Abstract
The integrity of the serotonin (5-HT) system is essential to normal respiratory and thermoregulatory control. Male and female transgenic mice lacking central 5-HT neurons (Lmx1bf/f/p mice) show a 50% reduction in the hypercapnic ventilatory response and insufficient heat generation when cooled (Hodges et al. 2008a; Hodges et al. 2008b). Lmx1bf/f/p mice also show reduced body temperatures (Tbody) and O2 consumption (V̇O2), and breathe less at rest and during hypoxia and hypercapnia when measured below thermoneutrality (24°C), suggesting a role for 5-HT neurons in integrating ventilatory, thermal and metabolic control. Here, the hypothesis that Pet-1 null mice, which retain 30% of central 5-HT neurons, will demonstrate similar deficits in temperature and ventilatory control was tested. Pet-1 null mice had fewer medullary tryptophan hydroxylase-immunoreactive (TPH+) neurons compared to wild type (WT) mice, particularly in the midline raphé. Female (but not male) Pet-1 null mice had lower baseline minute ventilation (V̇E), breathing frequency (f), V̇O2 and Tbody relative to female WT mice (P<0.05). In addition, V̇E and V̇E / V̇O2 were decreased in male and female Pet-1 null mice during hypoxia and hypercapnia (P<0.05), but only male Pet-1 null mice showed a significant deficit in the hypercapnic ventilatory response when expressed as % of control (P<0.05). Finally, male and female Pet-1 null mice showed significant decreases in Tbody when externally cooled to 4°C. These data demonstrate that a moderate loss of 5-HT neurons leads to a modest attenuation of mechanisms defending body temperature, and that there are gender differences in the contributions of 5-HT neurons to ventilatory and thermoregulatory control.
1. Introduction
Serotonin (5-HT) neurons provide neuromodulatory inputs to the respiratory control network, and play a major role in central chemoreception, most likely as CO2/pH chemoreceptors (Richerson 2004; Corcoran et al. 2009; Hodges et al. 2010a; Hodges et al. 2010b). 5-HT neurons also contribute to thermoregulatory control mechanisms, particularly during conditions that increase requirements for heat production and/or heat conservation (Morrison 2004a; Morrison 2004b; Madden et al. 2006). However, the exact nature of the contributions of 5-HT neurons to both respiratory and thermoregulatory control remains unclear.
One approach to determining the roles of 5-HT neurons in respiratory control has been through the study of transgenic mice with targeted mutations in specific aspects of the 5-HT system. For example, Pet-1 knockout (Pet-1 null) mice, which lack ~70% of 5-HT neurons throughout the classically-described B nuclei (Hendricks et al. 2003), display respiratory dysfunction (Erickson et al. 2007) and spontaneous bradycardias during early development (Cummings et al. 2009), as well as several abnormal behaviors as adults (Hendricks et al. 2003; Lerch-Haner et al. 2008). Neonatal mice with genetic deletion of Lmx1b in neurons expressing Pet-1 (Lmx1bf/f/p), which have a selective and severe deficit (>99%) in 5-HT neurons (Zhao et al. 2006), show frequent and severe apnea and high mortality (Hodges et al. 2009). Lmx1bf/f/p mice that survive to adulthood have fewer abnormalities in breathing at rest or under hypoxic conditions, but display a ~50% reduction in the hypercapnic ventilatory response (Hodges et al. 2008a; Hodges et al. 2008b).
The integrity of the 5-HT system appears to be particularly important during conditions that require defense of core body temperature against hypothermia. Heat generating (shivering and non-shivering thermogenesis) and retention (peripheral vasoconstriction) mechanisms cooperatively maintain core temperature in the face of environmental cooling. It has been shown that 5-HT neurons are activated with environmental (Martin-Cora et al. 2000) or hypothalamic (Nason et al. 2006) cooling, project to the intermediolateral cell column containing preganglionic sympathetic motor neurons (Cano et al. 2003), and can modulate shivering activity. Consistent with this, Lmx1bf/f/p mice lacking 5-HT neurons have a defect in brown adipose tissue activation and exhibit less shivering activity when externally cooled to 4°C (Hodges et al. 2008b). As a consequence, core body temperatures drop to below 30°C within 2 hours of cold exposure, despite apparently normal heat conservation mechanisms and thermosensory perception.
Interestingly, dysfunction of the 5-HT system reveals a differential contribution to respiratory and thermoregulatory control among males and females. For example, mice lacking the serotonin transporter (SERT), which presumably have an increase in extracellular 5-HT levels, display significant attenuation of the hypercapnic ventilatory response, and show an inability to maintain body temperatures in a 4°C environment (Li et al. 2008), similar to 5-HT neuron-deficient mice (Hodges et al. 2008b). However, the deficit in breathing during hypercapnia is specific to male SERT knockouts, and the drop in body temperature (−3°C) in the cold is specific to female SERT knockouts (Li et al. 2007). These and other data point to a differential contribution of 5-HT neurons to specific aspects of respiratory and thermoregulatory control mechanisms, which are dependent upon gender.
Importantly, various risk factors including gender, when combined with postulated defects in respiratory responses to hypoxia and hypercapnia, heart rate control, and arousal responses during sleep have all been postulated to contribute to sudden infant death syndrome (SIDS) (Hunt et al. 1987; Sridhar et al. 2003; Thach 2005; Kinney et al. 2009). Moreover, multiple abnormalities in the brainstem 5-HT system, including decreased 5-HT levels, decreased 5-HT1A receptor and serotonin transporter (SERT) binding, and an increase in the number of morphologically altered 5-HT neurons have been identified in SIDS cases (Paterson et al. 2006; Duncan et al. 2010). Collectively, these observations suggest that SIDS results from an important interaction between an abnormal 5-HT system, defects in cardiorespiratory and/or temperature control, the sleep/wake state, and other risk factors including gender.
Given the importance of further understanding the roles of 5-HT neurons in ventilatory and thermoregulatory control, the goals of these experiments were to characterize the effects of moderate (~70%) loss of 5-HT neurons in adult male and female adult Pet-1 null mice on: 1) the medullary distribution of 5-HT neurons, 2) breathing at rest, and during hypoxia and hypercapnia, and 3) thermoregulation in a cold environment.
2. Methods
All animals were housed and maintained in the Yale Animal Resource Center and all protocols approved by the Yale Animal Care and Use Committee.
2.1 Animal model
The generation of, and genotyping procedures for, Pet-1 null mice have previously been described (Hendricks et al. 2003). Age-matched WT (male = 15, female = 11) and Pet-1 null (male = 17, female = 19) mice were used in this study. Littermates were paired during testing when possible.
2.2. Immunohistochemistry and cell counts
Adult WT (male = 6, female = 3) and Pet-1 null (male = 5, female = 4) mice were anesthetized with sodium pentobarbital (80 mg/kg), and transcardially-perfused with phosphate buffered saline (PBS), and then 4% paraformaldehyde in phosphate buffer (PB). The brain was removed and post-fixed in 4% paraformaldehyde in PB for 24 hours, and then placed in 30% sucrose in PBS until no longer floating (2–4 days). The brainstem was then frozen (−80°C) until frozen-sectioned (20 μm) using a cryostat. All sections were adhered to coated slides for staining.
Brain sections from both WT and Pet-1 null mice were immunostained for tryptophan hydroxylase (TPH), the rate-limiting enzyme in 5-HT synthesis and marker for 5-HT neurons. Sections from both WT and Pet-1 null mice were always stained simultaneously in the same media to ensure that any potential differences in staining intensity and/or TPH+ cell numbers were not due to differences in the treatment of the sections. Sections were first washed with PBS for 5 minutes, and then immersed in 0.3% H2O2 in methanol for 30 minutes. Sections were then permeabilized in 0.4% Triton in PBS, blocked with 5% serum and 0.1% Triton in PBS, and then incubated in PBS with 2.5% serum, 0.1% Triton and a monoclonal primary antibody for TPH (1:2000, Sigma T0678 Clone WH-3) for >10 hours. The specificity of Clone WH-3 for the epitope sequences of mouse TPH-1 (ETVPWFP) and TPH-2 (EDVPWFP) has been well documented (Haycock et al. 2002; Ni et al. 2008), although TPH2 (and not TPH1) is nearly exclusively expressed in the brain (Patel et al. 2004). After washing 3 times in PBS for 10 minutes each, the sections were incubated for 30 minutes with an anti-mouse IgG secondary antibody (1:500, Vector Labs), washed in PBS for 30 minutes, and an ABC reaction using diaminobenzidine was performed (Vector Labs).
Counts of TPH+ neurons were from stained sections every 80 μm, beginning from −0.6 mm to 2.6 mm rostral to obex. Within each section, TPH+ neuron counts were made from two regions: a midline region described by a 0.5 mm-wide rectangle centered on the midline and extending from the ventral to dorsal surface (rectangle in upper panel in Figure 1A), and a second region which included all other TPH+ neurons outside the midline count region. All other TPH+ neurons counted were localized to the ventrolateral medulla (VLM). The VLM TPH+ neuron counts were made bilaterally and combined for the final VLM count.
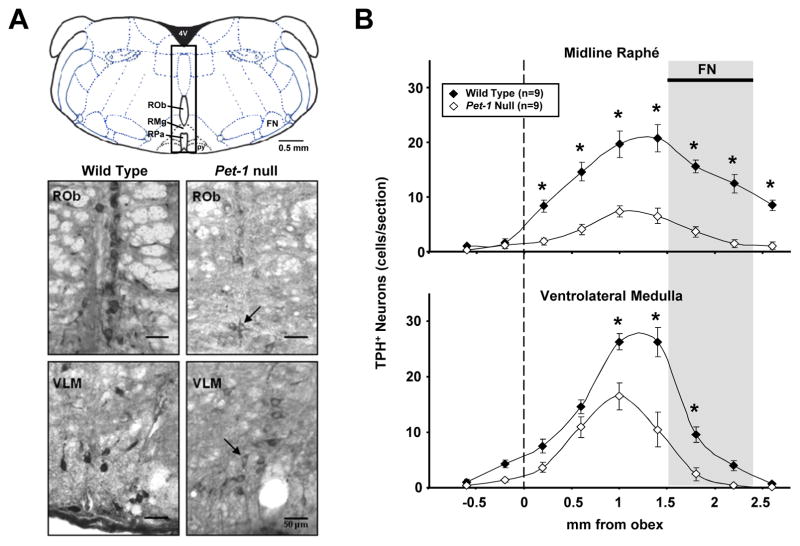
Figure 1.
A) A representative sketch (upper panel) of a section ~ −6.5mm from Bregma with an overlaying 0.5 mm rectangle centered on the midline, and extending to dorsal and ventral surfaces. This rectangle represents the midline count region, and includes the midline raphé nuclei (raphé obscurus, pallidus, and magnus). All other TPH+ neurons were included in the VLM region counts. Grayscale images of WT (lower left panels) and Pet-1 null mice (lower right panels) transverse medullary sections (20 μm) immunostained for TPH. Note that TPH+ 5-HT neurons were fewer in number, and generally lighter in staining intensity in Pet-1 null (arrows) compared to control mice in the midline and ventrolateral medullary (VLM) regions. B) The number of TPH+ neurons in WT (n=9) and Pet-1 null (n=9) brainstems along the midline (upper panel) and VLM regions (binned every 0.5 mm) are plotted using the obex (dotted line) as the reference point. Note that there were significantly fewer TPH+ 5-HT neurons in the midline (-73.2%) and VLM (-50.9%) regions in Pet-1 null mice.
2.3 Plethysmography
Ventilation was measured using standard flow-through plethysmography as described previously (Hodges et al. 2008a; Hodges et al. 2008b). Compressed gas mixtures contained: 21% O2 with 0, 3, 5, 7, or 10% CO2 (normoxic hypercapnia; balance N2), or 10% O2 (hypoxia; balance N2). Hypercapnia studies consisted of more than 20 minutes of baseline, followed by 10-minute exposures to 3, 5, 7, and 10% CO2, each separated by 10-minute baseline measurements. Similarly, hypoxia studies consisted of >20 minutes of baseline followed by 10 minutes of 10% O2. The plethysmograph was placed on a telemeter energizer/receiver (Model ER-4000, MiniMitter, Bend, OR) for continuous measurement of core body temperature in a subset of mice (see below). Air temperature (25–26 °C) and humidity (Omega HX-93AV, Omega Engineering Inc., Stamford, CT), animal temperature, and breathing-induced pressure oscillations (DC002NDR5, Honeywell International, Morristown, NJ) were measured continuously, sampled at 100 Hz, digitized using an A/D converter (PCI-6221, National Instruments, Austin, TX) and monitored/stored on disk using a custom-written data acquisition program (Matlab, The MathWorks, Natick, MA).
2.4 Telemetry Probe Implantation
The methods for implantation of telemetric temperature probes have been published (Hodges et al. 2008a; Hodges et al. 2008b). Briefly, a subset of WT (4 male, 5 female) and Pet-1 null (3 male, 7 female) mice were given pre-operative analgesia (meloxicam (1.0 mg/kg I.P.) or buprenorphine (0.1 mg/kg I.P.)) prior to induction and maintenance of anesthesia with 20% (v/v) isoflurane mixed with polyethylene glycol. Telemetric temperature probes (Emitter G2, MiniMitter, Bend, OR) were implanted into the abdomen using a ventral midline incision, and the wound was closed with 4-0 Ethilon suture (absorbable) and skin staples. Mice received 1.7 μg/ml meloxicam for 2 days, and were studied > 3 days post-op.
2.5 Cold challenge
Adult WT (male = 3, female = 1) and Pet-1 null (male = 4, female = 6) mice were implanted with telemetric temperature probes. The instrumented mice were then presented with a thermal challenge by placing them in a cold environment (4°C) for 4 hours after ≥ 30 minutes at room temperature, as described previously (Hodges et al. 2008b).
2.5 Data analysis
All data were analyzed off-line using custom-written software by an individual blind to the animal genotypes. All samples of continuous ventilatory data segments of 6–10 second duration that did not contain sighs, coughs, sniffing or movement artifacts were selected for analysis during minutes 2–10 of exposure to each gas mixture for the hypercapnia studies. Hypoxia data were analyzed during minutes 5–10 of the 10-minute exposure. Inspiratory time (TI, seconds), expiratory time (TE, seconds), inter-breath interval (IBI, seconds, used to calculate respiratory frequency (f), breaths·minute−1), standard deviation of IBI (seconds), tidal volume (VT, μl), and minute ventilation (V̇E, ml·minute−1, which is the product of VT and f), were calculated for all animals under all conditions.
An animal temperature of 37°C was applied to all data to calculate VT and V̇E (Drorbaugh et al. 1955), which along with V̇O2, were normalized to animal weight in grams. Telemetric temperature measures were obtained in a subset of WT and Pet-1 null mice while breathing 21% O2, and 10% O2 or 3%, 5%, 7%, or 10% CO2. Temperatures during these conditions were compared to determine the effects of gender and/or PET1 deletion.
2.5 Statistics
All data are presented as mean ± SEM. Due to significant differences in body weight between age-matched male and female mice (P<0.01), all statistical analyses were performed within sex to avoid complications of differences in, and normalization to body weight. Comparisons were made using a two-way ANOVA (SigmaPlot) with genotype and condition as factors, and subsequent analyses were followed by a Tukey post-hoc analysis for multiple comparisons. Valid pair-wise comparisons using either a paired t-test or t-test assuming unequal variances (MS Excel, Microsoft Corp.) were made, when appropriate. The threshold for significance was P<0.05.
3. Results
3.1. 5-HT neuron distribution in WT and Pet-1 null mice
Hendricks et al. previously showed that knockout of the Pet-1 gene significantly reduces the overall number of neurons expressing characteristics of adult 5-HT neurons by ~70% (Hendricks et al. 2003). Here, we performed 5-HT neuron counts after performing immunohistochemistry using a monoclonal anti-TPH antibody in WT and Pet-1 null mice to determine the relative distribution of 5-HT neurons within subsets of the medullary raphé populations. TPH+ neurons were present in all medullary raphé nuclei, including within the midline (obscurus, pallidus, and magnus) and lateral (parapyramidal or VLM) areas in both Pet-1 null and WT mice (Figure 1A). We did not observe TPH+ neurons outside of the expected locations for 5-HT neurons, as originally described by Dahlstrom and Fuxe (Dahlstrom et al. 1964) and later documented in Pet-1 null mice (Hendricks et al. 2003). However, the staining intensity of TPH+ neurons in Pet-1 null mice was consistently less intense (see arrows in lower panels of Figure 1A), which was not likely a result of procedural artifact as the WT and Pet-1 null sections were simultaneously stained by immersion in the same staining media.
In both WT and Pet-1 null mice there were few TPH+ neurons caudal to obex (opening of the central canal represented by dashed line in Figure 1B; ~7.4 mm caudal to Bregma), but TPH+ neuron counts increased to a peak near the caudal pole of the facial nucleus (FN), and declining more rostrally to regions beyond the pontomedullary border (~2.3 mm rostral to obex (~5.4 caudal to Bregma). There were significant effects of genotype (P<0.001), distance from obex (P<0.001) and their interaction (P<0.001) in the numbers of TPH+ neurons in WT and Pet-1 null mice. There were fewer TPH+ neurons in Pet-1 null mice in the midline raphé from 0.2 mm – 2.6 mm rostral to obex compared to WT mice (P<0.001). There were also fewer TPH+ neurons in the VLM region from 1.0 mm – 1.8 mm rostral to obex compared to WT mice (P < 0.001). Overall, there were 73.2% fewer midline TPH+ neurons compared to 50.9% fewer VLM TPH+ neurons, indicating an unequal contribution of Pet-1 to the development of these groups of 5-HT neurons. Finally, we found no obvious differences in TPH+ neuron counts among male and female Pet-1 null mice (male; n=5, female; n=4) within the midline or VLM count regions (P>0.05; data not shown).
3.2. Resting ventilation
Baseline physiologic measurements were obtained in age- and weight-matched male and female WT and Pet-1 null mice while breathing room air (RA; Table 1) before separate challenges with hypoxia or hypercapnia. These RA data were averaged for each animal, and the group means and S.E.M. are shown in Table 1. Also, Tbody was measured in a separate group of mice from that in which breathing was measured (during baseline and challenge conditions; see Methods).
Table 1.
Age, body weight, baseline ventilation and oxygen consumption in wild type and Pet-1 null mice
| Age (days) | Body weight (g) | f (min−1) | VT (μl min−1) | V̇E(ml min−1 g−1) | V̇O2(ml min−1 g−1) | Tbody† (°C) | V̇E/ V̇O2 | |
|---|---|---|---|---|---|---|---|---|
| Male | ||||||||
| WT (n = 11) | 173.6 ± 13.3 | 33.6 ± 1.8 | 177.9 ± 7.8 | 6.9 ± 0.3 | 1.24 ± 0.10 | 0.062 ± 0.005 | 37.2 ± 0.3 | 23.2 ± 0.7 |
| Pet-1 null (n = 13) | 174.7 ± 11.5 | 31.4 ± 1.0 | 168.1 ± 5.9 | 6.8 ± 0.3 | 1.13 ± 0.04 | 0.062 ± 0.004 | 37.4 ± 0.2 | 21.1 ± 1.1 |
| n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | |
| Female | ||||||||
| WT (n = 10) | 167.7 ± 19.3 | 25.6 ± 1.7 | 179.0 ± 6.0 | 8.6 ± 0.2 | 1.53 ± 0.45 | 0.071 ± 0.038 | 37.7 ± 0.1 | 23.4 ± 0.8 |
| Pet-1 null (n = 13) | 167.8 ± 15.9 | 22.8 ± 0.6 | 137.3 ± 3.7 | 8.6 ± 0.3 | 1.18 ± 0.44 | 0.061 ± 0.003 | 37.1 ± 0.1 | 21.0 ± 1.1 |
| n.s. | n.s. | ** | n.s. | ** | * | * | n.s. | |
Values are mean ± S.E.M.
P < 0.05,
P<0.001; t test applied within gender.
Tbody data obtained in a separate group of mice (see Methods).
f, breathing frequency, VT, tidal volume, V̇E, minute ventilation, V̇O2, oxygen consumption, Tbody, abdominal temperature, V̇E / V̇O2, ventilatory equivalent.
We found no differences between male WT and Pet-1 null mice in any baseline measures, including age, body weight, f, VT, V̇E, V̇O2, V̇E/ V̇O2 and Tbody (Table 1). In contrast, female Pet-1 null mice displayed decreased resting V̇E (P <0.05), which was a result of a lower resting f (P < 0.01). Resting V̇O2 was also lower in Pet-1 null females relative to female WT mice, such that the ventilatory equivalent (V̇E/ V̇O2) was unchanged. Female Pet-1 null mice also exhibited lower Tbody (P < 0.05) than female WT mice at rest during normoxia.
3.3 Ventilatory response to hypoxia (10% O2)
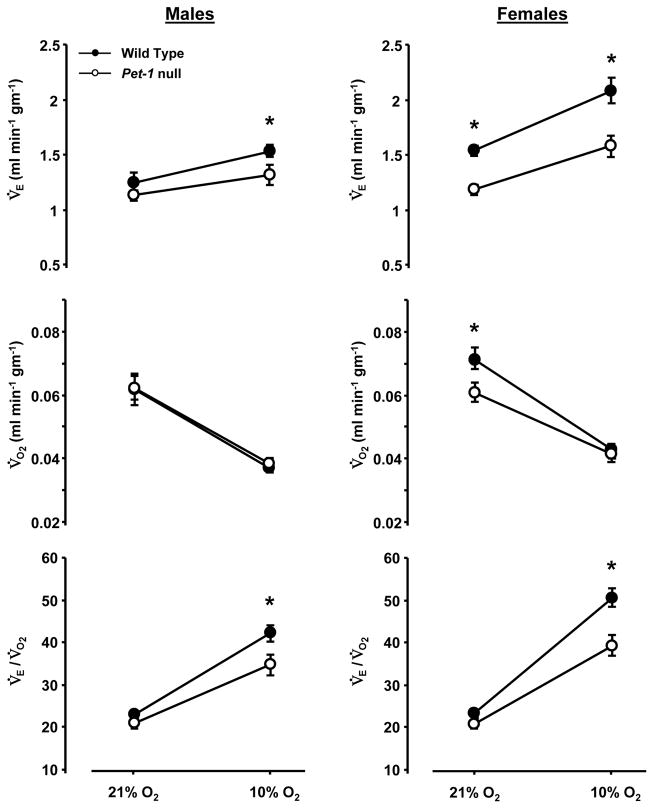
The ventilatory responses to poikilocapnic hypoxia were measured in Pet-1 null and WT male and female mice. Female Pet-1 null mice showed decreased V̇E (Figure 2) and f (P<0.001) at rest, and both males and females exhibited a lower V̇E while breathing 10% O2, but showed no differences in VT (P>0.05; data not shown) in either room air or hypoxia. V̇O2 was decreased in female Pet-1 null mice at rest, but male and female WT and Pet-1 null mice decreased V̇O2 to the same levels during hypoxia (Figure 2). Male and female WT and Pet-1 null mice had equal V̇E/ V̇O2 ratios while breathing room air, but V̇E/ V̇O2 was significantly decreased in both male and female Pet-1 null mice compared to WT mice during hypoxia. In addition, the percent increase in the V̇E/ V̇O2 ratio from rest to hypoxia was significantly lower in male (85.0 ± 7.5% compared to 108.3± 10.4%; P<0.05) and female (97.3 ± 10.0% compared to 123.4± 5.0%; P<0.05) Pet-1 null mice compared to WT mice, respectively. There were no differences in Tbody between WT and Pet-1 null mice during hypoxia (P>0.05). However, the decrease in Tbody from rest to the final 5 minutes of hypoxia tended to be greater in male (−0.66 ± 0.24 °C) and female (−0.93 ± 0.12 °C) Pet-1 null mice compared to male (−0.45 ± 0.1 °C) and female (−0.52 ± 0.18 °C) WT mice, but this difference did not reach statistical significance (P>0.05). Overall, these data indicate that both male and female Pet-1 null mice demonstrate an attenuated hypoxic ventilatory response.
Figure 2.
Differences in ventilation, oxygen consumption and their ratio in male (n=10) and female (n=10) WT and male (n=12) and female (n-13) Pet-1 null mice breathing room air (RA) or 10% O2. Male and female Pet-1 null mice (open symbols) displayed lower minute ventilation (V̇E; expressed as ml/min/gm), and V̇E / V̇O2 ratios while breathing 10% O2 compared to WT (closed symbols) mice, with no differences in (V̇O2).
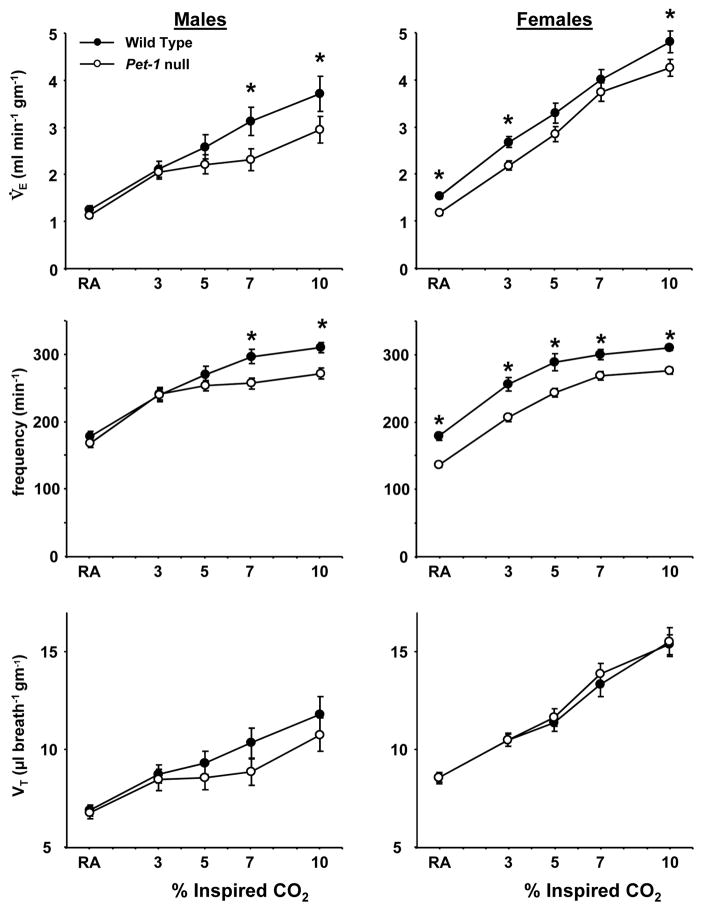
3.4 Ventilatory response to hypercapnia (3%, 5%, 7% and 10% CO2 in 21% O2)
In addition to hypoxia, male and female WT and Pet-1 null mice were also exposed to graded hypercapnia. Male Pet-1 null mice displayed a lower V̇E while breathing 7% and 10% CO2 compared to WT mice (P<0.05), due to a decreased f (P<0.05) (Figure 3). Similarly, female Pet-1 null mice displayed a decreased V̇E while breathing room air or 3%, or 10% CO2 (P<0.05), due solely to a decreased breathing frequency (P<0.05) at all levels of inspired CO2. There were no differences in VT among WT and Pet-1 null mice of either gender (Figure 3). Tbody was lower (P<0.01) in female Pet-1 null mice relative to WT females at all levels of hypercapnia tested, but was not different comparing male WT and Pet-1 null mice under all conditions (P>0.05).
Figure 3.
Differences in minute ventilation (V̇E; normalized to weight), breathing frequency (breaths/minute), and tidal volume (VT: normalized to weight) in male (n=11) and female (n=10) WT (closed symbols) and male (n=13) and female (n=13) Pet-1 null (open symbols) while breathing room air (RA) or 3%, 5%, 7%, or 10% CO2 in 21% O2. Male Pet-1 null mice showed lower V̇E and breathing frequency while breathing 7% and 10% CO2, and female Pet-1 null mice showed lower V̇E while breathing RA, and 3% and 10% CO2. VT in male and female Pet-1 null mice was not different from WT mice, indicating the lower V̇E was due to lower breathing frequency.
Due to the significant differences in baseline ventilation, V̇O2 and Tbody in the female Pet-1 null and WT mice (Table 1), the ventilatory response was also expressed as a percentage of the control V̇E breathing room air (RA; Figure 4). These data showed that the ventilatory response to higher levels of hypercapnia (7% and 10% CO2) was significantly blunted in male (P<0.01), but not female Pet-1 null mice relative to WT mice. Thus, normalizing the hypercapnic ventilatory response to the baseline revealed an apparent male-specific deficit in the hypercapnic ventilatory response.
Figure 4.
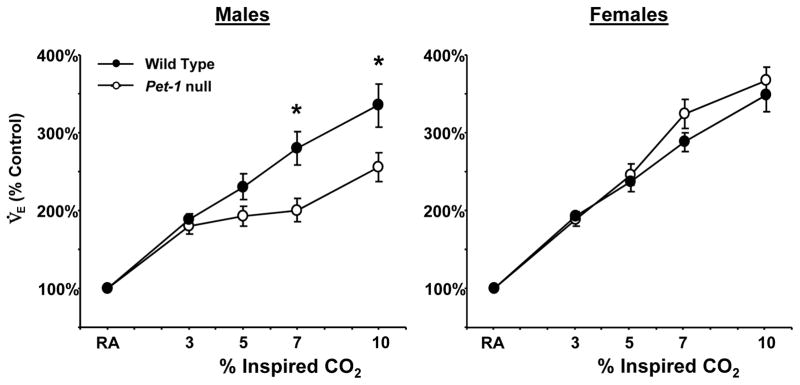
Differences in V̇E expressed as % of control in male (n=11) and female (n=10) WT (closed symbols) and male (n=13) and female (n=13) Pet-1 null (open symbols) mice breathing elevated inspired CO2. V̇E (% control) was lower in male Pet-1 null mice relative to WT mice during hypercapnia, but not different in female WT and Pet-1 null mice.
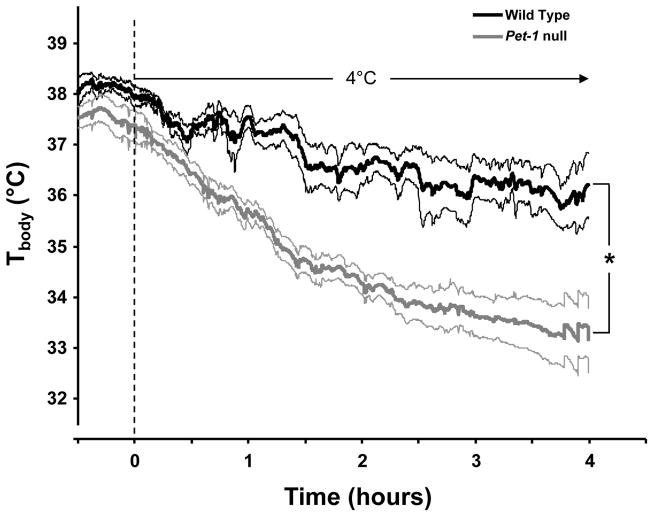
3.5 Maintenance of Tbody during an environmental cold challenge
Mice with near-complete loss of 5-HT neurons (Lmx1bf/f/p) completely fail to maintain Tbody in a cold (4°C) environment, and exhibit a lower Tbody at ~24°C (Hodges et al. 2008a; Hodges et al. 2008b). Tbody in Pet-1 null female (but not male) mice breathing room air is lower compared to female WT mice (P<0.05; Table 1), and is also lower during hypoxia and hypercapnia (P<0.05; data not shown). In contrast, male and female Pet-1 null mice both demonstrated an impaired ability to maintain Tbody compared to WT mice when exposed to an external cold challenge (Figure 5). The drop in Tbody during the cold challenge was not obviously different between male and female Pet-1 null mice, and thus the data were pooled (Figure 5). Tbody dropped in both WT and Pet-1 null mice when moved from room temperature to the cold (4°C; P<0.05), but the resulting decrease in Tbody was greater in the Pet-1 null mice (P<0.001; Figure 5). The drop in Tbody in Pet-1 null mice (−4°C in 4 hours) was also modest relative to that previously reported in transgenic mice lacking nearly all 5-HT neurons (more than −8°C in 1–2 hours) under the same conditions (Hodges et al. 2008b). These observations are consistent with the hypothesis that 5-HT neurons support cold-induced mechanisms of thermogenesis.
Figure 5.
Body temperature (Tbody; °C) in WT (black line; n=4) and Pet-1 null (gray line; n=10) mice measured continuously for 30 minutes before and during 4 hours of exposure to a 4°C environment. Mean Tbody (± SEM; indicated by thin lines of same colors) decreased significantly during the cold exposure in both WT and Pet-1 null mice (P<0.05), but Pet-1 null mice decreased more than WT mice (P<0.05). Data are pooled from male and female mice.
4. Discussion
Under conditions of thermoneutrality (30–31°C), adult male and female Lmx1bf/f/p mice with near-complete absence of 5-HT neurons display a 50% reduction in ventilation during hypercapnia, but breathe normally at rest and during hypoxia (Hodges et al. 2008b). At ambient temperatures typical of most laboratories and animal facilities (24°C), Lmx1bf/f/p mice also show reduced ventilation at rest and during hypoxia, which may be attributed to concomitant reductions in Tbody (Hodges et al. 2008a) due to a severely impaired thermogenic response to environmental cooling (Hodges et al. 2008b). Here we show that Pet-1 null mice, with a more modest loss of 5-HT neurons, display similar but less severe deficits during resting breathing, and in their responses to hypoxia and hypercapnia (measured at 24°C). However, some of these effects were sex-specific, where female Pet-1 null mice showed lower ventilation and Tbody at rest and during hypoxia and hypercapnia, but little effect on the response of ventilation to CO2 expressed as the percent change from baseline. Male Pet-1 null mice displayed normal resting breathing and Tbody, and attenuated ventilatory responses to hypoxia and high levels of hypercapnia independent of how the data were expressed. Both genders exhibited an inability to thermoregulate in cold conditions, suggesting that both genders have inherent dysfunction in temperature control when challenged.
Implicit in the conclusion that there are gender-specific differences in ventilation and temperature control among WT and Pet-1 null mice is that the differences arise due to a deficit in the knockouts, particularly the females. The alternative explanation is that WT females exhibited an abnormally high Tbody, for example at rest (Table 1), which allows for a statistically significant difference unrelated to the loss of Pet-1. The baseline V̇O2 measurements of WT males and WT females, and Tbody measurements in males in our study are in good agreement to those reported in mice by others (Li et al. 2008), but the Tbody values for the WT females in our study are apparently higher. However, irrespective of the differences in Tbody measurements, Pet-1 null females displayed a large deficit in minute ventilation due to a lower respiratory frequency at rest, which is unlikely due to differences in Tbody as it is nearly equal to male WT and Pet-1 null mice, and very close to that in other WT mice (Li et al. 2008). Considering that both female and male Pet-1 null mice show reduced ventilatory responses to hypoxia and large decreases in Tbody in the cold, the differences in ventilation at rest in female knockouts, and the apparent male-specific decrease in the hypercapnic ventilatory response (% control) leads to the conclusion that there are gender-specific deficits in respiratory control mechanisms in Pet-1 null mice.
What remains unclear is the neural substrate(s) that account for these gender-specific phenotypes in Pet-1 null mice. There are clear influences of gender on ventilatory control per se (Behan et al. 2008), some of which have been attributed to gender effects on the 5-HT system itself. Differences in 5-HT innervation and/or function among male and female rats have been reported or hypothesized (Behan et al. 2003; Barker et al. 2009), in addition to functional differences as evidenced by higher brain [5-HT] and TPH protein levels (Carlsson et al. 1988), and higher ratios of 5-HT/5-HIAA (an index of serotonergic activity) in the dorsal raphé nucleus in females compared to males (Dominguez et al. 2003). Receptors for female sex steroids are also differentially expressed within 5-HT neurons (Alves et al. 1998; Bethea et al. 2006; Bethea et al. 2008; Donner et al. 2009), and manipulating estradiol levels alter expression levels of Pet-1 and SERT mRNA (Rivera et al. 2009). Thus, there is the potential for female sex steroids directly enhancing 5-HT neuronal function in a gender-specific manner. Considering that both male and female mice lacking 5-HT neurons both exhibit severe deficits in the hypercapnic ventilatory response, it seems possible that the small numbers of 5-HT neurons in female Pet-1 null mice might be functioning more optimally, accounting for the male-specific deficits in the hypercapnic ventilatory response. However, this explanation is complicated by the observation that resting breathing is lower in female, but not male Pet-1 nulls, suggesting a more complicated mechanism. Regardless, we speculate that the remaining 5-HT neurons themselves might provide the neural substrate in manifesting the gender-dependent differences in respiratory control in Pet-1 null mice.
Despite the gender-specific effects on ventilation, there were no obvious differences in the distribution of, or numbers of 5-HT neurons among male and female Pet-1 null mice. There were regional differences in the remaining 5-HT neurons however, with a greater percentage of 5-HT neuron loss within the midline raphé compared to the VLM in Pet-1 null mice. This observation suggests that the generation or maintenance of midline raphé 5-HT neurons is potentially more dependent upon Pet-1 expression. Moreover, the differences in the numbers of surviving 5-HT neurons in the midline and VLM regions in Pet-1 null mice might translate to differences in physiologic phenotypes, given the functional heterogeneity among 5-HT neurons (Jacobs et al. 1992) and the potential differences in projection patterns among midline raphé (obscurus/pallidus/magnus) and VLM 5-HT neuron pools. To date, it remains unclear if there are specific subpopulations of 5-HT neurons that selectively innervate respiratory-related (Dobbins et al. 1994; Mulkey et al. 2007) or thermoregulatory (Cano et al. 2003) neural circuitry, although it may be relatively restricted to raphé pallidus in the case for thermoregulatory control (Morrison 2004b). Thus, any potential differences in projections or targets from the remaining 5-HT neurons could also provide a substrate for the gender-dependent phenotypes in Pet-1 null mice.
Cold exposure activates shivering, brown fat thermogenesis, and peripheral vasoconstriction. Efferent hypothalamic pathways for thermoregulation include medullary raphé neurons, which likely includes bulbospinal 5-HT neurons that project to spinal thermoeffector neurons driving brown fat metabolism (Cano et al. 2003). Consistent with this hypothesis, Lmx1bf/f/p mice fail to defend core Tbody in a cold (4°C) environment due to deficiencies in shivering and brown fat thermogenesis (Hodges et al. 2008b). Likewise, Pet-1 null mice also display an attenuated defense of Tbody when exposed to cold (and to a lesser extent during hypoxia), although the decrease in Tbody was comparatively modest to mice lacking 5-HT neurons. Core temperature in cold-exposed Pet-1 null mice fell to ~34.5°C within 2 hours of exposure, and to 33.5°C after 4 hours. There were also no obvious differences among male and female Pet-1 null mice in the ability to thermoregulate in the cold, despite the finding that female Pet-1 null mice had lower VO2 and Tbody under resting conditions. This may suggest that while the female knockouts may be better suited to respond robustly to a hypercapnic challenge, they are more vulnerable to problems with Tbody maintenance when unchallenged. Interestingly, these observations are comparable to those from SERT KO mice, where males exhibit deficits in the hypercapnic ventilatory response, and females show deficits in core temperature control in cold environments (Li et al. 2007; Li et al. 2008). These gender-specific phenotypes were not apparent in mice lacking central 5-HT neurons (Hodges et al. 2008b), allowing us to conclude that the activity of, and/or connectivity of the remaining 5-HT neurons to respiratory and thermoregulatory circuitry must contribute to these gender-related differences.
Increasing knowledge of the 5-HT system may lead to further insights into SIDS, given the evidence that SIDS involves dysfunction in respiratory and thermoregulatory control, multiple abnormalities in the 5-HT system, and a greater effect on males. The data presented here suggest that there may be important interactions with gender, respiratory and thermoregulatory control, and a dysfunctional 5-HT system, potentially due to the influences of sex hormones on 5-HT neuronal function. Gaining further insights into these gender-specific interactions of the effects of 5-HT system deficits on breathing and temperature control may provide further insights into the causal mechanisms of and male predominance in SIDS.
Acknowledgments
This work was supported by the Parker B. Francis Foundation (MRH) the NINDS, and the VAMC (G.B.R.).
References
- Alves SE, Weiland NG, Hayashi S, McEwen BS. Immunocytochemical localization of nuclear estrogen receptors and progestin receptors within the rat dorsal raphe nucleus. J Comp Neurol. 1998;391:322–334. [PubMed] [Google Scholar]
- Barker JR, Thomas CF, Behan M. Serotonergic projections from the caudal raphe nuclei to the hypoglossal nucleus in male and female rats. Respir Physiol Neurobiol. 2009;165:175–184. doi: 10.1016/j.resp.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behan M, Wenninger JM. Sex steroidal hormones and respiratory control. Respir Physiol Neurobiol. 2008;164:213–221. doi: 10.1016/j.resp.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behan M, Zabka AG, Thomas CF, Mitchell GS. Sex steroid hormones and the neural control of breathing. Respir Physiol Neurobiol. 2003;136:249–263. doi: 10.1016/s1569-9048(03)00086-7. [DOI] [PubMed] [Google Scholar]
- Bethea CL, Reddy AP. Effect of ovarian hormones on survival genes in laser captured serotonin neurons from macaques. J Neurochem. 2008;105:1129–1143. doi: 10.1111/j.1471-4159.2008.05213.x. [DOI] [PubMed] [Google Scholar]
- Bethea CL, Reddy AP, Smith LJ. Nuclear factor kappa B in the dorsal raphe of macaques: an anatomical link for steroids, cytokines and serotonin. J Psychiatry Neurosci. 2006;31:105–114. doi: 10.1016/j.yfrne.2006.03.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano G, Passerin AM, Schiltz JC, Card JP, Morrison SF, Sved AF. Anatomical substrates for the central control of sympathetic outflow to interscapular adipose tissue during cold exposure. J Comp Neurol. 2003;460:303–326. doi: 10.1002/cne.10643. [DOI] [PubMed] [Google Scholar]
- Carlsson M, Carlsson A. A regional study of sex differences in rat brain serotonin. Prog Neuropsychopharmacol Biol Psychiatry. 1988;12:53–61. doi: 10.1016/0278-5846(88)90061-9. [DOI] [PubMed] [Google Scholar]
- Corcoran AE, Hodges MR, Wu Y, Wang W, Wylie CJ, Deneris ES, Richerson GB. Medullary serotonin neurons and central CO2 chemoreception. Respir Physiol Neurobiol. 2009;168:49–58. doi: 10.1016/j.resp.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings KJ, Commons KG, Fan KC, Li A, Nattie EE. Severe spontaneous bradycardia associated with respiratory disruptions in rat pups with fewer brain stem 5-HT neurons. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1783–1796. doi: 10.1152/ajpregu.00122.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlstrom A, Fuxe K. Localization of monoamines in the lower brain stem. Experientia. 1964;20:398–399. doi: 10.1007/BF02147990. [DOI] [PubMed] [Google Scholar]
- Dobbins EG, Feldman JL. Brainstem network controlling descending drive to phrenic motoneurons in rat. J Comp Neurol. 1994;347:64–86. doi: 10.1002/cne.903470106. [DOI] [PubMed] [Google Scholar]
- Dominguez R, Cruz-Morales SE, Carvalho MC, Xavier M, Brandao ML. Sex differences in serotonergic activity in dorsal and median raphe nucleus. Physiol Behav. 2003;80:203–210. doi: 10.1016/j.physbeh.2003.07.012. [DOI] [PubMed] [Google Scholar]
- Donner N, Handa RJ. Estrogen receptor beta regulates the expression of tryptophan-hydroxylase 2 mRNA within serotonergic neurons of the rat dorsal raphe nuclei. Neuroscience. 2009;163:705–718. doi: 10.1016/j.neuroscience.2009.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drorbaugh JE, Fenn WO. A barometric method for measuring ventilation in newborn infants. Pediatrics. 1955;16:81–87. [PubMed] [Google Scholar]
- Duncan JR, Paterson DS, Hoffman JM, Mokler DJ, Borenstein NS, Belliveau RA, Krous HF, Haas EA, Stanley C, Nattie EE, Trachtenberg FL, Kinney HC. Brainstem serotonergic deficiency in sudden infant death syndrome. JAMA. 2010;303:430–437. doi: 10.1001/jama.2010.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson JT, Shafer G, Rossetti MD, Wilson CG, Deneris ES. Arrest of 5HT neuron differentiation delays respiratory maturation and impairs neonatal homeostatic responses to environmental challenges. Respir Physiol Neurobiol. 2007;159:85–101. doi: 10.1016/j.resp.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haycock JW, Kumer SC, Lewis DA, Vrana KE, Stockmeier CA. A monoclonal antibody to tryptophan hydroxylase: applications and identification of the epitope. J Neurosci Methods. 2002;114:205–212. doi: 10.1016/s0165-0270(01)00530-1. [DOI] [PubMed] [Google Scholar]
- Hendricks TJ, Fyodorov DV, Wegman LJ, Lelutiu NB, Pehek EA, Yamamoto B, Silver J, Weeber EJ, Sweatt JD, Deneris ES. Pet-1 ETS gene plays a critical role in 5-HT neuron development and is required for normal anxiety-like and aggressive behavior. Neuron. 2003;37:233–247. doi: 10.1016/s0896-6273(02)01167-4. [DOI] [PubMed] [Google Scholar]
- Hodges MR, Richerson GB. Interaction between defects in ventilatory and thermoregulatory control in mice lacking 5-HT neurons. Respir Physiol Neurobiol. 2008a;164:350–357. doi: 10.1016/j.resp.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges MR, Richerson GB. Medullary serotonin neurons and their roles in central respiratory chemoreception. Respir Physiol Neurobiol. 2010a doi: 10.1016/j.resp.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges MR, Richerson GB. The role of medullary serotonin (5-HT) neurons in respiratory control: contributions to eupneic ventilation, CO2 chemoreception, and thermoregulation. J Appl Physiol. 2010b;108:1425–1432. doi: 10.1152/japplphysiol.01270.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges MR, Tattersall G, Harris MB, McEvoy S, Richerson D, Deneris ES, Johnson RL, Chen ZF, Richerson GB. Defects in breathing and thermoregulation in mice with near-complete absence of central serotonin neurons. J Neurosci. 2008b;28:2495–2505. doi: 10.1523/JNEUROSCI.4729-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges MR, Wehner M, Aungst J, Smith JC, Richerson GB. Transgenic mice lacking serotonin neurons have severe apnea and high mortality during development. J Neurosci. 2009;29:10341–10349. doi: 10.1523/JNEUROSCI.1963-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt CE, Brouillette RT. Sudden infant death syndrome: 1987 perspective. J Pediatr. 1987;110:669–678. doi: 10.1016/s0022-3476(87)80001-x. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiol Rev. 1992;72:165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- Kinney HC, Richerson GB, Dymecki SM, Darnall RA, Nattie EE. Serotonin and the brainstem in the sudden infant death syndrome: A review. Annu Rev Pathol. 2009;4:517–550. doi: 10.1146/annurev.pathol.4.110807.092322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerch-Haner JK, Frierson D, Crawford LK, Beck SG, Deneris ES. Serotonergic transcriptional programming determines maternal behavior and offspring survival. Nat Neurosci. 2008 doi: 10.1038/nn.2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A, Nattie E. Serotonin transporter knockout mice have a reduced ventilatory response to hypercapnia (predominantly in males) but not to hypoxia. J Physiol. 2008;586:2321–2329. doi: 10.1113/jphysiol.2008.152231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A, Nattie EE. SERT knock-out mice have altered control of breathing and thermoregulation. FASEB J. 2007:21. [Google Scholar]
- Madden CJ, Morrison SF. Serotonin potentiates sympathetic responses evoked by spinal NMDA. J Physiol. 2006;577:525–537. doi: 10.1113/jphysiol.2006.116574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Cora FJ, Fornal CA, Metzler CW, Jacobs BL. Single-unit responses of serotonergic medullary and pontine raphe neurons to environmental cooling in freely moving cats. Neuroscience. 2000;98:301–309. doi: 10.1016/s0306-4522(00)00133-0. [DOI] [PubMed] [Google Scholar]
- Morrison SF. Activation of 5-HT1A receptors in raphe pallidus inhibits leptin-evoked increases in brown adipose tissue thermogenesis. Am J Physiol Regul Integr Comp Physiol. 2004a;286:R832–R837. doi: 10.1152/ajpregu.00678.2003. [DOI] [PubMed] [Google Scholar]
- Morrison SF. Central pathways controlling brown adipose tissue thermogenesis. News Physiol Sci. 2004b;19:67–74. doi: 10.1152/nips.01502.2003. [DOI] [PubMed] [Google Scholar]
- Mulkey DK, Rosin DL, West G, Takakura AC, Moreira TS, Bayliss DA, Guyenet PG. Serotonergic neurons activate chemosensitive retrotrapezoid nucleus neurons by a pH-independent mechanism. J Neurosci. 2007;27:14128–14138. doi: 10.1523/JNEUROSCI.4167-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nason MW, Jr, Mason P. Medullary raphe neurons facilitate brown adipose tissue activation. J Neurosci. 2006;26:1190–1198. doi: 10.1523/JNEUROSCI.4707-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni W, Geddes TJ, Priestley JR, Szasz T, Kuhn DM, Watts SW. The existence of a local 5-hydroxytryptaminergic system in peripheral arteries. Br J Pharmacol. 2008;154:663–674. doi: 10.1038/bjp.2008.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel PD, Pontrello C, Burke S. Robust and tissue-specific expression of TPH2 versus TPH1 in rat raphe and pineal gland. Biol Psychiatry. 2004;55:428–433. doi: 10.1016/j.biopsych.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Paterson DS, Trachtenberg FL, Thompson EG, Belliveau RA, Beggs AH, Darnall R, Chadwick AE, Krous HF, Kinney HC. Multiple serotonergic brainstem abnormalities in sudden infant death syndrome. JAMA. 2006;296:2124–2132. doi: 10.1001/jama.296.17.2124. [DOI] [PubMed] [Google Scholar]
- Richerson GB. Serotonergic neurons as carbon dioxide sensors that maintain pH homeostasis. Nat Rev Neurosci. 2004;5:449–461. doi: 10.1038/nrn1409. [DOI] [PubMed] [Google Scholar]
- Rivera HM, Oberbeck DR, Kwon B, Houpt TA, Eckel LA. Estradiol increases Pet-1 and serotonin transporter mRNA in the midbrain raphe nuclei of ovariectomized rats. Brain Res. 2009;1259:51–58. doi: 10.1016/j.brainres.2008.12.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridhar R, Thach BT, Kelly DH, Henslee JA. Characterization of successful and failed autoresuscitation in human infants, including those dying of SIDS. Pediatr Pulmonol. 2003;36:113–122. doi: 10.1002/ppul.10287. [DOI] [PubMed] [Google Scholar]
- Thach BT. The role of respiratory control disorders in SIDS. Respir Physiol Neurobiol. 2005;149:343–353. doi: 10.1016/j.resp.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Zhao ZQ, Scott M, Chiechio S, Wang JS, Renner KJ, Gereau RW, Johnson RL, Deneris ES, Chen ZF. Lmx1b is required for maintenance of central serotonergic neurons and mice lacking central serotonergic system exhibit normal locomotor activity. J Neurosci. 2006;26:12781–12788. doi: 10.1523/JNEUROSCI.4143-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]