Abstract
Biological activities of alkali extracted (Barium hydroxide: BE-480 kDa, Potassium hydroxide: KE1-1080 and KE2-40 kDa), purified arabinoxylans (AX) from the finger millet bran varying in their molecular weight, phenolic acid content, arabinose to xylose ratios were evaluated for their immune-stimulatory activities using murine lymphocytes and peritoneal exudate macrophages. All three purified AX displayed significant (p < 0.001) mitogenic activity and activation of macrophages including phagocytosis. Among these BE has shown higher enhancing lymphocyte proliferation (>2 fold) and macrophage phagocytosis than KE1 and KE2. The above results clearly documented that the immunostimulatory activity of arabinoxylans is directly proportional to the amount of ferulic acid content (0.11 mg/100 g), whereas molecular weight as well as arabinose/xylose ratio, did not have any bearing. Purified AX from the finger millet bran can be explored as a potent natural immunomodulator.
Keywords: Finger millet bran, Arabinoxylan, Immunomodulation, Mitogenic activity, Macrophage activation, Phagocytosis
Introduction
Arabinoxylans (AX) are major non-starch polysaccharides found in the cell wall of cereals, millets and pulses. These AX consists of α-L-arabinose and β-D-xylose in different ratios, however, their fine structure varies largely with respect to their botanical source, cultivar, as well as their location in various tissues of the grain. AX were isolated and characterized from wheat, rice, maize, rye, barley, oats, and higher plants (Izydorczyk and Biliaderis 1995; Kennedy et al. 1979). Anti-diabetic, anti-tumerogenic, atherosclerogenic effects, antioxidant as well as antimicrobial properties of AX were documented (Vincent and Liu 2000). Finger millet commonly referred as ragi is known to have high amount of AX. Conventionally, AX are classified into water-extractable (WE-AX) and water-unextractable (WU-AX) polysaccharides. WU-AX are retained in the cell wall by non-covalent (e.g. hydrogen bonds) and covalent interactions (e.g. ester and ether bonds, diferulic acid bridges) with other AX or other cell wall constituents (Cyran et al. 2004; Izydorczyk and Biliaderis 1995). The main parameters determining the structure of AX are the arabinose to xylose ratio (A/X ratio), the degree of substitution and its pattern of the arabinose residues along the xylan backbone. As arabinoxylans are complex, polydisperse and varies with respect to their structure, numerous isolation and fractionation techniques have been used to obtain more homogeneous fractions (Izydorczyk and Biliaderis 1995). Gruppen et al. (1991) introduced the use of barium hydroxide as a primary extractant for WU-AX. As barium ions form insoluble complexes with β-glucans, their use allows an enhanced separation of AX and β-glucans, resulting in the selective extraction of 80 % of the wheat flour WU-AX (Gruppen et al. 1991) or 50 % of the wheat bran WU-AX (Bergmans et al. 1996). The initiation of alkaline peeling (β-elimination) upon drastic alkaline conditions is prevented by the addition of sodium borohydride to the extract (Gruppen et al. 1992).
Finger millet, an important millet known for its several health benefits which in turn mainly attributed to its non-starch polysaccharides, among which AX is the most preponderant. We have previously isolated water unextractable arabinoxylans from the finger millet bran by sequential alkali extractions using barium hydroxide (BE) and potassium hydroxide (KE). These arabinoxylans were fractionated on DEAE Cellulose column using stepwise elution of water followed by ammonium carbonate (0.1–0.5 M) and alkali (0.1 and 0.2 alkali). The 0.1 M ammonium carbonate fractions varying in their molecular weight, phenolic acid content and arabinose to xylose ratios were resolved on Sephacryl S-400 gel filtration chromatography into one (BE) and two subfractions (KE1 and KE2) respectively and their homogeneity was proven by gel filtration, cellulose-acetate membrane electrophoresis and capillary electrophoresis (Prashanth and Muralikrishna 2014). To best of our knowledge, so far, no information exists on the immunological activities of finger millet bran AX. The functional attributes of AX may be related to the difference in the structure that in turn related to extraction procedures adopted. Immunomodulation of polysaccharides are related to their variation in structure (Tzianabos 2000). Hence it is of interest to study the in vitro immunomodulatory effects of purified finger millet bran AX (i.e. BE, KE1 and KE2) varying in molecular weight, phenolic acid content, arabinose to xylose ratios on immunoresponder cells, namely, murine lymphocytes, and peritoneal exudate cells.
Materials and methods
Materials
Finger millet (v. Indaf-15) seeds were procured from Vishweshwaraiah Canal (VC) farm of the University of Agricultural Sciences, Mandya, Karnataka, India. Inulin (from chicory root) was obtained from Sigma–Aldrich Co. Ltd., St. Louis, MO, USA. 3-(4,5-dimethylthiazol-2-yl) - 2,5-diphenyltetrazolium bromide A.R. (MTT) was a product of HiMedia Laboratories Ltd., Mumbai, India. Fetal bovine serum (FBS) was obtained from Sera- Lab (Sussex, England). Flat-bottom 96-well microtiter plates (MICROLON) were purchased from Greiner Bio-One GmbH, Frickenhausen, Germany. All the chemicals and reagents used are of analytical grade in the study.
Isolation of alkali soluble AX
AX from Finger millet bran was isolated and purified as described earlier by Prashanth and Muralikrishna (2014).
Bound phenolic acids and neutral sugar composition of purified AX
Bound phenolic acids from purified AX were extracted (Nordkvist et al. 1984) with slight modification (Subba Rao and Muralikrishna 2002). Purified AX were subjected for neutral sugar composition study by gas chromatography. For this, alditol acetate derivatives were prepared as described earlier (Sawardekar et al. 1965).
Experimental animals
In this study, the animals used were housed and maintained as per the ethical guidelines formulated and permitted by Institutional Animal Ethics Committee (IAEC) regarding standard commercial diet at ambient temperature in a hygienic environment. The handling, caring of animals and all experimental procedures involving in the study have been carried out in accordance with the ethical guidelines. Spleen was isolated from 4 to 8-week-old CFT Wistar rats (250 ± 2 g) for the isolation of splenocytes.
Preparation of cells from spleen
The rats were sacrificed by the cervical amputation under mild anesthesia; spleen was removed using aseptic techniques. The organs were passed through a sterilized stainless steel sieve (80 μm mesh) to obtain a single-cell suspension. Erythrocytes were destroyed by 0.85 % NH4Cl, 20 mM Tris–HCl buffer and the remaining cells were washed twice with PBS. The cells were suspended in RPMI-1640 medium supplemented with 10 % FBS, 100 U/mL streptomycin at a density of 2.0 × 106 cells/mL and cell count and viability were determined as followed.
Cell count and determination of viability
The isolated splenocytes from rat were counted using Trypan blue (0.2 %) stain. An aliquot (10 μL) of cell suspension was taken and diluted with 250 μL of diluent buffer (PBS with 1 % BSA) to which 10 μL of Trypan blue stain was added. The mixture was charged to an improved Neubauer (0.1 mm deep) Bright-Line hemocytometer (Reichert Scientific Instruments, Buffalo, NY, USA) using a clean fine pipette tip. The cells were observed under eyepiece (10 and 40×) and counted in the outer four chambers of the hemocytometer. The concentration of the cells were adjusted to 2 × 106 cells/mL splenocytes and used for cell proliferation assay.
Percentage viability of splenocytes in the prepared cell suspension was checked by Trypan blue exclusion method as described earlier (Chandrashekar and Venkatesh 2009; Clement and Venkatesh 2010). For cell viability determination, an aliquot of cell suspension was taken and mixed with an equal volume of 0.2 % Trypan blue, and kept at 25 °C for ~2 min. The cell suspension with Trypan blue was charged to a hemocytometer and observed under microscope. Cells, which are dead or partially damaged, appear dark blue (since they take up the dye) against a light blue background. The viable cells appear clear without any stain against the light blue background.
Measurement of cell proliferation
Cell proliferation or mitogenicity was measured by MTT assay (Mosmann 1983). Splenocytes (2 × 106 cells/mL) were cultured with purified samples (0.1, 1, 10, and 50 μg/mL), and polysaccharide mitogen, i.e., inulin (0.1, 1,10 and 50 μg/ mL) in 96-well plates for 24 and 72 h at 37 °C humidified atmosphere of 5 % CO2 in an incubator. After incubation period, 20 μL of 5 mg/mL sterile MTT solution (MTT dissolved in 0.1 M Tris-buffered saline and filtered to remove any insoluble matter) was added and incubation was done for an additional 4 h under the same conditions. After removing the culture plates, the samples were centrifuged at 750 g at 4 °C for 15 min. Supernatant was removed and the blue formazan crystals were resolubilized in 100 μL of 0.04 N acidic isopropanol under agitation. After dissolving the crystals, 100 μL of each sample was taken in microtiter plates which were then read in a microtiter plate reader (Model 680, Bio-Rad Laboratories Inc., Hercules, CA, USA) at 570 nm. Proliferation activity was represented as proliferative index in comparison to control.
Isolation of rat peritoneal exudates cells (PECs)
Peritoneal fluid from Wistar rat was harvested from the peritoneal cavity by infusing ice-cold sterile PBS containing 1 % BSA. After centrifugation (1000 rpm for 10 min), the cell pellets were suspended in RPMI-1640 supplemented with 10 % (v/v) FBS, streptomycin 100 U/mL and seeded in 96-well plate at a cell density of 7.5 × 104 cells/mL, containing various concentrations of purified arabinoxylans and polysaccharide mitogen i.e., inulin (0.1, 1, 10 and 50 μg) and allowed to culture in 5 % CO2 humidified incubator at 37 °C for 48 and 72 h. The cell viability was evaluated by Trypan blue exclusion test, and the macrophages proportion was determined by cell morphology under a microscope (Clement and Venkatesh 2010).
Determination of nitric oxide (NO)
NO release can be used as a quantitative index of macrophage activation. Nitrite concentration in the culture medium was determined by Griess reagent as an indicator of nitric oxide production (Morihara et al. 2002). The culture supernatant (100 μL volume) was incubated with 100 μL of Griess reagent [1 % sulfanilamide/ 0.1 % N-(1-naphthyl)-ethylenediamine di-hydrochloride/ 2.5 % H3PO4] at room temperature for 10 min. The absorbance of the chromophoric azo-derivative molecule was measured using the microplate reader at 540 nm. NO2ˉ was determined by using sodium nitrite as the standard.
Phagocytic activity of macrophages by purified AX
According to the method described by Roy and Rai (2009) phagocytic assay was performed. Briefly, peritoneal cells (1 × 105cells/mL) were flooded onto prewashed clean slides. The incubation of cells at 25 °C in a CO2 incubator for 90 min will lead to adherence of macrophages. Non-adherent cells were washed off with PBS, to which 1 mL of PBS containing purified arabinoxylans and inulin as a positive control (25 and 50 μg each) were added. After 2 h of incubation under the same condition slides were washed with sterile PBS. Yeast cell suspension was prepared by mixing 30 mg of Saccharomyces cerevisiae (commercial baker’s yeast) in 10 mL of PBS. Yeast cells were heat killed (at 80 °C for 15 min). The suspension was given three times washing with PBS and finally suspended with 4 % FBS in culture medium supplemented to get a concentration of ~1 × 107 cells/mL.
Each slide with adhered peritoneal macrophages was flooded with yeast cell suspension (heat-killed) to proceed with phagocytosis. After 2 h of incubation at 25 °C, the slides were rinsed three times in PBS, fixed in methanol, and stained with Geimsa. Stained phagocytic macrophages were counted visually under the microscope. Phagocytic activity was represented as phagocytic index in comparison to control.
Statistical analysis
Assays were performed in triplicate. Results were expressed as mean ± standard deviations (S.D). The statistical analysis was done using Microsoft Office Excel 2010. Single ANOVA was done to determine the statistical significance. A p- value of less than 0.05, 0.005 and 0.001 were taken as significance.
Results and discussion
Ferulic acid content and neutral sugar composition of purified AX
Bound phenolic acids relative percent ratio, ferulic acid content and arabinose/xylose ratios for BE, KE1 and KE2 respectively were provided in Table 1. Usually, the enzymatically extracted AX (Zhou et al. 2010) contain arabinose and arabinose linked ferulic acid moieties as substituents on the xylan backbone. However, in our study the alkali extracted purified AX have also shown ferulic acid contents (Table 1) unlike the alkali isolated ones from other sources (Zhou et al. 2010). The ferulic acid content was comparatively high (0.11 mg/100 g) in BE as compared to KE1 and KE2.
Table 1.
Ferulic acid content, bound phenolic acids relative percentage ratio and arabinose/xylose ratio of purified AX (BE, KE1 and KE2)
| Sample | Ferulic acid content (mg/100 g of purified AX) | Phenolic acids (relative percentage ratio) | Arabinose: Xylose | ||
|---|---|---|---|---|---|
| FA | FA | COA | CA | ||
| BE | 0.11 | 47 | 26 | 26 | 1:0.8 |
| KE1 | 0.004 | 18 | 52 | 30 | 1:0.8 |
| KE2 | 0.005 | 28 | 29 | 43 | 1:1.2 |
(FA, Ferulic acid; COA, Coumaric acid; CA, Caffeic acid; AX, Arabinoxylans)
Mitogenic activity of purified bran AX
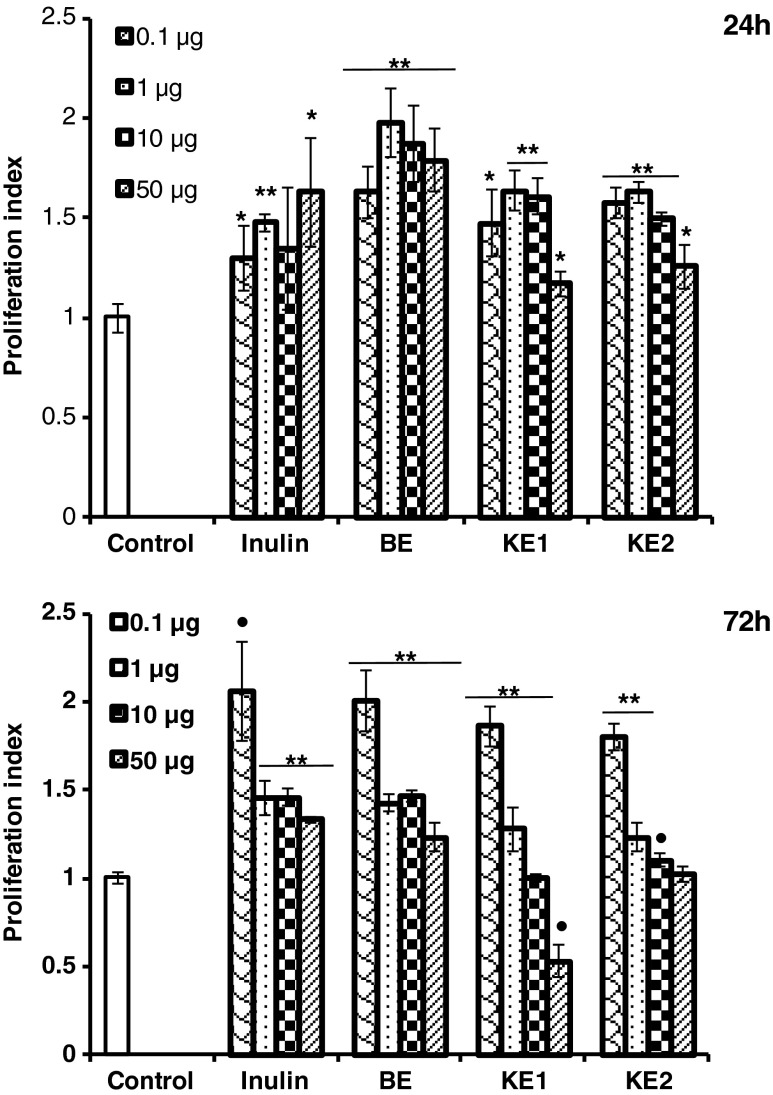
The mitogenic activity of AX purified from the finger millet bran towards rat splenocytes was studied in vitro (Fig. 1). Proliferation of lymphocyte response is used to evaluate the functional capacity of T- and B-lymphocyte immunity in vivo.). In the present study, Inulin (50 μg) was used as reference positive that showed 1.5 folds increase in mitogenic activity, in terms of proliferation index compared to untreated cells. BE (1 μg) showed two folds increase in mitogenic activity compared to untreated cells (Fig. 1). KE1 and KE2 were also significantly induced proliferation of murine splenocytes at 24 h of incubation for 0.1, 1 and 10 μg concentrations respectively. But after 72 h of incubation, 1, 10 and 50 μg concentration resulted in decreased mitogenic activity but 0.1 μg concentration retained activity (two folds compared to control) for BE, KE1 and KE2 respectively including for Inulin treated cells, indicated that mitogen activity is concentration and time dependent. Concanvalin A (ConA) stimulates T-cell and Lipopolysaccharides (LPS) are usually used to stimulate B-cell proliferation in vivo (Dai et al. 2009). However, in vitro assay with wheat bran AX revealed no significant changes in ConA- and LPS-stimulated splenocyte proliferation in previous studies (Zhou et al. 2010). Many polysaccharides are known to be T-cell independent B-cell stimulators in producing humoral response in laboratory animals (Taper and Roberfroid 2000; Kayse et al. 2003).
Fig. 1.
Mitogenic activity of purified arabinoxylans on rat splenocytes (2 × 106 cells/ml) for 24 and 72 h in vitro. The data are the means ± SD of triplicate samples (**p < 0.001, •p < 0.005, *p < 0.05)
Effect of purified AX on activation of macrophages
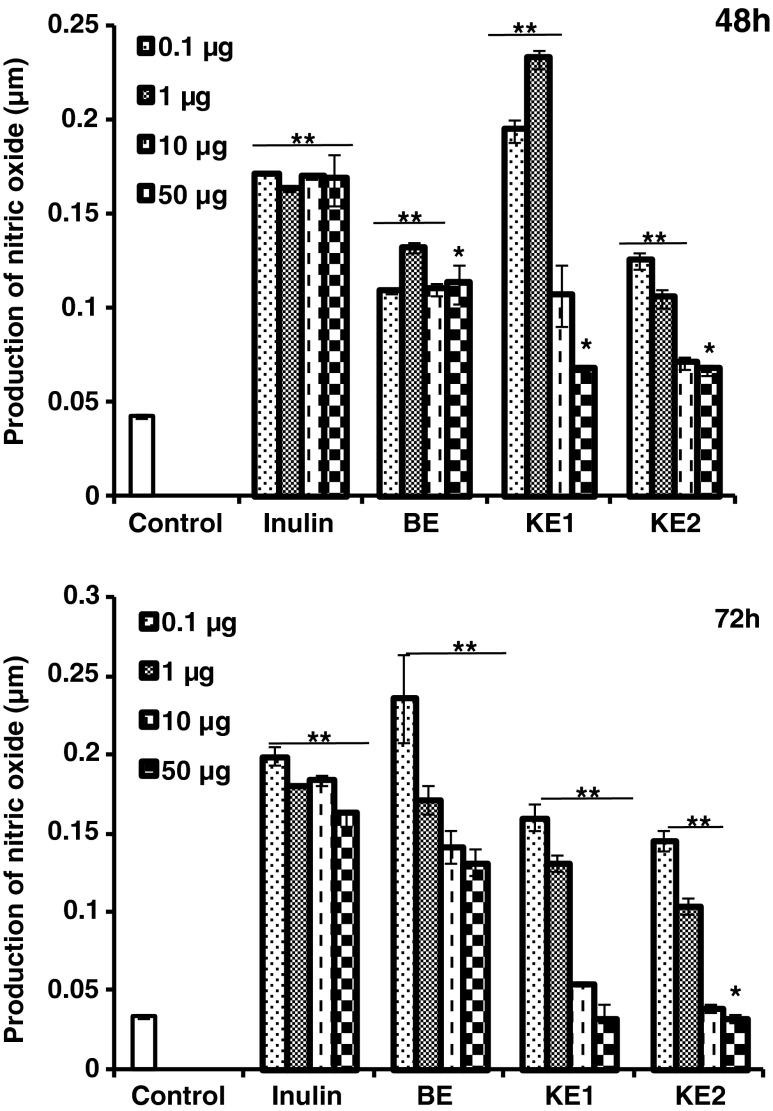
The release of NO from rat PECs in the presence of purified AX was measured at 48 and 72 h. At 48 h of incubation KE1 showed more than 4 fold increase in NO release compared to untreated cells at especially lower concentrations tested, namely, 0.1 and 1 μg (Fig. 2), whereas BE and KE2 showed around 2.5 fold and Inulin showed 3.5 fold increase at concentrations studied respectively. But at 72 h, the production of nitric oxide increased by 4.5 fold (p < 0.001) for 0.1 μg BE AX concentration compared to untreated cells. KE1 and KE2 showed decreased production, but in comparison with control, still significantly higher (p > 0.001). Inulin treatment maintained the similar threshold level of NO production even after 72 h for all the concentrations of Inulin studied. Hence, treatment with finger millet bran AX increased NO production in murine macrophage studied. Activated macrophages produce nitric oxide (NO) by endogenous action of nitric oxide synthase enzyme. It contributes to immune functions and in addition NO plays a vital role in the killing of intracellular microbial pathogens and tumoricidal activity (Ghoneum and Matsuura 2004).
Fig. 2.
Effect of purified arabinoxylans on nitric oxide production by peritoneal macrophages (2 × 106 cells/mL) in vitro. Nitrite was measured by the Griess reagent. The data are the means ± SD of triplicate samples (**p < 0.001, *p < 0.05)
Effect of purified AX on the phagocytic activity
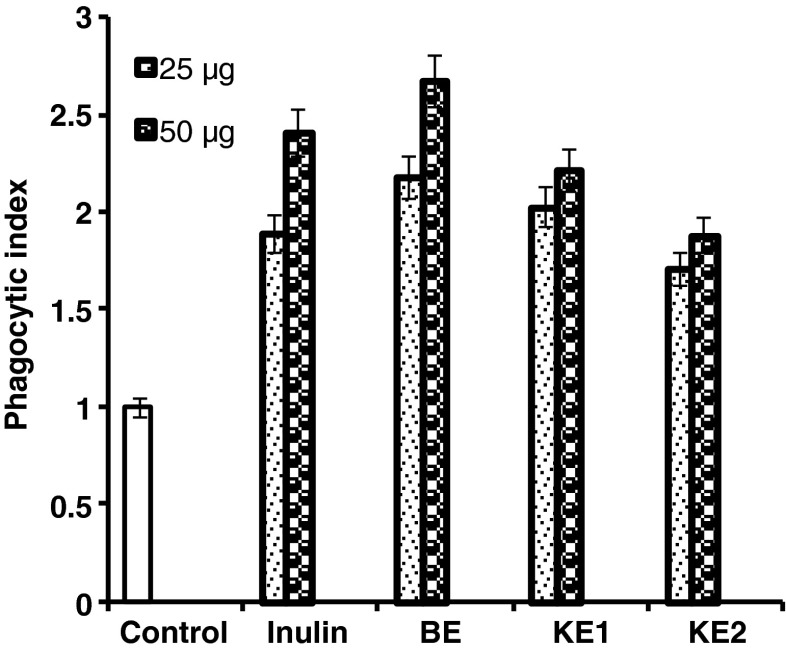
Phagocytosis was shown significantly high (p < 0.001) for sample isolated from the finger millet bran compared to control (Fig. 3). However, phagocytic activity was less significant (p < 0.01) in previously studied wheat bran and rice bran AX respectively (Zhou et al. 2010; Ghoneum and Matsuura 2004). AX activate macrophages to induce phagocytosis as depicted in Fig. 4. Macrophages present in rat peritoneal exudate cell may constitute an important arm of defense mechanism in immune response (Cavaillon 1994). Extensive evidence has shown to confirm the potential importance of macrophages in host defense mechanism.
Fig. 3.
Effect of phagocytosis of yeast cells by rat peritoneal macrophages in the presence of purified arabinoxylans (25 and 50 μg each). Phagocytosis by all purified arabinoxylans is significantly higher (p < 0.001) compared to control cells
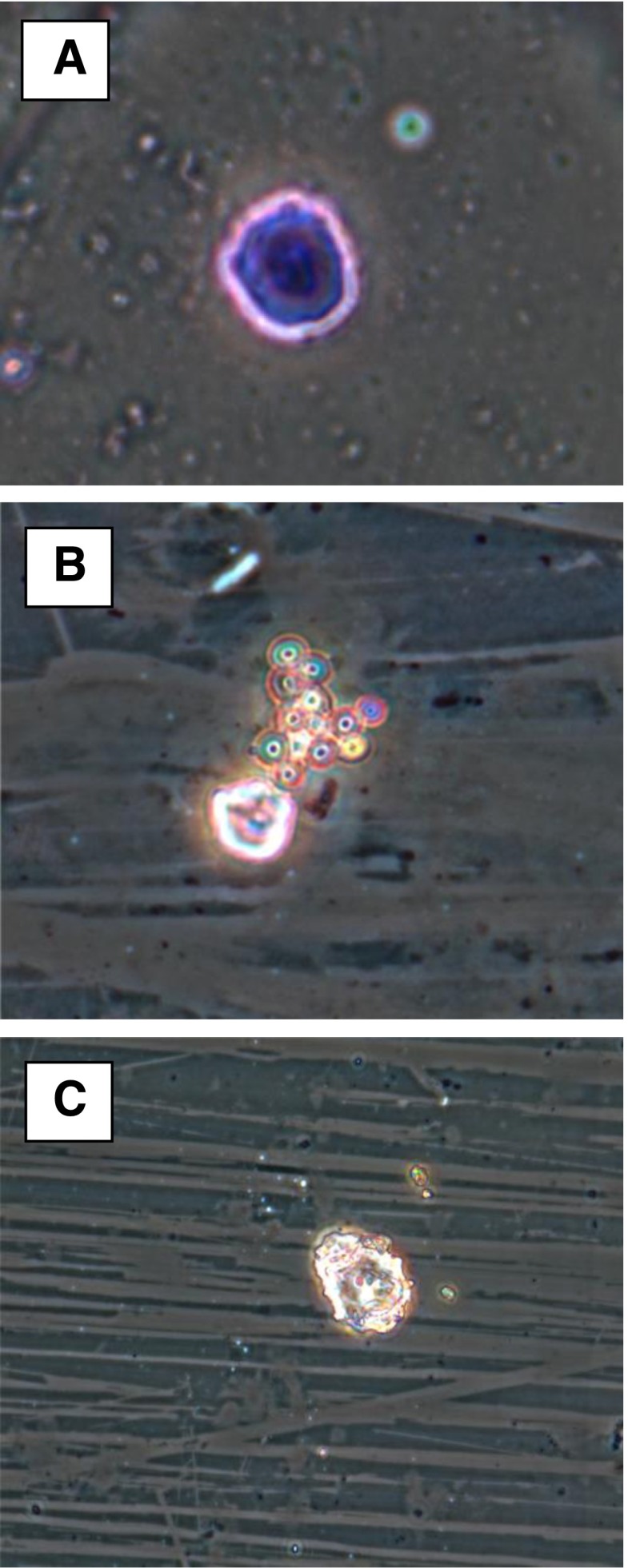
Fig. 4.
Microscopic observation of phagocytosis. Image a. rat peritoneal macrophage alone, Image b. phagocytosis of yeast cells by rat peritoneal macrophage in the presence of purified arabinoxylan and Image c. post phagocytic macrophage
The immune-enhancing function of finger millet bran AX may be less related to molecular weight and chemical composition. It is already known that dietary supplementation rich in polyphenols such as ferulic acid, gallic acid, and catechin appear to enhance immune cell functions (Alvarez et al. 2006), so presence of bound ferulic acid may be partly responsible for higher immunostimulating activity of BE. In the present study, the result obtained are in accordance with previously observed by Zhou et al. (2010) for enzyme treated AX in wheat bran.
Previous studies have shown prebiotics such as AX can also selectively stimulate beneficial microbes within the gut microbiota and may directly stimulate the immune system and enhance host defenses (Saulnier et al. 2009). The end-products of fermentation of prebiotics like short chain fatty acids (SCFAs) for instance butyrate, are capable of improving mucosal morphology by increasing mucin production and decreasing translocation by binding to SCFA receptors on immune cells within the gut lymphoid-associated tissue (Saulnier et al. 2009). However, further investigation about the relationship between the immune stimulating mechanisms of finger millet bran AX and its function as a prebiotic is part of our futuristic study.
Conclusion
In conclusion, purified AX from the finger millet bran has immunostimulatory activity by inducing cell proliferation and increased production of NO with the activation of macrophages including phagocytosis. Overall results revealed that the barium extracted AX has higher enhancing lymphocyte proliferation and macrophage phagocytosis than the potassium hydroxide extracted AX, may be due to presence of comparatively more ferulic acid content. Further results revealed that the molecular weight, as well as arabinose/xylose ratio of purified AX, does not have much importance in immunomodulation. AX extracted from the finger millet bran by alkaline aided procedures could be exploited as a good source of natural Immunomodulator for better health benefits.
Acknowledgments
The authors would like to thank Prof. Ram Rajasekharan, Director, Council of Scientific and Industrial Research-Central Food Technological Research Institute, Mysore for his constant encouragement. Smt. Savitha Prashanth, M.R. and Shruthi, R.R thank CSIR, New Delhi for senior research fellowships.
References
- Alvarez P, Alvarado C, Puerto M, Schlumberger A, Jiménez L, De la Fuente M. Improvement of leukocyte functions in prematurely aging mice after five weeks of diet supplementation with polyphenol-rich cereals. Nutrition. 2006;22:913–921. doi: 10.1016/j.nut.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Bergmans MEF, Beldman G, Gruppen H, Voragen AGJ. Optimisation of the selective extraction of (glucurono) arabinoxylans from wheat bran: use of barium and calcium hydroxide solution at elevated temperatures. J Cereal Sci. 1996;23:235–245. doi: 10.1006/jcrs.1996.0024. [DOI] [Google Scholar]
- Cavaillon JM. Cytokines and macrophages. Biomed Pharmacother. 1994;48:445–453. doi: 10.1016/0753-3322(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Chandrashekar PM, Venkatesh YP. Identification of the protein components displaying immunomodulatory activity in aged garlic extract. J Ethnopharmacol. 2009;124:384–390. doi: 10.1016/j.jep.2009.05.030. [DOI] [PubMed] [Google Scholar]
- Clement F, Venkatesh YP. Dietary garlic (Allium sativum) lectins, ASA I and ASA II, are highly stable and immunogenic. Int Immunopharmacol. 2010;10:1161–1169. doi: 10.1016/j.intimp.2010.06.022. [DOI] [PubMed] [Google Scholar]
- Cyran M, Courtin CM, Delcour JA. Heterogeneity in the fine structure of alkaliextractable arabinoxylans isolated from two rye flours with high and low breadmaking quality and their coexistence with other cell wall components. J Agri Food Chem. 2004;52:2671–2680. doi: 10.1021/jf030550r. [DOI] [PubMed] [Google Scholar]
- Dai ZY, Zhang H, Zhang YP, Wang HH. Chemical properties and immunostimulatory activity of a water-soluble polysaccharide from the clam of Hyriopsis cumingii Lea. Carbohydr Polym. 2009;77:365–369. doi: 10.1016/j.carbpol.2009.01.003. [DOI] [Google Scholar]
- Ghoneum M, Matsuura M. Augmentation of macrophages phagocytosis by modified arabinoxylan rice bran (MGM–3/ BioBran) Int J Immunopathol Pharmacol. 2004;17:283–292. doi: 10.1177/039463200401700308. [DOI] [PubMed] [Google Scholar]
- Gruppen H, Hamer RJ, Voragen AGJ. Water-unextractable cell wall material from wheat flour. I. Extraction of polymers with alkali. J Cereal Sci. 1991;16:41–51. doi: 10.1016/S0733-5210(09)80078-7. [DOI] [Google Scholar]
- Gruppen H, Hamer RJ, Voragen AGJ. Water-unextractable cell wall material from wheat flour. II. Fractionation of alkali-extracted polymers and comparison with water-extractable arabinoxylans. J Cereal Sci. 1992;16:53–67. doi: 10.1016/S0733-5210(09)80079-9. [DOI] [Google Scholar]
- Izydorczyk MS, Biliaderis CG. Cereal arabinoxylans: advances in structure and physicochemical properties. Carbohydr Polym. 1995;28:33–48. doi: 10.1016/0144-8617(95)00077-1. [DOI] [Google Scholar]
- Kayse O, Masihi KN, Kiderlen AF. Natural products and synthetic compounds as immunomodulators. Expert Rev Anti-Infect Ther. 2003;1:319–335. doi: 10.1586/14787210.1.2.319. [DOI] [PubMed] [Google Scholar]
- Kennedy JF, Sandhu JS, Southgate DAT. Structural data for the carbohydrate of ispaghula husk ex Plantago ovata Forsk. Carbohydr Res. 1979;75:265–274. doi: 10.1016/S0008-6215(00)84646-7. [DOI] [Google Scholar]
- Morihara N, Sumioka I, Moriguchi T, Uda N, Kyo E. Aged garlic extract enhances production of nitric oxide. Life Sci. 2002;71:509–517. doi: 10.1016/S0024-3205(02)01706-X. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Nordkvist E, Salomonsson AC, Aman P. Distribution of insoluble bound phenolic acids in barley grain. J Sci Food Agric. 1984;35:657–661. doi: 10.1002/jsfa.2740350611. [DOI] [Google Scholar]
- Prashanth SMR, Muralikrishna G. Arabinoxylan from finger millet (Eleusine coracana, v. Indaf 15) bran: purification and characterization. Carbohydr Polym. 2014;99:800–807. doi: 10.1016/j.carbpol.2013.08.079. [DOI] [PubMed] [Google Scholar]
- Roy B, Rai U. Genomic and non-genomic effect of cortisol on phagocytosis in freshwater teleost Channa punctatus: an in vitro study. Steroids. 2009;74:449–455. doi: 10.1016/j.steroids.2008.12.013. [DOI] [PubMed] [Google Scholar]
- Saulnier DM, Spinler JK, Gibson GR, Versalovic J. Mechanisms of probiosis and prebiosis: considerations for enhanced functional foods. Curr Opin Biotechnol. 2009;20:135–141. doi: 10.1016/j.copbio.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawardekar JS, Slonekar JM, Jeanes A quantitative determination of monosaccharides as their alditol acetates by gas liquid chromatography. Anal Chem. 1965;37:1602–1604. doi: 10.1021/ac60231a048. [DOI] [Google Scholar]
- Subba Rao MVSST, Muralikrishna G. Evaluation of the antioxidant properties of free and bound phenolic acids from native and malted finger millet (Ragi, Eleusine coracana Indaf-15) J Agric Food Chem. 2002;50:889–892. doi: 10.1021/jf011210d. [DOI] [PubMed] [Google Scholar]
- Taper HS, Roberfroid MB. Nontoxic potentiation of cancer chemotherapy by dietary oligofructose or inulin. Nutr Cancer. 2000;38:1–5. doi: 10.1207/S15327914NC381_1. [DOI] [PubMed] [Google Scholar]
- Tzianabos AO. Polysaccharide immunomodulators as therapeutic agents: structural aspects and biologic function. Clin Microbiol Rev. 2000;13:523–533. doi: 10.1128/CMR.13.4.523-533.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent ECO, Liu F. Immunomodulation and anti-cancer activity of polysaccharide-protein complexes. Curr Med Chem. 2000;7:715–729. doi: 10.2174/0929867003374705. [DOI] [PubMed] [Google Scholar]
- Zhou S, Liu X, Guo Y, Wang Q, Peng D, Cao L. Comparison of the immunological activities of arabinoxylans from wheat bran with alkali and xylanase-aided extraction. Carbohydr Polym. 2010;81:784–789. doi: 10.1016/j.carbpol.2010.03.040. [DOI] [Google Scholar]