Abstract
Seed cake protein (SCP) from Camellia oleifera was hydrolyzed by five commercial proteases (Flavorzyme, Trypsin, Neutrase, Papain, Alcalase). Amino acid composition, molecular weight distribution, antioxidant activity and functional property of the seed cake protein hydrolysates (SCPH) were investigated. Enzymatic hydrolysis improved protein solubility significantly but impaired the foaming and emulsifying property. Hydrolysate generated by alcalase had the highest hydrolysis degree (DH) and antioxidant activity, and displayed excellent protein solubility over wide range of pH, while hydrolysate prepared by flavorzyme showed better copper chelating capacity and emulsifying stability with low molecular weight distribution. Trypsin-treated SCPH showed better foaming property than original protein. The results indicated that enzyme type greatly influenced the molecular weight, functional property and antioxidant activity of SCPH. It was also found that electing appropriate protease and controlling the DH could be enhanced or reduced functional property according to actual applications.
Keywords: Camellia oleifera, Protein hydrolysate, Antioxidant activity, Functional property, Protease type
Introduction
Oxidation exists anytime and anywhere in our living surrounding which is fill of oxygen. Free radical is generated accompany with oxidation. In organism, free radical are cell-damaging, which can cause the damage of protein, mutation of DNA, oxidation in cell membrane phospholipids and modification in low density lipoproteins (Maritim et al. 2003). Oxidative stress named a condition in which the generation of free radical exceeded its elimination in organisms. Many studies have shown that oxidative stress contributed significantly to the development and the progression of various diseases, such as diabetes, cancer, neurodegenerative and cardiovascular diseases (Fiaschi and Chiarugi 2012). This brings about the need for synthetic and natural antioxidants, which can protect our bodies from various oxidative damages. Synthetic antioxidants display some toxic and hazardous effects despite cost-effective and efficient (Ito et al. 1985). Natural antioxidants from food resources, with no or little side effects, have been the focus of growing interest for their potential health benefits. Hence, the search for new sources of natural antioxidants is currently of major interest to researchers.
Several peptides from food sources have been found to possess antioxidant capacity, and their bioactive properties have been widely studied, especially animal food-derived proteins such as skin, fish, blood plasma (Sun et al. 2012; Choonpicharn et al. 2014; Kong et al. 2007), as well as some plant proteins like hemp, rice, and peanut (Zhao et al. 2012; Tang et al. 2009; Jamdar et al. 2010). These peptides were considered to be safe and healthy with high activity, easy absorption, low cost and no or little negative side effects, which commonly contained 2–20 amino acids with low molecular weight less than 6000 Da (Sarmadi and Ismail 2010). Their bioactive properties were largely related to enzymatic specificity, hydrolysis degree (DH), amino acid composition, structure and hydrophobicity (Sarmadi and Ismail 2010).
Camellia oleifera Abel., a theaceous evergreen shrub tree, was distributed and widely grew in the central and south China. The seed was used for producing edible oil (camellia oil) which was highly valued as “oriental olive oil” with rich unsaturated fatty acids. Camellia resource grew at high speed to meet domestic demand of superior edible oil and fat. A large amount of by-product, such as seed cake, was left in oil industry. Camellia seed cake was traditionally used for animal feeds, detergent, organic fertilizer, or fuel, but contained rich bioactive compounds such as polyphenol, saponin, protein, polysaccharide (He and Gu 1982). With 14–20 % crude protein, the seed cake should be treated as a valuable and low-cost source for the extraction of high-value, bioactive and nutritional plant protein.
Proteases were widely used to generate the food-derived hydrolysates/peptides due to their unique function, wide accessibility, and cost-effectiveness. In this study, we evaluated five commercially available proteases, namely alcalase, neutrase, trypsin, papain and flavourzyme, for producing antioxidant hydrolysates from the seed cake protein (SCP). Purpose of this work was to investigate antioxidant potential and functional property of enzymatic hydrolysates from SCP prepared by five proteases after their dependent hydrolysis process.
Materials and methods
Materials and reagents
Camellia seed cake was provided by Times biotech Co., Ltd. (Ya,an, China). The sample was cut into small fractions and further ground into fine powder in a high speed disintegrator and passed through a 40 mesh sieve. Alcalase, trypsin, neutrase, papain, flovorzyme, SephadexG-50, TNBS (2,4,6-trinitrobenzenesulphonic acid) and pyrocatechol violet were purchased from Kayon Biological technology Co., Ltd. (Shanghai, China). DPPH (1, 1-diphenyl-2-picrylhydrazyl) and Ferrozine were purchased from Aldrich-sigma chemical Co. (St. Louis. MO. USA). All other chemicals and regents used in the experiments were analytical grade.
Preparation of SCP
The resulting powder of the seed cake was extracted with hexane for 24 h, subsequently with 80 % (v/v) ethanol about every 1 h for five times at room temperature in order to remove the residual fatty acids, saponins and polyphenols. Protein was extracted by suspending the flour into distilled water (1:10, w/v) adjusted to pH 10.0 with 1 M NaOH, agitating for 30 min at 40 °C for two times and following by centrifugation at room temperature at 5000 g for 30 min. The supernatant was adjusted to pH 4.0−4.5 with 1 M HCl, and then centrifuged at 8000 g for 20 min. The precipitate was collected and freeze-dried.
Preparation of SCP hydrolysate (SCPH)
SCP was hydrolysed with various proteases at their respective optimum hydrolysis conditions according to the manufactures: (1) Flavourzyme: at pH 6.0 and 50 °C; (2) Trypsin: at pH 8.0 and 50 °C; (3) Neutrase: at pH 7.0 and 45 °C; (4) Papain: at pH 6.5 and 55 °C; (5) Alcalase: at pH 8.5 and 55 °C. The enzyme to substrate (SCP) ratio (E/S) was set at 2:100 (w/w). During the entire period of hydrolysis for 5 h, the pH of the slurry was constantly maintained by adding 1 M NaOH or 1 M HCl. The resulting hydrolysates were each adjusted to pH 7.0 and then heated in boiling water for 10 min to inactivate the enzyme. The follow step was centrifugated at 8000 g for 15 min to remove any impurities. The supernatant was then freeze-dried and stored at 20 °C for further use.
Determination of DH
DH was determined using the method of Benjakul and Morrissey (1997) though the reaction between α-amino group of sample and TNBS. The total α-amino groups obtained in sample 100 % hydrolysis by acid hydrolysis with 6 M HCl at 110 °C for 24 h.
Amino acid composition analysis
The SCPHs and SCP were hydrolysed with 6 M HCl for 24 h at 110 °C in a sealed tube. Amino acid composition, except the tryptophan, was measured by an automatic amino acid analyser (L-8800, Hitachi, Japan). The amino acid composition was reported as g/100 g protein.
Size exclusion chromatography (SEC)
Peptides fractions of SCPHs were separated by using sephadex G-50 column. 2 ml of sample solution was loaded on the Sephadex G-50 colunm (1.6 × 100 cm), and eluted by distilled water. Each 3 ml of fraction was collected at flow of 1 ml/min. The fractions were monitored at 280 nm.
Determination of functional properties
Protein solubility
The solubility was tested at different pH value following the method of Li et al. (2014) with a minor modification. Sample was dispersed in distilled water and adjusted to corresponding pH (a series of pH 2.0−11.0) by using 1 M HCl or 1 M NaOH. After stirring for 30 min at room temperature, the mixtures were centrifuged at 5000 g for 15 min. The supernatant was collected and subjected to the determination of the protein content (Lowry et al. 1951).
Foaming property
The foaming ability (FA) and foam stability (FS) of SCPHs were determined according to the method of Li et al. (2014). The samples were dispersed in distilled water to obtain the concentration of 3 mg/mL. 20 mL of solutions were homogenised in a 50 mL-cylinder at a speed of 10,000 rpm for 1 min. The total volume was measured at 0 and 30 min after whipping. FA was expressed as foam expansion at 0 min, and FS was expressed as foam expansion after 30 min of whipping. The equation was calculated as follows:
Where A represents volume after whipping (mL) and B represents volume before whipping (mL).
Emulsifying property
The emulsion activity index (EAI) and emulsion stability index (ESI) were determined according to the method of Li et al. (2014). Samples at 3 mg/mL were prepared using distilled water. Sample solution (6 mL) and oil (2 mL) were homogenized at a speed of 10,000 rpm for 1 min. The emulsions were pipetted out at 0 and 10 min and diluted with 0.1 % SDS to 100-fold. The mixture was mixed completely. The absorbance measured immediately (A0) and after 10 min (A10) of emulsification at 500 nm, and were used to calculate EAI and ESI as follows:
Where A0 and A10 represents the absorbance at time zero and 10 min, respectively; φ represents oil volume fraction; C represents protein concentration.
Determination of antioxidant activities
DPPH radical scavenging activity
The DPPH radical scavenging activity was determined as described by Wu et al. (2003) with a slight modification. 0.5 mL of sample solution with varying concentrations (0.3−10 mg/mL) was added 4.5 mL of 0.15 mM DPPH in 95 % ethanol. The mixture was mixed vigorously and placed in the dark for 30 min at room temperature. The absorbance of the resulting mixture was measured at 517 nm. Ascorbate (Vc) was the positive control. The equation was calculated as follow:
Where A0 represents the absorbance of the sample replaced by distilled water; As represents the absorbance of the sample; Ah represents the absorbance of the sample without adding DPPH solution.
Determination of reducing power
The reducing power was determined as described by Oyaizu (1986) with minor modifications. A series of SCPH solutions with concentrations (0.3−10 mg/mL) were prepared. 1 mL of sample was added with 2.5 mL of 0.2 M phosphate buffer (pH 6.6) and 2.5 mL of 1 % potassium ferricyanide. The mixtures were mixed vigorously and incubated in a water bath at 50 °C for 20 min. Subsequently, 2.5 mL of TCA was added to the mixture and centrifuged for 10 min at 3000 g. 2.5 mL of supernatant was diluted in 2.5 mL of distilled water and then mixed with 0.5 mL of 0.1 % ferric chloride. The absorbance of the resulting solution was measured at 700 nm. An increase in the absorbance of the mixture indicates an increase in the reducing power as measured by the reduction of ferric ions.
Determination of metal chelating capabilities
Iron chelating capability
Fe2+-chelating activity was determined by measuring the formation of the Fe2+-ferrozine complex (Decker and Welch 1990). A series of SCPH solutions with concentration (0.3−10 mg/mL) were prepared. 1 mL of sample was added with 3.7 mL of distilled water, and 0.1 mL of 2 mM FeCl2. The mixture was mixed vigorously. Ferrozine (0.2 mL, 5 mM) was added, and the mixture was then reacted for 20 min at room temperature. The absorbance was measured at 562 nm. Iron chelating activity was calculated as:
Where A0 represents the absorbance of the sample replaced by distilled water; As represents the absorbance of the sample.
Copper chelating capability
Cu2+-chelating activity was determined as described by Li et al. (2014) with slight modification. 4 mM pyrocatechol violet solution and 0.1 mg/mL CuSO4 solution was prepared respectively with Na acetate buffer (pH 6.0, 50 mM). 1 mL of sample was added with 2 mL of CuSO4 solution, then stirring completely. Subsequently, 0.5 mL of pyrocatechol violet solution was added the resulting solution. The mixture reacted for 20 min at room temperature. The absorbance was measured at 632 nm. Copper chelating capability was calculated as described above for iron.
Statistical analysis
All tests were done at least triplicate, and data was expessed as means ± standard deviation. Data were analysed by analysis of variance (ANOVA) using Origin Pro 8.0 statistics programme (OriginLab Corporation, Northampton, MA, USA), and statistical differences were defined as p < 0.05 by the Tukey test.
Results and analysis
Enzymatic hydrolysis
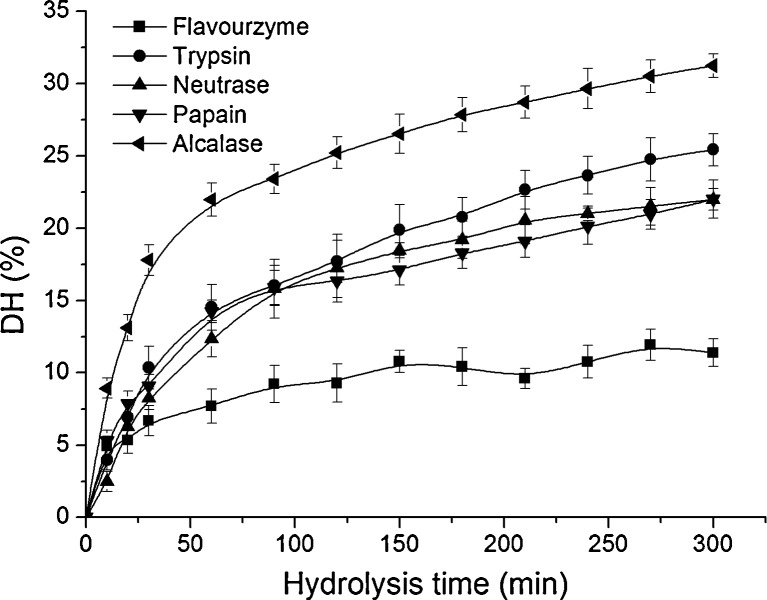
In this study, five proteases were added respectively at optimum conditions according to the manufactures, showing a typical hydrolysis curves (Fig. 1). All curves showed a high hydrolysis rate with SCP at 2∶100 (E/S) and concentration of 2 % during initial 90 min. Since then, the hydrolysis rate slowed down. This result indicated that the maximum protein cleavage occurred at first 90 min. The hydrolysis rate of alcalase was highest, following trypsin, neutrase and papain whose rates were similar at initial 90 min, while that of flavourzyme was the lowest. And at the end of hydrolysis process, the hydrolysis effect of alcalase was the highest with DH 31.25 %, and that of flavourzyme was the lowest with DH 11.32 %. The DHs of trypsin, neutrase and papain were 25.44, 21.92 and 22.13 %, respectively. These results denoted that the peptide bonds were easier cleaved by alcalase. The similar result was found previously for rice dreg protein (Zhao et al. 2012) and wheat gluten protein (Kong et al. 2007).
Fig. 1.
Time course of enzymatic hydrolysis of SCP with five enzymes represented by the DH
Amino acid composition analysis of SCP and SCPHs
Amino acids were considered to be important for the antioxidant activity of protein hydrolysates. For instance, Amino acids with aromatic residues could donate protons to electron deficient radicals (Sarmadi and Ismail 2010). The amino acid composition of SCP and SCPHs at 5 h of hydrolysis time was shown in Table 1. The data displayed that the favorite amino acids were Glu/Gln, Asp/Asn, Arg and Leu. SCPHs had a high content of charged amino acids with the range of 33.6−42.5 %. The content of hydrophobic amino acids was less than 21 %. These results implied that SCPHs might possess fine hydrophilism. In addition, comparing to SCP, the amino acid compositions of SCPHs changed slightly. There were some differentials in amino acid composition among five SCPHs, mainly attributed to the differences specifity of enzymes used.
Table 1.
Amino acid composition (g/100 g of protein) of SCP and SCPHs
| Amino acid | SCP | SCPHs prepared by five enzymes | ||||
|---|---|---|---|---|---|---|
| Flavourzyme | Trypsin | Neutrase | Papain | Alcalase | ||
| Asp | 7.87 ± 0.14 | 6.69 ± 0.08 | 6.52 ± 0.11 | 6.20 ± 0.12 | 6.69 ± 0.09 | 7.59 ± 0.05 |
| Thr | 2.26 ± 0.05 | 1.94 ± 0.07 | 1.89 ± 0.10 | 1.81 ± 0.12 | 1.97 ± 0.07 | 2.26 ± 0.09 |
| Ser | 3.47 ± 0.14 | 2.96 ± 0.06 | 2.88 ± 0.09 | 2.74 ± 0.10 | 2.97 ± 0.05 | 3.36 ± 0.08 |
| Glu | 21.49 ± 0.36 | 18.02 ± 0.22 | 17.83 ± 0.15 | 17.05 ± 0.17 | 18.35 ± 0.14 | 20.08 ± 0.26 |
| Gly | 3.02 ± 0.21 | 2.56 ± 0.10 | 2.46 ± 0.14 | 2.36 ± 0.07 | 2.60 ± 0.12 | 2.84 ± 0.16 |
| Ala | 4.07 ± 0.13 | 3.44 ± 0.15 | 3.33 ± 0.08 | 3.20 ± 0.07 | 3.47 ± 0.11 | 3.89 ± 0.12 |
| Cys | 0.25 ± 0.12 | 0.53 ± 0.06 | 0.43 ± 0.08 | 0.42 ± 0.06 | 0.47 ± 0.05 | 0.23 ± 0.10 |
| Trp | Not determined | |||||
| Val | 1.93 ± 0.10 | 1.65 ± 0.05 | 1.75 ± 0.07 | 1.48 ± 0.05 | 1.59 ± 0.09 | 1.74 ± 0.12 |
| Met | 1.39 ± 0.14 | 0.90 ± 0.10 | 1.18 ± 0.06 | 0.77 ± 0.08 | 0.75 ± 0.10 | 0.71 ± 0.07 |
| Ile | 2.84 ± 0.11 | 2.49 ± 0.05 | 2.40 ± 0.09 | 2.29 ± 0.06 | 2.51 ± 0.10 | 2.81 ± 0.12 |
| Leu | 5.91 ± 0.21 | 5.02 ± 0.19 | 4.96 ± 0.18 | 4.71 ± 0.10 | 5.07 ± 0.09 | 5.75 ± 0.14 |
| Tyr | 2.08 ± 0.05 | 1.67 ± 0.12 | 1.61 ± 0.07 | 1.53 ± 0.05 | 1.64 ± 0.06 | 1.62 ± 0.10 |
| Phe | 3.26 ± 0.14 | 2.65 ± 0.10 | 2.70 ± 0.07 | 2.59 ± 0.12 | 2.82 ± 0.12 | 3.22 ± 0.09 |
| Lys | 2.34 ± 0.10 | 1.94 ± 0.07 | 1.95 ± 0.12 | 1.86 ± 0.05 | 1.99 ± 0.10 | 2.26 ± 0.12 |
| His | 1.32 ± 0.09 | 1.07 ± 0.07 | 1.02 ± 0.10 | 0.98 ± 0.06 | 1.10 ± 0.07 | 1.28 ± 0.10 |
| Arg | 9.49 ± 0.26 | 8.12 ± 0.17 | 7.96 ± 0.14 | 7.52 ± 0.19 | 7.94 ± 0.22 | 8.83 ± 0.20 |
| Pro | 2.03 ± 0.07 | 1.87 ± 0.05 | 1.76 ± 0.09 | 1.53 ± 0.06 | 1.67 ± 0.09 | 1.97 ± 0.07 |
| % amino acid distribution | ||||||
| hydrophobic | 21.42 ± 0.57 | 18.02 ± 0.45 | 18.08 ± 0.37 | 16.57 ± 0.33 | 17.89 ± 0.39 | 20.09 ± 0.29 |
| (+) charged | 13.15 ± 0.27 | 11.14 ± 0.19 | 10.94 ± 0.15 | 10.36 ± 0.17 | 11.02 ± 0.19 | 12.37 ± 0.15 |
| (−) charged | 29.36 ± 0.49 | 24.72 ± 0.21 | 24.35 ± 0.14 | 23.25 ± 0.19 | 25.04 ± 0.09 | 27.67 ± 0.24 |
SEC analysis of SCPHs
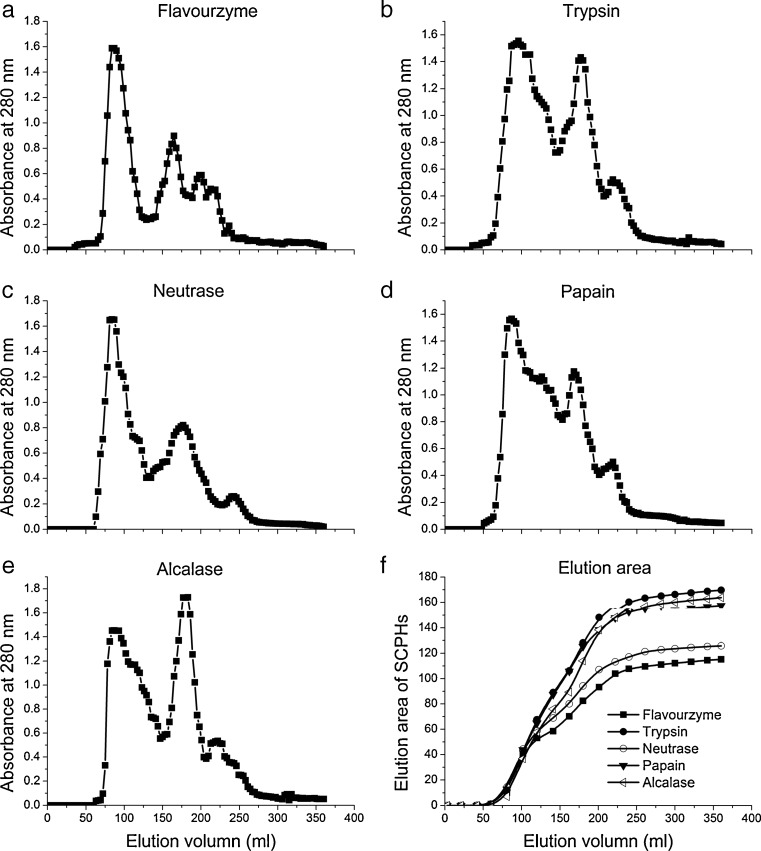
Molecular weight distributions of SCPHs obtained with five enzymes were shown in Fig. 2 by size exclusion chromatography (SEC) on Sephadex G-50. This column could separate the molecular weight (Mw) at the range of 1500–30 000 Da. As Fig. 2a–e shown, all preparations had a prominent peak at 90 mL, indicated that the high Mw of undigested protein was eluted primarily. And from Fig. 2f, there was a similar slope of the elution area curve before 90 mL. The result indicated that the undigested protein had approximately the same level for all preparations. There were some differentials among five preparations in terms of SEC profile yet. The prominent peak of trypsin and alcalase had similar peak height after 150 mL from Fig. 2d and e, and their elution area was non-significant. In addition, their peak height and elution area were higher than other preparations, indicated that trypsin and alcalase possessed high proteolytic activity to cleave the parent protein. High elution peak in SEC profile of hydrolysates was largely attributed to soluble aggregates from peptide fragments released during hydrolysis (Tang et al. 2009). There were a considerably obvious aggregate in preparation by alcalase and trypsin, while the aggregate peptide of flavourzyme and neutrase was limited around elution volume of 175 mL. Different proteases possessed their own specifity and proteolytic activity to the parent protein, and their hydrolysates resulted in the different molecular weight distribution.
Fig. 2.
SEC profiles of SCPHs obtained with different enzymes. Panels a–e referred to Flavourzyme, Trypsin, Neutrase, Papain and Alcalase respectively. Panel f was the elution area curve of SEC peak with elution volume through integral
Determination of functional properties
Protein solubility of SCP and SCPHs
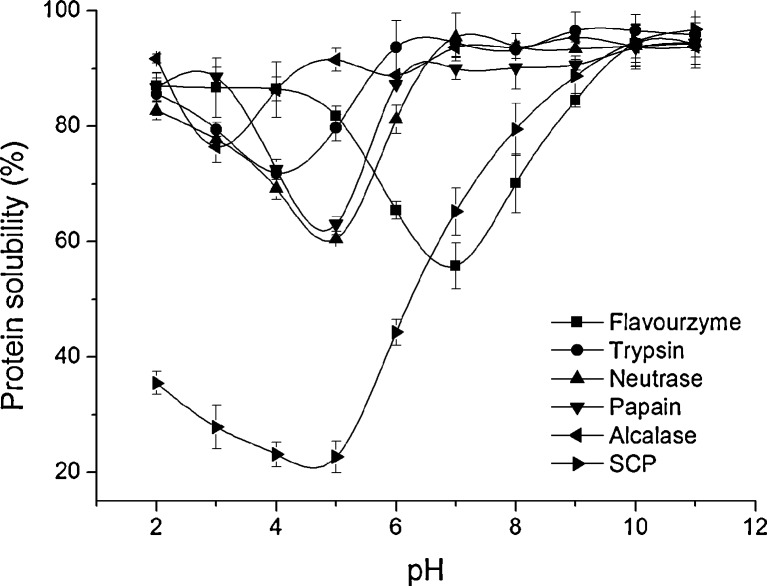
Solubility was one of the most important functional properties of protein hydrolysates and modified by hydrolysis process. The solubility of SCP and SCPHs prepared with various proteases for 5 h in the pH range of 2–11 was shown in Fig. 3. SCP showed the lower protein solubility than its hydrolysates, especially in acidic condition, indicated that enzymatic treatments could improve the solubility of SCP. All hydrolysates, except flavourzyme, had a good solubility more than 85 % under the condition of pH over 7. The lowest solubility was observed at their own isoelectric points (pI). The pI of SCPHs prepared by alcalase was around pH 3, trypsin around pH 4, flavorzyme around pH 7, neutrase and papain around pH 5. It seems that the pI of SCPH prepared by alkaline protease was lower than that by neutral and acidic protease. The cause maybe more negative charge residues released from parent protein treated by alkaline protease than that by others. In the range of pH 6–10, the solubility of flavourzyme-treated SCPH was noticeable lower than that of other SCPHs at the corresponding pH, possibly due to its low DH. The balance of hydrophilic and hydrophobic forces of peptides is another crucial influence on solubility increments (Gbogouri et al. 2004).
Fig. 3.
Protein solubility of SCPHs obtained with five enzymes at the range of pH 2.0–11.0
Foaming property of SCP and SCPHs
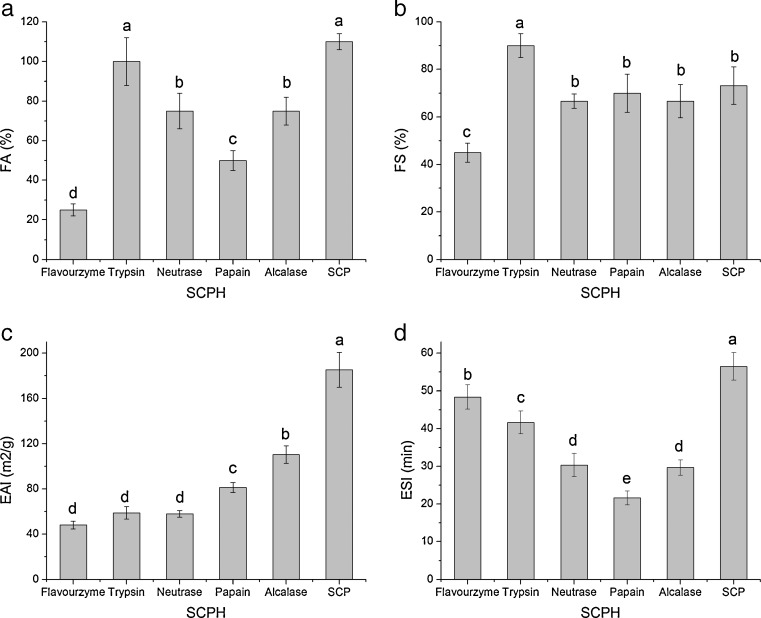
FA and FS of SCP and SCPHs were shown in Fig. 4a and b. FA of all preparations was less than 110 %. Foaming property of trypsin-treated hydrolysate showed the highest FA of 106 % and FS of 90.5 %, while that of Flavourzyme-treated hydrolysate with the lowest FA of 28 % and FS of 45.7 %. Generally, protein hydrolysates with high DH, which had the smaller peptides, migrated quickly to the interface, lowering the surface tension and give rise to the foam expansion (Intarasirisawat et al. 2012). Differences among all hydrolysates were depended on their enzyme specifity and proteolytic activity. SCP exhibited an excellent foaming property. Enzymatic hydrolysis reduced the foaming property of SCP, expect for trypsin which showed a better one. The results suggested that enzyme type was an import factor to determine functional property of protein hydrolysate. Beside enzyme type, there had many factors e.g., hydrolysis degree and acidic or basic surrounding to influence the functional property (Jamdar et al. 2010; Li et al. 2014).
Fig. 4.
Foaming properties, both foaming expansion activity (a) and foaming stability (b), and emulsifying properties, both emulsifying activity index (c) and emulsifying stability index (d) of SCPHs obtained with five enzymes. The different superscript letters denoted significant difference (p < 0.05)
Emulsifying property of SCP and SCPHs
The EAI and ESI of SCP and SCPHs prepared by various proteases at pH 7.0 were shown in Fig. 4c and d. Comparing with SCP, enzymatic hydrolysis impaired the emulsifying property. The EAI of SCPH prepared by alcalase and papain were higher than other SCPH prepared by other proteases, while their ESIs were relatively lower (p < 0.05). SCPH prepared by alcalase and papain with high DH mean more small peptides. Small peptides are more likely hydrophilic in nature, which cannot unfold and re-orient at the interface, and bring about the loss of emulsifying properties (Klompong et al. 2007; Intarasirisawat et al. 2012). High contents of larger molecular weight peptides contributed to the stability of emulsion (Mutilangi et al. 1996). Flavourzyme-treated SCPH had the lowest EAI, while had the highest ESI, mainly due to its low molecular weight distribution. The amphiphilicity of peptides also affected emulsifying properties (Klompong et al. 2007).
Determination of antioxidant activities
DPPH radical scavenging activity of SCP and SCPHs
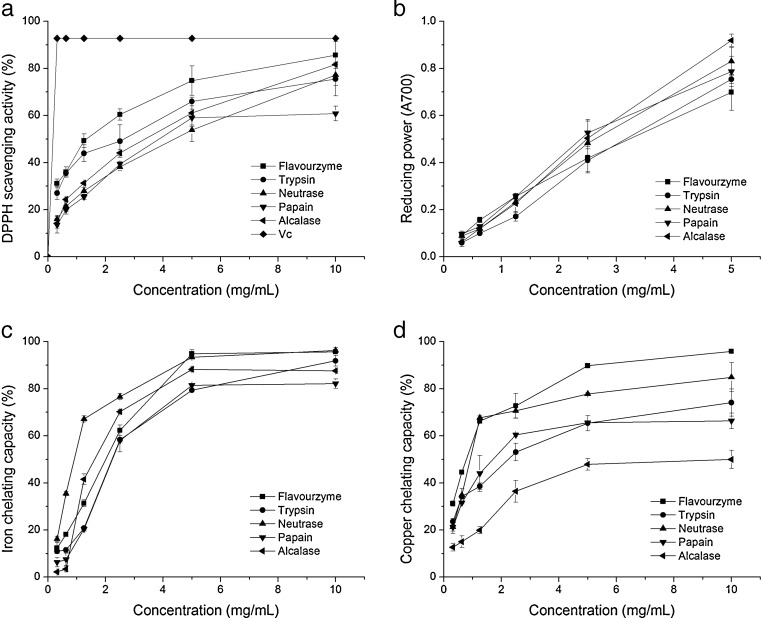
DPPH radical scavenging assay has been widely used to evaluate antioxidant property of various antioxidants (Klompong et al. 2007). The DPPH scavenging activities of five enzymatic hydrolysates for 5 h at various concentrations (0.3–10 mg/mL) was shown in Fig. 5a. Obviously, the activity of SCPHs was dose-increased independently. However, the effects of all hydrolysates were considerably lower than that of Vc (control). The term EC50 was used to evaluate the radical scavenging effects. Lower EC50 indicates higher free radical scavenging effect. The EC50 values for SCPH prepared by flavourzyme, trypsin, neutrase, papain and alcalase were estimated from Fig. 5a to be about 1.33, 2.63, 4.39, 3.85, 3.37 mg/mL, respectively, and apparently higher than that of Vc (<0.15 mg/mL). The difference of radical scavenging activities among hydrolysates maybe attributed to the amino acid compositions, molecular distribution, their structures and sequences of peptide (Sarmadi and Ismail 2010).
Fig. 5.
Antioxidant activities, both DPPH scavenging activity (a) and reducing power (b), and metal chelating capabilities, both iron chelating capability (c) and copper chelating capability (d) of SCPHs obtained with five enzymes at various concentrations
Reducing power of SCP and SCPHs
The reducing power of SCPH obtained by five different proteases for 5 h at various concentrations (0–5 mg/mL) is shown in Fig. 5b. The reducing power of SCPH increased with the higher concentrations, depending on the type of enzyme applied. The highest reducing power was observed for the hydrolysates obtained by alcalase, indicated that SCPH by Alcalase had more active amino acids or peptides to scavenge the free radicals. The lowest activity was obtained by flavourzyme. The difference in the reducing power maybe attributed to the specific peptide or amino acid composition (Wu et al. 2003). The data also suggested that SCPH could donate the electron to free radical.
Determination of metal chelating capacities SCP and SCPHs
Transition metal-ion can catalyze generation of reactive oxygen species which promote oxygen damage of cell and organ. Peptides can inhibit the negative effects caused metal ions though chelating them. The metal chelating capabilities of SCPHs as affected by the type of protease and concentration were depicted in Fig. 5c and d. Obviously, the metal chelating capabilities of SCPHs were increased with increasing concentration independently. All SCPHs possessed high iron chelating capacities over 80 % (>5 mg/mL). The EC50 values for hydrolysates prepared by flavourzyme, trypsin, neutrase, papain, alcalase were estimated from Fig. 5c to be about 2.00, 2.22, 0.912, 2.23, 1.67 mg/mL, respectively. Neutrase could be the optical protease to produce hydrolysate with great iron chelating capacity. Enzyme type and the DH greatly influenced the iron chelating capacity also has been reported in soy protein hydrolysate (Zhang et al. 2014).
In general, iron chelation was less efficient than copper chelation (Carrasco-Castilla et al. 2012), but in this study, it was opposite (except Flavourzyme). Concerning Cu2+ chelating capacity, flavourzyme-treated SCPH showed the highest capacity of 95.8 %, followed by nuetrase, trypsin, papain (84.9, 74.1, 66.3 % respectively) at 10 mg/mL, while the lowest capacity of SCPH prepared by alcalase just with 49.5 %. It seems that SCPH with higher DH had a lower copper chelating capacity (correlation coefficient −0.45). This results was coincided to the previous consequence reported (Li et al. 2014). Alcalase-treated SCPH had an excellent iron chelating capacity but pocessed poor copper chelating capacity. The cause might be attributed to the difference between the number of active sites chelating copper and ferrous ions.
Discussion
Functional properties of proteins are important in food applications. Most native proteins do not show functional properties desirably, thus their modification for improving these properties need to be addressed (Moure et al. 2006). Functional properties of proteins can be modified by physical, chemical and enzymatic treatments through changing protein structure. A compilation of the most interesting techno-functional properties of several products (meals, concentrates and isolates) obtained from oilseed on various operational conditions has been well reviewed (Moure et al. 2006). These oilseed proteins can be applied in food industry through increasing protein solubility and improving functional properties e.g., foaming, emulsifying and gelation property (Zhao et al. 2011). Enzymatic hydrolysis is an attractive tool to modify protein structure due to its mild reaction condition, easy control and minimal generation of byproduct (Mannheim and Cheryan 1992). Through enzymatic hydrolysis, functional property of oilseed proteins, especially protein solubility, can be improved (Zhao et al. 2011; Vioque et al. 2000), so were in our study. However, excessive hydrolysis impaired functional property such as foaming and emulsifying property (Jamdar et al. 2010; Kong et al. 2007). It is possible to enhance or reduce the functional property of protein hydrolysate prepared by depending on the protease type and the hydrolysis degree achieved (FitzGerald and O’Cuinn 2006). The limited enzymatic hydrolysis (generally DH < 10 %) improved the functional property of peanut and rapeseed protein, both foaming and emulsifying property (Zhao et al. 2011; Vioque et al. 2000), while a high hydrolysis degree reduced foaming and emulsifying property (Jamdar et al. 2010; Chabanon et al. 2007). Pepain-modified sesame protein isolates had better functional properties than original protein, especially in protein solubility, EAI and ESI (Bandyopadhyay and Ghosh 2002). Enzymatic modification enhanced the functional property of food protein has been well reviewed (Panyam and Kilara 1996). Protein hydrolyastes could be classified to determine their application through depending on the DH: (i) hyrolysates with a low DH (1–10 %) with improved the functional properties (mainly foaming and emulsifying property), (ii) hydrolysates with a variable DH that are used as flavourings, and (iii) extensive hydrolysates (>10 %) that are used as nutritional supplements (Rodríguez Patino et al. 2007; Panyam and Kilara 1996; Foegeding et al. 2002). In our study, enzyme type was an important factor to influence the functional property of protein hyrolysate, and trypsin was an excellent protease to generate protein hydrolysate with enhancing the foaming property of SCP. Electing appropriate protease and controlling the DH could be improved the functional properties of hydrolysates according to their actual applications.
Besides improving functional properties, enzymatic hydrolysis can be applied to generate bioactive peptides which exert several physiologic functions such as antioxidant (Li et al. 2014; Dey and Dora 2014), antimicrobial (McCann et al. 2006), hypocholesterolemic (Zhong et al. 2007), antihypertensive and antithrombotic (Choonpicharn et al. 2014) function. Antioxidant activity of peptides from food proteins has also well reviewed (Sarmadi and Ismail 2010). Antioxidant activity of protein hydrolysates influenced by several factors such as amino acid composition, amino acid sequence, and the length of peptide (Sarmadi and Ismail 2010; Wu et al. 2003). In our study, enzyme type and the DH greatly influenced antioxidant activity of protein hydrolysate.
Conclusion
The protein hydrolysates from Camellia oleifera seed cake were prepared by various enzymes. The amino acid composition, antioxidant activities and functional properties of SCPHs were evaluated. The resulting hydrolysates possessed a high content of charged amino acid composition. Hydrolysate prepared by alcalase showed the best solubility and relatively good antioxidant activity, while in the case of Trypsin, the best foaming property was observed. The emulsifying properties of SCPHs were influenced by enzyme type. SCPHs through enzymatic hydrolysis showed lower foaming and emulsifying properties than original SCP. All hysrolyates had an excellent antioxidant activity. Antioxidant activity of SCPHs was depended on the type of enzyme employed and the concentration. Trypsin-treated SCPH showed the highest iron chelating capability. Copper chelating capability was negatively correlated with the DH of hydrolysates. It was possible depending on electing enzyme type and the DH to generate hydrolysate products with improving functional property and enhancing antioxidant activity which determined their application.
Acknowledgments
The authors gratefully acknowledge the financial support by Department of Science and Technology Support Project (2013NZ0047) of Sichuan Province. The authors sincerely thank for the assistance of Xuejing Jia and Haoran Cheng.
References
- Bandyopadhyay K, Ghosh S. Preparation and characterization of papain-modified sesame (Sesamum indicum L.) protein isolates. J Agric Food Chem. 2002;50:6854–6857. doi: 10.1021/jf020320x. [DOI] [PubMed] [Google Scholar]
- Benjakul S, Morrissey MT. Protein hydrolysates from Pacific whiting solid wastes. J Agric Food Chem. 1997;45:3423–3430. doi: 10.1021/jf970294g. [DOI] [Google Scholar]
- Carrasco-Castilla J, Hernández-Álvarez AJ, Jiménez-Martínez C, Jacinto-Hernández C, Alaiz M, Girón-Calle J, Vioque J, Dávila-Ortiz G. Antioxidant and metal chelating activities of peptide fractions from phaseolin and bean protein hydrolysates. Food Chem. 2012;135:1789–1795. doi: 10.1016/j.foodchem.2012.06.016. [DOI] [PubMed] [Google Scholar]
- Chabanon G, Chevalot I, Framboisier X, Chenu S, Marc I. Hydrolysis of rapeseed protein isolates: kinetics, characterization and functional properties of hydrolysates. Process Biochem. 2007;42:1419–1428. doi: 10.1016/j.procbio.2007.07.009. [DOI] [Google Scholar]
- Choonpicharn S, Jaturasitha S, Rakariyatham N, Suree N, Niamsup H (2014) Antioxidant and antihypertensive activity of gelatin hydrolysate from Nile tilapia skin. J Food Sci Technol :1–6. doi:10.1007/s13197-014-1581-6 [DOI] [PMC free article] [PubMed]
- Decker EA, Welch B. Role of ferritin as a lipid oxidation catalyst in muscle food. J Agric Food Chem. 1990;38:674–677. doi: 10.1021/jf00093a019. [DOI] [Google Scholar]
- Dey S, Dora K. Antioxidative activity of protein hydrolysate produced by alcalase hydrolysis from shrimp waste (Penaeus monodon and Penaeus indicus) J Food Sci Technol. 2014;51:449–457. doi: 10.1007/s13197-011-0512-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiaschi T, Chiarugi P. Oxidative stress, tumor microenvironment, and metabolic reprogramming: a diabolic liaison. Int J Cell Biol. 2012 doi: 10.1155/2012/762825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FitzGerald RJ, O’Cuinn G. Enzymatic debittering of food protein hydrolysates. Biotechnol Adv. 2006;24:234–237. doi: 10.1016/j.biotechadv.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Foegeding EA, Davis JP, Doucet D, McGuffey MK. Advances in modifying and understanding whey protein functionality. Trends Food Sci Technol. 2002;13:151–159. doi: 10.1016/S0924-2244(02)00111-5. [DOI] [Google Scholar]
- Gbogouri G, Linder M, Fanni J, Parmentier M. Influence of hydrolysis degree on the functional properties of salmon byproducts hydrolysates. J Food Sci. 2004;69:C615–C622. doi: 10.1111/j.1365-2621.2004.tb09909.x. [DOI] [Google Scholar]
- He SN, Gu Y. The comprehensive utilization of camellia fruits. Am Camellia Yearbk. 1982;37:104–107. [Google Scholar]
- Intarasirisawat R, Benjakul S, Visessanguan W. Antioxidative and functional properties of protein hydrolysate from defatted skipjack (Katsuwonous pelamis) roe. Food Chem. 2012;135:3039–3048. doi: 10.1016/j.foodchem.2012.06.076. [DOI] [PubMed] [Google Scholar]
- Ito N, Fukushima S, Tsuda H. Carcinogenicity and modification of the carcinogenic response by BHA, BHT, and other antioxidants. Crit Rev Toxicol. 1985;15:109–150. doi: 10.3109/10408448509029322. [DOI] [PubMed] [Google Scholar]
- Jamdar S, Rajalakshmi V, Pednekar M, Juan F, Yardi V, Sharma A. Influence of degree of hydrolysis on functional properties, antioxidant activity and ACE inhibitory activity of peanut protein hydrolysate. Food Chem. 2010;121:178–184. doi: 10.1016/j.foodchem.2009.12.027. [DOI] [Google Scholar]
- Klompong V, Benjakul S, Kantachote D, Shahidi F. Antioxidative activity and functional properties of protein hydrolysate of yellow stripe trevally (Selaroides leptolepis) as influenced by the degree of hydrolysis and enzyme type. Food Chem. 2007;102:1317–1327. doi: 10.1016/j.foodchem.2006.07.016. [DOI] [Google Scholar]
- Kong X, Zhou H, Qian H. Enzymatic hydrolysis of wheat gluten by proteases and properties of the resulting hydrolysates. Food Chem. 2007;102(3):759–763. doi: 10.1016/j.foodchem.2006.06.062. [DOI] [Google Scholar]
- Li X, Shen S, Deng J, Li T, Ding C. Antioxidant activities and functional properties of tea seed protein hydrolysates (Camellia oleifera Abel.) influenced by the degree of enzymatic hydrolysis. Food Sci Biotechnol. 2014;23:2075–2082. doi: 10.1007/s10068-014-0282-2. [DOI] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Mannheim A, Cheryan M. Enzyme-modified proteins from corn gluten meal: preparation and functional properties. J Am Oil Chem Soc. 1992;69:1163–1169. doi: 10.1007/BF02637674. [DOI] [Google Scholar]
- Maritim AC, Sanders RA, Watkins JB. Diabetes, oxidative stress, and antioxidants: a review. J Biochem Mol Toxicol. 2003;17:24–38. doi: 10.1002/jbt.10058. [DOI] [PubMed] [Google Scholar]
- McCann KB, Shiell BJ, Michalski WP, Lee A, Wan J, Roginski H, Coventry MJ. Isolation and characterisation of a novel antibacterial peptide from bovine αS1-casein. Int Dairy J. 2006;16:316–323. doi: 10.1016/j.idairyj.2005.05.005. [DOI] [Google Scholar]
- Moure A, Sineiro J, Domínguez H, Parajó JC. Functionality of oilseed protein products: a review. Food Res Int. 2006;39:945–963. doi: 10.1016/j.foodres.2006.07.002. [DOI] [Google Scholar]
- Mutilangi W, Panyam D, Kilara A. Functional properties of hydrolysates from proteolysis of heat‐denatured whey protein isolate. J Food Sci. 1996;61:270–275. doi: 10.1111/j.1365-2621.1996.tb14174.x. [DOI] [Google Scholar]
- Oyaizu M. Studies on products of browning reaction: antioxidative activity of products of browning reaction. Jpn J Nutr. 1986;44:307–315. doi: 10.5264/eiyogakuzashi.44.307. [DOI] [Google Scholar]
- Panyam D, Kilara A. Enhancing the functionality of food proteins by enzymatic modification. Trends Food Sci Technol. 1996;7:120–125. doi: 10.1016/0924-2244(96)10012-1. [DOI] [Google Scholar]
- Rodríguez Patino JM, Miñones Conde J, Linares HM, Pedroche Jiménez JJ, Carrera Sánchez C, Pizones V, Rodríguez FM. Interfacial and foaming properties of enzyme-induced hydrolysis of sunflower protein isolate. Food Hydrocoll. 2007;21:782–793. doi: 10.1016/j.foodhyd.2006.09.002. [DOI] [Google Scholar]
- Sarmadi BH, Ismail A. Antioxidative peptides from food proteins: a review. Peptides. 2010;31:1949–1956. doi: 10.1016/j.peptides.2010.06.020. [DOI] [PubMed] [Google Scholar]
- Sun Q, Luo Y, Shen H, Li X, Yao L. Purification and characterisation of a novel antioxidant peptide from porcine haemoglobin hydrolysate. Int J Food Sci Technol. 2012;47:148–154. doi: 10.1111/j.1365-2621.2011.02820.x. [DOI] [Google Scholar]
- Tang C-H, Wang X-S, Yang X-Q. Enzymatic hydrolysis of hemp (Cannabis sativa L.) protein isolate by various proteases and antioxidant properties of the resulting hydrolysates. Food Chem. 2009;114:1484–1490. doi: 10.1016/j.foodchem.2008.11.049. [DOI] [Google Scholar]
- Vioque J, Sánchez-Vioque R, Clemente A, Pedroche J, Millán F. Partially hydrolyzed rapeseed protein isolates with improved functional properties. J Am Oil Chem Soc. 2000;77:447–450. doi: 10.1007/s11746-000-0072-y. [DOI] [Google Scholar]
- Wu H-C, Chen H-M, Shiau C-Y. Free amino acids and peptides as related to antioxidant properties in protein hydrolysates of mackerel (Scomber austriasicus) Food Res Int. 2003;36:949–957. doi: 10.1016/S0963-9969(03)00104-2. [DOI] [Google Scholar]
- Zhang M-N, Huang G-R, Jiang J-X. Iron binding capacity of dephytinised soy protein isolate hydrolysate as influenced by the degree of hydrolysis and enzyme type. J Food Sci Technol. 2014;51:994–999. doi: 10.1007/s13197-011-0586-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao G, Liu Y, Zhao M, Ren J, Yang B. Enzymatic hydrolysis and their effects on conformational and functional properties of peanut protein isolate. Food Chem. 2011;127:1438–1443. doi: 10.1016/j.foodchem.2011.01.046. [DOI] [Google Scholar]
- Zhao Q, Xiong H, Selomulya C, Chen XD, Zhong H, Wang S, Sun W, Zhou Q. Enzymatic hydrolysis of rice dreg protein: effects of enzyme type on the functional properties and antioxidant activities of recovered proteins. Food Chem. 2012;134:1360–1367. doi: 10.1016/j.foodchem.2012.03.033. [DOI] [PubMed] [Google Scholar]
- Zhong F, Liu J, Ma J, Shoemaker CF. Preparation of hypocholesterol peptides from soy protein and their hypocholesterolemic effect in mice. Food Res Int. 2007;40:661–667. doi: 10.1016/j.foodres.2006.11.011. [DOI] [Google Scholar]