Abstract
Wheat flour was replaced with mango ginger powder (MGP) at 0, 5, 10 and 15 %. Influence of MGP on rheological and baking characteristics was studied. Farinograph was used to study the mixing profile of wheat flour-MGP blend. Pasting profile of the blends namely gelatinization and retrogradation were carried out using micro-visco-amylograph. Test baking was done to obtain the optimum level of replacement and processing conditions. Sensory attributes consisting texture, taste, overall quality and breaking strength were assessed. Nutritional characterization of the soup sticks in terms of protein and starch in vitro digestibility, dietary fiber, minerals, polyphenols and antioxidant activity were determined using standard methods. With the increasing levels of MGP from 0 to 15 %, the farinograph water absorption increased from 60 to 66.7 %. A marginal increase in the gelatinization temperature from 65.4 to 66.2 °C was observed. Retrogradation of gelatinized starch granules decreased with the addition of MGP. The results indicated that the soup stick with 10 % MG had acceptable sensory attributes. The soup stick showed further improvement in terms of texture and breaking strength with the addition of gluten powder, potassium bromate and glycerol monostearate. The total dietary fiber and antioxidant activity of the soup sticks having 10 % MGP increased from 3.31 to 8.64 % and 26.83 to 48.06 % respectively as compared to the control soup sticks. MGP in soup sticks improved the nutritional profile.
Keywords: Mango ginger, Wheat flour, Rheology, Viscosity, Soup stick, Polyphenols
Introduction
Healthiness is considered one of the key-drivers in the food business in the present scenario since there is an increasing demand for healthy food products. Exploitation of nutritious and medicinally-valued plant foods can play an important role in meeting consumer demands. Development of fortified or other composite bakery products is the latest trend in bakery industry. The growing interest in these types of bakery products is due to their better nutritional properties.
Several epidemiological studies have established a link between phytochemicals and the range of biological activities that impart health benefits in human beings. Scientific research supports the biological activity of many of the phytochemicals more in their native forms. Amongst the phytochemicals, several groups of polyphenols (anthocyanins, proanthocyanidins, flavanones, isoflavones, resveratrol and ellagic acid), non-nutrient chemical and dietary constituents are currently used in the pharmaceutical industry. Spices are considered to be the storehouse of active phytochemicals (Policegoudra et al. 2011). Various spices belonging to the genus Curcuma are well-known for their multiple uses as medicines, cosmetics, dyes, flavourings and neutraceuticals. They originate from the Indo-Malayan region and are widely distributed in the tropics of Asia to Africa and Australia (Sasikumar 2007). Mango ginger (Curcuma amada Roxb.) is a unique spice having morphological resemblance to ginger. Curcuma amada in south Asian countries is known as mango ginger due to its particular aroma of raw mango. Jatoi et al. (2007) reported that little attention has been given to C. amada in spite of its valuable properties (e.g. antioxidant, antibacterial and antifungal) and rich chemical constituents. Curcuma amada is an untapped medicinal plant of the ginger family (Policegoudra et al. 2011). Rhizomes of the species are buff-colored outside with a light yellow or white inner epidermal layer, and are used in folklore medicines, in culinary preparations such as preserves, candy, pickles and sauce as a source of mango flavour and for the manufacture of oleoresin, essential oil, etc. (Gupta 2001). Pushpalatha and Sheela (2003) also analyzed the suitability of this spice for the preparation of value added products. Ground dried ginger is employed in a wide range of foodstuffs, especially in bakery products and desserts (Young et al. 2002).
Scientific research supports that the biological activities of phytochemicals impart health benefits in human beings. Since the endogenous fiber polysaccharide content in wheat flour is only 4 %, the incorporation of dietary fiber in wheat flour based product such as bread would raise the health profile of the final products (Scott et al. 2008). There are many scientific reports on the therapeutic properties of mango ginger and their potential pharmaceutical applications. However, scientific information on the use of this spice in common food products like bakery products is rare. Therefore, the present study was aimed at evaluating the effect of different levels of mango ginger powder on the rheological and baking characteristics of soup stick. In addition, the possibility to obtain nutritionally rich final product with good sensorial properties was studied.
Materials and methods
Commercial wheat flour was procured from the local market. Other ingredients such as compressed yeast (Tower brand, AB Mauri, Chennai, India), hydrogenated fat (Bunge India Pvt Ltd., Mumbai, India), salt (common food-grade sodium chloride), sugar, potassium bromate at 20 ppm (Qualigens Fine Chemicals, Mumbai, India), glycerol monostearate - GMS (Biocon India, Pvt Ltd, Bangalore, India) and vital gluten (Burn’s Philip, Pune, India) at 2 % level were also used. The GMS gel was prepared using emulsifier and water (1:4 ratios). Water was heated to 45 °C, GMS was added and then continuously stirred and cooled to obtain the gel. GMS was used at the level of 0.5 % on flour basis in the studies. All the chemicals used for extraction and spectrophotometry were of AR grade from Sigma-Aldrich (St Louis, MO, USA).
Processing of mango ginger
Fresh and healthy mango ginger (C. amada Roxb.) rhizomes were procured from the local market, Mysore, India available during autumn. Rhizomes were washed, sliced and dried in a Sakav Drier (Shirsat Electronics, Mumbai, India) at 50 °C for 8 h and powdered to a particle size of 140 μm (MGP).
Chemical characteristics of wheat flour
The chemical characteristics of wheat flour such as moisture (method 44–16), protein (method 46–10), crude fat (method 30–10), ash (method 08–01), protein (method 46–10), dry gluten (method 38–10), falling number (method 56–81B) and Zeleny’s sedimentation value (method 56–61A) were determined using standard methods of American Association of Cereal Chemists (AACC 2000).
Rheological characteristics
Blends were prepared by replacing wheat flour with MGP at 0, 5, 10 and 15 % levels and effect of MGP on the rheological characteristics of wheat flour dough was studied using farinograph (method 54–21) and amylograph (method22-10) according to standard AACC (2000) methods.
Preparation of soup stick
The following formulation was used for the preparation of soup stick: wheat flour-100 g; MGP (0, 5, 10 and 15 %) separately; compressed yeast-2 g; salt-1 g; sugar-1 g; hydrogenated fat- 5 g; cumin seeds (as a spice)- 0.5 g and water (optimum water absorption as determined with the farinograph). Soup sticks in quadruplicate were prepared by mixing the ingredients in a Hobart mixer (Model N-50, Hobart, GmbH, Offenburg, Germany) with a flat blade for 4 min at 61 rpm. The dough was fermented in a chamber maintained at 30 °C and 75 % relative humidity (RH) for 30 min, remixed, divided the dough into 15 g piece each, molded the dough piece into 19 cm long with approximate diameter of 10 mm, and again fermented for 15 min, at 30 °C, 85 % RH, and baked for 25 min at 200 °C, cooled and then packed.
Quality characteristics of soup sticks
The weight, diameter and length of soup stick were recorded. The breaking strength of soup stick was measured by following triple beam snap technique using a TAHDi (Stable Microsystems, Surrey, UK) according to the method mentioned by Gains (1991). The sample was rested on two supporting beams spread at a distance of 3 cm. Another beam connected to a moving part was brought down to break the soup stick at a cross head speed at 10 mm min-1 and load cell of 10 kg. Care was taken to see that the point of contact was equivalent from both the supporting beams. The peak force (g) at break, representing breaking strength, was recorded and the mean values of triplicates are expressed. The soup sticks were evaluated for sensory parameters namely color, appearance, texture, taste, flavor and overall acceptability on a 9-point hedonic scale by a panel of 6 judges according to the method of Hooda and Jood (2005).
Determination of soup stick anti-oxidant capacity
Sample extraction procedure
About 50 g each of wheat flour, mango ginger powder or soup stick was extracted using analytical grade methanol (50 ml) in a mechanical shaker at room temperature (25 ± 1 °C) for 24 h and filtered. The residue was again treated with the same solvent (25 ml) in a mechanical shaker for 30 min and filtered. The filtrate was concentrated to 10 ml in a rotary evaporator (Buchi Rotavapor R-124, Switzerland). Fresh methanol extracts were used to determine their ability to scavenge DPPH radicals and to assess the total phenolics content.
Determination of total phenolics content
The total phenolics content was determined according to Stoilova et al. (2007) with slight modifications. The method is based on the coloured reaction of phenolics with Folin-Ciocalteu reagent. Upon the reaction with phenols, Folin-Ciocalteu reagent is reduced to a blue coloured oxide. The intensity of the resulting colour was measured in a spectrophotometer at 750 nm. Analytical grade gallic acid was used as the standard. Twenty microliters (20 μl) of extract or standard solutions (0–500 mg/l) was added to 1.58 ml de-ionized water and 100 μl of Folin-Ciocalteau phenol reagents (10 %). After 5 min, 300 μl of 20 % sodium carbonate was added to the mixture and put in the dark for 1 h. Then the absorbance was measured at 750 nm. The concentration of total phenols was expressed as mg GAE/g of dry sample.
DPPH free radical scavenging activity test
The radical scavenging activity was determined according to Brand-Williams et al. (1995). Stable DPPH radical reaches the maximum absorbance at 517 nm and its color is purple. The change of this color into yellow is a result of pairing of an unpaired electron of a DPPH radical with the hydrogen of the antioxidant, thus generating reduced DPPH-H. Adding an antioxidant results in the decrease of absorbance, which is proportional to the concentration and antioxidant activity of the compound. Sixty microlitres (60 μl) of the extract was taken in test tubes. To this 40 μl of methanol and 5 ml of 0.1 mM solution of DPPH were added. The test tubes were shaken and kept in dark at 27 °C for 20 min. The absorbance was read and the percentage free DPPH radical scavenging activity was calculated from the following equation:
Statistical evaluation
Duncan’s New Multiple Range Test (Duncan 1955) was performed for the sensory parameters namely appearance, texture, taste and the overall quality using XLSTAT (Addinsoft, New York, NY, USA). A significance level of 5 % was adopted for the comparison.
Results and discussion
The wheat flour used in the study contained moisture−10.5 %; ash−0.5 %; dry gluten−8.5 %; Zeleny’s sedimentation value−21 ml; and falling number−418 s. The above results showed that the wheat flour selected for the study was of medium strong quality. MGP had moisture, ash and ether extract of 6.3, 5.9 and 2.0 % respectively (Table 1).
Table 1.
Chemical and physical characteristics of wheat flour and MGP
| Parameters | Wheat flour | MGP |
|---|---|---|
| Moisture (%) | 10.5 | 6.3 |
| Ash (%) | 0.5 | 5.9 |
| Ether extract (%) | 2.1 | 2.0 |
| Zeleny’s sedimentation value (ml) | 21.0 | ND |
| Falling number (s) | 418.50 | ND |
| Gluten (%) | 8.5 | ND |
All values expressed on a dry basis. ND not determined
Effect of MGP on the farinograph characteristics of wheat flour
The effect of variable amount of MGP on the mixing characteristics of wheat flour was measured by farinograph. The farinograph water absorption (FWA), which is the amount of water required for the dough to have a definite consistency increased from 60 % in the control wheat flour to 66.7 % with the addition of increasing levels of MGP from 5 to 15 % (Fig. 1a). Rosell et al. (2001) reported that the greater number of hydroxyl groups, which exist in the fiber structure, allows more water interaction through hydrogen bonding. The dough development time (DDT) increased from 1.4 to 14.4 min as the levels of MGP increased from 0 to 15 %. This could be due to the delayed formation of gluten owing to the presence of fiber in MGP. The dough stability (DS), which is an index of dough strength, was 5.4 min for the control. It was observed that dough stability also increased with increasing levels of MGP. The effect of mango ginger powder on the rheological characteristics of wheat flour has not been reported yet; however, Balestra et al. (2011) reported that addition of ginger powder in bread dough give rise to a network with higher density of cross-links. They also opined that the cross-linking could be due to the presence of ginger bioactives such as gingerol, dehydroginger-dione and shogaol (Daramola and Osanyinlusi 2006) which have both activated double bonds and hydroxyl/methoxy phenyl residues. A low effect of dietary fiber on dough viscoelastic behaviour is desirable, because it may indicate minor changes in soup stick making performance.
Fig. 1.
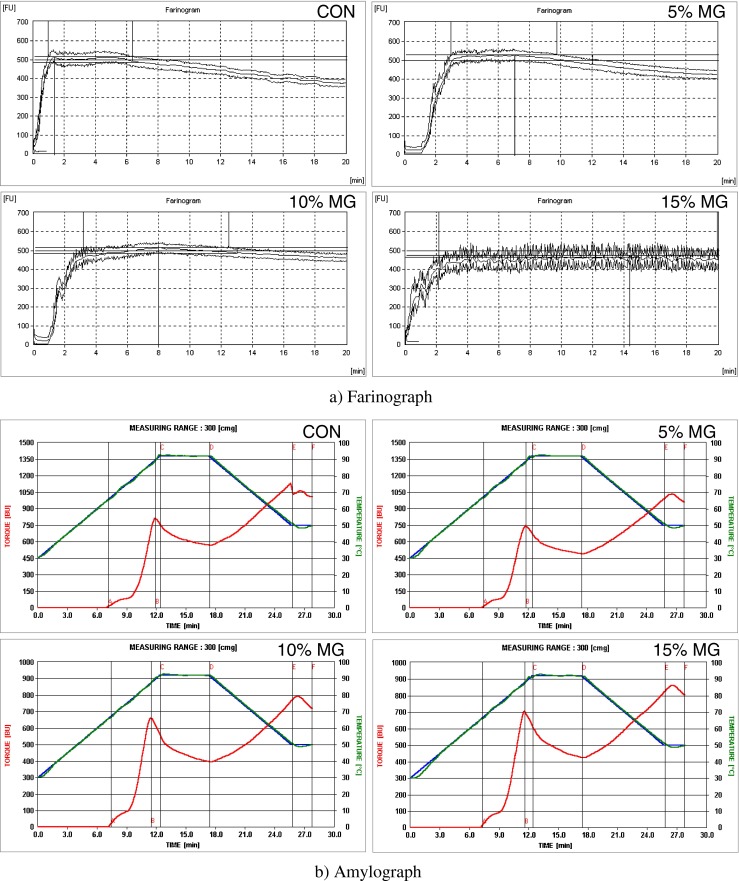
Rheological characteristics of wheat flour – mango ginger blends CON - Wheat flour (100 %); MG mango ginger
Effect of MGP on the pasting characteristics of wheat flour
The effect of MGP on the pasting characteristics of wheat flour is given in Fig. 1b). The gelatinization temperature provides an indication of minimum temperature required to cook. The pasting temperature of the control wheat flour and wheat flour with added MGP ranged between 65.4 to 66.8 °C. The peak viscosity (the highest viscosity of the paste during the heating phase) represents the ability of the starch granules to swell freely before their physical breakdown and also α- amylase activity. The peak viscosity of wheat flour was 815 BU and it showed a decrease with the addition of MGP. This reduction may be attributed to water being held from the starch granules by the MGP fiber and from a general reduction in starch content of pastes because of the replacement MGP fiber. A similar finding has been reported wherein the increment of ginger powder from 1 to 5 % on the cake flour made the gelatinization temperature lower (Yoon-Kyung et al. 2012). It was observed that the values of the amylographic breakdown which reflect the viscosity during cooking at 95 °C increased from 243 to 282 BU as the level of replacement of wheat flour with MGP increased from 0 to 15 %. The setback values which indicate the retrogradation of gelatinized starch granules decreased from 516 to 397 BU respectively with increase in MGP.
Effect of addition of MGP on the quality characteristics of soup sticks
The data on physico-sensory characteristics of soup stick is shown in Table 2 while Fig. 2 shows soup sticks with varying levels of mango ginger powder. The addition of MGP did not have any significant effect on the physical properties such as weight, diameter and length of the soup sticks as compared to that of the control. However, there was a change in the viscoelastic behavior of the dough as it was observed that mouldability of the soup sticks into cylindrical shape having 190 mm length decreased with the addition of MGP. This indicates that resistance to deformation increased owing to the interactions between fiber structure of MGP and the wheat proteins. A decrease in the length of soup sticks from 163.62 ± 1.29 to 160.1 ± 0.21 mm was seen with increasing levels of MGP from 0 to 15 % (Fig. 2). The texture of soup sticks measured in terms of breaking strength was 36.86 ± 3.6 g force for the control. The soup sticks became harder as seen in the increase in breaking strength values from 48.08 ± 5.56 to 66 ± 2.29 g force as the levels of replacement increased from 5 to 15 %.
Table 2.
Quality characteristics of soup sticks
| MGP (%) | Physical Parameters | Sensory Parameters | ||||||
|---|---|---|---|---|---|---|---|---|
| Weight* (g) | Diameter* (mm) | Length* (mm) | Breaking strength** (g) | Appearance (9) | Texture (9) | Taste (9) | Overall quality (9) | |
| 0 | 9.32 ± 0.08 | 10.3 ± 0.07 | 163.62 ± 1.29 | 36.9 ± 3.6 | 8.0a | 8.0a | 8.0a | 8.0a |
| 5 | 9.47 ± 0.08 | 10.3 ± 0.12 | 161.62 ± 0.96 | 48.1 ± 5.56 | 7.5ab | 7.0b | 7.0b | 7.0b |
| 10 | 9.45 ± 0.11 | 10.1 ± 0.05 | 161.25 ± 0.82 | 58.4 ± 5.49 | 7.5ab | 6.0c | 6.0c | 6.5c |
| 15 | 9.45 ± 0.11 | 10.0 ± 0.04 | 160.10 ± 0.21 | 66.0 ± 2.29 | 6.0c | 5.0d | 4.5d | 5.0d |
| 10 + Additives | 9.42 ± 0.14 | 10.3 ± 0.08 | 161.75 ± 0.82 | 45.3 ± 5.45 | 7.5ab | 7.0b | 6.5c | 7.0b |
Values are means ± standard deviation (n = 3, n** = 6); Means in the same column with different superscripts are significantly different (P ≤ 0.05) from each other; Additives: Gluten powder, Potassium bromate , Glycerol monostearate
Fig. 2.
Soup sticks with varying levels of mango ginger powder
The results of sensory analysis suggested that the addition of MGP in soup stick formulation did not have positive effect on sensory acceptability. Above 10 % substitution with MGP in the formulation, soup sticks had unappealing appearance and strong pungent mango ginger flavor, and therefore, were less acceptable. Shalini and Lakshmi Devi (2005) reported a similar observation in their evaluation on sensory characteristics of breads with ginger powder. They found that 10 % of ginger powder was well accepted. Hence, a 10 % MGP level of replacement was considered optimum and was treated with combination of gluten powder, potassium bromate and GMS. The soup sticks showed further improvement in terms of texture and reduction in breaking strength with the addition of additives in combination.
Nutritional characteristics of soup sticks
Nutritional characterization of the soup sticks in terms of protein and starch in vitro digestibility and dietary fiber were carried out. The data in Table 3 showed that wheat flour had total protein content of 10 ± 0.01 % with percent digestibility of 91.94 ± 0.15 5 % and total starch and In vitro digestibility of 73.16 ± 0.23 and 78.95 ± 0.12 % respectively. MGP had high protein digestibility (92.45 ± 0.16) and low starch digestibility (21.48 ± 0.14). The total dietary fiber content of wheat flour was 1.47 ± 0.04 %. It was observed that mango ginger powder had high dietary fiber of 40.37 ± 0.32 %. The total protein and starch content of control soup stick were 9.28 ± 0.02 and 65.63 ± 0.50 % with percent digestibility of 84.60 ± 0.23 and 39.05 ± 0.21 respectively. There was a marginal increase in the protein digestibility and a marginal decrease in starch profile of the soup stick having 10 % MGP as compared to control; however a two-fold increase in the dietary fiber content of the soup stick was seen.
Table 3.
Chemical characteristics of soup stick
| Samples | Total protein (%) | In vitro protein digestibility (%) | Total starch (%) | In vitro starch digestibility (%) | Soluble dietary fiber (%) | Insoluble dietary fiber (%) | Total dietary fiber (%) |
|---|---|---|---|---|---|---|---|
| Wheat flour | 10 ± 0.01 | 91.94 ± 0.15 | 73.16 ± 0.23 | 78.95 ± 0.12 | 0.3 ± 0.07 | 1.17 ± 0.03 | 1.47 ± 0.04 |
| MGP | 6.0 ± 0.01 | 92.45 ± 0.16 | 44.98 ± 0.21 | 21.48 ± 0.14 | 1.29 ± 0.09 | 39.08 ± 0.12 | 40.37 ± 0.32 |
| Control soup stick | 9.28 ± 0.02 | 84.60 ± 0.23 | 65.63 ± 0.50 | 39.05 ± 0.21 | 0.44 ± 0.01 | 2.87 ± 0.05 | 3.31 ± 0.20 |
| Optimized soup stick | 9.91 ± 0.02 | 85.06 ± 0.18 | 51.19 ± 0.12 | 33.48 ± 0.23 | 0.78 ± 0.01 | 7.86 ± 0.04 | 8.64 ± 0.51 |
MGP mango ginger powder; Values are means ± standard deviation (n = 3)
Mineral contents of soup stick
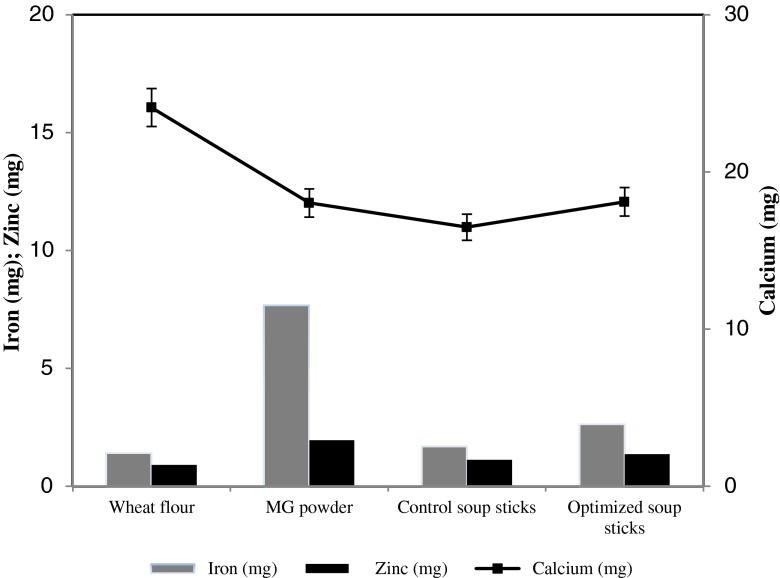
Wheat flour, MGP, control and optimized soup sticks were analyzed for minerals namely iron, zinc and calcium. As seen in Fig. 3, the iron, zinc and calcium content of wheat flour and MGP were 1.42 ± 0.02, 0.90 ± 0.01 and 24.09 ± 0.12 mg/100 g and 7.68 ± 0.10, 1.95 ± 0.04 and 18.02 ± 0.10 mg/100 g respectively. Addition of 10 % MGP increased the iron content of soup stick from 1.7 ± 0.03 to 2.64 ± 0.01 mg/100 g. Calcium content of control soup stick was 16.48 ± 0.08 mg/100 g which increased to 18.09 ± 0.12 mg/100 g in the optimized soup stick. No significant change in the zinc content was observed. Addition of MGP has resulted in the variation of mineral content in the control and the optimized soup sticks thereby improving the nutritional value of the optimized product.
Fig. 3.
Mineral content in raw materials and soup sticks
Total phenolics content and radical scavenging activity
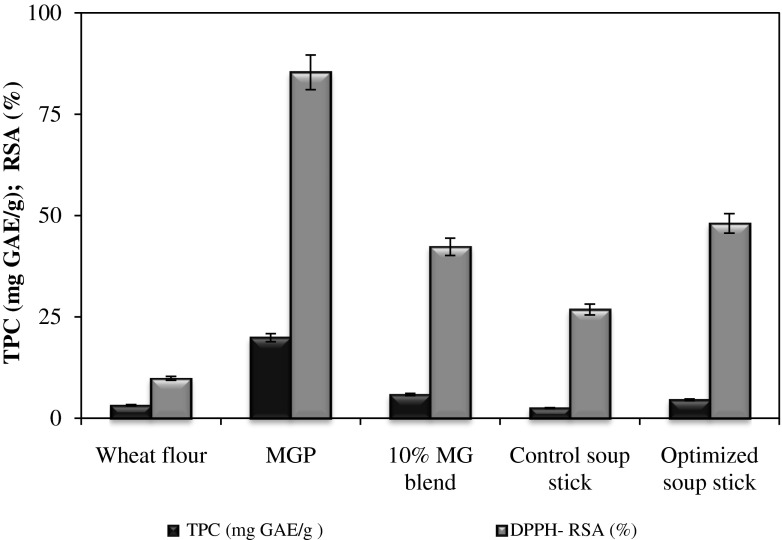
The total phenolics content (TPC) and the radical scavenging activity (RSA) of raw materials and soup sticks are shown in Fig. 4. The TPC and RSA values of wheat flour were 3.15 mg GAE/g DW and 9.81 % respectively. The higher values in MGP indicate that mango ginger is a good source of polyphenols. Replacement of wheat flour with 10 % MGP increased the TPC and RSA values to 5.81 mg GAE/g DW and 42.27 % respectively. These data indicate that mango ginger used at 10 % level increased the phenolics amount of nearly two-time compared to the quantity contained in the control soup stick. As expected, the ability to scavenge the DPPH radical from extracts of soup stick increased with the addition of MGP. Similar results were obtained in an investigation by Balestra et al. (2011) wherein 4.5 % ginger powder substitution level was considered optimum for bread making.
Fig. 4.
Total phenolics and radical scavenging activity in raw materials and soup sticks. MGP-Mango ginger powder; TPC total phenolic content; GAE gallic acid equivalent; DPPH diphenylpicrylhydrazyl; RSA radical scavenging activity
Conclusion
The study showed that addition of mango ginger powder at different levels (from 0 to 15 %) changes the mixing, pasting and baking characteristics of wheat flour. The dough with the highest level of MGP (15 %) showed the highest water absorption, dough development time and dough stability by farinograph. The results indicated that soup sticks at 10 % MG had acceptable sensory attributes. The pasting profile from amylograph indicated that retrogradation of gelatinized starch decreased with the addition of MGP. Sensory analysis indicated that 10 % of MGP could be included in soup stick formulation without any significant effect on the physico-sensory characteristics. The incorporation of MGP in formulation markedly increased the total phenolics content and the radical scavenging activity of soup stick extracts. Therefore, such a value added product would be helpful in promoting utilization of underutilized spice i.e. mango ginger. It can be concluded that it is important to choose an appropriate amount of mango ginger powder and processing parameters to obtain a healthy baked goods (high level of antioxidants).
References
- AACC (2000) Approved Methods of American Association of Cereal Chemists. The Association, St. Paul, MN
- Balestra F, Cocci E, Pinnavaia GG, Romani S. Evaluation of antioxidant, rheological and sensorial properties of wheat flour dough and bread containing ginger powder. LWT - Food Sci Technol. 2011;44(3):700–705. doi: 10.1016/j.lwt.2010.10.017. [DOI] [Google Scholar]
- Brand-Williams W, Cuveleir ME, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT - Food Sci Technol. 1995;28(1):25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- Daramola B, Osanyinlusi SA. Investigation on modification of cassava starch using active components of ginger roots (ZingiberofficinaleRoscoe) Afr J Biotechnol. 2006;5(10):917–920. [Google Scholar]
- Duncan DB. Multiple range and multiple F-test. Biometrics. 1955;11:1–42. doi: 10.2307/3001478. [DOI] [Google Scholar]
- Gains CS. Instrumental measurement of hardness of cookies and crackers. Cereal Foods World. 1991;36:991–994. [Google Scholar]
- Gupta VK. The wealth of India: first supplemented series (Raw materials) 2. New Delhi: Council of Scientific and Industrial Research, Pusa; 2001. pp. 259–260. [Google Scholar]
- Hooda S, Jood S. Organoleptic and nutritional evaluation of wheat biscuits supplemented with untreated and treated fenugreek flour. Food Chem. 2005;90:427–435. doi: 10.1016/j.foodchem.2004.05.006. [DOI] [Google Scholar]
- Jatoi SA, Kikuchi A, Gilani SA, Watanabe KN. Phytochemical, pharmacological and ethnobotanical studies in mango ginger (Curcuma amadaRoxb.;Zingiberaceae) Phytother Res. 2007;21:507–516. doi: 10.1002/ptr.2137. [DOI] [PubMed] [Google Scholar]
- Policegoudra RS, Aradhya SM, Singh L. Mango ginger (curcuma amadaroxb.) – a promising spice for phytochemicals and biological activities. J Biosci. 2011;36(4):739–748. doi: 10.1007/s12038-011-9106-1. [DOI] [PubMed] [Google Scholar]
- Pushpalatha PB, Sheela KB (2003) Value added products from mango ginger (Curcuma amada). Proc. National Seminar on New Perspectives in Spices, Medicinal and Aromatic Plants (Eds. Korikanthimath V. S., John Zachariah T., NirmalBabu K., Suseela Bhai R. and Kandiannan K), Goa. pp. 155 – 157
- Rosell CM, Rojas JA, Debarber B. Influence of hydrocolloids on dough rheology and bread quality. Food Hydrocoll. 2001;15:75–81. doi: 10.1016/S0268-005X(00)00054-0. [DOI] [Google Scholar]
- Sasikumar B. Genetic resources of Curcuma: diversity, characterization and utilization. Plant Genet Resour. 2007;3:230–25. doi: 10.1079/PGR200574. [DOI] [Google Scholar]
- Scott KP, Duncan SH, Flint HJ. Dietary fiber and the gut microbiota. Nutr Bull. 2008;33:201–11. doi: 10.1111/j.1467-3010.2008.00706.x. [DOI] [Google Scholar]
- Shalini D, Lakshami Devi N. Development and acceptability of breads incorporated with functional ingredients. J Food Sci Technol. 2005;42(6):539–54. [Google Scholar]
- Stoilova I, KrastanovA, Stoyanova A, Denev P, Gargova S (2007) Antioxidant activity of a ginger extract (Zingiberofficinale). Food Chem102 (3): 764–770
- Yoon-Kyung C, Jae-Joon L, Hyun-Joo L. Rheological properties of pound cake with ginger powder. Korean J Food Preserv. 2012;19(3):361–367. doi: 10.11002/kjfp.2012.19.3.361. [DOI] [Google Scholar]
- Young HY, Chiang CT, Huang YL, Pan FP, Chen GL. Analytical and stability studies of ginger preparations. J Food Drug Anal. 2002;10:149–153. [Google Scholar]