Abstract
Background
Several countries have implemented a mandatory folic acid fortification of wheat flour and selected grain products to increase folate intake of reproductive-aged women. Brazil implemented a folic acid fortification program in 2004. No previous studies have examined folate differences among Brazilian women following the mandate.
Objective
We evaluate differences in serum and red blood cell (RBC) folate concentrations between two samples of women of childbearing age from selective communities in Brazil, one tested before (N=116) and the other after the mandate (N=240).
Methods
We compared baseline folate levels from women enrolled into a prevention study shortly before the fortification mandate was implemented, to baseline levels of women from the same communities enrolled in the same study shortly after fortification began. Participants were women enrolled in a folate supplementation clinical trial, at a hospital specialized in treating craniofacial anomalies in the city of Bauru from January 29, 2004 to April 27, 2005. We only compared baseline folate levels before the women received OCPP folic acid supplements.
Results
Women enrolled after the fortification mandate had higher means of serum folate (20.3 versus 11.2 nmol/L; p < 0.001) and RBC folate (368.3 versus 177.6 nmol/L; p < 0.001) than women enrolled before the mandate. Differences in folate levels between the two groups remained after adjusting for several co-variables.
Conclusions
The results suggest that serum and RBC folate levels among women of childbearing age have increased after implementing the folic acid fortification mandate in Brazil.
Keywords: Folic acid, folate, neural tube defects, congenital anomalies, Brazil
INTRODUCTION
Several countries including the United States (US), Canada, Australia, Chile, Argentina, and Brazil have implemented mandatory folic acid fortification programs for flour and selected grain products in order to increase the folate intake of women of childbearing age and reduce the incidence of neural tube defects (NTDs) [1–5]. Other countries like New Zealand and Ireland are considering fortification mandates, but have opted to wait for additional research due to concerns about the safety of a population-wide increase in folate intake, especially related to increased risks of vitamin B12 deficiency masking [6] and cancer incidence, [7] although there is no robust and consistent evidence of such effects.
In the US population, blood folate levels significantly increased after the fortification program began [8]. Among women of childbearing age, both red blood cell (RBC) and serum folate levels increased and the percentage of women with low RBC and serum folate concentrations decreased to 5.1% and 0.8%, respectively [9]. Blood folate levels have also increased in other countries after fortification such as Canada, Australia, and Chile [3, 10–12].
The prevalence of NTDs has declined in the period following folic acid fortification programs [13–15]. In Brazil, NTD prevalence decreased by 22.6%, from 31.4 per 10,000 live births/stillbirths, before the fortification mandate, to 24.3 per 10,000 live births/stillbirths [15]. In the state of Sao Paulo (where our study sample resides as explained below), NTD prevalence declined by 35% among live births [16]. Folic acid has been suggested to play a role in preventing other birth defects such as oral clefts [17], but the evidence for such effects remains controversial.
Brazil initiated its mandatory fortification program in 2004. Beginning in June 2004, all wheat and corn flour mills were required to fortify flour by adding 150 microgram (mcg) of folic acid and 4.2 mg of iron for every 100 grams of wheat or corn flour [18, 19]. This dose is slightly higher than that of the fortification programs in US and Canada (140 mcg of folic acid per 100 grams of flour) but lower than that in Chile and Argentina. Therefore, one would expect increases in blood folate levels that are, at a minimum, close to those reported in these two countries. However, to the best of our knowledge, no previous studies have evaluated differences in serum and RBC folate levels following the fortification mandate in Brazil. Such an assessment is important since folate levels are one metric for evaluating the fortification program in Brazil, where use of folic acid containing supplements among women of childbearing age had been relatively low [20], suggesting a potentially high rate of folate deficiency, although population-based data on deficiency are non-existent for Brazil. To shed light on the effect of fortification on Brazilian women of childbearing age, we compared serum and RBC folate levels between women assessed before fortification was implemented and women assessed after fortification went into effect.
METHODS
Study Population, Procedures, and Measures
Our study sample included 356 women from the City of Bauru (state of Sao Paulo) in Brazil who participated in the Oral Cleft Prevention Program (OCPP). The OCPP was a randomized clinical trial aimed at assessing the effect of 4 mg versus 0.4 mg folic acid, taken daily during preconception and the first trimester of pregnancy, on oral cleft recurrence among women who themselves have or have had a child with an oral cleft without other birth defects [17, 21, 22]. The women included in our study were enrolled into the OCPP between January 29, 2004 and April 27, 2005, which covers the period before and after initiating the fortification program in Brazil. The women were identified through Hospital de Reabilitação de Anomalias Craniofaciais (HRAC), a hospital in the city of Bauru, Brazil that specializes in treating craniofacial anomalies, where they or their affected children had received care for oral clefting. Women of childbearing age who at the time of enrollment were not permanently sterilized, not pregnant, not taking medications for seizures, and not B12 deficient were eligible to participate. All study enrollment and data collection procedures were approved by all involved institutional IRBs, the local ethics committee at HRAC, and by the central research ethics committee in Brazil (CONEP).
Folate levels were measured in the study women at the time of their enrollment into the OCPP before they received the OCPP folic acid supplements (i.e. at baseline). We compared these baseline measurements between two groups of women. The first group was enrolled between January 29, 2004 and May 31, 2004 before the fortification program began. The second group was enrolled between June 1, 2004 and June 6, 2005 after the fortification program was implemented. Blood samples were collected in the morning in the communities where the study women lived. After collection, samples were transferred in portable refrigerators to the OCPP lab and processed on the day of collection. Blood folate measurements were done at the study site’s laboratory facility using the Immulite 2000 Immunoassay System. Laboratory test quality control was performed on all tests and results were double checked to assure correct data entry. At the time of enrollment, the study women were interviewed for several demographic and socioeconomic characteristics. All data were independently and systematically reviewed by the study Data Center at RTI International, an international research organization.
During the study period described above, 673 women were screened for eligibility into OCPP and 494 women were enrolled. A total of 124 screened women were found ineligible to participate in the OCPP, mostly due to sterilization of the woman or partner. A total of 356 subjects had non-missing values on both serum and RBC folate levels from the baseline tests and were included in our study. Of these, 116 women were enrolled before fortification began and 240 women were enrolled after fortification.
Data Analysis
Descriptive statistics were calculated for baseline demographic, socioeconomic, and health variables (maternal age, marital status, number of children, wanting more children, smoking cigarettes, drinking alcoholic beverages, contraception use, educational attainment, employment status, and frequency of gynecological visits) and for folate levels and compared between the two pre- and post-fortification. The t-tests and Wilcoxon tests were used to test differences in serum and RBC folate levels between the two study groups. T-tests and chi-square tests were used to compare the other variables.
In order to evaluate the need to adjust for potential confounders that can bias outcome differences between the pre- and post- fortification groups, we regressed serum and RBC folate levels using ordinary least squares (OLS) on the post-fortification group indicator and the demographic, socioeconomic, and health variables shown in Table 1, one at a time, for the subsample with complete data on all of these variables. None of these variables modified the coefficient of the post-fortification group indicator by more than 10%, suggesting that none were important confounders. However as a sensitivity check to evaluate the joint effects of all of these conceptually relevant variables on the outcome difference between the pre- and post-fortification groups, we estimated a regression model for folate levels that simultaneously adjusted for these variables. The specific variables that we jointly controlled for were: maternal age, marital status, number of children, wanting more children, smoking cigarettes, drinking alcoholic beverages, contraception use, educational attainment, employment status, and frequency of gynecological visits. The subsample with data on these covariables was 262 since some observations had missing data on one or more of these variables. Therefore, as a comparison to the adjusted model on this subsample with complete data on all covariables, we also show the results from an unadjusted model that was estimated in this subsample since the fortification period effect could differ from the main unadjusted effect estimated in the total sample (with data on folate levels) not only because of adjustment but because of being estimated in a subset of the total sample.
Table 1.
Summary Statistics Obtained at Baseline for Two Groups of Brazilian Women Subsequently Randomized in a Folic Acid Supplementation Trial
| Characteristic | Before Fortification | After Fortification | P-value |
|---|---|---|---|
| Maternal age (years) | 24.9 (5.46) | 27.2 (6.73) | 0.002 |
| Married | 0.05 | ||
| Yes | 69 [59.5] | 168 [70.0] | |
| No | 47 [40.5] | 72 [30.0] | |
| Number of children | 0.21 | ||
| 0 | 43 [37.1] | 73 [30.4] | |
| 1 | 39 [33.6] | 77 [32.1] | |
| 2 | 24 [20.7] | 61 [25.4] | |
| 3+ | 10 [8.6] | 29 [12.1] | |
| Smokes cigarettes | 0.43 | ||
| Yes | 17 [14.7] | 28 [11.7] | |
| No | 99 [85.3] | 212 [88.3] | |
| Drinks alcoholic beverages | 0.27 | ||
| Yes | 15 [12.9] | 42 [17.5] | |
| No | 101 [86.1] | 198 [82.5] | |
| Wants more children | 0.69 | ||
| Yes | 76 [67.5] | 152 [63.3] | |
| No | 40 [34.5] | 88 [36.7] | |
| Using contraceptive methods | 0.12 | ||
| Yes | 89 [76.7] | 165 [68.8] | |
| No | 27 [23.3] | 75 [31.3] | |
| Seeing a gynecologist | 0.003 | ||
| Less than once a year/never | 30 [25.9] | 49 [20.4] | |
| Once a year | 57 [49.1] | 109 [45.4] | |
| Once every 6 months | 29 [25.0] | 82 [34.2] | |
| Highest level of schooling | 0.26 | ||
| Fundamental | 32 [43.8] | 70 [36.1] | |
| Intermediate | 35 [48.0] | 95 [49.0] | |
| University plus | 6 [8.2] | 29 [15.0] | |
| Employed in the past month | 0.48 | ||
| Yes | 36 [49.3] | 105 [54.1] | |
| No | 37 [50.7] | 89 [45.9] |
Note: The Table reports summary statistics – means and standard deviations (SD) in parentheses for maternal age and Ns and percentages [%] in brackets for the categorical variables. The summary statistics are based on women with complete data on each variable. The 356 women included in the pre- and post-fortification periods had complete data on the folate variables. The number of women with complete data on the other variables ranged from 351 for maternal age to 267 for education and employment. Chi-square tests and t-tests were used to test differences in categorical and continuous variables, respectively, between the two study groups.
RESULTS
Table 1 describes the demographic, socioeconomic, and health characteristics of the two study groups. The two groups were similar on majority of the demographic, socioeconomic, and health characteristics including marital status, number of children, wanting more children, cigarette use, alcohol consumption, frequency of gynecological visits, contraceptive use, schooling attainment and employment status. The group tested before fortification was younger by about two years on average (mean age of 24.9 versus 27.2; p=0.0017), had a lower rate of married women (59.5% versus 70%; p=0.049) than the group tested after fortification, and had more gynecological visits (p=0.003).
Figure 1 displays the distributions of serum and RBC folate levels for the two groups including the median, interquartile range (25th and 75th percentiles), and distribution of values outside of the interquartile range. Table 2 reports means and standard deviations as well as statistical significance of difference between the two groups based on a t-test. Serum folate levels (nmol/L) ranged from 3 to 25.1 with a mean (standard deviation) of 11.2 (4.7) and a median of 10.7 in the group assayed before fortification began and from 6.3 to 52.2 with a mean (standard deviation) of 20.3 (7.9) and a median of 19 in the group assayed after fortification. The mean serum folate level was significantly higher among those tested after fortification (20.3 versus 11.2; p < 0.001). The median serum folate concentration was also significantly higher in the latter group (19 versus 10.7; p < 0.001).
Figure 1. Box Plots of Serum Folate and RBC Folate Levels by Fortification Period.
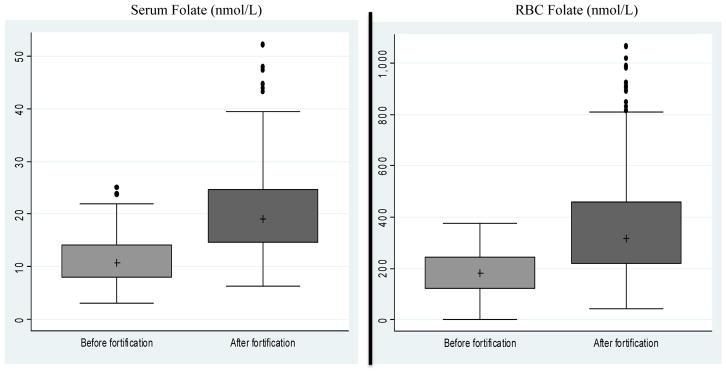
Notes: The median is represented by a plus (+). The inter-quartile range between the 25th and 75th percentiles is represented by the box. The lower whisker is equal to Q1-1.5*(Q3-Q1), where Q1 and Q3 are the values of the 25th and 75th percentiles, respectively. The upper whisker is equal to Q3+1.5*(Q3-Q1). Values outside of the whiskers are represented by dots. Median serum folate was 10.7 nmol/L before fortification and 19.0 nmol/L after fortification. The interquartile range for serum folate was 7.9–14.1 nmol/L before fortification and 14.6–24.8 nmol/L after fortification. Median RBC folate was 181.4 nmol/L before fortification and 315.4 nmol/L after fortification. The interquartile range for RBC folate was 122.3– 244.3 nmol/L before fortification and 220.6– 458.8 nmol/L after fortification.
Table 2.
Descriptive Statistics for Blood Folate Obtained at Baseline for Two Groups of Brazilian Women Subsequently Randomized in a Folic Acid Supplementation Trial
| Blood folate | Before Fortification (N=116) | After Fortification (N=240) | P-value |
|---|---|---|---|
| Serum folate (nmol/L) | 11.2 (4.66) | 20.3 (7.90) | <0.001 |
| Red blood cell (RBC) folate (nmol/L) | 177.6 (92.93) | 368.3 (212.12) | <0.001 |
Note: The Table reports summary statistics – means and standard deviations (SD) in parentheses. The summary statistics are based on 356 women had complete data on both serum and RBC folate levels. Group differences in means were evaluated using a t-test.
RBC folate levels (nmol/L) were higher in the group tested after fortification than the group tested before fortification. RBC folate ranged from 1.1 to 377.5 with a mean (standard deviation) of 177.6 (92.9) and a median of 181.4 in the group assayed before fortification, and from 42 to 1066.9 with a mean (standard deviation) of 368.3 (212.1) and a median of 315.4 in the group tested after fortification. The differences in mean and median RBC folate concentrations between the two groups were statistically significant (p < 0.001).
Table 3 reports the results of the OLS regressions comparing folate levels between the two study groups tested before and after fortification. We report results from the three models discussed above: The first (panel A) was estimated for the full sample with data on folate levels without adjustment for any covariables. The second (panel B) was estimated for the subset with complete data on all measured covariables (reported in Table 1) but was unadjusted and is reported for comparison to the third model (panel C), which adjusted for all these covariables. All regressions showed significantly higher means of serum and RBC folate levels in the group tested after fortification and the regression coefficients of the fortification periods were close across the three models.
Table 3.
Coefficients from the Ordinary Least Squares (OLS) Regression
| Variables | β (SE) from OLS for Folate Levels
|
|
|---|---|---|
| Serum folate | RBC folate | |
| A. Unadjusted model for full sample
|
||
| Tested after versus before fortification | 9.10*** (0.79) | 190.64*** (20.60) |
|
| ||
| Observations | 356 | 356 |
|
| ||
| B. Unadjusted model for subsample with data on all covariables
|
||
| Tested after versus before fortification | 9.40*** (1.01) | 186.93*** (25.41) |
|
| ||
| Observations | 262 | 262 |
|
| ||
| C. Adjusted model for subsample with data on all covariables
|
||
| Tested after versus before fortification | 8.67*** (1.04) | 180.14*** (26.78) |
|
| ||
| Observations1 | 262 | 262 |
Note: The Table reports the OLS regression coefficients (β) and standard errors (SE) in parentheses.
p < 0.01.
The adjusted model (Model C) controls for the following covariates: maternal age, marital status, number of children, wanting more children, smoking cigarettes, drinking alcoholic beverages, contraception use, educational attainment, employment status, and frequency of gynecological visits. The sample size declined to 262 observations when adjusting for these variables since some observations had missing data on one or more of these variables. As a reference, we also show the results from the unadjusted model (Model B) that was estimated in the subsample with complete data on all covariables (adjusted for in model C).
The unadjusted differences based on the full sample of 356 women were 9.1 and 190.64 nmol/L in mean serum and RBC folate levels, respectively (Table 3, panel A). When adjusting for all measured covariables at the same time, the pre-post fortification differences in serum and RBC folate levels were 8.7 and 180 nmol/L, respectively, and statistically significant (panel C). The unadjusted model for the subset with complete data on covariables showed virtually similar and statistically significant differences, with serum and RBC folate increasing by 9.4 and 187 nmol/L, respectively, in the post-fortification group (panel B). All in all, these results indicate that the main finding in our study was not sensitive to controlling for observed conceptually relevant covariables.
DISCUSSION
This analysis of data collected on a small convenience sample of Brazilian women of childbearing age suggests that serum and RBC folate levels have increased after fortification. These results provide a preliminary and descriptive evaluation of one of the main intended outcomes of the fortification program in Brazil, increasing folate levels among women of reproductive age. Observing a difference in RBC folate, which reflects a more chronic measure of folate nutritional status, suggests an improvement in dietary folate intake. The suggested increase in folate levels is consistent with the reported declines in NTD prevalence after the fortification in Brazil [15, 16].
Our study had some limitations. For example, we did not have data on use of supplements containing folic acid. An increase in the use of such supplements between pre- and post-fortification periods among women who had given birth to a child with a folate-related defect could potentially account for some of the observed differences in folate levels. However, >90% of women who were themselves affected with clefts and/or had children affected with clefts and enrolled in a later phase of the OCPP (within four years from the enrollment period for the women included in our analysis), when supplement data were collected, reported that they did not use vitamin supplements [17]. If anything, vitamin use rates have increased over these years due to greater awareness about folic acid effectiveness. Therefore, vitamin use rates were likely even lower than 10% during our study period. Furthermore, it is extremely unlikely that the pre- and post-fortification groups evaluated in this analysis differed significantly on vitamin use given the short period that separates them and that they are very similar on socioeconomic, demographic, and clinical factors that could associate with vitamin use.
In order to further evaluate the potential bias in our results due to lack of data on self-reported use of multivitamins and folic-acid supplements, we regressed baseline serum and RBC folate levels among a sample of about 1700 women similar in cleft risk to our study group and enrolled in the OCPP at a later phase when data on supplement use was collected (as noted earlier). We find that reporting use of multivitamins or folic acid containing supplements was associated with higher baseline serum levels by about 3.9 nmol/L on average, which is less than half of the difference in serum folate that we observe between the pre-post fortification groups. This indicates that even if none of the women in the pre-fortification group used multivitamins/folic-acid supplements at baseline and all women in the post-fortification group used these supplements (which obviously is an impossible scenario given the <10% rate of supplement use described above and the proximity between the pre- and post-fortification periods), not measuring self-reported use of supplements among women in this study could not explain the difference in serum folate-levels that we observe between the pre- and post-fortification groups. For RBC folate, we find that use of supplements is associated with an average increase by about 170 nmol/L, which is close to the difference in RBC folate-levels that we observe between the pre- and post-fortification groups. However again, for supplement use to explain the difference between these two groups, the rate of supplement use would have to have increased from 0% to 100% between the pre- and post-fortification periods, which is impossible given the low rates of supplement use in this population (<10%) and that the two periods are very close to each other. This evaluation strongly indicates that lacking data on vitamin use is unlikely to be a major source of bias in this analysis.
Since the baseline blood samples from women enrolled into the OCPP were assayed shortly after enrollment, the pre- and post-fortification samples were analyzed in different periods. Even though samples were tested using the same technology, comparing samples tested at different times (even with the same technology) may be confounded by batch effects. However, it is unlikely that any potential batch effects would explain the large differences we observed in folate levels between the two groups that are consistent with an effect from the fortification program.
It is also important to keep in mind that the studied women were all at high risk of giving birth to a child with a folate-related birth defect – either because they had already done so or were themselves affected with oral clefts. Consequently, their pre-fortification folate status was likely to have been lower than that of other population subgroups and the effect of fortification greater for them as well. However, women like the participants in our study comprise the group that fortification programs were designed to benefit. Our documentation of a large post- versus pre-fortification difference in folate status among OCPP participants adds importantly to the argument that a reduction in NTD occurrence post-fortification observed in Brazil [15] and other countries [11, 23–25] was mediated by a dramatic increase in folate status and decline in folate deficiency among high-risk women. Improving folate status in women of child-bearing age may have other benefits. These include protection against other birth defects [17, 26], allergies, and hyperallergenic responses [27], and maintaining health at old age [28].
As is the case for any population-level intervention program, there are concerns about potential adverse effects of folic acid fortification [29] that have reduced the enthusiasm of several countries to mandate fortification despite the above-mentioned benefits. One issue is the potential masking of vitamin B12 deficiency, although the evidence is generally mixed [30]. Previous studies have also shown mixed effects of folic acid use during pregnancy on child neurological development later in life [31]. Another potential issue is unmetabolized folic acid in serum; the average increase in daily consumption of 200 mcg of folate with the Brazilian mandate is unlikely to be problematic but increasing the level to 400 mcg is likely to lead to unmetabolised folic acid appearance [8, 9]. Evaluating the net benefits versus risks of folic acid fortification is beyond the scope of this study. However, investigating secondary outcomes of interest such as masking B12 deficiency, unmetabolized folic acid, supraphysiologic folate status, and child neurodevelopment specifically in the Brazilian population is important for future research.
Similar to other countries with mandated fortification programs, the fortification program in Brazil likely resulted in population-wide change in folate levels. Therefore, we recommend the use of stored pre-fortification serum or blood samples to assess the impact of fortification on folate levels in other population subgroups, as well as studies focused on the contribution of fortification to health outcomes other than NTDs.
Acknowledgments
Data collection was supported by grant U01HD040561 from the National Institute of Child Health and Human Development and the National Institute of Dental and Craniofacial Research (NIDCR) awarded as part of the Global Network for Women’s and Children’s Health Research and by NIH/NIDCR grant U01 DE017958.
Footnotes
Author disclosures: All authors have no conflicts of interest.
Statement of Authors Contribution: Drs. Chakrabory and Wehby conceived this paper and designed the analysis. Drs. Murray, Wehby, and Moretti-Ferreira designed the OCPP providing the data for this study. Mr. Goco and Ms. Moore oversaw data collection and management. Dr. Chakraborty, Dr. Nyarko, Ms. Moore, and Dr. Wehby contributed to the statistical analysis. All authors read and approved the manuscript.
Conflict of Interest: The authors declare that there are no conflicts of interest.
References
- 1.FDA. Food standards: amendment of standards of identity for enriched grain products to require addition of folic acid. 1996;61:8761. [Google Scholar]
- 2.Dietrich M, Brown CJP, Block G. The Effect of Folate Fortification of Cereal-Grain Products on Blood Folate Status, Dietary Folate Intake, and Dietary Folate Sources among Adult Non-Supplement Users in the United States. J Am Coll Nutr. 2005;24:266–274. doi: 10.1080/07315724.2005.10719474. [DOI] [PubMed] [Google Scholar]
- 3.Hertrampf E, Cortes F, Erickson JD, Cayazzo M, Freire W, Bailey LB, Howson C, Kauwell GP, Pfeiffer C. Consumption of folic acid-fortified bread improves folate status in women of reproductive age in Chile. The Journal of nutrition. 2003;133:3166–3169. doi: 10.1093/jn/133.10.3166. [DOI] [PubMed] [Google Scholar]
- 4.Castillo-Lancellotti C, Tur JA, Uauy R. Impact of folic acid fortification of flour on neural tube defects: a systematic review. Public Health Nutr. 2013;16:901–911. doi: 10.1017/S1368980012003576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rabovskaja V, Parkinson B, Goodall S. The Cost-Effectiveness of Mandatory Folic Acid Fortification in Australia. The Journal of nutrition. 2013;143:59–66. doi: 10.3945/jn.112.166694. [DOI] [PubMed] [Google Scholar]
- 6.Lawrence M. Assessing the case for mandatory folate fortification: policy-making in the face of scientific uncertainties. Australian and New Zealand Journal of Public Health. 2005;29:328–330. doi: 10.1111/j.1467-842x.2005.tb00202.x. [DOI] [PubMed] [Google Scholar]
- 7.Cole BF, Baron JA, Sandler RS, Haile RW, Ahnen DJ, Bresalier RS, McKeown-Eyssen G, Summers RW, Rothstein RI, Burke CA, Snover DC, Church TR, Allen JI, Robertson DJ, Beck GJ, Bond JH, Byers T, Mandel JS, Mott LA, Pearson LH, Barry EL, Rees JR, Marcon N, Saibil F, Ueland PM, Greenberg ER. Folic acid for the prevention of colorectal adenomas: a randomized clinical trial. JAMA. 2007;297:2351–2359. doi: 10.1001/jama.297.21.2351. [DOI] [PubMed] [Google Scholar]
- 8.Rader JI, Schneeman BO. Prevalence of neural tube defects, folate status, and folate fortification of enriched cereal-grain products in the United States. Pediatrics. 2006;117:1394–1399. doi: 10.1542/peds.2005-2745. [DOI] [PubMed] [Google Scholar]
- 9.Pfeiffer CM, Caudill SP, Gunter EW, Osterloh J, Sampson EJ. Biochemical indicators of B vitamin status in the US population after folic acid fortification: results from the National Health and Nutrition Examination Survey 1999–2000. The American Journal of Clinical Nutrition. 2005;82:442–450. doi: 10.1093/ajcn.82.2.442. [DOI] [PubMed] [Google Scholar]
- 10.Liu S, West R, Randell E, Longerich L, O’Connor KS, Scott H, Crowley M, Lam A, Prabhakaran V, McCourt C. A comprehensive evaluation of food fortification with folic acid for the primary prevention of neural tube defects. BMC pregnancy and childbirth. 2004;4:20. doi: 10.1186/1471-2393-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lindzon G, O’Connor DL. Folate during reproduction: the Canadian experience with folic acid fortification. Nutrition research and practice. 2007;1:163–174. doi: 10.4162/nrp.2007.1.3.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown RD, Langshaw MR, Uhr EJ, Gibson JN, Joshua DE. The impact of mandatory fortification of flour with folic acid on the blood folate levels of an Australian population. The Medical journal of Australia. 2011;194:65–67. doi: 10.5694/j.1326-5377.2011.tb04169.x. [DOI] [PubMed] [Google Scholar]
- 13.Boulet SL, Yang Q, Mai C, Kirby RS, Collins JS, Robbins JM, Meyer R, Canfield MA, Mulinare J. Trends in the postfortification prevalence of spina bifida and anencephaly in the United States. Birth Defects Res A Clin Mol Teratol. 2008;82:527–532. doi: 10.1002/bdra.20468. [DOI] [PubMed] [Google Scholar]
- 14.De Wals P, Fassiatou T, Margot IVA, Lowry RB, Jane AE, Michiel CVdH, Marian C, Soo-Hong U, Pamela Z, Barbara S, Bridget F, Nora SL, Theophile N. Spina bifida before and after folic acid fortification in Canada. Birth Defects Research Part A: Clinical and Molecular Teratology. 2008;82:622–626. doi: 10.1002/bdra.20485. [DOI] [PubMed] [Google Scholar]
- 15.Lopez-Camelo JS, Castilla EE, Orioli IM, Inagemp, Eclamc Folic Acid Flour Fortification: Impact on the Frequencies of 52 Congenital Anomaly Types in Three South American Countries. American Journal of Medical Genetics Part A. 2010;152A:2444–2458. doi: 10.1002/ajmg.a.33479. [DOI] [PubMed] [Google Scholar]
- 16.Fujimori E, Baldino CF, Sato APS, Borges ALV, Gomes MN. Prevalência e distribuição espacial de defeitos do tubo neural no Estado de São Paulo, Brasil, antes e após a fortificação de farinhas com ácido fólico. Cadernos de Saúde Pública. 2013;29:145–154. doi: 10.1590/s0102-311x2013000100017. [DOI] [PubMed] [Google Scholar]
- 17.Wehby GL, Felix TM, Goco N, Richieri-Costa A, Chakraborty H, Souza J, Pereira R, Padovani C, Moretti-Ferreira D, Murray JC. High dosage folic Acid supplementation, oral cleft recurrence and fetal growth. International journal of environmental research and public health. 2013;10:590–605. doi: 10.3390/ijerph10020590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanitaria ANDV. Consulta Publica no. 51. Diario Oficial da Uniao. 2002;111:70–71. [Google Scholar]
- 19.Ferreira AF, Giugliani R. Consumption of folic acid-fortified flour and folate-rich foods among women at reproductive age in South Brazil. Community genetics. 2008;11:179–184. doi: 10.1159/000113881. [DOI] [PubMed] [Google Scholar]
- 20.Wehby G, Castilla E, Lopez-Camelo J, Murray J. Predictors of multivitamin use during pregnancy in Brazil. International Journal of Public Health. 2009;54:78–87. doi: 10.1007/s00038-009-8103-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wehby GL, Norman G, Danilo M-F, Temis F, Antonio R-C, Carla P, Fernanda Q, Vila Camilla G, Rui P, Steve L, Tyler H, Hrishikesh C, Lorette J, Murray JC. Oral cleft prevention program (OCPP) BMC Pediatrics. 2012:12. doi: 10.1186/1471-2431-12-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chakraborty H, Wehby G, Goco N, Moore J, Kindem M, Vila-Nova C, Pereira R, Souza J, Viegas A, Felix T, Moretti-Ferreira D, Richieri-Costa A, Murray JC. Challenges and Implemented Solutions for the Oral Cleft Prevention Trial in Brazil. International Journal of Medicine and Public Health. 2011;1:9–16. [Google Scholar]
- 23.Hertrampf E, Cortes F. Folic acid fortification of wheat flour: Chile. Nutrition reviews. 2004;62:S44–48. doi: 10.1111/j.1753-4887.2004.tb00074.x. discussion S49. [DOI] [PubMed] [Google Scholar]
- 24.García-Fragoso L, García-García I, Rivera C. The use of folic acid for the prevention of birth defects in Puerto Rico. Ethnicity & disease. 2009;18(2 Suppl 2):168–171. [PMC free article] [PubMed] [Google Scholar]
- 25.Rasmussen SA, Erickson JD, Reef SE, Ross DS. Teratology: From science to birth defects prevention. Birth Defects Research Part A: Clinical and Molecular Teratology. 2009;85:82–92. doi: 10.1002/bdra.20506. [DOI] [PubMed] [Google Scholar]
- 26.Ionescu-Ittu R, Marelli AJ, Mackie AS, Pilote L. Prevalence of severe congenital heart disease after folic acid fortification of grain products: time trend analysis in Quebec, Canada. BMJ. 2009;338:b1673. doi: 10.1136/bmj.b1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsui EC, Matsui W. Higher serum folate levels are associated with a lower risk of atopy and wheeze. J Allergy Clin Immunol. 2009;123:1253–1259. e1252. doi: 10.1016/j.jaci.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McNulty H, Pentieva K, Hoey L, Strain JJ, Ward M. Nutrition Throughout Life: Folate. Int J Vitam Nutr Res. 2012;82:348–354. doi: 10.1024/0300-9831/a000130. [DOI] [PubMed] [Google Scholar]
- 29.Fajardo V, Varela-Moreiras G. Efficacy of Adding Folic Acid to Foods. Int J Vitam Nutr Res. 2012;82:177–186. doi: 10.1024/0300-9831/a000109. [DOI] [PubMed] [Google Scholar]
- 30.Morris MS, Jacques PF, Rosenberg IH, Selhub J. Folate and vitamin B-12 status in relation to anemia, macrocytosis, and cognitive impairment in older Americans in the age of folic acid fortification. The American Journal of Clinical Nutrition. 2007;85:193–200. doi: 10.1093/ajcn/85.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wehby GL, Murray JC. The effects of prenatal use of folic acid and other dietary supplements on early child development. Matern Child Health J. 2008;12:180–187. doi: 10.1007/s10995-007-0230-3. [DOI] [PubMed] [Google Scholar]


