Abstract
Purpose
Breast cancer mortality began declining in many Western countries during the late 1980s. We estimated the proportion of improvements in stage- and age-specific breast cancer survival in the United States explained by tumor size or estrogen receptor (ER) status.
Methods
We estimated hazard ratios for breast cancer–specific death from time of invasive breast cancer diagnosis in the National Cancer Institute's Surveillance, Epidemiology, and End Results 9 Registries Database from 1973 to 2010, with and without stratification by tumor size and ER status.
Results
Hazards from breast cancer–specific death declined from 1973 to 2010, not only in the first 5 years after diagnosis, but also thereafter. Stratification by tumor size explained less than 17% of the improvements comparing 2005 to 2010 versus 1973 to 1979, except for women age ≥ 70 years with local (49%) or regional (38%) disease. Tumor size usually accounted for more of the improvement in the first 5 years after diagnosis than later. Additional adjustment for ER status (positive, negative, or unknown) from 1990 to 2010 did not explain much more of the improvement, except for women age ≥ 70 years within 5 years after diagnosis.
Conclusion
Most stage-specific survival improvement in women younger than age 70 years old is unexplained by tumor size and ER status, suggesting a key role for treatment. In the first 5 years after diagnosis, tumor size contributed importantly for women ≥ 70 years old with local and regional stage, and stratification by tumor size and ER status explained even more of the survival improvement among women age ≥ 70 years.
INTRODUCTION
Breast cancer mortality rates began declining among young women in many Western countries during the 1980s,1,2 including the United States,3,4 even though breast cancer incidence rates increased.5–7 Screening patterns,8,9 treatments,10,11 and the proportion of estrogen receptor (ER) –positive cancers7,12 also changed.
Peto et al2 attributed breast cancer mortality improvements in the United Kingdom and United States to diagnosis and treatment. Screening patterns and adjuvant therapies changed in response to guidelines,8–11 consensus statements,13–15 and clinical evidence.16–20 Elaborate models that incorporated data from the Surveillance, Epidemiology, and End Results (SEER) Program of the National Cancer Institute on breast cancer incidence, survival, and many other factors indicated that both screening and adjuvant treatment contributed to improvements in mortality, but estimates were uncertain.21–23 Elkin et al24 studied secular trends in relative breast cancer survival in SEER from 1975 to 1999. By standardizing on tumor size, they showed that a substantial proportion of the improvement was attributable to decreases in tumor size within local and regional stage.
We extended the approach of Elkin et al24 to study improving breast cancer survival in the United States. Instead of relative survival, we used two measures, hazard rates for breast cancer–specific death and breast cancer–specific survival, after breast cancer diagnosis. In addition, we extended SEER case data from 1973 to 2010 and also examined ER status. We estimated how much of the secular improvement in the hazard of breast cancer–specific death could be explained by stratification on tumor size from 1973 to 2010 and by stratification on tumor size and ER status from 1990 to 2010. We discuss these findings in connection with screening, adoption of adjuvant treatments, and ER-specific cancer incidence.
METHODS
Data
We obtained invasive breast cancer incidence5,6 and mortality25 data from January 1, 1973, through December 31, 2010, from the National Cancer Institute's SEER 9 Registries Database, which covers approximately 10% of the US population. We analyzed breast cancer survival in all women diagnosed with invasive breast cancer during this period. We examined the following data obtained at diagnosis: age at diagnosis, year of diagnosis, tumor size (priority: operative report; physical examination; mammography), lymph nodal status (negative or positive), SEER historic stage A (local, regional, distant, or unknown), and ER status (positive, negative, or unknown). SEER recorded tumor size and historic stage A from 1973 onward, lymph nodal status from 1988, and ER expression from 1990. Survival information included vital status, cause of death, and survival time in months. We included only the first primary breast cancer.
Statistical Analysis
Most analyses focused on hazard rates for breast cancer–specific death and breast cancer–specific survival after diagnosis in SEER. Hazard ratios (HRs) for breast cancer–specific death were estimated using Cox proportional hazards models separately for times less than 5 years and ≥ 5 years after diagnosis. We added 0.5 to each survival time in months. For less than 5 years, follow-up ended at the earliest of 5.001 years, time at December 31, 2010, or time at death. A breast cancer death indicator was 1.0 if the time of death was the earliest of these times and the death was ascribed to breast cancer; otherwise, the indicator was 0. For ≥ 5 years after diagnosis, follow-up ended at the earliest of time at December 31, 2010, or time at death, and contributions to the partial likelihood only came from ≥ 5 years. Survival was analyzed within strata defined jointly by age at diagnosis (< 50, 50 to 69, or ≥ 70 years) and SEER stage. HRs compared with 1973 to 1979 for breast cancer–specific death were computed for 1980 to 1984, 1985 to 1989, 1990 to 1994, 1995 to 1999, 2000 to 2004, and 2005 to 2010. For analyses from 1990 to 2010, when ER data were available, the reference period was 1990 to 1994. The unadjusted HRs were compared with adjusted hazard ratios (HRadj) obtained by stratification on tumor size or on both tumor size and ER status. Age and year at diagnosis were categorized as described earlier, tumor size as less than 10, 10 to 19, 20 to 29, 30 to 39, 40 to 49, or ≥ 50 mm or unknown, and ER as positive, negative, or unknown. For a given age group, stage, and survival period, we measured the proportion of improvement explained in hazards for breast cancer–specific death and in breast cancer–specific 5-year survival. The percentage of improvement explained in the hazard of breast cancer–specific death is Δ1 = 100 × (HRadj – HR)/(1 – HR). The percentage of improvement explained in 5-year survival is Δ2 = 100 × [{Ŝ(t | C) − Ŝ(t | Cref)} − {Ŝadj(t | C) − Ŝadj(t | Cref)}] / {Ŝ(t | C) − Ŝ(t | Cref)}, where t = 5 for less than 5 years and t = 10 for analysis of 5-year survivors. Ŝ is a Kaplan-Meier estimate of 5-year breast cancer–specific survival, Ŝadj is a weighted average of tumor or tumor plus ER stratum-specific survival estimates, weighted by the stratum distribution at reference time Cref (ie, 1973 to 1979 or 1990 to 1994), and C is a later time interval. In the Data Supplement, Δ1 and Δ2 denote parameters rather than estimates. Jackknife estimates of variances were obtained for estimates (Data Supplement). Incidence and mortality rates per 100,000 woman-years were age standardized (direct method, 2000 US population). Analyses were performed with R version 3.0.0 (http://www.r-project.org).
RESULTS
The SEER 9 Registries included 543,171 women with first primary invasive breast cancer and 468,761,934 woman-years of follow-up from 1973 to 2010. Women diagnosed at age less than 50 years had larger tumors, a smaller proportion of local-stage disease, a higher rate of positive lymph nodes, and a higher rate of ER-negative disease (Data Supplement Table 1).
Improvement in HRs of Breast Cancer–Specific Death,1973 to 2010, and Adjustment for Tumor Size
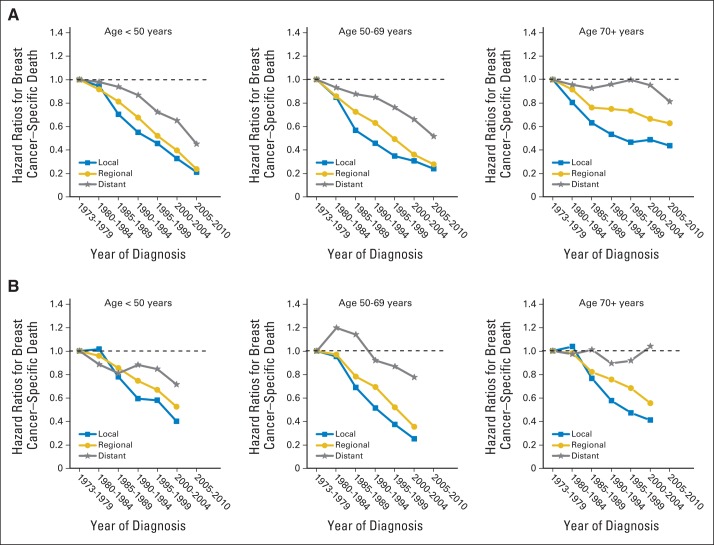
HRs compared with 1973 to 1979 for breast cancer–specific death are shown for three age-at-diagnosis strata for less than 5 years (Fig 1A) and ≥ 5 years after diagnosis (Fig 1B). In the first 5 years (Fig 1A), HRs decreased steadily in time for women younger than age 70, even among those with distant disease. In women age ≥ 70 years, improvements were confined to those with local or regional disease. Improvements were greater in younger than older women.
Fig 1.
Breast cancer–specific death hazard ratios are plotted against calendar time period, with referent 1973 to 1979. Plots are shown for three age-at-diagnosis strata and for (A) less than 5 years and (B) ≥ 5 years after diagnosis.
Figure 2A shows the percentage of the improvement in breast cancer–specific death hazard, comparing 2005 to 2010 versus 1973 to 1979, that is explained by stratification on tumor size in the first 5 years after diagnosis. For women younger than age 70 years, tumor size explained less than 17% of the improvement. For women age ≥ 70 years, the percentages explained were 49%, 39%, and 20% for local, regional, and distant disease, respectively. The percentages of improvement explained in breast cancer–specific 5-year survival were larger (86%, 65%, and 36% for local, regional, and distant disease, respectively) in women age ≥ 70 years, but still no larger than 33% in women younger than age 70 years (Data Supplement Table 2).
Fig 2.
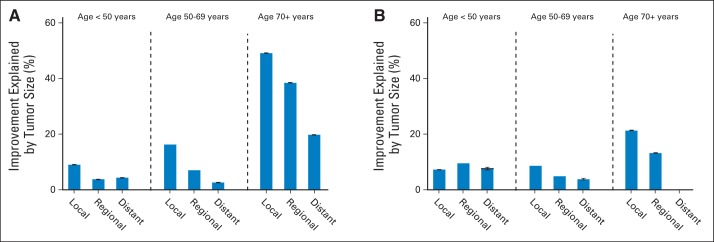
Percent improvement in breast cancer–specific death hazard ratios explained by stratification on tumor size. Data are shown for each combination of age group and stage at diagnosis: (A) 2005 to 2010 is compared with 1973 to 1979 for the first 5 years after diagnosis, and (B) 2000 to 2004 is compared with 1973 to 1979 for the period after 5 years after diagnosis. At the top of the bars, 95% CIs are indicated; no bar is shown if the CI includes zero.
After 5 years after diagnosis (Fig 1B), breast cancer–specific death HRs compared with 1973 to 1979 decreased over time for women with local or regional disease in all age groups. The HRs also decreased in women younger than age 50 years with distant disease. Figure 2B shows that the proportion of improvement explained is less than 10% in women younger than age 70 years and less than 22% in women age ≥ 70 years. The percentage of improvement in breast cancer–specific 5-year survival beginning 5 years after diagnosis that is explained is no larger than 25% for women younger than age 70 years and 24% for women age ≥ 70 years (Data Supplement Table 2; we do not mention estimates whose 95% CIs include 0).
Improvement in HRs of Breast Cancer-Specific Death,1990 to 2010, and Adjustment for Tumor Size Alone and for Tumor Size and ER Expression
HRs (compared with 1990 to 1994) for breast cancer–specific death in the first 5 years after diagnosis declined among women younger than age 70 years, even women with distant disease (Data Supplement Fig 1A). In women age ≥ 70 years, improvements were small and were not seen in women with distant disease. Figure 3A depicts the corresponding percentages of the improvements explained either by tumor size alone or by tumor size and ER status. Tumor size alone explained less than 10% of the improvement in women younger than age 70 years, and tumor size plus ER status explained less than 20%. In women age ≥ 70 years, tumor size explained as much as 46%, and tumor size plus ER status explained as much as 61%. Corresponding improvements in breast cancer–specific 5-year survival were less than 36% in women younger than age 70 years and less than 89% in women age ≥ 70 years (Data Supplement Table 3).
Fig 3.
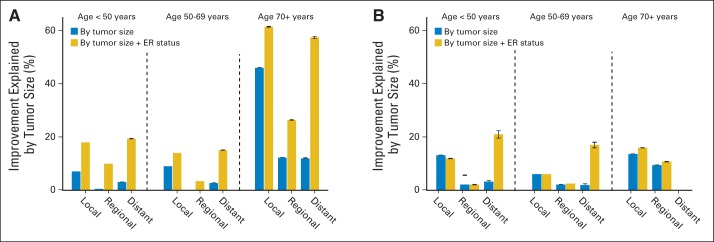
Percent improvement in breast cancer–specific death hazard ratios explained by stratification on tumor size and by stratification on tumor size and estrogen receptor (ER) status. Data are shown for each combination of age group and stage at diagnosis: (A) 2005 to 2010 is compared with 1990 to 1994 for the first 5 years after diagnosis, and (B) 2000 to 2004 is compared with 1990 to 1994 for the period after 5 years after diagnosis. At the top of the bars, 95% CIs are indicated; no bar is shown if the CI includes zero.
After 5 years after diagnosis, the HRs for breast cancer–specific death declined for women with local or regional disease (Data Supplement Fig 1B), and there was some improvement in women younger than age 70 years with distant disease. Changes in tumor size explained less than 14% of the improvement, regardless of age or stage (Fig 3B). Changes in tumor size and ER status explained 21% or less. Estimates of the percentage improvement in breast cancer–specific 5-year survival had wide confidence limits but were slightly larger than for breast cancer–specific death hazards (Data Supplement Table 3).
DISCUSSION
We applied survival methods to study secular trends in hazard rates for breast cancer–specific death and breast cancer–specific 5-year survival after diagnosis in SEER. These are not ecologic data but individual survival outcomes among women with first primary invasive breast cancer. We measured the proportion of improvement explained in breast cancer–specific death hazard rates and in breast cancer–specific 5-year survival and found the following results. Hazards from breast cancer–specific death declined consistently from 1973 to 2010, not only in the first 5 years after diagnosis, but also thereafter. Among women younger than age 70 years, stratification on tumor size explained less than 17% of the improvement in breast cancer–specific death hazards in the first 5 years comparing 2005 to 2010 versus 1973 to 1979. For women age ≥ 70 years, tumor size accounted for 49% of the improvement for local, 39% for regional, and 20% for distant stage in the first 5 years. Stratification on tumor size accounted for more improvement in the first 5 years after diagnosis than later. Improvements in hazards for breast cancer–specific death comparing 2005 to 2010 or 2000 to 2004 versus 1990 to 1994 were also impressive and could be adjusted for ER status as well as tumor size. Stratification on ER status and tumor size explained more than tumor size alone in 1990 to 2010. Adjustment for ER status and tumor size explained little among women younger than age 70 years, either within the first 5 years or thereafter. For women age ≥ 70 years, such adjustment explained 61% for local, 26% for regional, and 57% for distant stage within the first 5 years, but little thereafter.
Taken together, these results indicate that factors other than tumor size or ER status accounted for most of the stage-specific survival improvements in women younger than age 70 years. For women age ≥ 70 years, changes in tumor size explained much of the improvement from 1973 to 1979 to 2005 to 2010 in the first 5 years. After 5 years, neither tumor size alone nor tumor size and ER status explained much improvement in women age ≥ 70 years. Percent improvements explained in breast cancer–specific 5-year survival yielded similar conclusions (Data Supplement).
Our results describe survival improvements over time in each of nine categories defined by stage (local, regional, or distant) and age group (< 50, 50 to 69, or ≥ 70 years). However, it is challenging to relate these stage- and age-specific findings to trends in US breast cancer mortality rates because cancer screening has changed the distribution of age and stage at diagnosis and because changes in diagnostic methods may have altered the characteristics of patients within SEER stages in ways that are not captured by tumor size or ER status. For example, recently diagnosed women may have higher proportions of indolent tumors from length-biased sampling26 or overdiagnosis.27 Weighing against such explanations are the facts that improvements have been seen long after screening was well established and in women with distant disease at diagnosis, and our data excluded patients with in situ disease. Despite these concerns, it is tempting to interpret the stage- and age-specific improvements (shown in Fig 1 and Data Supplement Fig 1) in light of US data on factors such as use of adjuvant therapy and mammographic screening.
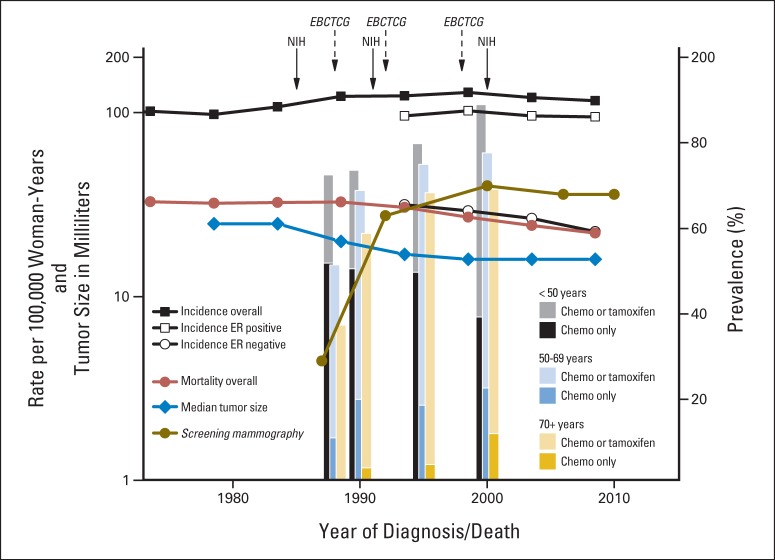
Figure 4 depicts various changes in the United States from 1973 to 2010. Overall age-adjusted breast cancer mortality rates were stable initially but decreased 30% from 33.5% in 1988 to 23.5% in 2010. Overall incidence rates increased through 2000 and declined slightly thereafter. ER-positive incidence rates have tracked with overall incidence, whereas ER-negative incidence rates have declined. Median tumor size decreased from 25 mm in the early 1980s to 16 mm by 2009. The proportion of women with mammographic screening within the previous 2 years increased from 29% in 1987 to approximately 70% from 2000 onward.8,9 From special studies in SEER,10,11 the percentages of women with invasive breast cancer who received adjuvant hormonal therapy and/or chemotherapy in 2000 were approximately 70% at age ≥ 70, 78% at age 50 to 69, and 88% at age 50 and younger. However, only approximately 10% of women age ≥ 70 years received chemotherapy alone in 2000, compared with 21% at age 50 to 59 and 40% at age less than 50 years.11
Fig 4.
Secular trends are shown for age-standardized (direct method, 2000 US population) breast cancer incidence (per 100,000 woman-years), for age-standardized estrogen receptor (ER)–positive incidence, for age-standardized ER-negative incidence, for age-standardized breast cancer mortality rates, and for median tumor size in millimeters. Data were obtained from the SEER 9 Registries Database for the years 1973 through 2010. Percent prevalence is displayed for adjuvant hormonal and/or chemotherapy and for screening mammography in the previous 2 years. Percent prevalence for chemotherapy was derived in SEER from Harlan et al10,11 and for screening mammography from the American Cancer Society's Facts and Figures.9,38 Publication dates for National Institutes of Health (NIH) Consensus Conferences13–15 and systemic reviews from the Early Breast Cancer Trialists' Collaborative Group (EBCTCG)16–20 are noted with solid and dashed arrows, respectively. The x-axis is on a linear time scale, the y-axis on the left is shown on a log scale, and the y-axis on the right is on a linear percentage scale. Chemo, chemotherapy.
The data in Figure 4 provide context and possible explanations for our findings. Changes in tumor size explained an appreciable proportion of the improvements in women age ≥ 70 years in the first 5 years after diagnosis (Fig 1A and Data Supplement). Screening, as well as palpation by health care providers and self-examination, may have contributed to decreases in tumor size, rendering surgical treatment more effective. In contrast, changes in tumor size explained little of the improvement in the first 5 years after diagnosis seen in women younger than age 70 years, who were treated more aggressively than older women. Consistent with this view is the fact that women younger than age 70 years with distant disease also showed important reductions in hazards for breast cancer–specific death (Fig 1A and Data Supplement); screening would not be expected to contribute much to improvements in women with distant disease. There were also important improvements in hazards for breast cancer–specific death beyond 5 years after diagnosis (Fig 1B and Data Supplement), but changes in tumor size explained little (Figs 2B and 3B).
From 1990 to 2010, breast cancers with ER-negative and ER-unknown status decreased in all age groups.7 Women with ER-negative and ER-unknown status have poorer survival than women with ER-positive cancer (unreported analyses). Among SEER patients with known ER status, the proportion of ER-positive patients increased in women younger than age 70 years but remained constant at 85% in women age ≥ 70 years (Data Supplement Table 4). Thus, the survival improvements explained by ER status among women younger than 70 years old (Fig 3) may reflect increasing proportions of ER-positive cancers and decreasing proportions of ER-negative and ER-unknown cancers. Among women age ≥ 70 years, the survival improvements explained by ER status probably reflect decreasing proportions of ER-unknown cancers (28.8% in 1990 to 1994 and 8.4% in 2005 to 2010).
Analyses for 1975 to 2000 from seven mathematical models attributed 28% to 65% of the reduction in the US breast cancer mortality rate in 2000 to screening mammography,22 with the balance, 72% to 35%, attributed to adjuvant therapy. An update that also used ER status attributed 38% to 52% to screening.23 It is difficult to compare our findings with these estimates of the proportion of improvements in overall US breast cancer mortality attributable to screening, because these models also incorporated information on the changing stage distribution. It is striking, however, how little of the stage- and age-specific improvements in women younger than age 70 years could be explained by changes in tumor size over time (Figs 2 and 3). The fact that tumor size explains little of the improvement in women younger than age 70 years suggests that treatment, rather than screening, accounts for most of the improvement in such women. Perhaps this fact is not evident when one analyzes overall US mortality rates.22,23
We assumed that improvements explained by stratification on tumor size reflect detection of smaller tumors by screening and consequent improved local control. However, smaller tumor size is temporally associated with decreasing ER-negative incidence, increasing proportions of ER-positive cancers, and decreasing proportions with unknown ER status (Fig 4 and Data Supplement Table 4). Hence survival improvements attributed to tumor size may partly reflect an increasing proportion of the more treatable ER-positive cancers and a decreasing proportion of the more difficult to manage ER-negative and ER-unknown cancers. Moreover, the extent of the mortality reduction from screening mammography is uncertain.28 In screening trials, mortality benefits lag behind initiation of screening by 10 to 14 years.29,30 US breast cancer mortality began to decline before widespread screening (Fig 4).31 In addition, mortality declines and screening began almost simultaneously in the United Kingdom, without the expected lag.2,32 Similar breast cancer mortality declines have been observed in European countries with and without national screening programs.33,34 Moreover, ecologic comparisons within Denmark failed to show greater mortality reductions in screened regions than in unscreened regions.35 Hence, our estimates of the proportions of survival improvements explained by tumor size may overestimate age- and stage-specific screening effects.
Study weaknesses include lack of individual treatment and screening data. ER data included unknowns and came from patient records and from many laboratories.36,37 Women within a given SEER stage may have changed over time in ways not measured by tumor size or ER status. The study had several strengths. It included a large representative sample of patients with invasive breast cancer, which permitted analyses within age-by-stage groups. Individual survival data, not ecologic data, drove these analyses. Compared with approaches based on complex models,22,23 we required few untestable assumptions. However, our assessments of the relative importance of screening and treatment are consequently less formal, and the interpretation of percent improvement explained by tumor size as a screening effect is open to question.
To summarize, women diagnosed with invasive breast cancer experienced large reductions in breast cancer–specific death hazards from 1973 to 2010, not only in the first 5 years after diagnosis, but also after 5 years. Improvements were evident throughout 1973 to 2010 and were also found in women younger than age 70 years with distant stage. Changes in tumor size explained less than 17% of the improvement in breast cancer–specific death hazards (33% of the improvement in breast cancer–specific 5-year survival) in women younger than age 70 years, suggesting that treatment played a key role. For women age ≥ 70 years, decreasing tumor size contributed to the improvements, suggesting an impact of screening or a change in inherent tumor characteristics. Better care associated with fewer patients with unknown ER status may have also improved the prognosis among women age ≥ 70 years in the first 5 years after diagnosis.
Footnotes
See accompanying editorial on page 2837
Supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Division of Cancer Epidemiology and Genetics (J-H.P., W.F.A., and M.H.G.) and by the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Science, ICT & Future Planning (Grant No. NRF-2013R1A1A1009737) (J.-H.P).
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: William F. Anderson, Mitchell H. Gail
Collection and assembly of data: William F. Anderson
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Improvements in US Breast Cancer Survival and Proportion Explained by Tumor Size and Estrogen-Receptor Status
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Ju-Hyun Park
No relationship to disclose
William F. Anderson
No relationship to disclose
Mitchell H. Gail
No relationship to disclose
REFERENCES
- 1.Hermon C, Beral V. Breast cancer mortality rates are levelling off or beginning to decline in many western countries: Analysis of time trends, age-cohort and age-period models of breast cancer mortality in 20 countries. Br J Cancer. 1996;73:955–960. doi: 10.1038/bjc.1996.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peto R, Boreham J, Clarke M, et al. UK and USA breast cancer deaths down 25% in year 2000 at ages 20-69 years. Lancet. 2000;355:1822. doi: 10.1016/S0140-6736(00)02277-7. [DOI] [PubMed] [Google Scholar]
- 3.Blot WJ, Devesa SS, Fraumeni JF., Jr Declining breast cancer mortality among young American women. J Natl Cancer Inst. 1987;78:451–454. [PubMed] [Google Scholar]
- 4.Tarone RE, Chu KC. Implications of birth cohort patterns in interpreting trends in breast cancer rates. J Natl Cancer Inst. 1992;84:1402–1410. doi: 10.1093/jnci/84.18.1402. [DOI] [PubMed] [Google Scholar]
- 5.National Cancer Institute. SEER*Stat Database: Incidence, SEER 9 Regs Research Data, Nov 2012 Sub (1973-2010), Katrina/Rita Population Adjustment, Linked to County Attributes, Total U.S., 1969-2011 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2013, based on the November 2012 submission, 2013. www.seer.cancer.gov.
- 6.National Cancer Institute. SEER*Stat Database: Incidence, SEER 18 Regs Research Data + Hurricane Katrina Impacted Louisianna Cases, Nov 2012 Sub (1973-2010 varying), Linked to County Attributes, Total U.S., 1969-2011 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2013, based on the November 2012 submission, 2013. www.seer.cancer.gov.
- 7.Anderson WF, Katki HA, Rosenberg PS. Breast cancer incidence in the United States: Current and future trends. J Natl Cancer Inst. 2011;103:1397–1402. doi: 10.1093/jnci/djr257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Swan J, Breen N, Graubard BI, et al. Data and trends in cancer screening in the United States: Results from the 2005 National Health Interview Survey. Cancer. 2010;116:4872–4881. doi: 10.1002/cncr.25215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American Cancer Society. Breast Cancer Facts & Figures 2013-2014. Atlanta, GA: American Cancer Society; 2014. [Google Scholar]
- 10.Harlan LC, Abrams J, Warren JL, et al. Adjuvant therapy for breast cancer: Practice patterns of community physicians. J Clin Oncol. 2002;20:1809–1817. doi: 10.1200/JCO.2002.07.052. [DOI] [PubMed] [Google Scholar]
- 11.Harlan LC, Clegg LX, Abrams J, et al. Community-based use of chemotherapy and hormonal therapy for early-stage breast cancer: 1987-2000. J Clin Oncol. 2006;24:872–877. doi: 10.1200/JCO.2005.03.5840. [DOI] [PubMed] [Google Scholar]
- 12.Anderson WF, Rosenberg PS, Petito L, et al. Divergent estrogen receptor-positive and -negative breast cancer trends and etiologic heterogeneity in Denmark. Int J Cancer. 2013;133:2201–2206. doi: 10.1002/ijc.28222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Consensus conference. Adjuvant chemotherapy for breast cancer. JAMA. 1985;254:3461–3463. [PubMed] [Google Scholar]
- 14.NIH consensus conference. Treatment of early-stage breast cancer. JAMA. 1991;265:391–395. [PubMed] [Google Scholar]
- 15.National Institutes of Health Consensus Development Panel. National Institutes of Health Consensus Development Conference statement: Adjuvant therapy for breast cancer, November 1-3, 2000. J Natl Cancer Inst Monogr. 2001;30:5–15. [PubMed] [Google Scholar]
- 16.Early Breast Cancer Trialists' Collaborative Group. Effects of adjuvant tamoxifen and of cytotoxic therapy on mortality in early breast cancer. An overview of 61 randomized trials among 28,896 women. N Engl J Med. 1988;319:1681–1692. doi: 10.1056/NEJM198812293192601. [DOI] [PubMed] [Google Scholar]
- 17.Early Breast Cancer Trialists' Collaborative Group. Systemic treatment of early breast cancer by hormonal, cytotoxic, or immune therapy. 133 randomised trials involving 31,000 recurrences and 24,000 deaths among 75,000 women. Lancet. 1992;339:1–15. [PubMed] [Google Scholar]
- 18.Early Breast Cancer Trialists' Collaborative Group. Systemic treatment of early breast cancer by hormonal, cytotoxic, or immune therapy: 133 randomised trials involving 31,000 recurrences and 24,000 deaths among 75,000 women. Lancet. 1992;339:71–85. [PubMed] [Google Scholar]
- 19.Early Breast Cancer Trialists' Collaborative Group. Polychemotherapy for early breast cancer: An overview of the randomised trials. Lancet. 1998;352:930–942. [PubMed] [Google Scholar]
- 20.Early Breast Cancer Trialists' Collaborative Group. Tamoxifen for early breast cancer: An overview of the randomised trials. Lancet. 1998;351:1451–1467. [PubMed] [Google Scholar]
- 21.Berry DA, Inoue L, Shen Y, et al. Modeling the impact of treatment and screening on U.S. breast cancer mortality: A Bayesian approach. J Natl Cancer Inst Monogr. 2006;36:30–36. doi: 10.1093/jncimonographs/lgj006. [DOI] [PubMed] [Google Scholar]
- 22.Berry DA, Cronin KA, Plevritis SK, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005;353:1784–1792. doi: 10.1056/NEJMoa050518. [DOI] [PubMed] [Google Scholar]
- 23.Munoz D, Near AM, van Ravesteyn NT, et al. Effects of screening and systemic adjuvant therapy on ER-specific US breast cancer mortality. J Natl Cancer Inst. 2014:106. doi: 10.1093/jnci/dju289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elkin EB, Hudis C, Begg CB, et al. The effect of changes in tumor size on breast carcinoma survival in the U.S.: 1975-1999. Cancer. 2005;104:1149–1157. doi: 10.1002/cncr.21285. [DOI] [PubMed] [Google Scholar]
- 25.National Cancer Institute. SEER*Stat Database: Mortality, All COD, Aggregated With State, Total U.S. (1969-2010), Katrina/Rita Population Adjustment, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2013, underlying mortality data provided by NCHS ( www.cdc.gov/nchs), 2013. www.seer.cancer.gov.
- 26.Zelen M, Feinleib M. On the theory of screening for chronic diseases. Biometrika. 1969;56:601–614. [Google Scholar]
- 27.Baker SG, Prorok PC, Kramer BS. Lead time and overdiagnosis. J Natl Cancer Inst. 2014:106. doi: 10.1093/jnci/dju346. [DOI] [PubMed] [Google Scholar]
- 28.Gotzsche PC, Jorgensen KJ. Screening for breast cancer with mammography. Cochrane Database Syst Rev. 2013;6:CD001877. doi: 10.1002/14651858.CD001877.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shapiro S, Venet W, Strax P, et al. Ten- to fourteen-year effect of screening on breast cancer mortality. J Natl Cancer Inst. 1982;69:349–355. [PubMed] [Google Scholar]
- 30.Baum M. Survival and reduction in mortality from breast cancer. Impact of mammographic screening is not clear. BMJ. 2000;321:1470. [PMC free article] [PubMed] [Google Scholar]
- 31.Feuer EJ, Wun LM. How much of the recent rise in breast cancer incidence can be explained by increases in mammography utilization? A dynamic population model approach. Am J Epidemiol. 1992;136:1423–1436. doi: 10.1093/oxfordjournals.aje.a116463. [DOI] [PubMed] [Google Scholar]
- 32.Forrest P. Report to the Health Ministers of England, Wales, Scotland, and Northern Ireland. London, United Kingdom: Department of Health and Social Security; 1986. Breast Cancer Screening. [Google Scholar]
- 33.Autier P, Boniol M, Gavin A, et al. Breast cancer mortality in neighbouring European countries with different levels of screening but similar access to treatment: Trend analysis of WHO mortality database. BMJ. 2011;343:d4411. doi: 10.1136/bmj.d4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Botha JL, Bray F, Sankila R, et al. Breast cancer incidence and mortality trends in 16 European countries. Eur J Cancer. 2003;39:1718–1729. doi: 10.1016/s0959-8049(03)00118-7. [DOI] [PubMed] [Google Scholar]
- 35.Jorgensen KJ, Zahl PH, Gotzsche PC. Breast cancer mortality in organised mammography screening in Denmark: Comparative study. BMJ. 2010;340:c1241. doi: 10.1136/bmj.c1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tarone RE, Chu KC. The greater impact of menopause on ER- than ER+ breast cancer incidence: A possible explanation (United States) Cancer Causes Control. 2002;13:7–14. doi: 10.1023/a:1013960609008. [DOI] [PubMed] [Google Scholar]
- 37.Anderson WF, Luo S, Chatterjee N, et al. Human epidermal growth factor receptor-2 and estrogen receptor expression, a demonstration project using the residual tissue repository of the Surveillance, Epidemiology, and End Results (SEER) program. Breast Cancer Res Treat. 2009;113:189–196. doi: 10.1007/s10549-008-9918-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Swan J, Breen N, Coates RJ, et al. Progress in cancer screening practices in the United States: Results from the 2000 National Health Interview Survey. Cancer. 2003;97:1528–1540. doi: 10.1002/cncr.11208. [DOI] [PubMed] [Google Scholar]