Abstract
Probiotic lactic acid bacteria are health promoters and have been traditionally consumed without the knowledge that they have beneficial properties. These bacteria mainly involve in secreting antimicrobials, enhance immune-modulatory effects, and preserve the intestinal epithelial barrier by competitively inhibiting the pathogenic organisms. The aim of this study was to investigate the in vitro probiotic properties of Lactobacillus pentosus, Lactobacillus plantarum, Lactobacillus plantarum ssp. argentoratensis, and Lactobacillus plantarum ssp. plantarum isolated from fermented Uttapam batter. The isolates produced bacteriocins that were effective against several pathogens. All the isolates exhibited tolerance to bile, gastric, and intestinal conditions. Beneficial properties like cholesterol assimilation and production of enzymes such as β-galactosidase, phytase and bile hydrolase varied among the isolates. Four isolates from each sub-species effectively adhered to Caco-2 cells and prevented pathogen adhesion. Using these strains, the soy milk was fermented, which exhibited higher antioxidant activity, 2,2-diphenylpicrylhydrazyl (DPPH) scavenging activity and decreased phytate content when compared to unfermented soy milk. Thus, these probiotic isolates can be successfully used for formulation of functional foods that thereby help to improvise human health.
Keywords: Antioxidant activity, Lactobacillus, Phytase, Probiotic, Soy milk fermentation, Uttapam batter
Introduction
Lactic acid bacteria (LAB) are a group of organisms that impose health benefits by live colonization in host cells or even by their antimicrobial metabolites through consumption of fermented food products. These bacteria are the normal flora of the gastrointestinal (GI) and urogenital tract and serve as potent probiotics to enhance health-modulating activities. World Health Organization (WHO) defined probiotics as “live microorganisms which when administered in adequate amounts confer health benefits on the host” (WHO/FAO 2002). Probiotic LAB confer several health benefits, which include synthesis of antimicrobial compounds, compete with pathogenic organisms for adhesion to intestinal epithelium, prevent infectious diseases and allergies (Sleator and Hill 2008), manage lactose intolerance, reduce serum cholesterol, provide anti-carcinogenic activity, and enhance immune function (Galdeano et al. 2007). They undergo a very hazardous journey through GI tract and colonize to jeopardize the survival of pathogenic microorganisms (Conway et al. 1987). Several preliminary in vitro tests are essential to qualify an organism to be probiotic such as resistance to gastric acidity, bile, and various antibiotics, and the ability to reduce pathogen adhesion to epithelium. Additional properties like cholesterol assimilation, absence of hemolytic activity, bile salt hydrolase, and β-galactosidase enzyme can expand the understanding of functional properties of these organisms. Nissen et al. (2009) proposed that the intake of functional foods (components include probiotics, prebiotics, polyunsaturated fatty acids (PUFA), antioxidants, vitamins, and minerals) could reduce the risk of several diseases. Lactobacillus plantarum has been widely used as a starter culture in fermentation of several food products like yogurt, cheese, pickles, kimchi, and sourdough (Hammes and Hertel 2006), the attribute of organisms from food extending to colonization in the intestine. Isolation and characterization of bacteriocinogenic (loss of activity after protease treatment) lactobacilli species from fermented Uttapam batter, an indigenous South Indian fermented food source, was studied earlier where Lactobacillus pentosus, Lactobacillus plantarum, Lactobacillus plantarum ssp. argentoratensis, and Lactobacillus plantarum ssp. plantarum were identified at sub-species level using molecular tools such as 16S rRNA and multiplex PCR (Saraniya and Jeevaratnam 2012). Soy milk is an economical protein and calorie-rich food consumed in several parts of the world. But unfermented soy milk contains high amount of phytate, an anti-nutrient factor that prevents absorption of minerals. Most of the earlier published works focused on the growth of LAB, pH and isoflavone content in soy milk but did not detail the reduction of phytate content in the soy milk (Chen et al. 2013; Li et al. 2012). Hence, an attempt was made in this study to evaluate the probiotic properties of Lactobacillus pentosus, Lactobacillus plantarum, Lactobacillus plantarum ssp. argentoratensis, and Lactobacillus plantarum ssp. plantarum in vitro and the phytate content and antioxidant properties in fermented soy milk using these isolates as compared to unfermented soy milk.
Methodology
Strains and antibacterial assay
Potent isolates were isolated from fermented Uttapam batter and identified using 16S rRNA gene analysis and were deposited in GenBank, NCBI. Antibacterial activity assay using cell-free supernatant concentrate (CFSC) adjusted to pH 6.0 was carried out against intestinal pathogens (Table 1) by agar well diffusion assay (Saraniya and Jeevaratnam 2012). The pathogens used were obtained from the Microbial Type Culture Collection (MTCC), at Institute of Microbial Technology, Chandigarh, India, and maintained in MRS broth for LAB isolates and soya bean casein digest broth for non-LAB organisms and stored at −20 °C with 30 % glycerol.
Table 1.
Antibacterial spectrum of the eight potent isolates against LAB and pathogens
| Isolates | SJ65 | SJ5 | SJ22 | SJ6 | SJ37 | SJ40 | SJ33 | SJ9 |
|---|---|---|---|---|---|---|---|---|
|
Lactobacillus rhamnosus
MTCC 1408 |
10 ± 1 | 12 ± 1 | 12 ± 1 | 11 ± 1 | 10 ± 1 | 12 ± 1 | 11 ± 2 | 12 ± 2 |
|
Enterococcus faecalis
MTCC 439 |
11 ± 1 | 10 ± 1 | 11 ± 1 | 11 ± 1 | 10 ± 1 | 10 ± 2 | 11 ± 2 | 12 ± 2 |
|
Staphylococcus aureus
MTCC 737 |
18 ± 3 | 17 ± 2 | 17 ± 3 | 16 ± 2 | 16 ± 4 | 16 ± 3 | 15 ± 3 | 18 ± 3 |
|
Listeria monocytogenes
MTCC 657 |
20 ± 2 | 14 ± 3 | 15 ± 2 | 15 ± 2 | 15 ± 3 | 10 ± 2 | 14 ± 2 | 15 ± 2 |
|
Bacillus cereus
MTCC 1272 |
20 ± 2 | 20 ± 3 | 15 ± 2 | 18 ± 3 | 15 ± 3 | 18 ± 4 | 17 ± 2 | 19 ± 4 |
|
Vibrio parahemolyticus
MTCC 451 |
17 ± 3 | 16 ± 3 | 15 ± 2 | 16 ± 2 | 14 ± 4 | 15 ± 2 | 16 ± 2 | 16 ± 3 |
|
Aeromonas hydrophila ssp. hydrophilia
MTCC 1739 |
19 ± 2 | 17 ± 2 | 18 ± 3 | 17 ± 2 | 14 ± 3 | 18 ± 4 | 17 ± 3 | 19 ± 3 |
|
Escherichia coli
MTCC 728 |
18 ± 3 | 16 ± 3 | 16 ± 2 | 14 ± 2 | 19 ± 3 | 13 ± 2 | 18 ± 4 | 15 ± 2 |
SJ65 – Lactobacillus pentosus, SJ5 and SJ22 – Lactobacillus plantarum, SJ6, SJ33, SJ37, SJ40 – Lactobacillus plantarum ssp. argentoratensis, SJ9 – Lactobacillus plantarum ssp. plantarum. Inhibition zone expressed in mm inclusive of well diameter 6 mm. Values are mean ± SD of 3 independent experiments performed in duplicates
Bile tolerance test
Bile tolerance test was carried out by growing the lactobacilli isolates in MRS broth containing 0, 0.1, 0.3 and 0.5 % of oxgall bile (Himedia, India) for 24 h at 37 °C. The growth of cells was monitored at 560 nm for every 1 h till 8 h and after 24 h. The delay in growth was calculated against time, which was used as a measure of bile tolerance (de Valdez and de Taranto 2001).
Screening of isolates for tolerance to various pH and simulated gastric and intestinal juice
Initial screening of isolates for tolerance to various initial pH (2.0, 3.0, 7.0) was done. The isolates were grown in MRS broth and the cells were pelleted in sterile condition. The cells of 108 colony forming units (cfu) were suspended in sterile saline solution, the pH of which was adjusted with 1 M HCl and incubated at 37 °C for 0, 2, and 4 h, respectively. The cell suspension was then plated on MRS agar, incubated at 37 °C, and the cfu was calculated. Further the isolates were subjected to in vitro simulated gastric and intestinal tolerance test. The cells were washed with saline, and the cells (108 cfu/ml) were suspended in saline adjusted to pH 2.0 with 3 mg/ml of pepsin for gastric tolerance and by addition of 0.3 % bile salt and 1 mg/ml pancreatin adjusted to pH 8.0 for intestinal tolerance. Cell suspension in saline with pH 7.0 serves as control. After 0, 2, 4, and 6 h of incubation at 37 °C, the cells were serially diluted and plated on MRS agar and the cfu was calculated (Osmanagaoglu et al. 2010).
Bacterial adhesion to hydrocarbons (BATH assay)
The in vitro bacterial cell adhesion to hydrocarbons was determined by the method of Doyle and Rosenberg (1995) with a few modifications. Isolates grown in MRS broth were centrifuged and the pellet was washed twice and suspended in phosphate buffer (pH 7.0) adjusted to 1.0 A560 using spectrophotometer (Shimadzhu, Japan). Equal volume of solvent (hexadecane and xylene) and bacterial suspension was added and vortexed for 2 min. The tubes were allowed to stand at 37 °C for 30 min for the separation of two phases. The aqueous phase (A) was carefully removed and the OD at 560 nm was measured. The percentage of cell surface hydrophobicity (%H) of the strain adhering to solvents was calculated using the formula,
where ODI and ODF are initial and final absorbance.
Auto-aggregation and co-aggregation property
For auto- and co-aggregation, cells were pelleted by centrifugation at 8,000 g for 10 min and OD adjusted to 1.0 A560 using spectrophotometer and washed twice in 50 mM phosphate buffer (pH 7.0). The auto- and co-aggregation were performed as described by Osmanagaoglu et al. (2010). For co-aggregation studies, the isolates were incubated along with Lactobacillus rhamnosus (MTCC 1408), Lactobacillus plantarum (MTCC 6161), Escherichia coli (MTCC 728), and Listeria monocytogenes (MTCC 657). The percentage of auto-aggregation and co-aggregation was calculated using the formula,
where ODI and ODF are initial and final absorbance.
Adhesion to human epithelial carcinoma cell line Caco-2
The colonic adenocarcinoma cells (Caco-2) were procured from NCCS (National Centre for Cell Sciences), Pune, India. The cells were maintained in minimal essential medium (MEM) with 10 % fetal bovine serum (Sigma, India), 25 mM HEPES buffer, 20 U/ml penicillin, and 100 μg/ml of streptomycin. For adhesion assay, 105 cells were transferred to six-well plate to obtain confluence after a period of 15 days. The bacterial cells (108 cfu/ml) were suspended in supplement-free MEM, added to Caco-2 cells, and incubated for 90 min. After incubation, the cells were fixed with 2 % formaldehyde, stained with 0.1 % acridine orange, and visualized under fluorescence microscope. For pathogen exclusion assay, the Caco-2 cells were treated with isolates as described above, followed by addition of Listeria monocytogenes (108 cfu/ml in supplement-free MEM), incubated for an additional 60 min, and stained with 0.1 % acridine orange. The cells were collected and plated on either MRS agar, incubated anaerobically or Listeria enrichment broth (Himedia, Mumbai) for the selective enumeration of lactic acid bacteria and Listeria monocytogenes respectively. The exclusion percentage was determined using the formula,
where AI is the initial cfu and AF is the final cfu of Listeria monocytogenes after incubation (Osmanagaoglu et al. 2010).
Antibiotic susceptibility test
The isolates were checked for antibiotic susceptibility in MRS soft agar, inoculated using 108 cfu/ml with respective cultures that were allowed for solidification at room temperature. The commercial antibiotic disc (Himedia, India) was placed on to the agar and incubated at 37 °C for 24 h, and the resistance or sensitivity was noted according to the CLSI/NCCLS standard (CLSI 2011).
Cholesterol assay
MRS broth having 0.3 % bile oxgall was sterilized and to this a final concentration of 100 μg/ml of sterile cholesterol was added (MRS-CHO) and inoculated with 1 % (v/v) with an overnight culture of the respective isolates. Uninoculated MRS-CHO broth was also processed in the same way as the control. Cholesterol estimation was done spectrophotometrically by o-pthalaldehyde method (de Valdez and de Taranto 2001).
where C is the control (MRS-CHO without any inoculums) and T is the test (MRS-CHO inoculated with isolates).
Detection of bile salt hydrolase activity
The presence of bile hydrolase enzyme in the Lactobacillus strains was detected by culturing the bacteria on MRS agar containing 0.5 % (w/v) taurodeoxycholic acid (TDCA) and incubated under anaerobic conditions for 72 h. Deconjugation of taurodeoxycholate results in a white precipitate of deoxycholate in the area of bacterial colonies (Dashkevicz and Feighner 1989).
β-galactosidase activity
The isolates were evaluated for β-galactosidase activity by growing in MRS broth having lactose, and the cells were recovered by centrifugation at 8,000 g for 10 min. The cells were washed with saline, suspended in Z buffer (60 mM Na2HPO4, 40 mM NaH2PO4, and 50 mM β-mercaptoethanol pH 7.0), and permeabilized with toluene. The permeabilized cells were incubated with ortho-nitrophenyl-β-galactoside (4 mg/ml) at 37 °C for 15 min and the reaction was stopped by addition of 1 % sodium bicarbonate. The absorbance was read at 550 and 420 nm, respectively, and the enzyme unit was expressed in Miller units (Vinderola and Reinheimer 2003).
where the absorbance at 420 nm denotes the end product color, while the absorbance at 560 nm denotes the correction factor for cell turbidity.
Hemolytic activity in blood agar
Hemolysis in blood agar for the isolates was evaluated using blood agar base supplemented with 5 % of human blood as detailed by Gao et al. (2012). Escherichia coli for α-hemolysis and Streptococcus pyogenes for β-hemolysis were used as positive control.
Screening of phytase production
The isolates were screened initially for phytase production as described by Anastasio et al. (2010). The isolates were spotted in modified MRS agar containing phytate and incubated at 37 °C for 48 h. The clearing of zone around the spot after incubation with 2 % cobalt chloride solution for 20 min indicates positive for phytase production.
Phytase assay
The isolate was grown in phytate-containing Chalmers broth for 24 h at 35 °C and the CFS was collected by centrifugation at 10,000 g for 15 min. One milliliter of CFS was mixed with 0.6 ml of reaction mixture (3 mM phytate in 0.2 M sodium acetate buffer pH 4.5) and incubated at 50 °C for 15 min. The reaction was terminated by addition of 1 ml of 10 % TCA. The released inorganic phosphate was measured by addition of 0.75 ml of coloring reagent prepared freshly (4 volumes of ammonium molybdate in 5.5 % sulfuric acid and 1 volume of 2.7 % ferrous sulfate solution). The absorbance was measured at 700 nm. Phytase activity was measured in μmol of inorganic phosphate released from phytate. The Chalmers broth was used as negative control. One unit of phytase is defined as the amount of enzyme required to produce 1 μmol of inorganic phosphate per minute under assay conditions (Anastasio et al. 2010).
Preparation and fermentation of soy milk
Soybeans were soaked in three volumes of distilled water at ambient temperature for 10 h. The hydrated beans were dehulled manually and ground with distilled water with a ratio of 1:5 w/v and filtered through double layer cheesecloth. The resultant soy milk in the filtrate was sterilized at 121 °C for 15 min. The isolate was grown in MRS broth until the cells attained mid log phase and harvested by centrifugation at 8,000 g for 10 min. After washing, the cells were suspended in sterile saline (0.85 % NaCl). Five milliliter of soy milk was inoculated with 2 % (v/v) of the isolates and incubated at 35 °C for 18 h. Uninoculated soy milk was used as control.
Estimation of phytate content
The phytate content in fermented and unfermented soy milk was extracted with trichloroacetic acid and precipitated with ferric salt. The iron content in the precipitate was determined using potassium thiocyanate and the phytate phosphorous was calculated assuming the constant 4Fe:6P molar ratio (Sadasivam and Manickam 2008).
Antioxidant activity of fermented and unfermented soy milk
2, 2-diphenylpicrylhydrazyl (DPPH) scavenging activity
An aliquot of 0.5 ml of sample was mixed with 1 ml of methanolic DPPH (final concentration: 0.1 mmol/L) and incubated in dark for 30 min. After incubation, the absorbance was read at 517 nm against reagent blank (Marazza et al. 2012). The DPPH scavenging activity was expressed in percentage using the formula given below:
Reducing power property
For evaluating the reducing power, 1 ml of PBS (phosphate buffered saline), 1 ml of 1 % (w/v) potassium ferricyanide, and 0.5 ml of sample were mixed together and incubated at 50 °C for 30 min. Then 1 ml of 10 % (w/v) TCA was added and the mixture was centrifuged at 8,000 g for 10 min. To 2 ml of supernatant, 0.4 ml of 0.1 % (w/v) of ferric chloride wad added and vortexed. After incubation for 10 min at room temperature, the absorbance was measured at 700 nm. The higher the absorbance of reaction mixture, higher the reducing power (Marazza et al. 2012).
Statistical analysis
The results are expressed as mean and standard deviation of 3 independent experiments performed in duplicate. The statistical analysis was performed using OriginPro v8. The results were compared using analysis of variance (ANOVA) by Turkey’s pot-hoc test and p < 0.05 was considered as statistically significant.
Results and Discussion
The isolates from Uttapam batter were identified as Lactobacillus pentosus, Lactobacillus plantarum, Lactobacillus plantarum ssp. plantarum, and Lactobacillus plantarum ssp. argentoratensis with accession numbers JN573616 to JN573623 (Saraniya and Jeevaratnam 2012). These isolates were evaluated for probiotic and beneficial properties to assess their suitability for the formulation of functional foods and biomedical applications. The present study also intends to establish the phytate content and antioxidant properties in fermented soy milk, studied using the Uttapam batter isolates. Thus identification of strains having antimicrobial, probiotic and other beneficial potential from different ecological niches and utilization of those collections of strains together for the formulation of functional foods would be advantageous to humans.
Antibacterial spectrum against LAB and pathogenic organisms
The antimicrobial activity (Table 1) of Uttapam batter LAB isolates was minimal or no activity against LAB indicators signifying that the isolates do not pose any threat to other LAB strains, while these isolates showed effective inhibitory activity against some intestinal Gram-positive and Gram-negative pathogens suggesting effective antimicrobial activity against pathogens. Earlier study showed that the eight isolates were bacteriocinogenic in nature as there was loss of activity after protease treatment (Saraniya and Jeevaratnam 2012).
Bile, simulated gastric, and intestinal tolerance test
Probiotic bacteria have to essentially prevail through stress environments, acidic stomach, and then through hydrolytic activity of enzymes including bile salt in the small intestine. Morelli (2000) proposed that the pH of stomach varies between pH 1.5 and 3.0. In cases of starvation and stress conditions, the pH usually declines to 1.5, while it increases to 3.0 after meal. Initial screening showed the eight isolates were tolerant to acidic and neutral pH. The isolates showed tolerance to simulated gastric and intestinal conditions with minimal decrease in cfu (Table 2). In addition, the isolates were resistant to 0.3 % bile exhibiting delay in growth of 1 to 7 h (Table 3), which was similar to the observations made by Morelli (2000) where the growth of Lactobacillus in the presence of oxgall is not species-dependent but strain-dependent. This study also showed that tolerance varied among the organisms, indicating that it was mostly strain-dependent.
Table 2.
Simulated gastric and intestinal tolerance of the eight isolates
| Isolates | Conditions | Log cfu/ml | |||
|---|---|---|---|---|---|
| 0 | 2 | 4 | 6 h incubation | ||
| SJ65 | Control | 9.30 ± 0.39 | 9.31 ± 0.37 | 9.19 ± 0.15 | ND |
| Artificial gastric juice | 9.52 ± 0.50 | 7.53 ± 0.47 | 6.88 ± 0.82 | ND | |
| Artificial intestinal juice | 9.27 ± 0.48 | ND | 8.66 ± 0.33 | 8.12 ± 0.41 | |
| SJ9 | Control | 9.44 ± 0.28 | 9.26 ± 0.36 | 9.17 ± 0.36 | ND |
| Artificial gastric juice | 9.37 ± 0.49 | 7.64 ± 0.29 | 7.14 ± 0.56 | ND | |
| Artificial intestinal juice | 9.26 ± 0.27 | ND | 8.15 ± 0.20 | 7.76 ± 0.28 | |
| SJ5 | Control | 9.44 ± 0.61 | 9.62 ± 0.21 | 9.00 ± 0.64 | ND |
| Artificial gastric juice | 9.34 ± 0.50 | 7.94 ± 0.14 | 6.65 ± 0.39 | ND | |
| Artificial intestinal juice | 9.41 ± 0.33 | ND | 8.08 ± 0.26 | 7.05 ± 0.34 | |
| SJ22 | Control | 9.46 ± 0.37 | 9.15 ± 0.57 | 9.17 ± 0.47 | ND |
| Artificial gastric juice | 9.11 ± 0.49 | 7.33 ± 0.39 | 7.31 ± 0.32 | ND | |
| Artificial intestinal juice | 9.09 ± 0.27 | ND | 8.34 ± 0.39 | 8.47 ± 0.53 | |
| SJ6 | Control | 9.34 ± 0.39 | 9.20 ± 0.24 | 9.21 ± 0.40 | ND |
| Artificial gastric juice | 9.79 ± 0.15 | 7.51 ± 0.33 | 6.87 ± 0.09 | ND | |
| Artificial intestinal juice | 9.36 ± 0.31 | ND | 7.91 ± 0.16 | 8.07 ± 0.64 | |
| SJ33 | Control | 9.40 ± 0.42 | 9.52 ± 0.39 | 9.26 ± 0.40 | ND |
| Artificial gastric juice | 9.64 ± 0.29 | 7.45 ± 0.30 | 7.14 ± 0.40 | ND | |
| Artificial intestinal juice | 9.51 ± 0.62 | ND | 8.52 ± 0.28 | 7.41 ± 0.32 | |
| SJ37 | Control | 9.16 ± 0.37 | 9.14 ± 0.59 | 9.34 ± 0.49 | ND |
| Artificial gastric juice | 8.61 ± 0.22 | 8.34 ± 0.38 | 7.56 ± 0.21 | ND | |
| Artificial intestinal juice | 8.28 ± 0.36 | ND | 8.01 ± 0.25 | 7.80 ± 0.17 | |
| SJ40 | Control | 9.69 ± 0.19 | 9.24 ± 0.53 | 9.51 ± 0.33 | ND |
| Artificial gastric juice | 8.30 ± 0.38 | 8.68 ± 0.29 | 7.30 ± 0.72 | ND | |
| Artificial intestinal juice | 8.20 ± 0.56 | ND | 8.06 ± 0.36 | 8.02 ± 0.44 | |
Values are mean ± SD of three independent experiments performed in duplicate. ND – not determined
Table 3.
Survival in intestinal condition, Cell surface properties and Adhesion to intestinal cells by the eight isolates from Uttapam batter
| SJ65 | SJ9 | SJ5 | SJ22 | SJ6 | SJ33 | SJ37 | SJ40 | |
|---|---|---|---|---|---|---|---|---|
| Bile tolerance @ | 4 h | 2 h | 2 h | 2 h | 1 h | 6 h | 1 h | 7 h |
| Cholesterol assimilation* | 73 ± 3a | 71 ± 2a | 56 ± 5b | 60 ± 4b | 44 ± 3c | 48 ± 5c | 47 ± 6c | 44 ± 4c |
| β-Galactosidase (MU)# | 2225 ± 153a | 1574 ± 92b | 835 ± 70c | 1436 ± 124b | 1747 ± 29b | 1416 ± 161b | 1849 ± 336b | 1749 ± 81b |
| Bile Salt hydrolase | + | – | – | – | + | + | + | + |
| Hemolysis | – | – | – | – | – | – | – | – |
| Hydrophobicity* | ||||||||
| Xylene | 69 ± 2a | 77 ± 4b | 11 ± 2c | 94 ± 3d | 40 ± 3e | 40 ± 6e | 94 ± 2d | 32 ± 4e |
| Hexadecane | 61 ± 3a | 65 ± 3a | 33 ± 2b | 70 ± 3c | 35 ± 3b | 53 ± 3d | 92 ± 3e | 56 ± 2d |
| Auto-aggregation* | 63 ± 1a | 69 ± 2b | 48 ± 4c | 55 ± 5c | 55 ± 4c | 57 ± 5c | 76 ± 3d | 62 ± 1a |
| Co-aggregation* | ||||||||
| Lactobacillus rhamnosus | 57 ± 3a | 55 ± 2a | 69 ± 2b | 55 ± 3a | 50 ± 3c | 45 ± 2c | 57 ± 4a | 55 ± 3a |
| Lactobacillus plantarum | 61 ± 5a | 58 ± 4a | 49 ± 3b | 60 ± 4a | 46 ± 4c | 65 ± 2a | 59 ± 2a | 51 ± 1c |
| Listeria monocytogenes | 67 ± 4a | 61 ± 5a | 41 ± 6b | 63 ± 4a | 53 ± 5c | 56 ± 5a | 68 ± 5a | 39 ± 3b |
| Escherichia coli | 63 ± 4a | 65 ± 5a | 50 ± 4b | 57 ± 3a | 64 ± 4a | 61 ± 3a | 69 ± 3a | 48 ± 3b |
| Caco-2 cell adhesion* | 17.6 ± 2.9a | 15.6 ± 3.4a | ND | 14.9 ± 2.8a | ND | ND | 17.3 ± 4.8a | ND |
| Pathogen exclusion* | 84 ± 4a | 76 ± 5a | ND | 64 ± 2b | ND | ND | 79 ± 3a | ND |
Values are mean ± SD of three independent experiments performed in duplicate. ND – not determined
@ Time delay for bile tolerance was calculated as time taken to increase OD560 by 0.3 units
* expressed in %
# expressed in miller units
a,b,c,d,e Means within the same row bearing different superscripts differ significantly (p < 0.05, ANOVA test)
Aggregation studies for the promising isolates
It is a desirable quality for a probiotic to adhere and aggregate on to the host cell to elicit the beneficial effects. Cesena et al. (2001) proposed that the isolates that have the ability to aggregate are generally hydrophobic in nature and can withstand to survive well in the intestinal tract. The isolates were evaluated for hydrophobicity using solvents xylene and hexadecane, and all isolates showed varying hydrophobicity (Table 3). Maximal hydrophobicity was shown by isolate SJ37. A correlation was observed between BATH assay and autoaggregation results wherein Lactobacillus plantarum ssp. argentoratensis SJ37 showed maximal hydrophobicity and aggregation. The hydrophobicity and the autoaggregation ability are two independent factors that are essentially required for adhesion. All the eight isolates showed effective co-aggregation against the viable pathogens Listeria monocytogenes and Escherichia coli, with >50 % aggregation property, while an earlier study on co-aggregation using Lactobacillus rhamnosus LC-705 and Lactobacillus fermentum ME-3 showed 29 and 25 %, respectively, against Bifidobacterium vulgatus whereas Bifidobacterium longum 46 and Bifidobacterium lactis 420 showed only 5 and 4 %, respectively, against Bifidobacterium vulgates (Collado et al. 2008).
Antibiogram for the eight isolates
Lactobacilli group of organisms naturally exhibits resistance to wide range of antibiotics, and the susceptibility testing of each strain is essential (Charteris et al. 1998). The eight isolates were studied for susceptibility/resistance to various groups of antibiotics, and the results are presented in Table 4. Isolates SJ22, SJ37 and SJ65 were found to be resistant to most of the antibiotics, while the other isolates were susceptible to most of the protein synthesis inhibitors and some cell wall synthesis inhibitors. However, all isolates were resistant to nucleic acid synthesis inhibitors except Co-trimoxazole and Nitrofurantoin. Earlier reports have shown that LAB has intrinsic antibiotic resistance genes to antibiotics like tetracycline (tet), erythromycin (erm), chloramphenicol (cat), and vancomycin (van). These resistances are usually due to mutation at chromosomal or plasmid level and the genes are transferred either to the same group or to the pathogens by the mechanism of horizontal gene transfer (Ashraf and Shah 2011; Todorov et al. 2012). Resistance to aminogylcosides and nucleic acids inhibitors varied among the isolates, while all showed resistance to lipopolysaccharide inhibitor.
Table 4.
Antibiotic sensitivity/resistance properties for the eight LAB isolates
| Antibiogram | SJ5 | SJ22 | SJ6 | SJ33 | SJ37 | SJ40 | SJ9 | SJ65 |
|---|---|---|---|---|---|---|---|---|
| Cell wall synthesis inhibitors | ||||||||
| Penicillin (10 mcg) | S | S | S | S | S | S | S | S |
| Amphicillin (10 mcg) | S | S | S | S | S | S | S | S |
| Cefuroxime (30 mcg) | S | S | S | S | S | S | S | S |
| Ceftriaxone (30 mcg) | S | S | S | S | S | S | S | S |
| Cephalexin (30 mcg) | S | R | R | S | R | R | S | R |
| Cepharadine (30 mcg) | S | R | R | S | R | R | S | R |
| Cephaloridine (30 mcg) | S | S | R | S | S | S | S | R |
| Cloxacillin (5 mcg) | S | R | R | S | S | R | S | R |
| Mecillinam (30 mcg) | R | S | R | S | R | R | R | R |
| Protein synthesis inhibitors | ||||||||
| Chloramphenicol (25 mcg) | S | S | S | S | S | S | S | S |
| Tetracycline (10 mcg) | S | S | S | S | S | S | S | S |
| Erythromycin (10 mcg) | S | S | S | S | S | S | S | S |
| Amikacin (10 mcg) | S | S | S | S | R | S | S | R |
| Gentamycin (30 mcg) | S | R | S | S | R | R | S | R |
| Lincomycin (10 mcg) | S | R | S | S | R | S | S | R |
| Streptomycin (10 mcg) | S | S | S | R | R | S | S | R |
| Nucleic acid inhibitors | ||||||||
| Ciprofloxacin (1 mcg) | R | R | R | R | R | R | R | R |
| Co-trimoxazole (25 mcg) | S | S | S | S | S | S | S | S |
| Nalidixic acid (30 mcg) | R | R | R | R | R | R | R | R |
| Norfloxacin (300 mcg) | R | R | R | R | R | R | R | R |
| Nitrofurantoin (300 mcg) | S | S | S | S | S | S | S | S |
| Lipopolysacharide inhibitor | ||||||||
| Colistin (10 mcg) | R | R | R | R | R | R | R | R |
R Resistance, S Sensitive; mcg microgram
Beneficial properties of the isolates
Beneficial properties like the presence of β-galactosidase and bile salt hydrolase activity, cholesterol sequestering ability, and absence of hemolysis are presented in Table 3. Microbial β-galactosidase is currently of interest because it is utilized for bioprocess technology. β-galactosidase (lactase) is an enzyme that hydrolyzes glycosidic bonds, and the insufficiency of this enzyme leads to a condition called lactose intolerance with clinical symptoms like abdominal cramps and diarrhea. In this study, β-galactosidase production ranged between 835 and 2225 MU, where the maximal production was by Lactobacillus pentosus SJ65 followed by Lactobacillus plantarum ssp. plantarum SJ9. Earlier, Wang et al. (2010) showed maximal β-galactosidase production by Lactobacillus paracasei F08 and Lactobacillus acidophilus C11 isolated from infant feces and pickled cabbage. Bile acids are synthesized from cholesterol and conjugated with either glycine or taurine to form bile salts. The intestinal bacteria produce bile salt hydrolase enzyme and prevent bile salt toxicity (De Smet et al. 1995). The BSH-negative cells are more prone to bile salt toxicity through intracellular acidification (Begley et al. 2006). In this study, out of eight isolates Lactobacillus pentosus SJ65 and Lactobacillus plantarum ssp. argentoratensis SJ6, SJ33, SJ37, and SJ40 showed the presence of bile hydrolase in the medium containing TDCA, while Lactobacillus plantarum SJ5, SJ22 and Lactobacillus plantarum ssp. plantarum SJ9 did not show bile salt hydrolase activity.
Probiotics are known to have hypocholesterolemic effects by its ability to decrease cholesterol level in small intestine and help to prevent coronary heart disease. In this study, maximal cholesterol assimilation was observed by Lactobacillus pentosus SJ65, followed by Lactobacillus plantarum ssp. plantarum SJ9 with 73 and 71 %. Kimoto et al. (2002) have demonstrated the cholesterol-lowering ability by probiotics and the probable mechanisms involved. Cholesterol can be removed either by the process of assimilation or through deconjugation of the bile acids facilitating excretion or by cell surface binding with both live and dead cells of probiotic bacteria. They have also proposed that the incidence of cholesterol binding by dead cells was less compared to that of live cells. Although several debates still persist regarding the function of bile salt hydrolase activity and cholesterol assimilation by these bacteria, yet these assays are performed additionally to understand the functionality of these organisms to human health. The eight probiotic isolates were non-hemolytic, when grown in blood agar medium, and hence they can be used safely in animal and human applications that may lead to formulation of functional foods.
Caco-2 cell adhesion for the promising isolates
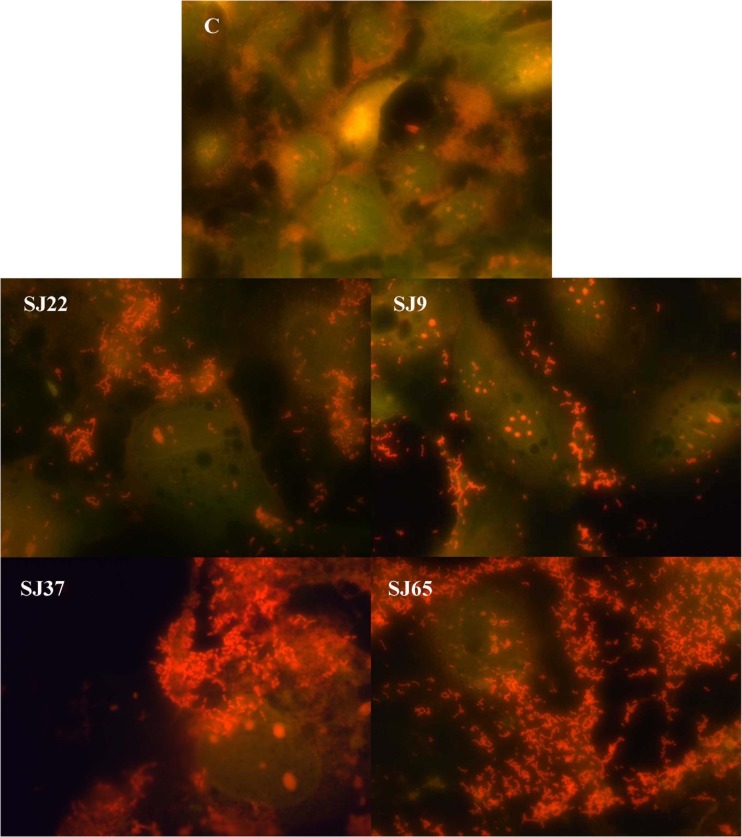
Adherence of bacteria to the hydrophobic surface is an essential property, which leads to aggregation on to the host cell. Adhesion to intestine is an effective property that allows colonization in GI tract. Based on the acid and bile tolerance, aggregation and beneficial properties, four potent isolates from each sub-species were chosen for cell adhesion analysis using Caco-2 cell lines (Fig. 1). In this study, adhesion of the isolates on to cell lines varied between 10 and 20 % (Table 3), while there was not any significant difference among isolates. An earlier study showed 6 to 8 % adhesion for Lactobacillus plantarum (Tuomola and Salminen 1998). In addition, the prevention of pathogen adhesion by the isolates showed 75 to 85 % exclusion by each sub-species, wherein maximal prevention of pathogen adhesion (84 %) was observed by Lactobacillus pentosus SJ65 against Listeria monocytogenes. Earlier studies also demonstrated that Lactobacillus acidophilus LB inhibited the adhesion of Escherichia coli, Salmonella typhimurium, and Listeria monocytogenes, while Lactobacillus casei ssp. rhamnosus Lcr35 against Escherichia coli and Klebsiella pneumoniae and also Lactobacillus acidophilus and Lactobacillus casei inhibited Helicobacter pylori NCTC 11637 using Caco-2 cell lines (Forestier et al. 2001; Midolo et al. 1995). Interactions between probiotics and intestinal mucus are very essential because they help to potentially prevent the adhesion of enteropathogenic organisms. An adherent probiotic bacterium effectively mediates several functions like competitive exclusion of pathogenic bacteria and modulation of immune function by interacting with gut-associated lymphoid tissue (GALT). The possible mechanisms by which pathogen adhesion is prevented could be by either specific blockage on cell receptors or steric interactions.
Fig. 1.
Adhesion of lactobacillus on to cell lines stained with acridine orange and observed under fluorescent microscope. C – Control; SJ22 – Lactobacillus plantarum; SJ9 – Lactobacillus plantarum ssp. plantarum; SJ37 – Lactobacillus plantarum ssp. argentoratensis; SJ65 – Lactobacillus pentosus
Phytase and antioxidant activity in unfermented and fermented soy milk by probiotic isolates
Phytate is a well-known anti-nutrient factor that prevents/reduces bioavailability of minerals like iron, calcium, magnesium, and zinc, and proteins in the human intestine. The phosphate in phytate cannot be absorbed by animals because they lack phytase enzyme. Phytase acts by hydrolyzing phytate into myoinositol and phosphate, thereby eliminating its anti-nutritional effects (Greiner and Konietzny 2006). The phytase produced by the microbial source, especially by LAB, is having more commercial applications. The isolates produced phytase at varied concentration; the isolate Lactobacillus pentosus SJ65 produced maximum (Table 5). The normal level of phytate content in soy beans varies from 9.2 to 16.7 mg/g of dry matter (Chen et al. 2013), which limits the usage of raw soy milk. Microbial fermentation of soy milk helps to degrade and enhance the nutritional value of soy milk. Among the four isolates, Lactobacillus pentosus SJ65 has decreased 66 % of phytate content in fermented soy milk as compared to the unfermented soy milk (Table 5), which was more than that produced by Leuconostoc mesenteroides KC51 isolated from Kimchi, which degraded only 50 % of phytate after 18 h of incubation (Oh and In 2009). Apart from the phytase activity removing anti-nutritional effects, these isolates also caused enhanced antioxidant activity in the final product (Table 5). The antioxidant-rich foods have shown highly beneficial effect on human health. Among the isolates, Lactobacillus pentosus SJ65 exhibited 87 % DPPH radical scavenging property and maximum reducing power (A700: 1.74). Thus value addition can be achieved in the soy milk fermented with these LAB isolates. The enhanced antioxidant activity of fermented soy milk may be because of increased free phenolic content. The isoflavone, a predominant phenolic compound in soy milk, is metabolized by the bacteria and converted into health promoting compounds that have higher antioxidant activity (Chen et al. 2013). Telang et al. (2010) have also shown that approximately 50 % increased the concentration of daidzein and genistein, metabolic products of isoflavone, in fermented soy milk as compared to unfermented soy milk. Moreover, the fermentation by LAB leads to degradation of the soy milk protein into tripeptides and amino acids, which along with phenolic content exhibited synergistic antioxidant activity (Saito et al. 2003). As the probiotic isolate Lactobacillus pentosus SJ65 degrades phytate and exhibits greater antioxidant activity than other isolates, it can be used as the starter culture in cereal-based fermentation and supplied with diet.
Table 5.
Phytase and antioxidant activity in unfermented and fermented soy milk by probiotic isolates
| Isolates | Phytase production * (Units/ml) |
Phytate content # (%) |
DPPH scavenging$ (%) |
Reducing property @ (A700) |
|---|---|---|---|---|
| Unfermented soy milk | NIL | 100 | 71 ± 5.64 | 1.15 ± 0.04 |
| Fermented soy milk | ||||
| SJ9 | 1.8 ± 0.03 | 56 ± 4.98 | 74 ± 3.64 | 1.36 ± 0.05 |
| SJ22 | 1.8 ± 0.07 | 58 ± 4.26 | 76 ± 4.65 | 1.52 ± 0.06 |
| SJ37 | 1.5 ± 0.06 | 47 ± 5.61 | 72 ± 2.57 | 1.32 ± 0.03 |
| SJ65 | 2.1 ± 0.08 | 66 ± 2.27 | 87 ± 3.68 | 1.74 ± 0.04 |
Values are mean ± SD of three independent experiments performed in duplicate
* expressed as units/ml phytase was defined as amount of enzyme required to produce 1 μmol of inorganic phosphate per minute under assay conditions
# expressed as percentage. % of phytate = [(unfermented soy milk – fermented soy milk)/unfermented soy milk)] × 100
$ expressed as percentage % DPPH scavenging = [1 – (A517 of sample/A517 of control)] × 100
@ expressed as absorbing units at 700 nm
Conclusion
In conclusion, evidence from in vitro evaluation of the Lactobacilli from fermented Uttapam batter, a south Indian food source, could reckon in selection and the use of most efficient probiotic isolate Lactobacillus pentosus SJ65, which enhanced the nutritional status of fermented soy milk and needs to be further evaluated using animal models leading to formulations of functional foods beneficial to mankind.
Acknowledgments
The author A. Saraniya, acknowledges the Indian Council of Medical Research (ICMR), New Delhi, for funding the study in the form of fellowship (3/1/3/JRF-2011/HRD-11-21148).
Conflicts of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Anastasio M, Pepe O, Cirillo T, Palomba S, Blaiotta GA, Villani F. Selection and use of phytate-degrading LAB to improve cereal-based products by mineral solubilization during dough fermentation. J Food Sci. 2010;75:28–35. doi: 10.1111/j.1750-3841.2009.01402.x. [DOI] [PubMed] [Google Scholar]
- Ashraf R, Shah NP. Antibiotic resistance of probiotic organisms and safety of probiotic dairy products. Review. Int Food Res J. 2011;18:837–853. [Google Scholar]
- Begley M, Hill C, Gahan CGM. Bile salt hydrolase activity in probiotics. Appl Environ Microbiol. 2006;72:1729–1738. doi: 10.1128/AEM.72.3.1729-1738.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesena C, Morelli L, Alander M, Siljander T, Tuomola E, Salminen S, Mattila-Sandholm T, Vilpponen-Salmela T, von Wright A. Lactobacillus crispatus and its non-aggregating mutant in human colonization trials. J Dairy Sci. 2001;84:1001–1010. doi: 10.3168/jds.S0022-0302(01)74559-6. [DOI] [PubMed] [Google Scholar]
- Charteris WP, Kelly PM, Morelli L, Collins JK. Antibiotic susceptibility of potentially probiotic Lactobacillus species. J Food Prot. 1998;61:1636–1643. doi: 10.4315/0362-028x-61.12.1636. [DOI] [PubMed] [Google Scholar]
- Chen L, Madl RL, Vadlani PV, Li L, Wang W. Value - added products from soybean: removal of anti-nutritional factors via bioprocessing. In: El-Shemy HA, editor. Soybean - bio-active compounds. Croatia: InTech; 2013. pp. 161–180. [Google Scholar]
- CLSI . Performance standards for antimicrobial susceptibility testing; twenty-first informational supplement. Pennsylvania: Clinical and Laboratory Standards Institute; 2011. [Google Scholar]
- Collado MC, Meriluoto J, Salminen S. Adhesion and aggregation properties of probiotic and pathogen strains. Eur Food Res Technol. 2008;226:1065–1073. doi: 10.1007/s00217-007-0632-x. [DOI] [Google Scholar]
- Conway PL, Gorbach SL, Goldin BR. Survival of lactic acid bacteria in the human stomach and adhesion to intestinal cells. J Dairy Sci. 1987;70:1–12. doi: 10.3168/jds.S0022-0302(87)79974-3. [DOI] [PubMed] [Google Scholar]
- Dashkevicz MP, Feighner SD. Development of a differential medium for bile salt hydrolase-active Lactobacillus spp. Appl Environ Microbiol. 1989;55:11–16. doi: 10.1128/aem.55.1.11-16.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Smet I, Van Hoorde L, Vande Woestyne M, Christiaens H, Verstraete W. Significance of bile salt hydrolytic activities of Lactobacilli. J Appl Bacteriol. 1995;79:292–301. doi: 10.1111/j.1365-2672.1995.tb03140.x. [DOI] [PubMed] [Google Scholar]
- de Valdez GF, de Taranto MP. Probiotic properties of Lactobacilli: cholesterol reduction and bile salt hydrolase activity. In: Spencer JFT, de Spencer ALR, editors. Food microbiology protocols. New Jersey: Humana Press; 2001. pp. 173–182. [Google Scholar]
- Doyle RJ, Rosenberg M. Measurement of microbial adhesion to hydrophobic substrates. Methods Enzymol. 1995;253:542–550. doi: 10.1016/S0076-6879(95)53046-0. [DOI] [PubMed] [Google Scholar]
- Forestier C, De Champs C, Vatoux C, Joly B. Probiotic activities of Lactobacillus casei rhamnosus: in vitro adherence to intestinal cells and antimicrobial properties. Res Microbiol. 2001;152:167–173. doi: 10.1016/S0923-2508(01)01188-3. [DOI] [PubMed] [Google Scholar]
- Galdeano CM, LeBlanc M, Vinderola G, Bibas MEB, Perdigón G. Proposed model: mechanisms of immunomodulation induced by probiotic bacteria. Clin Vaccine Immunol. 2007;14:485–492. doi: 10.1128/CVI.00406-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Li D, Liu S, Liu Y. Probiotic potential of Lactobacillus sake C2 isolated from traditional Chinese fermented cabbage. Eur Food Res Technol. 2012;234:45–51. doi: 10.1007/s00217-011-1608-4. [DOI] [Google Scholar]
- Greiner R, Konietzny U. Phytase for food application. Food Technol Biotechnol. 2006;44:125–140. [Google Scholar]
- Hammes WP, Hertel C (2006) The genera Lactobacillus and Carnobacterium, in: Dworkin M, Falkow S, Rosenberg E, Schleifer K.-H, Stackebrandt E (Eds.). The Prokaryotes, New York, 4:320–403
- Kimoto H, Ohmomo S, Okamoto T. Cholesterol removal from media by Lactococci. J Dairy Sci. 2002;85:3182–3188. doi: 10.3168/jds.S0022-0302(02)74406-8. [DOI] [PubMed] [Google Scholar]
- Li H, Yan L, Wang J, Zhang Q, Zhou Q, Sun T, Chen W, Zhang H. Fermentation characteristics of six probiotic strains in soymilk. Ann Microbiol. 2012;62:1473–1483. doi: 10.1007/s13213-011-0401-8. [DOI] [Google Scholar]
- Marazza JA, Nazarenob MA, de Gioria GS, Garroa MS. Enhancement of the antioxidant capacity of soymilk by fermentation with Lactobacillus rhamnosus. J Funct Foods. 2012;4:594–601. doi: 10.1016/j.jff.2012.03.005. [DOI] [Google Scholar]
- Midolo PD, Lambert JR, Hull R, Luo F, Grayson ML. In vitro inhibition of Helicobacter pylori NCTC 11637 by organic acids and lactic acid bacteria. J Appl Bacteriol. 1995;79:475–479. doi: 10.1111/j.1365-2672.1995.tb03164.x. [DOI] [PubMed] [Google Scholar]
- Morelli L. In vitro selection of probiotic lactobacilli: a critical appraisal. Curr Issues Intest Microbiol. 2000;1:59–67. [PubMed] [Google Scholar]
- Nissen L, Chingwaru W, Sgorbati B, Biavati B, Cencic A. Gut health promoting activity of new putative probiotic/protective Lactobacillus spp. strains: a functional study in the small intestinal cell model. Int J Food Microbiol. 2009;135:288–294. doi: 10.1016/j.ijfoodmicro.2009.08.027. [DOI] [PubMed] [Google Scholar]
- Oh N, In M. Phytate degradation by Leuconostoc mesenteroides KC51 cultivation in soymilk African. J Biotechnol. 2009;8:3023–3026. [Google Scholar]
- Osmanagaoglu O, Kiran F, Ataoglu H. Evaluation of in vitro probiotic potential of Pediococcus pentosaceus OZF isolated from human breast milk. Probiotics Antimicrob Proteins. 2010;2:162–174. doi: 10.1007/s12602-010-9050-7. [DOI] [PubMed] [Google Scholar]
- Sadasivam S, Manickam A. Biochemical Methods. 3. New Delhi: New Age; 2008. [Google Scholar]
- Saito K, Jin D, Ogawa T, Muramoto K. Antioxidative properties of tripeptide libraries prepared by the combinatorial chemistry. J Agric Food Chem. 2003;51:3668–3674. doi: 10.1021/jf021191n. [DOI] [PubMed] [Google Scholar]
- Saraniya A, Jeevaratnam K. Molecular characterization of bacteriocinogenic Lactobacillus species isolated from fermented Uttapam batter. Biosci Biotechnol Res Asia. 2012;9:417–421. doi: 10.13005/bbra/1017. [DOI] [Google Scholar]
- Sleator RD, Hill C. New frontiers in probiotic research. Lett Appl Microbiol. 2008;46:143–147. doi: 10.1111/j.1472-765X.2007.02293.x. [DOI] [PubMed] [Google Scholar]
- Telang AM, Joshi VS, Sutar N, Thorat BN. Enhancement of biological properties of soymilk by fermentation. Food Biotechnol. 2010;24:375–387. doi: 10.1080/08905436.2010.524489. [DOI] [Google Scholar]
- Todorov SD, LeBlanc JG, Franco BDGM. Evaluation of the probiotic potential and effect of encapsulation on survival for Lactobacillus plantarum ST16Pa isolated from papaya. World J Microbiol Biotechnol. 2012;28:973–984. doi: 10.1007/s11274-011-0895-z. [DOI] [PubMed] [Google Scholar]
- Tuomola EM, Salminen SJ. Adhesion of some probiotic and dairy Lactobacillus strains to Caco-2 cell cultures. Int J Food Microbiol. 1998;41:45–51. doi: 10.1016/S0168-1605(98)00033-6. [DOI] [PubMed] [Google Scholar]
- Vinderola CG, Reinheimer JA. Lactic acid starter and probiotic bacteria: a comparative “in vitro” study of probiotic characteristics and biological barrier resistance. Food Res Int. 2003;36:895–904. doi: 10.1016/S0963-9969(03)00098-X. [DOI] [Google Scholar]
- Wang C-Y, Lin P-R, Ng C-C, Shyu Y-T. Probiotic properties of Lactobacillus strains isolated from the feces of breast-fed infants and Taiwanese pickled cabbage. Anaerobe. 2010;16:578–585. doi: 10.1016/j.anaerobe.2010.10.003. [DOI] [PubMed] [Google Scholar]
- WHO/FAO (2002) Working group report on drafting guidelines for the evaluation of probiotics in food. London, Ontario, Canada