Abstract
In the current study, effects of fermentation on physicochemical and functional properties of brown rice flour (BRF) were investigated. Fermentation conditions were optimized using response surface methodology to achieve moderate acidity (pH 5–6), specifically pH 5.5 of brown rice batter with time, temperature and yeast concentration as the independent variables. The results indicated that brown rice batter was well fermented to maintain pH 5.5 at optimum conditions of 32 °C for 6.26 h using 1 % yeast concentration. Fermentation at moderate acidity significantly increased the levels of protein, total ash, insoluble fiber, soluble fibre, minerals, phenolics, antioxidants, resistant starch, riboflavin, pyridoxine, nicotinic acid, γ-tocotrienol, and δ-tocotrienol. However, it reduced the contents of γ-oryzanol, γ-tocopherol, α-tocopherol, phytic acid, amylose and total starch. Foaming capacity, foaming stability, oil holding capacity, gelatinization temperatures, enthalpy and whiteness of BRF were increased after fermentation. In contrast, its swelling power, water solubility index, hot paste viscosity, breakdown, and setback significantly decreased. Microstructure of BRF was also influenced, where its starch granules released from its enclosed structure after fermentation. This investigation shows evidence that yeast fermentation modified the functionality of BRF and can be used as a functional food ingredient.
Keywords: Brown rice flour, Yeast fermentation, Moderate acidity, Optimization, Physicochemical properties, Functional properties
Introduction
Epidemiological evidence indicated that consumption of whole cereal products reduced incidence of chronic diseases such as cardiovascular disease (Katcher et al. 2008; Liu et al. 2000) and type 2 diabetes (Sun et al. 2010). The health effects associated with consumption of whole grains are due to the significant bioactive components that concentrate in the outer layers of the grain. Recently, increased consumer awareness towards healthy life style has indirectly increased the demand for functional cereal products. However, one of the limitations of whole grain utilization in the development of functional cereal products is the non-appealing sensory properties of the end product as contributed by the grain outer layers or bran fraction.
Rice (Oryza sativa L.) is a popular crop in Asia and many parts of the world. It is widely consumed as polished grains, where its bran and germ are removed during milling. The milling process causes considerable reduction in rice micronutrients since they are located in the bran and germ fractions. The bran contains almost 65 % of the nutrients that include fibres, minerals, vitamins and phenolics (Lerma-García et al. 2009).
Several of the rice bran components such as phenolic acids, tocopherols, tocotrienols, polyphenols, oryzanols and fibres are reported to possess biological activities (Aguilar-Garcia et al. 2007). However, utilization of non-milled or brown rice as a staple food is not popular due to its dark colour and hard texture, even after cooking. Brown rice flour, however, has been used to produce functional whole cereal products, such as bread (Moroni et al. 2009). Nevertheless, the bran fraction negatively affected the sensory qualities of the bread. Therefore, modifications of BRF to produce whole cereal products are required to reduce the negative effects associated with the bran fraction.
Fermentation of cereals with yeast and lactic acid bacteria has been reported to relief the negative effects of the bran and improve texture, flavour and structure of whole wheat and rye breads (Katina et al. 2005). However, less attention has been paid to study the influence of fermentation on the nutritional and functional properties of whole grain flour. Biochemical changes associated with microbial metabolism and enzyme actions during fermentation are the main factors affecting the nutritional and textural properties of the fermented product (Kwon et al. 2010). Functional properties of whole grain flour play a significant part in the physical performance of its end product during processing and storage stability. These functional properties include viscosity, water and oil holding capacities and foaming activity.
The beneficial influences of fermentation mainly depend on the microbe types and fermentation conditions. The degree of acidity is one of the parameters that should be taken into consideration to avoid the detrimental effect of high acidity on the structure of fermented whole grain product. It is expected that high acidity causes higher proteolysis which in turn decrease the volume and increase the sourness of the product. Flander et al. (2011) reported no negative effects on the quality of oat-wheat bread associated with using optimized mild acidity fermented flour.
To our knowledge, there is a lack of information regarding the influence of fermentation on the functional and nutritional properties of BRF. Therefore, the objectives of this study were to optimize the fermentation conditions for BRF to achieve moderate acidity of its batter, and to evaluate the effect of yeast fermentation at moderate acidity on the physicochemical and functional properties of the fermented flour.
Materials and methods
Materials
MR219 rice with its bran intact (Eco-brown, Malaysia) and baker’s yeast (Eagle QS6540 2801 0001, China) were obtained from a supermarket in Selangor, Malaysia. The yeast used was selected based on a preliminary study, which compared its activity to that of Saf-levure (S.I. Lesaffre 59703, Mareq, France) and Mauriban (AB MAURI Vietnam Limited, Vietnam) yeasts (data not shown). The brown rice grains were ground into flour using a FOSS Tecator grinder (CyclotechTM 1093, Hoganas, Sweden) to pass through a 500 μm sieve. The flour was packaged under vacuum and stored at 4 °C in a polyethylene bag. The chemicals were obtained from Merck (Darmstadt, Germany) and Sigma-Aldrich (USA) and they were of analytical grade.
Optimization of fermentation conditions
Response surface methodology (RSM) with central composite design (CCD) was applied to demonstrate the effects of the independent variables including time (6–24 h), temperature (25–35 °C) and baker’s yeast concentration (1–5 %) on the dependent variables, titratable acidity and pH. The twenty runs based on CCD and the six centre points are shown in Table 1. The BRF (50 g), 36 mL of water and a specific amount of baker’s yeast were mixed in a beaker and the mixture was fermented according to the experimental design in a fermenting chamber (Binder 10–01536, Germany). Brown rice batter fermented at the optimum conditions was then dried at 50 °C for 3 h and ground using a FOSS Tecator grinder (CyclotechTM 1093, Hoganas, Sweden) to pass through a 500 μm sieve to obtain fermented brown rice flour (FBRF).
Table 1.
Experimental and fitted values of responses and P-value of validation of fermented brown rice batter
| pH | TTA | ||||||
|---|---|---|---|---|---|---|---|
| Blocks | Temperature (°C) | Time (h) | Yeast concentration (%) | EV | FV | EV | FV |
| 3(C) | 30.0000 | 15.0000 | 3.00000 | 6.38 | 6.25899 | 6.25 | 5.92903 |
| 3 | 35.0000 | 15.0000 | 3.00000 | 5.02 | 4.62792 | 8.15 | 8.76765 |
| 3 | 25.0000 | 15.0000 | 3.00000 | 5.39 | 5.02577 | 6.85 | 6.90042 |
| 3 | 30.0000 | 24.0000 | 3.00000 | 5.01 | 4.95562 | 8.55 | 8.45439 |
| 3(C) | 30.0000 | 15.0000 | 3.00000 | 6.30 | 6.25899 | 5.50 | 5.92903 |
| 3 | 30.0000 | 6.0000 | 3.00000 | 6.62 | 5.91806 | 4.75 | 5.51368 |
| 3 | 30.0000 | 15.0000 | 5.00000 | 6.34 | 6.18840 | 5.59 | 6.13872 |
| 3 | 30.0000 | 15.0000 | 1.00000 | 6.35 | 6.32957 | 5.95 | 5.71934 |
| 2 | 26.9382 | 9.4887 | 4.22474 | 5.32 | 5.78691 | 6.25 | 5.69531 |
| 2 | 33.0618 | 20.5113 | 4.22474 | 4.83 | 4.95391 | 8.75 | 8.63955 |
| 2(C) | 30.0000 | 15.0000 | 3.00000 | 6.34 | 6.25899 | 5.85 | 5.92903 |
| 2 | 26.9382 | 20.5113 | 1.77526 | 5.31 | 5.28399 | 7.15 | 7.23930 |
| 2(C) | 30.0000 | 15.0000 | 3.00000 | 6.34 | 6.25899 | 5.80 | 5.92903 |
| 2 | 33.0618 | 9.4887 | 1.77526 | 5.34 | 5.62973 | 6.85 | 6.58193 |
| 1 | 33.0618 | 9.4887 | 4.22474 | 5.24 | 5.54328 | 7.60 | 6.83875 |
| 1 | 26.9382 | 9.4887 | 1.77526 | 5.55 | 5.87336 | 5.30 | 5.43850 |
| 1 | 33.0618 | 20.5113 | 1.77526 | 4.88 | 5.04036 | 8.45 | 8.38273 |
| 1(C) | 30.0000 | 15.0000 | 3.00000 | 6.34 | 6.25899 | 6.05 | 5.92903 |
| 1(C) | 30.0000 | 15.0000 | 3.00000 | 6.34 | 6.25899 | 5.85 | 5.92903 |
| 1 | 26.9382 | 20.5113 | 4.22474 | 5.13 | 5.19754 | 7.45 | 7.49612 |
| P-value = 1 | P-value = 1 | ||||||
EV experimental value, FV fitted value by the software, (C) centre points, TTA Titratable acidity
Determination of acidity
Titratable acidity (TTA) was determined following the method described by Kati et al. (2004). A 10 g of batter was dispersed in 100 mL of distilled water and titrated with 0.1 N NaOH until pH 8.5 was obtained. The amount of NaOH needed corresponded to TTA value in mL. A pH meter (DELTA 320, Shanghai, China) was used to determine the pH of the fermented brown rice batter.
Determination of proximate and fibre compositions
Proximate composition of BRF and FBRF, which include moisture, protein, and lipid contents were determined according to AOAC methods 934.01, 920.87 and 923.05, respectively (AOAC 2005a, b). Air oven method was used to determine the moisture content till constant weight was reached. The protein content was determined following the micro-Kjeldahl method using a FOSS protein analyzer (KjeltecTM 8400, Hoganas, Sweden) and a factor of 5.95 was used as the nitrogen conversion. Lipid content was determined using the Soxhlet extraction method by using a FOSS fat analyzer (SoxtecTM 2050, Hoganas, Sweden) and petroleum ether was used as the extraction solvent. Total ash was determined according to ISO method 2171 (ISO 1993). The AOAC method 993.19 (AOAC 2005a, b) was followed to determine total fibre, insoluble fibre and soluble fibre FOSS Fibertech (FibertecTM 1023, Hoganas, Sweden).
Determination of mineral content
A flame atomic absorption spectrophotometer (Perkin Elmer AAnalyst 400, Shelton, USA) was used to measure calcium, iron, magnesium and zinc contents of BRF and FBRF according to AOAC method 985.35 (AOAC 2005a, b). While, phosphorus content was measured using a UV–vis spectrophotometer (PerkinElmer, Shelton, USA) with ammonium-vanadomolybdate reagent based on AOAC method 995.11 (AOAC 2005a, b).
Determination of phytic acid
A rapid method as described by Wu et al. (2010) was adopted with a slight modification in terms of the extraction period. Briefly, 0.06 g of BRF or FBRF was extracted with 10 mL of 0.2 N HCL at room temperature for 3 h. The mixture was centrifuged at 2000 g for 10 min. The supernatant (0.5 μL) was pipetted into a test tube and 1 mL of ammonium iron sulphate was added, which was prepared by dissolving 0.2 g of NH4Fe (SO4)2.12H2O into 100 mL of 2 mol/L HCL and the volume was made to 1000 mL with distilled water. The mixture was then incubated at 100 °C for 30 min. A 2 mL of (1 % v/v) 2′,2-bipyridine solution was added to the mixture after cooling to ambient temperature and the absorbance was immediately recorded at 519 nm against distilled water using a UV–vis spectrophotometer (Perkin Elmer, Shelton, USA). The results were expressed as μg phytic acid per g sample using a standard curve prepared by diluting stock solution of phytic acid sodium salt hydrate.
Total phenolic content (TPC) assay
Folin-Ciocalteau method described by Beta et al. (2005) with slight modifications was used to determine the total phenolic content (TPC) of BRF and FBRF. Sample (0.20 g) was mixed with 4 mL of 80 % aqueous methanol, sonicated for 12 min at 25 °C and then centrifuged at 2000 g for 10 min. The extraction was repeated two times. Folin-Ciocalteau reagent was freshly prepared by diluting 1 mL of the concentrated reagent to 10 mL with 80 % aqueous methanol. A 1.5 mL of the diluted reagent was added to 200 μL of the combined supernatant. After 5 min, 1.5 mL sodium carbonate (6 % w/v) was added to the mixture. The absorbance was determined at 725 nm after incubation for 1 h in a dark cupboard. The results were expressed as mg gallic acid equivalent (GAE) per g sample.
Ferric reducing ability power (FRAP) assay
The antioxidant activities (AA) of BRF and FBRF were determined using FRAP method. The extraction method was similar as described in section 2.7. The supernatant (300 μL) was mixed with 2.85 mL of stock solution (10:1:1) consisting of 300 mM acetate buffer pH 3.6, 10 mM TPTZ (2,4,6 Tri(2-Pyridyl)s-triazine) and 20 mM ferric chloride which was prepared freshly, and warmed at 37 °C for 5 min. The mixture was then incubated at 37 °C for 30 min in the dark. The absorbance was recorded at 593 nm and the results were expressed as mmol trolox equivalent (TE) per g sample.
Vitamin E and γ-oryzanol determinations
Extraction and determination of both vitamin E (tocopherols and tocotrienols) and total γ-oryzanol were carried out according to the method described by Aguilar-Garcia et al. (2007). A high performance liquid chromatograph (HPLC) equipped with a fluorescence detector (Agilent Technologies 1200 Series, Waldbronn, Germany) was used to determine the extracted tocopherols, tocotrienols and γ-oryzanols. Stock solutions of tocopherols, tocotrienols and γ-oryzanols were prepared according to Ye et al. (1998). Data were collected and interpreted using ChemStation B.0201 software (Waldbronn, Germany).
Vitamin B determination
Riboflavin, nicotinic acid and pyridoxine were determined by adopting the procedure described by AACC method 86–90.01 (AACC 1999) with some modifications in sample extraction. Sample (0.5 g) was hydrolyzed with 5 mL of 1 N NaOH for 1 h at 50 °C. After hydrolysis, the pH of the mixture was adjusted to 6.8 using 0.1 N HCl (Maria et al. 2008). Thiamine extraction, however, was performed using 0.1 N HCl for 30 min at 100 °C and after cooling, the pH of the extract was adjusted to 6.8 using 0.1 N NaOH according to AOAC method 953.17 (AOAC 2005a, b). The extracts were centrifuged at 2000 g for 15 min and filtered through 0.45 μm membrane filters into vials for HPLC analysis. HPLC equipped with a UV detector (Waters 2489 UV/Visible Detector and Empower software, Milford, USA) was used for the vitamin B measurements.
Total starch and resistant starch determinations
Total starch and resistant starch were determined according to the procedure supplied by the Resistant Starch Assay Kit (Megazyme International Ireland Ltd. Co., Wicklow, Ireland).
Amylose content determination
The amylose content of BRF and FBRF was determined using FlAstar 5000 Analyzer equipped with 5027 sampler (FOSS TecatorTM, Sweden) following the AACC method 61–03.01 (AACC 2001). The results were expressed as percentages by mass by referring to the calibration curve prepared using rice flour with medium level amylose as a standard (Fluka, Sigma) by using a SoFIA software (FOSS TecatorTM, Sweden).
Water and oil holding capacities measurements
Water holding capacity (WHC) and oil holding capacity (OHC) were measured by placing 1 g of sample into a pre-weighed centrifuge tube. A 10 mL of either distilled water or corn oil was added and the mixture in the tubes was vortexed for 2 min. The suspension was allowed to stand for 30 min at ambient temperature and then centrifuged at 4000 g for 25 min. After decanting the excess water or oil, the tubes were inverted to drain. The difference between the initial and final weights was taken as the ability of sample to hold either water or oil (Elkhalifa et al. 2005).
Foaming capacity and stability measurements
In order to evaluate the foaming capacity (FC) and foaming stability (FS) of BRF and FBRF, 2 g of each sample was mixed with 50 mL of distilled water using a high pressure homogenizer (ultra-TURRAX T25 Basic, Staufan, Germany) at 6000 rpm for 2 min. The mixture was transferred to a measuring cylinder and FC was measured by taking the foam volume. After 1 h, the foam volume was measured again indicating FS (Maninder et al. 2007).
Water solubility index measurement
Two and a half grams of BRF or FBRF was placed in a centrifuge tube, mixed with 30 mL of distilled water and vortexed for 5 min. The mixture was heated at 90 °C for 15 min, left to cool at room temperature (25 °C) and then centrifuged at 3000 g for 10 min. The supernatant was decanted into pre-weighed dish which in turn was evaporated overnight at 110 °C. The water solubility index (WSI) was expressed as percentage of weight of dissolved solids in the supernatant to weight of the original sample (Elkhalifa et al. 2005).
Swelling power measurement
Swelling power (SP) measurement was performed according to Lin et al. (2011) with slight modifications. One gram of sample was placed into a pre-weighed centrifuge tube and mixed with 50 mL of distilled water using a magnetic stirrer for 5 min. The mixture was heated at 80 °C for 30 min in a water bath and centrifuged at 1040 g for 15 min after cooling to room temperature. The supernatant was removed and the tube with the sediment was re-weighed. The increase in sample weight was taken as its SP.
Color measurement
Color of the flours was examined using a Hunter Lab (Minolta chromameter, CR-300, Japan). Chroma values were given as L, a and b. L represents a measure of lightness, while the yellowness/blueness and redness/greenness are indicated by the b and a values, respectively. Calibration of the chromameter was performed using a standard plate and its L, a and b values were 94.1, 0.3129 and 0.3189, respectively. Total color difference (ΔE) of the samples was estimated by using the following equation:
Pasting properties determination
Pasting properties of BRF and FBRF were measured according to AACC method 61–02 (AACC 1995) using a rapid viscosity analyser (Newport Scientific Pty. Ltd. Warriewood, Australia).
Thermal properties determination
Differential scanning calorimeter (DSC 823, Mettler-Toledo, Switzerland) was used to determine the thermal properties of BRF and FBRF. A 5 mg of sample was loaded into a DSC aluminum pan (4 μL) and deionised water was added to reach 1: 3 sample to water ratio. The pan was then sealed and left for conditioning at room temperature for 1 h before being heated from 27 to 120 °C at a rate of 10 °C/min. An empty pan was used as a reference. The onset temperature (TO), peak temperature (TP), conclusion temperature (TC) and transition enthalpy (ΔH) were recorded from the curve using STARe to evaluate the thermal characteristics of the sample (Lin et al. 2011).
Microscopic analysis
Microstructure of BRF and FBRF was analyzed using a scanning electron microscope (JEOL-JSM-6400 SEM, Japan). Flour samples were mounted on circular aluminium stubs, coated with gold and examined at an accelerating potential of 15 kV.
Statistical analyses
To achieve the optimum conditions for moderate acidity of FBRF (pH 5.5), RSM was used to form the experimental design and to analyse the results using Minitab 14 software (USA). According to the highest R2, adjusted R2, number of significant term, lowest P-value of regression, highest P-value of lack of fit and lowest error of model terms, linear square was the appropriate module for both pH and TTA. One way analysis of variance (ANOVA) and Tukey’s multiple range tests with a confidence interval of 95 % were applied to report the significant differences between the obtained results.
Results and discussion
Optimum conditions for production of moderate acidity batter
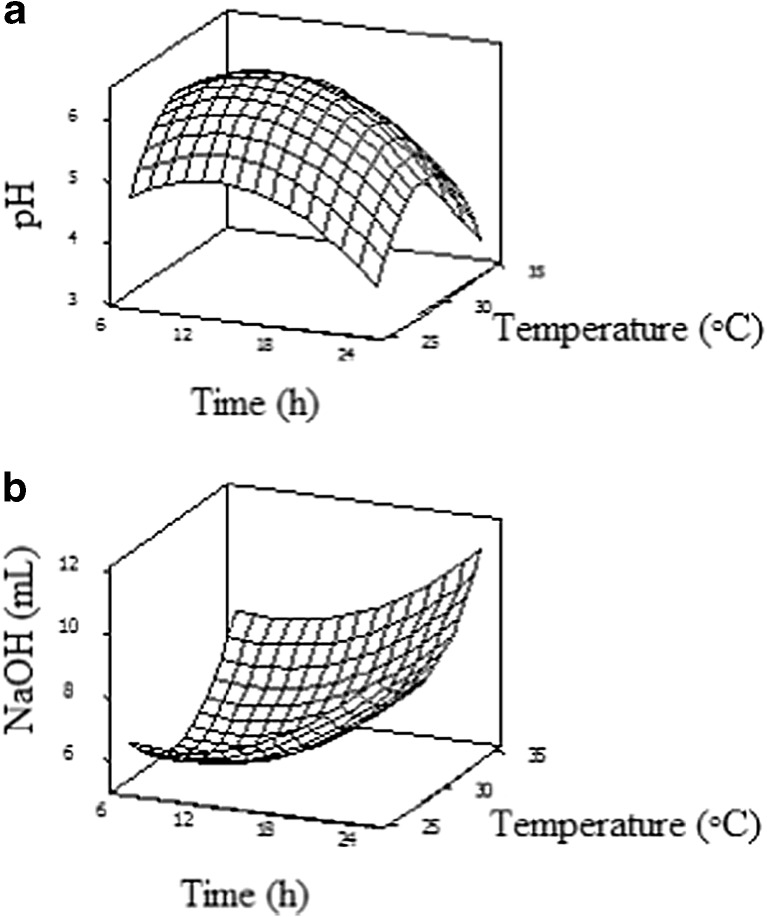
The acidity of fermented products has a significant effect on their taste, flavour, shelf life and overall quality. The effects of time, temperature and yeast concentration were evaluated using RSM with CCD. The regression coefficients for the response variables, with the corresponding R2, adjusted R2 and P-value are shown in Table 2. The results showed that temperature and time had significant quadratic effects on acidity measured either as pH or TTA (Fig. 1). Between the fermentation conditions, the temperature demonstrated the most significant effect (P < 0.05) on the brown rice batter acidity as indicated by recording the lowest P-value (Table 2). Yeast concentration had no significant effect on TTA and pH in the tested range. The pH of brown rice batter ranged from 4.38 to 6.62, while TTA varied from 4.75 to 8.75 mL 0.1 N NaOH/10 g batter (Table 1). Titratable acidity and pH of yeast fermented brown rice batter can be predicted by the following equations:
Table 2.
Parameters of regression coefficient, R2, R adjusted and P-value for the final reduced modules of the pH and titratable acidity (TTA) of fermented brown rice batter
| Modules | Symbol | Full module | Reduced module | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter estimate | P-value | Parameter estimate | P-value | ||||||
| pH | TTA | pH | TTA | pH | TTA | pH | TTA | ||
| Linear | Y | 0.1489 | 0.3277 | 0.866 | 0.836 | −0.0353 | 0.1048 | 0.554 | 0.288 |
| Te | 3.4742 | −4.3643 | 0.000 | 0.001 | 3.3974 | −4.3853 | 0.000 | 0.000 | |
| Ti | 0.3516 | −0.1046 | 0.103 | 0.768 | 0.2510 | −0.2274 | 0.004 | 0.074 | |
| Quadratic | Te2 | −0.0582 | 0.0767 | 0.000 | 0.001 | −0.0573 | 0.0762 | 0.000 | 0.000 |
| Ti2 | −0.0104 | 0.0132 | 0.003 | 0.017 | −0.0101 | 0.0130 | 0.001 | 0.005 | |
| Y2 | −0.0787 | 0.0471 | 0.153 | 0.612 | – | – | – | – | |
| Interaction | Te*Ti | −0.0033 | −0.0022 | 0.582 | 0.833 | – | – | – | – |
| Te*Y | 0.0087 | −0.0067 | 0.743 | 0.888 | – | – | – | – | |
| Ti*Y | 0.0019 | −0.0204 | 0.900 | 0.448 | – | – | – | – | |
| R2 | 92.2 % | 92.8 % | 89.4 % | 91.9 % | |||||
| R2 –adjusted | 81.5 % | 82.9 % | 83.2 % | 87.2 % | |||||
Te temperature, Ti time, Y yeast concentration, TTA titratable acidity
Fig. 1.
Response surface plots for the effect of time and temperature on pH (a) and titratable acidity (TTA) (b) of fermented brown rice batter
Where Ti: time, Te: temperature and Y: yeast concentration.
The optimum condition of the desirable milder acidity (pH 5.5) was achieved at 32 °C for 6.26 h using 1 % yeast concentration. Moderate acidification can cause moderate amylolytic and proteolytic hydrolyses that can improve the quality of products such as bread specifically in its texture and volume. Thus, milder acidity of FBRF may provide technological advantage since high acidity may cause negative effects on the flavor and texture of products (Kati et al. 2004). In addition, it could allow using high quantity of FBRF in the end product as an ingredient.
Proximate and fiber compositions
Proximate composition of BRF and FBRF are presented in Table 3. The findings showed that yeast fermentation significantly increased the protein content. The cause might be related to the accumulation of the microbial cells. This result is in agreement with that of other studies that reported noteworthy increase in protein content of cereals and legumes after natural fermentation for 4 days (Shekib 1994). Another study also reported an increase in protein concentration as a result of natural fermentation of maize in the first 12 h of the fermentation period (Yousif and El Tinay 2000). A study conducted on yeast fermented rice bran protein concentrate indicated that its protein content significantly increased compared to unfermented and naturally fermented samples. The authors attributed the increase in protein content to the rise in microorganism biomass caused by yeast proliferation and then creation a new protein due to accumulation of microbial cells during fermentation (Chinma et al. (2014)). There were also significant increases in the total ash, soluble fiber and insoluble fiber in BRF after fermentation. The correct mechanism associated with this finding is still not clear. However, it could be because of acidification and enzymatic actions involved in the cell wall degradation and increased solubility of arabinoxylans. Moreover, acid hydrolysis of dietary fiber has been indicated during bread making (Katina et al. 2007). Lipid content of FBRF, however, was slightly decreased.
Table 3.
Proximate and nutritional compositions of brown rice flour (BRF) and fermented brown rice flour (FBRF)
| Parameter | BRF | FBRF |
|---|---|---|
| Moisture (%) | 9.76 ± 0.01a | 11.38 ± 0.37b |
| Protein (%) | 7.70 ± 0.00a | 8.70 ± 0.01b |
| Ash (%) | 1.13 ± 0.01a | 1.21 ± 0.01b |
| Lipid (%) | 2.58 ± 0.01a | 2.56 ± 0.02a |
| Carbohydrates | ||
| Soluble fibre (%) | 1.12 ± 0.01a | 2.33 ± 0.04b |
| Insoluble fibre (%) | 1.35 ± 0.04a | 1.57 ± 0.04b |
| Resistant starch (%) | 3.10 ± 0.12a | 4.31 ± 0.11b |
| Total starch (%) | 65.57 ± 0.49a | 58.24 ± 0.98b |
| Amylose content (%) | 23.89 ± 0.07a | 23.46 ± 0.12b |
| Minerals | ||
| Phosphorus (%) | 16.90 ± 0.13a | 19.87 ± 0.05b |
| Magnesium (μg/g) | 19.70 ± 0.12a | 22.86 ± 0.05b |
| Zinc (μg/g) | 14.24 ± 0.35a | 19.08 ± 0.11b |
| Calcium (μg/g) | 105.75 ± 1.48a | 120.35 ± 0.07b |
| Iron (μg/g) | 5.09 ± 0.12a | 5.74 ± 0.14b |
| Vitamins | ||
| Thiamine (μg/g) | ND | ND |
| Riboflavin (μg/g) | 0.24 ± 0.00a | 8.92 ± 0.09b |
| Nicotinic acid (μg/g) | 6.87 ± 0.01a | 7.52 ± 0.06b |
| Pyridoxine (μg/g) | 0.12 ± 0.00a | 0.60 ± 0.00b |
| α-tocopherol (μg/g) | 4.03 ± 0.01a | 3.99 ± 0.01a |
| γ-tocopherol (μg/g) | 2.95 ± 0.02a | 2.85 ± 0.02b |
| δ-tocopherol (μg/g) | 0.76 ± 0.00a | 0.78 ± 0.01a |
| α-tocotrienol (μg/g) | 2.52 ± 0.05a | 2.59 ± 0.01a |
| γ-tocotrienol (μg/g) | 10.31 ± 0.16a | 11.07 ± 0.13b |
| δ-tocotrienol (μg/g) | 1.22 ± 0.02a | 1.36 ± 0.01b |
| Antioxidant activity | ||
| TPC (mg GAE/g) | 1.11 ± 0.01a | 1.24 ± 0.00b |
| FRAP (mmol TE/g) | 1.03 ± 0.01a | 1.18 ± 0.01b |
| Others | ||
| Phytic acid (μg/g) | 128.71 ± 0.04a | 75.56 ± 1.84b |
| γ-oryzanols (μg/g) | 266.40 ± 2.82b | 206.64 ± 4.21b |
Represented values are means ± standard deviations of three replicates
Values with the same superscript letter within a row are not significantly different (P > 0.05)
ND Not detected, GAE Gallic acid equivalent, TE Trolox equivalent, TPC Total phenolic content, FRAP Ferric reducing ability power
Total phenolic content (TPC)
Fermentation caused a significant increase of the extractable TPC in FBRF by 13 % (Table 3). The TPC elevation in this study corroborates the earlier finding about L. rhamnosus and S. cerevisiae which were used to examine the effect of fermentation for 24 h on cereals by Dordević et al. (2010) who demonstrated an increase in TPC of those cereals. Moore et al. (2007) also reported significant increase in soluble phenolic content which include ferulic acids, p-coumaric, and syringic after fermentation of hard wheat bran for 48 h using three commercial bakerʼs yeast. The drop in the pH value during fermentation may be the main cause that affects the degradation of grain structure and also the activated or secreted enzymes could release the phenolic compounds, since phenolic compounds are partly responsible for insoluble cell wall matrix. It was pointed out that, bound phenolics could be released as a result of enzymatic treatment (Bartolome and Gómez-Cordovés 1999). Total phenolic content was determined using Folin-Ciocalteu method which is not specific and has limitations. Thus, additional organic materials that were produced during fermentation which may react with Folin-Ciocalteu reagent such as amino acids, acetic acid, and pyridoxine can overestimate TPC. Besides, this may also reflect the enzymatic actions specifically proteolysis during fermentation (Katina et al. 2007).
Ferric reducing ability power (FRAP)
It is well known that brown rice possesses high antioxidant activity. In the current study, fermentation caused a further significant increase in its antioxidant activity by 15 % as indicated by its FRAP value (Table 3). A different study reported that incubation for 24 h had no considerable effect on FRAP values of some cereals fermented with L. rhamnosus and S. Cerevisiae (Dordević et al. 2010). Another study demonstrated that yeast fermented rice bran protein concentrate had higher FRAP value than that of naturally fermented and non-fermented rice bran protein concentrates. The authors found that yeast fermented rice bran protein concentrate had also higher contents of sulphur-containing amino acids (cysteine and methionine) than naturally fermented and non-fermented samples (Chinma et al. 2014). It was established that some residues related to sulphur and acidic amino acids strongly contribute to ferric reducing ability power of proteins (Chinma et al. 2014). Accordingly, the increment in antioxidant activity of FBRF could be related to the increase in bioactive substances or to the changes in protein composition and functionality.
Vitamin B content
Brown rice is a good source of vitamin B complex. Statistically, fermentation significantly increased riboflavin, pyridoxine and nicotinic acid contents of FBRF (Table 3). This findings support that of previous research that indicated fermentation for 6 h with yeast enhanced the riboflavin and pyridoxine concentrations of wheat bread (Batifoulier et al. 2005). Mahgoub et al. (1999) demonstrated that fermentation period more than 6 h reduced thiamine and riboflavin contents in wheat bread. Improvement in niacin and pyridoxine content were also observed in Turkish cereal food fermented traditionally for 4 days with yogurt as a source of lactic acid bacteria (Ekinci 2005). It is reported that using enzyme protocol in vitamin B determination increased their content due to the release of their bound forms from starch and protein (Ndaw et al. 2000). Thus, enzyme interactions with starch and protein during BRF fermentation would have increased the vitamin content in FBRF. Thiamine was not detected in BRF and even after fermentation. It has been reported that vitamin B content in cereals varied according to varieties, and growing conditions (Adrian and Petit 1970; Davis et al. 1981).
Vitamin E and γ-oryzanol contents
Brown rice is considered a good and rich source of vitamin E isomers and γ-oryzanols. Significant increases in γ-tocotrienol and δ-tocotrienol were noticed after fermentation of BRF. In contrast, γ-tocopherol and α-tocopherol were significantly reduced (Table 3). A different study found that total tocopherol in soy germ decreased after 6 h fermentation with three different cultures (Hubert et al. 2008). A decrease in tocopherol and tocotrienol contents after cereal fermentation was also reported by Katina et al. (2005). As shown in Table 3, total γ-oryzanol decreased significantly after fermentation. The decrease in total γ-oryzanol might be related to the drop in the pH that could reduce the stability of these components.
Mineral and phytic acid contents
Whole grains are a good source of some minerals such as zinc, calcium, magnesium, iron and phosphorus. However, their availability is limited by the presence of phytic acid which has the ability to chelate divalent and trivalent minerals (Lopez et al. 2001). Significant increases in zinc, phosphorus, magnesium, iron and calcium levels were observed after fermentation which is consistent with the increase in total ash, whereas, significant reduction was observed in phytic acid content by almost 41 % after fermentation (Table 3). Reduction in the pH value during fermentation might have activated the phytase enzyme which degraded phytic acid complex and released the minerals (Katina et al. 2005). It has been reported that moderate drop in the pH to 5.5 could decrease phytate content during fermentation and increase mineral content (Lopez et al. 2001). Fermentation of cassava tuber also exhibited an enhancement in calcium content as reported by Oyewole and Ayo Odunfa (1989). Another study illustrated significant decline in phytate quantity in rice throughout fermentation (Liang et al. 2008). However, there was no considerable effect on zinc solubility that may provide indication of zinc interaction with other rice component such as fibre (Liang et al. 2008). A study reported that ash content of yeast fermented rice bran protein were significantly greater than that of unfermented and naturally fermented. It can be pointed out that higher ash content could increase mineral solubility and bioavailability (Chinma et al. (2014)). It appears that fermentation has the capability to improve mineral bioavailability and reduce anti-nutritive substances.
Total starch, resistant starch and amylose contents
Total starch content of BRF was reduced after fermentation as shown in Table 3. The drop in the pH to 5.5 during fermentation activated α-amylase (data not shown), which breakdown the starch to maltose and glucose, in addition to its consumption by the yeast (Martínez-Anaya 2003). Amylose content of FBRF was also significantly decreased but it was still in the same range as normal amylose content of the BRF. The reduction in amylose content might be due to the breakdown of its chain by the active α-amylase during fermentation. Resistant starch content was significantly increased after fermentation, which might corroborate with the increase in insoluble fiber content.
Water and oil holding capacities
Water holding capacity (WHC) shows the ability of a material to imbibe water in limited water environment. Table 4 shows that fermentation slightly reduced WHC of FBRF from 2.04 to 2.02 g/g. This was in close agreement with the findings of a study on fermented sorghum flour, where natural fermentation for 8, 16 and 24 h slightly reduced its WHC (Elkhalifa et al. 2005). However, fermented and non-fermented brown rice flour still had higher WHC than fluted pumpkin flour (0.37 g/g) (Elkhalifa et al. 2005) and marama bean flour (1.5 g/g) (Maruatona et al. 2010).
Table 4.
Functional properties of brown rice flour (BRF) and fermented brown rice flour (FBRF)
| Parameter | BRF | FBRF |
|---|---|---|
| Water holding capacity (g/g) | 2.04 ± 0.01ª | 2.02 ± 0.01ª |
| Oil holding capacity (g/g) | 0.80 ± 0.00ª | 0.95 ± 0.00b |
| Foaming capacity (%) | 60.50 ± 1.40ª | 76.00 ± 0.00b |
| Foaming stability (%) | 56.50 ± 2.21a | 70.50 ± 3.54b |
| Water solubility index (%) | 2.71 ± 0.71ª | 2.34 ± 0.00b |
| Swelling power (g/g) | 5.77 ± 0.18a | 5.05 ± 0.01b |
Represented values are means ± standard deviations of three replicates
Values with the same superscript letter within a row are not significantly different (P > 0.05)
Fermentation significantly increased OHC from 0.8 to 0.95 g/g sample (Table 4). Oil retention is associated with protein non-polar side chains that could attach to the hydrophobic groups in oil. Therefore, this finding suggests that protein alterations occurred during fermentation and enhanced its ability to absorb more oil or due to the increase in protein content (Table 4). Early study indicated that the ability of soy protein to absorb oil increased with increasing protein concentration (Maruatona et al. 2010). Similar observation has been found when comparing oil absorption capacity of defatted cupuassu seed flour to its protein concentrate (Carvalho et al. 2006). The reason for this is still not clear, but it may be related to physical entrapment of the oil by the protein or the increase in hydrophobic groups. As indicated by Chinma et al. (2014) rice bran protein concentrate fermented with yeast possessed higher OHC and greater quantity of non-polar amino acids than naturally and non-fermented samples, which might support the previous predictions. Oil holding capacity is an important property in retaining flavour, improving mouth-feel and palatability of a food product. Thus, FBRF could be used in baking products where high amount of oil is needed.
Foaming capacity (FC) and foaming stability (FS)
The current study indicated that FC and FS increased significantly after fermentation (Table 4). FC is mainly related to protein solubility (Nakai 1983). Therefore, the possible explanation for this might be due to a form of new protein functional properties after fermentation. Increasing its surface active characteristics, enhancing protein solubility and its rapid adsorption around the interfacial area were examples of these functional properties. Moreover, fermentation could also improve protein-protein interaction and promotes creation of multilayer viscoelastic film that increased foam stability by offering resistance to the bubbles from collapsing (Adebowale and Lawal 2004). BRF and FBRF showed higher foaming abilities compared to sorghum, cowpea and marama bean flours (Maruatona et al. 2010; Elkhalifa et al. 2005). It was also observed that foaming ability and stability of rice bran protein concentrate increased after yeast fermentation compared to non-fermented rice bran protein concentrate (Chinma et al. 2014). This property provides an opportunity for FBRF to be used in gluten-free products such as sponge cake.
Water solubility index (WSI) and swelling power (SP)
In spite of starch and protein hydrolyzation during fermentation, solubility of FBRF was significantly decreased (Table 4). This finding was unexpected and suggests that fermentation with the conditions employed could reduce the quantity of soluble macromolecules or change its functionality. Also, this result could be explained by the yeast consumption of the soluble materials during fermentation. Since solubility provides indication of the available soluble matters in the media, another possible explanation could be that FBRF has greater quantity of hydrophobic groups. The decrease in FBRF solubility suggests using it in dough formulations.
Swelling power (SP) is an aspect of water uptake measurement during starch gelatinization. SP of FBRF was lower than that BRF (Table 4). Starch contributes the major component in BRF and its structure has been reported to affect the functional properties of the flour such as swelling behaviour (Wang et al. 2010). Thus, the reduction in the swelling ability of the flour might be due to the reduction in starch content and the occurred changes in its structure. It has been reported that increase in starch SP is associated with higher quantity of amylopectin short chains, however long chains have the opposite effect (Sasaki and Matsuki 1998). The results were in accordance with earlier observation where swelling power of naturally fermented corn starch decreased compared to the control sample (Yang and Tao 2008).
Colour characteristics
Fermentation improved the whiteness of FBRF, where it recorded higher L value compared to the reference sample (Table 5). Lu et al. (2005) reported that natural fermentation enhanced the whiteness of rice flour. Moreover, Chinma et al. (2014) found that the colours of yeast and naturally fermented rice bran protein concentrates were significantly lighter than that of non-fermented sample. The ΔE indicates the extent of the total colour difference. The greater the ΔE the higher the colour difference which was observed for BRF in comparison to that of FBRF. However, it does not give indication about how the colour differs. Colour is an important quality of the food products and it is one of the limitations of BRF usage. Yeast fermentation process significantly decreased the darkness of FBRF.
Table 5.
Thermal properties, pasting properties and colour characteristics of brown rice flour (BRF) and fermented brown rice flour (FBRF)
| Parameter | BRF | FBRF |
|---|---|---|
| Thermal properties | ||
| Onset temperature (°C) | 74.77 ± 0.07a | 75.63 ± 0.11b |
| Peak temperature (°C) | 77.99 ± 0.04a | 79.29 ± 0.13b |
| End temperature (°C) | 81.70 ± 0.10a | 83.26 ± 0.17b |
| Enthalpy ΔH (J/g) | 4.56 ± 0.21a | 6.43 ± 0.10b |
| Pasting properties | ||
| Pasting temperature (°C) | 88.05 ± 0.01a | 87.98 ± 0.04a |
| Peak viscosity (RVU) | 170.50 ± 0.71a | 151.28 ± 1.58b |
| Breakdown (RVU) | 64.50 ± 0.35a | 53.25 ± 0.64b |
| Final viscosity (RVU) | 335.90 ± 0.14a | 227.00 ± 1.44b |
| Setback (RVU) | 551.90 ± 0.28a | 504.00 ± 0.28b |
| Colour parameters | ||
| L | 76.14 ± 0.08a | 77.27 ± 0.06b |
| a | 0.47 ± 0.04a | 0.43 ± 0.01a |
| b | 8.31 ± 0.00a | 8.51 ± 0.01b |
| ΔE | 19.66 ± 0.07a | 18.72 ± 0.05b |
Represented values are means ± standard deviations of three replicates
Values with the same superscript letter within a row are not significantly different (P > 0.05)
Pasting properties
The pasting properties of BRF, such as peak viscosity, breakdown, final viscosity and setback decreased as a result of yeast fermentation (Table 5). This could be due to endogenous or yeast enzyme degradation of the macromolecules such as starch, which significantly decreased. Pasting temperature of BRF also decreased after fermentation, however, the decrease was not significant. The pasting properties obtained during the current study are consistent with those of Wang et al. (2000) who found that peak viscosity of rice flour decreased when the pH dropped to 4.10. They explained that starch granules became more fragile and break easily due to acidification. The pasting properties results were also in agreement with that of previous research that reported setback and breakdown of rice flour decreased after lactic acid fermentation (Yang and Tao 2008). Similar observations were reported when corn starch was steeped in lactic acid aqueous phase (Haros et al. 2004). It was also reported that fermentation mainly affected the amorphous area which is composed of amylose and short chains of amylopectin (Lu et al. 2005). Short chains of amylopectin promote swelling due to their ability to form perfect crystalline structure, which lead to greater peak viscosity (Yang and Tao 2008). Therefore, it can be explained that the decrease in peak viscosity is related to the decrease in starch content and also might be due to alterations in the degree of polymerization and amylopectin branching architecture (Vandeputte et al. 2003; Jane et al. 1999). Breakdown value of BRF reduced after fermentation. This could be correlated with the decline in leaching out of starch polymers especially amylose content, where it significantly decreased. The decrease in the setback could be also associated with the reduction in amylose content. Setback is a measure of starch retrogradation (Chiang and Yeh 2002). Therefore, lower setback value after fermentation implies a delay in retrogradation phenomenon in the final product.
Thermal properties
The current study indicated significant increases in the onset temperature (TO), peak temperature (TC), conclusion temperature (TC) and enthalpy (∆H) of BRF after fermentation (Table 5). In contrast, Lu et al. (2005) reported significant decrease in the gelatinization temperatures of naturally fermented rice. As discussed earlier, changes in the starch composition had occurred during fermentation. These changes include decrease in the amylose content and might also involve alterations in crystalline to amorphous areas ratio and molecular structure of amylopectin that significantly impacted the gelatinization temperatures (Hagenimana et al. 2005). It is known that fermentation mainly affects the amorphous area. Starches with high amylose content and longer average chain molecules usually demonstrate higher transition temperatures (Hagenimana et al. 2005), however, amylose content decreased in the current study. High transition temperatures were due to large degree of crystallinity that increased the structural stability of the starch granules towards gelatinization. It seems possible that the increase in enthalpy after fermentation suggests changes in the crystallinity and more energy was needed for its disruption (Chang et al. 2006). Furthermore, it is reported that proteins heat stability is measured by the balance of non-polar and polar amino acids, where proteins with higher quantities of non-polar amino acids possess greater denaturation temperature (Kaur and Singh 2007). In this study, it was observed that the ability of BRF to absorb oil increased after fermentation, which suggested an increase in non-polar side chain proteins that might also lead to an increase in the enthalpy.
Microstructure
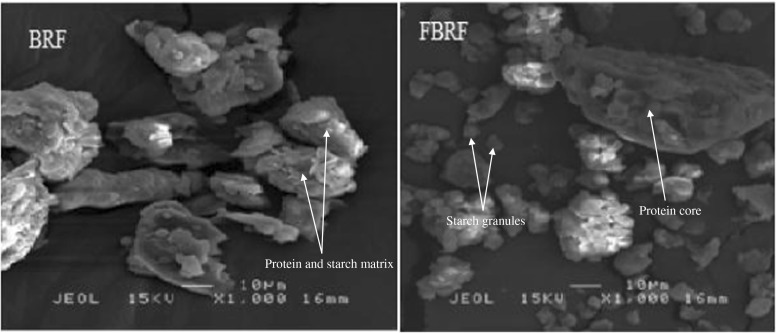
As presented in Fig. 2, starch granules in BRF were completely embedded in a compact protein matrix. Scanning electron micrographs of FBRF at 1000 magnification indicated a release of starch granules that were strongly packed in the enclosed structure. In addition, there were large and small polyhedral granules and the surface of flour granules was not smooth and they were irregular. The image of FBRF also shows a protein core which is left after fermentation and obviously illustrates the alveolar structures where the starch granules were blocked in the BRF. Similar structure was observed for fermented and non-fermented sorghum flour (Elkhalifa et al. 2006). They reported that proteolytic actions occurred during fermentation and this influenced the protein coat which disappeared in FBRF. Fermented sample also showed aggregated starch granules. This phenomenon may be related to weak starch-starch interactions because of fermentation (Singh et al. 2010). Starch is the main component of rice flours and therefore, it is expected to be responsible for most of rice flour functional properties. Some physicochemical and functional properties such as SP and WHC were found to be significantly correlated with the granule size and structure of starches isolated from several plant sources (Singh et al. 2003). Thus, reduction in starch and changes in starch structure as a result of fermentation can be one of the reasons for the observed alterations in the physicochemical and functional properties of BRF.
Fig. 2.
Microstructure of brown rice flour (BRF) and fermented brown rice flour (FBRF)
Conclusions
The current study indicated that fermentation of BRF with 1 % baker’s yeast at 32 °C for 6.26 h was effective in modifying its physicochemical and functional properties. Fermentation process at moderate acidity provided considerable improvements in antioxidant activity and nutritional value, where the levels of protein, ash, soluble fiber, vitamins, and minerals were significantly enhanced. The reductions in WHC and WSI, and the increases in FC and OHC suggest using FBRF in dough formulations such as non-gluten bread and cake. Lower ability to absorb water is also an advantageous property for preparation of thinner gruels. In addition, fermentation could eliminate the drawback of the dark colour of BRF, where the whiteness of the FBRF increased. The alteration which occurred in thermal profile, pasting profile and microstructure of FBRF could reflect the enzymatic actions on the macrocomponents of FBRF especially protein and starch. From these results FBRF is more nutritious than BRF and it can be also utilized as a functional food ingredient.
Acknowledgments
The authors are grateful to Universiti Putra Malaysia and Padiberas Nasional Berhad (BERNAS) for supporting this research.
Conflict of interest
The authors report no conflicts of interest.
Contributor Information
Muna Ilowefah, Email: mona.milad2005@gmail.com.
Jamilah Bakar, Email: jamilah@putra.upm.edu.my.
Hasanah M. Ghazali, Email: hasanah@putra.upm.edu.my
Ahmed Mediani, Email: medianiahmed47@gmail.com.
Kharidah Muhammad, Phone: +603-89468394, Email: kharidah@putra.upm.edu.my.
References
- Adebowale KO, Lawal OS. Comparative study of the functional properties of bambarra groundnut (Voandzeia subterranean), jack bean (Canavalia ensiformis) and mucuna bean (Mucuna pruriens) flours. Food Res Int. 2004;37:355–365. doi: 10.1016/j.foodres.2004.01.009. [DOI] [Google Scholar]
- Adrian J, Petit L. Vitamins of cereals and their evolution during technologic treatment. Ann Nutr Metab. 1970;24:B131–B168. [PubMed] [Google Scholar]
- Aguilar-Garcia C, Gavino G, Baragaño-Mosqueda M, Hevia P, Gavino VC. Correlation of tocopherol, tocotrienol, γ-oryzanol and total polyphenol content in rice bran with different antioxidant capacity assays. Food Chem. 2007;102:1228–1232. doi: 10.1016/j.foodchem.2006.07.012. [DOI] [Google Scholar]
- AOAC (2005) Official Methods of Analysis, 18th ed, Method 920.87, 934.01, 923.05, 993.19, 985.35, 995.11. Association of Official Analytical Chemists, Washington, DC
- AOAC (2005) Official Methods of Analysis, 18th ed, Method 953.17. Thiamine (vitamin B1) in grain products. Association of Official Analytical Chemists, Washington
- Bartolome B, Gómez-Cordovés C. Barley spent grain: release of hydroxycinnamic acids (ferulic and p‐coumaric acids) by commercial enzyme preparations. J Sci Food Agric. 1999;79:435–439. doi: 10.1002/(SICI)1097-0010(19990301)79:3<435::AID-JSFA272>3.0.CO;2-S. [DOI] [Google Scholar]
- Batifoulier F, Verny M, Chanliaud E, Rémésy C, Demigné C. Effect of different breadmaking methods on thiamine, riboflavin and pyridoxine contents of wheat bread. J Cereal Sci. 2005;42:101–108. doi: 10.1016/j.jcs.2005.03.003. [DOI] [Google Scholar]
- Beta T, Nam S, Dexter JE, Sapirstein HD. Phenolic content and antioxidant activity of pearled wheat and roller-milled fractions. Cereal Chem. 2005;82:390–393. doi: 10.1094/CC-82-0390. [DOI] [Google Scholar]
- Carvalho AV, García NHP, Amaya-Farfán J. Physico-chemical properties of the flour, protein concentrate, and protein isolate of the cupuassu (Theobroma grandiflorum Schum) seed. J Food Sci. 2006;71:S573–S578. doi: 10.1111/j.1750-3841.2006.00156.x. [DOI] [Google Scholar]
- Chang Y, Lin C, Chen J. Characteristics of mung bean starch isolated by using lactic acid fermentation solution as the steeping liquor. Food Chem. 2006;99:794–802. doi: 10.1016/j.foodchem.2005.07.060. [DOI] [Google Scholar]
- Chiang PY, Yeh AI. Effect of soaking on wet-milling of rice. J. Cereal Sci. 2002;35:85–94. doi: 10.1006/jcrs.2001.0419. [DOI] [Google Scholar]
- Chinma CE, Ilowefah M, Shammugasamy B, Ramakrishnan Y, Muhammad K. Chemical, antioxidant, functional and thermal properties of rice bran proteins after yeast and natural fermentations. Int. J. Food Sci. Technol. 2014;49:2204–2213. doi: 10.1111/ijfs.12533. [DOI] [Google Scholar]
- Davis K, Cain R, Peters L, Le Tourneau D, McGinnis J. Evaluation of the nutrient composition of wheat. II. Proximate analysis, thiamin, riboflavin, niacin and pyridoxine. Cereal Chem. 1981;58:116–120. [Google Scholar]
- Dordević TM, Šiler-Marinković SS, Dimitrijević-Branković SI. Effect of fermentation on antioxidant properties of some cereals and pseudo cereals. Food Chem. 2010;119:957–963. doi: 10.1016/j.foodchem.2009.07.049. [DOI] [Google Scholar]
- Ekinci R. The effect of fermentation and drying on the water-soluble vitamin content of tarhana, a traditional Turkish cereal food. Food Chem. 2005;90:127–132. doi: 10.1016/j.foodchem.2004.03.036. [DOI] [Google Scholar]
- Elkhalifa AEO, Schiffler B, Bernhardt R. Effect of fermentation on the functional properties of sorghum flour. Food Chem. 2005;92:1–5. doi: 10.1016/j.foodchem.2004.05.058. [DOI] [Google Scholar]
- Elkhalifa AEO, Bernhardt R, Bonomi F, Iametti S, Pagani MA, Zardi M. Fermentation modifies protein/protein and protein/starch interactions in sorghum dough. Eur. Food Res. Technol. 2006;222:559–564. doi: 10.1007/s00217-005-0124-9. [DOI] [Google Scholar]
- Flander L, Suortti T, Katina K, Poutanen K. Effects of wheat sourdough process on the quality of mixed oat-wheat bread. LWT Food Sci. Technol. 2011;44:656–664. doi: 10.1016/j.lwt.2010.11.007. [DOI] [Google Scholar]
- Hagenimana A, Pu P, Ding X. Study on thermal and rheological properties of native rice starches and their corresponding mixtures. Food Res Int. 2005;38:257–266. doi: 10.1016/j.foodres.2004.05.009. [DOI] [Google Scholar]
- Haros M, Perez OE, Rosell CM. Effect of steeping corn with lactic acid on starch properties. Cereal Chem. 2004;81:10–14. doi: 10.1094/CCHEM.2004.81.1.10. [DOI] [Google Scholar]
- Hubert J, Berger M, Nepveu F, Paul F, Daydé J. Effects of fermentation on the phytochemical composition and antioxidant properties of soy germ. Food Chem. 2008;109:709–721. doi: 10.1016/j.foodchem.2007.12.081. [DOI] [PubMed] [Google Scholar]
- AACC International (1995) Approved Methods of Analysis, 9th Ed. Method 61–02.01 Detremination of the pasting properties of rice with Rapid Visco-Analyser. 1995. First Approval 10-26-94. AACC International, St. Paul
- AACC International (1999) Approved Methods of Analysis, 11th Ed. Method 86–90.01.B-Vitamin in Vitamin Concentrates by HPLC. Approved November 3. AACC International, St. Paul
- AACC International (2001) Approved Methods of Analysis, 10th ed. Methods 61–03.01. Amylograph method for milled rice. AACC International, St. Paul
- ISO 2171 (1993) International standard, cereals and milled cereal products- determination of total ash
- Jane J, Chen YY, Lee LF, McPherson AE, Wong KS, Radosavljevic M, et al. Effects of amylopectin branch chain length and amylose content on the gelatinization and pasting properties of starch. Cereal Chem. 1999;76:629–637. doi: 10.1094/CCHEM.1999.76.5.629. [DOI] [Google Scholar]
- Katcher HI, Legro RS, Kunselman AR, Gillies PJ, Demers LM, Bagshaw DM, et al. The effects of a whole grain-enriched hypocaloric diet on cardiovascular disease risk factors in men and women with metabolic syndrome. Am J Clin Nutr. 2008;87:79–90. doi: 10.1093/ajcn/87.1.79. [DOI] [PubMed] [Google Scholar]
- Kati K, Kaisa P, Karin A. Influence and interactions of processing conditions and starter culture on formation of acids, volatile compounds, and amino acids in wheat sourdoughs. Cereal Chem. 2004;81:598–610. doi: 10.1094/CCHEM.2004.81.5.598. [DOI] [Google Scholar]
- Katina K, Arendt E, Liukkonen K, Autio K, Flander L, Poutanen K. Potential of sourdough for healthier cereal products. Trends Food Sci. Technol. 2005;16:104–112. doi: 10.1016/j.tifs.2004.03.008. [DOI] [Google Scholar]
- Katina K, Laitila A, Juvonen R, Liukkonen K, Kariluoto S, Piironen V, et al. Bran fermentation as a means to enhance technological properties and bioactivity of rye. Food Microbiol. 2007;24:175–186. doi: 10.1016/j.fm.2006.07.012. [DOI] [PubMed] [Google Scholar]
- Kaur M, Singh N. Characterization of protein isolatesfrom different Indian chickpea (Cicer arietinum) cultivars. Food Chem. 2007;102:366–374. doi: 10.1016/j.foodchem.2006.05.029. [DOI] [Google Scholar]
- Kwon DY, Kim HJ, Park S. Antidiabetic effects of fermented soybean products on type 2 diabetes. Nutr Res. 2010;30:1–13. doi: 10.1016/j.nutres.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Lerma-García MJ, Herrero-Martínez JM, Simó-Alfonso EF, Mendonça CRB, Ramis-Ramos G. Composition, industrial processing and applications of rice bran γ-oryzanol. Food Chem. 2009;115:389–404. doi: 10.1016/j.foodchem.2009.01.063. [DOI] [Google Scholar]
- Liang J, Han B, Nout MJR, Hamer RJ. Effects of soaking, germination and fermentation on phytic acid, total and in vitro soluble zinc in brown rice. Food Chem. 2008;110:821–828. doi: 10.1016/j.foodchem.2008.02.064. [DOI] [PubMed] [Google Scholar]
- Lin Q, Xiao H, Fu X, Tian W, Li L, Yu F. Physico-chemical properties of flour, starch, and modified starch of two rice varieties. Agric. Sci. China. 2011;10:960–968. doi: 10.1016/S1671-2927(11)60082-5. [DOI] [Google Scholar]
- Liu S, Manson JE, Stampfer MJ, Rexrode KM, Hu FB, Rimm EB, et al. Whole grain consumption and risk of ischemic stroke in women: A prospective study. JAMA. 2000;284:1534–1540. doi: 10.1001/jama.284.12.1534. [DOI] [PubMed] [Google Scholar]
- Lopez HW, Krespine V, Guy G, Messager A, Demigne C, Remesy C. Prolonged fermentation of whole wheat sourdough reduces phytate level and increases soluble magnesium. J Agric Food Chem. 2001;49:2657–2662. doi: 10.1021/jf001255z. [DOI] [PubMed] [Google Scholar]
- Lu Z, Li L, Min W, Wang F, Tatsumi E. The effects of natural fermentation on the physical properties of rice flour and the rheological characteristics of rice noodles. Int J Food Sci Technol. 2005;40:985–992. doi: 10.1111/j.1365-2621.2005.01032.x. [DOI] [Google Scholar]
- Mahgoub SEO, Ahmed BM, Ahmed MMO, Agib EN. Effect of traditional Sudanese processing of kisra bread andhulu-mur drink on their thiamine, riboflavin and mineral contents. Food Chem. 1999;67:129–133. doi: 10.1016/S0308-8146(99)00074-6. [DOI] [Google Scholar]
- Maninder K, Sandhu KS, Singh N. Comparative study of the functional, thermal and pasting properties of flours from different field pea (Pisum sativum L.) and pigeon pea (Cajanus cajan L.) cultivars. Food Chem. 2007;104:259–267. doi: 10.1016/j.foodchem.2006.11.037. [DOI] [Google Scholar]
- Maria PR, Nicula A, Socaciu C. The optimization of extraction and HPLC analysis of vitamins B from yeast products. Bull UASVM Agric. 2008;65:324–328. [Google Scholar]
- Martínez-Anaya MA. Associations and interactions of micro-organisms in dough fermentations: effects on dough and bread characteristics. In: Kulp K, Lorenz K, editors. Handbook of dough fermentations. New York: Marcel Dekker; 2003. p. 63.195. [Google Scholar]
- Maruatona GN, Duodu KG, Minnaar A. Physicochemical, nutritional and functional properties of marama bean flour. Food Chem. 2010;121:400–405. doi: 10.1016/j.foodchem.2009.12.054. [DOI] [Google Scholar]
- Moore J, Cheng Z, Hao J, Guo G, Liu J, Lin C, Yu L. Effects of solid-state yeast treatment on the antioxidant properties and protein and fiber compositions of common hard wheat bran. J Agric Food Chem. 2007;55:10173–10182. doi: 10.1021/jf071590o. [DOI] [PubMed] [Google Scholar]
- Moroni AV, Dal Bello F, Arendt EK. Sourdough in gluten-free bread-making: an ancient technology to solve a novel issue. Food Microbiol. 2009;26:676–684. doi: 10.1016/j.fm.2009.07.001. [DOI] [PubMed] [Google Scholar]
- Nakai S. Structure-function relationships of food proteins: with an emphasis on the importance of protein hydrophobicity. J Agric Food Chem. 1983;31:676–683. doi: 10.1021/jf00118a001. [DOI] [Google Scholar]
- Ndaw S, Bergaentzle M, Aoudea-Werner D, Hasselmann C. Extraction procedures for the liquid chromatographic determination of thiamine, riboflavin and vitamin B6 in foodstuffs. Food Chem. 2000;71:129–138. doi: 10.1016/S0308-8146(00)00135-7. [DOI] [Google Scholar]
- Oyewole OB, Ayo Odunfa S. Effects of fermentation on the carbohydrate, mineral, and protein contents of cassava during “fufu” production. J. Food Compos. Anal. 1989;2:170–176. doi: 10.1016/0889-1575(89)90078-1. [DOI] [Google Scholar]
- Sasaki T, Matsuki J. Effect of wheat starch structure on swelling power. Cereal Chem. 1998;75:525–529. doi: 10.1094/CCHEM.1998.75.4.525. [DOI] [Google Scholar]
- Shekib LA. Nutritional improvement of lentils, chick pea, rice and wheat by natural fermentation. Plant Foods Hum Nutr. 1994;46:201–205. doi: 10.1007/BF01088991. [DOI] [PubMed] [Google Scholar]
- Singh N, Singh J, Kaur L, Sodhi NS, Gill BS. Morphological, thermal and rheological properties of starches from different botanical sources. Food Chem. 2003;80:219–231. doi: 10.1016/S0308-8146(02)00416-8. [DOI] [Google Scholar]
- Singh S, Singh N, Ezekiel R, Kaur A. Effects of gamma-irradiation on the morphological, structural, thermal and rheological properties of potato starches. Carbohydr Polym. 2010;83:1521–1528. doi: 10.1016/j.carbpol.2010.09.063. [DOI] [Google Scholar]
- Sun Q, Spiegelman D, Van Dam RM, Holmes MD, Malik VS, Willett WC, et al. White rice, brown rice, and risk of type 2 diabetes in US men and women. Arch Intern Med. 2010;170:961–969. doi: 10.1001/archinternmed.2010.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandeputte GE, Derycke V, Geeroms J, Delcour JA. Rice starch. II Structure aspects provide insight into swelling and pasting properties. J Food Sci. 2003;38:53–59. [Google Scholar]
- Wang H, Sun D, Zeng Q, Lu Y. Effect of pH, corn starch and phosphates on the pasting properties of rice flour. J Food Eng. 2000;46:133–138. doi: 10.1016/S0260-8774(00)00077-7. [DOI] [Google Scholar]
- Wang B, Wang L, Li D, Wei Q, Adhikari B. The rheological behavior of native and high-pressure homogenized waxy maize starch pastes. Carbohydr Polym. 2010;88:481–489. doi: 10.1016/j.carbpol.2011.12.028. [DOI] [Google Scholar]
- Wu P, Zhao T, Tian JC. Phytic acid contents of wheat flours from different mill streams. Agric. Sci. China. 2010;9:1684–1688. doi: 10.1016/S1671-2927(09)60266-2. [DOI] [Google Scholar]
- Yang Y, Tao W. Effects of lactic acid fermentation on FT-IR and pasting properties of rice flour. Food Res Int. 2008;41:937–940. doi: 10.1016/j.foodres.2007.10.011. [DOI] [Google Scholar]
- Ye L, Landen W, Jr, Lee J, Eitenmiller R. Vitamin E content of margarine and reduced fat products using a simplified extraction procedure and HPLC determination. J. Liq. Chromatogr. Relat. Technol. 1998;21:1227–1238. doi: 10.1080/10826079808006596. [DOI] [Google Scholar]
- Yousif NE, El Tinay AH. Effect of fermentation on protein fractions and in vitro protein digestibility of maize. Food Chem. 2000;70:181–184. doi: 10.1016/S0308-8146(00)00069-8. [DOI] [PubMed] [Google Scholar]