Abstract
Secreted ferritin is the major iron storage and transport protein in insects. Here we characterize the message and protein expression profiles of yellow fever mosquito (Aedes aegypti) ferritin heavy chain homologue (HCH) and light chain homologue (LCH) subunits in response to iron and bacterial challenge. In vivo experiments demonstrated tissue specific regulation of HCH and LCH expression over time post-blood meal (PBM). Transcriptional regulation of HCH and LCH was treatment specific, with differences in regulation for naïve versus mosquitoes challenged with heat-killed bacteria (HKB). Translational regulation by iron regulatory protein (IRP) binding activity for the iron responsive element (IRE) was tissue specific and time-dependent PBM. However, mosquitoes challenged with HKB showed little change in IRP/IRE binding activity compared to naïve animals. The changes in ferritin regulation and expression in vivo were confirmed with in vitro studies. We challenged mosquitoes with HKB followed by a blood meal to determine the effects on ferritin expression, and demonstrate a synergistic, time-dependent, regulation of expression for HCH and LCH.
Keywords: Aedes aegypti, bacteria, ferritin, iron, IRP, mosquito
Introduction
Female mosquitoes must blood feed for oogenesis, and as a consequence, they are exposed to a high iron load, and potentially, to blood-borne pathogens. Mosquitoes can be infected by bacteria, fungi, viruses and parasites. The yellow fever vector, Aedes aegypti, also transmits dengue. WHO currently estimates 40% of the world's population are now at risk from dengue, and there are approximately 50 million dengue infections each year (WHO, 2009a). While approximately 900 million people are at risk for yellow fever in Africa and Latin America; 200 000 people contract the disease worldwide annually (WHO, 2009b).
Mosquito immune response to pathogens induces ovarian follicular epithelial cell resorption during oocyte development and decreases egg production in the African malaria mosquito, Anopheles gambiae (Jiang et al., 2009). Reduced access to iron also decreases egg numbers (Kogan, 1990). Proteins involved in the processing of dietary iron in A. aegypti may play a significant role in the mosquito immune response and ovarian follicular cell absorption supporting the importance of further research into iron metabolism in this vector (Fallon & Sun, 2001; Hua et al., 2007; Jiang et al., 2009; Magalhaes et al., 2008; Yu et al., 2007).
Since iron is an essential nutrient for many organisms, including pathogens, a novel method of sequestering iron during infection is needed to ensure the survival of the organism and to compromise the viability of the invading pathogen (Ganz, 2009; Ong et al., 2006; Wang & Cherayil, 2009; Weinberg & Miklossy, 2008). One mechanism is the iron-withholding strategy of the innate immune response (Ganz, 2009; Ong et al., 2006; Wang & Cherayil, 2009; Weinberg & Miklossy, 2008). In insects, hemolymph ferritin is found in mg/L concentration (Ong et al., 2006; Yoshiga et al., 1997). Current evidence supports that secreted ferritin serves as the primary iron transport protein in insects (Geiser et al., 2009; Nichol et al., 2002; Zhou et al., 2007). Zhou et al. (2007) found radiolabeled iron fed to A. aegypti in a blood meal is absorbed, and the iron transported to tissues, particularly ovaries and eggs, by secreted ferritin. Insect ferritin consists of 24 heavy and light chain subunits, homologues of the vertebrate heavy and light chains (HCH and LCH, respectively), configured as a sphere that can hold up to 4500 atoms of iron per molecule (Hamburger et al., 2005; Kalgaonkar & Lonnerdal, 2009). This property makes it a very efficient transport protein for acquiring iron from the diet when available and needed for rapid egg production. Since ferritin serves this role in insects, we hypothesized that it would be responsive to infections as an iron-withholding protein.
Insects are able to defend themselves against a variety of pathogens through the rapid induction of their innate immune response by humoral and cell-mediated mechanisms (Bartholomay et al., 2004; Dong et al., 2006; Fallon & Sun, 2001; Hoffmann, 1997; Hughes, 1998; Lowenberger, 2001; Masova et al., 2010). This response involves induced expression and secretion of antibacterial and antifungal molecules (i.e. defensin, cecropin, transferrin (Tsf), and lysozyme (Bartholomay et al., 2004; Bartholomay et al., 2007; Castillo et al., 2006; Dimopouloset al., 2000; Hillyer et al., 2003a; Hillyer et al., 2003b; Magalhaes et al., 2008). These factors are a critical component of the humoral response of insects to fight infection. It takes as little as three hours to up-regulate the abundant expression of defensin in insects following bacterial challenge; the expression of defensin can plateau 12 to 36 hours post infection and remain elevated for 21 days (Cho et al., 1996; Fallon & Sun, 2001; Gao et al., 1999; Hoffmann, 1997; Lowenberger, 2001; Masova et al., 2010). Defensins are expressed in insect fat body and secreted into the hemolymph (Blandin et al., 2002; Cho et al., 1997; Eggleston et al., 2000; Masova et al., 2010). They are also expressed in the gut of mosquitoes as well as the salivary glands (Blandin et al., 2002; Dixit et al., 2008; Dong et al., 2006; Hoffmann, 1997; Luplertlop et al., 2011). Expression of defensin can occur at any developmental stage in mosquitoes (Eggleston et al., 2000; Lowenberger, 2001; Lowenberger et al., 1999).
Many insect antimicrobial peptide gene promoter regions have several κB-like motifs including that for A. aegypti and A. gambiae defensin (Antonova et al., 2009; Eggleston et al., 2000; Meredith et al., 2006; Xi et al., 2008). Cis-regulatory elements that bind NF-κB factors also are found in the promoter regions of the mosquito ferritin heavy and light chains (Dunkov & Georgieva, 1999; Ong et al., 2006; Pham et al., 2003; Pham & Chavez, 2005; Recalcati et al., 2008). We hypothesized that an increase in transcription of the insect ferritin HCH and LCH subunits could occur as part of the immune response.
In mammals, translation of both the ferritin light and heavy chain subunits is activated by cytokines and synthesis is increased via disassociation of trans-acting RNA poly(C)-binding proteins from the acute box, a cis-acting translational enhancer, downstream of the iron responsive element (IRE) in the 5’UTR of the message for either of the subunits (Rogers, 1996; Thomson et al., 2005; Thomson et al., 1999). The IRE allows translational control of subunit synthesis by iron through the action of the iron regulatory proteins (IRPs). Under intracellular low iron conditions, IRP binding to the IRE effectively blocks ferritin subunit translation. IRP/IRE binding activity is decreased during an immune response allowing increased ferritin subunit synthesis. Translational control by cytokines is not independent from iron-regulated translation of the subunit mRNA transcripts through the IRP/IRE interaction (Rogers, 1996; Thomson et al., 2005; Thomson et al., 1999).
In insects, one or both ferritin subunits have a 5’UTR IRE in the mRNA sequence which can respond to intracellular iron availability by either binding insect IRP under low intracellular iron to inhibit ferritin subunit translation or disassociating from insect IRP with high intracellular iron to increase ferritin subunit translation (Birney et al., 2004; Du et al., 2000; Geiser et al., 2006; Hajdusek et al., 2009; Nichol & Locke, 1999; Zhang et al., 2001; Zhang et al., 2002). The effect of the immune response on IRP/IRE binding activity in insects has only been demonstrated in A. gambiae where exposure to LPS increased binding activity in Sua1B cells. Of the two ferritin subunits characterized from A. aegypti, HCH has a 5’UTR IRE (Fiuza et al., 1996; Geiseret al., 2003) and since insect IRP/IRE binding activity in response to immune challenge has been examined in a singular insect (Zhang et al., 2002), translational regulation of ferritin by IRP during an immune response is difficult to predict, warranting further analysis.
Much is known about the immune response in mosquitoes (Bartholomay et al., 2004; Bartholomay et al., 2010; Dimopoulos et al., 2001; Fallon & Sun, 2001; Hillyer et al., 2003a; Lowenberger, 2001; Michel & Kafatos, 2005; Osta et al., 2004; Schnitger et al., 2007; Vernick et al., 2005; Xi et al., 2008) and the effect of the response on fecundity (Hua et al., 2007; Jiang et al., 2009; Yu et al., 2007). However, the regulation of ferritin and IRP in infection and following a blood meal is less clear. We report challenging mosquitoes with heat-killed bacteria (HKB) followed by a blood meal to determine the effects of these treatments in tandem on ferritin expression, and we demonstrate a synergistic, time-dependent, up-regulation of expression for HCH and LCH, as well as decreased IRP/IRE binding activity with iron and HKB challenge.
Materials and methods
Mosquito Rearing
Aedes aegypti (UGAL/Rockefeller strain) adult mosquitoes were routinely maintained at 27°C, 70%–80% relative humidity with a photoperiod of 16:8 h (L:D) on 10% sucrose solution ad libitum. As previously described (Zhou et al., 2009), several hundred females four days old were injected with 69 nL of autoclaved gram-positive Bacillus subtilis suspension (6.9 × 105 bacteria/animal; heat-killed bacteria (HKB)) in mosquito saline (150 mmol/L NaCl, 4 mmol/L KCl, 3 mmol/L CaCl2, 1.8 mmol/L NaHCO3, 0.6 mmol/L MgCl2, 25 mmol/L HEPES, pH 7.0) or 69 nL of mosquito saline as a naïve control. The injected mosquitoes were allowed to recover for 3 h and then fed on porcine blood administered by membrane feeding. Three independent samples of 15 female mosquitoes for each group were drawn at random from each treatment population at each time interval of 0, 24 and 72 h PBM; for each sample, fat body (FB), midgut (MG) and ovary (OV) tissues were dissected into and pooled in RLT Buffer (Qiagen) containing β-ME for total RNA extraction or disruption buffer (10 mmol/L Tris-HCl, pH 8.0, 10 mmol/L KCl, 10 mmol/L NaCl, freshly added 2× Protease Inhibitor Cocktail Set I (Calbiochem) and 0.5 mM dithiothreitol) for protein analysis and frozen in liquid nitrogen, stored at −80°C.
Cell culture
Aedes aegypti (L.) epithelial-like, larval cells (CCL-125) were obtained from the American Type Culture Collection (ATCC). The cells were maintained as previously described (Geiser et al., 2007). Briefly, at the start of each experiment the complete medium (75% DMEM high glucose (Invitrogen), 25% Sf-900 II SFM (Invitrogen),15% heat-inactivated fetal bovine serum (Gemini Bio-Products) and 1% of 100× the antibiotic-antimycotic formula provided in the Invitrogen kit (Invitrogen)) was removed and the cells were washed twice with Hank's Balanced Salt Solution (HBSS; Invitrogen). Serum-free, antibiotic-antimycotic-free medium was placed on the cells and the cells were incubated for 1 h (28°C with 5% CO2). Following this incubation the medium was replaced with fresh serum-free, antibiotics/antimycotics-free medium. An autoclaved bacterial mixture of B. subtilis and gram-negative E. coli (100 bacteria/cell) in HBSS was prepared (HKB). Experiments were conducted on thirty-six flasks of passage 85 subcultured CCL-125 cells. Flasks of cells taken at random were used for each treatment in an experiment and three experiments were conducted. Immune challenged cells were treated with HKB and HBSS (H), 100 μmol/L ferric ammonium citrate (F or FAC, Sigma, 18.3% iron, 1 μg Fe/μg FAC) dissolved in HBSS or 100 μmol/L FAC with 100 μM deferoxamine mesylate salt (F/D or FAC/DES, Sigma) prepared in HBSS. Naïve cells were treated with HBSS, FAC or FAC/DES and additional HBSS, the vehicle for the HKB, was added to make the treatment volume the same for all flasks. Cells were incubated for 4 or 22 h at 28°C in vented 75 cm2 tissue culture flasks (Corning Incorporated). Since all cells at the time of harvest did not adhere, the medium was removed from the flask of cells and transferred to a 15 mL conical tube and centrifuged at 900 g for 10 min, 4°C. The supernatant was removed, flash frozen in liquid nitrogen and stored at −80°C for subsequent use. The remaining cells in the flasks were scraped into 5 mL HBSS, added to the cell pellet from the medium and suspended. The cell suspension was centrifuged at 900 g for 10 min, 4°C. The supernatant was removed and the cells were suspended in 5 mL fresh HBSS. One ml (~1.5 × 106 cells) of the cell suspension was taken from each sample and microcentrifuged at 9000 g for 2 min, 4°C. The supernatant was removed and the cell pellet was frozen in liquid nitrogen and stored at −80°C for RNA isolation. Aliquots of cells were immediately taken to perform cell viability; total cell number and calcein quench assays. The remaining cells were centrifuged at 900 g for 10 min, 4°C. The supernatant was removed and the cell pellet was suspended in 250 μL hypotonic buffer (10 mmol/L HEPES, pH 7.9, 1.5 mmol/L MgCl2, 10 mmol/L KCl, freshly added 2× Protease Inhibitor Cocktail Set I (Calbiochem) and 0.5 mmol/L dithiothreitol), transferred to a 1.5 mL microcentrifuge tube and frozen in liquid nitrogen for cell lysis.
Cell viability and cell number
Cell viability was determined in triplicate for all flasks by CellTiter 96® Aqueous One Solution Cell Proliferation Assay per manufacturer's instructions (Promega Corporation). This assay measures the mitochondrial activity produced by metabolically active cells. Total cell number was measured in triplicate for all flasks by the LIVE/DEAD® Viability/Cytotoxicity Assay per manufacturer's instructions (Molecular Probes).
Iron determination
Calcein fluorescent quench by iron was used to measure iron uptake into cells as previously described (Geiser et al., 2007). Fluorescent quench was measured in triplicate for all flasks by the LIVE/DEAD® Viability/Cytotoxicity Assay per manufacturer's instructions (Molecular Probes) by adding calcein to a final concentration of 1 μmol/L and incubating for 45 min, 28°C.
Real-Time RT-PCR
Total RNA was isolated from ~1.5× 106 CCL-125 cells and mosquito tissues using the RNeasy® Mini Kit (Qiagen Inc.). Equal volume of each purified total RNA sample was treated with DNase (Invitrogen) according to the manufacturer's instructions for 30 min at 37°C and for 10 min at 65°C. Equal volume of each DNase-treated total RNA sample was used for real-time RT-PCR. Reverse transcription was done according to the manufacturer's instructions using M-MuLV Reverse Transcriptase from the First Strand cDNA Synthesis Kit (Fermentas, Inc.). The primers for the PCR reactions were designed to obtain specific PCR products of similar size for the ORF of each message: HCH (198 bp): 5′-ccaggcccaggaacaaacag-3′ and 3′-tcaaaaagaaggtgcggcgg-5′; LCH (173 bp): 5′-ttcaccgcccagttttcctca-3′ and 3′-ctagaggctgttccggac-5′; Defensin A1 (DefA1; 211 bp): 5′-tgtcatttgtttcctggctctg-3′ and 3′-gcaaccactatcacgaacacga-5′; IRP (115 bp): 5′-aatcgaaggacagcgtgaag-3′ and 3′-ccacagccaccataacttcg-5′; S7 (184 bp): 5′-ggagatgaactcggacctga-3′ and 3′-caagaggccgttcgtgca-5′. Real-time RT-PCR reactions were conducted using iQ™ SYBR® Green Supermix (BIORAD) with the buffers provided, at: 94°C, 3 min, 1 cycle; 94°C, 10 sec; 60°C, 30 sec and 72°C, 30 sec, 40 cycles; with a melt curve over a temperature range starting at 55°C and ending at 95°C in a MyiQ™ Cycler (BIORAD). PCR product quality was monitored using post-PCR melt curve analysis. A standard curve for each product, performed simultaneously with experimental samples, demonstrated the experimental samples fell within the linear range. Data analysis of each sample run twice (duplicates) for fold change was quantified using the Pfaffl (2001) method to calculate for relative quantification. The data are reported as fold change relative to a baseline of 1 established from the expression of these messages in HBSS-treated naïve cells incubated for either 4 or 22 h during the in vitro HKB treatment experiment or naïve females 0 h PBM during the in vivo HKB challenge experiment. All PCR products were cloned and sequenced to determine that the product sequence represents that of the desired message.
Protein extracts
Animal tissue samples suspended in ~150 μL disruption buffer were frozen at −80°C, thawed on ice and homogenized, three times. The tissue samples were then centrifuged at 100000× g for 30 min, 4°C; after spinning the supernatant was transferred to a new tube labeled cytoplasmic extract and the pellet in the original tube was suspended in 100 μL disruption buffer and labeled membrane extract. Cell media samples were concentrated five-fold by centrifuging samples in Centricon® Ultracel YM-30 centrifugal filter devices (Millipore) at 5000 g for ~20 min, 4°C. Cell extracts were prepared as previously described with some modification (Geiser et al., 2009). Briefly, cell pellets suspended in hypotonic buffer were frozen at −80°C, thawed on ice and homogenized, three times. Animal tissue, and cell extracts and medium protein concentrations were determined by the method of Bradford (1976). Membrane and cytoplasmic extracts from OV were recombined after determining the protein content of the membranes was low. The membrane extracts from FB and MG were further processed by Triton extraction to release proteins from the membranes (Patton et al., 2005). Briefly, Triton X-100 (Sigma) was added to the membrane samples to a concentration of 0.5% (v/v) in disruption buffer with 2× Protease Inhibitor Cocktail Set I (Calbiochem), incubated for 1 h at 4°C and centrifuged at 20000× g for 5 min, 4°C. Cytoplasmic and membrane extracts from MG were treated with methyl ethyl ketone (MEK) to remove the remaining heme from the blood meal that could potentially interfere with protein detection in the samples. Briefly, MEK was added to samples 3:1 (v/v), vortexed, allowed to incubate for 10 min at RT, centrifuged at 20000× g for 5 min at RT and the aqueous, lower phase was collected. The concentrated media samples, cell and animal tissue extracts were frozen in small aliquots and held at −80°C until use.
Western blots
Proteins were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) 17% homogeneous slab gels, run at 60 volts overnight (>1000 Vh), 4°C. Cell extracts were loaded at 15 g total protein and concentrated media samples at 6 g total protein. Animal tissue equivalents were loaded as follows: OV extracts (2 animals), FB cytoplasmic extracts (4 animals for 0 and 72 h; 1 animal for 24 h) , FB membrane extracts (4 animals), MG cytoplasmic extracts (2 animals) and MG membrane extracts (1 animal for 24 h, 2 animals for 0 and 72 h). Ferritin and IRP bands were normalized to actin as an internal control for cell extracts and FB cytoplasmic and membrane extracts. However, since no internal control protein is available for use for cell culture medium, and OV and MG actin was not detectable, purified FLAG with bacterial alkaline phosphatase (25 ng media samples; 75 ng OV and MG samples; Sigma) was added as an exogenous loading control. Proteins were transferred to nitrocellulose membranes in the Electrophoretic Blotting System (C.B.S. Scientific Company). Efficient transfer of proteins was confirmed by SYPRO® Ruby protein blot stain (BIORAD) and Kaleidoscope molecular weight markers (BIORAD). The nitrocellulose membranes were cut for analysis of the individual proteins. The ferritin nitrocellulose membranes were blocked overnight in 4.0% non-fat dry milk, 25 mmol/L phosphate, 25 mmol/L acetate buffer, 0.02% sodium azide pH 7.0 at 4°C. After blocking, the nitrocellulose membranes were incubated with anti-A. aegypti ferritin-specific rabbit serum (1:2000 v/v, a kind gift from Dr. John Law) diluted in blocking buffer for 2 h, RT. The nitrocellulose membranes were washed three times in 25 mmol/L phosphate, 25 mmol/L acetate, 0.02% sodium azide, pH 7.0 and developed with anti-rabbit alkaline phosphatase conjugate antibody (1:1000 v/v; Jackson Immuno) according to the manufacturer's methods (BIORAD). The IRP, FLAG and actin membranes were blocked overnight in 10 mmol/L phosphate-buffered saline and 0.05% Triton X-100 (PTX) with 3% BSA and 7% non-fat dry milk at 10°C. Membranes were incubated with either anti-KLH-conjugated-IRP-epitope-specific rabbit serum (1:2000 v/v; CKNQDLEFERNKERF; Sigma Genosys), anti-FLAG-specific rabbit serum (1:4000 v/v; Sigma) or anti-β-actin-specific mouse serum (1:2000 v/v; Abcam) diluted in PTX with 3% BSA for 2 h, RT. The membranes were washed three times and developed with either anti-rabbit alkaline phosphatase conjugate antibody (1:1000 v/v; Jackson Immuno) or anti-mouse alkaline phosphatase conjugate antibody (1:1000 v/v; Jackson Immuno). Digital images were accessed using the VersaDoc™ 3000 Imaging System (BIORAD) and quantified with Quantity One 4.5.1 software (BIORAD). Data were analyzed by densitometry of a defined volume of all the ferritin subunits, IRP, FLAG and actin proteins and corrected for background. Ferritin or IRP concentrations were evaluated by taking their volumes in ratio to an exogenous (FLAG) or endogenous (actin) loading control. FB, OV and MG samples volumes were corrected for the number of animal tissue equivalents. Volume units are arbitrarily assigned.
Electrophoretic mobility shift assays (EMSA)
EMSA were performed as described by Schalinske and Eisenstein (1996) with modifications for mosquito samples (Geiser et al., 2007; Geiser et al., 2006; Zhang et al., 2002). IRP1/IRE interactions were measured by incubating a molar excess of radio-labeled probe with cell or tissue extract. Briefly, 2.5 μg of cell extract or animal tissue equivalents (1 animal for OV extracts, 1 animal for FB cytoplasmic extract, 1 animal for FB membrane extracts, 1 animal for MG cytoplasmic and membrane extracts combined) and 50 fmol of Aedes ferritin heavy chain IRE [32P] labeled-transcript were incubated in 37.5 mmol/L KCl-Hepes, 12.5 mmol/L DTT, 1.9 mmol/L MgCl2, 6.3% glycerol and 0.6 U RNase Inhibitor (Invitrogen) for 20 min at RT. RNase T1 (6 Units) was added and the mixture incubated for 10 min at RT. Finally, heparin (100 μg) was added and the sample incubated for 10 min at RT. All samples were run on 6.5% polyacrylamide gels, and IRP/IRE binding activity was assessed by autoradiography exposure to Blue XB-1 film (Eastman Kodak Company). Digital images were accessed using a VersaDoc™ 3000 Imaging System (BIORAD) and quantified with Quantity One 4.5.1 software (BIORAD). Data were analyzed by peak density of defined bands and corrected for background. Density units are arbitrarily assigned.
Data analysis
Protein extracts of samples were analyzed by Western blot or EMSA and combined data for a given time and treatment was reported as the mean and standard deviation (StD). For real time PCR, RNA extracts were analyzed twice, and for a given time and treatment both results were included in calculations of the mean and StD. Significance of time differences (0, 24, 72 h PBM) in animals or iron treatments (H, F, F/D) in cells was determined by one-way analysis of variance (ANOVA) using the Tukey's multiple comparisons test. Treatment differences (Naïve, HKB Challenge) in means for a given time point or iron treatment were determined by a one-tailed unpaired t-test for comparison of selected data sets (Graph Pad Software, Inc.). All cell culture experiments were conducted in triplicate and the data for a given variable were analyzed at the same time.
Results
Heat-killed bacteria induce an immune response in CCL-125 cells and mosquito tissues
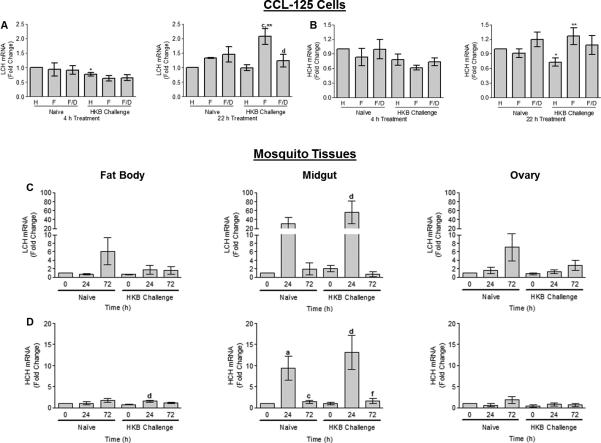
Challenge with heat-killed bacteria (HKB) provoked an immune response in CCL-125 cells and female yellow fever mosquitoes as demonstrated by an increase in message levels of the antimicrobial peptide defensin A1 (DefA1) in cells and tissues. HKB challenge up-regulated DefA1 message of CCL-125 cells at 4 h (P < 0.008) and 22 h (P <0.03) and confirmed the presence of an immune response (Fig. 1A). Iron-treatment with 100 μmol/L ferric ammonium citrate (FAC) alone also increased DefA1 at 22 h (P < 0.05). DefA1 in mosquitoes increased in fat body (FB) with time post-blood meal (PBM) by 24 h in both naïve and HKB challenged animals. The response was significantly greater in HKB challenge relative to that of naïve animals at 24 h PBM (P <0.005; Fig. 1B). Mosquitoes challenged with HKB also showed a significant increase in DefA1 message at 24 h relative to that of naïve mosquitoes for both midgut (MG; P <0.04) and ovary (OV; P =0.067; Fig. 1B). DefA1 increased by 24 h in all three tissues, but remained elevated at 72 h only in FB. Thus, HKB challenge in cells and mosquitoes induces an immune response as measured by an increase in DefA1 mRNA where FB is the primary site of transcript expression.
Fig. 1.
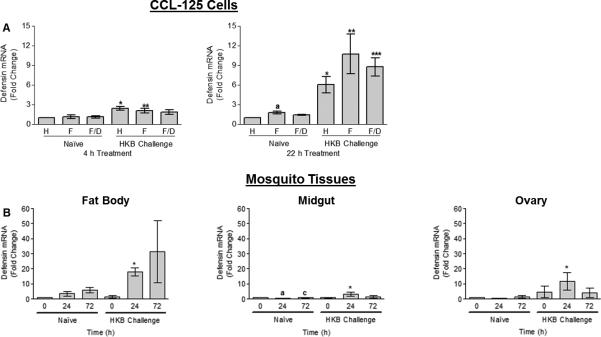
Quantification of defensin A1 (DefA1) transcript expression by real-time RT-PCR in CCL-125 cells (A) and female yellow fever mosquito, Aedes aegypti, tissues (B) exposed to iron or a blood meal and heat-killed bacteria (HKB). (A) CCL-125 cells were treated with HBSS (H), 100 μmol/L FAC (F) or 100 μmol/L FAC/ 100 μmol/L DES (F/D) as either naïve or HKB challenged cells. Cells were collected at 4 and 22 h post-treatment. *SD relative to naïve HBSS-treated cells 4 h (P < 0.008) and 22 h post-treatment (P < 0.02). **SD relative to naïve FAC-treated cells 4 h (P = 0.056) and 22 h post-treatment (P < 0.03). aSD relative to naïve 22 h HBSS-treated cells (P < 0.05). ***SD relative to naïve 22 h FAC/DES-treated cells (P < 0.007). (B) Female mosquitoes were injected with either mosquito saline (naïve) or heat-killed bacteria (HKB challenge) 3 h prior to blood feeding. Fat body (FB), midgut (MG) and ovary (OV) tissues were collected at 0, 24 and 72 h post-blood meal (PBM) and processed. *Significantly different (SD) relative to 24 h PBM naïve animals in FB (P < 0.005), MG (P < 0.04) and OV (P = 0.067). aSD relative to naïve animals 0 h PBM (P < 0.01). cSD relative to naïve animals 24 h PBM (P < 0.05). Graphed data represent means ± standard deviation (StD) of three independent cell culture samples and animal samples.
HKB challenge differentially regulates ferritin transcript expression in CCL-125 cells and mosquito tissues
HKB challenge in the presence of iron significantly up-regulated LCH message at 22 h relative to levels for cells treated with either HKB or FAC alone (P <0.02), and the addition of a ferric iron chelator, 100 μmol/L deferoxamine mesylate salt (DES), reduced the up-regulation (P <0.05) indicating iron was partially responsible for this effect (Fig. 2A). HCH message also showed significant up-regulation for cultures treated with HKB and iron relative to those treated with iron alone (P =0.05; Fig. 2B). At 22 h, HCH message was only partially reduced by the addition of DES indicating that HKB and iron synergistically up-regulated this message (Fig. 2B). The observed regulation of LCH and HCH transcript expression was not a result of detrimental effects of the treatments on these cells since differences in cell numbers were less than 10% as a result of any treatment (Fig. 1SA). Although the addition of iron reduced viability by not more than 20% in both naïve and HKB challenged cells, this condition is resolved by 22 h (Fig. 1SB). Calcein quench analyses confirmed uptake of iron into the cells by 4 h and retention at 22 h in the presence or absence of HKB and showed that HKB challenge did not alter the levels of the iron pool accessible by calcein (Fig. 1SC).
Fig. 2.
Quantification of ferritin light chain (LCH) and heavy chain homologue (HCH) transcript expression by real-time RT-PCR in CCL-125 cells (A–B) and female A. aegypti tissues (C–D) exposed to iron or a blood meal and HKB. (A–B) CCL-125 cells were treated with H, F or F/D as either naïve or HKB challenged cells. Cells were collected at 4 and 22 h post-treatment and processed. (A) CCL-125 cell LCH mRNA levels. *SD relative to naïve HBSS-treated cells (P < 0.002). cSD relative to HKB HBSS-treated cells (P < 0.01). **SD relative to naïve FAC-treated cells (P < 0.02). dSD relative to HKB FAC-treated cells (P < 0.05). (B) CCL-125 cell HCH mRNA levels. *SD relative to naïve HBSS-treated cells (P < 0.009). **SD relative to naïve FAC-treated cells (P = 0.05). (C–D) Female mosquitoes were either naïve or HKB challenged 3 h prior to blood feeding. FB, MG and OV tissues were collected at 0, 24 and 72 h PBM and processed. (C) Female mosquito tissues LCH mRNA levels. dSD relative to HKB treated animals 0 h PBM (P = 0.057). (D) Female mosquito tissues HCH mRNA levels. dSD relative to HKB animals 0 h PBM in FB (P < 0.05) and MG (P < 0.05). aSD relative to naïve animals 0 h PBM (P < 0.05). cSD relative to naïve animals 24 h PBM (P < 0.05). fSD relative to HKB animals 24 h PBM (P < 0.05). Graphed data represent means ± StD of three independent cell culture samples and animal samples.
Comparison of the means for naïve and HKB challenged mosquitoes showed a synergistic increase for MG HCH and LCH message following blood feeding with HKB challenge. In MG, ferritin LCH message increased with time PBM and peaked at 24 h (Fig. 2C). Using 0 h for naïve mosquitoes as controls, HKB challenged MG LCH message doubled at 0 h, increased significantly to 56-fold by 24 h, and then declined by 72 h. The increase in LCH message appeared to be greater for HKB challenged mosquitoes versus naïve at 24h PBM; however values did not reach significance. Message levels for the ferritin HCH also increased with time PBM for naïve and infected animals; within either group the increase reached significance at 24 h (9-fold increase in naïve mosquitoes and a 12-fold increase in HKB challenged mosquitoes), then declined significantly by 72 h. A comparison of infected to naïve mosquitoes at each time interval suggested a greater positive message response occurred in infected animals, but values did not reach significance (Fig. 2D). Thus, both HCH and LCH messages show a positive response to iron exposure and to bacterial challenge. MG is the primary site of ferritin subunit transcription and message levels for either the LCH or HCH PBM were an order of magnitude greater than those for FB or OV (Fig. 2C–D). HCH and LCH messages also generally increased over time in FB and OV, and reached significance for the HCH at 24 h for FB for HKB challenged mosquitoes relative to 0 h for the same treatment group.
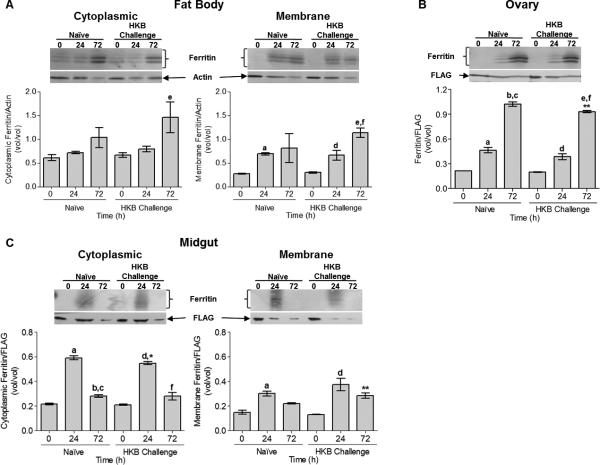
Mosquito ferritin is up-regulated in tissues PBM; HKB challenge and iron cause synergistic up-regulation of ferritin expression in CCL-125 cells
Ferritin protein levels were examined in three tissues. FB cytoplasmic ferritin increased by 72 h PBM, and was greatest for animals treated with HKB (Fig. 3A). FB membrane ferritin increased significantly 24 h PBM in naïve (P = 0.053) and HKB challenged (P < 0.05) animals and levels were sustained at 72 h in naïve animals and further increased in animals treated with HKB (Fig. 3A). Thus, despite that LCH and HCH message levels declined by 72 h in FB for animals treated with HKB, protein levels were maintained or increased in this tissue. In OV, ferritin increased with time PBM, was maximal at 72 h (P < 0.0001) and HKB challenge appeared to reduce this response at 72 h PBM (P < 0.02; Fig. 3B). Ferritin increased in MG in both cytoplasmic (P < 0.001) and membrane (P < 0.01) fractions 24 h PBM and decreased by 72 h (Fig. 3C). Levels of membrane ferritin were greater for mosquitoes treated with HKB 72 h PBM (P = 0.053) than for naïve animals indicating a synergistic up-regulation of ferritin by blood feeding and HKB treatment (Fig. 3C) for this tissue. We conclude from these data that ferritin protein expression is tissue specific and sensitive to blood feeding and HKB challenge.
Fig. 3.
Tissue expression of ferritin protein from female A. aegypti exposed to a blood meal and HKB was analyzed by Western blot. Female mosquitoes were naïve or challenged with HKB 3 h prior to blood feeding. FB, MG and OV tissues were collected at 0, 24 and 72 h PBM and processed. (A) FB cytoplasmic and membrane ferritin protein levels. eSD relative to HKB animals 0 h PBM for cytoplasmic (P = 0.052) and membrane extracts (P < 0.001). aSD relative to naïve animals 0 h PBM (P = 0.053). dSD relative to HKB animals 0 h PBM (P < 0.05). fSD relative to HKB animals 24 h PBM (P < 0.05). (B) OV tissue ferritin protein levels. aSD relative to naïve animals 0 h PBM (P < 0.01). bSD relative to naïve animals 0 h PBM (P <0.001). cSD relative to naïve animals 24 h PBM (P < 0.001). dSD relative to HKB animals 0 h PBM (P < 0.01). eSD relative to HKB animals 0 h PBM (P < 0.001). fSD relative to HKB animals 24 h PBM (P < 0.001). (C) MG cytoplasmic and membrane ferritin protein levels. aSD relative to naïve animals 0 h PBM for cytoplasmic (P < 0.001) and membrane extracts (P < 0.01). bSD relative to naïve animals 0 h PBM (P < 0.05). cSD relative to naïve animals 24 h PBM (P < 0.001). dSD relative to HKB animals 0 h PBM for cytoplasmic (P < 0.001) and membrane extracts (P < 0.01). fSD relative to HKB animals 24 h PBM (P < 0.001). Cytoplasmic ferritin levels are lower in HKB animals 24 h PBM (*P = 0.052) relative to naïve animals and membrane ferritin levels are greater in HKB animals 72 h PBM (**P = 0.052) relative to naïve animals. A representative Western blot is shown above the graphed data. Graphed data represent means ± StD of three independent animal samples.
In CCL-125 cells, ferritin increased significantly with time for all treatments (Fig. 4A). By 22 h, ferritin was greatest for cells treated with HKB and iron together (P < 0.02; Fig. 4A). Secreted ferritin also was greatest for cells treated with HKB and iron at 22 h (P < 0.02; Fig. 4B). From these data we conclude, that challenge of CCL-125 cells with HKB in the presence of iron provokes a synergistic up-regulation of ferritin that is reflected by an increase in cell-associated and secreted protein.
Fig. 4.
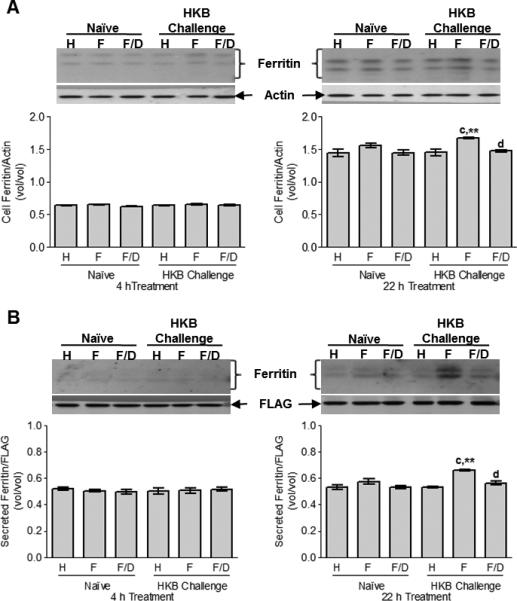
CCL-125 cell-associated expression (A) and secretion (B) of ferritin protein with exposure to iron and HKB was analyzed by Western blot. CCL-125 cells were treated with H, F or F/D as either naïve or HKB challenged cells. Cells and medium were collected at 4 and 22 h post-treatment and processed. (A) Cell-associated ferritin levels. cSD relative to HKB HBSS-treated cells (P < 0.01). **SD relative to naïve FAC-treated cells (P < 0.02). dSD relative to HKB FAC-treated cells (P < 0.05). (B) Cell secreted ferritin levels in the media. cSD relative to HKB HBSS-treated cells (P < 0.001). **SD relative to naïve FAC-treated cells (P < 0.02). dSD relative to HKB FAC-treated cells (P < 0.01). A representative Western blot is shown above the graphed data. Data represent means ± StD of three independent cell culture samples.
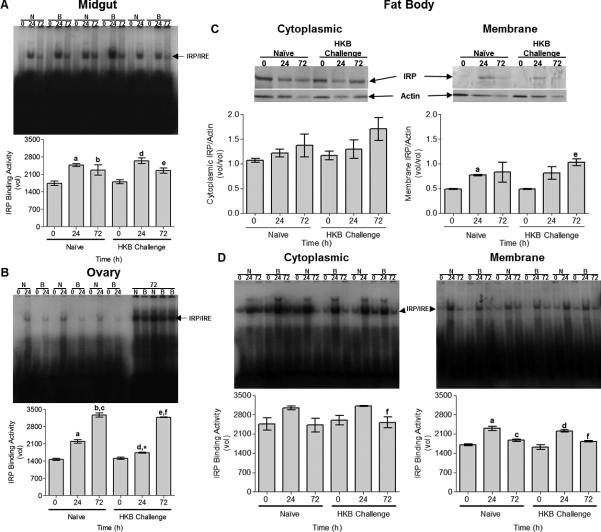
Mosquito IRP/IRE binding activity increases early in midgut and fat body and later in ovary PBM
We have previously demonstrated in CCL-125 cells that IRP/IRE binding activity is generally not responsive to iron dose or chelation (Geiser et al., 2007; Geiser et al., 2006). We also confirmed that IRP expression is not sensitive to these treatments by a separate set of experiments (Fig. 2SA–B; Geiser et al., 2009). However, IRP/IRE binding activity is a sensitive measure of active IRP protein in tissues. We have previously shown that reducing agents, such as β-mercaptoethanol, do not alter binding activity of mosquito IRP (Geiser et al., 2006), which is consistent with earlier studies characterizing Drosophila IRP (Gray et al., 1996). Thus, we quantify only total available binding activity in these samples.
MG IRP/IRE binding activity increased at 24 h PBM, and was sustained at 72 h (Fig. 5A). Evaluation of MG IRP transcript indicated message increased 24 h PBM, but then declined (Fig. 3SA). This implies that a change occurs in the protein that allows sustained IRP/IRE binding activity despite decreased transcript levels at 72 h PBM. In MG, neither IRP/IRE binding activity nor IRP message levels were responsive to HKB treatment. In OV, IRP/IRE binding activity increased with time PBM and was unresponsive to HKB treatment (Fig. 5B). OV IRP message levels increase 72 h PBM and HKB treatment reduced this response (Fig. 3SA).
Fig. 5.
Tissue iron regulatory protein (IRP)/ iron responsive element (IRE) binding activity (A–B, D) and FB IRP expression (C) from female A. aegypti exposed to a blood meal and HKB was analyzed by electrophoretic mobility shift assay (EMSA) and Western blot, respectively. Female mosquitoes were naïve or challenged with HKB 3 h prior to blood feeding. FB, MG and OV tissues were collected at 0, 24 and 72 h PBM and processed. (A) MG tissue IRP/IRE binding activity. aSD relative to naïve animals 0 h PBM (P < 0.01). bSD relative to naïve animals 0 h PBM (P < 0.05). dSD relative to HKB animals 0 h PBM (P < 0.01). eSD relative to HKB animals 0 h PBM (P < 0.05). (B) OV tissue IRP/IRE binding activity. aSD relative to naïve animals 0 h PBM (P < 0.001). bSD relative to naïve animals 0 h PBM (P < 0.001). cSD relative to naïve animals 24 h PBM (P < 0.001). dSD relative to HKB animals 0 h PBM (P < 0.01). *SD relative to naïve animals 24 h PBM (P < 0.002). eSD relative to HKB animals 0 h PBM (P < 0.001). fSD relative to HKB animals 24 h PBM (P < 0.001). (C) FB cytoplasmic and membrane IRP expression levels. aSD relative to naïve animals 0 h PBM (P =0.054). eSD relative to HKB animals 0 h PBM (P < 0.02). A representative Western blot is shown above the graphed data. (D) FB cytoplasmic and membrane IRP/IRE binding activity. fSD relative to HKB animals 24 h PBM in cytoplasmic (P = 0.057) and membrane extracts (P < 0.01). aSD relative to naïve animals 0 h PBM (P < 0.01). cSD relative to naïve animals 24 h PBM (P < 0.05). dSD relative to HKB animals 0 h PBM (P < 0.001). Each tissue EMSA gel autoradiographs are shown above the graphed data. Graphed data represent means ± StD of three independent animal samples.
For FB, we evaluated protein and IRP/IRE binding activity in each fraction. Cytoplasmic IRP increased with time PBM, while membrane IRP increased and stabilized 24 h PBM (Fig. 5C). FB IRP levels were unresponsive to HKB challenge. IRP/IRE binding activity also increased in cytoplasmic and membrane fractions 24 h PBM, but decline by 72 h and was unresponsive to HKB treatment (Fig. 5D). In FB, IRP mRNA levels increased and were maximal by 72 h PBM (P < 0.05) and as with the other tissues HKB treatment reduced this response (P < 0.002; Fig. 3SA). In summary, IRP/IRE binding activity and IRP expression increase PBM in FB, MG and OV, but that each tissue follows a different time course. IRP/IRE binding activity and FB IRP is unresponsive to HKB treatment PBM in all tissues we examined. However, IRP message up-regulation PBM is reduced in each tissue by HKB.
HKB challenge and iron decreases IRP/IRE binding activity with no effect on IRP expression in CCL-125 cells
In CCL-125 cells, IRP increased with culture time, but was not responsive to stimulation with iron or HKB (Fig. 6A). Cell IRP/IRE binding activity was reduced by HKB challenge in the presence of iron at 4 h relative to cells treated with either HKB or iron. The administration of DES with FAC partially mitigated this effect (Fig. 6B). By 22 h, HKB challenge reduced IRP/IRE binding activity in the presence (P <0.03) or absence (P <0.04) of iron treatment compared to that of naïve cells (Fig. 6B). Neither HKB challenge nor iron-treatment altered cell IRP mRNA levels by 4 h (Fig. 3SB). By 22 h, iron administration down-regulated IRP message, while levels were unresponsive to HKB challenge (Fig. 3SB). While IRP expression increased with culture time, it was unresponsive to stimulation with iron or HKB. HKB challenge reduces IRP/IRE binding activity and has an early effect in the presence of iron. With time, the iron effect is stabilized and HKB down-regulation of IRP/IRE binding activity is sustained in the presence or absence of iron. This could be a factor in the up-regulation of HCH synthesis in response to HKB challenge in the presence of iron.
Fig. 6.
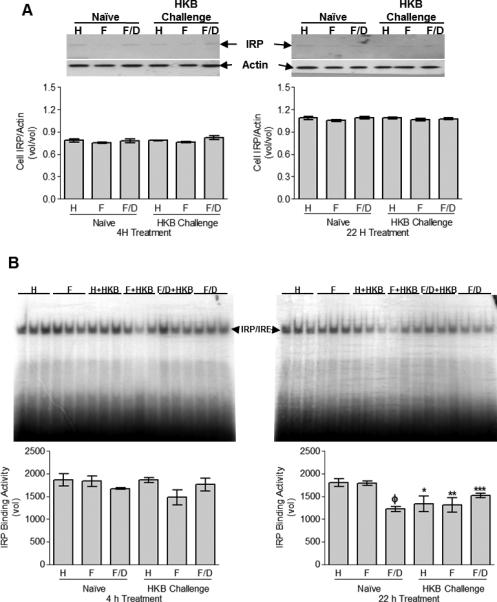
CCL-125 cell-associated IRP expression (A) and IRP/IRE binding activity (B) with exposure to iron and HKB was analyzed by Western blot and EMSA, respectively. CCL-125 cells were treated with H, F or F/D as either naïve or HKB challenged cells. Cells were collected at 4 and 22 h post-treatment and processed. (A) Cell-associated IRP protein levels. A representative Western blot is shown above the graphed data. (B) Cell-associated IRP/IRE binding activity. ΦSD relative to naïve HBSS (P < 0.01) and FAC-treated cells (P < 0.01). *SD relative to naïve HBSS-treated cells (P < 0.04). **SD relative to naïve FAC-treated cells (P < 0.03). ***SD relative to naïve FAC/DES-treated cells (P < 0.008). The EMSA gel autoradiographs are shown above the graphed data. Graphed data represent means ± StD of three independent cell culture samples.
Discussion
Mosquitoes are exposed to a variety of pathogens (i.e. viruses, bacteria, parasites, fungi). For these insects to survive and to thrive in this hostile environment an adequate immune response is required at all developmental stages. Insects are equipped with a highly efficient immune system that is activated quickly after a pathogenic invasion and encompasses many defensive strategies including phagocytic cells in the hemolymph; opsonization, clotting and melaninization propagated by proteolytic cascades, and induced synthesis of potent antimicrobial peptides (Chenet al., 2010). Several insightful reviews have been published discussing the various aspects of insect immunity including those focused on vectors of human disease, such as mosquitoes (Abraham & Jacobs-Lorena, 2004; Chen et al., 2010; Christophides et al., 2004; Fallon & Sun, 2001; Lowenberger, 2001; Michel & Kafatos, 2005; Osta et al., 2004). However, relatively few focus on the role of iron metabolism during the course of an immune response. Hematophagic insects consume a blood meal for a variety of reasons including egg development. Blood feeding provides the nutrients required for oogenesis, yet this practice is inherently dangerous to insects in that they are exposed to pathogens, oxidative stress and a high concentration of heme in the blood meal (Sanders et al., 2003). Here we examined the effects of an immune response to HKB on ferritin, the major insect iron storage and transport protein, in A. aegypti larval cells and adult female tissues.
Our results indicate that the synthesis of DefA1 is a product of HKB challenge and not iron treatment, in CCL-125 cells as well as adult tissues where the magnitude of DefA1 mRNA up-regulation is substantially greater for tissues from infected animals relative to those from naïve animals. It has previously been observed that DefA1 expression in Aedes FB is similar in blood-fed and non-blood-fed mosquitoes (Xiao et al., 2010). In HKB challenged mosquitoes, FB DefA1 transcript increased over time PBM, this has been demonstrated previously and is expected since DefA1 is one of several antimicrobial peptides produced by the FB in response to pathogens (Gao et al., 1999; Magalhaes et al., 2008; Meredith et al., 2006). In HKB challenged mosquitoes, a differential expression pattern for DefA1 mRNA response is observed in MG and OV that likely reflects that these tissues are not typically responsible for the majority of immune defense, and are generally dedicated to processing the nutrients from the blood-meal for the purpose of oogenesis. This also may indicate that limited resources are dedicated to mounting an immune response in the MG and OV tissues in order to promote fecundity. This phenomenon has been reported in A. gambiae inoculated with lipopolysaccharide (LPS; (Jiang et al., 2009)), mosquito vector for Western Equine encephalitis (Culex tarsalis) infected with West Nile virus (Yu et al., 2007), and A. aegypti orally infected with dengue virus serotype 2 (Hua et al., 2007). Thus, data support that the synthesis of DefA1 in tissues is sensitive to HKB challenge, while the blood meal has little effect on transcript expression.
As reported, ferritin is the major protein for sequestering and trafficking iron in insects and expression is induced by the availability of iron in cells and tissues. Previous work in mosquitoes showed that iron treatment provokes an increase in ferritin expression (Li et al., 2002). In order to confirm this and to determine whether iron was involved in the mechanism for the regulation of ferritin expression in response to blood feeding and HKB challenge; we evaluated ferritin expression of mosquito cells following treatment with iron, HKB or both. In CCL-125 cells, both the LCH and HCH subunit transcripts are increased by iron exposure. HKB challenge with iron supplementation significantly up-regulates transcripts for both subunits. In MG, LCH and HCH transcripts are increased at 24 h PBM and subsequently decreased by 72 h PBM. Whereas, FB and OV tissues demonstrate an increase in both subunit transcripts in naïve animals over time PBM; this pattern is suppressed in the HKB challenged mosquitoes. The findings in bumblebee (Bombus ignitus) demonstrated HCH message is up-regulated during conditions of wounding; bacterial challenge and iron overload (Wang et al., 2009). Transcription of B. ignitus HCH is regulated in a time-dependent manner as well (Wang et al., 2009).
The complex picture of ferritin message expression in mosquitoes could be a product of several regulatory schemes. Ferritin transcript expression is clearly a function of tissue specific regulation and MG LCH and HCH transcripts are an order of magnitude higher 24 h PBM than FB and OV. The MG is the major tissue for ferritin expression and secretion that is responsible for detoxifying and processing iron from the blood meal and transporting it to other tissues (Zhou et al., 2007). The transcriptional regulation of both subunits is complicated by the head-to-head orientation of the LCH and HCH on the same chromosome and both share a common promoter sequence with no intervening insulator (Geiser et al., 2003). This suggests the expression of the Aedes ferritin genes are most likely coordinately controlled (Pham & Chavez, 2005).
The influence of the immune response on the transcriptional regulation of the Aedes LCH and HCH may account for some of the differential regulation observed between naïve and HKB challenged cells and tissues. Further, it has been demonstrated in Aedes as well as Anopheles mosquitoes that the primary cis-regulatory elements (CRE) responsible for the induced expression of defensin in immune regulation bind NFκB (nuclear factor κ B) and C/EBP (CCAAT/enhancer binding protein) (Meredith et al., 2006) while NF-IL6 (nuclear factor interleukin 6), ICRE (interferon consensus response elements), NF-ELAM1 (nuclear factor endothelial leukocyte adhesion molecule 1), HNF-5 (hepatic nuclear factor 5) and GATA may also bind putative CREs identified in the defensin promoter (Cho et al., 1997; Eggleston et al., 2000). Several canonical immune-related CREs are identified in the shared promoter regions of the Aedes and Drosophila LCH and HCH genes that could interact with GATA, C/EBP, NFκB/crel (Relish), and IRFs (interferon regulatory factors) (Fig. 4S; Dunkov & Georgieva, 1999; Phamet al., 2003; Pham & Chavez, 2005). Other CREs in the shared promoter also could influence transcript regulation. Canonical moieties that bind transcription factors involved in the response to metals and ecdysone also have been identified in both the Aedes and Drosophila ferritin shared promoters (Fig. 4S; Dunkov & Georgieva, 1999; Pham et al., 2003; Pham & Chavez, 2005).
A clear picture of the impact of the innate immune response on the regulation of Aedes LCH and HCH genes requires an examination of protein expression. The divergent expression of ferritin for different tissues undoubtedly reflects the timing of iron delivery from the blood meal, the levels of iron delivery from the blood meal and the role of the tissue in iron homeostasis. Within 72 h PBM, absorption of iron from the diet is complete and unabsorbed iron is excreted accounting for the decline in ferritin in MG at this time point (Zhou et al., 2007). Our data confirm that the high levels of ferritin in MG 24 h PBM reflect the requirement for ferritin as an iron delivery protein as well as early exposure of this tissue to the high iron levels of the blood meal. More than half of absorbed iron is transported to the ovaries and eggs, while the remainder is distributed among the other tissues (Zhou et al., 2007). Thus, the peripheral tissues show an increase in ferritin at a later time when sufficient iron is delivered to the tissue to induce ferritin for iron storage. Once iron storage in ferritin is achieved we might expect that the primary stimulus for sustaining message levels would be reduced and message levels would decline while protein levels would be sustained or increased. Ferritin message levels are likely more sensitive to iron levels than protein levels, as the latter reflects not only storage requirements, but also synthesis, degradation and secretion. Ferritin is a relatively stable protein (Jensen et al., 2002; Jensen et al., 2003).
We evaluated ferritin in either the cytoplasmic or membrane compartments for MG and FB because our previous work established expression in both compartments in mosquito cells (Geiser et al., 2007; Geiser et al., 2009). Ferritin found in cell cytoplasm in insects probably functions to sequester and to store iron reducing potential oxidative stress and making iron available when needed as a cofactor in newly synthesized proteins. Whereas ferritin in cell membrane compartments could serve a role in long term iron storage, but more likely provides secreted ferritin for iron transport among tissues as needed. In keeping with these hypotheses, ferritin in MG compartments followed a time course reflecting iron levels PBM for this tissue, while ferritin expression in FB suggests this tissue could be the primary site of longer term iron storage for mosquitoes. We did not evaluate carcass as part of this work, and thus, the primary site of iron storage in mosquitoes remains inconclusive.
The variable response of ferritin expression and regulation observed in vivo was further analyzed in vitro with cell culture experiments to observe the mechanistic regulatory effects of HKB challenge in the presence of iron. Both the in vivo and in vitro work support a synergistic effect of HKB challenge and iron in up-regulation of ferritin. This was observed in cells and in animals, and supports the hypothesis that ferritin is cytoprotective, and in hemolymph could serve a role in sequestering iron away from pathogens in the hemocoel. This is consistent with our previous work that showed that ferritin is up-regulated as a cytoprotective compound (Geiseret al., 2003; Geiser et al., 2006), as well as that of others who demonstrated increased ferritin secreted into the humoral fluids of the invertebrate, Amphioxus (Branchiostoma belcheri), when exposed to iron and lipopolysaccharide (LPS) challenge (Li et al., 2008). Although up-regulation of ferritin for iron delivery PBM is an early response, ferritin up-regulation by HKB challenge in cells or animals occurs later. This could support a role for ferritin in sequestering iron from pathogens. This hypothesis is supported by findings of others in flesh fly Sarcophaga bullata (Masova et al., 2010), sea bass Dicentrarchus labrax (Ishiguro et al., 2007), red flour beetle Tribolium castaneum (Vila et al., 2005) and sand flies Lutzomyia longipalpis (Yuan et al., 2006).
We found that IRP/IRE binding activity in FB, OV and MG was unresponsive to HKB challenge and, in fact, was up-regulated following blood feeding. These data are similar to our earlier in vitro work where iron treatment of CCL-125 cells did not provoke a reduction in IRP/IRE binding activity (Geiser et al., 2007; Geiser et al., 2006). In contrast to the IRP1 of mammals, the mosquito IRP does not form an iron sulfur cluster and is neither down-regulated nor degraded in response to increasing iron levels (Fig. 2SA–B; Geiser et al., 2007; Geiser et al., 2006). Differential regulation of IRP by cell compartment has been demonstrated in mammals (Patton et al., 2005). Although IRP levels in FB were greater in the cytoplasm than membranes, expression in response to blood feeding and HKB challenge followed a similar time course. However, the changes in IRP protein were not reflected in IRP/IRE binding activity for the cytoplasmic and membrane compartments in this tissue implying a change in the mosquito IRP allowing for a reduction in IRP/IRE binding activity 72 h PBM for both naïve and HKB challenged mosquitoes in the presence of increasing levels of protein. The results in mosquitoes were similar to observations in vitro (mosquito cells), where IRP protein expression increased overtime for all treatments; however, while IRP/IRE binding activity is refractory to HKB challenge in mosquito tissues, CCL-125 cell binding activity decreases in the presence of HKB. This evidence supports that IRP/IRE binding activity does not necessarily reflect IRP message or protein levels and implies a modification in the protein that regulates binding activity (Vardhanet al., 2009). The unique behavior of mosquito IRP could be related to post-translational modifications, specifically phosphorylation of the protein. Mammalian IRP1/ 2 are phosphoproteins. Potential PKC phosphorylation sites in the mosquito IRP are found at residues 157-159 and Ser 711 (Zhang et al., 2002), sites known to be phosphorylated in mammals (Brownet al., 1998; Eisenstein, 2000; Eisenstein et al., 1993; Schalinske & Eisenstein, 1996).
Complicating the picture for ferritin regulation in insects is the finding that the Drosophila HCH transcript has two different sequences, one with an IRE and one without, that occur by alternative splicing of the 5’-UTR (Dunkov & Georgieva, 1999). On examination, the HCH sequence predicts a similar configuration for the A. aegypti transcript that also shows two putative sequences, one with an IRE in the 5’-UTR and one without. Although splicing would influence the IRE presence, the ORF would remain unaffected. We have not yet completed RACE analysis of these transcripts to confirm that splicing occurs and under what conditions. Nonetheless, the expression of the HCH transcript without an IRE could allow for increased translation of this subunit even in the face of increased IRP/IRE binding activity and could explain the up-regulation of ferritin in cells and tissues exposed to HKB or iron in the presence of sustained or increased IRP/IRE binding activity. The A. aegypti LCH transcript has no IRE and regulation of expression is at the transcriptional level (Geiser et al., 2003). The unpredictable responsiveness of IRP to HKB challenge lends support to the notion that although this protein can repress HCH translation, in insects control of ferritin synthesis is primarily transcriptional and the synergistic effects we observed in ferritin up-regulation with iron and HKB challenge are most likely mediated by transcriptional control factors (Dunkov & Georgieva, 1999; Pham et al., 2003; Pham & Chavez, 2005). In any case, taken together, available evidence supports that the roles of IRP in mosquitoes have yet to be elucidated, and that its functions and response in these animals clearly differs from those for mammals (Geiser et al., 2007; Geiser et al., 2006; Zhang et al., 2002).
We have characterized the message and protein expression profiles of ferritin subunits and IRP from the invertebrate disease vector, A. aegypti, in response to iron and bacterial challenge through in vivo and in vitro experimentation. Mammalian ferritin acts as a positive acute phase protein in reaction to infection, mosquito ferritin responds similarly. However, the regulatory pathway is not as defined or well understood in mosquitoes and requires further investigation into the activity of CREs in the shared ferritin promoter, the implications of alternatively spliced variants of the HCH subunit and the unique behavior of mosquito IRP to elucidate the role of iron and immunity in A. aegypti.
Supplementary Material
Acknowledgments
This work was supported by funds from the National Institutes of Health, National Institute of General Medical Sciences (GM056812), the Agricultural Experiment Station and the College of Agriculture and Life Sciences at the University of Arizona.
Footnotes
Disclosure
All authors declare there are no relationships, financial or otherwise, that may pose a conflict of interest.
References
- Abraham EG, Jacobs-Lorena M. Mosquito midgut barriers to malaria parasite development. Insect Biochemistry & Molecular Biology. 2004;34:667–671. doi: 10.1016/j.ibmb.2004.03.019. [DOI] [PubMed] [Google Scholar]
- Antonova Y, Alvarez KS, Kim YJ, Kokoza V, Raikhel AS. The role of NF-kappaB factor REL2 in the Aedes aegypti immune response. Insect Biochemistry and Molecular Biology. 2009;39:303–314. doi: 10.1016/j.ibmb.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barray S, Devauchelle G. Study of the structural polypeptides of Chilo suppressalis iridescent virus (Iridovirus type 6) Canadian journal of microbiology. 1979;25:841–849. doi: 10.1139/m79-124. [DOI] [PubMed] [Google Scholar]
- Bartholomay LC, Cho WL, Rocheleau TA, Boyle JP, Beck ET, Fuchs JF, Liss P, Rusch M, Butler KM, Wu RC, Lin SP, Kuo HY, Tsao IY, Huang CY, Liu TT, Hsiao KJ, Tsai SF, Yang UC, Nappi AJ, Perna NT, Chen CC, Christensen BM. Description of the transcriptomes of immune response-activated hemocytes from the mosquito vectors Aedes aegypti and Armigeres subalbatus. Infection and Immunity. 2004;72:4114–4126. doi: 10.1128/IAI.72.7.4114-4126.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholomay LC, Mayhew GF, Fuchs JF, Rocheleau TA, Erickson SM, Aliota MT, Christensen BM. Profiling infection responses in the haemocytes of the mosquito, Aedes aegypti. Insect Molecular Biology. 2007;16:761–776. doi: 10.1111/j.1365-2583.2007.00773.x. [DOI] [PubMed] [Google Scholar]
- Bartholomay LC, Waterhouse RM, Mayhew GF, Campbell CL, Michel K, Zou Z, Ramirez JL, Das S, Alvarez K, Arensburger P, Bryant B, Chapman SB, Dong Y, Erickson SM, Karunaratne SH, Kokoza V, Kodira CD, Pignatelli P, Shin SW, Vanlandingham DL, Atkinson PW, Birren B, Christophides GK, Clem RJ, Hemingway J, Higgs S, Megy K, Ranson H, Zdobnov EM, Raikhel AS, Christensen BM, Dimopoulos G, Muskavitch MA. Pathogenomics of Culex quinquefasciatus and meta-analysis of infection responses to diverse pathogens. Science. 2010;330:88–90. doi: 10.1126/science.1193162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birney E, Clamp M, Durbin R. GeneWise and Genomewise. Genome Research. 2004;14:988–995. doi: 10.1101/gr.1865504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blandin S, Moita LF, Kocher T, Wilm M, Kafatos FC, Levashina EA. Reverse genetics in the mosquito Anopheles gambiae: targeted disruption of the Defensin gene. EMBO Reports. 2002;3:852–856. doi: 10.1093/embo-reports/kvf180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brown NM, Anderson SA, Steffen DW, Carpenter TB, Kennedy MC, Walden WE, Eisenstein RS. Novel role of phosphorylation in Fe-S cluster stability revealed by phosphomimetic mutations at Ser-138 of iron regulatory protein 1. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:15235–15240. doi: 10.1073/pnas.95.26.15235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo JC, Robertson AE, Strand MR. Characterization of hemocytes from the mosquitoes Anopheles gambiae and Aedes aegypti. Insect Biochemistry and Molecular Biology. 2006;36:891–903. doi: 10.1016/j.ibmb.2006.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HF, Huang SS, Zhang YZ, Zeng X, Huang ZH. Control efficacy of Trichogramma japonicum against Chilo suppressalis and Chilaraea auricilia. Journal of Applied Ecology. 2010;21:743–748. in Chinese. [PubMed] [Google Scholar]
- Cho WL, Fu TF, Chiou JY, Chen CC. Molecular characterization of a defensin gene from the mosquito, Aedes aegypti. Insect Biochemistry and Molecular Biology. 1997;27:351–358. doi: 10.1016/s0965-1748(97)00017-9. [DOI] [PubMed] [Google Scholar]
- Cho WL, Fu YC, Chen CC, Ho CM. Cloning and characterization of cDNAs encoding the antibacterial peptide, defensin A, from the mosquito, Aedes aegypti. Insect Biochemistry and Molecular Biology. 1996;26:395–402. doi: 10.1016/0965-1748(95)00108-5. [DOI] [PubMed] [Google Scholar]
- Christophides GK, Vlachou D, Kafatos FC. Comparative and functional genomics of the innate immune system in the malaria vector Anopheles gambiae. Immunological Reviews. 2004;198:127–148. doi: 10.1111/j.0105-2896.2004.0127.x. [DOI] [PubMed] [Google Scholar]
- Dimopoulos G, Casavant TL, Chang S, Scheetz T, Roberts C, Donohue M, Schultz J, Benes V, Bork P, Ansorge W, Soares MB, Kafatos FC. Anopheles gambiae pilot gene discovery project: identification of mosquito innate immunity genes from expressed sequence tags generated from immune-competent cell lines. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:6619–6624. doi: 10.1073/pnas.97.12.6619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimopoulos G, Muller HM, Levashina EA, Kafatos FC. Innate immune defense against malaria infection in the mosquito. Current Opinion in Immunology. 2001;13:79–88. doi: 10.1016/s0952-7915(00)00186-2. [DOI] [PubMed] [Google Scholar]
- Dixit R, Sharma A, Patole MS, Shouche YS. Molecular and phylogenetic analysis of a novel salivary defensin cDNA from malaria vector Anopheles stephensi. Acta tropica. 2008;106:75–79. doi: 10.1016/j.actatropica.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Dong Y, Aguilar R, Xi Z, Warr E, Mongin E, Dimopoulos G. Anopheles gambiae immune responses to human and rodent Plasmodium parasite species. PLoS Pathogensens. 2006;2:e52. doi: 10.1371/journal.ppat.0020052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Foissac X, Carss A, Gatehouse AMR, Gatehouse JA. Ferritin acts as the most abundant binding protein for snowdrop lectin in the midgut of rice brown planthoppers (Nilaparvata lugens). Insect Biochemistry and Molecular Biology. 2000;30:297–305. doi: 10.1016/s0965-1748(99)00130-7. [DOI] [PubMed] [Google Scholar]
- Dunkov BC, Georgieva T. Organization of the ferritin genes in Drosophila melanogaster. DNA and Cell Biology. 1999;18:937–944. doi: 10.1089/104454999314791. [DOI] [PubMed] [Google Scholar]
- Eggleston P, Lu W, Zhao Y. Genomic organization and immune regulation of the defensin gene from the mosquito, Anopheles gambia. Insect Moloecular Biology. 2000;9:481–490. doi: 10.1046/j.1365-2583.2000.00212.x. [DOI] [PubMed] [Google Scholar]
- Eisenstein RS. Iron regulatory proteins and the molecular control of mammalian iron metabolism. Annual Review of Nutrition. 2000;20:627–662. doi: 10.1146/annurev.nutr.20.1.627. [DOI] [PubMed] [Google Scholar]
- Eisenstein RS, Tuazon PT, Schalinske KL, Anderson SA, Traugh JA. Iron-responsive element-binding protein. Phosphorylation by protein kinase C. Journal of Biological Chemistry. 1993;268:27363–27370. [PubMed] [Google Scholar]
- Fallon AM, Sun D. Exploration of mosquito immunity using cells in culture. Insect Biochemistry and Molecular Biology. 2001;31:263–278. doi: 10.1016/s0965-1748(00)00146-6. [DOI] [PubMed] [Google Scholar]
- Fiuza L, Nielsen-Leroux C, Goze E, Frutos R, Charles J. Binding of Bacillus thuringiensis Cry1 toxins to the midgut brush border membrane vesicles of Chilo suppressalis (Lepidoptera: Pyralidae): Evidence of Shared Binding Sites. Applied and Environmental Microbiology. 1996;62:1544–1549. doi: 10.1128/aem.62.5.1544-1549.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganz T. Iron in innate immunity: starve the invaders. Current Opinion in Immunology. 2009;21:63–67. doi: 10.1016/j.coi.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Hernandez VP, Fallon AM. Immunity proteins from mosquito cell lines include three defensin A isoforms from Aedes aegypti and a defensin D from Aedes albopictus. Insect Molecular Biology. 1999;8:311–318. doi: 10.1046/j.1365-2583.1999.83119.x. [DOI] [PubMed] [Google Scholar]
- Geiser DL, Chavez CA, Flores-Munguia RF, Winzerling JJ, Pham DQ. Aedes aegypti ferritin: A cytotoxic protector against iron and oxidative challenge? European Journal of Biochemistry. 2003;270:1–8. doi: 10.1046/j.1432-1033.2003.03709.x. [DOI] [PubMed] [Google Scholar]
- Geiser DL, Mayo JJ, Winzerling JJ. The unique regulation of Aedes aegypti larval cell ferritin by iron. Insect Biochemistry and Molecular Biology. 2007;37:418–429. doi: 10.1016/j.ibmb.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Geiser DL, Shen MC, Mayo JJ, Winzerling JJ. Iron loaded ferritin secretion and inhibition by CI-976 in Aedes aegypti larval cells. Comparative Biochemistry and Physiology B. 2009;152:352–363. doi: 10.1016/j.cbpb.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiser DL, Zhang D, Winzerling JJ. Secreted ferritin: mosquito defense against iron overload? Insect Biochemistry and Molecular Biology. 2006;36:177–187. doi: 10.1016/j.ibmb.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Gray NK, Pantopoulos K, Dandekar T, Ackrell AC, Hentze MW. Translational regulation of mammalian and Drosophila citric acid cycle enzymes via iron-responsive elements. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:4925–4930. doi: 10.1073/pnas.93.10.4925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajdusek O, Sojka D, Kopacek P, Buresova V, Franta Z, Sauman I, Winzerling J, Grubhoffer L. Knockdown of proteins involved in iron metabolism limits tick reproduction and development. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:1033–8. doi: 10.1073/pnas.0807961106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamburger AE, West APJ, Hamburger ZA, Hamburger P, Bjorkman PJ. Crystal structure of a secreted insect ferritin reveals a symmetrical arrangement of heavy and light chains. Journal of Molecular Biology. 2005;349:558–569. doi: 10.1016/j.jmb.2005.03.074. [DOI] [PubMed] [Google Scholar]
- Hillyer JF, Schmidt SL, Christensen BM. Hemocyte-mediated phagocytosis and melanization in the mosquito Armigeres subalbatus following immune challenge by bacteria. Cell Tissue Research. 2003a;313:117–127. doi: 10.1007/s00441-003-0744-y. [DOI] [PubMed] [Google Scholar]
- Hillyer JF, Schmidt SL, Christensen BM. Rapid phagocytosis and melanization of bacteria and Plasmodium sporozoites by hemocytes of the mosquito Aedes aegypti. Journal of Parasitology. 2003b;89:62–69. doi: 10.1645/0022-3395(2003)089[0062:RPAMOB]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Hoffmann JA. Immune responsiveness in vector insects. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:11152–11153. doi: 10.1073/pnas.94.21.11152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua H, Lu Q, Cai M, Xu C, Zhou DX, Li X, Zhang Q. Analysis of rice genes induced by striped stemborer (Chilo suppressalis) attack identified a promoter fragment highly specifically responsive to insect feeding. Plant Molecular Biology. 2007;65:519–530. doi: 10.1007/s11103-007-9185-4. [DOI] [PubMed] [Google Scholar]
- Hughes AL. Protein phylogenies provide evidence of a radical discontinuity between arthropod and vertebrate immune systems. Immunogenetics. 1998;47:283–296. doi: 10.1007/s002510050360. [DOI] [PubMed] [Google Scholar]
- Ishiguro S, Li Y, Nakano K, Tsumuki H, Goto M. Seasonal changes in glycerol content and cold hardiness in two ecotypes of the rice stem borer, Chilo suppressalis, exposed to the environment in the Shonai district, Japan. Journal of Insect Physiology. 2007;53:392–397. doi: 10.1016/j.jinsphys.2006.12.014. [DOI] [PubMed] [Google Scholar]
- Jensen LJ, Gupta R, Blom N, Devos D, Tamames J, Kesmir C, Nielsen H, Staerfeldt HH, Rapacki K, Workman C, Andersen CA, Knudsen S, Krogh A, Valencia A, Brunak S. Prediction of human protein function from post-translational modifications and localization features. Journal of Molecular Biology. 2002;319:1257–1265. doi: 10.1016/S0022-2836(02)00379-0. [DOI] [PubMed] [Google Scholar]
- Jensen LJ, Gupta R, Staerfeldt HH, Brunak S. Prediction of human protein function according to Gene Ontology categories. Bioinformatics. 2003;19:635–642. doi: 10.1093/bioinformatics/btg036. [DOI] [PubMed] [Google Scholar]
- Jiang X, Qu M, Denholm I, Fang J, Jiang W, Han Z. Mutation in acetylcholinesterase1 associated with triazophos resistance in rice stem borer, Chilo suppressalis (Lepidoptera: Pyralidae). Biochemical and Biophysical Research Communications. 2009;378:269–272. doi: 10.1016/j.bbrc.2008.11.046. [DOI] [PubMed] [Google Scholar]
- Kalgaonkar S, Lonnerdal B. Receptor-mediated uptake of ferritin-bound iron by human intestinal Caco-2 cells. The Journal of Nutritional Biochemistry. 2009;20:304–311. doi: 10.1016/j.jnutbio.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogan PH. Substitute blood meal for investigating and maintaining Aedes aegypti (Diptera: Culicidae). Journal of Medical Entomology. 1990;27:709–712. doi: 10.1093/jmedent/27.4.709. [DOI] [PubMed] [Google Scholar]
- Li M, Saren G, Zhang S. Identification and expression of a ferritin homolog in amphioxus Branchiostoma belcheri: evidence for its dual role in immune response and iron metabolism. Comparative Biochemistry and Physiology B. 2008;150:263–270. doi: 10.1016/j.cbpb.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Li YP, Goto M, Ding L, Tsumuki H. Diapause development and acclimation regulating enzymes associated with glycerol synthesis in the Shonai ecotype of the rice stem borer larva, Chilo suppressalis walker. Journal of Insect Physiology. 2002;48:303–310. doi: 10.1016/s0022-1910(01)00177-9. [DOI] [PubMed] [Google Scholar]
- Lowenberger C. Innate immune response of Aedes aegypti. Insect Biochemistry and Molecular Biology. 2001;31:219–229. doi: 10.1016/s0965-1748(00)00141-7. [DOI] [PubMed] [Google Scholar]
- Lowenberger CA, Smartt CT, Bulet P, Ferdig MT, Severson DW, Hoffmann JA, Christensen BM. Insect immunity: molecular cloning, expression, and characterization of cDNAs and genomic DNA encoding three isoforms of insect defensin in Aedes aegypti. Insect Molecular Biology. 1999;8:107–118. doi: 10.1046/j.1365-2583.1999.810107.x. [DOI] [PubMed] [Google Scholar]
- Luplertlop N, Surasombatpattana P, Patramool S, Dumas E, Wasinpiyamongkol L, Saune L, Hamel R, Bernard E, Sereno D, Thomas F, Piquemal D, Yssel H, Briant L, Misse D. Induction of a peptide with activity against a broad spectrum of pathogens in the Aedes aegypti salivary gland, following infection with Dengue Virus. PLoS Pathogensens. 2011;7:e1001252. doi: 10.1371/journal.ppat.1001252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magalhaes T, Oliveira IF, Melo-Santos MA, Oliveira CM, Lima CA, Ayres CF. Expression of defensin, cecropin, and transferrin in Aedes aegypti (Diptera: Culicidae) infected with Wuchereria bancrofti (Spirurida: Onchocercidae), and the abnormal development of nematodes in the mosquito. Experimental Parasitology. 2008;120:364–371. doi: 10.1016/j.exppara.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Masova A, Sanda M, Jiracek J, Selicharova I. Changes in the proteomes of the hemocytes and fat bodies of the flesh fly Sarcophaga bullata larvae after infection by Escherichia coli. Proteome Science. 2010;8:1. doi: 10.1186/1477-5956-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith JM, Munks RJ, Grail W, Hurd H, Eggleston P, Lehane MJ. A novel association between clustered NF-kappaB and C/EBP binding sites is required for immune regulation of mosquito Defensin genes. Insect Molecular Biology. 2006;15:393–401. doi: 10.1111/j.1365-2583.2006.00635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel K, Kafatos FC. Mosquito immunity against Plasmodium. Insect Biochemistry and Molecular Biology. 2005;35:677–689. doi: 10.1016/j.ibmb.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Nichol H, Law JH, Winzerling JJ. Iron metabolism in insects. Annual Review of Entomology. 2002;47:535–559. doi: 10.1146/annurev.ento.47.091201.145237. [DOI] [PubMed] [Google Scholar]
- Nichol H, Locke M. Secreted ferritin subunits are of two kinds in insects molecular cloning of cDNAs encoding two major subunits of secreted ferritin from Calpodes ethlius. Insect Biochemistry & Molecular Biology. 1999;29:999–1013. doi: 10.1016/s0965-1748(99)00076-4. [DOI] [PubMed] [Google Scholar]
- Ong ST, Ho JZ, Ho B, Ding JL. Iron-withholding strategy in innate immunity. Immunobiology. 2006;211:295–314. doi: 10.1016/j.imbio.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Osta MA, Christophides GK, Vlachou D, Kafatos FC. Innate immunity in the malaria vector Anopheles gambiae: comparative and functional genomics. Journal of Experimental Biology. 2004;207:2551–2563. doi: 10.1242/jeb.01066. [DOI] [PubMed] [Google Scholar]
- Patton SM, Pinero DJ, Surguladze N, Beard J, Connor JR. Subcellular localization of iron regulatory proteins to Golgi and ER membranes. Journal of Cell Science. 2005;118:4365–4373. doi: 10.1242/jcs.02570. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Research. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham DQ-D, Shaffer JJ, Chavez CA, Douglass PL. Identification and mapping of the promoter for the gene encoding the ferritin heavy-chain homologue of the yellow fever mosquito Aedes aegypti. Insect Biochemistry & Molecular Biology. 2003;33:51–62. doi: 10.1016/s0965-1748(02)00167-4. [DOI] [PubMed] [Google Scholar]
- Pham DQ, Chavez CA. The ferritin light-chain homologue promoter in Aedes aegypti. Insect Molecular Biology. 2005;14:263–270. doi: 10.1111/j.1365-2583.2005.00556.x. [DOI] [PubMed] [Google Scholar]
- Recalcati S, Invernizzi P, Arosio P, Cairo G. New functions for an iron storage protein: the role of ferritin in immunity and autoimmunity. Journal of Autoimmunity. 2008;30:84–89. doi: 10.1016/j.jaut.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Rogers JT. Ferritin translation by interleukin-1 and interleukin-6 - the role of sequences upstream of the start codons of the heavy and light subunit genes. Blood. 1996;87:2525–2537. [PubMed] [Google Scholar]
- Sanders HR, Evans AM, Ross LS, Gill SS. Blood meal induces global changes in midgut gene expression in the disease vector, Aedes aegypti. Insect Biochemistry & Molecular Biology. 2003;33:1105–1122. doi: 10.1016/s0965-1748(03)00124-3. [DOI] [PubMed] [Google Scholar]
- Schalinske KL, Eisenstein RS. Phosphorylation and activation of both iron regulatory proteins 1 and 2 in HL-60 cells. Journal of Biological Chemistry. 1996;271:7168–7176. doi: 10.1074/jbc.271.12.7168. [DOI] [PubMed] [Google Scholar]
- Schnitger AK, Kafatos FC, Osta MA. The melanization reaction is not required for survival of Anopheles gambiae mosquitoes after bacterial infections. Journal of Biological Chemistry. 2007;282:21884–21888. doi: 10.1074/jbc.M701635200. [DOI] [PubMed] [Google Scholar]
- Thomson AM, Cahill CM, Cho HH, Kassachau KD, Epis MR, Bridges KR, Leedman PJ, Rogers JT. The acute box cis-element in human heavy ferritin mRNA 5′–untranslated region is a unique translation enhancer that binds poly(C)-binding proteins. Journal of Biological Chemistry. 2005;280:30032–30045. doi: 10.1074/jbc.M502951200. [DOI] [PubMed] [Google Scholar]
- Thomson AM, Rogers JT, Leedman PJ. Iron-regulatory proteins, iron-responsive elements and ferritin mRNA translation. The International Journal of Biochemistry and Cell Biology. 1999;31:1139–1152. doi: 10.1016/s1357-2725(99)00080-1. [DOI] [PubMed] [Google Scholar]
- Vardhan H, Bhengraj AR, Jha R, Singh Mittal A. Chlamydia trachomatis alters iron-regulatory protein-1 binding capacity and modulates cellular iron homeostasis in HeLa-229 cells. Journal of Biomedicine & Biotechnology. 2009;2009:342032. doi: 10.1155/2009/342032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernick KD, Oduol F, Lazzaro BP, Glazebrook J, Xu J, Riehle M, Li J. Molecular genetics of mosquito resistance to malaria parasites. Current Topics in Microbiology & Immunology. 2005;295:383–415. doi: 10.1007/3-540-29088-5_15. [DOI] [PubMed] [Google Scholar]
- Vila L, Quilis J, Meynard D, Breitler JC, Marfa V, Murillo I, Vassal JM, Messeguer J, Guiderdoni E, San Segundo B. Expression of the maize proteinase inhibitor (mpi) gene in rice plants enhances resistance against the striped stem borer (Chilo suppressalis): effects on larval growth and insect gut proteinases. Plant Biotechnology Journal. 2005;3:187–202. doi: 10.1111/j.1467-7652.2004.00117.x. [DOI] [PubMed] [Google Scholar]
- Wang D, Kim BY, Lee KS, Yoon HJ, Cui Z, Lu W, Jia JM, Kim DH, Sohn HD, Jin BR. Molecular characterization of iron binding proteins, transferrin and ferritin heavy chain subunit, from the bumblebee Bombus ignitus. Comparative Biochemistry and Physiology B. 2009;152:20–27. doi: 10.1016/j.cbpb.2008.09.082. [DOI] [PubMed] [Google Scholar]
- Wang L, Cherayil BJ. Ironing out the wrinkles in host defense: interactions between iron homeostasis and innate immunity. Journal Innate Immunity. 2009;1:455–464. doi: 10.1159/000210016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe H, Aratake, Kayamura T. Serial passage of a nuclear polyhedrosis virus of the silkworm, Bombyx mori, in larvae of rice stem borer, Chilo suppressalis. Journal of Invertebrate Pathology. 1975;25:11–17. doi: 10.1016/0022-2011(75)90280-3. [DOI] [PubMed] [Google Scholar]
- Weinberg ED, Miklossy J. Iron withholding: A defense against disease. JAD. 2008;13:451–463. doi: 10.3233/jad-2008-13409. [DOI] [PubMed] [Google Scholar]
- WHO . Dengue and dengue haemorrhagic fever. Fact sheet N°117 ed. World Health Organization; 2009a. [Google Scholar]
- WHO . Yellow fever. Fact sheet N°100 ed. World Health Organization; 2009b. [Google Scholar]
- Xi Z, Ramirez JL, Dimopoulos G. The Aedes aegypti toll pathway controls dengue virus infection. PLoS Pathogens. 2008;4:e1000098. doi: 10.1371/journal.ppat.1000098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao HJ, Mou FC, Zhu XF, Xue FS. Diapause induction, maintenance and termination in the rice stem borer Chilo suppressalis (Walker). Journal of Insect Physiology. 2010;56:1558–1564. doi: 10.1016/j.jinsphys.2010.05.012. [DOI] [PubMed] [Google Scholar]
- Yoshiga T, Hernandez VP, Fallon AM, Law JH. Mosquito transferrin, an acute-phase protein that is up-regulated upon infection. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:12337–12342. doi: 10.1073/pnas.94.23.12337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu YS, Xue S, Wu JC, Wang F, Yang GQ. Changes in levels of juvenile hormone and molting hormone in larvae and adult females of Chilo suppressalis (Lepidoptera: Pyralidae) after imidacloprid applications to rice. Journal of Economic Entomology. 2007;100:1188–1193. doi: 10.1603/0022-0493(2007)100[1188:cilojh]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Yuan Z, Li F, Hu X, Zhang Z. Geostatistical analysis on temporal patterns of Chilo suppressalis population. Journal of Applied Ecology. 2006;17:673–677. [PubMed] [Google Scholar]
- Zhang D, Albert DW, Kohlhepp P, Dq DP, Winzerling JJ. Repression of Manduca sexta ferritin synthesis by IRP1/IRE interaction. Insect Molecular Biology. 2001;10:531–539. doi: 10.1046/j.0962-1075.2001.00293.x. [DOI] [PubMed] [Google Scholar]
- Zhang D, Dimopoulos G, Wolf A, Minana B, Kafatos FC, Winzerling JJ. Cloning and molecular characterization of two mosquito iron regulatory proteins. Insect Biochemistry and Molecular Biology. 2002;32:579–589. doi: 10.1016/s0965-1748(01)00138-2. [DOI] [PubMed] [Google Scholar]
- Zhou G, Kohlhepp P, Geiser D, Frasquillo Mdel C, Vazquez-Moreno L, Winzerling JJ. Fate of blood meal iron in mosquitoes. Journal of Insect Physiology. 2007;53:1169–1178. doi: 10.1016/j.jinsphys.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou G, Velasquez LS, Geiser DL, Mayo JJ, Winzerling JJ. Differential regulation of transferrin 1 and 2 in Aedes aegypti. Insect Biochemistry and Molecular Biology. 2009;39:234–244. doi: 10.1016/j.ibmb.2008.12.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.