Abstract
Dysregulated calcium signaling has been increasingly implicated in tumor dissemination and progression. In a recent study, we investigated the mechanism underlying calcium-mediated invasion and metastasis in melanoma and discovered that hyperactive Ca2+ oscillation in melanoma cells enhanced invasion and metastasis by promoting invadopodium formation and extracellular matrix remodeling.
Keywords: calcium oscillation, STIM1, Orai1, invadopodia, melanoma invasion
Abbreviations
- ECM
extracellular matrix
- HCC
hepatocellular carcinoma
- MT1-MMP
membrane-type 1 matrix metalloprotease
- SOCE
store-operated calcium entry
Gaining invasiveness and overcoming the physical barriers imposed by the extracellular matrix (ECM) are the first, and arguably most critical, steps of tumor metastasis.1 In addition to facilitating dissemination, remodeling of the ECM is essential for tumor growth and the establishment of metastatic niches by malignant cells.2 Invadopodia of malignant cancer cells are actin-rich, ECM-degrading membrane protrusions that are critical for tumor invasion and metastasis.1 In a recent study, we investigated the regulation of invadopodium formation and metastasis by Ca2+ signaling in melanoma.3 We unexpectedly discovered that hyperactive Ca2+ signaling in metastatic melanoma cells is organized in the form of oscillatory waves to orchestrate invadopodia assembly and melanoma invasion.3
In recent years, dysregulated Ca2+ signaling has been increasingly implicated in cancer invasion and metastasis, yet the underlying mechanism remained largely unclear.4,5 To gain mechanistic insight into Ca2+-mediated invasion and metastasis, we examined the role of Ca2+ signaling in invadopodium formation in melanoma cells and discovered that blocking store-operated calcium (SOC) channel signaling significantly decreased invadopodium number and activity. Accompanying the assembly of invadopodia was an oscillatory Ca2+ signal mediated by the SOC channel proteins STIM1 and ORAI1. Interestingly, disruption of the Ca2+ oscillation by either blocking store operated calcium entry (SOCE; which decreased cytosolic Ca2+ concentration), or by inducing constitutive calcium entry with thapsigargin or the ionophore A-23187 (which increased cytosolic Ca2+ concentration) similarly inhibited invadopodium assembly and melanoma invasion, signifying the importance of temporal Ca2+ signal coding during metastatic dissemination.
By screening a panel of protein kinases, we identified the non-receptor tyrosine kinase Src as a downstream effector of SOCE. The notion that SOCE regulates invadopodium assembly through Src is further supported by the rescue of defective invadopodium formation in STIM1 knockdown melanoma cells by constitutively active v-Src, and the abrogation of STIM1-mediated invadopodium assembly by the Src inhibitor dasatinib. The fact that constitutive Ca2+ influx induced by thapsigargin and A-23187 was a robust activator of Src begs the question: Why do melanoma cells use an oscillatory Ca2+ signal instead of a steady Ca2+ increase?
Ca2+ is a notoriously versatile second messenger. It is estimated that hundreds of genes in the human genome contain Ca2+ binding EF-hand or C2 domains.6 The specificity and versatility of Ca2+ signaling relies on the intricate spatial and temporal coding of cytosolic Ca2+ concentration.6 By compartmentalizing Ca2+ signals into spatial-temporal patterns, cells are able to activate selective downstream signaling events at a defined time and subcellular location.6 It is believed that the frequency and amplitude of Ca2+ oscillations serve as digital signals that selectively activate threshold-dependent downstream events. The tight control of cytosolic Ca2+ is critical not only for signaling specificity but also for cell survival, since a prolonged and uncontrolled global increase in cytosolic Ca2+ is toxic to the cell and eventually leads to cell death.7 By organizing SOCE signals in the form of Ca2+ oscillation, melanoma cells are able to provide the Ca2+ signal necessary for invadopodium assembly and ECM remodeling over an extended period of time without causing cytotoxicity (Fig. 1A). In contrast, although constitutive Ca2+ influx induced by thapsigargin and A23187 robustly increases Src activity it might also indiscriminately activate hundreds of other Ca2+-dependent signaling pathways, which eventually reduce melanoma cell fitness and inhibit melanoma invasion (Fig. 1B).
Figure 1.
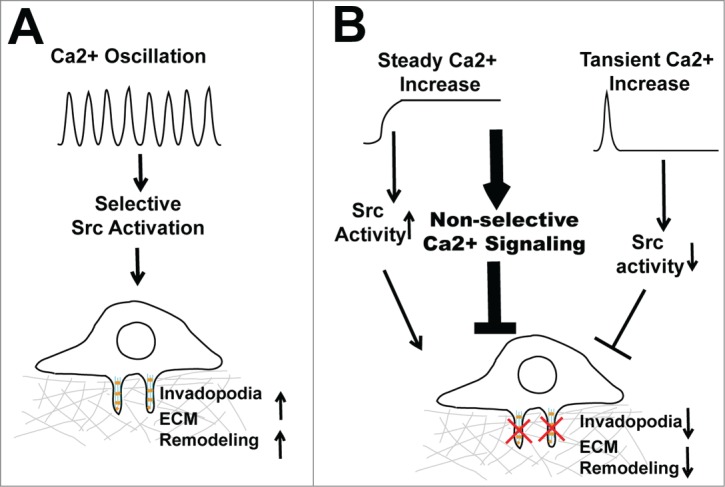
Regulation of invadopodia by Ca2+ oscillation. (A) Hyperactive store-operated calcium entry (SOCE) increases Ca2+ oscillation frequency and amplitude, which selectively activates Src to promote invadopodium assembly and extracellular matrix remodeling. (B) Disruption of Ca2+ oscillation, either by a constitutive increase in cytosolic Ca2+ or by blockage of SOCE, inhibits invadopodium formation and melanoma invasion.
It is also possible that melanoma invasion and invadopodium assembly require a coordinated cycle of Ca2+ peaks and valleys, as recently demonstrated in mast cell exocytosis by Wollman and Meyer.8 Ca2+ oscillation in antigen-activated mast cells drives the cyclic assembly and disassembly of cortical actin. Newly assembled cortex actin serves as a carrier to capture secretory vesicles, whereas disassembly of cortical actin allows the passage of vesicles to facilitate membrane fusion. Intriguingly, we discovered that recycling of membrane type 1 matrix metalloprotease (MT1-MMP, also known as MMP14) to the plasma membrane required SOCE, blockage of which resulted in entrapment of MT1-MMP in endosomes.3 It would be interesting to determine whether SOCE-mediated Ca2+ oscillation coordinates the recycling of endocytosed MT1-MMP to the plasma membrane.
Of note, tumor-promoting Ca2+ oscillation has also recently been observed in esophageal squamous cell carcinoma and hepatocellular carcinoma (HCC).9,10 Overexpression of Orai1 in esophageal carcinoma cells is responsible for hyperactive Ca2+ oscillation, which promotes cancer cell motility and proliferation in vitro and tumor growth in a xenograft model.10 In HCC the voltage-gated calcium channel subunit CACNA2D1 was found to be a marker for recurrent disease. Recurrent HCC cells had higher expression of α2δ1 and hyperactive Ca2+ oscillation, which could be inhibited by a blocking antibody targeting CACNA2D1.10 These observations, together with our recent finding, suggest that Ca2+ oscillation might be a signaling mechanism that is commonly hijacked by malignant cells to facilitate cancer progression. Future investigation in this area will likely significantly advance our understanding of how deregulated Ca2+ signaling promotes cancer malignancy.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work is supported by the National Cancer Institute (R01175741).
References
- 1.Nurnberg A, Kitzing T, Grosse R. Nucleating actin for invasion. Nat Eev Cancer 2011; 11(3):177–87; PMID:21326322; http://dx.doi.org/ 10.1038/nrc3003 [DOI] [PubMed] [Google Scholar]
- 2.Hotary KB, Allen ED, Brooks PC, Datta NS, Long MW, Weiss SJ. Membrane type I matrix metalloproteinase usurps tumor growth control imposed by the three-dimensional extracellular matrix. Cell. 2003; 114(1):33-45; PMID:12859896; http://dx.doi.org/ 10.1016/S0092-8674(03)00513-0 [DOI] [PubMed] [Google Scholar]
- 3.Sun J, Lu F, He H, Shen J, Messina J, Mathew R, Wang D, Sarnaik AA, Chang WC, Kim M, et al.. STIM1- and Orai1-mediated Ca2+ oscillation orchestrates invadopodium formation and melanoma invasion. J Cell Biol. 2014; 207(4):535-48, http://dx.doi.org/ 10.1083/jcb.201407082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prevarskaya N, Skryma R, Shuba Y. Calcium in tumour metastasis: new roles for known actors. Nat Rev Cancer. 2011; 11(8):609-18; PMID:21779011; http://dx.doi.org/ 10.1038/nrc3105 [DOI] [PubMed] [Google Scholar]
- 5.Wang JY, Sun J, Huang MY, Wang YS, Hou MF, Sun Y, He H, Krishna N, Chiu SJ, Lin S, et al.. STIM1 overexpression promotes colorectal cancer progression, cell motility and COX-2 expression. Oncogene. 2014; PMID:25381814; http://dx.doi.org/ 10.1038/onc.2014.366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clapham DE, Calcium signaling. Cell. 2007; 131(6):1047-58; PMID:18083096; http://dx.doi.org/ 10.1016/j.cell.2007.11.028 [DOI] [PubMed] [Google Scholar]
- 7.Orrenius S, Zhivotovsky B, Nicotera P. Regulation of cell death: the calcium-apoptosis link. Nat Rev Mol Cell Biol. 2003; 4(7):552-65; PMID:12838338; http://dx.doi.org/ 10.1038/nrm1150 [DOI] [PubMed] [Google Scholar]
- 8.Wollman R, Meyer T. Coordinated oscillations in cortical actin and Ca2+ correlate with cycles of vesicle secretion. Nat Cell Biol 2012; 14(12):1261-9; PMID:23143397; http://dx.doi.org/ 10.1038/ncb2614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu H, Zhang H, Jin F, Fang M, Huang M, Yang CS, Chen T, Fu L, Pan Z. Elevated Orai1 expression mediates tumor-promoting intracellular Ca2+ oscillations in human esophageal squamous cell carcinoma. Oncotarget. 2014; 5(11):3455-71; PMID:24797725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao W, Wang L, Han H, Jin K, Lin N, Guo T, Chen Y, Cheng H, Lu F, Fang W, et al.. 1B50-1, a mAb raised against recurrent tumor cells, targets liver tumor-initiating cells by binding to the calcium channel alpha2delta1 subunit. Cancer Cell 2013; 23(4):541-56; PMID:23597567; http://dx.doi.org/ 10.1016/j.ccr.2013.02.025 [DOI] [PubMed] [Google Scholar]


