Abstract
In this study, an optimisation of extraction of sulphur volatile compounds (SVCs) has been performed using Central Composite Design. The conditions of the highest amount of eluated peaks and total peaks area have been treated. Factors such as coating of fiber for SPME (Solid Phase Microextraction), extraction temperature and extraction time have been optimised. The SVCs have shown the optimal extraction using a DVB/CAR/PDMS (divinylbenzene/carboxen/polydimethylsiloxane) fiber at 73 °C during 50 min. Furthermore, a pre-incubation step lasting 20 min at the extraction temperature has been used. In total, 12 samples have been investigated at the mentioned optimal conditions, eight from the Alliaceae and four from the Brassicaceae family. The highest number of SVCs (24) has been identified in the sample of chive. The most frequently identified compound found in 11 of 12 samples has been dimethyl trisulphide.
Keywords: Sulphur volatile compounds, Headspace, Solid-phase microextraction, Alliaceae, Brassicaceae, Gas chromatography/mass spectrometry
Introduction
The aroma of food is affected by various kinds of constituents, among which sulphur containing compounds are a significant group due to their abundance and aromatic impact. Sulphur is an essential element occurring particularly in two amino acids - cysteine and methionine. Decomposition products of those organic molecules are volatile hydride- and alkyl-sulphur constituents such as thiols, sulphides (disulphides, trisulphides etc.). In a trace level these compounds can be responsible for specific aroma causing odour problems such as pungency in eyes and nose (Lanzotti 2006).
The SVCs have been examined in a quite wide scale of publications, especially in samples belonging to genus Allium. Numbers of methods have been used for isolation of these constituents from the samples. For example, liquid-liquid extraction (Yabuki et al. 2010), hydrodistillation (Calvo-Gomez et al. 2004; Godevac et al. 2008) or ultrasonic assisted extraction methods (Kimbaris et al. 2006) were used. A lot of works focused on extraction of volatile compounds of onion and garlic using headspace solid phase microextraction (HS-SPME) (Colina-Coca et al. 2013; Jarvenpaa et al. 1998; Mondy et al. 2002; Teyssier et al. 2001).
The SVCs might be also presented in family Brassicaceae. However, the volatile compounds have not been so commonly examined in this family. For instance volatiles of mustard were analysed using SPME (Brassica juncea (L.) Coss.) (Zhao et al. 2007) and hydrodistillation was used for radish aroma profile analysis (Blazevic and Mastelic 2009).
Plants belonging to these two families are often edible and therefore are usually used in a cuisine all over the world, especially in the northern hemisphere. Lot of them are also known for having antimicrobial or anticancer effects. Furthermore, they are medicinal plants often used in both a folk medicine and a pharmaceutical industry (Lanzotti 2006; Yabuki et al. 2010).
One of the methods for extraction of the SVCs is the above-mentioned HS-SPME. It is a simple, inexpensive, relatively fast and easily automated method integrating sampling, extraction, pre-concentration and sample introduction into a single solvent-free step. However, SPME is quite sensitive to experimental conditions such as extraction time, heating temperature, sample volume, concentration, sample matrix and its uniformity, desorption conditions or choice of fiber (thickness and polarity) (Kumar et al. 2008; Mester and Sturgeon 2005; Qiu et al. 2014).
The objective of the present investigation has been an optimisation of the extraction of the SVCs from chive in order to compare sulphur aroma profile of plants belonging to families Alliaceae and Brassicaceae. The automated HS-SPME has been used for the extaction. Separation, detection and identification have been carried out by gas chromatography/mass spectrometry system (GC-MS).
Materials and methods
Plant material
Ten various kinds of plants, in which sulphur volatile compounds were expected, have been purchased from a local market or cultivated by local growers. These plants belonging among vegetables and are often used as food or medicaments are listed in Table 1. Regarding the sample of scallion two different parts - root and leaves – have been examined because both of them are edible and applicable in the cuisine. Otherwise, from the other samples, only roots have been analysed. Although, leaves are often also edible it is not so common to use them in the cuisine. All these plants grow in the Czech Republic and therefore all of the samples have Czech origin.
Table 1.
List of plant material
| Family Alliaceae | Family Brassicaceae | ||
|---|---|---|---|
| Sample | Latin name | Sample | Latin name |
| Chive | Allium schoenoprasum L. | Horseradish | Cochlearia armoracia L. |
| Onion | Allium cepa L. | Radish | Raphanus sativus L. |
| Garlic | Allium sativum L. | Kohlrabi | Brassica oleracea L. var. gongylodes |
| Shallot | Allium cepa L. var. aggregatum | Broccoli | Brassica oleracea L. var. italica |
| Leek | Allium porrum L. | ||
| Scallion | Allium fistulosum L. | ||
Chemicals
n-Alkane mixture standard solution (C8-C20) was purchased from Sigma-Aldrich (Prague, Czech Republic) in concentrations of 40 mg · l−1 dissolved in n-hexane.
Distilled water was purified using a SG Ultra Clear UV water purification system (SG, Hamburg, Germany).
Sample preparation
Fresh parts (leaves or roots) of the samples have been washed with purified water and diseased parts have been removed. After that the samples have been cut into small pieces and homogenised by dispersant Ultra Turrax DI 25 basic (IKA-WERKE, Staufen, Germany). A 0.5 g ±1 % of homogenised sample has been placed into 20 mL headspace vial and closed by a cap with a Teflon septum.
Experimental design
All statistical procedures have been performed using the Statistica CZ software, version 12 (StatSoft CR, Prague, Czech Republic).
A central composite design (CCD) approach has been used for modelling the obtained responses. Each parameter has been estimated at five levels, namely ±1, ±α, and one central point replicated twice.
Two extraction variables considered for this experiment were extraction temperature (X1 with coded levels x1) and extraction time (X2 with coded levels x2). Table 2 summarises the whole design consisted of 10 experimental points, which have been used to estimate effects of each factor on the extraction efficiency.
Table 2.
Central composite design-coded independent variables (x1, x 2), corresponding experimental conditions (X1, X 2) and results represented by total peaks area and number of peaks for DVB/CAR/PDMS fiber
| Run | Extraction temperature [°C] x1 (X1) | Extraction time [min] x 2 (X 2) | Total peaks area | Number of peaks | ||
|---|---|---|---|---|---|---|
| Observed | Predicted | Observed | Predicted | |||
| 1 | 1 (70) | 1 (50) | 9.95E + 08 | 9.83E + 08 | 137 | 132 |
| 2 | −1 (50) | 1 (50) | 8.75E + 08 | 8.44E + 08 | 128 | 126 |
| 3 (c) | 0 (60) | 0 (40) | 8.91E + 08 | 8.94E + 08 | 126 | 126 |
| 4 (c) | 0 (60) | 0 (40) | 8.97E + 08 | 8.94E + 08 | 127 | 126 |
| 5 | −α (46) | 0 (40) | 7.21E + 08 | 7.34E + 08 | 120 | 119 |
| 6 | 0 (60) | −α (26) | 7.53E + 08 | 7.18E + 08 | 123 | 121 |
| 7 | −1 (50) | −1 (30) | 6.54E + 08 | 6.69E + 08 | 112 | 115 |
| 8 | α (74) | 0 (40) | 9.68E + 08 | 9.52E + 08 | 129 | 132 |
| 9 | 0 (60) | α (54) | 9.08E + 08 | 9.39E + 08 | 127 | 131 |
| 10 | 1 (70) | −1 (30) | 8.07E + 08 | 8.42E + 08 | 129 | 129 |
α for rotatability is 1.41 (coded value)
SPME equipment
In this study, three different fibers have been compared for their suitability and efficiency regarding the extraction of SVCs. Those were 85 μm Polyacrylate (PA); 75 μm Carboxen/Polydimethylsiloxane (CAR/PDMS); 50/30 μm StableFlex DVB/CAR/PDMS. SPME holder for an automatic sampling and all fibers were purchased from Supelco (Bellefonte, PA, USA). Before use, new SPME fibers were conditioned by heating in hot injection port of a gas chromatograph according to the producer’s instructions. In addition, fibers were conditioned at 200 °C for 2 min prior to each sample extraction.
Chromatographic analysis
A gas chromatograph, model GC-17A, coupled with mass spectrometry detector QP 5050A (Shimadzu, Kyoto, Japan) and PAL-Combi auto-sampler (CTC Analytics AG, Zwingen, Switzerland) equipped with an auto-sampling device for SPME, a device for conditioning and agitating of the vial with the sample. Injections have been performed in split mode 1:5. The GC-MS system has been equipped with a capillary column SLB-5 ms with length 30 m, 0.25 mm inner diameter and 0.25 μm film thickness (J&W Scientific, Folsom, CA, USA). Helium 5.0 (Linde Gas a.s., Prague, Czech Republic) was used as the carrier gas at a constant linear velocity of 30 cm/s. The injector and the interface temperature were maintained at 250 °C. The column temperature has been programmed as follows: the initial temperature was 55 °C (4 min) then increased at a rate of 6.5 °C/min up to 250 °C (2 min). The mass spectrometer was operated in the full scan mode over a mass range of m/z 45–500 and in the electron ionization (EI) mode (70 eV). The linear retention indices (RI) were calculated for a homologous series of n-alkanes (C8-C20) injected under the same GC-MS conditions. The SVCs have been identified by comparing the results obtained with the reference mass spectra from the NIST library using the criterion at least 85 % similarity for the mass spectra and by comparing with linear retention index (if available).
Results and discussion
Firstly, an optimisation procedure for HS-SPME method has been carried out. The optimisation was composed of examination of SPME fiber, extraction temperature and extraction time. The experiments were evaluated by the number of peaks and the sum of all peak areas detected during analysis.
Optimisation of extraction
Selection of SPME fiber
Commercially available fibers are coated with a polymeric film that could have different thickness and polarity. The thickness of the film affects both the equilibrium time and sensitivity of the method. More effective extraction is carried out with a thicker fiber coating due to its capacity and transfer into the GC injector without losses. Also, the thicker fibers should be used for analytes with a low molecular weight because of easy migration of the analytes. On the other hand, the thinner fibers are able to extract and desorb compounds with a high boiling point and a mass weight in relatively short times and at high temperatures (Otles et al. 2009; Pawliszyn 2011; Wardencki et al. 2004). Three different SPME fibers (see 2.5) have been examined in the present study in order to find the optimal one for the extraction of the SVCs according to the stationary phases of individual fibres, producer’s recommendations (Sigma-Aldrich 2014) and literature (Jirovetz et al. 2002). The comparison has been performed at temperatures 50, 60 and 70 °C for 30, 40 and 50 min. For this evaluation sample of chive was used. Total peaks area (TPA) and number of peaks (NoP) in chromatograms were used for determination of the suitability of individual fibers.
Table 3 shows results of tested SPME fibers for extracting volatiles compounds from the sample of chive. It is clear that analysis with DVB/CAR/PDMS fiber has given higher total signal and higher number of peaks in chromatograms than the other examined fibres and this is also applicable for sulphur compounds identified in individual chromatograms. Therefore, DVB/CAR/PDMS fiber has been chosen as the optimal for the extraction of the SVCs in the mentioned real sample. Furthermore, on average about 20 % of all peaks in chromatograms have been sulphur compounds and this has been similar for all three examined fibers. The average relative peak area of SVCs has ranged from 60 % (75 μm CAR/PDMS fiber) via 68 % (50/30 μm DVB/CAR/PDMS fiber) to 69 % (85 μm PA fiber).
Table 3.
Results of HS-SPME/GC-MS analysis with use of three different SPME fibres. TPA total peaks area, NoP number of peaks
| Extraction temperature [°C], time [min] | 50/30 μm DVB/CAR/PDMS | 75 μm CAR/PDMS | 85 μm PA | |||
|---|---|---|---|---|---|---|
| NoP (sulphur compounds) | TPA (sulphur compounds) | NoP (sulphur compounds) | TPA (sulphur compounds) | NoP (sulphur compounds) | TPA (sulphur compounds) | |
| 50, 30 | 112 (22) | 6,6E + 08 (4,6E + 08) | 83 (17) | 4,0E + 08 (2,4E + 08) | 92 (18) | 3,8E + 08 (2,8E + 08) |
| 50, 50 | 128 (22) | 9,0E + 08 (6,4E + 08) | 85 (17) | 4,1E + 08 (2,9E + 08) | 96 (19) | 4,6E + 08 (3,3E + 08) |
| 60, 40 | 126 (22) | 8,8E + 08 (6,5E + 08) | 96 (17) | 4,0E + 08 (2,3E + 08) | 112 (20) | 5,5E + 08 (3,8E + 08) |
| 70, 30 | 129 (23) | 8,3E + 08 (5,2E + 08) | 112 (17) | 5,2E + 08 (2,8E + 08) | 102 (20) | 5,1E + 08 (3,6E + 08) |
| 70, 50 | 137 (24) | 1,2E + 09 (7,3E + 08) | 113 (18) | 6,3E + 08 (3,6E + 08) | 112 (20) | 6,4E + 08 (3,9E + 08) |
Statistical evaluation of SPME
Design of experiments has covered 10 experiments for DVB/CAR/PDMS fiber in the temperature range from 46 (−α) to 74 (+α) °C and the extraction time from 26 (−α) to 54 (+α) minutes, while pre-incubation time has been maintained constant at the temperature of the extraction for 20 min. All the optimisation experiments have been performed with the sample of chive. In the statistical software Statistica 12 there are options to build different polynomial models which refer to a linear modelling and second-order modelling. Second-order modelling provides models which have advantages in better predictions of response. Thus, the second-ordered polynomial equations have been constructed for the response variables (total peaks area (TPA) and number of peaks (NoP)) related to the experimental conditions, i. e. Eq. (1) for TPA and Eq. (2) for NoP.
| 1 |
| 2 |
where X1 is extraction temperature (°C), and X2 is extraction time (min).
The significant factors (p-value less than 0.05 at confidence level 95 %) of the equations were the linear terms of the extraction temperature and extraction time (Eq. (1)), and linear term of the extraction temperature (Eq. (2)). All these factors had positive effects on response variables. The experimental values correlate well with the values predicted by the models (shown in Table 2). The square roots of the determination coefficient of the models (R2) with linear terms, quadratic terms, and interaction between linear terms were 0.9521 for TPA and 0.8198 for NoP. R2 considering only linear effects in the model was 0.8962 for TPA and 0.7748 for NoP, and increases to 0.9495 for TPA and 0.7783 for NoP with introduction of quadratic terms.
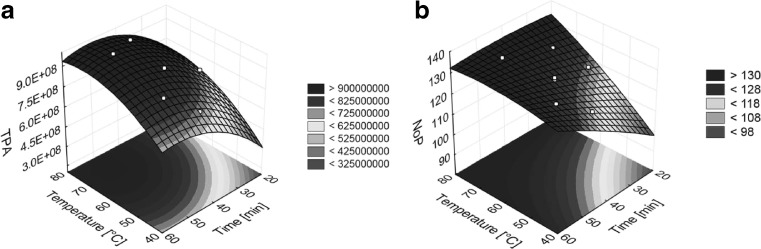
The response surfaces obtained by displaying Eqs. (1) and (2) in three-dimensional plots are presented in the Fig. 1a for TPA, and 1b for NoP. Each of graphs represents dependency of the response variables on independent variables. The effects of parameters on the TPA and NoP and interaction between them can be estimated from the biaxial contour plot in the base of three-dimensional plot and from the shape of three-dimensional surface, and it can be noted that both independent variables have similar effects on TPA and NoP. Dark colour indicates increase of TPA (Fig. 1a) and NoP (Fig. 1b) and surroundings of optimum conditions. White points on the surface of three-dimensional plots show areas of individual models. In the case of TPA, critical values of independent variables lie within inside appropriate model, and on the border conditions in the case of NoP.
Fig. 1.
Response surface plots showing effects of extraction temperature and extraction time on total peak areas (a) and number of peaks (b)
Response surface models (Fig. 1a and b) provide similar critical values of independent variables in maximum of three-dimensional plots in ranges 73–74 °C and 50–56 min. Therefore, we decided to use critical values of TPA model (73 °C, 50 min) as optimum extraction conditions due higher square root of the determination coefficient of the model (R2 = 0.9521). This value indicates, that less than 5 % of the total variations could not be explained by the model.
Analysis of real samples and identification of SVCs
As it is obvious in the Table 4, in a half of the samples (6) the SVCs constitute majority of the aroma profiles (more than 50 % relative area (to the total area)). Those are chive (61.98 %), shallot (53.24 %), garlic (66.31 %), leek (66.11 %), scallion leaves - sample 2 (61.58 %) and horseradish (95.05 %).
Table 4.
Individual sulphur volatile compounds identified in the real samples
| RI | Molecular formula | Chive | Onion | Shallot | Garlic | Leek | Scallion root – sample 1 | Scallion leafs - sample 1 | Scallion leafs - sample 2 | Kohlrabi | Broccoli | Radish | Horseradish | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbonyl sulphide | 687 | COS | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | 0.15a |
| Sulphur dioxide | 688 | SO2 | 0.09a | n.i. | n.i. | n.i. | 0.19a | 0.45a | 0.55a | 0.19a | n.i. | n.i. | n.i. | n.i. |
| Dimethyl sulphide | 697 | C2H6S | n.i. | n.i. | n.i. | 0.02a | n.i. | 0.55a | n.i. | n.i. | 0.02b | 2.59 b | 2.54b | 0.03a |
| CS2 | 700 | CS2 | 0.13a | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. |
| Allyl mercaptan | 707 | C3H6S | n.i. | n.i. | n.i. | 0.18a | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. |
| Methylthiirane | 709 | C3H6S | 0.51a | 0.17a | n.i. | 0.02a | 0.47a | 1.19a | 1.09a | 0.51a | n.i. | n.i. | n.i. | n.i. |
| Dimethyl disulphide | 763 | C2H6S2 | 0.88b | 0.04a | 0.09a | 0.03b | 0.43b | n.i. | n.i. | 0.29a | 0.05b | 1.48b | 19.06 b | n.i. |
| 3,3-Thiobis(1-propene) | 861 | C6H10S | n.i. | n.i. | n.i. | 0.15a | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. |
| Allyl isothiocyanate | 891 | C4H5NS | n.i. | n.i. | n.i. | 0.03a | 0.09b | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | 21.38b |
| 2,4-Dimethylthiophene | 908 | C6H8S | 0.05b | 0.02b | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. |
| 2,5-Dimethylthiophene | 908 | C6H8S | n.i. | n.i. | 0.02a | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. |
| 3,4-Dimethylthiophene | 908 | C6H8S | n.i. | n.i. | 0.22b | n.i. | 0.03b | n.i. | n.i. | 0.15a | n.i. | n.i. | n.i. | n.i. |
| Methyl 2-propenyl disulphide | 920 | C4H8S2 | 0.06a | 0.35b | n.i. | 0.90b | 0.06a | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. |
| Methyl propyl disulphide | 935 | C4H10S2 | 3.17b | n.i. | n.i. | n.i. | 3.91b | n.i. | 0.73a | 2.23a | n.i. | n.i. | 1.36a | n.i. |
| Methyl-1-propenyl disulphide | 943 | C4H8S2 | 3.51b | 1.45b | 2.52b | 0.19b | 1.57a | 2.18a | 0.15a | 2.09a | n.i. | n.i. | 0.66a | n.i. |
| Isobutyl isothiocyanate | 956 | C5H9NS | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | 0.03b |
| Dimethyl trisulphide | 977 | C2H6S3 | 10.49 b | 2.93b | 5.69a | 0.39b | 0.54b | 1.63a | 0.63a | 6.32a | 2.12b | 0.90b | 9.16b | n.i. |
| 3-Butenyl isothiocyanate | 984 | C5H7NS | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | 0.26a |
| S-Methyl Methanethiosulphonate | 998 | C2H6OS2 | 0.53a | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. |
| 2-butyl isothiocyanate | 999 | C5H9NS | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | 0.05b |
| 3-Methylbutyl isothiocyanate | 1061 | C6H11NS | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | 0.13a | n.i. | n.i. | 0.42b |
| 4-(Methylthio)-butanonitrile | 1091 | C5H9NS | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | 4.90b | n.i. | n.i. | n.i. |
| Diallyl disulphide | 1093 | C6H10S2 | n.i. | n.i. | n.i. | 0.05b | 0.11a | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. |
| Pentyl isothiocyanate | 1099 | C6H11NS | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | 0.06b |
| Propyl cis-1-propenyl disulphide | 1114 | C6H12S2 | n.i. | 0.57a | 0.42b | n.i. | 1.10a | 0.33a | 0.36a | n.i. | n.i. | n.i. | n.i. | n.i. |
| Dipropyl disulphide | 1115 | C6H14S2 | 7.77b | 2.54b | 2.31a | n.i. | 20.21 b | 3.06a | 17.97 a | 9.12 a | 0.12a | n.i. | n.i. | n.i. |
| Propyl trans-1-propenyl disulphide | 1123 | C6H12S2 | 5.95a | 1.06b | 0.98b | n.i. | 10.63a | 4.72a | 7.58a | 6.47a | 0.04a | n.i. | n.i. | n.i. |
| 3-Ethyl-1,2-dithi-4-ene | 1127 | C6H10S2 | 0.42b | 0.72a | 0.68a | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. |
| 3-Ethyl-1,2-dithi-5-ene | 1132 | C6H10S2 | 0.73b | 1.04a | 1.25a | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. |
| Methyl (methylthio)methyl disulphide | 1137 | C3H8S3 | 0.79a | n.i. | n.i. | 0.05a | n.i. | n.i. | n.i. | n.i. | 0.02a | n.i. | 0.61a | n.i. |
| Methyl 2-propenyl trisulphide | 1148 | C4H8S3 | 0.52a | 0.55a | 0.53a | 9.70b | 0.19a | 0.32a | n.i. | 0.30a | n.i. | n.i. | n.i. | n.i. |
| Methyl propyl trisulphide | 1161 | C4H10S3 | 5.27a | 2.94b | 3.17a | 0.02a | n.i. | 6.17a | n.i. | 8.76a | 0.02a | n.i. | 0.42a | n.i. |
| Dithioallyl propionate | 1165 | C6H10S2 | 0.51a | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. |
| 4-Methylpentyl isothiocyanate | 1165 | C7H13NS | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | 0.05a | n.i. | n.i. | 0.08a |
| Methyl cis-1-propenyl trisulphide | 1171 | C4H8S3 | 4.53b | 6.71b | 8.49b | 0.09b | 0.79b | 2.15b | n.i. | 5.54b | 0.03b | n.i. | n.i. | n.i. |
| Methyl trans-1-propenyl trisulphide | 1176 | C4H8S3 | 6.20b | 8.72 b | 11.03b | 0.14b | 1.11b | 2.96b | 0.28b | 7.25b | 0.03b | n.i. | n.i. | n.i. |
| 3-Vinyl-[4H]-1,2-dithiin | 1202 | C6H8S2 | n.i. | n.i. | n.i. | 0.71b | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. |
| 2-Vinyl-[4H]-1,3-dithiin | 1230 | C6H8S2 | n.i. | n.i. | n.i. | 2.45b | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. |
| Dimethyl tetrasulphide | 1230 | C2H6S4 | 2.07a | 8.62a | 13.19 a | n.i. | 0.29a | 1.44a | n.i. | 2.82a | 14.58 a | n.i. | 2.34a | n.i. |
| Benzothiazole | 1243 | C7H5NS | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | 0.23a | n.i. | n.i. | n.i. | n.i. | n.i. |
| 3-(Methylthio)propyl isothiocyanate | 1320 | C5H9NS2 | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | 4.69b | n.i. | n.i. | 0.18a |
| Di-2-propenyl trisulphide | 1336 | C6H10S3 | n.i. | n.i. | n.i. | 46.76 b | 0.06a | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. |
| Dipropyl trisulphide | 1339 | C6H14S3 | 3.57a | 1.84b | 0.99b | n.i. | 14.89a | 8.44 a | 7.82a | 8.75a | 0.02a | n.i. | n.i. | n.i. |
| Propenyl propyl trisulphide | 1353 | C6H12S3 | 4.12a | n.i. | n.i. | n.i. | 0.51b | n.i. | n.i. | 0.60a | n.i. | n.i. | n.i. | n.i. |
| 3,5-Diethyl-1,2,4-trithiolane | 1353 | C4H8S3 | n.i. | n.i. | n.i. | 1.45b | 8.93a | n.i. | 5.22a | n.i. | n.i. | n.i. | 0.98a | n.i. |
| Benzyl thiocyanate | 1377 | C8H7NS | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | 2.19b |
| 2-phenethylisothiocyanate | 1495 | C9H9NS | n.i. | n.i. | n.i. | 0.1a | n.i. | n.i. | n.i. | n.i. | 11.94b | n.i. | n.i. | 70.19 b |
| Methyl-3,4-dimethyl-2-thienyl disulphide | 1512 | C7H10S2 | 0.11a | 0.50a | 0.22a | n.i. | n.i. | n.i. | n.i. | 0.19a | n.i. | n.i. | n.i. | n.i. |
| (3-isothiocyanato-propyl)-benzene | 1589 | C10H11NS | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | 0.03a |
| Di-2-propenyl tetrasulphide | 1597 | C6H10S4 | n.i. | n.i. | 1.44a | 2.89b | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. |
| Proportion of SVCs in the aroma profile of the sample (%) | 61.98 | 40.77 | 53.24 | 66.31 | 66.11 | 35.59 | 42.61 | 61.58 | 38.76 | 4.97 | 37.13 | 95.05 | ||
| Total sulphur compounds | 24 | 18 | 18 | 21 | 21 | 14 | 12 | 17 | 16 | 3 | 9 | 13 | ||
The most abundant compound for the individual samples is in bold
n.i. not identified compound, RI linear retention index
a – compounds identified using mass spectral library
b – compounds identified using library and by compliance with published information (RI, mass spectrum)
Totally, in this work 50 SVCs have been identified at least in one of the samples. The most common identified compounds have been dimethyl trisulphide that occurred in all samples except horseradish, and dimethyl disulphide found in 10 samples. These constituents are typical representatives of sulphur containing plants which have been published in quiet wide numbers of research that deal with aroma profiles of those plants (Colina-Coca et al. 2013; Jabalpurwala et al. 2010; Jirovetz et al. 2002; Ma et al. 2011; Yabuki et al. 2010). The other very often identified compounds have been methyl trans-1-propenyl disulphide and methyl trans-1-propenyl trisulphide found in 9 samples (see Table 4).
Alliaceae family
Eight of all the examined samples belong to the Alliaceae family (see Table 1). The aroma profile of this family has been observed in a wide scale of publications. For instance, volatiles of chive were extracted from headspace and then analysed by gas chromatography (Mann et al. 2011). Nevertheless, only 5 SVCs were identified. Those were four variously substituted disulphides, and dimethyl thiophene. On the other hand, in another publication, the SVCs were extracted from Chinese chive (Allium tuberosum Rottler) by diethyl ether and subsequently analysed by GC-MS (Yabuki et al. 2010). In total, 11 compounds were identified, mostly sulphides. None of the authors mentioned percentage of individual SVCs. In the present research 24 SVCs were identified, eight of them are in compliance with one or both mentioned publications. The most abundant SVC has been dimethyl trisulphide (10.49 %).
Onion and garlic are quite often examined matrixes, particularly due to their world-wide cuisine utilisation. For instance, volatile compounds of processed onion were analysed by headspace gas chromatography–mass spectrometry (HS-GC-MS) (Colina-Coca et al. 2013). In that work it was able to identify 18 volatile compounds, 12 of them were SVCs. The most dominant SVC was dipropyl disulphide (48 %). In comparison to the present work, 17 SVCs were identified and 8 of them are in compliance with the research referred by Colina-Coca. The most abundant SVCs were methyl trans-1-propenyl trisulphide (8.72 %) and dimethyl tetrasulphide (8.62 %).
Another work dealt with comparing distillation and ultrasonic assisted extraction methods for isolation of volatiles contained in garlic followed by GC-MS (Kimbaris et al. 2006). In total, 30 volatile components were identified at least by one method. Sulphur was contained in 27 of them, the vast majority were sulphides. The most abundant SVCs were diallyl disulphide (28.4 %) and diallyl trisulphide (20.4 %). In the present work, 21 SVCs were identified, also mostly sulphides. Although 11 SVCs were identified as the same in both, the present and Kimbaris’s, research, Kimbaris did not refer any isothiocyanate. In the present work 2 isothiocyanates were identified.
An enzyme-assisted extraction for examination of quality of garlic volatile oil was used (Sowbhagya et al. 2009). In this type of extraction enzymes were applied to garlic prior to steam distillation/hydrodistillation. The analysis of volatile compounds was performed using GS-MS. Ileven SVCs were identified, none isothiocyanate. Eight of them are in compliance with the present research. Furthermore, the most abundant SVC was di-2-propenyl trisulphide that generated about 50 % of total odour in the present and also in Sowbhagya’s research.
There are not many works dealing with the aroma profile of shallot, leek and scallion. The first named plant or its organosulphides, respectively, were examined in a recent work by Tocmo. (Tocmo et al. 2014). Shallot was studied as a potential dietary source of organic polysulphides. Shallot oil was obtained and subsequently compared using two different extraction methods, hydrodistillation and extraction using anhydrous diethyl ether. The second one showed better results related to the number of eluted peaks. In total, 19 volatile compounds were identified, from which 14 were SVCs. Six of them are in compliance with the present work where 19 SVCs were identified. As was expected, the most dominant SVCs were the same as in the sample of onion; dimethyl tetrasulphide (13.19 %) and methyl trans-1-propenyl trisulphide (11.03 %).
Aroma profile of leek was studied by GC-MS (Schreyen et al. 1976). In total, 67 compounds were observed in leek essential oil and 22 of them were SVCs (particularly sulphides, thiophene derivatives and thiols). In comparison to the present work, 21 SVCs were identified; seven are in compliance with Schreyen’s work. The most abundant compound was dipropyl disulphide (21.21 %).
None research concerning the aroma profile of scallion have been available. In the present work, two samples of scallion were compared. From one of them (sample 1) both parts, root and leaves, and from the other one (sample 2) only leaves were examined. Sample 1 was purchased from a local market while sample 2 was harvested by a local grower. As is seen in the table 4, sample 2 contained more SVCs (17) than root (14) or leaves (12) of the sample 1. This could be caused particularly by different growing conditions.
Brassicaceae family
With regard to the occurrence of the observed volatile constituents this family has given various results. In the sample of broccoli only three SVCs were identified. Those were sulphide components, namely dimethyl sulphide (2.59 %), dimethyl disulphide (1.48 %) and dimethyl trisulphide (2.12 %). As is obvious, in this sample SVCs generate only approximately 5 % of total aroma. Those three sulphides were identified also in the work of Vidal-Aragon (Vidal-Aragon et al. 2009) where aromatic compounds of seven broccoli cultivars were analysed by an automatic headspace analyser. In total, there were identified 45 constituents from which eight belong to the SVCs. The most abundant SVCs were dimethyl disulphide and methanethiol. Nevertheless, Vidal-Aragon figured out that in 5 cultivars the SVCs formed major aroma profile (more than 50 % of total aroma). Regarding the fact, the sample of broccoli tested in the present work contained only 5 % of SVCs, it could origin from the Parthenon cultivar (19 % of SVCs, according to the work of Aragon).
Volatile compounds of kohlrabi were extracted with modified Likens and Nickerson apparatus using triply distilled 2-methylbutane (MacLeod and MacLeod 1990). The extracts were subsequently analysed by GC-MS. According to that work, the SVCs provided a high proportion of kohlrabi volatiles (about 37 % w/w). In total, 16 SVCs were identified. The major compound was dimethyl trisulphide (25 % w/w). In the present work, 17 SVCs were identified. The most abundant was dimethyl tetrasulphide (14.58 %), while dimethyl trisulphide generated only 6.32 % of the total aroma. However, only six SVCs were identified as the same in both, the present and the mentioned research.
Hydrodistillation in a Clevenger-type apparatus was used for the extraction of volatile compounds in three kinds of radish (Blazevic and Mastelic 2009). The subsequent analysis was carried out using GC-MS system. The SVCs formed 32.6 % of black, 36.9 % of white and 20.5 % of total aroma of red radish. Furthermore, in total 15 SVCs were identified at least in one of the samples. Those were 10 isothiocyanates, 3 sulphides and 2 others. The major SVC was 4-(methylthio)butyl isothiocyanates in all samples. In the present research, nine SVCs were identified, mostly sulphides. The most dominant SVCs were methyl-1-propenyl sulphide (19.06 %) and dimethyl trisulphide (9.16 %).
Horseradish is a plant with very strong odour. In a recent work of Tomsone, SPME (PDMS/CAR/DVB fiber was chosen) in connection with GC-MS was employed for extraction and analysis of volatile compounds of horseradish (Tomsone et al. 2013). According to that research, horseradish contains 0.2–1.0 % of essential oil composed mainly of allylisothiocyanate and diallylsulphide. In total, there were identified 9 SVCs from which allylisothiocyanate was the most abundant constituent (64–82 %). In the present research, 13 SVCs were identified and moreover these compounds generated more than 95 % of the total aroma of horseradish. The most dominant SVCs were 2-phenethylisothiocyanate (70.19 %) and dimethyl disulphide (19.06 %). Seven SVCs are in compliance with the work of Tomsone.
Conclusion
The HS-SPME coupled with GC-MS is a rapid, simple, sensitive and solventless method. Results presented in this work show that the both Alliaceae and Brassicaceae families include relatively wide range aroma profiling compounds. For the extraction of sulphur volatile compounds the optimisation using Central Composite Design has been carried out. The sulphur volatile compounds of 12 real samples (8 from the Alliaceae and 4 from the Brassicaceae family) have been extracted using a DVB/CAR/PDMS fiber that has shown the best efficiency for the extraction of those compounds. The optimal conditions have been 73 °C and 50 min (see Fig. 1). The highest number of sulphur constituents has been identified in the sample of chive (24); on the other hand, the least number of those compounds has been identified in broccoli (3). In the half of the samples, sulphur volatile compounds have constituted more that 50 % of total aroma profile.
References
- Blazevic I, Mastelic J. Glucosinolate degradation products and other bound and free volatiles in the leaves and roots of radish (Raphanus sativus L.) Food Chem. 2009;113:96–102. doi: 10.1016/j.foodchem.2008.07.029. [DOI] [Google Scholar]
- Calvo-Gomez O, Morales-Lopez J, Lopez MG. Solid-phase microextraction-gas chromatographic-mass spectrometric analysis of garlic oil obtained by hydrodistillation. J Chromatogr A. 2004;1036:91–93. doi: 10.1016/j.chroma.2004.02.072. [DOI] [PubMed] [Google Scholar]
- Colina-Coca C, Gonzalez-Pena D, Vega E, de Ancos B, Sanchez-Moreno C. Novel approach for the determination of volatile compounds in processed onion by headspace gas chromatography–mass spectrometry (HS GC-MS) Talanta. 2013;103:137–144. doi: 10.1016/j.talanta.2012.10.022. [DOI] [PubMed] [Google Scholar]
- Godevac D, Vujisic L, Mojovic M, Ignjatovic A, Spasojevic I, Vajs V. Evaluation of antioxidant capacity of Allium ursinum L. volatile oil and its effect on membrane fluidity. Food Chem. 2008;107:1692–1700. doi: 10.1016/j.foodchem.2007.10.017. [DOI] [Google Scholar]
- Jabalpurwala F, Gurbuz O, Rouseff R. Analysis of grapefruit sulphur volatiles using SPME and pulsed flame photometric detection. Food Chem. 2010;120:296–303. doi: 10.1016/j.foodchem.2009.09.079. [DOI] [Google Scholar]
- Jarvenpaa EP, Zhang ZY, Huopalahti R, King JW. Determination of fresh onion (Allium cepa L.) volatiles by solid phase microextraction combined with gas chromatography mass spectrometry. Z Lebensm Unters Forsch A-Food Res Technol. 1998;207:39–43. doi: 10.1007/s002170050292. [DOI] [Google Scholar]
- Jirovetz L, Buchbauer G, Ngassoum MB, Geissler M. Analysis of the headspace aroma compounds of the seeds of the Cameroonian “garlic plant” Hua gabonii using SPME/GC/FID, SPME/GC/MS and olfactometry. Eur Food Res Technol. 2002;214:212–215. doi: 10.1007/s00217-001-0481-y. [DOI] [Google Scholar]
- Kimbaris AC, Siatis NG, Daferera DJ, Tarantilis PA, Pappas CS, Polissiou MG. Comparison of distillation and ultrasound-assisted extraction methods for the isolation of sensitive aroma compounds from garlic (Allium sativum) Ultrason Sonochem. 2006;13:54–60. doi: 10.1016/j.ultsonch.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Kumar A, Gaurav MAK, Tewary DK, Singh B. A review on development of solid phase microextraction fibers by sol–gel methods and their applications. Anal Chim Acta. 2008;610:1–14. doi: 10.1016/j.aca.2008.01.028. [DOI] [PubMed] [Google Scholar]
- Lanzotti V. The analysis of onion and garlic. J Chromatogr A. 2006;1112:3–22. doi: 10.1016/j.chroma.2005.12.016. [DOI] [PubMed] [Google Scholar]
- Ma YK, Song DD, Wang ZF, Jiang JK, Jiang T, Cui FJ, Fan XB. Effect of ultrahigh pressure treatment on volatile compounds in garlic. J Food Process Eng. 2011;34:1915–1930. doi: 10.1111/j.1745-4530.2009.00502.x. [DOI] [Google Scholar]
- MacLeod G, MacLeod AJ. The glucosinolates and aroma volatiles of green kohlrabi. Phytochemistry. 1990;29:1183–1187. doi: 10.1016/0031-9422(90)85425-F. [DOI] [Google Scholar]
- Mann RS, Rouseff RL, Smoot JM, Castle WS, Stelinski LL. Sulfur volatiles from Allium spp. affect Asian citrus psyllid, Diaphorina citri Kuwayama (Hemiptera: Psyllidae), response to citrus volatiles. Bull Entomol Res. 2011;101:89–97. doi: 10.1017/S0007485310000222. [DOI] [PubMed] [Google Scholar]
- Mester Z, Sturgeon R. Trace element speciation using solid phase microextraction. Spectrochim Acta B Atomic Spectrosc. 2005;60:1243–1269. doi: 10.1016/j.sab.2005.06.013. [DOI] [Google Scholar]
- Mondy N, Duplat D, Christides JP, Arnault I, Auger J. Aroma analysis of fresh and preserved onions and leek by dual solid-phase microextraction-liquid extraction and gas chromatography–mass spectrometry. J Chromatogr A. 2002;963:89–93. doi: 10.1016/S0021-9673(02)00221-2. [DOI] [PubMed] [Google Scholar]
- Otles S, Lecoq O, Dodds JA. Dry particle high-impact coating of biopowders: coating strength. Part Sci Technol. 2009;27:352–361. doi: 10.1080/02726350902993987. [DOI] [Google Scholar]
- Pawliszyn J. Handbook of solid phase microextraction. 1. London: Elsevier; 2011. [Google Scholar]
- Qiu R, Qu D, Hardy GES, Trengove R, Agarwal M, Ren YL. Optimization of headspace solid-phase microextraction conditions for the identification of phytophthora cinnamomi rands. Plant Dis. 2014;98:1088–1098. doi: 10.1094/PDIS-12-13-1258-RE. [DOI] [PubMed] [Google Scholar]
- Schreyen L, Dirinck P, Vanwassenhove F, Schamp N. Volatile flavor components of leek. J Agric Food Chem. 1976;24:336–341. doi: 10.1021/jf60204a056. [DOI] [Google Scholar]
- Sigma-Aldrich (2014) Selection Guide for Supelco SPME Fibers. http://www.sigmaaldrich.com/analytical-chromatography/sample-preparation/spme/selecting-spme-fiber.html. Accessed 01 June 2014
- Sowbhagya HB, Purnima KT, Florence SP, Rao AGA, Srinivas P. Evaluation of enzyme-assisted extraction on quality of garlic volatile oil. Food Chem. 2009;113:1234–1238. doi: 10.1016/j.foodchem.2008.08.011. [DOI] [Google Scholar]
- Teyssier C, Amiot MJ, Mondy N, Auger J, Kahane R, Siess MH. Effect of onion consumption by rats on hepatic drug-metabolizing enzymes. Food Chem Toxicol. 2001;39:981–987. doi: 10.1016/S0278-6915(01)00056-4. [DOI] [PubMed] [Google Scholar]
- Tocmo R, Lin Y, Huang DJ. Effect of processing conditions on the organosulfides of shallot (allium cepa L. Aggregatum group) J Agric Food Chem. 2014;62:5296–5304. doi: 10.1021/jf500739n. [DOI] [PubMed] [Google Scholar]
- Tomsone L, Kruma Z, Galoburda R, Talou T. Composition of volatile compounds of horseradish roots (armoracia rusticana L.) depending on the genotype. Proc Latv Univ Agric. 2013;29:1–10. doi: 10.2478/plua-2013-0001. [DOI] [Google Scholar]
- Vidal-Aragon C, et al. Productive characteristics and volatile compounds of seven broccoli cultivars. Ital J Food Sci. 2009;21:17–28. [Google Scholar]
- Wardencki W, Michulec M, Curylo J. A review of theoretical and practical aspects of solid-phase microextraction in food analysis. Int J Food Sci Technol. 2004;39:703–717. doi: 10.1111/j.1365-2621.2004.00839.x. [DOI] [Google Scholar]
- Yabuki Y, et al. Characterisation of volatile sulphur-containing compounds generated in crushed leaves of Chinese chive (Allium tuberosum Rottler) Food Chem. 2010;120:343–348. doi: 10.1016/j.foodchem.2009.11.028. [DOI] [Google Scholar]
- Zhao DY, Tang J, Ding XL. Analysis of volatile components during potherb mustard (Brassica juncea, Coss.) pickle fermentation using SPME-GC-MS. Lwt-Food Sci Technol. 2007;40:439–447. doi: 10.1016/j.lwt.2005.12.002. [DOI] [Google Scholar]