Abstract
An inverse association between coffee and Parkinson's disease (PD) has been reported. However, it remains uncertain why some but not all coffee drinkers are less susceptible to PD. We considered the possibility of a pharmaco-genetic effect. In our study, we included 1,208 subjects (446 case-unaffected sibling pairs and 158 case-unrelated control pairs) recruited from an ongoing study of the molecular epidemiology of PD in the Upper Midwest (USA). We collected information on lifetime coffee drinking and we studied two genes: ADORA2A, which encodes the major receptor activity of caffeine in the brain (variants rs5751876 and rs3032740), and CYP1A2, which encodes the major rate-limiting step of caffeine metabolism (variants rs35694136 and rs762551). We did not observe significant associations of coffee drinking or of the genetic variants with PD susceptibility, either independently or jointly, in the sample overall and in most strata. Our study neither supports the hypothesis that coffee protects against PD nor provides evidence for a pharmacogenetic effect.
Keywords: Parkinson's disease, coffee, genes
An inverse association of coffee drinking with Parkinson's disease (PD) has been reported.1–6 However, this association has not always been replicated,7–10 and in some studies has been observed primarily in men1 or to a lesser extent in women.4 Avoidance of coffee may be a manifestation of premorbid PD personality, or caffeine in coffee may exert a neuroprotective effect.11–14 Assuming that coffee has a protective effect, it is unclear why some coffee drinkers are protected against PD and others are not, and why men are more protected than woman.
The individual variability and between-studies variability in the effect of coffee may be due to gene-environment interactions. Yet to date, there has been only one limited study of coffee, caffeine-related genes, and PD.15 The identification of genetic modifiers of the putative neuroprotective effect could inform the design of clinical trials of coffee or caffeine in PD, and might provide additional insights on the pathophysiology of the disease.16 Therefore, the aims of this study were to study the main and joint effects of coffee, caffeine-related genes, and PD.
PATIENTS AND METHODS
Study Sample
All subjects were recruited as part of an ongoing study of the molecular epidemiology of PD. Cases were patients with PD referred sequentially to the Department of Neurology of the Mayo Clinic in Rochester, MN, during the 10-year period from June 1, 1996 to May 31, 2006. Cases resided in Minnesota or in one of the surrounding four states (Wisconsin, Iowa, North Dakota, and South Dakota). Control subjects consisted primarily of unaffected siblings17 of PD cases who screened negative for PD or parkinsonism via telephone interview,18 or siblings who screened positive but were confirmed to be free of parkinsonism at clinical examination. We also recruited unrelated controls from the same geographic region. Controls of age 65 or older were randomly selected from the Centers for Medicare and Medicaid Services (CMS) lists. Controls younger than 65 years were selected using random digit dialing, according to standard techniques.19,20 All unrelated controls screened negative for PD or parkinsonism via telephone interview; unrelated controls screening positive could not be examined and were excluded from the study. All examinations (cases and siblings screening positive) were performed in a standardized fashion by neurologists specialized in Movement Disorders, and employing a detailed protocol for clinical assessment.
Exposure Assessment
Exposures were obtained by direct interview (or by proxy for deceased or incapacitated subjects) using a structured questionnaire that was administered via telephone by trained research assistants. To reduce interview and recall biases, interviewers were kept unaware of the case or control status of subjects, and the subjects (or their proxies) were kept unaware of the study hypotheses. The information collected was for the time period spanning from birth to the age at onset of PD in cases or the same age in matched controls (see later). The lag time between recruitment of cases and controls and their interview was kept to a minimum of 2 to 3 weeks to avoid intercurrent deaths or loss of interest in the study. Cases and controls were asked to provide information about coffee drinking, including number of cups, (quantified as small cups or regular coffee cup of about 6 ounces, or one shot of espresso; or as medium or large cup or coffee mug of about 12 ounces, or two shots of espresso) that they drank per day, with details about periods of life with different amounts. Subjects were instructed to not consider decaffeinated coffee in their responses. Information on tea and sodas was also collected: subjects were asked to provide similarly detailed information on both hot and iced tea, but they were asked to not consider herbal tea in their answers. Similarly, only caffeinated sodas were investigated.
Candidate Susceptibility Genes
We selected two genes for consideration in this study. Both genes encode proteins that are related to caffeine responsiveness or metabolism in humans. The ADORA2A gene encodes the adenosine receptor A2a.
This receptor is the major target of caffeine in the central nervous system and it is abundant in the basal ganglia, the motor control pathway perturbed in patients with PD.21 The cytochrome P450 1A2 gene (CYP1A2) encodes an enzyme responsible for caffeine N3-demethylation. This enzyme is the major rate-limiting step of caffeine metabolism in humans, converting almost 90% of caffeine to paraxantine.22
Blood Sampling, DNA Extraction, and Genotyping
The study was approved by the Institutional Review Board of the Mayo Clinic in Rochester, MN. After written informed consent, a blood sample was obtained directly from each subject who underwent a clinical assessment or via mail-in kit for subjects who did not undergo a clinical assessment. Blood was processed for DNA extraction via the Puregene procedure (Gentra Systems®). The ADORA2A and CYP1A2 SNPs were analyzed using a chip-based platform (Nanogen®, San Diego, CA), employing previously described methods.23
We genotyped two single nucleotide polymorphisms (SNPs) within the ADORA2A gene and two within the CYP1A2 gene that occurred with greater than 1% frequency in Caucasian European subjects.24–26 ADORA2A rs5751876 is a synonymous polymorphism (Tyr to Tyr substitution in exon 4 at amino acid position 361). ADORA2A rs3032740 is characterized by an insertion of a train of thymidines in intron 3, which seems to reduce protein expression.27 CYP1A2 rs35694136 is positioned in the 5′ promoter region and CYP1A2 rs762551 is positioned in the first intron of the gene; both SNPs are haplotype tagging.28
Data Analyses
We matched cases to a single unaffected sibling of the same sex (when able) and then of closest age at study. For cases without an available sibling, we matched an unrelated control for sex, age (±2 years), and self-reported ethnicity. Hardy-Weinberg equilibrium was assessed for each SNP (in controls), and linkage disequilibrium (LD) maps were built for the genes of interest.29 For analyses of PD susceptibility, we employed conditional logistic regression to study the main and joint effects of coffee and genes. We considered autosomal dominant, autosomal recessive, and log-additive coding schemes for each SNP and calculated odds ratios (ORs) and 95% confidence intervals (95% CIs). All analyses were done using SAS version 8.2 (SAS Institute, Cary, NC), and statistical tests were performed at the two-tailed alpha level of 0.05.
Quality Measures and Reliability Study
Recruitment of cases, matching of controls, telephone screening interviews of unaffected siblings and unrelated controls to rule out PD, physical examinations of cases or siblings screening positive for PD, and risk factors interviews of all subjects were performed in a standardized manner by trained personnel. Interviewers were kept masked to the clinical status of the interviewed subjects. We also performed a reliability study of coffee exposure as measured via telephone interview. Specifically, we randomly selected 20 pro-bands, 20 unaffected siblings, and 20 unrelated controls from our sample and conducted a second risk factor assessment via telephone interview. The second interview was performed by an interviewer other than the one who performed the initial one (by design). The median lag time between the first and the second interview was 4.8 months (range 2.3–14.6). DNA extraction and genotyping were performed with standardized techniques and without knowing the clinical status of the subjects.
RESULTS
We included 604 case–control pairs (446 case-unaffected sibling pairs and 158 case-unrelated control pairs), for whom data were available for coffee and at least one SNP. Table 1 summarizes demographic characteristics and ancestry information of our sample. More than 85% of eligible PD cases participated in the study (all provided DNA and underwent risk factor interviews). The participation rates among siblings were 92% for the risk factor interview and 81% for the blood sample donation. The participation rate among unrelated controls for both the interview and the blood sample was 57%. Information was obtained by proxies in 9.1% of the cases and in 2.5% of controls. Spouses were the main source of proxy information (70%), followed by the offspring (14.3%) and the siblings of the subjects (7.1%). For two cases information was obtained by a brother- or sister-in-law (2.9%), and only in one case each (1.4%) by a parent, a niece or nephew, a son- or daughter-in-law, and a stepchild. The average time between PD diagnosis and the time of the risk factor interview was 5.6 years.
TABLE 1 Demographic characteristics of Parkinson's disease cases, siblings, and unrelated controls
| Case–sibling pairs |
Case-unrelated control pairs |
All case–control pairs |
||||
|---|---|---|---|---|---|---|
| General characteristics | PD cases | Sibling controls | PD cases | Unrelated controls | PD cases | All controls |
| Total sample, n (%)a | 446 | 446 | 158 | 158 | 604 | 604 |
| Men | 273 (61.2) | 219 (49.1) | 93 (58.9) | 93 (58.9) | 336 (60.6) | 312 (51.7) |
| Women | 173 (38.8) | 227 (50.9) | 65 (41.1) | 65 (41.1) | 238 (39.4) | 292 (48.3) |
| Age at onset, median (range) | 59.4 (30.6–86.9) | – | 58.3 (36.7–69.7) | – | 59.1 (30.6–86.9) | – |
| Age at study, median (range) | 66.0 (32.8–91.4) | 64.6 (32.0–90.4) | 63.1 (44.3–70.8) | 62.4 (44.9–66.8) | 64.7 (32.8–91.4) | 63.9 (32.0–90.4) |
| Region of origin of parents, n (%)b | ||||||
| Both parents of European origin | 383 (85.9) | 369 (82.7) | 121 (76.6) | 137 (86.7) | 504 (83.4) | 506 (83.8) |
| Both parents Northern European | 147 (38.4) | 97 (26.3) | 39 (32.2) | 38 (27.7) | 186 (36.9) | 135 (26.7) |
| Both parents Central European | 106 (27.7) | 140 (37.9) | 37 (30.6) | 47 (34.3) | 143 (28.4) | 187 (37.0) |
| Both parents Southern European | 3 (0.8) | 3 (0.8) | 1 (0.8) | 1 (0.7) | 4 (0.8) | 4 (0.8) |
| Both parents European, mixed region | 127 (33.2) | 129 (35.0) | 44 (36.4) | 51 (37.2) | 171 (28.3) | 180 (35.6) |
| Only one parent of European origin | 38 (8.5) | 46 (10.3) | 29 (18.4) | 11 (7.0) | 67 (11.1) | 57 (9.4) |
| One parent declared “American” | 17 (3.8) | 16 (3.6) | 10 (6.3) | 7 (4.4) | 27 (4.5) | 23 (3.8) |
| Both parents declared “American” | 17 (3.8) | 18 (4.0) | 5 (3.2) | 5 (3.2) | 22 (3.6) | 23 (3.8) |
| Both parents Asian | 1 (0.2) | 2 (0.4) | 1 (0.6) | 0 (0.0) | 2 (0.3) | 2 (0.3) |
| Both parents Mexican | 1 (0.2) | 1 (0.2) | 0 (0.0) | 0 (0.0) | 1 (0.2) | 1 (0.2) |
| Both parents African | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Unknown | 4 (0.9) | 9 (2.0) | 1 (0.6) | 2 (1.3) | 5 (0.8) | 11 (1.8) |
446 case–sibling pairs and 158 case-unrelated control pairs used in conditional logistic regression analyses for ADORA2A rs3032740, CYP1A2 (rs35694136 and rs762551), and coffee. Each member of the pair contained complete coffee data and at least 1 SNP genotyped.
Region of origin of parents was self-reported by subjects. Note that Parkinson's disease cases and their siblings were not always in agreement. “Northern European” includes Scandinavian, Swedish, Norwegian, Finnish, Danish, Irish, or British origin. “Central European” includes French, Belgian, Dutch, Swiss, Luxemburgian, German, Austrian, Hungarian, Polish, Czechoslovakian, or Russian origins. “Southern European” includes Italian, Spanish, Portuguese, Greek, or Yugoslavian origins.
Coffee drinking was not associated with PD susceptibility, either overall (ever/never) or by number of cups per day (Table 2). Adjusting our analyses for the level of education (years of school in quartiles) and cigarette smoking (ever/never) did not change the results (data not shown). On the other hand, cigarette smoking (ever/never) was inversely associated with PD susceptibility in our sample, overall (OR: 0.49, 95% CI: 0.37–0.64, P < 0.0001) and in several strata (data not shown). No significant differences in the pattern of coffee use in cases and controls at different periods of life were found; both groups showed a stable intake of coffee over time (data not shown). Agreement between the first and second telephone risk factor interview was 90% for ever versus never coffee drinking (κ = 0.72, 95% CI: 0.51, 0.93). For age-started drinking coffee and coffee-cup/years, the intraclass correlation coefficient was 0.87 for both variables.
TABLE 2.
Risk of Parkinson's disease: Conditional logistic regression analysis for coffee, adjusted for smoking
| Coffee ever/never |
N (%) |
Model** |
Model** |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample or stratum | N* | Yes | No | OR (95% CI) coffee ever/never | P value | OR (95% CI) smoke ever/never | P value | OR (95% CI) coffee cups per day | P value | OR (95% CI) smoke ever/never | P value |
| Cases, all | 604 | 490 (81.1) | 114 (18.9) | 1.13 (0.81–1.57) | 0.49 | 0.48 (0.36–0.63) | <0.0001 | 1.00 (0.76–1.31) | 0.99 | 0.49 (0.37–0.65) | <0.0001 |
| Controls, all | 604 | 501 (82.9) | 103 (17.1) | ||||||||
| Cases, female | 189 | 154 (81.5) | 35 (18.5) | 1.00 (0.57–1.74) | 0.99 | 0.55 (0.32–0.93) | 0.03 | 1.26 (0.78–2.05) | 0.35 | 0.51 (0.30–0.88) | 0.02 |
| Controls, female | 189 | 157 (83.1) | 32 (16.9) | ||||||||
| Cases, male | 263 | 218 (82.9) | 45 (17.1) | 1.18 (0.82–1.86) | 0.52 | 0.54 (0.35–0.81) | 0.003 | 0.76 (0.51–1.13) | 0.17 | 0.57 (0.38–0.85) | 0.006 |
| Controls, male | 263 | 214 (81.4) | 49 (18.6) | ||||||||
| Cases | 446 | 364 (81.6) | 82 (18.4) | 1.24 (0.82–1.86) | 0.30 | 0.33 (0.23–0.48) | <0.0001 | 1.05 (0.76–1.46) | 0.78 | 0.34 (0.24–0.50) | <0.0001 |
| Controls, siblings | 446 | 373 (83.6) | 73 (16.4) | ||||||||
| Cases | 158 | 126 (79.7) | 32 (20.3) | 1.08 (0.58–2.01) | 0.81 | 0.85 (0.52–1.39) | 0.52 | 0.97 (0.58–1.64) | 0.92 | 0.87 (0.53–1.42) | 0.57 |
| Controls, unrelated | 158 | 128 (81.0) | 30 (19.0) | ||||||||
N, number of pairs.
All models were adjusted for age at study (continuous variable) and gender, as appropriate. Coffee cups per day were coded continuously. Models adjusted for education were similar (data not shown).
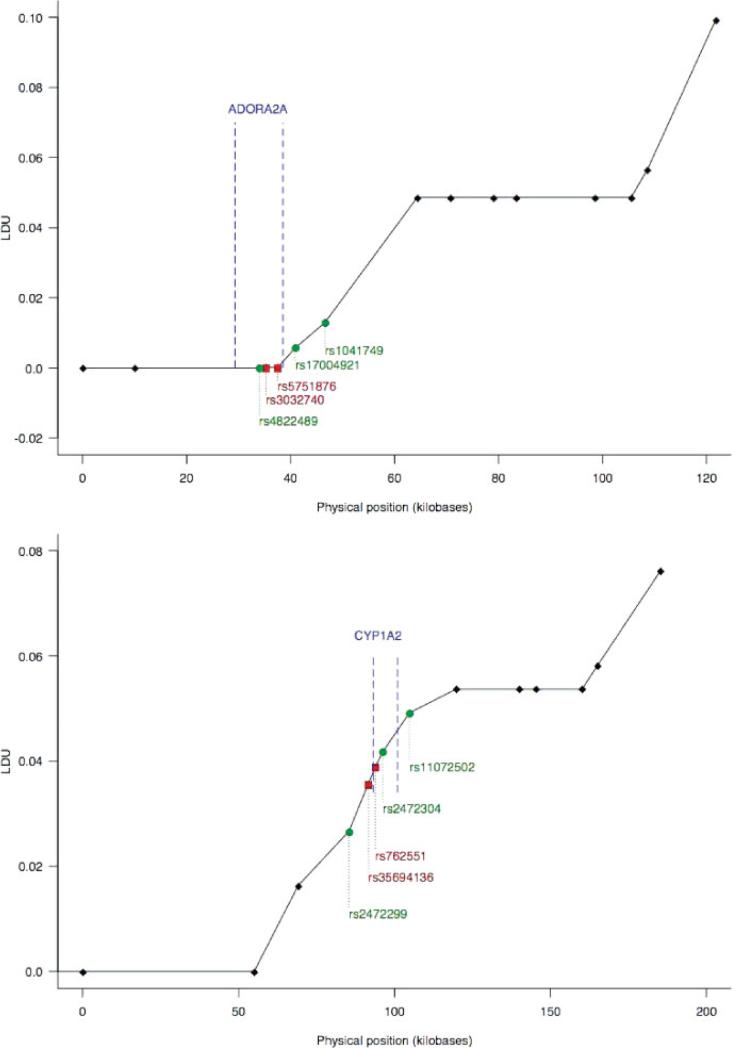
All four SNPs of ADORA2A and CYP1A2 were in Hardy-Weinberg equilibrium in controls. For a smaller group of subjects, we also had data available for additional SNPs in the two candidate genes, because those subjects were also included in a whole-genome association study of PD reported elsewhere.30 Using information for the additional SNPs, we constructed LD maps under the Malecot model using LDMAP29 for the region surrounding the ADORA2A and the CYP1A2 loci. The two ADORA2A SNPs selected for this study (rs5751876 and rs3032740) were in LD (see Fig. 1). On the other hand, for the CYP1A2 SNPs (rs35694136 and rs762551) there was less LD between the two SNPs and with other regions of the gene (see Fig. 1). For this reason, to study the association of CYP1A2 and ADORA2A loci with PD, we analyzed data for both CYP1A2 SNPs but only for ADORA2A rs3032740, as the function of that SNP has been defined.28
FIG. 1.
Linkage disequilibrium (LD) maps for ADORA2A (top panel) and CYP1A2 (bottom panel) genes. A “flat” line between two markers indicates a region of LD. When a gene lies completely within a flat line segment, it is adequately mapped by any SNP marker within that segment. SNPs designated by boxes and red color were genotyped as part of this study, and SNPs designated by circles and green color were genotyped as part of a whole-genome association study of PD.30 SNPs are displayed from left to right in 5′ to 3′ direction. LDU, linkage disequilibrium units.
None of the three SNPs were associated with PD susceptibility in the sample overall, either before or after adjusting for coffee and smoking (Table 3). However, findings were significant for the ADORA2A SNP rs3032740 in men (OR: 0.71, 95%; CI: 0.52–0.96; P = 0.03). No global differences in haplotype frequencies were revealed in two-locus haplotype analyses for the CYP1A2 gene. No significant interactions were observed for coffee and any of the SNPs, after adjusting for age at study and gender (Table 4).
TABLE 3.
Risk of Parkinson's disease: Conditional logistic regression analyses for informative SNPs in caffeine-related genes
| Genotype, n (%)** |
Model*** |
|||||
|---|---|---|---|---|---|---|
| Gene, SNP | N* | 1/1 | 1/2 | 2/2 | OR (95% CI) | P-value*** |
| ADORA2A, rs3032740 | ||||||
| Cases, all | 565 | 223 (39.5) | 263 (46.5) | 79 (14.0) | 0.84 (0.62–1.14) | 0.26 |
| Controls, all | 565 | 203 (35.9) | 268 (47.4) | 94 (16.6) | ||
| CYP1A2, rs35694136 | ||||||
| Cases, all | 455 | 397 (87.3) | 55 (12.1) | 3 (0.7) | 0.69 (0.41–1.18) | 0.17 |
| Controls, all | 455 | 389 (85.5) | 65 (14.3) | 1 (0.2) | ||
| CYP1A2, rs762551 | ||||||
| Cases, all | 391 | 207 (52.9) | 150 (38.4) | 34 (8.7) | 0.73 (0.49–1.10) | 0.13 |
| Controls, all | 391 | 194 (49.6) | 166 (42.5) | 31 (7.9) | ||
N = number of pairs.
1/1 = homozygous for the more common (“wild type”) allele, 2/2 = homozygous for the less common (minor) allele; 1/2 = heterozygous for the minor allele.
Model = conditional logistic regression models adjusted for age at study (continuous variable), gender, and smoking. Models assume autosomal dominant genetic effect (2/2 or 1/2 vs. 1/1); autosomal recessive and log additive effects were also consistent (data not shown).
TABLE 4.
Susceptibility to Parkinson's disease: Joint effect models for coffee drinking and caffeine-related genes
| Model** |
|||
|---|---|---|---|
| Subjects, n* | HR (95% CI) | P-value | |
| Coffee and ADORA2A rs3032740 | |||
| No coffee, no variant | 90 | 1.00 (Reference) | – |
| No coffee, variant | 116 | 0.90 (0.50–1.65) | 0.74 |
| Coffee, no variant | 336 | 0.97 (0.58–1.61) | 0.89 |
| Coffee, variant | 558 | 0.81 (0.49–1.33) | 0.40 |
| Coffee and CYP1A2 rs35694136 | |||
| No coffee, no variant | 130 | 1.00 (Reference) | – |
| No coffee, variant | 15 | 1.93 (0.54–6.94) | 0.31 |
| Coffee, no variant | 656 | 1.06 (0.70–1.59) | 0.79 |
| Coffee, variant | 109 | 0.74 (0.38–1.42) | 0.37 |
| Coffee and CYP1A2 rs762551 | |||
| No coffee, no variant | 61 | 1.00 (Reference) | – |
| No coffee, variant | 60 | 0.77 (0.36–1.65) | 0.50 |
| Coffee, no variant | 340 | 0.93 (0.52–1.66) | 0.81 |
| Coffee, variant | 321 | 0.70 (0.37–1.32) | 0.28 |
Subjects were cases, unaffected siblings, and unrelated controls (matched case–control pairs) for which complete data on coffee drinking and genotypes were available.
All models were adjusted for gender and age at study (continuous variable), as appropriate. Coffee was coded dichotomously as ever or never. Each genetic variant was coded assuming an autosomal dominant genetic effect. Autosomal recessive and log-additive effects were also consistent (data not shown).
DISCUSSION
In this study we neither observed a significant inverse association of coffee use (ever/never) with PD nor a dose effect or an association in men only. To date, the inverse association of coffee drinking with PD has been assessed mainly in population-based case-unrelated control studies or in cohort studies.1–5 By contrast, our study is different because it is primarily family-based. For studies testing genetic hypotheses, the use of unaffected siblings as controls is well established as a method to circumvent possible population stratification bias.17 Our use of unaffected siblings as controls for this study of exposures to coffee (as well as caffeine-related genes) is also justified by published family-based and discordant twin-based studies of exposures to smoking and PD,31,32 and more recently of coffee and PD.33 Those family-based studies yielded results similar to the results of population-based case–control studies. However, consistent with our study, a Swedish twins study and some population-based studies also failed to observe a significant inverse association between caffeine intake and PD susceptibility8–10 or disease-modifying effects.34 Some of these negative studies were not included in the extensive meta-analysis of coffee and PD published by Hernan et al.,7 because they were published after January 1, 2002.
Our choice of caffeine-related genes was well justified because ADORA2A encodes the receptor at which caffeine exerts its effect in the central nervous system, and CYP1A2 encodes the rate-limiting enzyme in caffeine metabolism. However, SNPs were selected according to the literature accessible before 1999 and prior to the availability of the International Haplotype Map (www.hapmap.org). Our LD maps demonstrated adequate linkage disequilibrium mapping of the ADORA2A locus (except perhaps at the 3′-end), but limited mapping of the CYP1A2 locus. Therefore, our negative findings for the SNPs that we genotyped need to be interpreted with caution. It is reassuring that one whole-genome association study of PD that included three SNPs for CYP1A2 and three SNPs for ADORA2A,30 and another whole-genome association study of PD that included one SNP for ADORA2A,35 also revealed no associations with PD. In addition, Tan et al.15 failed to report any main effects of ADORA2A 2592C>Tins (now coded as rs3032740) on PD susceptibility or any joint effects with coffee (consistent with our study).
Despite negative results, our study has a number of strengths. Our sample size (cases and matched controls) was large and of sufficient size to detect main and joint effects. Our controls were preferentially unaffected siblings, thus limiting possible confounding due to population stratification.36 We extended the study of the joint effects of coffee and caffeine-related genes to include CYP1A2, which encodes the major rate limit- ing step of caffeine metabolism, in addition to ADORA2A (which encodes the caffeine receptor in the brain). We were also able to compare findings from this study with the findings from two whole-genome association studies of PD, and we were able to use overlapping data from our study and from one of the whole-genome association studies30 to construct detailed linkage disequilibrium maps.
Our study also has limitations. Our sample was not population-based. However, cases were recruited prospectively and resided in a geographically defined region. We have previously shown that the demographic and clinical characteristics were similar for PD patients residing in Olmsted County, MN, (a defined population) or within a 120 mile radius of the County (about one half of the cases). However, the cases residing in the broader five-state region tended to be younger (possibly biasing toward increased heritability and genetic effects).37 Our controls were primarily unaffected siblings; our intention was to limit possible confounding due to population ethnic stratification. However, unaffected sibling controls can be over-matched for genetic and environmental factors, leading to false negative findings. For this reason, we performed sensitivity analyses, which showed that the ORs for the explanatory variables were similar when restricting the sample to case-unaffected sibling pairs or to case-unrelated control pairs only.
The exposure assessments were interview-based and the information collected was for the time period spanning from birth to the time of the study for cases and controls. Recall bias may have compromised the validity of the study, or PD may have changed coffee drinking habits in cases (personality changes or altered smell and taste early after disease onset). Therefore, we restricted our analyses to exposures preceding the age at onset of PD in cases or the same age in matched controls. Our study did not consider the possible effects of passive exposures to coffee during gestation. We limited our analyses to caffeinated coffee. It is possible that chemicals in coffee other than caffeine are associated with PD. Our subjects drank other beverages that contained caffeine, such as tea and sodas. However, we performed separate analyses for tea or soda drinking and found that they were not significantly associated with PD (data not shown). In addition, we did not assess possible hormonal influences that might confound studies of gene-environment interactions in PD.38,39 For this reason, we also performed analyses stratified by or adjusted for gender.
Genotyping was performed only for two SNPs each within the two selected genes, and the LD mapping of the two gene loci was not complete. Nevertheless, our findings converged with those of two published whole-genome association studies of PD that considered additional SNPs in the genes.31,35 One or more pieces of information regarding genetic or environmental factors were occasionally missing from either or both members of a case–control pair. This limited the number of pairs available for the matched pair analyses and could have limited the statistical power of the study, particularly for the case-control pairs.
In summary, our study neither supports the hypothesis that coffee protects against PD nor provides evidence for pharmacogenetic effects. Although our study had strengths, it also had weaknesses that warrant caution in the interpretation of our findings and warrant additional consideration of the hypotheses. Future studies of the association of coffee and PD might consider genomic pathways-based analyses of additional caffeine responsiveness and metabolism genes, sources of caffeine in addition to coffee, and exposures during gestation as well as later in life.40
Acknowledgments
This study was supported by the National Institutes of Health (grants NS 33978 and ES 10751).
Footnotes
Potential conflict of interest: None reported.
REFERENCES
- 1.Benedetti MD, Bower JH, Maraganore DM, et al. Smoking, alcohol, and coffee consumption preceding Parkinson's disease: a case-control study. Neurology. 2000;55:1350–1358. doi: 10.1212/wnl.55.9.1350. [DOI] [PubMed] [Google Scholar]
- 2.Ross GW, Abbott RD, Petrovitch H, et al. Association of coffee and caffeine intake with the risk of Parkinson disease. JAMA. 2000;283:2674–2679. doi: 10.1001/jama.283.20.2674. [DOI] [PubMed] [Google Scholar]
- 3.Ascherio A, Zhang SM, Hernán MA, et al. Prospective study of caffeine consumption and risk of Parkinson's disease in men and women. Ann Neurol. 2001;50:56–63. doi: 10.1002/ana.1052. [DOI] [PubMed] [Google Scholar]
- 4.Hu G, Bidel S, Jousilahti P, Antikainen R, Tuomilehto J. Coffee and tea consumption and the risk of Parkinson's disease. Mov Disord. 2007;22:2242–2248. doi: 10.1002/mds.21706. [DOI] [PubMed] [Google Scholar]
- 5.Powers KM, Kay DM, Factor SA, et al. Combined effects of smoking, coffee, and NSAIDs on Parkinson's disease risk. Mov Disord. 2008;23:88–95. doi: 10.1002/mds.21782. [DOI] [PubMed] [Google Scholar]
- 6.Sääksjärvi K, Knekt P, Rissanen H, Laaksonen MA, Reunanen A, Männistö S. Prospective study of coffee consumption and risk of Parkinson's disease. Eur J Clin Nutr. 2008;62:908–915. doi: 10.1038/sj.ejcn.1602788. [DOI] [PubMed] [Google Scholar]
- 7.Hernan MA, Takkouche B, Caamaño-Isorna F, Gestal-Otero JJ. A meta-analysis of coffee drinking, cigarette smoking, and the risk of Parkinson's disease. Ann Neurol. 2002;52:276–284. doi: 10.1002/ana.10277. [DOI] [PubMed] [Google Scholar]
- 8.Preux PM, Condet A, Angelade C, et al. Parkinson's disease and environmental factors. Matched case-control study in the Limousin region, France. Neuroepidemiology. 2000;19:333–337. doi: 10.1159/000026273. [DOI] [PubMed] [Google Scholar]
- 9.Checkoway H, Powers K, Smith-Weller T, Franklin GM, Long-streth WT, Jr, Swanson PD. Parkinson's disease risks associated with cigarette smoking, alcohol consumption, and caffeine intake. Am J Epidemiol. 2002;155:732–738. doi: 10.1093/aje/155.8.732. [DOI] [PubMed] [Google Scholar]
- 10.Wirdefeldt K, Gatz M, Pawitan Y, Pedersen NL. Risk and prospective factors for Parkinson's disease: a study in Swedish twins. Ann Neurol. 2005;57:27–33. doi: 10.1002/ana.20307. [DOI] [PubMed] [Google Scholar]
- 11.Kartzinel R, Shoulson I, Calne DB. Studies with bromocriptine. III. Concomitant administration of caffeine to patients with idiopathic parkinsonism. Neurology. 1976;26:741–743. doi: 10.1212/wnl.26.8.741. [DOI] [PubMed] [Google Scholar]
- 12.Stromberg U, Waldeck B. Behavioural and biochemical interaction between caffeine and L-dopa. J Pharm Pharmacol. 1973;25:302–308. doi: 10.1111/j.2042-7158.1973.tb10012.x. [DOI] [PubMed] [Google Scholar]
- 13.Menza MA, Golbe LI, Cody RA, Forman NE. Dopamine-related personality traits in Parkinson's disease. Neurology. 1993;43:505–508. doi: 10.1212/wnl.43.3_part_1.505. [DOI] [PubMed] [Google Scholar]
- 14.Tomer R, Aharon-Peretz J. Novelty seeking and harm avoidance in Parkinson's disease: effects of asymmetric dopamine deficiency. J Neurol Neurosurg Psychiatry. 2004;75:972–975. doi: 10.1136/jnnp.2003.024885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tan EK, Lu ZY, Fook-Chong SMC, et al. Exploring an interaction of adenosine A2A receptor variability with coffee and tea intake in Parkinson's disease. Am J Med Gen Part B. 2006;141:634–636. doi: 10.1002/ajmg.b.30359. [DOI] [PubMed] [Google Scholar]
- 16.Lin JH. Pharmacokinetic and pharmacodynamic variability: a daunting challenge in drug therapy. Curr Drug Metab. 2007;8:109–136. doi: 10.2174/138920007779816002. [DOI] [PubMed] [Google Scholar]
- 17.Maraganore DM. Blood is thicker than water. The strength of family-based case-control studies. Neurology. 2005;64:408–409. doi: 10.1212/01.WNL.0000152585.76852.9C. [DOI] [PubMed] [Google Scholar]
- 18.Rocca WA, Maraganore DM, McDonnell SK, Schaid DJ. Validation of a telephone questionnaire for Parkinson's disease. J Clin Epidemiol. 1998;51:517–523. doi: 10.1016/s0895-4356(98)00017-1. [DOI] [PubMed] [Google Scholar]
- 19.Hartge P, Brinton LA, Rosenthal JF, Cahill JI, Hoover RN, Waksberg J. Random digit dialing in selecting a population-based control group. Am J Epidemiol. 1984;120:825–833. doi: 10.1093/oxfordjournals.aje.a113955. [DOI] [PubMed] [Google Scholar]
- 20.Potthoff RF. Telephone sampling in epidemiologic research: to reap the benefits, avoid the pitfalls. Am J Epidemiol. 1994;139:967–978. doi: 10.1093/oxfordjournals.aje.a116946. [DOI] [PubMed] [Google Scholar]
- 21.Svenningsson P, Le Moine C, Fisone G, Fredholm BB. Distribution, biochemistry and function of striatal adenosine A2A receptors. Prog Neurobiol. 1999;59:355–396. doi: 10.1016/s0301-0082(99)00011-8. [DOI] [PubMed] [Google Scholar]
- 22.Butler MA, Iwasaki M, Guengerich FP, Kadlubar FF. Human cytochrome P-450PA (P-450IA2), the phenacetin O-deethylase, is primarily responsible for the hepatic 3-demethylation of caffeine and N-oxidation of carcinogenic arylamines. Proc Natl Acad Sci USA. 1989;86:7696–7700. doi: 10.1073/pnas.86.20.7696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sohni YR, Cerhan JR, O'Kane D. Microarray and microfluidic methodology for genotyping cytokine gene polymorphisms. Hum Immunol. 2003;64:990–997. doi: 10.1016/s0198-8859(03)00174-5. [DOI] [PubMed] [Google Scholar]
- 24.Chida M, Yokoi T, Fukui T, Kinoshita M, Yokota J, Kamataki T. Detection of three genetic polymorphisms in the 5′-flanking region and intron 1 of human CYP1A2 in the Japanese population. Jpn J Cancer Res. 1999;90:899–902. doi: 10.1111/j.1349-7006.1999.tb00832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sachse C, Brockmoller J, Bauer S, Roots I. Functional significance of a C–>A polymorphism in intron 1 of the cytochrome P450 CYP1A2 gene tested with caffeine. Br J Clin Pharmacol. 1999;47:445–449. doi: 10.1046/j.1365-2125.1999.00898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deckert J, Nothen MM, Franke P, et al. Systematic mutation screening and association study of the A1 and A2a adenosine receptor genes in panic disorder suggest a contribution of the A2a gene to the development of disease. Mol Psychiatry. 1998;3:81–85. doi: 10.1038/sj.mp.4000345. [DOI] [PubMed] [Google Scholar]
- 27.Alsene K, Deckert J, Sand P, de Wit H. Association between A2a receptor gene polymorphisms and caffeine-induced anxiety. Neuropsychopharmacology. 2003;28:1694–1702. doi: 10.1038/sj.npp.1300232. [DOI] [PubMed] [Google Scholar]
- 28.Sachse C, Bhambra U, Smith G, et al. Polymorphisms in the cytochrome P450 CYP1A2 gene (CYP1A2) in colorectal cancer patients and controls: allele frequencies, linkage disequilibrium and influence on caffeine metabolism. J Clin Pharmacol. 2003;55:68–76. doi: 10.1046/j.1365-2125.2003.01733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maniatis N, Collins A, Xu CF, et al. The first linkage disequilibrium (LD) maps: delineation of hot and cold blocks by diplo-type analysis. Proc Natl Acad Sci USA. 2002;99:2228–2233. doi: 10.1073/pnas.042680999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maraganore DM, de Andrade M, Lesnick TG, et al. High-resolution whole-genome association study of Parkinson disease. Am J Hum Genet. 2005;77:685–693. doi: 10.1086/496902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scott WK, Zhang F, Stajich JM, Scott BL, Stacy MA, Vance JM. Family-based case-control study of cigarette smoking and Parkinson disease. Neurology. 2005;64:442–447. doi: 10.1212/01.WNL.0000150905.93241.B2. [DOI] [PubMed] [Google Scholar]
- 32.Tanner CM, Goldman SM, Aston DA, et al. Smoking and Parkinson's disease in twins. Neurology. 2002;58:581–588. doi: 10.1212/wnl.58.4.581. [DOI] [PubMed] [Google Scholar]
- 33.Hancock DB, Martin ER, Stajich JM, et al. Smoking, caffeine, and nonsteroidal anti-inflammatory drugs in families with Parkinson disease. Arch Neurol. 2007;64:576–580. doi: 10.1001/archneur.64.4.576. [DOI] [PubMed] [Google Scholar]
- 34.Kandinov B, Giladi N, Korczyn AD. The effect of cigarette smoking, tea, and coffee consuption on the progression of Parkinson's disease. Parkinsonism Relat Disord. 2007;13:243–245. doi: 10.1016/j.parkreldis.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 35.Fung HC, Scholz S, Matarin M, et al. Genome-wide genotyping in Parkinson's disease and neurologically normal controls: first stage analysis and public release of data. Lancet Neurol. 2006;5:911–916. doi: 10.1016/S1474-4422(06)70578-6. [DOI] [PubMed] [Google Scholar]
- 36.Khoury MJ, Beaty TH, Cohen BH. Fundamentals of genetic epidemiology. Oxford University Press; New York: 1993. [Google Scholar]
- 37.Rocca WA, Peterson BJ, McDonnell SK, et al. The Mayo Clinic family study of Parkinson's disease: study design, instruments, and sample characteristics. Neuroepidemiology. 2005;24:151–167. doi: 10.1159/000083612. [DOI] [PubMed] [Google Scholar]
- 38.Marchetti B, Serra PA, L'Episcopo F, et al. Hormones are key actors in gene x environment interactions programming the vulnerability to Parkinson's disease: glia as a common final pathway. Ann N Y Acad Sci. 2005;1057:296–318. doi: 10.1196/annals.1356.023. [DOI] [PubMed] [Google Scholar]
- 39.Rocca WA, Bower JH, Maraganore DM, et al. Increased risk of parkinsonism in women who underwent oophorectomy before menopause. Neurology. 2008;70:200–209. doi: 10.1212/01.wnl.0000280573.30975.6a. [DOI] [PubMed] [Google Scholar]
- 40.Lesnick TG, Papapetropoulos S, Mash DC, et al. A genomic pathway approach to a complex disease: axon guidance and Parkinson disease. PLoS Genetics. 2007;3:e98. doi: 10.1371/journal.pgen.0030098. [DOI] [PMC free article] [PubMed] [Google Scholar]