Abstract
Resin from Pistacia terebinthus tree was used for the immobilization of L. casei ATCC 393 cells. The encapsulated L. casei cells biocatalysts were added as adjuncts during yogurt production at 45 °C and probiotic viability was assessed during storage at 4 °C. For comparison reasons yogurt with free L. casei cells were prepared. The effect of encapsulated bacteria as adjuncts in yogurt on pH, lactic acid, lactose and other physicochemical parameters were studied for 60 storage days at 4 °C. Samples were also tested for the microbiological and organoleptic characteristics during storage at 4 °C. Encapsulation matrix seems to sustain the viability of embedded L. casei cells at levels more than 7 logcfug−1 after 60 days of storage at 4 °C. Furthermore, the absence of pathogens such as Salmonella, Staphylococci, Enterobacteriaceae and coliforms in the produced yogurts is noteworthy where spoilage microorganisms such as yeasts and molds seem to affect yogurt quality only in absence of Pistacia terebinthus resin. The effect of the resin on production of aroma-related compounds responsible for yogurt flavor was also studied using the solid phase microextraction gas chromatography/mass spectrometry technique. Alpha and beta- pinene were the major aroma compounds detected in produced yogurts (over 60 % of total aromatic compounds detected). Yogurts with immobilized cells on P.terebintus resin had a fine aroma and taste characteristic of the resin.
Keywords: Pistacia terebinthus resin, Immobilization, L. casei, Yogurt, GC-MS
Introduction
During the last decades nutritionists, academics and even consumers all over the world have recognized fermented dairy products as being beneficial to human health. Yogurt, in particular, is traditionally produced by milk inoculation with cultures composed of Streptococcus thermophilus and Lactobacillus delbrueckii subsp. bulgaricus. However, lately yogurt has been enriched with various species of Bifidobacteria and Lactobacillus (Gilliland et al. 2002; Granato et al. 2010; He et al. 2008; Picot and Lacroix 2004) in order to serve as carrier food for probiotic cultures.
Several scientists have associated the nutritional benefits of yogurt with the high population of lactic acid bacteria in the product (Plessas et al. 2012; Shah et al. 1995). For that reason, many countries have established minimum values of viable lactic acid bacteria for yoghurts and/ or fermented milks during shelf life ranging from 1 × 106 to 5 × 108 colony forming units (CFU) per gram (Birollo et al. 2000; Shah et al. 1995; Vinderola and Reinheimer 2003).
Nevertheless, yogurt is a metabolically active product that presents modifications throughout its self-life impairing the quality of the product. Postacidification is one of the changes in yogurts which is claimed to affect the viability of probiotic lactic acid bacteria (Dave and Shah 1998). Therefore, several methods have been proposed to improve viability of probiotic bacteria, among which, encapsulation appears to be the most promising (Cai et al. 2014; Champagne et al. 1992; Picot and Lacroix 2004; Sathyabama et al. 2014). Although many immobilization supports have been proposed in fermentation systems, various natural materials, such as fruit pieces, gluten pellets, brewer’s spent grains and delignified cellulosic residues were investigated and successfully applied as immobilization supports in wine making, brewing, whey and milk fermentations (Bosnea et al. 2009; Kopsahelis et al. 2007, 2012; Tsakiris et al. 2004).
Pistacia terebinthus (Anacardiaceae family) is a resin producing tree that mainly grows on dry rock slopes and hill sides or in pine forests in Mediterranean region. Especially in Cyprus, Pistacia terebinthus resin is called Pafos’ pissa and is a major contributor in the local economy. Since ancient times, the importance of the Pistacia resin was known and its products were among the valued trading commodities (Stern et al. 2008). The resins was extensively used in Turkish folk medicine for the treatment of sunstroke, peptic ulcer and asthma (Tuzlaci and Aymaz 2001) while the crude extracts, essential oils and some triterpenoid constituents are also used in treatments of eczema, paralysis, diarrhea, throat infections, renal stones, jaundice, asthma and stomach-ache, and also as an astringent, anti-inflammatory, antipyretic, antibacterial, antiviral, pectoral and stimulant (Duru et al. 2003; Lamiri et al. 2001; Mouhajir et al. 2001). The resin is also traditionally used as chewing gum and as food additive (Tuzlaci and Aymaz 2001).
The aim of this study was to evaluate Pistacia terebinthus resin (Pafos’ Pissa) as encapsulation material for Lactobacillus casei cells and examine the effects of encapsulated L. casei cells on Pafos’ Pissa when added fresh or freeze dried in yogurts. For first time Pistacia terebinthus resin was used for Lactobacillus species immobilization taking in account its above biological activities and flavor. An additional objective was to evaluate the viability of L. casei cells in the produced yogurts during their refrigerated storage and examine the volatile profile of the produced yogurts.
Materials and methods
Culture growth and cell immobilization
Lactobacillus casei ATCC 393 (DSMZ, Germany) was grown at 37 °C for 72 h in MRS Broth (Merck, Darmstadt, Germany) under anaerobic conditions. L. casei cells were harvested by centrifugation at 4,165 g for 10 min at 20 °C (SIGMA 3 K12, Bioblock Scientific, Laborzentrifugen GmbH, Osterode, Germany). Pistacia terebinthus resin (Pafos’ Pissa) was used as encapsulation material. Prior use, pissa was freeze-dried overnight at 135.10−3 mbar and at −45 °C in a Freeze Drying System, Freezone 4.5 (Labconco, Kansas City, Missouri, USA), so that the structure opens and creates holes where the cells can be entrapped. Cell encapsulation was carried out by introducing 50 g of the support along with 8 g (wet weight) of L. casei cells in 2 L MRS broth (Merck, Darmstadt, Germany) and the mixture was allowed to ferment at 37 °C for 48 h without agitation. When immobilization was complete (glucose in the liquid culture was <1 g/L), the fermented liquid was decanted and the immobilized biocatalyst was washed twice with sterile Ringer’s solution for removal of free cells. The produced biocatalysts were then used as adjuncts in yogurt production.
Yoghurt production
Five hundred fifty mL of commercial homogenized and pasteurized milk containing 3.7 % fat and 13.2 % total solids was heat treated to 90 °C for 10 min, cooled down to 45 °C and divided into 5 equal portions (100 mL each). The first batch (C: control) was inoculated with yogurt culture (5 % inoculum, Streptococcus thermophilus and L. delbruecki subsp. bulgaricus, 1:1 proportion), the second (FLC) was inoculated with yogurt culture plus L. casei ATCC 393 (5 % inoculum), the third (IM 0.3), fourth (IM 0.6) and fifth (IM 1.2) with yogurt culture (5 %) plus encapsulated L. casei cells on pissa pieces (0.3, 0.6 and 1.2 g), respectively. All batches were poured into plastic cups (100 mL) and incubated at 42–45 °C until pH drops to 4.8. The yogurts were then transferred to cold storage (4 °C ±1) and stored for 60 days. Samples were selected and analyzed from each batch at several time intervals after production.
Chemical and physical properties
pH
The pH values of milk and yogurts were measured using a digital pH meter by direct immersion of the electrode (ΕPI-BION SENTRON pH-System 1001).
Lactose and lactic acid determination
For lactose and lactic acid determination, five grams of yoghurt sample were diluted with water to a total volume of 200 mL, mixed well and centrifuged at 4,125 g for 10 min (Shimadzu Application news, No L213), then the solution was used for lactose and lactic acid determination.
Lactose was determined by high performance liquid chromatography, using a Shimadzu chromatograph with a SCR-101 N stainless steel column, a LC-9A pump, a CTO-10A oven at 60 °C and a RID-6A refractive index detector. Three times distilled water was used as mobile phase with a flow rate of 0.8 mL/min and 1-butanol was used as an internal standard. Samples of 0.5 and 2.5 mL of a 1 % (v/v) solution of 1-butanol were diluted to 50 mL and 40 μL were injected directly to the column. Lactose concentrations were calculated using standard curves.
Lactic acid was determined by high performance liquid chromatography, using a Shimadzu chromatograph with a Shim-pack IC-A1 stainless steel column, a LC-10A pump, a CTO-10A oven at 40 °C and a CDD-6A conductivity detector. A solution of 2.5 mM phthalic acid and 2.4 mM Tris (hydroxymethyl) aminomethane (pH 4.0) in three times distilled water was used as mobile phase with a flow rate of 1.5 mL/min. Samples of 0.25 mL were diluted to 25 mL and 50 μL were injected directly to the column. Lactic acid concentrations were calculated using standard curves.
Syneresis
Syneresis was measured according to Keogh and O’Kennedy (1998) with modifications. The samples were centrifuged at 350 g for 10 min under 4 °C in triplicate. The syneresis percentage was calculated by the milk whey mass separated from the gel network during centrifugation divide by the initial yogurt mass, multiplied by 100 (Granato et al. 2014).
Microbiological analyses
Ten-gram portions of yoghurt samples were diluted to 100 mL with sterilized Ringer solution ¼ strength, mixed with a stomacher (Bagmixer 400, Model VW, Interscience). Subsequent dilutions were made in sterilised Ringer’s solution ¼ strength.
Viable counts for streptococci, lactobacilli, moulds and yeasts, coliforms, enterobacteria, salmonella and staphylococcus were performed in duplicate by pour plating 1 or 0.1 mL of appropriate dilutions on the selective media for each species and according to instructions given by manufacturer. Viable counts of total mesophilic aerobic bacteria were enumerated on plate count agar (PCA) (Fluka) after incubation at 37 °C for 72 h, coliform counts were enumerated on Violet red bile agar (Fluka) after incubation at 30 °C for 24 h, total enterobacteriaceae were enumerated on Violet red bile glucose agar (Fluka) after incubation at 37 °C for 24 h. Streptococcus thermophilus was determined on M-17 agar (Fluka) following incubation at 30 °C for 72 h, L. delbrueckii subsp. bulgaricus was determined on MRS agar acidified at 5.2 pH. Staphylococcus counts were determined on Baird Parker agar (Fluka) after incubation at 37 °C for 48 h, salmonella counts on Brilliant green agar (Fluka) after incubation at 37 °C for 24–48 h, and yeasts and molds were determined by plating on Potato Dextrose Agar (Fluka) after incubation at 30 °C for 72 h. Finally, viable L. casei counts were determined on MRS agar – vancomycin agar (Fluka) after at 37 °C incubation for 48–72 h, according to Tharmaraj and Shah (2003). After the appropriate incubation period, plates with 30–300 colonies were selected for enumeration. The original count in the sample was expressed as logcfu per gram of yoghurt.
Solid phase microextraction (SPME) gas chromatography–mass spectrometry (GC–MS) analysis
Samples of yogurts were studied for volatile by products composition and terpenoid content using SPME GC–MS analysis. Ten milliliters of each sample and 3.0 g NaCl were introduced into a 20 mL headspace vial fitted with a Teflon-lined septum sealed with an aluminum crimp seal, through which the SPME syringe needle (bearing a 2-cm fibre coated with 50/30 mm Divinylbenzene/Carboxen on poly-dimethyl-siloxane bonded to a flexible fused silica core, Supelco, Bellefonte, PA, USA) was introduced. The container was then thermostated at 60 °C for 45 min. The absorbed volatile analytes were then analyzed by GC–MS (Shimadzu GC-17A, MS QP5050, capillary column Supelco CO Wax-10 60 m, 0.32 mm i.d., 0.25 μm film thickness). Helium was used as carrier gas at a flow rate of 1.8 mL/min. Oven temperature was set at 35 °C for 6 min, followed by a temperature gradient of 2 °C/min to 60 °C, held constant for 5 min, raised to 200 °C at 5 °C/min, and then to 250 °C at 25 °C/min with a final isothermal period of 6 min. The injector was operated in splitless mode. Injector and detector temperatures were 280 °C and 250 °C, respectively. The mass spectrometer was operated in the electron impact mode with the electron energy set at 70 eV and mass range m/z 29–400. The identification was effected by comparing the retention times with those of authentic compounds, by mass spectra of the authentic compounds generated in the laboratory, by mass spectra obtained from NIST107, NIST21 and SZTERP libraries, and by determining kovats’ retention indexes and comparing with those reported in the literature. Kovats’ retention indexes (KI) were determined by injection of a standard mixture containing the homologous series of normal alkanes (C7–C32) in pure hexane under exactly the same experimental conditions, as described above (Kopsahelis et al. 2009).
Electron microscope investigation (SEM)
Pieces of encapsulated L. casei were washed with synthetic medium and dried over night at room temperature. The pieces of P. terebinthus resin with immobilized biocatalyst were coated with gold in a Balzers SCD 004 Sputter coater (Bal-Tec, Schalksmühle, Germany) for 2 min. Later, the samples were examined in a JSM-6300 scanning electron microscope (JEOL, Tokyo, Japan), operated at an accelerating voltage of 20 kV. Scanning electron micrographs were obtained at several magnifications.
Experimental design and statistical analysis
In the experiments conducted, the effect of encapsulated of L. casei in physicochemical and microbiological properties of yogurts as well as L. casei viability during storage for 60 days was studied. The experiments and the preliminary sensory evaluation were designed and analyzed statistically by ANOVA. Duncan’s multiple range tests was used to determine significant differences among results (coefficients, ANOVA tables and significance) were computed using SPSS v.8.5.
Results and discussion
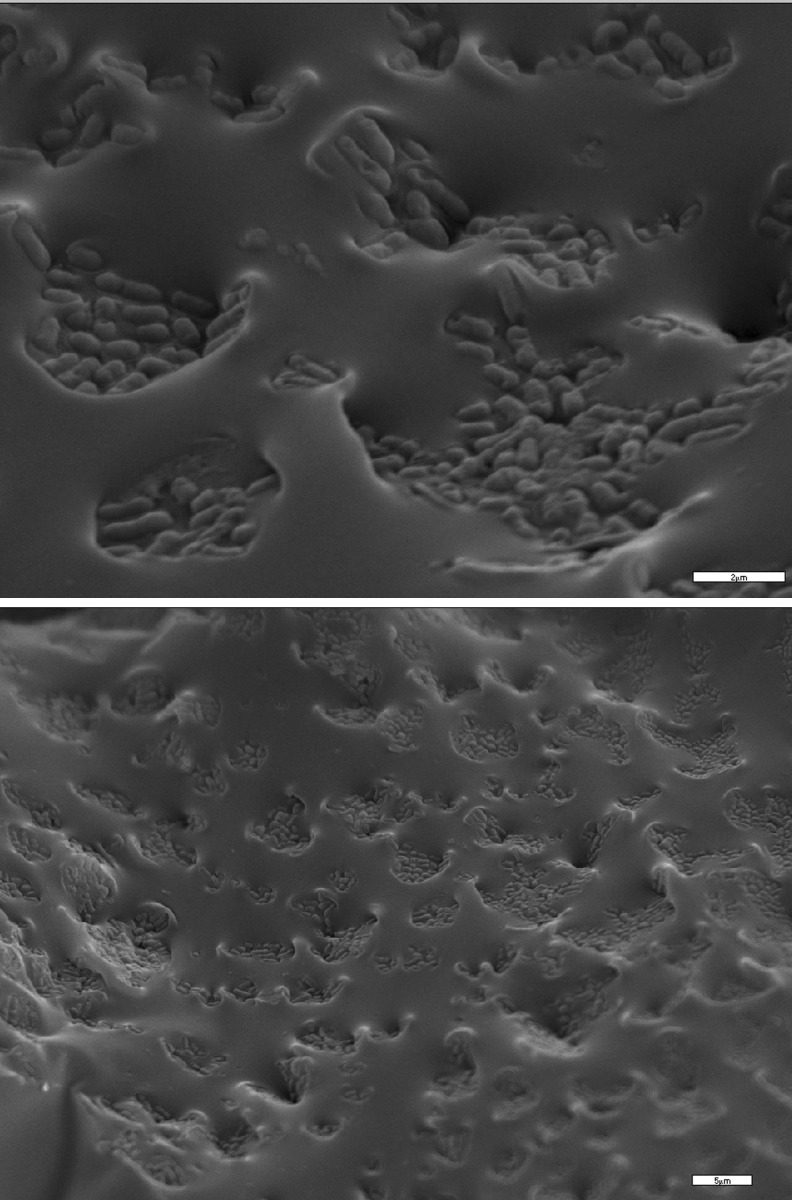
Pistacia terebinthus resin, also known as Pafos’ pissa was assessed as encapsulation matrix for Lactobacillus casei cells. Pissa is a well-known commercial product in Cyprus and is normally used in beverages and cooking because of the intense aroma. From the electron micrograph it is obvious L. casei cells were entrapped within the viscous resin matrix (Fig. 1). Yogurts were prepared using the traditional set method because it’s simple and it favors the better survival of probiotic microorganisms in comparison with methods where adjuncts are added later in production (Gardini et al. 1999; Hull et al. 1984). Regarding the need for careful selection of potential probiotic strains in yogurts like products, L. casei ATCC393 strain was selected based on the in vitro screening of the survival in simulated GI tract conditions (Choi et al. 2006) as well as in the in vivo survival of the strain (Foligne et al. 2007). Yogurts with free L. casei cells were also prepared for comparison reasons.
Fig. 1.
Electron micrographs of encapsulated L.casei cells in Pistacia resin
Effect of added enriched materials on yogurts post acidification during storage for 60 days at 4 °C
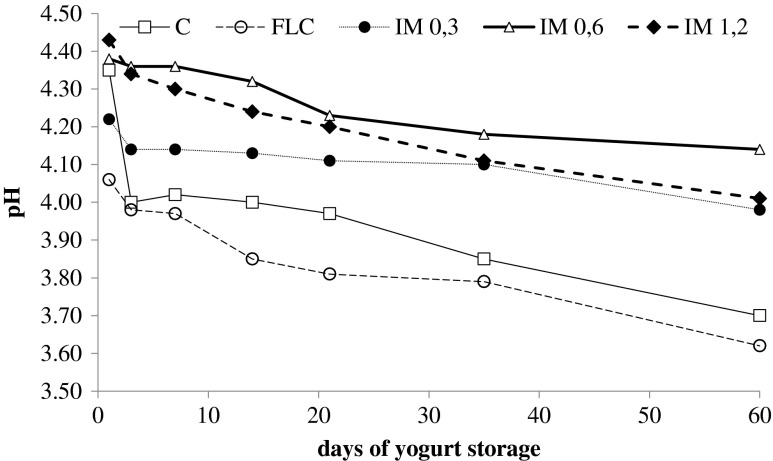
Acidification trend in yogurt fermentation is presented in Fig. 2. The lowest pH values were observed for yogurts with free L. casei cells (FLC). After 60 days of refrigerated storage, yogurts produced with free L. casei cells as adjunct had the lowest pH value (3.62). Yogurts with only starter cultures of Streptococcus thermophilus and Lactobacillus bulgaricus (Control) also had low pH value after 60 days of storage. However, no significant difference (P < 0.05) were found between yogurts with free L. casei and control yogurt. Postacidification effect was observed in all yogurts (Fig. 2). However, L. casei has a pH- resistant character and usually can survive post- acidification effects. In case of yogurts produced with the addition of encapsulated L.casei in Pissa there was a delay in decrease of pH during storage so pH remains at acceptable organoleptic levels (4–4,2). This is a noteworthy result for the use of Pafos’ pissa as carrier of L.casei cells and addition in yogurt.
Fig. 2.
Post acidification in produced yogurts during storage at 4 °C for 60 days
Lactose assimilation and lactic acid production in produced yogurts
Increase in lactic acid concentration was generally observed during 60 storage days with simultaneous decrease of lactose (Table 1). As expected, storage time significantly affects total acidity levels in all samples. Significant difference (P > 0.05) in the amount of lactic acid was observed within the yogurts produced with free L. casei and control yogurt, while no significant differences were observed in yogurts with encapsulated L. casei (0.3 g/0.6 g and 1.2 g of pissa). Lactic acid production was detected in all yogurts ranged in acceptable levels (1.43–1.63 g lactic acid per 100 g yogurt) at 60 storage days. Slower rates were observed in yogurt lactic acid production with encapsulated cells in comparison with free cells in yogurt production (Table 1). The hard and viscous matrix of pissa probably does not permit the exchange of nutrients and fermentation by-products. It is widely known that growth of microorganisms in milk leads to reduction in pH. The lactose accumulation that is observed in all samples is probably consumed by L. bulgaricus and free L. casei cells more quick than by encapsulated L. casei. So we believe that at low temperature 4 °C the pissa is more inflexible than the one at higher temperatures and the matrix encloses the cells in such way that they reduce their metabolism due to mechanical stress. The slower metabolic rates help L. casei cells remain more viable within the matrix. No significant differences were observed in lactose accumulation within the samples.
Table 1.
Lactose and lactic acid concentrations of produced yogurts during storage
| Yogurt | Storage time (days) | Lactose (g/100 g yogurt) | Lactic acid (g/100 g yogurt) |
|---|---|---|---|
| C. Yogurt without L. casei | 1 | 2.50 | 0.79 |
| 14 | 2.43 | 1.13 | |
| 21 | 2.34 | 1.23 | |
| 35 | 2.27 | 1.29 | |
| 60 | 1.12 | 1.43 | |
| FC Yogurt with free L. casei cells | 1 | 2.53 | 1.05 |
| 14 | 2.00 | 1.37 | |
| 21 | 1.91 | 1.45 | |
| 35 | 1.84 | 1.59 | |
| 60 | 1.81 | 1.63 | |
| IM0.3 Yogurt with 0.3 g encapsulated L. casei | 1 | 2.43 | 0.73 |
| 14 | 1.83 | 1.11 | |
| 21 | 1.66 | 1.30 | |
| 35 | 1.70 | 1.41 | |
| 60 | 1.69 | 1.48 | |
| IM0.6 Yogurt with 0.6 g encapsulated L. casei | 1 | 2.50 | 0.70 |
| 14 | 2.30 | 0.99 | |
| 21 | 2.31 | 1.27 | |
| 35 | 2.30 | 1.47 | |
| 60 | 2.06 | 1.51 | |
| IM1.2 Yogurt with 1.2 g encapsulated L. casei | 1 | 2.57 | 0.79 |
| 14 | 2.37 | 0.97 | |
| 21 | 2.35 | 1.24 | |
| 35 | 2.31 | 1.50 | |
| 60 | 2.02 | 1.58 |
Yogurt syneresis
Syneresis or spontaneous whey separation on the surface of yogurt is regarded as a defect. This problem can be reduced or eliminated by increasing the level of milk solids to ∼ 15 %, by the use of stabilizers, or by adding exopolysaccharide (EPS)-producing starter cultures (Amatayakul et al. 2006; Tamime and Deeth 1980).
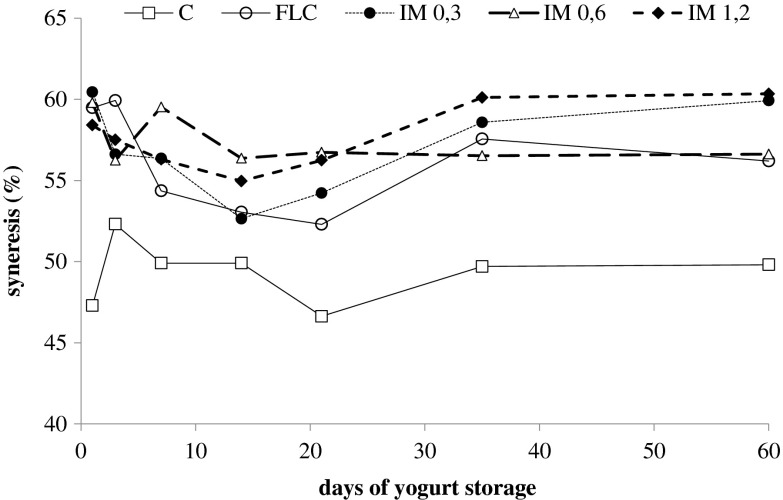
Figure 3 shows the syneresis in the produced yogurts during storage for 60 days. In all cases, syneresis values are elevated, probably because no extra solids were added during production. Mani-López et al. (2014), have studied syneresis in commercial yogurts and in probiotic yogurt produced in their laboratory. They concluded that commercial yogurts appeared to have less syneresis than the probiotic yogurts. The probiotic yogurts had syneresis values over 40 %. It is noteworthy that, they did not add any additional solids in the matrix of the produced yogurts.
Fig. 3.
Syneresis of yogurts during storage at 4 °C for 60 days ( C,
C,  FLC,
FLC,  IM 0.3,
IM 0.3,  IM 0.6,
IM 0.6,  IM 1.2)
IM 1.2)
The addition of solids, such as whey proteins, increases the interaction within the proteins that create the gel network, resulting in increment of the gel stability; thus reducing syneresis. Furthermore, the slow growth of probiotic bacteria in yogurts due to the lack of proteolytic enzymes, lead in a product with poor rheological characteristics and increased syneresis (Dave and Shah 1998). Amatayakul et al. (2006), reported syneresis values over 45 in yogurts with 9 and 14 % total solids that were fermented with exopolysaccharide (EPS)-producing starter cultures. (González-Martínez et al. 2002) reported syneresis values of 23 and 36 % in yogurts that whey proteins were added.
Viability of L. casei during storage
Food presents a good vehicle to deliver probiotics in the intestine, while maintaining viable probiotic cell counts at high level by the end of the expiration date is required for most health benefits. Table 2 shows the starter’s populations L. bulgaricus ssp. delbrueckii and S. thermophilus as well the population of the adjunct L. casei during yogurt storage at 4 °C for 60 days. L. casei was added into the yogurt as free cells or encapsulated in pissa matrix during yogurt production at 45 °C. In all cases, at the beginning of the experiment yogurts showed counts of starters higher than 108 CFU g−1.
Table 2.
Microbiological profile of yogurts
| Yogurt | Storage (days) | St. thermophilus | L. bulgaricus | L. casei |
|---|---|---|---|---|
| Log CFU/g | Log CFU/g | Log CFU/g | ||
| C | 1 | 9.06 | 9.02 | 0 |
| 14 | 9.02 | 8.30 | 0 | |
| 21 | 8.64 | 8.08 | 0 | |
| 35 | 8.03 | 7.79 | 0 | |
| 60 | 7.47 | 7.34 | 0 | |
| FC | 1 | 9.28 | 9.18 | 8.99 |
| 14 | 9.15 | 8.84 | 7.92 | |
| 21 | 9.05 | 8.84 | 7.83 | |
| 35 | 8.87 | 8.66 | 7.57 | |
| 60 | 8.81 | 8.58 | 7.09 | |
| IM0.3 | 1 | 9.78 | 9.38 | 8.75 |
| 14 | 9.28 | 9.08 | 8.28 | |
| 21 | 9.22 | 9.00 | 8.20 | |
| 35 | 9.22 | 8.89 | 8.18 | |
| 60 | 8.74 | 8.60 | 8.08 | |
| IM0.6 | 1 | 9.33 | 9.27 | 8.86 |
| 14 | 8.98 | 8.67 | 8.64 | |
| 21 | 8.96 | 8.44 | 8.58 | |
| 35 | 8.95 | 8.40 | 8.46 | |
| 60 | 8.67 | 8.37 | 8.18 | |
| IM1.2 | 1 | 9.53 | 9.12 | 9.10 |
| 14 | 9.26 | 8.88 | 8.93 | |
| 21 | 9.20 | 8.84 | 8.84 | |
| 35 | 9.00 | 8.77 | 8.71 | |
| 60 | 8.95 | 8.63 | 8.33 |
L. casei population level in all produced yogurts at the first storage day ranged from 8.75 to 9.10 logcfu g−1, while after 35 days of storage at 4 °C ranged from 7.57 to 8.71 logcfu g−1 and after 60 days of storage ranged from 7.09 to 8.33 logcfu g−1. Generally, after 35 days of storage all populations are reduced, however yogurts with encapsulated L. casei show higher populations than free cells.
The effect of the adjunct addition (free or encapsulated) on foodborne pathogens and spoilage microorganisms
It is noteworthy that no-growth of Salmonella, of staphylococci, Enterobacteriaccae and coliforms was observed in all samples. However, yeast appeared in yogurts produced only with starter culture and free L. casei after 60 days of storage. Even though, yeasts are not involved in the fermentation process during yogurt production, but they are a major cause of spoilage of the final product (Fleet 1990). No yeast growth was observed in the samples with encapsulated L. casei. The resin matrix is rich in phenolics that probably have some antimicrobial effect, thus protect the produced yogurts from pathogenic and spoilage microorganisms.
Volatile profile of yogurts produced with encapsulated L. casei in resin matrix
Volatile compounds of yogurts produced after incorporation of 0.3 g of encapsulated L. casei per 100 g of yogurt, were determined by SPME/ GC-MS (Table 3). The majority of the determined compounds were monoterpenes comprising more than 60 % of the total volatiles. A-pinene was the major volatile compound detected (~58 % of total compounds) followed by d-limonene, terpinolene, α-terpineol and myrtenol. Monoterpenoids, except for the improvement of yogurt aroma, take part in the inhibition of spoilage microorganisms growth. As mentioned by Kotan et al. (2007), oxygenated monoterpernes exhibit a variable degree of antibacterial activity against 63 bacterial strains. In addition, the essential oils of three Pistacia species, containing mainly α-pinene, β-pinene, limonene and α-terpineol, inhibited the growth of three agricultural pathogenic fungi (Duru et al. 2003). Probably, the detected terpenes (Table 3) with the Pafos’ pissa polyphenols inhibited the growth of pathogenic bacteria.
Table 3.
Chemical composition of volatile compounds in yogurts (% of the total compounds) produced with incorporation of 0.3 g of encapsulated L. casei per 100 g of yogurt
| Compound | Identification method | Kovats index | Kovats index ref | yogurt containing free L.casei | yogurt containing immobilized L.casei in pissa Pafou | % of the total determined organic compounds |
|---|---|---|---|---|---|---|
| Monoterpene compounds | ||||||
| α-pinene | KI, MS | 1,025 | 1,027d | Nd | + | 58,5 |
| 1,020g | ||||||
| 1,019a | ||||||
| Camphene | KI, MS | 1,063 | 1,053b | Nd | + | 0,6 |
| 1,080j | ||||||
| Beta-pinene | KI, MS | 1,105 | 1,113c | Nd | + | 1,5 |
| 1,108a | ||||||
| 3-carene | KI, MS | 1,138 | 1,114e | Nd | + | 0,4 |
| 1,143g | ||||||
| Beta-myrcene | KI, MS | 1,152 | 1,157b | Nd | + | 1,1 |
| 1,149d | ||||||
| 1,158j | ||||||
| 2-carene | MS | 1,164 | N.A. | Nd | + | 0,1 |
| D-limonene | KI, MS | 1,180 | 1,191d | Nd | + | 2,8 |
| 1,189e | ||||||
| Beta-phellandrene | KI, MS | 1,188 | 1,197d | Nd | + | 0,1 |
| o-cymene | MS | 1,250 | N.A. | Nd | + | 1,7 |
| Oxygenated monoterpenes | ||||||
| Eucalyptol | KI, MS | 1,196 | 1,200d | Nd | + | 0,4 |
| Terpinolene | KI, MS | 1,259 | 1,271h | Nd | + | 2,9 |
| Camphenol | MS | 1,482 | N.A. | Nd | + | 0,6 |
| Linalool | KI, MS | 1,531 | 1,544d | Nd | + | 0,7 |
| Bornyl acetate | KI, MS | 1,571 | 1,574h | Nd | + | 2,1 |
| 4 terpineol | KI, MS | 1,593 | 1,602a | Nd | + | 1,1 |
| 1,593c | ||||||
| Pinocarveol | MS | 1,651 | Ν.Α. | Nd | + | 1,4 |
| Verbenol | MS | 1,671 | N.A. | Nd | + | 2,4 |
| a- terpineol | KI, MS | 1,688 | 1,691h | Nd | + | 5,2 |
| 1,700d | ||||||
| 5,8,10 undecatrien-3-ol | MS | 1,716 | N.A. | Nd | + | 3,3 |
| Melilotal | MS | 1,783 | N.A. | Nd | + | 0,4 |
| Myrtenol | KI, MS | 1,787 | 1,789h | Nd | + | 2,9 |
| p-cymene-8-ol | KI, MS | 1,842 | 1,846h | Nd | + | 5,7 |
| Aromatic organic compounds | ||||||
| Toluene | KI, MS | 1,038 | 1,039d | Nd | + | 0,8 |
| 1,035a | ||||||
| Benzene-butyl | MS | 1,120 | N.A. | Nd | + | 0,6 |
| Isopropenyltoluene | MS | 1,423 | N.A. | Nd | + | 2,8 |
MS positive identification by mass spectra obtained from NIST107, NIST21 and SZTERP libraries, Nd Not detected, N.A Not available
a(Shiratsuchi et al. 1993)
b(Gardeli et al. 2008)
c(Högnadóttir and Rouseff 2003)
d(Prompona et al. 2012)
e(Kandylis et al. 2010)
f(Vichi et al. 2007)
g(Lee et al. 2005)
h(Goodner 2008)
Conclusions
The production of a novel probiotic yogurt by introducing L. casei encapsulated in Pistacia terebinthus resin was assessed in the present study. The obtained results showed that encapsulation favored the viability of L. casei in refrigerated storage of yogurts at levels more than 7 log CFU g−1 after 60 days of storage at 4 °C. In yogurt with immobilized cells, the pH values remained at acceptable organoleptic levels (4–4,2). The Pafos’ pissa gave a fine aroma and taste, and pathogenic microorganisms were not detected. Pissa is a promising support for probiotic bacteria since the viscous matrix seem to protect the bacteria from the acidic environment of yogurts.
Acknowledgments
Schoina V. would like to thank the State Scholarships Foundation (ΙΚΥ) for the financial support in the frame of her PhD thesis.
References
- Amatayakul T, Sherkat F, Shah NP. Syneresis in set yogurt as affected by EPS starter cultures and levels of solids. Int J Dairy Technol. 2006;59:216–221. doi: 10.1111/j.1471-0307.2006.00264.x. [DOI] [Google Scholar]
- Birollo GA, Reinheimer JA, Vinderola CGV. Viability of lactic acid microflora in different types of yoghurt. Food Res Int. 2000;33:799–805. doi: 10.1016/S0963-9969(00)00101-0. [DOI] [Google Scholar]
- Bosnea LA, Kourkoutas Y, Albantaki N, Tzia C, Koutinas AA, Kanellaki M. Functionality of freeze-dried L. casei cells immobilized on wheat grains. LWT Food Sci Technol. 2009;42:1696–1702. doi: 10.1016/j.lwt.2009.05.011. [DOI] [Google Scholar]
- Cai S, Zhao M, Fang Y, Nishinari K, Phillips GO, Jiang F. Microencapsulation of Lactobacillus acidophilus CGMCC1.2686 via emulsification/internal gelation of alginate using Ca-EDTA and CaCO3 as calcium sources. Food Hydrocoll. 2014;39:295–300. doi: 10.1016/j.foodhyd.2014.01.021. [DOI] [Google Scholar]
- Champagne CP, Gaudy C, Poncelet D, Neufeld RJ. Lactococcus lactis release from calcium alginate beads. Appl Environ Microbiol. 1992;58:1429–1434. doi: 10.1128/aem.58.5.1429-1434.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SS, Kim Y, Han KS, You S, Oh S, Kim SH. Effects of Lactobacillus strains on cancer cell proliferation and oxidative stress in vitro. Lett Appl Microbiol. 2006;42:452–458. doi: 10.1111/j.1472-765X.2006.01913.x. [DOI] [PubMed] [Google Scholar]
- Dave RI, Shah NP. Ingredient supplementation effects on viability of probiotic bacteria in yogurt. J Dairy Sci. 1998;81:2804–2816. doi: 10.3168/jds.S0022-0302(98)75839-4. [DOI] [PubMed] [Google Scholar]
- Duru ME, Cakir A, Kordali S, Zengin H, Harmandar M, Izumi S, Hirata T. Chemical composition and antifungal properties of essential oils of three Pistacia species. Fitoterapia. 2003;74:170–176. doi: 10.1016/S0367-326X(02)00318-0. [DOI] [PubMed] [Google Scholar]
- Fleet GH. Yeasts in dairy-products. J Appl Bacteriol. 1990;68:199–211. doi: 10.1111/j.1365-2672.1990.tb02566.x. [DOI] [PubMed] [Google Scholar]
- Foligne B, et al. Correlation between in vitro and in vivo immunomodulatory properties of lactic acid bacteria. World J Gastroenterol. 2007;13:236–243. doi: 10.3748/wjg.v13.i2.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardeli C, Vassiliki P, Athanasios M, Kibouris T, Komaitis M. Essential oil composition of Pistacia lentiscus L. and Myrtus communis L.: Evaluation of antioxidant capacity of methanolic extracts. Food Chem. 2008;107:1120–1130. doi: 10.1016/j.foodchem.2007.09.036. [DOI] [Google Scholar]
- Gardini F, Lanciotti R, Guerzoni ME, Torriani S. Evaluation of aroma production and survival of Streptococcus thermophilus Lactobacillus delbrueckii subsp bulgaricus and Lactobacillus acidophilus in fermented milks. Int Dairy J. 1999;9:125–134. doi: 10.1016/S0958-6946(99)00033-3. [DOI] [Google Scholar]
- Gilliland SE, Reilly SS, Kim GB, Kim HS. Viability during storage of selected probiotic lactobacilli and bifidobacteria in a yogurt-like product. J Food Sci. 2002;67:3091–3095. doi: 10.1111/j.1365-2621.2002.tb08864.x. [DOI] [Google Scholar]
- González-Martínez C, Becerra M, Cháfer M, Albors A, Carot JM, Chiralt A. Influence of substituting milk powder for whey powder on yoghurt quality. Trends Food Sci Technol. 2002;13:334–340. doi: 10.1016/S0924-2244(02)00160-7. [DOI] [Google Scholar]
- Goodner KL. Practical retention index models of OV-101, DB-1, DB-5, and DB-wax for flavor and fragrance compounds. LWT Food Sci Technol. 2008;41:951–958. doi: 10.1016/j.lwt.2007.07.007. [DOI] [Google Scholar]
- Granato D, Branco GF, Cruz AG, Faria JDAF, Shah NP. Probiotic dairy products as functional foods comprehensive. Rev Food Sci Food Saf. 2010;9:455–470. doi: 10.1111/j.1541-4337.2010.00120.x. [DOI] [PubMed] [Google Scholar]
- Granato D, de Araújo Calado VM, Jarvis B. Observations on the use of statistical methods in food science and technology. Food Res Int. 2014;55:137–149. doi: 10.1016/j.foodres.2013.10.024. [DOI] [Google Scholar]
- He T, et al. Effects of yogurt and bifidobacteria supplementation on the colonic microbiota in lactose-intolerant subjects. J Appl Microbiol. 2008;104:595–604. doi: 10.1111/j.1365-2672.2007.03579.x. [DOI] [PubMed] [Google Scholar]
- Högnadóttir Á, Rouseff RL. Identification of aroma active compounds in orange essence oil using gas chromatography–olfactometry and gas chromatography–mass spectrometry. J Chromatogr A. 2003;998:201–211. doi: 10.1016/S0021-9673(03)00524-7. [DOI] [PubMed] [Google Scholar]
- Hull RR, Roberts AV, Mayes JJ (1984) Survival of Lactobacillus acidophilus in yoghurt. Aus J Dairy Technol 39
- Kandylis P, Drouza C, Bekatorou A, Koutinas AA. Scale-up of extremely low temperature fermentations of grape must by wheat supported yeast cells. Bioresour Technol. 2010;101:7484–7491. doi: 10.1016/j.biortech.2010.04.031. [DOI] [PubMed] [Google Scholar]
- Keogh MK, O’Kennedy BT. Rheology of stirred yogurt as affected by added milk fat, protein and hydrocolloids. J Food Sci. 1998;63:108–112. doi: 10.1111/j.1365-2621.1998.tb15687.x. [DOI] [Google Scholar]
- Kopsahelis N, Kanellaki M, Bekatorou A. Low temperature brewing using cells immobilized on brewer’s spent grains. Food Chem. 2007;104:480–488. doi: 10.1016/j.foodchem.2006.11.058. [DOI] [Google Scholar]
- Kopsahelis N, Nisiotou A, Kourkoutas Y, Panas P, Nychas GJE, Kanellaki M. Molecular characterization and molasses fermentation performance of a wild yeast strain operating in an extremely wide temperature range. Bioresour Technol. 2009;100:4854–4862. doi: 10.1016/j.biortech.2009.05.011. [DOI] [PubMed] [Google Scholar]
- Kopsahelis N, Bosnea L, Kanellaki M, Koutinas AA (2012) Volatiles formation from grape must fermentation using a cryophilic and thermotolerant yeast. Applied Biochemistry and Biotechnology:1–16 [DOI] [PubMed]
- Kotan R, Kordali S, Cakir A. Screening of antibacterial activities of twenty-one oxygenated monoterpenes Zeitschrift Fur Naturforschung C-a. J Biosci. 2007;62:507–513. doi: 10.1515/znc-2007-7-808. [DOI] [PubMed] [Google Scholar]
- Lamiri A, Lhaloui S, Benjilali B, Berrada M. Insecticidal effects of essential oils against Hessian fly Mayetiola destructor (Say) Field Crop Res. 2001;71:9–15. doi: 10.1016/S0378-4290(01)00139-3. [DOI] [Google Scholar]
- Lee S-J, Umano K, Shibamoto T, Lee K-G. Identification of volatile components in basil (Ocimum basilicum L.) and thyme leaves (Thymus vulgaris L.) and their antioxidant properties. Food Chem. 2005;91:131–137. doi: 10.1016/j.foodchem.2004.05.056. [DOI] [Google Scholar]
- Mani-López E, Palou E, López-Malo A. Probiotic viability and storage stability of yogurts and fermented milks prepared with several mixtures of lactic acid bacteria. J Dairy Sci. 2014;97:2578–2590. doi: 10.3168/jds.2013-7551. [DOI] [PubMed] [Google Scholar]
- Mouhajir F, Hudson JB, Rejdali M, Towers GHN. Multiple antiviral activities of endemic medicinal plants used by Berber peoples of Morocco. Pharm Biol. 2001;39:364–374. doi: 10.1076/phbi.39.5.364.5892. [DOI] [Google Scholar]
- Picot A, Lacroix C. Encapsulation of bifidobacteria in whey protein-based microcapsules and survival in simulated gastrointestinal conditions and in yoghurt. Int Dairy J. 2004;14:505–515. doi: 10.1016/j.idairyj.2003.10.008. [DOI] [Google Scholar]
- Plessas S, Bosnea L, Alexopoulos A, Bezirtzoglou E. Potential effects of probiotics in cheese and yogurt production: a review. Eng Life Sci. 2012;12:433–440. doi: 10.1002/elsc.201100122. [DOI] [Google Scholar]
- Prompona K-D, Kandylis P, Tsakiris A, Kanellaki M, Kourkoutas Y. Application of alternative technologies for elimination of artificial colorings in alcoholic beverages produced by citrus medica and potential impact on human health. Food Nutr Sci. 2012;3:959–969. doi: 10.4236/fns.2012.37127. [DOI] [Google Scholar]
- Sathyabama S, Ranjith Kumar M, Bruntha Devi P, Vijayabharathi R, Brindha Priyadharisini V. Co-encapsulation of probiotics with prebiotics on alginate matrix and its effect on viability in simulated gastric environment. LWT Food Sci Technol. 2014;57:419–425. doi: 10.1016/j.lwt.2013.12.024. [DOI] [Google Scholar]
- Shah NP, Lankaputhra WEV, Britz ML, Kyle WSA. Survival of lactobacillus acidophilus and bifidobacterium bifidum in commercial yoghurt during refrigerated storage. Int Dairy J. 1995;5:515–521. doi: 10.1016/0958-6946(95)00028-2. [DOI] [Google Scholar]
- Shiratsuchi H, Shimoda M, Minegishi Y, Osajima Y. Isolation and identification of volatile flavor compounds in nonfermented coarse-cut sausage. Flavor as a quality factor of nonfermented sausage. J Agric Food Chem. 1993;41:647–652. doi: 10.1021/jf00028a027. [DOI] [Google Scholar]
- Stern B, Heron C, Tellefsen T, Serpico M. New investigations into the Uluburun resin cargo. J Archaeol Sci. 2008;35:2188–2203. doi: 10.1016/j.jas.2008.02.004. [DOI] [Google Scholar]
- Tamime AY, Deeth HC. Yogurt - technology and biochemistry. J Food Prot. 1980;43:939–977. doi: 10.4315/0362-028X-43.12.939. [DOI] [PubMed] [Google Scholar]
- Tharmaraj N, Shah NP. Selective enumeration of lactobacillus delbrueckii ssp. bulgaricus, streptococcus thermophilus, lactobacillus acidophilus, bifidobacteria, lactobacillus casei, lactobacillus rhamnosus, and propionibacteria. J Dairy Sci. 2003;86:2288–2296. doi: 10.3168/jds.S0022-0302(03)73821-1. [DOI] [PubMed] [Google Scholar]
- Tsakiris A, Bekatorou A, Psarianos C, Koutinas AA, Marchant R, Banat IM. Immobilization of yeast on dried raisin berries for use in dry white wine-making. Food Chem. 2004;87:11–15. doi: 10.1016/j.foodchem.2003.10.010. [DOI] [Google Scholar]
- Tuzlaci E, Aymaz PE. Turkish folk medicinal plants. Part IV: gonen (Balikesir) Fitoterapia. 2001;72:323–343. doi: 10.1016/S0367-326X(00)00277-X. [DOI] [PubMed] [Google Scholar]
- Vichi S, Guadayol JM, Caixach J, López-Tamames E, Buxaderas S. Comparative study of different extraction techniques for the analysis of virgin olive oil aroma. Food Chem. 2007;105:1171–1178. doi: 10.1016/j.foodchem.2007.02.018. [DOI] [Google Scholar]
- Vinderola CG, Reinheimer JA. Lactic acid starter and probiotic bacteria: a comparative “in vitro” study of probiotic characteristics and biological barrier resistance. Food Res Int. 2003;36:895–904. doi: 10.1016/S0963-9969(03)00098-X. [DOI] [Google Scholar]