absence and presence of cysteine, as a function of substrate concentration in Lineweaver-Burk plot. The Km and Vmax values were 28.6 mM and 3333.3 GDU/g and 25 mM and 5000 GDU/g respectively, in the absence and presence of cysteine (Fig. 1a), which emphasized the enhanced gelatin hydrolysis with V-type activation in the presence of cysteine as depicted in Fig. 2a.
Fig. 1.
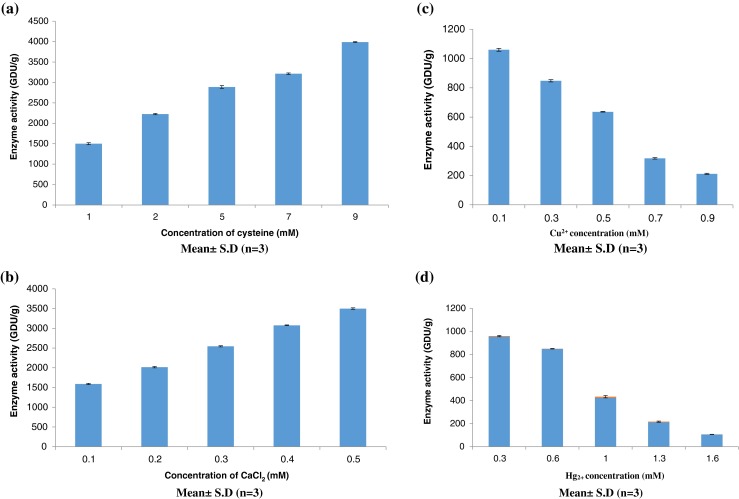
a) Effect of cysteine (1, 2, 5, 7 and 9 mM), (b) Ca2+ (0.1, 0.2, 0.3, 0.4 and 0.5 mM), (c) Cu2+ (0.1 mM, 0.3 mM, 0.5 mM, 0.7 mM, 0.9 mM), and (d) Hg2+ (0.3 mM, 0.6 mM, 1.0 mM, 1.3 mM, 1.6 mM) on the hydrolysis of gelain by bromelain extracted from Ananas comosus
Fig. 2.
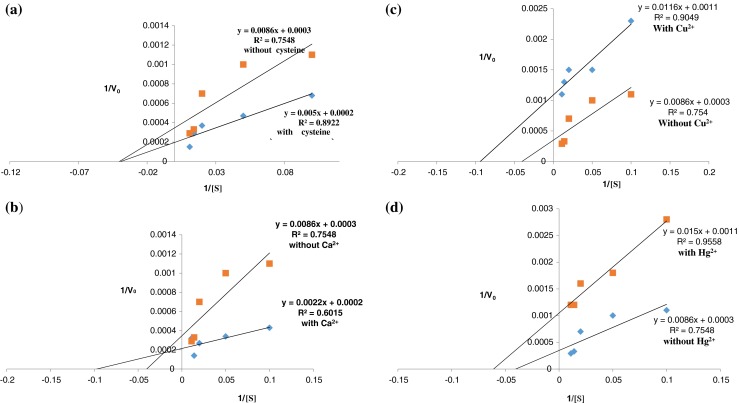
Lineweaver burk plots for the hydrolysis of gelatin in the presence of (a) cystiene, (b) Ca2+, (c) Cu2+, and (d) Hg2+ by bromelain extracted from fruit Ananas comosus. The same experiments were repeated atleast three times (Values are mean of three observations)
The effects of different concentrations of Ca2+ on fruit bromelain activity implied that the trend of the response to increasing ion concentration was consistent. These results corroborate the earlier observations ((Kaul et al. 2002; Wang et al. 2009), which implied that calcium ions promote the bromelain activity by stabilizing the secondary structure of enzyme. The hydrolysis of gelatin was enhanced by 37.52 % at 0.3 mM-0.5 mM Ca2+ ions (Fig. 1b). Interestingly, both K and V-type activations were observed with Km = 11 mM and Vmax = 5000 GDU/g in the presence of Ca2+ ions compared to Km = 28.6 mM and Vmax = 3333.3 GDU/g in controls (Fig. 2b). However, the coefficient of determination for lineweaver burk plot for Ca2+ i.e. R2 is 0.6015, which suggests about 60 % of the activation in bromelain from fruit Ananas comosus could be attributed to the presence of Ca2+ in reaction mixture. This is a low but reasonable value, and indicates a real relationship. The structure activity relationship of bromelain in the presence of Ca2+ ions may also be influenced by factors like electrostatic shielding of charge, salt effects on water structure, specific and nonspecific binding of protein molecule to ions present in the microenvironment of the enzyme (Haq et al. 2005).
Bromelain activity is inhibited by wide range of compounds like H2O2, potassium ferricyanide, potassium permanganate, trans-epoxysu- ccinyl-L-leucylamido {4-guanidino} butane, and metals like mercury, silver, copper, cobalt, zinc, etc. (McGarvey and Christoffersen 1992; Shukor et al. 2008; Padma et al. 2012). The inhibitory effect of Cu2+ on the gelatin hydrolysis activity of fruit bromelain is illustrated in Fig.1c. The enzyme activities were 848 GDU/g and 636 GDU/g at 0.3 mM and 0.5 mM Cu2+ ions. The protease activity was further reduced to 318 GDU/g and 212 GDU/g at 0.7 mM and 0.9 mM Cu2+ ions, implying that protease activity decreased markedly (66.6 %) as Cu2+ ions concentration was increased from 0.5 to 0.9 mM (Fig. 1c). The observed inhibitory effects of Cu2+ are in agreement to the earlier reports that Cu2+ inhibits the papain-like cysteine proteases by forming coordination bond with catalytic sulfhydryl group (Jiang et al. 2007; Shukor et al. 2008; Marshall and Golden 2012; Masdor and Said 2011). Lineweaver Burk plot for the reaction in sodium acetate buffer containing 0.6 mM Cu2+ suggested that Km and Vmax of enzyme were reduced from 28.6 mM to 3333.3 GDU/g in controls to 10.5 mM and 909 GDU/g in the presence of Cu2+, implying uncompetitive type of inhibition (Fig. 2c).
As depicted in Fig. 1d, the protease activities were 848 GDU/g and 424 GDU/g at 0.6 mM and 1 mM Hg2+ ions. Gelatin hydrolysis by fruit bromelain was further reduced to 212 GDU/g and 106 GDU/g at 1.3 mM and 1.6 mM Hg2+ ions which suggested that 50 % protease activity was reduced as the concentration of Hg2+ was enhanced from 1.3 to 1.6 mM in sodium acetate buffer. These results suggest that Hg2+ ions inhibited the fruit bromelain activity in concentration dependent manner. These reports corroborate the earlier contention that Hg2+ is a potent inhibitor of cysteine proteases (Jiang et al. 2007; Shukor et al. 2008; Marshall and Golden 2008). An uncompetitive inhibition was observed when the fruit bromelain activity was assayed in the presence of Hg2+ (Fig. 2d), which was in contrast to earlier observations (Marshall and Golden 2008), which suggested a change in Vmax with unaltered Km i.e. non-competitive inhibition of caesin hydrolysis by bromelain from Morinda citrifolia in the presence of HgCl2.
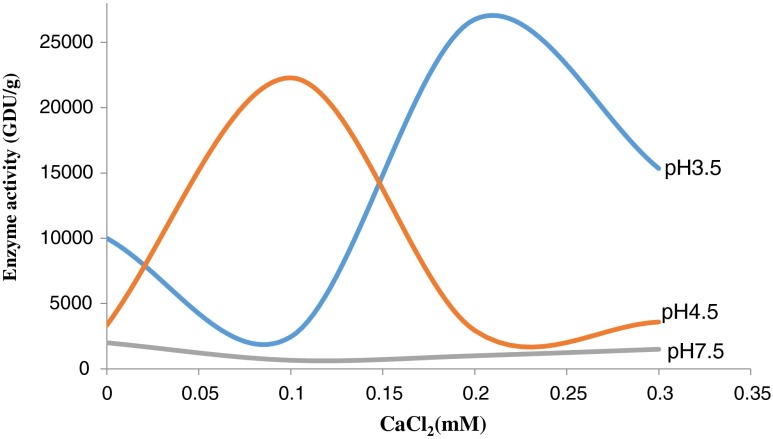
Fig. 3 illustrates the effects of 0.05–0.3 mM Ca2+ ions on fruit bromelain activity as the function of pH. At pH 3.5, 0.2 mM Ca2+ stimulated the enzyme activity by 167.59 % with subsequent decrease in enzyme activity at 0.3 mM Ca2+ ions. At pH 4.5, addition of 0.1 mM Ca2+ activated the enzyme by 568.5 %, but further increase to 0.3 mM markedly reduced the enzyme activity. However, no apparent change was observed in thiol protease activity at pH 7.5 (Fig 3).
Fig. 3.
pH dependent effect of Ca2+ ions on Bromelain (values are mean of 2 preparations). Enzyme assays were done in duplicate
Data showing the effects of divalent ions i.e. Ca2+, Cu2+ and Hg2+ on kinetic parameters of fruit bromelain as the function of pH is given in Tables 2, 3 and 4. As shown in Table 2, at pH 3.5, Km and Vmax were 11.5 mM and 5000 GDU/g at 0.5 mM Ca2+ ions compared to 15 mM and 10000 GDU/g at 0 mM Ca2+ ions, implying uncompetitive inhibition. However, both K-and V-type activations of enzyme were observed in the presence of Ca2+ at pH 4.5 and 7.5. In the presence of 0.5 mM Ca2+ at pH 4.5, Vmax increased to 5000 GDU/g from 3333.3 GDU/g (controls), but Km was reduced to 11 mM in the presence of 0.5 mM Ca2+ ions from 28.6 mM in controls (Table 2). Similarly, at pH 7.5, the activation of bromelain by Ca2+ ions was observed, with Vmax increased from 2000 GDU/g to 3333.3 GDU/g in presence of 0.5 mM Ca2+ with reduced Km i.e. 6.33 mM at 0.5 mM Ca2+ compared to 29.4 mM at 0 mM Ca2+ ions, suggesting both K and V- type activation.
Table 2.
Effect of Ca2+ on kinetic parameters of Bromelain at pH (3.5, 4.5, and 7.5)
| pH | Ca2+ = 0 mM | Ca2+ = 0.5 mM | ||
|---|---|---|---|---|
| Km (mM) | Vmax (GDU/g) | Km (mM) | Vmax (GDU/g) | |
| 3.5 | 15 ± 0.47 | 10000 ± 6.0 | 11.5 ± 0.05* | 5000 ± 5.03* |
| 4.5 | 28.6 ± 0.2 | 3333.3 ± 0.58 | 11 ± 0.100* | 5000 ± 1.15* |
| 7.5 | 29.4 ± 0.1 | 2000 ± 1.15 | 6.33 ± 0.01* | 3333.3 ± 0.75* |
Mean ± S.D (n = 3), p* < 0.001 vs control
Table 3.
Effect of Cu2+on kinetic parameters of Bromelain at pH (3.5, 4.5, and 7.5)
| pH | Cu2+ = 0 mM | Cu2+ = 0.6 mM | ||
|---|---|---|---|---|
| Km (mM) | Vmax (GDU/g) | Km (mM) | Vmax (GDU/g) | |
| 3.5 | 15 ± 0.47 | 10000 ± 6.0 | 35 ± 0.11* | 1000 ± 0.76* |
| 4.5 | 28.6 ± 0.2 | 3333.3 ± 0.58 | 11 ± 1.0* | 1000 ± 2.0* |
| 7.5 | 29.4 ± 0.1 | 2000 ± 1.15 | 15 ± 0.2* | 500 ± 1.39* |
Mean ± S.D (n = 3), p* < 0.001 vs control
Table 4.
Effect of Hg2+ on kinetic parameters of Bromelain at pH (3.5, 4.5, and 7.5)
| pH | Hg2+ = 0 mM | Hg2+ = 0.6 mM | ||
|---|---|---|---|---|
| Km (mM) | Vmax (GDU/g) | Km (mM) | Vmax (GDU/g) | |
| 3.5 | 15 ± 0.47 | 10000 ± 6.0 | 2.0 ± 0.1* | 1000 ± 2.0* |
| 4.5 | 28.6 ± 0.2 | 3333.3 ± 0.58 | 15 ± 0.17* | 1000 ± 0.87* |
| 7.5 | 29.4 ± 0.1 | 2000 ± 1.15 | 35 ± 0.2* | 1000 ± 3.0* |
Mean ± S.D (n = 3), Mean ± S.D (n = 3), p* < 0.001 vs control
Table 3 presents data on the effects of Cu2+ on kinetic parameters of fruit bromelain as the function of pH. At pH 3.5, Km was increased to 28.6 mM at 0.6 mM Cu2+ compared to 15 mM at 0 mM Cu2+. However, Vmax remained unchanged implying competitive type of inhibition for bromelain by Cu2+ at pH 3.5. However, at pH 4.5, both the Km and Vmax were reduced to 10.5 mM and 909 GDU/g in the presence of Cu2+ compared to 28.6 mM and 3333.3 GDU/g respectively (controls). Similarly, at pH 7.5, Km and Vmax were reduced to 15 mM and 500 GDU/g at 0.6 mM Cu2+ compared to 29.4 mM and 2000 GDU/g at 0 mM Cu2+. Thus uncompetitive inhibition by Cu2+ was observed at pH 4.5 and 7.5.
The effects of bromelain inhibitor i.e. Hg2+ ions were also analyzed as the function of pH and results obtained are shown in Table 4. At pH 3.5, Km and Vmax for fruit bromelain were decreased from 15 mM to 10000 GDU/g in controls to 2 mM and 1000 GDU/g in the presence of Hg2+ ions. However, at pH 4.5, Km and Vmax were 15 mM and 1000 GDU/g at 0.6 mM Hg2+ compared to 28.6 mM and 3333.3 GDU/g under control conditions, implying uncompetitive inhibition. At pH 7.5, Km was increased to 35 mM from 29.4 mM, with reduced Vmax i.e. 1000 GDU/g at 0.6 mM Hg2+ compared to controls (2000 GDU/g). However, Marshall and Golden (2008) suggested a non-competitive inhibition of bromelain from Morinda citrifolia at pH 7.1.
The present data implies that metal ion interactions with fruit bromelain from Ananas comosus are pH dependent Allen et al. (1978) suggested that ionizable groups which contribute towards papain catalysis are imidazole of His-159, thiol of Cys-25 and carboxyl of Asp-158 and papain displays several ionic pathways including acylation, deacylation or both, depending upon the ionic form of the active site, which is pH-dependent. Kinetic studies with stem bromelain by Valle et al. (2008) suggested that at pH 5–8, the enzyme has hydrogen bond between thiol and imidazole functional group (i.e. Cys-His) and is critical for its catalytic activity. It is well known that cysteine protease activities are sensitive to transition metal ions ((Jiang et al. 2007; Shukor et al. 2008; Marshall and Golden 2008; Masdor and Said 2011). Since Cu2+ and Hg2+ ions have been shown to bind at catalytic site of cysteine proteases (Jiang et al. 2007), and bromelain exhibits similar structure for active site to other cysteine proteases, the enzyme kinetics suggest that the metal ions interaction with fruit bromelain in Ananas comosus are influenced by pH, which may be due to ionization of some key amino acid residues in catalytic site. Nevertheless, future investigations are necessary to identify the metal binding sites of fruit bromelain, which could provide an insight into the mechanism of modulation of the Ananas comosus bromelain activity by metal ions.
Acknowledgments
The authors are grateful to the management of Lovely Professional University, Phagwara (India) for providing facilities to conduct the laboratory work.
Conflict of interest
There is no conflict of interest among authors of this publication.
Footnotes
Research highlights
1. We examined the kinetic behavior of fruit bromelain in the presence of activators i.e. cysteine and Ca2+, and inhibitors i.e. Cu2+ and Hg2+.
2. Bromelain activity is enhanced with V-type activation in the presence of cysteine, however, both K and V-type activations are observed in the presence of Ca2+ ions.
3. Cu2+ and Hg2+ ions inhibit the enzyme uncompetitively.
4. We examined the effects of divalent ions i.e. Ca2+, Cu2+ and Hg2+ on kinetic parameters of bromelain as the function of pH.
5. Metal ion interactions with bromelain from Ananas comosus are pH dependent.
References
- Allen KG, Stewart JA, Johnson PE, Wettlaufer DG (1978) Identification of the functional ionic groups of papain by pH/rate profile analysis. Eur J Biochem 87:575–582 [DOI] [PubMed]
- Bala M, Ismail NA, Mel M, Jami MS, Salleh HM, Amid A. Bromelain production: current trends and perspective. Arch Des Sci. 2012;65(11):369–399. [Google Scholar]
- Bala M, Mel M, Jami MS, Amid A, Salleh HM. Kinetic studies on recombinant stem bromelain. Adv in Enz Res. 2013;1(3):52–60. doi: 10.4236/aer.2013.13006. [DOI] [Google Scholar]
- Beuth J. Proteolytic enzyme therapy in evidence-based complementary oncology: fact or fiction? Integr Cancer Ther. 2008;7(4):311–316. doi: 10.1177/1534735408327251. [DOI] [PubMed] [Google Scholar]
- Brien S, Lewith G, Walker A, Hicks SM, Middleton D. Bromelain as a treatment for osteoarthritis: a review of clinical studies. Evid-Based Complem Altern med: eCAM. 2004;1(3):251–257. doi: 10.1093/ecam/neh035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland WW. The statistical analysis of enzyme kinetic data. Adv Enzymol Relat Areas Mol Biol. 1967;29:1–32. doi: 10.1002/9780470122747.ch1. [DOI] [PubMed] [Google Scholar]
- Dubey VK, Pande M, Singh BK, Jagannadham MV. Papain-like proteases: applications of their inhibitors. Int J Genet Eng. 2013;1(3):042–050. [Google Scholar]
- Esnault E. Beer stabilization with papain. Brew Guard. 1995;124:47–49. [Google Scholar]
- Fitzhugh DJ, Shan S, Dewhirst MW, Hale LP. Bromelain treatment decreases neutrophil migration to sites of inflammation. Clin Immunol. 2008;128(1):66–74. doi: 10.1016/j.clim.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspani L, Limiroli E, Ferrario P, Bianchi M. In vivo and in vitro effects of bromelain on PGE (2) and SP concentrations in the inflammatory exudate in rats. Pharmacol Res. 2002;65:83–86. doi: 10.1159/000056191. [DOI] [PubMed] [Google Scholar]
- Gautam SS, Mishra KS, Dash V, Goyal Amit K, Rath G. Comparative study of extraction, purification and estimation of bromelain from stem and fruit of pineapple plant Thai. J Pharm Sci. 2010;34:67–76. [Google Scholar]
- Godfrey T, West S. Industrial enzymes. 2. New York: Stocton Press; 1996. [Google Scholar]
- Gortner WA, Singleton V. Chemical and physical development of the pineapple fruit III: nitrogenous and enzyme constituents. J Food Sci. 1965;30(1):24–29. doi: 10.1111/j.1365-2621.1965.tb00256.x. [DOI] [Google Scholar]
- Hale LP, Greer PK, Trinh CT, James CL. Proteinase activity and stability of natural bromelain preparations. J Immunol. 2005;5(4):783–793. doi: 10.1016/j.intimp.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Haq SK, Rasheedi S, Sharma P, Ahmed B, Khan RH. Influence of salts and alcohols on the conformation of partially folded intermediate of stem bromelain at low pH. Int J of Biochem Cell Bio. 2005;37:361–374. doi: 10.1016/j.biocel.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Harrach T, Eckert K, Maurer HR, Machleidt I, Machleidt W, Nuck R. Isolation and characterization of two forms of an acidic bromelain stem proteinase. J Protein Chem. 1998;17(4):351–361. doi: 10.1023/A:1022507316434. [DOI] [PubMed] [Google Scholar]
- Hatano K, Sawano Y, Tanokura M. Structure-function relationship of bromelain isoinhibitors from pineapple stem. Biol Chem. 2002;383:1151–1156. doi: 10.1515/BC.2002.126. [DOI] [PubMed] [Google Scholar]
- Hebbar HU, Sumana B, Raghavarao KSMS. Use of reverse micellar systems for the extraction and purification of bromelain from pineapple waste. Bioresour Technol. 2008;99:4896–4902. doi: 10.1016/j.biortech.2007.09.038. [DOI] [PubMed] [Google Scholar]
- Jiang J, X-Da Y, Wang K. Inhibition of cysteine protease papain by metal ions and poysulfide complexex, especially mercuric ions. J Chin Pharma Sci. 2007;16:1–8. [Google Scholar]
- Kaul P, Sathish HA, Prakash V. Effect of metal ions on structure and activity of papain from Carica papaya. Nahrung. 2002;46(1):2–6. doi: 10.1002/1521-3803(20020101)46:1<2::AID-FOOD2>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Ketnawa S, Chaiwut P, Rawdkeun S. Extraction of bromelain from pineapple peel. Food Sci Tech Int. 2011;4(17):395–402. doi: 10.1177/1082013210387817. [DOI] [PubMed] [Google Scholar]
- Khan RH, Rasheedi S, Haq SK. Effect of pH, temperature and alcohols on the stability of glycosylated and deglycosylated stem bromelain. J Biosci. 2003;28:709–714. doi: 10.1007/BF02708431. [DOI] [PubMed] [Google Scholar]
- Koh J, Kang SM, Kim SJ, Cha MK, Kwon YJ. Effect of pineapple protease on the characteristics of protein fibers. Fibers Polym. 2006;7:180–185. doi: 10.1007/BF02908264. [DOI] [Google Scholar]
- Lowry OH, Osebrough JN, Farr AL, Randall RJ. Protein measurement with the Folin-phenol reagents. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Marshall S, Golden J. Characterization of bromelain from Morinda citrifolia (Noni) J Sci Res. 2012;4(2):445–456. [Google Scholar]
- Masdor NA, Said NAM. Partial purification of crude stem bromelain improves its sensitivity as a protease inhibitive assay for heavy metals. Aust J Basic Appl Sci. 2011;5(10):1295–1298. [Google Scholar]
- Maurer HR. Bromelain: biochemistry, pharmacology and medical use. Mol Life Sci. 2001;58:1234–1245. doi: 10.1007/PL00000936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarvey DJ, Christoffersen RE. Characterization and kinetic parameters of ethylene-forming enzyme from avocado fruit. J Biol Chem. 1992;267:5964–5967. [PubMed] [Google Scholar]
- Moodie P. Gelatin digestion unit analytical method. New York: Enzyme Development Corporation; 2001. [Google Scholar]
- Ota S, Moore S, Stein WH. Preparation and chemical properties of purified stem and fruit bromelains. Biogeosciences. 1964;3(2):180–185. doi: 10.1021/bi00890a007. [DOI] [PubMed] [Google Scholar]
- Padma PS, Jayakumar K, Mathai V, Chintu S, Sarath BK. Immobilization and kinetic studies of bromelain: a plant cysteine protease from pineapple (Ananas comosus) plant parts. Int J Med Health Sci. 2012;3:10–14. [Google Scholar]
- Rowan AD, Buttle DJ, Barrett AJ. The cysteine proteinases of the pineapple plant. Biochem J. 1990;266:869–875. [PMC free article] [PubMed] [Google Scholar]
- Shukor MY, Masdor N, Baharom NA, Jamal JA, Abdullah MPA, Shamaan NA, Syed MA. An inhibitive determination method for heavy metals using bromelain, a cysteine protease. Appl Biochem Biotechnol. 2008;144(3):283–291. doi: 10.1007/s12010-007-8063-5. [DOI] [PubMed] [Google Scholar]
- Valle DM, Bruno M, Laura LMI, Caffini NO, Cantera AMB. Granulosain I, and a cysteine Prote-ase isolated from ripe fruits of solanum granuloso-lep-rosum (Solanaceae) Protein J. 2008;27:267–275. doi: 10.1007/s10930-008-9133-4. [DOI] [PubMed] [Google Scholar]
- Walsh G. Protein biochemistry and biotechnology. New York: John Wiley and Sons; 2002. [Google Scholar]
- Wang X, Liu Z, Hu X, Huang H (2009) Effects of Ca ~ (2+) on thermo stability and secondary structure of bromelain. Chin Food Addit doi:cnki:sun:spkj.0.2009-01-049
- Yasuda Y, Takahashi N, Murachi T. The composition and structure of carbohydrate moiety of stem bromelain. Biogeosciences. 1970;9:25–32. doi: 10.1021/bi00803a004. [DOI] [PubMed] [Google Scholar]