Abstract
1,2-Cyclohexane dicarboxylic acid, diisononyl ester (DINCH) is a complex mixture of nine carbon branched-chain isomers. It has been used in Europe since 2002 as a plasticizer to replace phthalates such as di(2-ethylhexyl)phthalate (DEHP) and diisononyl phthalate (DINP). Urinary concentrations of the oxidative metabolites of DINCH, namely cyclohexane-1,2-dicarboxylic acid-monocarboxy isooctyl ester (MCOCH); cyclohexane-1,2-dicarboxylic acid-mono(oxo-isononyl) ester (MONCH); and cyclohexane-1,2-dicarboxylic acid-mono(hydroxy-isononyl) ester (MHNCH), can potentially be used as DINCH exposure biomarkers. The concentrations of MCOCH, MONCH and MHNCH were measured by online solid phase extraction-high performance liquid chromatography-tandem mass spectrometry in urine collected in 2000 (n=114), 2001 (n=57), 2007 (n=23), 2009 (n=118), 2011 (n=94) and 2012 (n=121) from convenience groups of anonymous U.S. adult volunteers with no known DINCH exposure. None of the DINCH metabolites were detected in samples collected in 2000 and 2001. Only one sample collected in 2007 had measureable concentrations of DINCH metabolites. The detection rate for all three metabolites increased from 2007 to 2012. The presence of oxidative metabolites of DINCH in urine suggests that these oxidative metabolites can be used as DINCH biomarkers for exposure assessment even at environmental exposure levels.
Keywords: DINCH, Plasticizers, Phthalates, Exposure, Oxidative metabolites
1. Introduction
1,2-Cyclohexane dicarboxylic acid, diisononyl ester is also known as di(isononyl) cyclohexane-1,2-dicarboxylate (DINCH). It is a complex mixture of linear and branched nine carbon dialkyl chain isomers of 1,2-cyclohexane dicarboxylic acid. DINCH is manufactured by catalytic hydrogenation of the aromatic ring of diisononyl phthalate (DINP) and commercialized as Hexamoll® DINCH. Since 2002, Hexamoll® DINCH has been used primarily as a plasticizer in polyvinylchloride plastics (up to 40%) and as an impact modifier in polystyrene (max 3%) in Europe (EFSA, 2006). DINCH is specifically used in human contact applications such as toys, medical devices, and food packaging (EFSA, 2006). In 2006, the European Food Safety Authority approved DINCH for a wide variety of food contact applications. DINCH is considered a safe alternative to di(2-ethylhexyl) phthalate (DEHP) and DINP (Crespo et al., 2007), whose use has been restricted in the United States and some European countries because of concerns regarding potential human toxicity (Consumer Product Safety Commission, 2012; European Council for Plasticisers and Intermediates, 2012). Because of the growing global demand for alternative plasticizers, however, the production of DINCH is on the rise (BASF, 2011), and human exposure to DINCH is expected to occur.
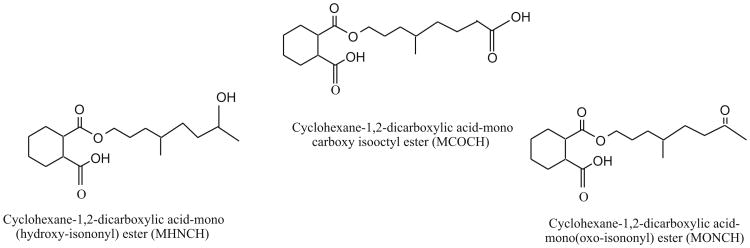
Toxicological studies in animals have shown no evidence of developmental or reproductive toxicity or endocrine disruptive properties of DINCH (EFSA, 2006). But at high doses, thyroid hyperplasia and signs of renal toxicity have been reported in rats (EFSA, 2006). Several oxidative metabolites of DINCH have been identified in DINCH-dosed rats (Silva et al., 2012) and have been used as biomarkers of exposure to DINCH in humans (Koch et al., 2013b; Schutze et al., 2012). In this study, we measured three of these oxidative metabolites: cyclohexane-1,2-dicarboxylic acid-monocarboxy isooctyl ester (MCOCH), cyclohexane-1,2-dicarboxylic acid-mono(oxo-isono-nyl) ester (MONCH), and cyclohexane-1,2-dicarboxylic acid-mono (hydroxy-isononyl) ester (MHNCH) (Fig. 1), in urine samples collected in 2000—2012 from convenience samples of U.S. adults with no known DINCH exposure. Because DINCH is used as a replacement plasticizer for DEHP and DINP applications, we also measured mono-(2-ethy-5-carboxyhexyl) phthalate (MECPP), a metabolite of DEHP, and mono-carboxyoctyl phthalate (MCOP), a metabolite of DINP. We hypothesized that we could use urinary concentrations of DINCH metabolites to assess the internal dose of DINCH in humans because urinary concentrations of nonpersistent chemicals or their metabolites have been used frequently as exposure biomarkers (Calafat et al., 2006; Needham et al., 2004; Sexton et al., 2004).
Fig. 1.
DINCH metabolites proposed as biomarkers for exposure assessment in humans. Structures shown represent only one of the potential isomers.
2. Materials and methods
2.1. Chemicals
Cyclohexane-1,2-dicarboxylic acid, mono hydroxyisononyl ester (MHNCH) and D4-MHNCH were generous gifts from Dr. Holger Koch (University of Bochum, Germany). We purchased MECPP from Cambridge Isotope laboratories, Inc. (Andover, MA). 13C6-MECPP, MCOP and 13C6-MCOP were purchased from CanSyn (Ontario, Canada). Acetonitrile (HPLC grade), water (HPLC grade), and methanol (99.8%, HPLC grade) were purchased from Honeywell Burdick & Jackson (Muskegon, MI). β-glucuronidase (Escherichia coli-K12) was purchased from Roche Biomedical (Mannheim, Germany). All chemicals and reagents were used without further purification.
2.2. Analytical method
The analytical method for measuring DINCH and phthalate oxidative metabolites in urine, adapted from previous methods (Kato et al., 2005; Silva et al., 2007), is presented in detail in the Supporting information. Briefly, urine (0.1 mL) was spiked with an internal standard solution containing deuterium- or 13C-labeled analogs of the target metabolites and a buffered β-glucuronidase solution (E. coli-K12; 25 μL, pH 6.5/1 M), and incubated for a minimum of 120 min. The target analytes in the spiked urine were extracted using online solid phase extraction, chromatographically resolved by high performance liquid chromatography and detected using negative ion electrospray-ionization tandem mass spectrometry.
We did not attempt to separate the individual isomers of the DINCH or DINP metabolites. The isomers of the metabolites were eluted as undefined cluster of peaks (Fig. 2) and, for quantification, we integrated the whole area under the cluster of peaks (Silva et al., 2012, 2006a). We quantified MONCH and MCOCH using the MHNCH calibration curve with D4-MHNCH as the internal standard. Therefore, the reported concentrations for MONCH and MCOCH are only semi-quantitative in nature.
Fig. 2.
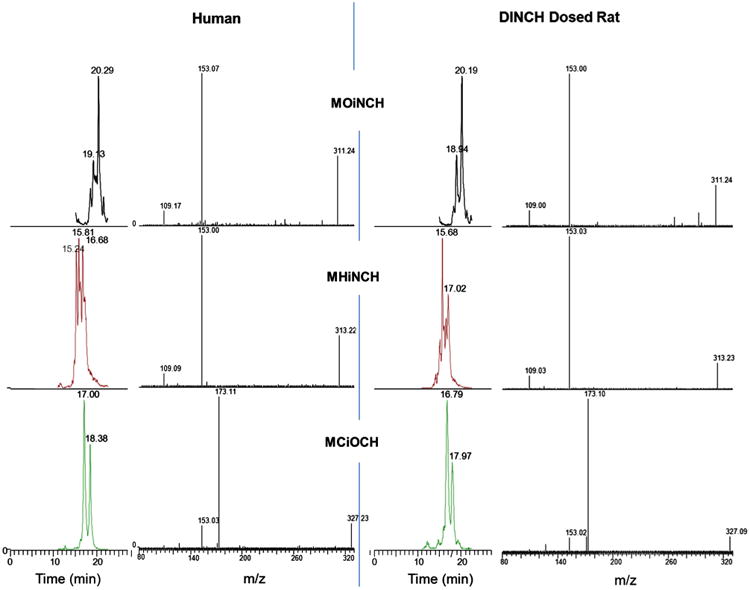
Chromatographic separation and mass spectra of three DINCH metabolites from human urine (this work) and DINCH-dosed rat urine (Silva et al., 2012). The identical retention times and the mass spectra of the metabolites between human urine and dosed rat urine confirm the positive identification of DINCH metabolites. Multiple isomers of each metabolite eluted with close retention times.
The identity of each metabolite peak in human urine was confirmed by matching the mass spectra obtained from DINCH-dosed rat urine (Fig. 2). The limits of detection (LODs) were 0.4 ng/mL for the three DINCH metabolites and 0.2 ng/mL for MECPP and MCOP. The enzymatic hydrolysis step was omitted during sample preparation to measure the free metabolite concentrations. Subsets of samples with analyte concentrations at least >3*LOD were analyzed for free metabolite concentrations to increase the accuracy of the quantification of free metabolites. Samples with concentrations of DINCH metabolites ranging from LOD to 3*LOD were not analyzed for free metabolite species. Correlation analysis between metabolites were performed with levels >LOD.
2.3. Subjects
The urine samples analyzed for this study were archived samples (stored at -70 °C) collected anonymously in Atlanta, GA in 2000, 2001, 2007, 2009, 2011, and 2012 from a demographically diverse group of U.S. male and female adults with no known DINCH exposure. No personal information from the subjects was available. Samples were collected between 8:00 am and 5:00 pm and were not necessarily first-morning voids. The Centers for Disease Control and Prevention (CDC) Internal Review Board approved the collection of the urine for the development and validation of analytical methods at CDC. A waiver of informed consent was requested under 45 CFR 46.116(d).
3. Results and discussion
In experimental animals, DINCH is less toxic than the high molecular weight phthalate plasticizers DEHP and DINP; thus DINCH is considered a versatile replacement for these chemicals (EFSA, 2006). The potential health effects of DINCH in humans are currently unknown.
High molecular weight phthalates, such as DEHP and DINP, metabolize extensively before their excretion in urine (Koch and Calafat, 2009; Silva et al., 2006b). Some of these metabolites are unique for the parent chemical. Thus, some metabolites have been used as exposure biomarkers for DEHP (e.g., MECPP) and DINP (e.g., MCOP) (Koch et al., 2007; Silva et al., 2006a). Similarly, MHNCH, MCOCH, and MONCH are unique DINCH metabolites and as such can be used as biomarkers of DINCH exposure (Koch et al., 2013b; Silva et al., 2012).
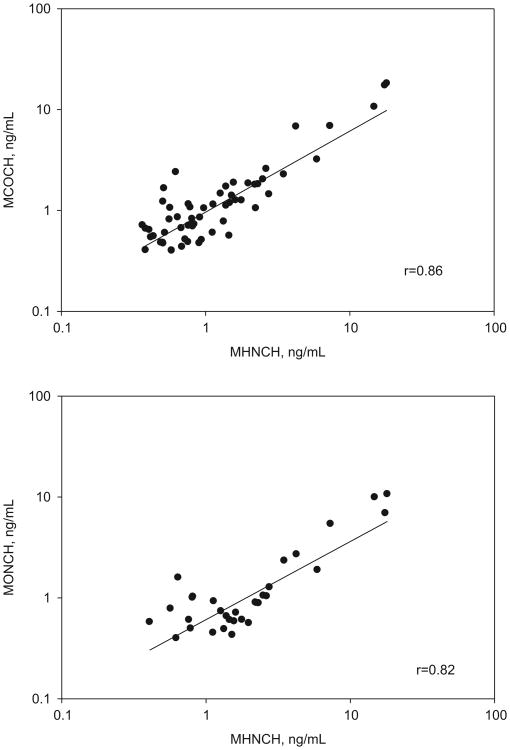
In this study, the concentrations of MHNCH, MCOCH, and MONCH were measured in urine collected in 2000, 2001, 2007, 2009, 2011, and 2012 from convenience samples of U.S. male and female adults. We observed a wide range of concentrations of DINCH metabolites (Table 1). As expected, no DINCH metabolites were detected in urine collected in 2000 and 2001 because commercialization of DINCH started in Europe in 2002 (EFSA, 2006). We detected DINCH metabolites in only one of the samples collected in 2007; the frequency of detection of DINCH metabolites increased to almost 10% in the samples collected in 2009 [MHNCH (8%), MCOCH (8%), MONCH (9%)] and to approximately 15% in those collected in 2011 [MHNCH (13%), MCOCH (15%), MONCH (15%)]. In the samples collected in 2012, the detection frequency and magnitude of the concentrations were higher than in earlier years (Table 1). Because the detection rate was <50% for all three DINCH metabolites in all the years examined, we did not calculate geometric mean concentrations. Nevertheless, because all three metabolites of DINCH originated from a common precursor, their concentrations correlated well (r>0.8, Fig. 3).
Table 1.
Distribution of urinary concentrations (in ng/mL) of DINCH, DEHP and DINP metabolites.a
| Urinary metabolite | Year | N | Select percentiles | Frequency of detection (%) | |||
|---|---|---|---|---|---|---|---|
|
| |||||||
| 50th | 75th | 90th | 95th | ||||
| MHNCH | 2000 | 114 | <LOD | <LOD | <LOD | <LOD | 0 |
| 2001 | 57 | <LOD | <LOD | <LOD | <LOD | 0 | |
| 2007 | 23 | <LOD | <LOD | <LOD | <LOD | 4 | |
| 2009 | 118 | <LOD | <LOD | <LOD | 0.5 | 8 | |
| 2011 | 94 | <LOD | <LOD | 0.6 | 1.5 | 13 | |
| 2012 | 121 | <LOD | <LOD | 0.6 | 1.4 | 19 | |
| MCOCHb | 2000 | 114 | <LOD | <LOD | <LOD | <LOD | 0 |
| 2001 | 57 | <LOD | <LOD | <LOD | <LOD | 0 | |
| 2007 | 23 | <LOD | <LOD | <LOD | <LOD | 4 | |
| 2009 | 118 | <LOD | <LOD | <LOD | 0.4 | 8 | |
| 2011 | 94 | <LOD | <LOD | 0.5 | 1.2 | 15 | |
| 2012 | 121 | <LOD | <LOD | 0.8 | 2.4 | 21 | |
| MONCHb | 2000 | 114 | <LOD | <LOD | <LOD | <LOD | 0 |
| 2001 | 57 | <LOD | <LOD | <LOD | <LOD | 0 | |
| 2007 | 23 | <LOD | <LOD | <LOD | <LOD | 4 | |
| 2009 | 118 | <LOD | <LOD | <LOD | 0.5 | 9 | |
| 2011 | 94 | <LOD | <LOD | <LOD | 0.4 | 15 | |
| 2012 | 121 | <LOD | <LOD | 0.6 | 1 | 16 | |
| MCOP | 2000 | 114 | 2.5 | 4.1 | 7.2 | 12.2 | 97 |
| 2001 | 57 | 2.0 | 3.9 | 5.3 | 7.0 | 97 | |
| 2007 | 23 | 4.7 | 9.5 | 26.1 | 51.3 | 96 | |
| 2009 | 118 | 19.9 | 71.4 | 199 | 408 | 100 | |
| 2011 | 94 | 15.6 | 45.2 | 115 | 158 | 100 | |
| 2012 | 121 | 27.4 | 65.6 | 157 | 237 | 100 | |
| MECPP | 2000 | 114 | 20.3 | 37.9 | 72.2 | 152 | 100 |
| 2001 | 57 | 21.2 | 39.1 | 109 | 206 | 96 | |
| 2007 | 23 | 28.3 | 84.5 | 138 | 149 | 96 | |
| 2009 | 118 | 20.8 | 66.1 | 279 | 383 | 100 | |
| 2011 | 94 | 10.8 | 19.2 | 46.9 | 68.5 | 100 | |
| 2012 | 121 | 11.7 | 21.4 | 47.2 | 70.2 | 100 | |
LOD-limit of detection; LOD=0.4 ng/mL for MONCH, MHNCH and MCOCH, and 0.2 ng/mL for MCOP and MECPP.
Concentrations of MONCH and MCOCH are only semi-quantitative based on MHNCH standards.
Fig. 3.
Correlation analyses of urinary concentrations of DINCH metabolites. r represents the Pearson correlation coefficient. Solid line represents the regression line.
Glucuronidation facilitates urinary excretion of xenobiotics (Silva et al., 2003). MONCH and MHNCH excreted fully glucuroni-dated (>99%). However, for MCOCH, the most polar of the three DINCH metabolites measured, the percentage excreted as the glucuronide conjugate was considerably lower (65%) than for the other metabolites.
The urinary concentrations of MHNCH, MONCH, and MCOCH were lower than the concentrations of the DEHP and DINP metabolites, MECPP and MCOP, respectively (Table 1). Furthermore, the urinary concentrations of DINCH metabolites did not correlate with the urinary concentrations of either MECPP or MCOP (r<0.1, p>0.05). The lack of correlation between DINCH and phthalate metabolites in these subjects may indicate a difference between the sources of DINCH exposure and the two phthalates examined. Interestingly, the urinary concentrations of MCOP—a DINP metabolite—increased from 2000 to 2012 (Table 1). In contrast, we observed a decline in the urinary concentrations of MECPP—a DEHP metabolite—during the same time period. Similarly, a German retrospective human biomonitor-ing study reported a decline in urinary concentrations of DEHP metabolites and an increase in urinary concentrations of DINP metabolites (Wittassek et al., 2007).
In a recent study, three human volunteers, who were orally administered DINCH, excreted 23.7% of DINCH as the non-specific metabolite cyclohexane-1,2-dicarboxylic acid, 2% as MONCH, 10.7% as MHNCH and 2% as MCOCH (Koch et al., 2013a). These data suggest that the metabolism of DINCH is different from that of phthalates because, unlike DINCH, DEHP and DINP mostly excrete as their oxidative metabolites (Koch and Calafat, 2009). Taken together, the findings above suggest that until 2012, environmental exposure to DINCH in the United States was likely lower than exposure to DEHP and DINP. Further, based on urinary concentrations of the respective metabolites, exposures to DINCH and DINP appear to have increased since the early 2000s, while exposure to DEHP may have decreased over the same period. For DINP and DEHP, data from the U.S. National Health and Nutrition Examination Survey (NHANES) also show similar trends in geometric mean concentrations of MCOP and MECPP (Table 2), (CDC, 2013), thus suggesting a parallel decrease in DEHP exposure and increase in DINP exposure.
Table 2.
Geometric mean concentrations (in ng/mL) of urinary mono-(carboxyoctyl)phtha-late (MCOP) and mono-(2-ethyl-5-carboxypentyl)phthalate (MECPP) for the US population from the National Health and Nutrition Examination Survey (CDC, 2013).
| Urinary metabolite | Year | N | Select percentiles | Geometric mean | |||
|---|---|---|---|---|---|---|---|
|
| |||||||
| 50th | 75th | 90th | 95th | ||||
| MCOP | 2005—2006 | 2548 | 5.10 | 10.9 | 25.5 | 54.4 | 5.39 |
| 2007—2008 | 2604 | 6.40 | 14.9 | 34.6 | 63.0 | 6.79 | |
| 2009—2010 | 2749 | 11.8 | 33.1 | 83.3 | 160 | 12.6 | |
| MECPP | 2003—2004 | 2605 | 33.0 | 71.8 | 168 | 339 | 34.7 |
| 2005—2006 | 2548 | 35.6 | 79.7 | 211 | 386 | 38.6 | |
| 2007—2008 | 2604 | 31.2 | 69.5 | 153 | 308 | 33.3 | |
| 2009—2010 | 2749 | 20.4 | 39.9 | 78.4 | 127 | 20.7 | |
In summary, we measured urinary concentrations of three oxidative metabolites of DINCH, a replacement plasticizer for DINP and DEHP, in urine collected in 2000—2012 from several convenience samplings of U.S. adults. The metabolites of DINCH were not detected in any of the samples collected in 2000—2001, which was before DINCH commercial introduction in 2002 in the European market. In contrast, the detection of DINCH metabolites increased in samples collected from 2007 to 2012. These data suggest that DINCH oxidative metabolites serve as effective biomarkers to assess environmental exposures to DINCH.
Supplementary Material
Footnotes
Appendix A. Supporting information: Supplementary information associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.envres.2013.05.007.
Disclaimer: The use of trade names is for identification purposes only and does not constitute endorsement by the U.S. Department of Health and Human Services or the Centers for Disease Control and Prevention. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
References
- BASF. BASF Plasticizer Hexamoll® DINCH Grows from Strength to Strength. 2011 Available from:< http://www.basf.com/group/pressrelease/P-11-365>.
- Calafat AM, Ye XY, Silva MJ, Kuklenyik Z, Needham LL. Human exposure assessment to environmental chemicals using biomonitoring. Int J Androl. 2006;29:166–170. doi: 10.1111/j.1365-2605.2005.00570.x. [DOI] [PubMed] [Google Scholar]
- CDC. National Report on Human Exposure to Environmental Chemicals. Centers for Disease Control and Prevention, National Center for Environmental Health, Division of Laboratory Sciences; Atlanta, GA: 2013. [Google Scholar]
- Consumer Product Safety Commission. Products Containing Certain Phtha-lates. [accessed 22.04.13];2012 Available from:< http://www.cpsc.gov/about/cpsia/sect108.html>.
- Crespo JE, Balart R, Sanchez L, Lopez J. Substitution of di(2-ethylhexyl) phthalate by di(isononyl) cyclohexane-1,2-dicarboxylate as a plasticizer for industrial vinyl plastisol formulations. J Appl Polym Sci. 2007;104:1215–1220. [Google Scholar]
- EFSA. Opinion of the scientific panel on food additives, flavourings, processing aids and materials in contact with food (AFC) on a request related to a 12th list of substances for foold control materials. EFSA J. 2006:395–401. [Google Scholar]
- European Council for Plasticisers and Intermediates. Review of Recent Scientific Data on Di-isononyl Phthalate (DINP) and Risk Characterisation for its Use in Toys and Childcare Articles 2012 [Google Scholar]
- Kato K, Silva MJ, Needham LL, Calafat AM. Determination of 16 phthalate metabolites in urine using automated sample preparation and online preconcentration/high-performance liquid chromatography/tandem mass spectrometry. Anal Chem. 2005;77:2985–2991. doi: 10.1021/ac0481248. [DOI] [PubMed] [Google Scholar]
- Koch HM, Calafat AM. Human body burdens of chemicals used in plastic manufacture. Philos Trans R Soc B—Biol Sci. 2009;364:2063–2078. doi: 10.1098/rstb.2008.0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch HM, Muller J, Angerer J. Determination of secondary, oxidised di-iso-nonylphthalate (DINP) metabolites in human urine representative for the exposure to commercial DINP plasticizers. J Chromatogr B. 2007;847:114–125. doi: 10.1016/j.jchromb.2006.09.044. [DOI] [PubMed] [Google Scholar]
- Koch HM, Schutze A, Palmke C, Angerer J, Bruning T. Metabolism of the plasticizer and phthalate substitute diisononyl-cyclohexane-1,2-dicarboxylate (DINCH) in humans after single oral doses. Arch Toxicol. 2013a;87:799–806. doi: 10.1007/s00204-012-0990-4. [DOI] [PubMed] [Google Scholar]
- Koch HM, Schtze A, Palmke C, Angerer J, Brning T. Metabolism of the plasticizer and phthalate substitute diisononyl-cyclohexane-1,2-dicarboxylate (DINCH-«) in humans after single oral doses. Arch Toxicol. 2013b;87:799–806. doi: 10.1007/s00204-012-0990-4. [DOI] [PubMed] [Google Scholar]
- Needham LL, Barr D, Patterson DG, Pirkle JL. Biomonitoring: an integral part of exposure analysis. Neurotoxicology. 2004;25:666. [Google Scholar]
- Schutze A, Palmke C, Angerer J, Weiss T, Bruning T, Koch HM. Quantification of biomarkers of environmental exposure to di(isononyl)cyclo-hexane-1,2-dicarboxylate (DINCH) in urine via HPLC-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2012;895—896:123–130. doi: 10.1016/j.jchromb.2012.03.030. [DOI] [PubMed] [Google Scholar]
- Sexton K, Needham LL, Pirkle JL. Human biomonitoring of environmental chemicals. Am Scientist. 2004;92:38–45. [Google Scholar]
- Silva MJ, Furr J, Preau JL, Samandar E, Gray L, Calafat AM. Identification of potential biomarkers of exposure to di(isononyl)cyclohexane-1,2-dicarbox-ylate (DINCH), an alternative for phthalate plasticizers. J Expo Sci Environ Epidemiol. 2012;22:204–211. doi: 10.1038/jes.2011.43. [DOI] [PubMed] [Google Scholar]
- Silva MJ, Barr DB, Reidy JA, Kato K, Malek NA, Hodge CC, et al. Glucuronidation patterns of common urinary and serum monoester phthalate metabolites. Arch Toxicol. 2003;77:561–567. doi: 10.1007/s00204-003-0486-3. [DOI] [PubMed] [Google Scholar]
- Silva MJ, Reidy JA, Preau JL, Needham LL, Calafat AM. Oxidative metabolites of diisononyl phthalate as biomarkers for human exposure assessment. Environ Health Perspect. 2006a;114:1158–1161. doi: 10.1289/ehp.8865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva MJ, Samandar E, Preau JL, Needham LL, Calafat AM. Urinary oxidative metabolites of di(2-ethylhexyl) phthalate in humans. Toxicology. 2006b;219:22–32. doi: 10.1016/j.tox.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Silva MJ, Samandar E, Preau JL, Reidy JA, Needham LL, Calafat AM. Quantification of 22 phthalate metabolites in human urine. J Chromatogr B. 2007;860:106–112. doi: 10.1016/j.jchromb.2007.10.023. [DOI] [PubMed] [Google Scholar]
- Wittassek M, Wiesmuller GA, Koch HM, Eckard R, Dobler L, Muller J, et al. Internal phthalate exposure over the last two decades—a retrospective human biomonitoring study. Int J Hyg Environ Health. 2007;210:319–333. doi: 10.1016/j.ijheh.2007.01.037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.