Abstract
Purpose
A 2006 randomized trial demonstrated a 16-month survival benefit with intraperitoneal and intravenous (IP/IV) chemotherapy administered to patients who had ovarian cancer, compared with IV chemotherapy alone, but more treatment-related toxicities. The objective of this study was to examine the use and effectiveness of IP/IV chemotherapy in clinical practice.
Patients and Methods
Prospective cohort study of 823 women with stage III, optimally cytoreduced ovarian cancer diagnosed at six National Comprehensive Cancer Network institutions. We examined IP/IV chemotherapy use in all patients diagnosed between 2003 and 2012 (N = 823), and overall survival and treatment-related toxicities with Cox regression and logistic regression, respectively, in a propensity score–matched sample (n = 402) of patients diagnosed from 2006 to 2012, excluding trial participants, to minimize selection bias.
Results
Use of IP/IV chemotherapy increased from 0% to 33% between 2003 and 2006, increased to 50% from 2007 to 2008, and plateaued thereafter. Between 2006 and 2012, adoption of IP/IV chemotherapy varied by institution from 4% to 67% (P < .001) and 43% of patients received modified IP/IV regimens at treatment initiation. In the propensity score–matched sample, IP/IV chemotherapy was associated with significantly improved overall survival (3-year overall survival, 81% v 71%; hazard ratio, 0.68; 95% CI, 0.47 to 0.99), compared with IV chemotherapy, but also more frequent alterations in chemotherapy delivery route (adjusted rates discontinuation or change, 20.4% v 10.0%; adjusted odds ratio, 2.83; 95% CI, 1.47 to 5.47).
Conclusion
Although the use of IP/IV chemotherapy increased significantly at National Comprehensive Cancer Network centers between 2003 and 2012, fewer than 50% of eligible patients received it. Increasing IP/IV chemotherapy use in clinical practice may be an important and underused strategy to improve ovarian cancer outcomes.
INTRODUCTION
Several randomized clinical trials have demonstrated that intraperitoneal and intravenous (IP/IV) chemotherapy improves survival in women with optimally resected, stage III ovarian cancer, compared with IV chemotherapy alone.1–3 In 2006, the National Cancer Institute (NCI) issued a rare Clinical Announcement encouraging IP/IV chemotherapy use after the Gynecologic Oncology Group (GOG) conducted a randomized trial, GOG-172, that demonstrated a 16-month improvement in median overall survival.
To date, however, few studies have examined the impact of this announcement on the use of IP/IV chemotherapy in clinical practice or investigated whether the survival benefit in GOG-172 is representative of outcomes outside of clinical trials. This is important because fewer than 3% of adult patients with cancer enroll onto clinical trials, and trial participation may be associated with better survival outcomes,4,5 raising concerns about the generalizability of these findings.6
Several factors have been identified as potential barriers to integration of IP/IV chemotherapy into practice,7,8 including treatment-related toxicities, the absence of a standard regimen, patients' preferences, and the inconvenience of an inpatient regimen.8–10 In addition, retrospective studies have documented higher rates of extra-abdominal cancer recurrences, raising concerns about whether IP/IV chemotherapy provides effective systemic control.11,12 Finally, some physicians may believe that alternate chemotherapy regimens (eg, dose-dense paclitaxel) offer comparable survival with fewer toxicities.13
In this study, we examined the use of IP/IV chemotherapy over time at six comprehensive cancer centers where structural barriers to IP/IV chemotherapy administration (eg, trained nursing staff and resource intensity)7,14 should be rare. In addition, we sought to characterize factors associated with IP/IV chemotherapy use and the regimens delivered, because physicians frequently modify the GOG-172 regimen to minimize toxicities.7,10,15 Finally, we examined outcomes associated with IP/IV chemotherapy, including chemotherapy completion, treatment-related toxicities, site(s) of first recurrence, and overall survival. We hypothesized that IP/IV chemotherapy would be associated with improved survival compared with IV chemotherapy, despite frequent modifications to the GOG-172 regimen.
PATIENTS AND METHODS
Data Source
The National Comprehensive Cancer Center Network (NCCN) Ovarian Cancer Outcomes Database Project was created in 2005 to examine the adoption and dissemination of IP/IV chemotherapy before the publication of GOG-1721 and the NCI Clinical Announcement in 2006.9 Between October 2005 and June 2012, data were prospectively collected on all patients with newly diagnosed ovarian, fallopian, or primary peritoneal cancers who received all or part of their care at six institutions: City of Hope Comprehensive Cancer Center, Dana-Farber/Brigham and Women's Cancer Center, Fox Chase Cancer Center, Ohio State University Comprehensive Cancer Center, The University of Texas MD Anderson Cancer Center, and the University of Michigan Comprehensive Cancer Center. To allow for a comparison of IP/IV chemotherapy use before and after the NCI Clinical Announcement, patient data from October 2003 and September 2005 were abstracted retrospectively.
Medical record abstraction was performed by clinical research associates who received training and support from centralized project staff, resulting in high reliability at audit.16,17 Abstracted data included sociodemographic (eg, age, race/ethnicity, income, and zip code), clinical (eg, Charlson comorbidity index18 and GOG performance status), tumor (eg, stage, grade, histology, and disease sites at diagnosis and recurrence), and treatment (eg, surgical procedures and chemotherapy—agents, route, and first and last date of treatment) characteristics, residual disease, and vital status. Detailed data were collected longitudinally until death for all patients who continued being seen at participating centers. Vital status was confirmed by the National Death Index. The institutional review board at each center approved the overall project, and the Dana-Farber/Harvard Cancer Center's Office for Human Research Studies deemed this study exempt from review.
Study Cohorts
We identified two cohorts of patients with stage III, optimally cytoreduced ovarian cancer (defined by GOG-172 criteria as ≤ 1 cm gross residual disease).1 In cohort 1, we examined IP/IV chemotherapy use between 2003 and 2012. In cohort 2, we examined factors associated with IP/IV versus IV chemotherapy and compared outcomes of IP/IV versus IV chemotherapy among patients diagnosed after 2006. We excluded clinical trial participants to minimize selection bias4,5 and more accurately capture true clinical practice after the NCI Clinical Announcement. For cohort 1, we identified 823 eligible patients who received adjuvant chemotherapy within 90 days of surgery.19 We excluded patients who received neoadjuvant chemotherapy, were missing a chemotherapy route, or were lost to follow-up or died within 90 days of surgery. For cohort 2, we identified 498 patients from cohort 1 who were diagnosed after January 2006 and treated outside of clinical trials. The study cohort flow diagram is displayed in Figure 1.
Fig 1.
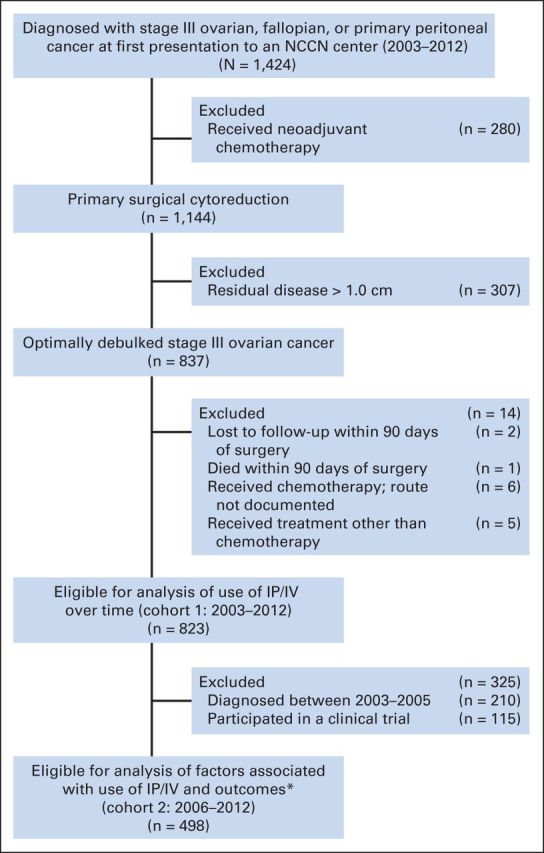
Study cohort. IP/IV, intraperitoneal and intravenous; NCCN, National Comprehensive Cancer Network. (*) Propensity scores were overlapping (within 0.5%) for all but one patient who received IP/IV chemotherapy. For the rest of the patients who received IP/IV chemotherapy (n = 201), we obtained one matching patient who received IV chemotherapy using single nearest-neighbor without replacement.
Outcomes
Our primary outcomes of interest were as follows: the proportion of patients receiving IP/IV chemotherapy and overall survival, defined as survival months from the first chemotherapy dose until death or the end of the observation period (February 15, 2013); patients alive after this date were censored. Secondary outcomes included chemotherapy completion, treatment-related toxicities, and site(s) of first disease recurrence. Treatment-related toxicities were defined from the chemotherapy start date until 90 days after completion.20 Medical records were abstracted to obtain the reason for chemotherapy discontinuation (eg, planned therapy ended, toxicity, and cancer progression), and whether additional chemotherapy was delivered. Sites of first disease recurrence were abstracted from notes, imaging, and pathology reports, and categorized as intra-abdominal or distant recurrences.
Independent Variables
For the analysis of factors associated with IP/IV chemotherapy, the independent variables of interest included age at diagnosis, race, Hispanic ethnicity, insurance, income (derived from Census 2000 data linked by patient zip code), GOG performance status, Charlson comorbidity score,18 primary cancer site, substage, grade, histology, residual disease after surgery, NCCN institution, and year of surgery. For the outcomes analysis, the independent variable of interest was chemotherapy route (ie, IP/IV or IV) at treatment initiation. IP/IV chemotherapy was defined by the GOG-172 regimen, with other combinations classified as modifications.
Analyses
In cohort 1, we tested for changes in the proportion of patients receiving IP/IV versus IV chemotherapy over time using the Cochran-Armitage test for trend. Among patients who received IP/IV chemotherapy, we examined the proportion who received the GOG-172 regimen, treatment on a clinical trial, or a modified regimen. For cohort 2, we used multivariable logistic regression to examine associations between receipt of IP/IV versus IV chemotherapy and patients' sociodemographic, disease, and institutional characteristics. We included all variables of interest regardless of statistical significance, except for insurance because of collinearity with age.
Outcomes analyses were conducted in a propensity score–matched sample (n = 402; 201 patient pairs matched using single nearest neighbor without replacement) to balance measureable confounders between those who received IP/IV and IV chemotherapy; all variables described in Table 1 were used to calculate each patient's propensity of receiving IP/IV chemotherapy. Cox proportional hazards regression was used to examine associations between chemotherapy route and overall survival, with a robust variance estimator to correct for clustering within the matched pairs.21 Conditional logistic regression for matched groups was used to assess associations between chemotherapy route and treatment-related toxicities, reasons for treatment discontinuation or modification, and recurrence site(s).
Table 1.
Characteristics of Patients Receiving IP/IV or IV Chemotherapy at NCCN Centers (2006-2012)
| Characteristic | No. (%) | Unadjusted |
Adjusted |
|||
|---|---|---|---|---|---|---|
| Receiving IP/IV Chemotherapy (%) | P | Odds Ratio | 95% CI | P | ||
| Overall | 498 (100) | 41 | ||||
| Age, years | < .001 | < .001 | ||||
| 18-54 | 144 (29) | 49 | Reference | — | ||
| 55-64 | 185 (37) | 45 | 0.81 | 0.48 to 1.39 | ||
| 65-74 | 117 (23) | 37 | 0.46 | 0.25 to 0.86 | ||
| > 74 | 52 (10) | 12 | 0.11 | 0.04 to 0.32 | ||
| Race | .19 | .70 | ||||
| White | 456 (92) | 41 | Reference | — | ||
| Non-white | 42 (8) | 31 | 0.85 | 0.36 to 1.99 | ||
| Ethnicity | .88 | .60 | ||||
| Non-Hispanic | 480 (96) | 41 | Reference | — | ||
| Hispanic | 18 (4) | 39 | 0.71 | 0.20 to 2.52 | ||
| Income* | .32 | .16 | ||||
| 1 (lowest) | 125 (25) | 34 | Reference | — | ||
| 2 | 125 (25) | 39 | 1.86 | 0.99 to 3.48 | ||
| 3 | 124 (25) | 44 | 1.86 | 0.99 to 3.52 | ||
| 4 (highest) | 124 (25) | 44 | 1.78 | 0.92 to 3.45 | ||
| GOG performance status | .20 | .36 | ||||
| 0 | 350 (70) | 41 | Reference | — | ||
| 1-2 | 70 (14) | 33 | 0.88 | 0.41 to 1.88 | ||
| Unknown | 78 (16) | 47 | 0.58 | 0.27 to 1.23 | ||
| Charlson score | < .001 | .002 | ||||
| 0 | 381 (77) | 45 | Reference | — | ||
| 1 | 46 (9) | 15 | 0.20 | 0.08 to 0.53 | ||
| 2+ | 71 (14) | 31 | 0.55 | 0.29 to 1.06 | ||
| Primary site | .05 | .42 | ||||
| Ovarian | 405 (81) | 42 | Reference | — | ||
| Fallopian | 42 (8) | 48 | 1.50 | 0.68 to 3.31 | ||
| Peritoneal | 51 (10) | 25 | 0.73 | 0.33 to 1.63 | ||
| Stage | .55 | .78 | ||||
| III† | 14 (3) | 43 | 0.72 | 0.18 to 2.81 | ||
| IIIA | 30 (6) | 30 | 0.63 | 0.23 to 1.71 | ||
| IIIB | 52 (10) | 46 | 1.08 | 0.50 to 2.30 | ||
| IIIC | 402 (81) | 41 | Reference | — | ||
| Histology | .10 | .16 | ||||
| Serous | 378 (76) | 43 | Reference | — | ||
| Nonserous | 120 (24) | 34 | 0.68 | 0.39, 1.17 | ||
| Grade | .22 | .30 | ||||
| I-II‡ | 97 (19) | 35 | Reference | — | ||
| III | 401 (81) | 42 | 1.37 | 0.75 to 2.49 | ||
| Residual disease | .64 | .61 | ||||
| None | 183 (37) | 42 | Reference | — | ||
| ≤ 1 cm | 145 (29) | 42 | 0.75 | 0.42 to 1.35 | ||
| Optimal | 170 (34) | 38 | 0.93 | 0.53 to 1.64 | ||
| Institution | < .001 | < .001 | ||||
| 1 | § | 67 | 3.30 | 1.15 to 9.47 | ||
| 2 | § | 63 | 1.44 | 0.66 to 3.13 | ||
| 3 | § | 4 | 0.03 | 0.01 to 0.13 | ||
| 4 | § | 20 | 0.20 | 0.08 to 0.46 | ||
| 5 | § | 31 | 0.31 | 0.13 to 0.71 | ||
| 6 | § | 49 | Reference | — | ||
| Year of surgery | .12 | .07 | ||||
| 2006 | 85 (17) | 33 | Reference | — | ||
| 2007-2008 | 159 (32) | 48 | 1.89 | 0.98 to 3.63 | ||
| 2009-2010 | 164 (33) | 38 | 0.96 | 0.49 to 1.89 | ||
| 2011-2012 | 90 (18) | 39 | 1.03 | 0.48 to 2.24 | ||
Abbreviations: GOG, Gynecologic Oncology Group; IP, intraperitoneal; IV, intravenous; NCCN, National Comprehensive Cancer Network.
Derived from the Census 2000 data linked to patient by zip code. Income was unknown for 21 patients (4%), and was imputed on the basis of the median income of patients separately by institution.
Includes 14 patients with unknown substage.
Includes 21 patients with unknown grade.
Absolute numbers are suppressed to protect the identity of individual institutions.
Matching on the propensity score achieved reasonable balance for all measured confounders with the exception of institution (Appendix Table A1, online only). Institution was not significantly associated with overall survival on univariable analysis (P = .16, data not shown). Sensitivity analyses were performed to consider the impact of excluding each individual institution from the survival model to ensure that the results were not explained by observations at a single institution. Additional analyses were performed to confirm that the results of the propensity score analysis were robust to different methods (ie, regression adjustment, stratification, and inverse probability weights). A two-sided P < .05 was considered statistically significant. Statistical analyses were performed with Stata version 13.1 (StataCorp LP, College Station, TX).
RESULTS
Use of IP/IV Chemotherapy Over Time
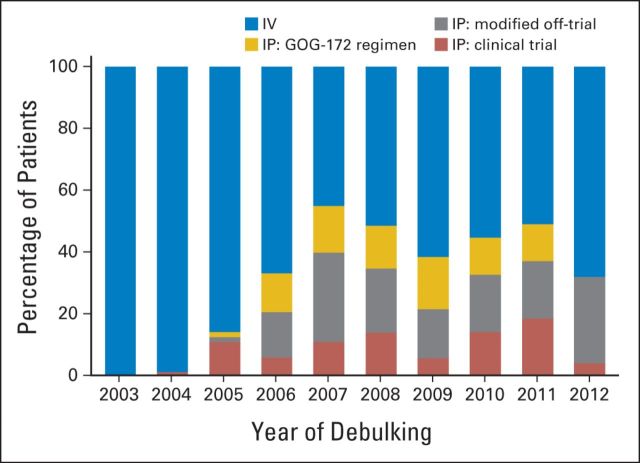
Between 2003 and 2012, 287 (35%) of 823 patients received IP/IV chemotherapy. Figure 2 shows the use of IP/IV chemotherapy over time. From 2003 to 2006, the annualized use of IP/IV chemotherapy increased from 0% to 33% (Ptrend < .001). IP/IV use increased to nearly 50% from 2007 to 2008, but plateaued thereafter (Ptrend = .79 for 2006 to 2012). Among patients receiving IP/IV chemotherapy, 29% received the GOG-172 regimen without modification, 28% were enrolled onto clinical trials, and 43% received modified regimens at treatment initiation (Appendix Table A2, online only). The proportion of patients who received modified regimens varied by institution from 7% to 87% (P < .001) and frequently involved reductions in dose of IP cisplatin or an IV drug substitution (eg, docetaxel for paclitaxel).
Fig 2.
Proportion of patients treated with intraperitoneal (IP) or intravenous (IV) chemotherapy over time at National Comprehensive Cancer Network centers, 2003 to 2012. GOG, Gynecologic Oncology Group.
Factors Associated With Use of IP/IV Chemotherapy
Table 1 shows the characteristics of patients receiving IP/IV versus IV chemotherapy after the NCI Clinical Announcement, excluding trial participants (cohort 2). Overall, 202 (41%) of 498 eligible patients received IP/IV chemotherapy. In adjusted analyses, patients who were younger and with fewer comorbidities more often received IP/IV versus IV chemotherapy (both P ≤ .002). There were no significant differences in IP/IV chemotherapy use by race, ethnicity, substage, or extent of residual disease. Rates of IP/IV use varied significantly by institution (P < .001; unadjusted range, 4% to 67%).
Chemotherapy Completion
Of the 202 women who started IP/IV chemotherapy, 44% completed six or more cycles and 89% were classified as completing planned therapy, compared with 91% of patients receiving IV chemotherapy. Women received a median of five IP/IV cycles (interquartile range, 3 to 6), and completion rates did not differ by receipt of GOG-172 versus modified regimens (P = .83; Appendix Table A3, online only).
Chemotherapy-Related Toxicities and Site(s) of First Disease Recurrence
As shown in Table 2, anemia rates were significantly higher in the IP/IV versus IV chemotherapy group (adjusted rates, 10.9% v 5.0%; adjusted odds ratio [AOR], 2.20; 95% CI, 1.04 to 4.65), as were hospitalizations (adjusted rates, 14.4% v 10.0%; AOR, 1.47; 95% CI, 0.82 to 2.64), although this latter difference was not statistically significant. Clinical complications did not otherwise differ by route. Compared with those receiving IV chemotherapy, women who received IP/IV chemotherapy were more likely to change treatments because of treatment-related toxicities (adjusted rates, 20.4% v 10.0%; AOR, 2.83; 95% CI, 1.47 to 5.47) or for other reasons (eg, IP port catheter complication; adjusted rates, 8.5% v 4.0%; AOR, 2.14; 95% CI, 0.87 to 5.26), although this latter difference was not statistically significant. Of the 41 patients who discontinued IP/IV chemotherapy because of toxicities, 13 switched to a new IP/IV chemotherapy regimen, 22 switched to IV chemotherapy, and 6 discontinued adjuvant treatment.
Table 2.
Rates of Toxicities, Chemotherapy Discontinuation or Change, and Site(s) of Disease Recurrence by Route of Chemotherapy (2006-2012)
| Event | Propensity Score–Matched Sample |
||||
|---|---|---|---|---|---|
| IV, % (n = 201) | IP/IV, % (n = 201) | Odds Ratio for IP/IV v IV | 95% CI | P | |
| Toxicity | |||||
| Hospitalization | 10.0 | 14.4 | 1.47 | 0.82 to 2.64 | .19 |
| IP catheter complication* | 0.5 | 13.4 | 27.0 | 3.67 to 199.0 | .001 |
| Diarrhea | 1.0 | 3.0 | 3.00 | 0.61 to 15.9 | .18 |
| Nausea and vomiting | 3.5 | 5.0 | 1.50 | 0.53 to 4.21 | .44 |
| Dehydration | 3.0 | 4.5 | 1.50 | 0.53 to 4.21 | .44 |
| Febrile neutropenia | 2.0 | 2.0 | 1.00 | 0.25 to 4.00 | .99 |
| Anemia | 5.0 | 10.9 | 2.20 | 1.04 to 4.65 | .04 |
| Thrombocytopenia | 2.0 | 1.5 | 0.75 | 0.17 to 3.35 | .71 |
| Infection† | 3.5 | 4.5 | 1.29 | 0.48 to 3.45 | .62 |
| Neuropathy | 29.9 | 28.4 | 0.92 | 0.58 to 1.46 | .72 |
| Reason for discontinuation or change in delivery‡ | |||||
| Planned therapy ended | 84.1 | 70.1 | Reference | — | |
| Cancer progression | 2.0 | 1.0 | 0.50 | 0.09 to 2.73 | .42 |
| Toxicity | 10.0 | 20.4 | 2.83 | 1.47 to 5.47 | .002 |
| Other | 4.0 | 8.5 | 2.14 | 0.87 to 5.26 | .10 |
| Disease recurrence§ | |||||
| Intra-abdominal | 70.6 | 41.2 | Reference | — | — |
| Distant | 29.4 | 58.8 | 3.14 | 1.34 to 7.36 | .008 |
Abbreviations: IP, intraperitoneal; IV, intravenous.
Complications include blockage, infection, or leak.
Infections requiring hospitalization.
Odds ratio determined from multinomial logistic regression with planned therapy ended as the base outcome variable. Other includes the following: IP port catheter complication (n = 14), patient/family preferences (n = 4), drug shortage (n = 1), comorbidity (n = 1; patient needed hernia surgery), and unknown (n = 7).
Among 102 patients (51 matched pairs) with documented disease recurrence.
Women treated with IP/IV chemotherapy had higher odds of presenting with distant disease at first recurrence (adjusted rates, 58.8% v 29.4%; AOR, 3.14; 95% CI, 1.34 to 7.36), compared with IV chemotherapy.
Survival Outcomes
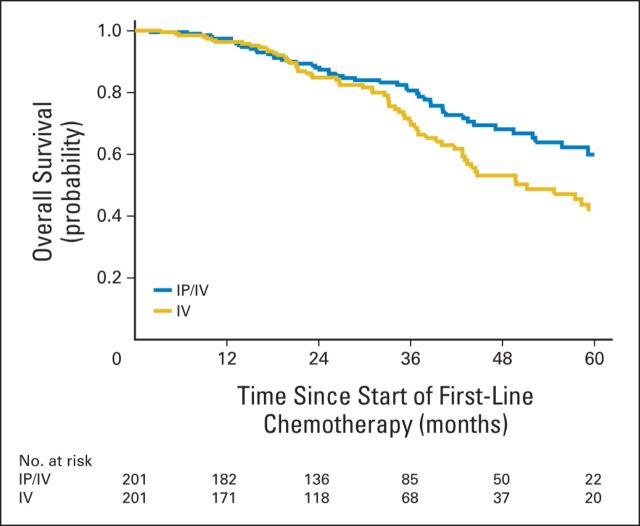
Survival curves for the propensity-matched sample are shown in Figure 3. At a median follow-up period of 32 months, 114 patients had died (51 v 63 patients who received IP/IV v IV chemotherapy, respectively). The 3-year overall survival for IP/IV chemotherapy was 81% (95% CI, 73% to 86%) compared with 71% (95% CI, 62% to 78%) for IV chemotherapy.
Fig 3.
Overall survival with propensity score–matched sample for National Comprehensive Cancer Network patients with optimally cytoreduced, stage III ovarian cancer by first-line chemotherapy administration with intraperitoneal or intravenous (IP/IV) chemotherapy, 2006 to 2012.
As shown in Table 3, patients who received IP/IV chemotherapy had a significantly improved overall survival, compared with IV chemotherapy (hazard ratio [HR], 0.68; 95% CI, 0.47 to 0.99), after matching on the propensity score. In addition, the superiority of IP/IV chemotherapy was supported by three alternative propensity score–adjusted models and conventional multivariable adjustment.
Table 3.
Overall Survival of Patients Treated With IP/IV Versus IV Chemotherapy (2006-2012)
| Model | Hazard Ratio | 95% CI | P |
|---|---|---|---|
| Unadjusted model | 0.66 | 0.47 to 0.92 | .02 |
| Propensity score–adjusted models | |||
| Matching 1:1 (201 matched pairs) | 0.68 | 0.47 to 0.99 | .047 |
| Inverse probability weights | 0.65 | 0.46 to 0.92 | .02 |
| Regression adjustment | 0.66 | 0.44 to 0.98 | .04 |
| Stratification | 0.64 | 0.42 to 0.98 | .04 |
| Multivariable-adjusted model* | 0.62 | 0.41 to 0.94 | .03 |
Abbreviations: IP, intraperitoneal; IV, intravenous.
Adjusting for all variables that were included in the propensity score model.
In a sensitivity analysis, the adjusted HRs ranged from 0.61 to 0.76 on excluding each institution one by one, indicating that the survival benefit observed was not driven by outcomes at a single institution. For example, on exclusion of institution 3, where 4% of patients received IP/IV chemotherapy, the adjusted HR was 0.70 (95% CI, 0.48 to 1.02); because of the decreased sample size, this result did not meet conventional levels of statistical significance (P = .06).
DISCUSSION
In this prospective study of the use and effectiveness of IP/IV chemotherapy in 823 patients at six major cancer centers, we found that fewer than 50% of eligible patients received IP/IV chemotherapy over time, even after the NCI Clinical Announcement, despite a clear survival benefit. In addition, we observed marked variation in the adoption of IP/IV chemotherapy by institution and significant heterogeneity in the IP/IV regimens used at treatment initiation. Indeed, 43% of patients received modified regimens. Despite widespread treatment heterogeneity, we observed a significant survival benefit associated with IP/IV versus IV chemotherapy and relatively few differences in treatment-related toxicities between groups, suggesting IP/IV chemotherapy is feasible to use in clinical practice.
To our knowledge, this is the first prospective, multi-institutional, comparative effectiveness study to demonstrate that IP/IV chemotherapy is associated with significantly improved survival among women with optimally debulked, stage III ovarian cancer, outside of a clinical trial. These results extend two previous studies that demonstrated infrequent use of IP/IV chemotherapy among patients treated in an integrated health care network and Medicare beneficiaries.22,23 Consistent with these studies, we found that patient characteristics, including younger age and fewer medical comorbidities, are associated with receipt of IP/IV chemotherapy. In addition, our study extends these results by including patients who closely matched the GOG-172 eligibility criteria and examining clinical outcomes, including survival and treatment-related toxicities, with stringent adjustment for relevant prognostic factors.24
The magnitude of the survival benefit associated with IP/IV chemotherapy we detected is similar to results from randomized clinical trials.1–3,9 We observed a relative risk of death in the IP/IV versus IV group of 0.68 (95% CI, 0.47 to 0.99), comparable to that observed in GOG-114 (0.76 [95% CI, 0.61 to 0.96]), GOG-172 (0.75 [95% CI, 0.58 to 0.97]), and a pooled analysis of six randomized trials.1,2,9,25 Although randomized trials have demonstrated a clear survival benefit with IP/IV chemotherapy in clinical trials, only 41% of eligible patients at six academic centers received it, suggesting that IP/IV chemotherapy may be currently underused.
Reassuringly, although women treated with IP/IV chemotherapy were more likely to switch chemotherapy routes because of treatment-related toxicities compared with IV chemotherapy, their adjuvant chemotherapy completion rates were not lower overall (IP/IV v IV chemotherapy, 89% v 91%). Similarly, we did not observe higher rates of neuropathy, dehydration, or infections reported in GOG-172, although we may have been underpowered to detect these differences.1,26 This may be because providers modified IP/IV regimens to reduce toxicities or added prophylactic supportive care (eg, IV fluids and growth factors). The heterogeneity in IP/IV regimens we observed confirms that physicians have not adopted a standard IP/IV chemotherapy regimen.
Consistent with previous observational studies, women who received IP/IV chemotherapy were more likely to develop distant metastases at first disease recurrence, instead of experiencing intra-abdominal relapses.11,12 This suggests that IP/IV chemotherapy treats disease effectively within the anatomic regions of drug distribution but may compromise systemic disease control. Alternatively, these women may avoid early intra-abdominal recurrences but present with distant recurrences because they experience relapse later in time.
Our finding that IP/IV chemotherapy use varied between such similar academic institutions is surprising, particularly because patients' sociodemographic and disease characteristics are relatively uniform across NCCN centers.17 This may be partially explained by institutional differences in clinical trial participation. For example, although use of IP/IV chemotherapy was lower at institution 3 than at others, when it was administered, it was usually administered on a clinical trial. Specifically, although 19% of eligible patients received IP/IV chemotherapy between 2006 and 2012 at institution 3 overall (both on and off trials), 87% of these patients were treated on a clinical trial and thus excluded from our study. In contrast, overall IP/IV chemotherapy use ranged from 24% to 68% at other institutions, and the proportion treated on trial varied from 0% to 30%.
Our results suggest that the use of IP/IV chemotherapy may also be influenced by local culture and clinical practice leaders' enthusiasm for treatments and clinical trials.27,28 This does not discount the importance of patient preferences.29 Rather, it suggests that women may receive substantially different treatment, depending on where they seek care, even within NCCN centers, and that additional interventions may be required to ensure that IP/IV chemotherapy decision making is more uniform and patient centered. Future studies should directly examine how clinicians' attitudes toward IP/IV chemotherapy are associated with use across centers.
Our study has several limitations. First, we were unable to compare survival differences between different IP/IV chemotherapy regimens because of the number of adaptations observed. Second, the database did not capture dose-dense paclitaxel because the study started in late 2005, before evidence demonstrating improved survival in Japanese populations with dose-dense paclitaxel was available.13,30 However, if dose-dense paclitaxel use increased over time, survival in the IV chemotherapy group should have improved, making it more difficult to detect a survival benefit with IP/IV chemotherapy. We expect that this question will be more definitively answered in two randomized clinical trials, the iPocc Trial31 and GOG-252.32 Third, the database did not differentiate between inpatient and outpatient administration of IP/IV chemotherapy because prospective data supporting the feasibility of outpatient administration of IP/IV chemotherapy were not published until 2009. Also, we were unable to ascertain progression-free survival (PFS) accurately, although PFS may be more prone to bias than overall survival in clinical practice because it is directly dependent on the frequency of surveillance testing (eg, CA125 or computed tomographic scans).33 Future studies should examine PFS using a uniform method to monitor disease progression at regular intervals, similar to clinical trials.
Although we expect our results to be more generalizable than clinical trials, they may not be representative of clinical practice outside of academic institutions because most are high-volume hospitals, a factor associated with improved survival.34,35 Finally, in this observational study, we cannot exclude the possibility that selection bias contributed to our findings. For example, if healthier patients were more likely to receive IP/IV chemotherapy than less healthy patients, that could explain the survival benefit we observed. Although we could not adjust for unobserved confounders, our rich clinical data and statistical analyses allowed adjustment for key clinical variables with important prognostic implications (eg, performance status, comorbid disease, and extent of residual disease).24
In conclusion, our findings suggest that the use of IP/IV chemotherapy at NCCN centers increased significantly after the publication of GOG-172 and the NCI Clinical Announcement. However, fewer than 50% of eligible women received IP/IV chemotherapy overall, and the integration of IP/IV chemotherapy into clinical practice varied significantly among institutions. Despite frequent modifications to the GOG-172 regimen, we found that use of IP/IV chemotherapy in clinical practice is feasible and associated with improved survival compared with IV chemotherapy, consistent with results from randomized trials. Together, these findings suggest that IP/IV is an important and possibly underused, evidence-based treatment strategy for improving outcomes in ovarian cancer.
Acknowledgment
We acknowledge the following people for their contributions to this project: all of the study participants; the Ovarian Cancer Collaborative Outcomes Research Database for material and administrative support; Janet Files for administrative and organizational assistance; Layla Rouse and Monir Corona for assistance with development of the study database; and the National Comprehensive Cancer Center for providing support for the outcomes database infrastructure and management.
Appendix
Table A1.
Patient Characteristics After Matching on the Propensity Score for Treatment with IP/IV Chemotherapy versus IV Chemotherapy
| Characteristic | IV (n = 201) | IP/IV (n = 201) | Standardized Difference, %* |
|
|---|---|---|---|---|
| Before Propensity Score Matching | After Propensity Score Matching | |||
| Age, years | 32.5 | 11.2 | ||
| 18-54 | 59 (29) | 70 (35) | ||
| 55-64 | 72 (36) | 82 (41) | ||
| 65-74 | 59 (29) | 43 (21) | ||
| > 74 | 11 (5) | 6 (3) | ||
| Race | 12.3 | 5.8 | ||
| White | 185 (92) | 188 (94) | ||
| Nonwhite | 16 (8) | 13 (6) | ||
| Ethnicity | 1.3 | 2.6 | ||
| Non-Hispanic | 193 (96) | 194 (97) | ||
| Hispanic | 8 (4) | 7 (3) | ||
| Income | 5.9 | 8.0 | ||
| 1 (lowest) | 59 (29) | 43 (21) | ||
| 2 | 49 (24) | 48 (24) | ||
| 3 | 47 (23) | 55 (27) | ||
| 4 (highest) | 46 (23) | 55 (27) | ||
| GOG performance status | 3.9 | 0.2 | ||
| 0 | 140 (70) | 141 (70) | ||
| 1-2 | 25 (12) | 23 (11) | ||
| Unknown | 36 (18) | 37 (18) | ||
| Charlson score | 37.7 | 12.0 | ||
| 0 | 163 (81) | 172 (86) | ||
| 1 | 14 (7) | 7 (3) | ||
| 2+ | 24 (12) | 22 (11) | ||
| Primary site | 26.0 | 15.1 | ||
| Ovarian | 163 (81) | 169 (84) | ||
| Fallopian | 16 (8) | 19 (9) | ||
| Peritoneal | 22 (11) | 13 (6) | ||
| Stage | 2.1 | 6.3 | ||
| III | 4 (2) | 6 (3) | ||
| IIIA | 12 (6) | 9 (4) | ||
| IIIB | 18 (9) | 23 (11) | ||
| IIIC | 167 (83) | 163 (81) | ||
| Histology | 15.1 | 11.9 | ||
| Serous | 150 (75) | 160 (80) | ||
| Nonserous | 51 (25) | 41 (20) | ||
| Grade | 11.4 | 5.2 | ||
| I-II | 38 (19) | 34 (17) | ||
| III | 163 (81) | 167 (83) | ||
| Residual disease | 2.4 | 1.7 | ||
| None | 71 (35) | 77 (38) | ||
| ≤ 1 cm | 61 (30) | 61 (30) | ||
| Optimal | 69 (34) | 63 (31) | ||
| Institution | 95.5 | 50.2 | ||
| 1 | † | † | ||
| 2 | † | † | ||
| 3 | † | † | ||
| 4 | † | † | ||
| 5 | † | † | ||
| 6 | † | † | ||
| Year of surgery | 11.4 | 9.0 | ||
| 2006 | 38 (19) | 28 (14) | ||
| 2007-2008 | 60 (30) | 76 (38) | ||
| 2009-2010 | 70 (35) | 63 (31) | ||
| 2011-2012 | 33 (16) | 34 (17) | ||
Abbreviations: GOG, Gynecologic Oncology Group; IP, intraperitoneal; IV, intravenous.
In general, variables with standardized differences of less than 10% are considered to have negligible differences between treatment groups.
Absolute numbers are suppressed to protect the identity of individual institutions.
Table A2.
IP/IV Regimens by Institution From 2003 to 2012 (cohort 1)
| Regimen | Overall % (N = 287) | Patients Receiving IP/IV by Institution (%)* |
|||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | ||
| IP/IV with GOG-172, %† | 29 | 48 | 49 | 0 | 63 | 13 | 0 |
| IP/IV on clinical trial, % | 28 | 19 | 27 | 87 | 30 | 0 | 30 |
| Modified IP/IV regimen, %‡ | 43 | 32 | 24 | 13 | 7 | 87 | 70 |
| IV drug substitution | 23 | 3 | 0 | 0 | 4 | 17 | 66 |
| IP drug substitution | 1 | 10 | 0 | 0 | 0 | 0 | 0 |
| IP dose reduction | 16 | 16 | 24 | 13 | 0 | 70 | 0 |
| Other | 2 | 3 | 0 | 0 | 4 | 0 | 4 |
Abbreviations: GOG, Gynecologic Oncology Group; IP, intraperitoneal; IV, intravenous.
Absolute numbers are suppressed to protect the identity of individual institutions.
GOG-172 regimen is IV paclitaxel, 135 mg/m2, on day 1, IP cisplatin, 100 mg/m2, on day 2, and IP paclitaxel, 60 mg/m2, on day 8.
Examples include IV drug substitution (eg, docetaxel instead of paclitaxel), IP dose reduction (eg, cisplatin, 75 instead of 100 mg/m2), IP drug substitution (eg, carboplatin for cisplatin), or combined adjustments (eg, IV docetaxel and IP carboplatin).
Table A3.
Toxicities and Number of IP Chemotherapy Cycles Delivered by Regimen From 2006 to 2012 Off Trial (cohort 2)
| Variable | % Total (N = 202) | % GOG-172 Regimen (n = 81) | % Modified Regimens (n = 121) |
|---|---|---|---|
| No. of IP cycles delivered | |||
| 1-2 | 21 | 21 | 20 |
| 3-4 | 19 | 17 | 21 |
| 5+ | 60 | 62 | 59 |
| Toxicity | |||
| Hospitalization | 14.4 | 19.8 | 10.7 |
| IP catheter complication* | 13.4 | 12.3 | 14.0 |
| Diarrhea | 3.0 | 3.7 | 2.5 |
| Nausea and vomiting | 5.0 | 6.2 | 4.1 |
| Dehydration | 4.5 | 7.4 | 2.5 |
| Febrile neutropenia | 2.0 | 3.7 | 0.8 |
| Anemia | 10.9 | 14.8 | 8.3 |
| Thrombocytopenia | 1.5 | 2.5 | 0.8 |
| Infection† | 4.5 | 3.7 | 5.0 |
| Neuropathy | 28.2 | 32.1 | 25.6 |
| Reason for discontinuation or chemotherapy change | |||
| Planned therapy ended | 70.3 | 67.9 | 71.9 |
| Cancer progression | 1.0 | 2.5 | 0.0 |
| Toxicity | 20.3 | 24.7 | 17.4 |
| Other | 8.4 | 4.9 | 10.7 |
NOTE. There were no significant differences between groups by regimen (GOG-172 v modified regimen) for number of IP cycles delivered, toxicities, or reason for discontinuation or change in chemotherapy regimen (all P > .09) by Fisher's exact test.
Abbreviations: GOG, Gynecologic Oncology Group; IP, intraperitoneal.
Complications include blockage, infection, or leak.
Infections requiring hospitalization.
Footnotes
Listen to the podcast by Dr Armstrong at www.jco.org/podcasts
Processed as a Rapid Communication manuscript.
Supported by the National Cancer Institute Grant No. K07 CA166210 (to A.A.W.).
Presented, in part, at the 50th Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, May 30-June 3, 2014.
The funding organizations had no role in the preparation, review, or approval of the manuscript.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: Alexi A. Wright, Jane C. Weeks
Financial support: Alexi A. Wright, Ursula A. Matulonis, Jane C. Weeks
Administrative support: Dana E. Milne, Joyce C. Niland, Jane C. Weeks, David M. O'Malley
Provision of study materials or patients: Alexi A. Wright, Mihaela C. Cristea, Jennifer J. Griggs, Charles F. Levenback, Gina Mantia-Smaldone, Ursula A. Matulonis, Jane C. Weeks, David M. O'Malley
Collection and assembly of data: Alexi A. Wright, Dana E. Milne, Joyce C. Niland, Jane C. Weeks
Data analysis and interpretation: Alexi A. Wright, Angel Cronin, Michael A. Bookman, Robert A. Burger, David E. Cohn, Mihaela C. Cristea, Jennifer J. Griggs, Nancy L. Keating, Charles F. Levenback, Gina Mantia-Smaldone, Ursula A. Matulonis, Larissa A. Meyer, Joyce C. Niland, Jane C. Weeks, David M. O'Malley
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Use and Effectiveness of Intraperitoneal Chemotherapy for Treatment of Ovarian Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Alexi A. Wright
No relationship to disclose
Angel Cronin
No relationship to disclose
Dana E. Milne
No relationship to disclose
Michael A. Bookman
Employment: McKesson, US Oncology Research
Honoraria: Genentech
Consulting or Advisory Role: Genentech, Boehringer Ingelheim, AstraZeneca, AbbVie, Sanofi, Novartis, ImmunoGen, Endocyte, Gradalis
Robert A. Burger
Consulting or Advisory Role: Amgen, Boehringer Ingelheim, Genentech, Janssen Pharmaceuticals, Oxigene, Roche
Travel, Accommodations, Expenses: Genentech
David E. Cohn
No relationship to disclose
Mihaela C. Cristea
No relationship to disclose
Jennifer J. Griggs
No relationship to disclose
Nancy L. Keating
No relationship to disclose
Charles F. Levenback
No relationship to disclose
Gina Mantia-Smaldone
No relationship to disclose
Ursula A. Matulonis
No relationship to disclose
Larissa A. Meyer
Research Funding: CSL Behring (I), GORE (I), Medtronic (I)
Travel, Accommodations, Expenses: AstraZeneca
Joyce C. Niland
No relationship to disclose
Jane C. Weeks
No relationship to disclose
David M. O'Malley
Honoraria: AstraZeneca, Genentech, Janssen Pharmaceuticals, Eisai
REFERENCES
- 1.Armstrong DK, Bundy B, Wenzel L, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354:34–43. doi: 10.1056/NEJMoa052985. [DOI] [PubMed] [Google Scholar]
- 2.Alberts DS, Liu PY, Hannigan EV, et al. Intraperitoneal cisplatin plus intravenous cyclophosphamide versus intravenous cisplatin plus intravenous cyclophosphamide for stage III ovarian cancer. N Engl J Med. 1996;335:1950–1955. doi: 10.1056/NEJM199612263352603. [DOI] [PubMed] [Google Scholar]
- 3.Markman M, Bundy BN, Alberts DS, et al. Phase III trial of standard-dose intravenous cisplatin plus paclitaxel versus moderately high-dose carboplatin followed by intravenous paclitaxel and intraperitoneal cisplatin in small-volume stage III ovarian carcinoma: An intergroup study of the Gynecologic Oncology Group, Southwestern Oncology Group, and Eastern Cooperative Oncology Group. J Clin Oncol. 2001;19:1001–1007. doi: 10.1200/JCO.2001.19.4.1001. [DOI] [PubMed] [Google Scholar]
- 4.Unger JM, Barlow WE, Martin DP, et al. Comparison of survival outcomes among cancer patients treated in and out of clinical trials. J Natl Cancer Inst. 2014;106:dju002. doi: 10.1093/jnci/dju002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peppercorn JM, Weeks JC, Cook EF, et al. Comparison of outcomes in cancer patients treated within and outside clinical trials: Conceptual framework and structured review. Lancet. 2004;363:263–270. doi: 10.1016/S0140-6736(03)15383-4. [DOI] [PubMed] [Google Scholar]
- 6.Bleyer A. In and out, good and bad news, of generalizability of SWOG treatment trial results. J Natl Cancer Inst. 2014;106:dju027. doi: 10.1093/jnci/dju027. [DOI] [PubMed] [Google Scholar]
- 7.Naumann RW, Sukumvanich P, Edwards RP. Practice patterns of intraperitoneal chemotherapy in women with ovarian cancer. Gynecol Oncol. 2009;114:37–41. doi: 10.1016/j.ygyno.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 8.Cristea M, Han E, Salmon L, et al. Practical considerations in ovarian cancer chemotherapy. Ther Adv Med Oncol. 2010;2:175–187. doi: 10.1177/1758834010361333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Cancer Institute. NCI issues clinical announcement for preferred method of treatment for advanced ovarian cancer. http://ctep.cancer.gov/highlights/docs/clin_annc_010506.pdf.
- 10.Barlin JN, Dao F, Bou Zgheib N, et al. Progression-free and overall survival of a modified outpatient regimen of primary intravenous/intraperitoneal paclitaxel and intraperitoneal cisplatin in ovarian, fallopian tube, and primary peritoneal cancer. Gynecol Oncol. 2012;125:621–624. doi: 10.1016/j.ygyno.2012.03.027. [DOI] [PubMed] [Google Scholar]
- 11.Tanner EJ, Black DR, Zivanovic O, et al. Patterns of first recurrence following adjuvant intraperitoneal chemotherapy for stage IIIC ovarian cancer. Gynecol Oncol. 2012;124:59–62. doi: 10.1016/j.ygyno.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 12.Esselen KM, Rodriguez N, Growdon W, et al. Patterns of recurrence in advanced epithelial ovarian, fallopian tube and peritoneal cancers treated with intraperitoneal chemotherapy. Gynecol Oncol. 2012;127:51–54. doi: 10.1016/j.ygyno.2012.05.026. [DOI] [PubMed] [Google Scholar]
- 13.Katsumata N, Yasuda M, Takahashi F, et al. Dose-dense paclitaxel once a week in combination with carboplatin every 3 weeks for advanced ovarian cancer: A phase 3, open-label, randomised controlled trial. Lancet. 2009;374:1331–1338. doi: 10.1016/S0140-6736(09)61157-0. [DOI] [PubMed] [Google Scholar]
- 14.Alhayki M, Hopkins L, Le T, et al. Intraperitoneal chemotherapy for advanced epithelial ovarian cancer: A Canadian perspective. Int J Gynecol Cancer. 2006;16:1761–1765. doi: 10.1111/j.1525-1438.2006.00713.x. [DOI] [PubMed] [Google Scholar]
- 15.Konner JA, Grabon DM, Gerst SR, et al. Phase II study of intraperitoneal paclitaxel plus cisplatin and intravenous paclitaxel plus bevacizumab as adjuvant treatment of optimal stage II/III epithelial ovarian cancer. J Clin Oncol. 2011;29:4662–4668. doi: 10.1200/JCO.2011.36.1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kho ME, Lepisto EM, Niland JC, et al. Reliability of staging, prognosis, and comorbidity data collection in the National Comprehensive Cancer Network (NCCN) non-Hodgkin lymphoma (NHL) multicenter outcomes database. Cancer. 2008;113:3209–3212. doi: 10.1002/cncr.23911. [DOI] [PubMed] [Google Scholar]
- 17.Weeks JC, Uno H, Taback N, et al. Interinstitutional variation in management decisions for treatment of 4 common types of cancer: A multi-institutional cohort study. Ann Intern Med. 2014;161:20–30. doi: 10.7326/M13-2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 19.Wright JD, Herzog TJ, Neugut AI, et al. Effect of radical cytoreductive surgery on omission and delay of chemotherapy for advanced-stage ovarian cancer. Obstet Gynecol. 2012;120:871–881. doi: 10.1097/AOG.0b013e31826981de. [DOI] [PubMed] [Google Scholar]
- 20.Hassett MJ, O'Malley AJ, Pakes JR, et al. Frequency and cost of chemotherapy-related serious adverse effects in a population sample of women with breast cancer. J Natl Cancer Inst. 2006;98:1108–1117. doi: 10.1093/jnci/djj305. [DOI] [PubMed] [Google Scholar]
- 21.Austin PC. The use of propensity score methods with survival or time-to-event outcomes: Reporting measures of effect similar to those used in randomized experiments. Stat Med. 2014;33:1242–1258. doi: 10.1002/sim.5984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bowles EJ, Wernli KJ, Gray HJ, et al. Diffusion of intraperitoneal chemotherapy in women with advanced ovarian cancer in community settings 2003-2008: The effect of the NCI clinical recommendation. Front Oncol. 2014;4:43. doi: 10.3389/fonc.2014.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fairfield KM, Murray K, LaChance JA, et al. Intraperitoneal chemotherapy among women in the Medicare population with epithelial ovarian cancer. Gynecol Oncol. 2014;134:473–477. doi: 10.1016/j.ygyno.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 24.Landrum LM, Java J, Mathews CA, et al. Prognostic factors for stage III epithelial ovarian cancer treated with intraperitoneal chemotherapy: A Gynecologic Oncology Group study. Gynecol Oncol. 2013;130:12–18. doi: 10.1016/j.ygyno.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elit L, Oliver TK, Covens A, et al. Intraperitoneal chemotherapy in the first-line treatment of women with stage III epithelial ovarian cancer: A systematic review with metaanalyses. Cancer. 2007;109:692–702. doi: 10.1002/cncr.22466. [DOI] [PubMed] [Google Scholar]
- 26.Wenzel LB, Huang HQ, Armstrong DK, et al. Health-related quality of life during and after intraperitoneal versus intravenous chemotherapy for optimally debulked ovarian cancer: A Gynecologic Oncology Group study. J Clin Oncol. 2007;25:437–443. doi: 10.1200/JCO.2006.07.3494. [DOI] [PubMed] [Google Scholar]
- 27.Rogers EM. Diffusion of Innovation. New York, NY: The Free Press; 2003. [Google Scholar]
- 28.Chassin MR. Explaining geographic variations: The enthusiasm hypothesis. Med Care. 1993;31:YS37–YS44. doi: 10.1097/00005650-199305001-00006. [DOI] [PubMed] [Google Scholar]
- 29.Havrilesky LJ, Alvarez Secord A, Ehrisman JA, et al. Patient preferences in advanced or recurrent ovarian cancer. Cancer. 2014;120:3651–3659. doi: 10.1002/cncr.28940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Katsumata N, Yasuda M, Isonishi S, et al. Long-term results of dose-dense paclitaxel and carboplatin versus conventional paclitaxel and carboplatin for treatment of advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer (JGOG 3016): A randomised, controlled, open-label trial. Lancet Oncol. 2013;14:1020–1026. doi: 10.1016/S1470-2045(13)70363-2. [DOI] [PubMed] [Google Scholar]
- 31.Gynecologic Oncology Trial & Investigation Consortium. Intraperitoneal therapy for ovarian cancer with carboplatin trial (iPocc) Clinicaltrials.gov identifier NCT01506856. https://clinicaltrials.gov/ct2/show/NCT01506856.
- 32.National Cancer Institute. Bevacizumab and intravenous or intraperitoneal chemotherapy in treating patients with stage II-III ovarian epithelial cancer, fallopian tube cancer, or primary peritoneal cancer. Clinicaltrials.gov identifier NCT00951496. https://clinicaltrials.gov/show/NCT00951496.
- 33.Salani R, Backes FJ, Fung MF, et al. Posttreatment surveillance and diagnosis of recurrence in women with gynecologic malignancies: Society of Gynecologic Oncologists recommendations. Am J Obstet Gynecol. 2011;204:466–478. doi: 10.1016/j.ajog.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 34.Bristow RE, Chang J, Ziogas A, et al. High-volume ovarian cancer care: Survival impact and disparities in access for advanced-stage disease. Gynecol Oncol. 2014;132:403–410. doi: 10.1016/j.ygyno.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 35.Cliby WA, Powell MA, Al-Hammadi N, et al. Ovarian cancer in the United States: Contemporary patterns of care associated with improved survival. Gynecol Oncol. 2015;136:11–17. doi: 10.1016/j.ygyno.2014.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]