Abstract
This study aimed to evaluate impingement drying (ID) as a rapid drying method to dry wet apple pomace (WAP) and to investigate the fortification of dried apple pomace flour (APF) or WAP in bakery and meat products. ID at ~110 °C reduced the moisture content of apple pomace from 80 % (wet basis) to 4.5 % within 3 h, compared with 24 h to 2.2 % using 40 °C forced-air drying and ~60 h to 2.3 % using freeze drying. Furthermore, ID enhanced the extractable phenolic compounds, allowing for a 58 % increase in total phenolic content (TPC) compared with wet pomace, a 110 % and 83 % higher than TPC in forced-air dried and freeze dried samples, respectively. The 15–20 % APF-fortified cookies were found to be ~44–59 % softer, ~30 % more chewy, and ~14 % moister than those of the control. WAP-fortified meat products had significantly higher dietary fiber content (0.7–1.8 % vs. 0.1–0.2 % in control) and radical scavenging activity than that of the control. These results suggest that impingement drying is a fast and effective method for preparing dried APF with highly retained bioactive compounds, and apple pomace fortified products maintained or even had improved quality.
Keywords: Apple pomace, Impingement dry, Dietary fibers, Functional bakery and meat products
Introduction
Apple pomace is the primary byproduct generated from the production of apple juice (Lu and Yeap Foo 2000), with conventional juice processing generating over 300 g of pomace for every liter of juice (Kaushal et al. 2002). It was estimated that about 1.3 million metric tons of apple pomace are produced in more than 500 juice processing plants in the United States per year (Chatanta et al. 2007), and about $10 million in the US alone was spent annually on the disposal of apple pomace (Shalini and Gupta 2010). Despite being a byproduct, apple pomace contains high amount of bioactive compounds, such as dietary fiber and polyphenols, which can provide high value for its applications in food and other fields for generating value-added products, such as enzymes, single cell protein, aroma compounds, ethanol, organic acids, polysaccharides, and mushrooms (Vendruscolo et al. 2008).
Currently, the primary usage of apple pomace is livestock feed. Some efforts have been made for increasing the value-added usage of apple pomace, such as producing pectin (Canteri-Schemin et al. 2005; Garna et al. 2007) and adding in different types of bakery products (Masoodi and Chauhan 1998; Rupasinghe et al. 2008; Sivam et al. 2011; Sudha et al. 2007; Wang and Thomas 1989), but few if any have seen widespread adoption due to storage/spoilage issues of the wet pomace. Fresh (wet) apple pomace is highly perishable due to its high moisture (66.4–78.2 %) and sugars including glucose, fructose, sucrose, and xylose (48.0–62.0 %) (Joshi and Attri 2006; Kennedy et al. 1999; Smock and Neuber 1950). While refrigeration and freezing can be applied to extend storage time, the energy and space required make them not economically feasible and practical. The other possible solution is drying by reducing the moisture contents to ~10 %, providing longer storage period and reducing storage space and transportation costs, but is not without its own concerns.
The biggest concern with drying apple pomace is that the bioactive compounds in apple pomace are sensitive to heat and oxygen. This phenomena is not unique to apple pomace, and as such different drying methods have been studied in various fruit pomace and vegetables (Bai et al. 2013; Choicharoen et al. 2009; Constenla et al. 2002; de Torres et al. 2010; Khanal et al. 2010; Raghavan and Orsat 2007; Tseng and Zhao 2012; Yemmireddy et al. 2013). Khanal et al. (2010) reported that drying grape and blueberry pomaces at lower temperature for longer time (40 °C for 72 h) did not cause significant losses in anthocyanins, while higher temperature drying (125 °C) for a much shorter time (8 h) led to 70 % reduction. Similarly, the bioactive compounds of garlic decreased with increasing drying temperature, even at relatively mild range (40 to 60 °C) (Mendez et al. 2005). Likewise, the amount of volatile compounds, phenolic contents, anthocyanins, and flavonols in grape skins were significantly lower in oven-dried samples in contrast to freeze-dried samples (de Torres et al. 2010) and our previous study in wine grape pomace also found that freeze drying retains the highest level of bioactive compounds and DPPH radical scavenging activity compared with 40 °C conventional, vacuum oven, and 25 °C ambient air drying methods (Tseng and Zhao 2012). While freeze drying seems ideal, it can result in quality loss, as Tambunan et al. (2001) indicated that the quality of the freeze-dried pomace is slightly lower than that of fresh material, but still remain considerably higher than that of oven dried samples. Still, the biggest drawback for both forced-air and freeze drying methods is the amount of time required, as much as 48 and 60 h, respectively, to reduce moisture content to ~5–6 % (Tseng and Zhao 2012). Plus, freeze drying is 4–8 times more expensive than hot-air drying depending on the type of the raw materials, the capacity of the plant, duration of cycle, etc. (Ratti 2001), thus not economically feasible for drying low value byproducts.
Impingement drying (ID) offers “a novel alternative to flash dryers for high-moisture particulate materials” (Choicharoen et al. 2009). ID uses high velocity streams of hot air across the product being dried, which are then circulated back across the heating element. These intensive streams of hot air result in a very efficient drying, which has been shown to be significantly faster than forced-air drying for blueberries (Yemmireddy et al. 2013). Further, like all drying processes, there is a relationship between temperature and drying time, with an 85 °C ID taking about 50 % longer than a 107 °C ID (Yemmireddy et al. 2013). Even partial treatment with hot air impingement has also been shown to have beneficial effects, with one study showing that a 90 s treatment at 110 °C not only extensively decreased drying time, but also effectively inhibited enzymatic browning, making it an ideal pretreatment method for enhancing both the drying kinetics and quality of raisins (Bai et al. 2013). Since enzymatic browning is primarily caused by polyphenol oxidase and peroxidase (Kader et al. 1997), the ability of short ID treatment to inactivate the enzymes makes it quite attractive as a means of more quickly producing a dried apple pomace with improved bioactive properties.
Apple pomace flour (APF) or wet apple pomace (WAP) can partially substitute wheat flour or meat in bakery or meat products, respectively to enhance dietary fiber and bioactive compounds in the products. This innovative approach to create functional food items could not only increase the value of the byproduct from apple juice processing, but also allows commonly consumed products with enhanced health benefits.
This study investigated the feasibility of impingement drying as a means of producing dried apple pomace flour, comparing it with the conventional forced-air and freeze drying by determining the drying rate and quantifying the total phenolic contents, and radical scavenging activity along with dietary fiber profiles in dried pomace samples. In addition, the effect of partial substitution of wheat flour with APF and meat content with WAP on the textural, physicochemical, and biological properties was studied.
Materials and methods
Preparation of dried apple pomace
WAP which had not been treated by pectic enzymes was obtained from a local juice processor (Hood River Juice Co., Hood River, OR, USA), and frozen immediately at −24 °C until usage. Frozen apple pomace was subjected to three different drying treatments: forced air drying at 40 °C (Model STM 40, Precision Scientific Inc., Buffalo, NY, USA), ID at 110 °C (Lincoln Impinger, Foodservice Product Inc. Cleveland, OH, USA), and freeze drying at −55 °C at an absolute pressure of 17.33 Pa (Model 651 m-9WDF20, Hull Corp., Hatboro, PA, USA). The forced-air and freeze drying conditions were chosen based on our previous studies in wine grape pomace (Tseng and Zhao 2012), while the ID conditions were selected after preliminary trials on apple pomace. Water loss (%) was monitored during forced-air and impingement drying, but not freeze drying due to practical constraints.
Impingement dried pomace was ground into flour using a laboratory mill (Thomas-Wiley model 4, Thomas Scientific, NJ, USA) fitted with a 0.5 mm screen. Apple pomace flour (APF) was vacuum-packed using a FoodSaver 1075 (Tilia Inc., USA) in multi-ply bag (FoodSaver Roll, Tilia Inc., USA) and stored in a −24 °C freezer before further use.
Total extractable pectin
Prior to assay, all pomace samples were dried in an environmental chamber (T10RS, Tenney Environmental, Williamsport, PA, USA) at 10 % RH and 70 °C for 48 h to reach final moisture content of ~8.5 %.
For determining the total extractable pectin (TEP), extractable pectins were fractionated into water soluble pectin (WSP), chelator soluble pectin (CSP), and hydroxide soluble pectin (HSP) (Silacci and Morrison 1990). Pomace powder, 1.0 g, was homogenized (PT 10–35, Kinematica, Littau, Switzerland) in 20 g deionized water for 10 min, which was then filtrated (Whatman No. 1 filter paper). WSP was obtained from the precipitation by adding ethanol (95 %) to the filtrate (5:1) and allowing it to stand overnight in the refrigerator. The residue was boiled with 95 % ethanol for 10 min, followed by three successive extractions with 50 mL of 20 mM Na2-EDTA at pH 8.0. The suspension was filtered and the filtrates were combined for CSP. The residue was extracted again with 50 mL of 50 mM NaOH for 15 min at room temperature and filtered for HSP. For measuring the fractionized WSP, CSP, and HSP, a 250 μL of boric acid-sodium chloride solution (prepared with 3 g of boric acid and 2 g of sodium chloride in 100 mL distilled water) and 250 μL of collected samples (or standard) were mixed with 4 mL of 96 % H2SO4 and incubated at 70 °C for 40 min. A 200 μL of dimethyphenol reagent (100 mg of 3, 5-dimethyphenol solubilized in 100 mL of glacial acetic acid; Sigma Chemical Co., St. Louis, MO, USA) was then added and mixed, and the absorbance of the mixture was read at 400 nm and 450 nm, respectively using a spectrophotometer (Model UV160U, Shimadzu Corporation, Kyoto, Japan). WSP, CSP, and HSP was quantified as galacturonic acid equivalents (GUAE), and the sum of quantified WSP, CSP, and HSP were presented as TEP (AOAC 2007).
Dietary fiber profiling
Soluble dietary fiber (SDF) was determined as the sum of uronic acid (UA) and neutral sugar (NS), and insoluble dietary fiber (IDF) as the sum of klason lignin (KL), UA, and NS following the method of Deng et al. (2011). Results were expressed as percentage (%) dry mass (DM).
To separate SDF and IDF, 0.5 g of dried pomace sample was defatted by two successive extractions with 25 mL of petroleum ether for 10 min in an ultrasonic water bath at room temperature; the liquid and solid phases being separated by vacuum filtration using 11 μm filter paper (Whatman No. 1, GE Healthcare Life Sciences, Piscataway, NJ, USA). The retentate was extracted three times using 80 % ethanol in order to remove any soluble lower molecular weight saccharides. The resulting residue was dried at 40 °C for 16 h, followed by treatment with 0.0275 mL of protease (P-5459, Sigma Chemical Co., MO, USA) in 0.05 M phosphate buffer (pH 7.5) at 60 °C for 30 min (AOAC 1985; Bravo and Saura-Calixto 1998). After enzyme treatment, the mixture was filtered through a fresh 11 μm filter paper with the permeate being saved for the determination of SDF. The retentate was then washed with two portions of 10 mL deionized water, and the permeate was combined with the previously saved permeate for SDF determination. The solid material remaining on the filter paper was washed once with 95 % ethanol and then twice with acetone before drying at 40 °C for 16 h (Prosky et al. 1985). This dried residue was used for IDF analysis.
For determining SDF, NS content was analyzed based on the anthrone method, in which 1 mL of sample solution was thoroughly mixed with 2 mL of 75 % H2SO4 and 4 mL of anthrone reagent (0.5 g anthrone (Alfa Aesar, Ward Hill, MA, USA) solubilized in 250 mL of 75 % H2SO4 and incubated at 100 °C for 15 min before cooling to room temperature. The absorbance of the resulting hydrolysate at 578 nm was measured using a spectrophotometer after Gerhardt et al. (1994). Absorbance values were converted to glucose equivalent (GE) using a calibration curve prepared with a D-glucose (Sigma Chemical Co., St. Louis, MO, USA). Aliquots of the same hydrolysate were also used to determine UA fraction of SDF, quantified as GUAE using the same method described above for TEP determination.
To determine IDF, the dry residue was subjected to two successive steps of hydrolysis, in which the first hydrolysis was carried by adding 3 mL of 72 % sulfuric acid to the residue and incubated at 30 °C for 1 h, followed by the second hydrolysis of the residue with 86 mL of 2.5 % sulfuric acid in an autoclave at 121 °C for 1 h. The hydrolyzed mixture was then filtered using fritted crucible (Pyrex®30 mL M, Corning, Inc., USA), and the permeate was subjected to the same assays for UA and NS as described above. The retentate in the crucible was used for the determination of KL based on mass measurement after drying the residue at 105 °C for 16 h and subtracted the mass after ashing for 5 h at 525 °C. IDF was calculated as the total of NS, UA, and KL in the hydrolyzed mixture.
Analysis of bioactive compounds
Samples were extracted three times using 70 % acetone/0.1 % HCl/29.9 % water (v/v/v) at a solvent to pomace powder ratio of 4:1 (v/w) (Deng et al. 2011). To aid in extraction, the mixture was placed in ultrasonic unit (Branson B-220H, SmithKline Co., USA) for 60 min, and centrifuged at 10,000 g for 15 min. The supernatant was decanted and the subsequent extraction performed. The supernatants from each extraction were combined and the organic solvents were evaporated under vacuum using a rotary evaporator (Roto-vap, Brinkman Instruments, Westbury, NY) at 40 °C. Extract volume was standardized to 200 mL using deionized water and 1.5 mL aliquots of the standardized solutions were stored in a −24 °C freezer until analysis.
Total phenolics content (TPC) was measured by Folin-Ciocalteu colorimetric assay as described by Singleton and Rossi (1965) by reacting the diluted extract with Folin-Ciocalteu reagent (Sigma Chemical Co., MO, USA) for 10 min, and then incubating with 20 % NaCO3 in a 40 °C water bath for 15 min. Absorbance was measured spectrometrically at 765 nm. Gallic acid (Sigma Chemical Co., USA) was used to construct a standard curve, and results are expressed as mg gallic acid equivalents (GAE)/100 g DM.
Radical scavenging activity (RSA) was evaluated using 1,1-diphenyl-2-picryhydrazyl (DPPH) radical (Brand-Williams et al. 1995) by reacting the diluted extract with a standardized DPPH solution (9 mg DPPH in 100 mL of methanol) for 10 min at room temperature. Ascorbic acid (Mallinckrodt Baker Inc., St. Louis, MO, USA) was applied as a standard and the results are expressed as mg ascorbic acid equivalents (AAE)/100 g DM at absorbance of 517 nm.
Preparation of bakery products
Two different amounts (15 and 20 %) of apple pomace were incorporated in the bakery products based on our preliminary studies by considering the minimal impact on the appearance, color and texture of the products. Cookies were prepared by following the formula and procedures from Nishibori and Kawasihi (1992) with the formula for control as: 135 g wheat flour, 60 g sugar, 75 g butter, 30 g eggs, and 1.5 g baking soda. A 15 and 20 % APF were substituted for wheat flour. The cookies were round in shape with diameter of 30 mm and thickness of 5 mm and baked in an electric oven at 150 °C for 10 min.
Muffins were prepared using the formulation described in the Better Homes and Garden New Cook Book (2012) with the formula of control as: 259.30 g fine wheat flour, 74.25 g sugar, 10 g baking powder, 1.43 g salt, 60 g eggs, 182.25 g milk, and 54.42 g cooking oil. Again, 15 and 20 % APF were substituted for wheat flour. Dry ingredients (flour, sugar, baking powder, and salt) and wet ingredients (egg, milk, and oil) were mixed thoroughly in separate bowls. The contents of the two bowls were then combined and stirred lightly. Portions of the resultant batter were poured into muffin liners about 2/3 full and baked for 26 min at 175 °C, with a rotation of the pan occurring halfway through the baking time in order to ensure more even cooking. The muffins were allowed to cool in the pan for 10 min, before removal from the liners and final cooling on an elevated wire rack. Prepared bakery products were packed in the zip-lock bags and stored at room conditions for 1 day, and then used for physicochemical property analysis.
Preparation of meat products
Two different amounts (10 and 20 %) of apple pomace were incorporated into the meat products based on the same reasons as stated for the bakery products. Chicken patties were prepared by following the formulation and procedure of (Naveena et al. 2006; Verma et al. 2010) with slight modification. The basic formula of chicken patties was: 75.14 g lean meat, 2.00 g sodium chloride, 0.50 sodium hexametaphosphate, 0.015 g sodium nitrite, 6.50 g ice flake, 1.50 g egg white, 7.00 g oil, 0.75 g carrageenan, 0.10 sodium alginate, and 2.00 g wheat flour. WAP of 10 and 20 % was substituted for chicken meat. Chicken meat was cut into small cubes and minced using a food processor. Precisely weighed ingredients were mixed for 2 min in a mixer, manually formed into an approximately 50 (diameter) ×10 mm (thickness) patty, cooked for 35 min to achieve an internal temperature of about 85 °C, and stored at 4 °C for quality analysis.
Beef jerky was prepared as follows: WAP and ground beef was passed through ¼” plate equipped grinder. Ground beef (454 g) were cured by mixed with commercially available spices consisting of salt, sugar, sodium nitrite, propylene glycol, and silicon dioxide (NESCO®/American Harvest®, WI, US) and added with mixed ingredients including dextrose, spices, salt, natural hickory smoke flavor, monosodium glutamate, hydrolyzed soy protein, dehydrated onion, dehydrated garlic, caramel color, soybean oil, and silicon dioxide (NESCO®/American Harvest®, WI, US). A 10 or 20 % WAP was substituted for ground beef (w/w). All cured muscle sample mixture were dried by using impingement drying oven (~110 °C) for 30 min. After cooling down in the ambient temperature, physicochemical and textural properties were measured in the same day. Samples were also vacuum packaged (FoodSaver Vacuum Sealer 1075, Tilia Inc., USA) (FoodSaver Roll, Tilia Inc., USA) and stored at −24 °C for bioactive compounds assay.
Physicochemical properties of bakery and meat products
Color of each baked products were measured using a colorimeter (Minolta, Model EC-10, Konica Minolta Holdings, Tokyo, Japan) by randomly taken on the top crust. The color parameters, L* (lightness), a* (redness), and b* (yellowness) were recorded, and color change (ΔE) of APF-fortified products in comparison with the control ones was calculated as
where L2, a2, and b2 were color values of APF-fortified baked goods, and L1, a1, and b1 were for non-fortified controls.
Texture analysis was conducted on a TA-XT2 Texture Analyzer fitted with a 25 kg load cell (Stable Micro Systems, Texture Technologies Corp., Scarsdale, NY, USA). Muffins and cookies were tested for firmness and springiness. Muffin samples were prepared by cutting 2.0 cm wide vertical cross sections outwards from the center of each muffin, while cookies were tested as a whole, with both samples being subjected to a single compress and hold test using a 25 mm diameter cylindrical probe (Texture technologies Corp., Model TA-3) as described by Acosta et al. (Acosta et al. 2011). Samples were compressed 25 % of their initial height at a probe speed of 1.0 mm/s and then held for 60 s. Firmness was calculated as the maximum force achieved at the first compression in grams, while springiness was determined as the ratio of the force at 60 s to the maximum force. For chicken patties, the textural profile analysis (TPA) was performed using central cores of two pieces of each sample, which were compressed twice to 50 % of the original height, allowing 5 s between the two compression cycles. The following parameters were quantified: hardness (N, maximum force required to compress the sample), springiness (ability of the sample to recover its original form after deforming force was removed), adhesiveness (N/s, area under the abscissa after the first compression), and cohesiveness (extent to which the sample could be deformed prior to rupture) (Bourne 2002). For beef jerkies, shear force was measured by a knife blade with sharp 45° chisel end (TA-42). Maximum force measured to cut the sample was expressed as grams (g).
Experimental design and statistical analysis
A completely randomized design (CRD) with three different drying methods (40 °C forced-air drying, freeze drying, and 110 °C impingement drying) and three different APF substitution levels in each bakery and meat product (0, 15, and 20 % for cookies and muffins and 0, 10, and 20 % for chicken patty and beef jerky) were applied. All the experiments were performed in triplicate, and the resultant data were analyzed for statistical significance via multi-way analysis of variance (ANOVA), with post hoc testing using least significant difference (LSD) by means of statistical software (SAS v 9.2, The SAS Institute, USA). Results were considered to be different if p < 0.05.
Results and discussion
Dietary fiber profiling of apple pomace
TEP including HSP, CSP, and WSP are reported in Table 1. TEP of apple pomace was ~6.60 %, with CSP and HSP predominating, ~2.69 and 2.33 %, respectively as compared with WSP (~1.59 %). It should be noted that the amount of TEP depends on the analytical method employed. Enzymatic assay (AOAC 2007) and total pectin yield analysis (Canteri-Schemin et al. 2005) were the two common methods with the late one allowing for the measurement of tightly bound pectin that are less able to be hydrolyzed by the former method. Hence, TEP measured in this study using the enzymatic assay was relatively lower than that obtained from previous studies. According to Garna and others (2007), extraction at lower pH, higher temperature, and longer time exerted higher pectin yield (8.9 g/100 g DM) in comparison with that at relatively milder conditions (2.9 g/100 g DM). Part of these differences could also be explained by the differences in particle size, as at least one previous study demonstrated that smaller particle sizes increase the pectin yield, with pomace flour having much higher value (9.73 g/100 g DM) than unground pomace (6.13 g/100 g DM) (Canteri-Schemin et al. 2005).
Table 1.
Dietary fiber (DF) profiling of dried apple pomace+
| Compositions of dietary fiber | ||||
| Uronic acid (%) | Neutral sugar (%) | Klason lignin (%) | Total amounts (%) | |
| Soluble DF (SDF) | 0.15 ± 0.08 | 1.64 ± 0.58 | – ++ | 1.79 ± 0.63 |
| Insoluble DF (IDF) | 12.38 ± 2.65 | 23.50 ± 3.30 | 24.04 ± 2.85 | 59.92 ± 6.52 |
| Total amounts (%) | 12.53 ± 2.60 | 25.14 ± 3.30 | 24.04 ± 2.85 | 61.71 ± 6.06 |
| Compositions of extractable pectin | ||||
| Water soluble pectin (WSP, %) | Chelator soluble pectin (CSP, %) | Hydroxide soluble pectin (HSP, %) | Total amounts (%) | |
| Extractable pectin (%) | 1.59 ± 0.10 | 2.69 ± 0.29 | 2.33 ± 0.07 | 6.60 ± 0.25 |
| Total dietary fiber (TDF, %) | 68.32 ± 3.44 | |||
+Apple pomace sample was dried at 10 % RH and 70 °C for 48 h to reach final moisture content of ~8.5 %
++ Klason lignin was not able to be determined in soluble dietary fiber
TDF, SDF, and IDF values of dried apple pomace are presented in Table 1. TDF was 68.3 % which was similar to the 64.5 % found in pomace from cider processing (Bibbin-Martinez et al. 2011) and within the range of 60.7–89.8 % found in pomace of three different apple cultivars (Royal Gala, Granny Smith, and Liberty) (Figuerola et al. 2005). IDF content of our samples was ~59.9 % and comprised of roughly equal levels of NS and KL (~23.5 % and ~24.0 %, respectively) with slightly lower level of uronic acid (UA) (~12.4 %). The IDF value in this study agreed with those in the literatures, in which the IDF content of pomace from cider processing was 54.5 % (Bibbin-Martinez et al. 2011) and that from different apple cultivars (again, Royal Gala, Granny Smith, and Liberty) ranged from 56.5 to 81.6 % (Figuerola et al. 2005). SDF content in our samples was approximately 1.79 %, consisting primarily of NS (~1.64 %) and low level of UA (~0.15 %), and these values were much lower than previously reported ones, such as 8.0 % in apple pomace from cider processing (Bibbin-Martinez et al. 2011), and 4.1–14.3 % in pomace from Royal Gala, Granny Smith, and Liberty apples (Figuerola et al. 2005). These differences might be due to the different processing methods applied to extract the juice, particularly whether enzymes were used to aid the extraction. Apples were not subjected to enzymatic treatment before juicing in this study, thus water-soluble fiber (i.e. pectin) was more readily transferred into juice part instead of remain in pomace (solubilized in juice first, but precipitated later). Hence, apple pomace could have less amount of water soluble fiber. i.e., pectin. The study by Figuerola et al. (2005) used apples which had been cored prior to juicing, while the study by Bibbin-Martinez et al. (2011) relied upon commercial by-products which could have been treated with enzymes to improve juice extraction. Further, given the range of values across the cultivars (where known), it was entirely possible that cultivar composition also contributed to the differences seen.
Impacts of different drying methods
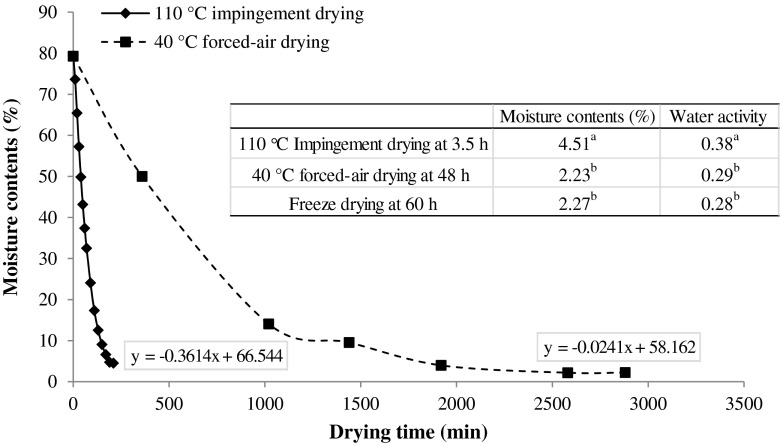
The rate of water loss (%) of pomace during forced-air drying (48 h) and ID (4 h) are illustrated in Fig. 1. In the regression equation, the slope of ID (0.3614) was significantly greater than that of forced-air dry (0.0241), demonstrating that ID had much faster drying rate than that of forced-air drying. Although MC and Aw of ID samples were slightly higher than those of forced-air and freeze dried ones (Fig. 2), the Aw range (0.28–0.38) of all samples were significantly lower than 0.75, a critical value for the prevention of phytochemical degradation during storage and for inhibition of growth of most microorganisms (Lavelli and Corti 2011). Hence, even though there were some variations between the three drying methods, all produced a desirable MC and Aw, and ID could do so with a significantly reduced drying time, thus allowing for lower cost than other two methods.
Fig. 1.
Rate of water loss (%) during drying of apple pomace using different drying methods. The final moisture content and water activity are illustrated in the table; Means preceded by the same small letter in the same column were not significantly different (P > 0.05)
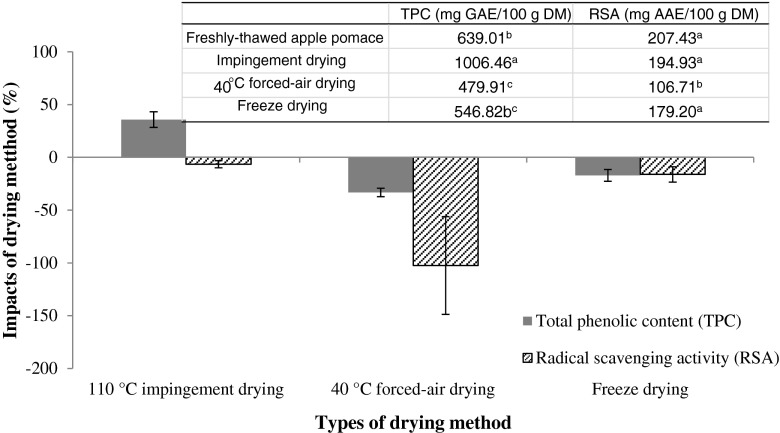
Fig. 2.
Impacts of different drying methods on total phenolic content (TPC) and radial scavenging activity (RSA) of dried apple pomace. Change of TPC and RSA was calculated as the decreasing or increasing amount of TPC and RSA in dried apple pomace in comparison with freshly-thawed, undried sample. In the table, means preceded by the same small letter in the same column were not significantly different (P > 0.05)
Figure 2 shows the effect of drying methods on the retained amount of TPC and RSA of dried apple pomace in comparison with that of fresh samples. TPC of the ID samples was significantly higher (~1006.46 mg GAE/100 g DM) than that of forced-air dried (~479.91 mg GAE/100 g DM) and freeze dried (~546.82 mg GAE/100 g DM). In addition, ID appeared to significantly enhance TPC, resulting in 58 % higher value than that of fresh pomace, and 110 % and 83 % higher than that of forced-air and freeze dried samples, respectively. This phenomenon had also been seen in other dried fruit byproducts, with a heat treatment of 150 °C for 60 min increasing the TPC of citrus peels 131 to 143 %, depending on whether the extracts were prepared using ethanol or water (Jeong et al. 2004). One explanation for this increase was that many phenolic compounds exist in complex linkages with other components, typically through ester, ether, or acetal bonds (Robbins 2003), and high temperature treatments can potentially liberate these bound phenolics, making them more available for extraction. Xu et al. (2006) were even able to measure this phenomenon, finding that after heat treatment of citrus peels, the free fraction of phenolic acids increased, whereas ester, glycoside, and ester bound fractions decreased.
RSA of dried pomace samples were significantly lower compared with fresh pomace, regardless of the type of drying method (Fig. 3), but the ID samples showed markedly higher level of activity (~194.93 mg AAE/100 g DM) than either forced-air dried (~106.71 mg AAE/100 g DM) or freeze dried (~179.20 mg AAE/100 g DM), consistent with the TPC results. Hence, the impingement drying helped retain the high amount of TPC and RSA in comparison with forced-air and freeze drying methods.
Fig. 3.
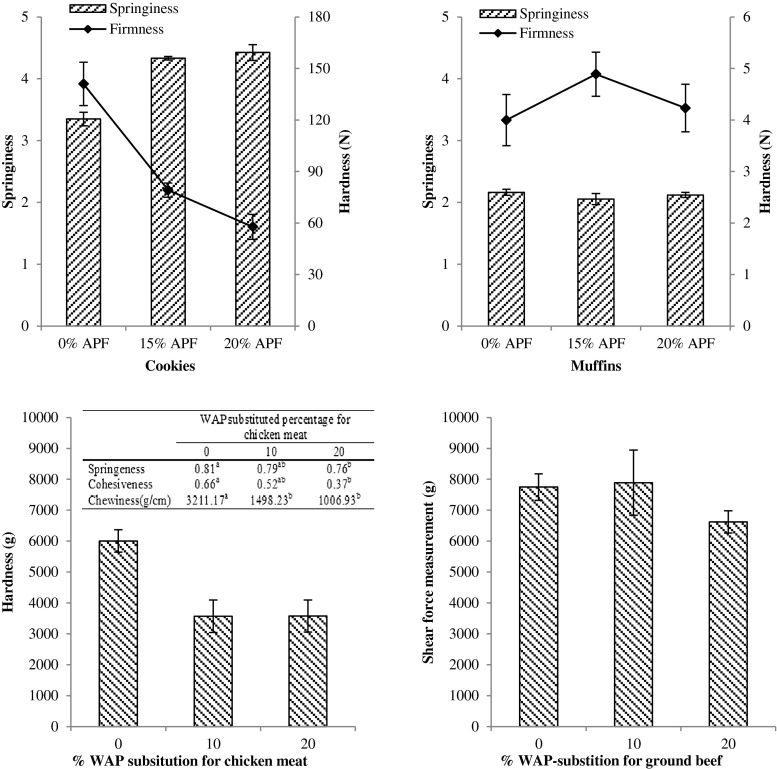
Textural properties of bakery and meat products fortified with different percentages of apple pomace flour (APF) or wet apple pomace (WAP); Bakery and meat products were fortified with APF and WAP, respectively
Characteristics of bakery products fortified with apple pomace flour
Color values of APF-fortified bakery products are shown in Table 2. Cookies fortified with 15 and 20 % APF were significantly darker (possessed a lower L value) than non-fortified ones, and this trend became more marked with increasing percentages of APF. Redness (a* value) was significantly higher in the APF-fortified samples compared with the non-fortified ones, but there was no significant difference between the two levels of fortification. Compared with the non-fortified cookies, yellowness (b* value) of 15 % APF-fortified cookies was significantly lower, but that of 20 % APF-fortified one was significantly higher. The largest color change (ΔE) of APF-fortified cookies in comparison with non-fortified one was found in 20 % APF fortification. These color changes could be due to one or both of the following reasons. First, the original color (light brown) of APF was much darker than that of wheat flour, which could translate into a darker brown color in the final baked product. Secondly, apple pomace had higher level of sugar compared with wheat flour, allowing for the increased caramelization and Maillard reaction during baking. In muffins, the similar trends were seen for both lightness and redness.
Table 2.
Color values of bakery and meat products fortified with different percentages of apple pomace flour (APF) or wet apple pomace (WAP) and color differences (ΔE) from those without APF or WAP
| Types of products | Level of APF or WAP substitution (%) | L | a | b | ΔE ++ |
|---|---|---|---|---|---|
| Cookie* | 0 | 73.3a + | 0.9b | 27.7b | –+++ |
| 15 | 44.7b | 13.5a | 24.1c | 14.7b | |
| 20 | 37.1c | 14.3a | 29.1a | 31.3a | |
| Muffin* | 0 | 74.6a | 1.4b | 27.3ab | – |
| 15 | 40.5b | 15.6a | 27.8a | 37.0b | |
| 20 | 34.7c | 15.9a | 24.8b | 42.6a | |
| Chicken patty** | 0 | 65.80a | 11.73b | 33.73a | – |
| 10 | 51.90b | 18.63a | 32.80a | 15.91a | |
| 20 | 48.23b | 19.87a | 31.10a | 19.69a | |
| Beef jerky** | 0 | 28.50a | 26.40a | 13.67b | – |
| 10 | 31.23a | 22.43b | 15.90ab | 5.39a | |
| 20 | 32.03a | 20.17b | 16.40a | 7.79a |
+ Means preceded by the same small letter in the same column within each food product were not significantly different (P > 0.05)
++ ΔE = ; L1, a1, and b1 are for control and L2, a2, and b2 are for each treatment
+++The value is not available for the control
* Baked products were fortified with apple pomace flour
** Meat products were fortified with wet apple pomace
Textural properties of APF-fortified bakery products are reported in Fig. 3. APF-fortified cookies were significantly softer with higher springiness compared with the control. This result was consistent with the report of Min et al. (2010) that cookies fortified with 10–30 % apple derived pectin-enriched material (PEM) have significantly lower hardness than non-fortified one. One possible explanation might be due to the low amount of moisture available in pomace. Since APF had lower MC (~5 %) than that of wheat flour (~14 %), substitution of wheat flour at 10–20 % level might impact the protein and/or carbohydrate structures, resulting in softer cookies. This could also help explain why our observations were contrary to the finding of Ajila et al. (2008) that fortifying cookies with mango peel powders at MC of ~10 % increased the hardness of the cookies since at this MC level, mango peel powders could provide enough water to form the carbohydrate structures in comparison with apple pomace. In addition, the dietary fiber in APF exhibited high water-retaining ability, which could account for much of the enhanced springiness in the APF-fortified cookies. For muffins, moisture levels could also explain the reasons behind the lack of any significant difference in the textural properties between non-fortified and APF-fortified ones (Fig. 3), since muffin was made from batters, rather than dough, and as such contained significantly higher level of moisture (due primarily to the eggs and milk in their formulae). These differences in moisture meant that the complex structures were likely not as compact, since the components that made up those structures were not competing for limited amounts of water with which to bond.
Characteristics of meat products fortified with whole apple pomace
Color values of WAP-fortified chicken patties and beef jerkies are also reported in Table 2. Chicken patties fortified with 10 and 20 % APF were significantly darker corresponding with the lower L values than non-fortified ones, whereas there was no further decrease with increasing substitution level. Redness (a* value) was significantly higher in WAP-fortified samples than non-fortified ones, but there was no significant difference between the two substitution levels. The color change (ΔE) of 10 and 20 % WAP-fortified chicken patties were 15.91 and 19.69, respectively. As previously mentioned in bakery products, these changes could be due to the original color (light brown) of WAP and high level of sugar inducing Maillard and caramelization reactions. For WAP-fortified beef jerkies, 20 % WAP substitution induced significant change in redness and yellowness in comparison with control, and the color change (ΔE) of WAP-fortified samples was 5.39 and 7.79 for 10 and 20 % WAP substitution, respectively.
Textural properties of WAP-fortified meat products are reported in Fig. 3. For chicken patties, WAP substitution significantly lowered hardness, springiness, cohesiveness, and chewiness in comparison with control. Similar trend was found in WAP-fortified beef jerkies, showing significantly lower shear force than control. These results were probably due to the dietary fiber contents in WAP for dissociating food matrix, and were consistent with the previous studies reporting significantly lower hardness of cooked sausage and restructured pork fortified with dietary fibers (Cáceres et al. 2004; Todd et al. 1989).
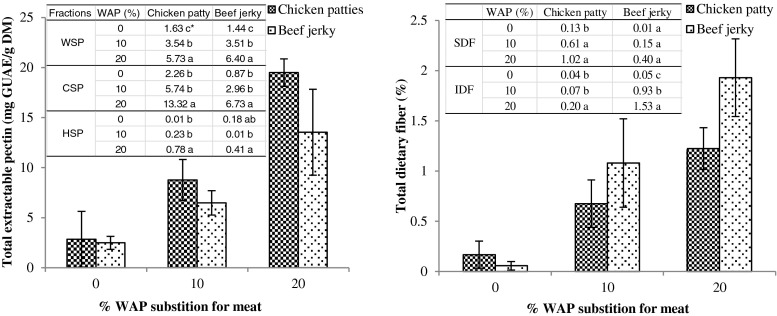
TEP and TDF of WAP-fortified meat products are reported in Fig. 4. TEP of WAP-fortified samples was significantly improved in comparison with control, and was more marked with higher WAP substitution level. Each fraction of pectin including WSP, CSP, and HSP also showed increasing trend with increasing substitution levels. Likewise, TDF including SDF and IDF were significantly higher in WAP fortified meat products in comparison with control. Hence, it was assumed that valuable pectin and dietary fiber contents from apple pomace are successfully transferred to meat products.
Fig. 4.
Total extractable pectin and dietary fiber contents of meat products fortified with different percentages of wet apple pomace (WAP); * Means (3 replications) with the different letters in the same column within each measurement are significantly different (P < 0.05)
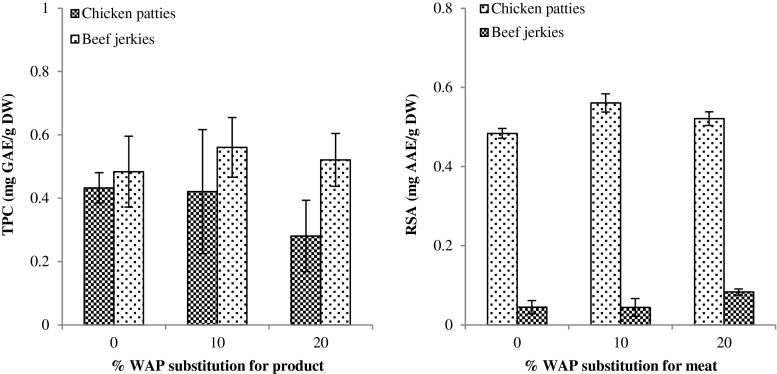
TPC and RSA of WAP fortified meat products were determined in comparison with control (Fig. 5). There was no significant difference in TPC between control and WAP-fortified chicken patty or beef jerky as it shows high variances between prepared samples. Folin-ciocalteu (FC) reagent for analyzing TPC could not only detect phenolic contents, but also associated protein or sugar contents since it could react with any reducing substances. Hence, FC assay was not representative for determining TPC in meat products due to high quantity of protein content and various kinds of additives. In contrast, 10 and 20 % WAP-fortified chicken patties and 20 % WAP-fortified beef jerkies exerted significantly higher RSA than control. Based on RSA results, it was assumed that TPC of WAP-fortified meat products is also improved in comparison with non-fortified ones as RSA is closely related to TPC.
Fig. 5.
Total phenolic contents (TPC) and radical scavenging activity (RSA) of meat products fortified with different percentages of wet apple pomace (WAP); TPC of WAP was 6.39 mg GAE/g dry weight (DW); RSA of WAP was 2.07 mg AAE/g DW; GAE and AAE indicate gallic acid and acetic acid equivalents, respectively
Conclusions
Impingement dry required significantly less drying time (~1/8 and 1/20) than that of forced-air and freeze dry, respectively for reducing the moisture content <10 % and water activity <0.5 to ensue longer shelf-life and microbial safety of the product at ambient storage conditions. The dried apple pomace obtained from the impingement dry possessed higher amount of total phenolic content and correlating radical scavenging activity than those of the other two drying methods. Hence, the impingement drying may be employed in the commercial drying process to reduce the production time and cost.
The partial substitution of apple pomace flour (APF) for wheat flour in cookies and muffins had no detrimental effects on the physicochemical and textural properties of the products in comparison with the control. Wet apple pomace directly substituted to meat products reduced their firmness, but was able to enhance the dietary fiber, pectin, and radical scavenging activity. APF or WAP, therefore, can be used as a good source of dietary fiber and polyphenols in various types of food applications for health promotion. Sensory evaluation and shelf-life study for various pomace fortified food products are under the way in our continuous efforts to develop more pomace fortified health promotion food products.
Acknowledgments
The authors gratefully thank the financial support of the Hood River Juice Company (Hood River, OR, USA) on this project, as well as the supply of the apple pomace samples.
References
- Acosta K, Cavender G, Kerr WL. Sensory and physical properties of muffins made with waxy whole wheat flour. J Food Qual. 2011;34:343–351. doi: 10.1111/j.1745-4557.2011.00401.x. [DOI] [Google Scholar]
- Ajila CM, Leelavathi K, Prasada Rao UJS. Improvement of dietary fiber content and antioxidant properties in soft dough biscuits with the incorporation of mango peel powder. J Cereal Sci. 2008;48:319–326. doi: 10.1016/j.jcs.2007.10.001. [DOI] [Google Scholar]
- AOAC Changes in methods. J Assoc Off Anal Chem. 1985;68(43):A14–A43. [Google Scholar]
- AOAC. 994.13 . Official Methods of Analysis of AOAC International. 17. Washington: AOAC; 2007. [Google Scholar]
- Bai J-W, Sun D-W, Xiao H-W, Mujumdar AS, Gao Z-J (2013) Novel high-humidity hot air impingement blanching (HHAIB) pretreatment enhances drying kinetics and color attributes of seedless grapes. Innov Food Sci Emerg Technol 20:230–237
- Better, Homes, and, Gardens (2012) Better Homes and Gardens New Cook Book
- Bibbin-Martinez MD, Encisco-Chavez B, Galicia SBN, Hernandez DC (2011) Soluble dietary fiber generation from apple pomace. Int Congr Eng Food (ICEF). Poster Presentation Athens Greece
- Bourne M (2002) Food texture and viscosity: concept and measurement. 2nd edn. Academic Press, San Diago California
- Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT - Food Sci Technol. 1995;28:25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- Bravo L, Saura-Calixto F. Characterization of dietary fiber and the in vitro indigestible fraction of grape pomace. Am J Enol Vitic. 1998;49:135–141. [Google Scholar]
- Cáceres E, Garcia ML, Toro J, Selgas MD. The effect of fructooligosaccharides on the sensory characteristics of cooked sausages. Meat Sci. 2004;68:87–96. doi: 10.1016/j.meatsci.2004.02.008. [DOI] [PubMed] [Google Scholar]
- Canteri-Schemin MH, Fertonani HCR, Waszczynskyj N, Wosiacki G. Extraction of pectin from apple pomace. Braz Arch Biol Tehcnol. 2005;48:259–266. doi: 10.1590/S1516-89132005000200013. [DOI] [Google Scholar]
- Chatanta D, Attri C, Gopa K, Devi M, Gupta G, Bhalla T. Bioethanol production from apple pomace left after juice extraction. Int J Microbiol. 2007;5:2. [Google Scholar]
- Choicharoen K, Devahastin S, Soponronnarit S. Performance and energy consumption of an impinging stream dryer for high-moisture particulate materials. Drying Technol. 2009;28:20–29. doi: 10.1080/07373930903423608. [DOI] [Google Scholar]
- Constenla D, Ponce AG, Lozano JE. Effect of pomace drying on apple pectin. LWT - Food Sci Technol. 2002;35:216–221. doi: 10.1006/fstl.2001.0841. [DOI] [Google Scholar]
- de Torres C, Díaz-Maroto MC, Hermosín-Gutiérrez I, Pérez-Coello MS. Effect of freeze-drying and oven-drying on volatiles and phenolics composition of grape skin. Anal Chim Acta. 2010;660:177–182. doi: 10.1016/j.aca.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Deng Q, Penner MH, Zhao Y. Chemical composition of dietary fiber and polyphenols of five different varieties of wine grape pomace skins. Food Res Int. 2011;44:2712–2720. doi: 10.1016/j.foodres.2011.05.026. [DOI] [Google Scholar]
- Figuerola F, Hurtado ML, Estvez AM, Chiffelle I, Asenjo F. Fibre concentrates from apple pomace and citrus peel as potential fibre sources for food enrichment. Food Chem. 2005;91:395–401. doi: 10.1016/j.foodchem.2004.04.036. [DOI] [Google Scholar]
- Garna H, Mabon N, Robert C, Cornet C, Nott K, Legros H, Wathelet B, Paquot M. Effect of extraction conditions on the yield and purity of apple pomace pectin precipitated but not washed by alcohol. J Food Sci. 2007;72:C001–C009. doi: 10.1111/j.1750-3841.2006.00227.x. [DOI] [PubMed] [Google Scholar]
- Gerhardt P, Wood WA, Krieg NR, Murray R (1994) Methods for general and molecular bacteriology. ASM Press, Washington DC, pp 518
- Jeong S-M, Kim S-Y, Kim D-R, Jo S-C, Nam KC, Ahn DU, Lee S-C. Effect of heat treatment on the antioxidant activity of extracts from citrus peels. J Agric Food Chem. 2004;52:3389–3393. doi: 10.1021/jf049899k. [DOI] [PubMed] [Google Scholar]
- Joshi VK, Attri D. Solid state fermentation of apple pomace for the production of value added products. Indian J Nat Prod Resour. 2006;5:289–296. [Google Scholar]
- Kader F, Rovel B, Girardin M, Metche M. Mechanism of browning in fresh highbush blueberry fruit (Vaccinium corymbosum L). Role of blueberry polyphenol oxidase, chlorogenic acid and anthocyanins. J Sci Food Agric. 1997;74:31–34. doi: 10.1002/(SICI)1097-0010(199705)74:1<31::AID-JSFA764>3.0.CO;2-9. [DOI] [Google Scholar]
- Kaushal NK, Joshi VK, Sharma RC. Effect of stage of apple pomace collection and the treatment on the physical-chemical and sensory qualities of pomace papad (fruit cloth) J Food Sci Technol. 2002;39:388–393. [Google Scholar]
- Kennedy M, List D, Lu Y, Foo LY, Newman RH, Sims IM, Bain PJS, Hamilton B, Fenton G. Apple pomace and products derived from apple pomace: Uses, composition and analysis. In: Linskens H, Jackson J, editors. Anal. Plant Waste Mater. Berlin: Springer; 1999. pp. 75–119. [Google Scholar]
- Khanal RC, Howard LR, Prior RL. Effect of heating on the stability of grape and blueberry pomace procyanidins and total anthocyanins. Food Res Int. 2010;43:1464–1469. doi: 10.1016/j.foodres.2010.04.018. [DOI] [Google Scholar]
- Lavelli V, Corti S. Phloridzin and other phytochemicals in apple pomace: stability evaluation upon dehydration and storage of dried product. Food Chem. 2011;129:1578–1583. doi: 10.1016/j.foodchem.2011.06.011. [DOI] [Google Scholar]
- Lu Y, Yeap Foo L. Antioxidant and radical scavenging activities of polyphenols from apple pomace. Food Chem. 2000;68:81–85. doi: 10.1016/S0308-8146(99)00167-3. [DOI] [Google Scholar]
- Masoodi FA, Chauhan GS. USE OF APPLE POMACE AS A SOURCE OF DIETARY FIBER IN WHEAT BREAD. J Food Process Preserv. 1998;22:255–263. doi: 10.1111/j.1745-4549.1998.tb00349.x. [DOI] [Google Scholar]
- Mendez L, Makhlouf J, Ratti C (2005) Garlic quality properties during constant convective drying. In: Orsat V, Raghavan GSV, Kudra T (eds) In Proc. 3rd inter-american drying conference. McGill University, Montreal, Paper D-6
- Min B, Bae IY, Lee HG, Yoo S-H, Lee S. Utilization of pectin-enriched materials from apple pomace as a fat replacer in a model food system. Bioresour Technol. 2010;101:5414–5418. doi: 10.1016/j.biortech.2010.02.022. [DOI] [PubMed] [Google Scholar]
- Naveena BM, Muthukumar M, Sen AR, Babji Y, Murthy TRK. Quality characteristics and storage stability of chicken patties formulated with finger millet flour (ELEUSINE CORACANA) J Muscle Foods. 2006;17:92–104. doi: 10.1111/j.1745-4573.2006.00039.x. [DOI] [Google Scholar]
- Nishibori S, Kawasihi S. Effect of vaious sugars on the quality of baked cookies. Cereal Chem. 1992;69:160–163. [Google Scholar]
- Prosky L, Asp N, Fudra I, DeVries J, Schweizer T, Harland B. Determination of total dietary fiber in foods and food products: collaborative study. J Assoc Off Anal Chem. 1985;68:677–679. [PubMed] [Google Scholar]
- Raghavan GSV, Orsat V. Recent advances in drying of biomaterials for superior quality bioproducts. Asia-P ac J Chem Eng. 2007;2:20–29. doi: 10.1002/apj.51. [DOI] [Google Scholar]
- Ratti C. Hot air and freeze-drying of high-value foods: a review. J Food Eng. 2001;49(4):311–319. doi: 10.1016/S0260-8774(00)00228-4. [DOI] [Google Scholar]
- Robbins RJ. Phenolic acids in foods: an overview of analytical methodology. J Agric Food Chem. 2003;51:2866–2887. doi: 10.1021/jf026182t. [DOI] [PubMed] [Google Scholar]
- Rupasinghe HPV, Wang L, Huber GM, Pitts NL. Effect of baking on dietary fibre and phenolics of muffins incorporated with apple skin powder. Food Chem. 2008;107:1217–1224. [Google Scholar]
- Shalini R, Gupta DK. Utilization of pomace from apple processing industries: a review. J Food Sci Technol. 2010;47:365–371. doi: 10.1007/s13197-010-0061-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silacci MW, Morrison JC. Changes in pectin content of cabernet sauvignon grape berries during maturation. Am J Enol Vitic. 1990;41(2):111–115. [Google Scholar]
- Singleton VL, Rossi JA., Jr Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic. 1965;16:144–158. [Google Scholar]
- Sivam AS, Sun-Waterhouse D, Waterhouse GIN, Quek S, Perera CO. Physicochemical properties of bread dough and finished bread with added pectin fiber and phenolic antioxidants. J Food Sci. 2011;76:H97–H107. doi: 10.1111/j.1750-3841.2011.02086.x. [DOI] [PubMed] [Google Scholar]
- Smock RM, Neuber AM (1950) Apples and Apple products, Volume 2 of economic crops, Apple products. In (pp. 440). New York: Interference
- Sudha ML, Baskaran V, Leelavathi K. Apple pomace as a source of dietary fiber and polyphenols and its effect on the rheological characteristics and cake making. Food Chem. 2007;104:686–692. doi: 10.1016/j.foodchem.2006.12.016. [DOI] [Google Scholar]
- Tambunan AH, Yudistira K, Hernani Freeze drying characteristics of medicinal herbs. Drying Technol. 2001;19:325–331. doi: 10.1081/DRT-100102907. [DOI] [Google Scholar]
- Todd SL, Cunningham FE, Claus JR, Schwenke JR. Effect of dietary fiber on the texture and cooking characteristics of restructured pork. J Food Sci. 1989;54:1190–1192. doi: 10.1111/j.1365-2621.1989.tb05951.x. [DOI] [Google Scholar]
- Tseng A, Zhao Y. Effect of different drying methods and storage time on the retention of bioactive compounds and antibacterial activity of wine grape pomace (Pinot Noir and Merlot) J Food Sci. 2012;77:H192–H201. doi: 10.1111/j.1750-3841.2012.02840.x. [DOI] [PubMed] [Google Scholar]
- Vendruscolo F, Albuquerque PM, Streit F, Esposito E, Ninow JL. Apple Pomace: a versatile substrate for biotechnological applications. Crit Rev Biotechnol. 2008;28:1–12. doi: 10.1080/07388550801913840. [DOI] [PubMed] [Google Scholar]
- Verma AK, Sharma BD, Banerjee R. Effect of sodium chloride replacement and apple pulp inclusion on the physico-chemical, textural and sensory properties of low fat chicken nuggets. LWT - Food Sci Technol. 2010;43:715–719. doi: 10.1016/j.lwt.2009.12.006. [DOI] [Google Scholar]
- Wang HJ, Thomas RL. Direct use of apple pomace in bakery products. J Food Sci. 1989;54:618–620. doi: 10.1111/j.1365-2621.1989.tb04665.x. [DOI] [Google Scholar]
- Xu G, Ye X, Chen J, Liu D. Effect of Heat Treatment on the Phenolic Compounds and Antioxidant Capacity of Citrus Peel Extract. J Agric Food Chem. 2006;55:330–335. doi: 10.1021/jf062517l. [DOI] [PubMed] [Google Scholar]
- Yemmireddy VK, Chinnan MS, Kerr WL, Hung Y-C. Effect of drying method on drying time and physico-chemical properties of dried rabbiteye blueberries. LWT - Food Sci Technol. 2013;50:739–745. doi: 10.1016/j.lwt.2012.07.011. [DOI] [Google Scholar]