Abstract
Purpose
To describe photoreceptor structure and recovery following macular hole (MH) closure with pars plana vitrectomy (PPV) using adaptive optics scanning light ophthalmoscopy (AOSLO) and spectral domain optical coherence tomography (SD-OCT).
Methods
A pilot imaging study of eyes undergoing PPV for MH was conducted. Imaging with SD-OCT and AOSLO was performed at varying time points following PPV.
Patients
4 eyes from 4 subjects undergoing PPV for MH.
Results
Despite successful MH closure, disruption of the foveal inner segment ellipsoid zone (EZ) was seen in all patients when imaged at a mean of 5.9 months following PPV. Disruption of the photoreceptor mosaic was seen using AOSLO at locations corresponding to regions of EZ disruption on SD-OCT. Cone density immediately surrounding these disruptions was normal, except for 1 patient. In 2 patients who were imaged serially up to 17 months after PPV, recovery of cone cells within regions of mosaic disruption could be detected over time.
Conclusion
Photoreceptor disruption exists even after apparent MH closure. Remodeling of the foveal cone mosaic continues for many months following surgery, perhaps accounting for the delayed post-operative improvements of visual acuity in some patients. SD-OCT and AOSLO are useful tools for monitoring photoreceptor recovery following surgical closure of MH.
Keywords: Adaptive optics, Macular hole, Optical coherence tomography, Photoreceptor
INTRODUCTION
Macular hole (MH) is an idiopathic or traumatic full thickness defect of the neural retina in the foveal region. Management involves pars plana vitrectomy (PPV) surgery with stripping of preretinal membranes and intraocular gas tamponade. While PPV achieves MH closure in over 90% of cases, some patients suffer permanent vision loss despite successful surgery [1]. The variable visual recovery seen in these patients may be explained through an improved understanding of the cellular changes following closure of MH.
Adaptive optics scanning light ophthalmoscopy (AOSLO) and spectral domain optical coherence tomography (SD-OCT) enable non-invasive examination of retinal anatomy and photoreceptor structure. Recently, these imaging tools have been used to study subjects with MH [1–3]. Studies have reported a correlation between postoperative visual impairment and disruption of the photoreceptor layers on SD-OCT, namely the external limiting membrane (ELM) and inner segment ellipsoid zone (EZ) [1]. Unfortunately, the lateral resolution of SD-OCT is not sufficient to resolve the photoreceptors on a cellular scale, precluding direct correlations between the sampling mosaic and visual function. This shortcoming of SD-OCT is illustrated by studies in which AOSLO imaging resolved disruptions in the photoreceptor mosaic that were not visible with SD-OCT [4]. Recent findings using AOSLO to image patients following surgical closure of the MH with PPV include “dark areas” within the macula; however, the integrity of the central-most foveal cones was not able to be imaged or assessed [2,3]. Furthermore, longitudinal imaging with AOSLO of patients with closed MH following PPV has not been described to our knowledge. Thus, many questions remain regarding the changes in the photoreceptor mosaic following surgical MH closure. Here, we used AOSLO to examine foveal photoreceptor structure in patients following PPV for MH, and compared these findings to those from SD-OCT. For the first time in this patient population, we show cone photoreceptor structure at the foveal center and demonstrate recovery of cone photoreceptor structure over time using longitudinal imaging.
MATERIAL AND METHODS
Human Subjects
We conducted a pilot study of eyes undergoing PPV surgery for MH at the Medical College of Wisconsin, Milwaukee. Research was approved by the Institutional Review Board at the Medical College of Wisconsin and was conducted in accordance with the tenets of the Declaration of Helsinki. Informed consent was obtained from all patients.
Spectral Domain Optical Coherence Tomography (SD-OCT)
Post-operative images of the macula were obtained using a Bioptigen SD-OCT (Bioptigen, Inc., Durham, NC). Line scan sets were acquired (1000 A-scans/B-scan; 100 repeated B-scans) through the closed macular hole, and this location was confirmed based on inspection of an additional high-density volume scan. Scans were registered and averaged to increase signal to noise ratio. The volumetric scans (1000 A-scans/B-scan, 100 B-scans/volume) provided dense sampling throughout the macula, enabling more precise comparison between SD-OCT findings and those from AOSLO. The appearance of the outer retinal layers (ELM, EZ) was qualitatively assessed by 2 graders (WJW & JC).
Foveal pit morphology measurements assessing change in the depth and diameter of the fovea of subject PK_0858 were obtained by applying methods previously described by Dubis et al [5]. These measurements were calculated using volumetric images of the macula obtained with a Cirrus HD-OCT (Carl Zeiss Meditec, Dublin, CA).
Adaptive Optics Scanning Light Ophthalmoscopy (AOSLO)
Images of the photoreceptor mosaic, including the area of the surgically closed macular hole, were acquired using a previously described AOSLO [6]. Images were obtained using an Inphenix 775-nm superluminescent diode with a 12-nm full-width-at-half-maximum bandwidth. The subject was instructed to fixate at different positions to allow imaging of different retinal locations. Individual image sequences contained 150 frames.
Intraframe distortions within the AOSLO retinal images were corrected as previously described [6]. Registration of frames within a given image sequence was performed using a “strip” registration method [7]. Once all the frames were registered, the 40 frames with the highest normalized cross correlation to the reference frame were averaged, increasing the signal to noise ratio. The final images were then montaged using commercial software (Adobe Photoshop; Adobe Systems, San Jose, CA), and the final montage was manually registered to a color fundus image using alignment of blood vessels. Cone density was measured from subjects at various parafoveal regions of interest (ROIs) following the methods described by Garrioch et al [8].
RESULTS
Four eyes from four subjects underwent imaging with SD-OCT and AOSLO on average 5.9 months (range, 1.2 – 6.4 months) following PPV for idiopathic full thickness MH. A second imaging session was obtained on average 15.5 months (range, 12–17.5 months) following PPV in three subjects.
In initial AOSLO images for all four cases, areas of photoreceptor mosaic disruption were seen at the fovea. While these areas corresponded to regions of EZ disruption on SD-OCT, there were differences, which are outlined below.
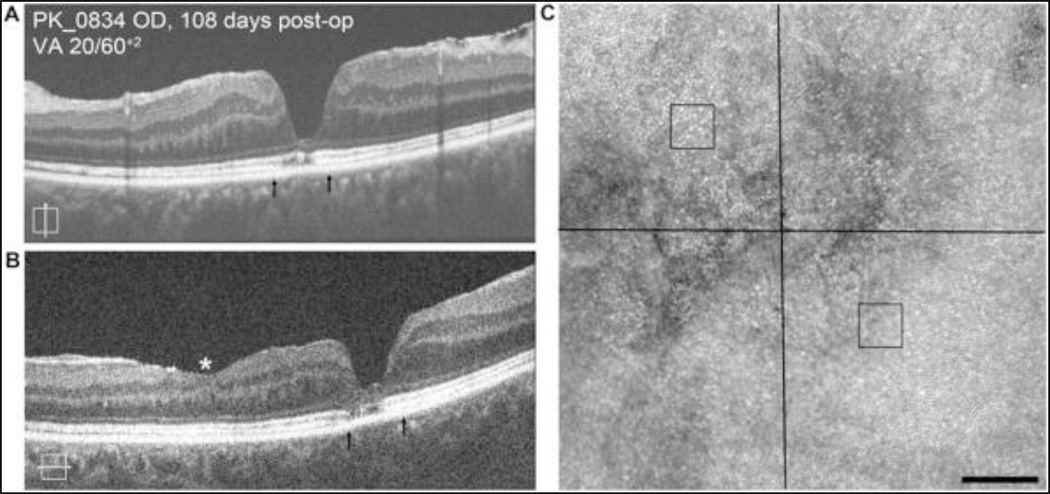
One subject (PK_0834) who was imaged at 108 days postoperatively with a visual acuity of 20/60+2, demonstrated only a mild disruption of cone mosaic at the fovea on AOSLO even though SD-OCT revealed advanced loss of the outer nuclear layer (ONL) and patchy disruption of the EZ at the foveal center (Figure 1). Cone density was measured at two ROIs adjacent to this disruption, each being 0.6 degrees from the foveal center. The infero-nasal ROI had a density of 59,504 cones/mm2 which is within 2 standard deviations of the normal mean at this eccentricity [8]. In contrast, the supero-temporal ROI had a density of 44,959 cones/mm2, which is greater than 2 standard deviations below the normal mean at this eccentricity [8]. This heterogeneous recovery of the cone mosaic is not resolvable from the SD-OCT images.
Figure 1.
Imaging of subject PK_0834 at 108 days following MH closure. Vertical SD-OCT B-scan (A) reveals patchy disruption of EZ band along with advanced loss of the ONL at the base of the fovea. Similar changes are seen in horizontal B-scan (B). Arrows depict area imaged with AOSLO. Asterisk (*) denotes region of iatrogenic surgical thinning of inner retinal layer. AOSLO imaging reveals disruption of photoreceptor mosaic corresponding to regions of SD-OCT abnormalities (C). Cone density was measured at two ROIs adjacent to this disruption, each being 0.6 degrees from the foveal center (black squares). The infero-nasal ROI had a density of 59,504 cones/mm2 which is within 2 standard deviations of the normal mean at this eccentricity [8]. In contrast, the supero-temporal ROI had a density of 44,959 cones/mm2, which is greater than 2 standard deviations below the normal mean at this eccentricity [8]. Scale bars = 100 µm.
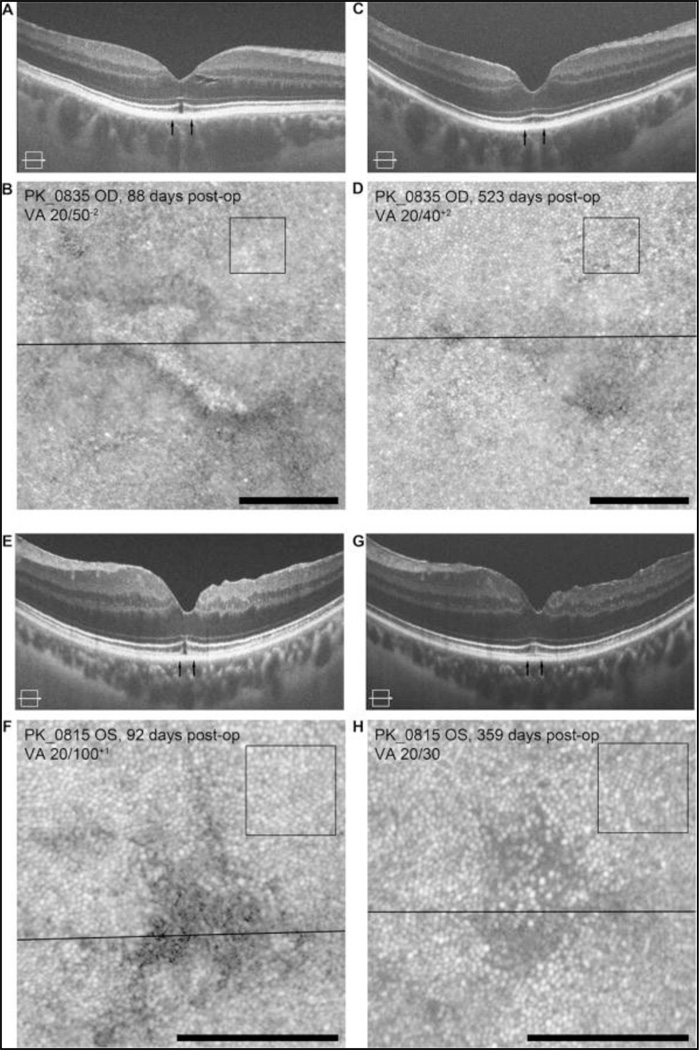
The regions of cone mosaic disruption were shown to recover over time in two patients. In subject PK_0835, we observed a focal EZ disruption and increased intensity of the ELM at the fovea on SD-OCT at about 3 months after surgery (Figure 2A). The corresponding AOSLO image of the cone mosaic revealed diffuse disruption of the cone mosaic immediately adjacent to this lesion (Figure 2B), with a reflective structure within the area of the EZ disruption. At 17 months after surgery, there was clear restoration of the EZ at the fovea on SD-OCT (Figure 2C) and improvement in the appearance of the cone mosaic in the AOSLO image (Figure 2D). During this time period, visual acuity improved from 20/50 to 20/40. In order to assess whether the resolution of the foveal mosaic was due to "shifting" of the surrounding photoreceptors, we measured cone density at the same retinal eccentricity at the two time points and found the difference in density (77,025 cones/mm2 versus 73,719 cones/mm2) to be within the expected measurement error. In subject PK_0815, we observed a similar EZ lesion on SD-OCT as the previous subject and the cone mosaic images showed a corresponding hyporeflective area at about 3 months after PPV (Figure 2E, F). At 1 year after surgery, the EZ disruption resolved on SD-OCT images and numerous cones became visible within the previously hyporeflective area on AOSLO (Figure 2G, H). During this time period, visual acuity improved from 20/100 to 20/30. As with PK_0835, cone density measurements taken from perifoveal region at the two time points were not significantly different (75,372 cones/mm2 versus 70,413 cones/mm2), suggesting that the “appearance” or resolution of cone structure at the fovea was not due to "shifting" or migration of nearby cones into this area. In both PK_0835 and PK_0815, the cone density measurements in the regions surrounding the foveal center were within the expected normal range as well, highlighting the focal nature of the cone mosaic disruptions following surgical MH closure.
Figure 2.
Longitudinal imaging reveals dynamic recovery of photoreceptor structure. In subject PK_0835 at 3 months post-operatively, SD-OCT image shows a focal EZ lesion and increased intensity of the ELM (A). Corresponding AOSLO image shows a well-circumscribed area of photoreceptor disruption (B). Images of the same patient 17 months post-operatively shows improved cone structure on AOSLO (C) and corresponding resolution of foveal EZ disruption on SD-OCT (D). In subject PK_0815 at 3 months post-operatively, SD-OCT images show a similar EZ lesion (E) and cone mosaic images showed a corresponding hyporeflective area (F). Nine months later, the EZ lesion resolved (G) and cones became visible within the hyporeflective area on AOSLO (H). In both cases, serial cone densities in regions adjacent to EZ disruption (black squares) were within expected repeatability [8], consistent with no significant change in the mosaic adjacent to the EZ disruption. Scale bars = 100 µm.
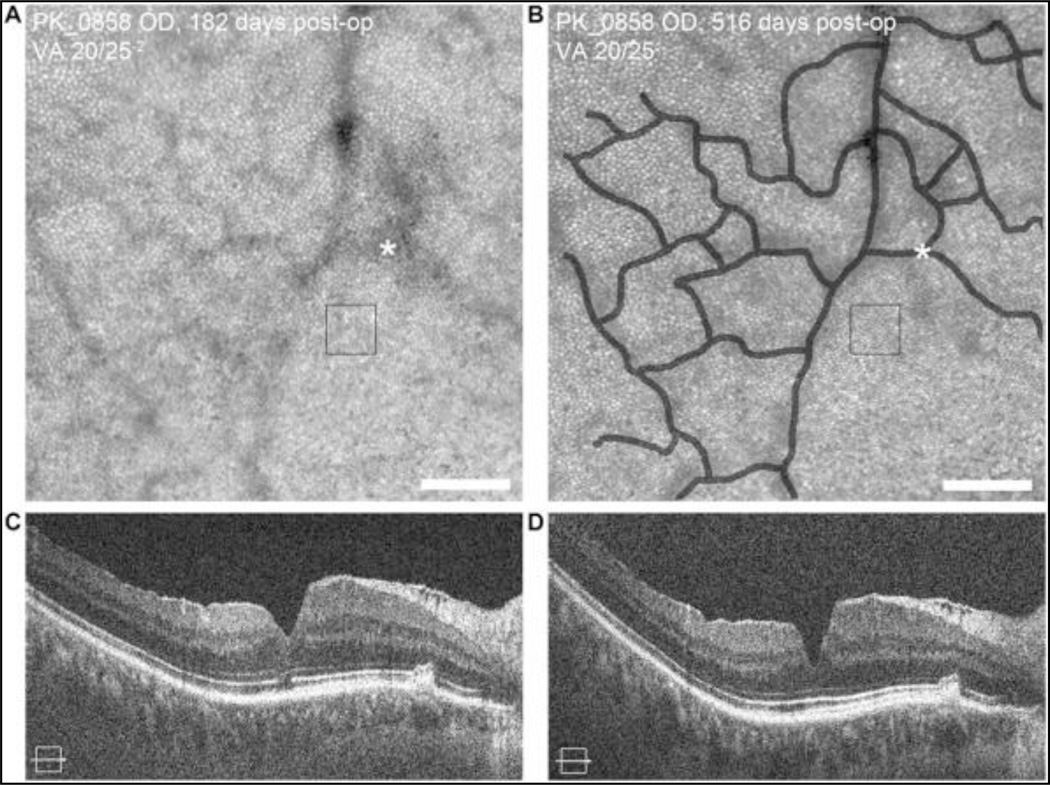
Post-operative changes of the inner retina were also observed. Patterns of retinal vessel shadows in AOSLO images of the cone mosaic changed in one subject (PK_0858) when imaged at 6 months and 17 months following surgery (Figure 3). Over this same period, the foveal pit depth increased from 113 microns to 136 microns, and the width of the pit decreased from 1097 microns to 961 microns. Serial cone density measurements (47,273 cones/mm2 versus 49,587 cones/mm2) at a ROI were within the expected repeatability of the measurement suggesting that the vascular and foveal changes observed are restricted to the inner retina without involvement of the photoreceptors.
Figure 3.
Longitudinal imaging reveals inner-retina remodeling over time. AOSLO image from the superior retina of subject PK_0858 approximately 6 months post-operatively shows a complete contiguous cone mosaic (A), with the foveal center at the lower right corner of the image. Subsequent imaging of same patient approximately 17 months post-operatively shows alterations in the retinal blood vessel shadows in the AOSLO image (B). The vessels comprising the foveal avascular zone in (A) were manually traced and are shown as a transparent overlay in panel (B). The asterisk in the two images shows a common vessel intersection, highlighting the altered contour between imaging sessions. Cone density measured at a single ROI near this location at both time points (black squares) were within expected repeatability [8], consistent with no significant change in the mosaic. SD-OCT images at the same time points (C, D) reveal a change in foveal pit morphology, consistent with inner retinal remodeling (see text). Scale bars = 100 µm.
DISCUSSION
This report demonstrates the utility of AOSLO for assessing photoreceptor structure in patients with MH closure following PPV. Consistent with previous reports [2,3], we identified persistent disruptions of the foveal cone mosaic despite closure of the MH and anatomic success following PPV. However, in contrast to prior reports which describe persistent large hyporeflective “dark areas” in the macula, we observed residual cone photoreceptors even at the foveal center. Some subjects had cone mosaics of normal density in retinal areas immediately adjacent to areas of focal disruption at the foveal center. Reasons for our ability to identify cone photoreceptor structures, albeit disrupted, at the foveal center when others could not are unclear, but may involve differences in imaging technique, data processing, and devices between groups. We utilized a logarithmic display for our AOSLO images, which allows better visualization of weakly reflecting cells.
We also demonstrate with longitudinal AOSLO imaging that these disruptions of the foveal cone mosaic can improve over time but may be delayed in some cases. This finding corroborates prior studies of visual function where delayed recovery of visual acuity can occur over extended periods of time, in some cases for as long as 2–3 years [9]. We believe the improvements in foveal photoreceptor mosaic observed here are likely due to recovery of cones within the area of disruption as opposed to "shifting" of neighboring cells given the stability of serial cone density measurements in areas of retina adjacent to these disruptions. Although structural recovery has been documented with SD-OCT [1,4,10] the resolution of commercial SD-OCT is not sufficient to resolve individual photoreceptors, and consequently is limited in its ability to analyze the relationship between cone structure and visual function.
This study identified one important disconnect between AOSLO and SD-OCT in subject (PK_0834), and the authors are not aware that this finding has been previously reported. Subject PK_0834 had a fairly intact foveal photoreceptor mosaic on AOSLO imaging but demonstrated near absence of the ONL and disruption of the EZ at the base of the fovea on SD-OCT (Figure 1). This finding contradicts previous reports that an intact ONL is necessary for restoration of the photoreceptor microstructure [10]. It is possible that this finding may reflect post-operative changes whereby closure of the MH results in lateral displacement of photoreceptor nuclei. If that is true, then it suggests that ONL thickness alone may not be a good marker for photoreceptor structure when trying to correlate structure and function in these patients. However, more subjects need to be imaged in order to study this further.
CONCLUSIONS
In summary, AOSLO and SD-OCT are complementary methods for assessing photoreceptor structure following MH closure. AOSLO provides cellular resolution and visualization of the cone mosaic, and can demonstrate changes in photoreceptor structure over time. AOSLO provides information that is not available with any other current imaging modality, including SD-OCT. While this study sheds light on various aspects of retinal structures following MH closure, further studies with more subjects imaged pre- and post-operatively are needed to elucidate better understanding of the relationship between photoreceptor structure and visual prognosis.
Table 1.
Preoperative and Postoperative Visual Acuities
| Subject No. (Age) |
Preoperative Visual Acuity |
Visual Acuity at First Imaging Session (Days After Surgery) |
Visual Acuity at Second Imaging Session (Days After Surgery) |
|---|---|---|---|
| PK_0815 (59 years) | 20/100 | 20/100+1 (92 days after Surgery) | 20/30 (359 days after surgery) |
| PK_0834 (55 years) | 20/60 | 20/60+2 (108 days after Surgery) | NA |
| PK_0835 (58 years) | 20/125 | 20/50−2 (88 days after Surgery) | 20/40+2 (523 days after surgery) |
| PK_0858 (58 years) | 20/40 | 20/25−2 (182 days after Surgery) | 20/25 (516 days after surgery) |
SUMMARY STATEMENT.
Adaptive optics scanning light ophthalmoscopy (AOSLO) is a valuable tool for evaluating photoreceptor structure and recovery following surgical closure of macular hole. It demonstrates persistent disruption of the photoreceptor mosaic with subsequent remodeling for months following surgery, perhaps accounting for the delayed post-operative improvements of visual acuity in some patients.
ACKNOWLEDGMENTS
The authors wish to thank Phyllis Summerfelt for her assistance in recruiting patients and acquiring images.
Supported by NIH grants R01EY017607 and P30EY001931, The E. Matilda Ziegler Foundation for the Blind, Thomas M. Aaberg, Sr. Retina Research Fund, Foundation Fighting Blindness, RD and Linda Peters Foundation, and an unrestricted departmental grant from Research to Prevent Blindness, Inc., New York, NY. This investigation was conducted in a facility constructed with support from Research Facilities Improvement Program, Grant Number C06RR016511, from the National Center for Research Resources, National Institutes of Health. The sponsors or funding organizations had no role in the design or conduct of this research. A. Dubra holds a Career Award at the Scientific Interface from the Burroughs Wellcome Fund and is the recipient of a Career Development Award from Research to Prevent Blindness (RPB).
Footnotes
The author(s) declare(s) that there is no financial conflict of interests regarding the publication of this article.
References
- 1.Itoh Y, Inoue M, Rii T, Hiraoka T, Hirakata A. Correlation between length of foveal cone outer segment tips line defect and visual acuity after macular hole closure. Ophthalmology. 2012;119:1438–1446. doi: 10.1016/j.ophtha.2012.01.023. [DOI] [PubMed] [Google Scholar]
- 2.Ooto S, Hangai M, Takayama K, Ueda-Arakawa N, Hanebuchi M, Yoshimura N. Photoreceptor damage and foveal sensitivity in surgically closed macular holes: An adaptive optics scanning laser ophthalmoscopy study. Am J Ophthalmol. 2012;154:174–186. doi: 10.1016/j.ajo.2012.01.031. [DOI] [PubMed] [Google Scholar]
- 3.Yokota S, Ooto S, Hangai M, et al. Objective assessment of foveal cone loss ratio in surgically closed macular holes using adaptive optics scanning laser ophthalmoscopy. PLoS ONE. 2013;8(5):e63786. doi: 10.1371/journal.pone.0063786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hansen SO, Cooper RF, Dubra A, Carroll J, Weinberg DV. Selective cone photoreceptor injury in acute macular neuroretinopathy. Retina. 2013;33:1650–1658. doi: 10.1097/IAE.0b013e31828cd03a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dubis AM, McAllister JT, Carroll J. Reconstructing foveal pit morphology from optical coherence tomography imaging. Br J Ophthalmol. 2009;93(9):1223–1227. doi: 10.1136/bjo.2008.150110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dubra A, Sulai Y, Norris JL, et al. Noninvasive imaging of the human rod photoreceptor mosaic using a confocal adaptive optics scanning ophthalmoscope. Biomed Opt Express. 2011;2:1864–1876. doi: 10.1364/BOE.2.001864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dubra A, Harvey Z. Lecture Notes in Computer Science (including subseries Lecture Notes in Artificial Intelligence and Lecture Notes in Bioinformatics) Vol. 6204. LNCS; 2010. Registration of 2D Images from Fast Scanning Ophthalmic Instruments; pp. 60–71. [Google Scholar]
- 8.Garrioch R, Langlo C, Dubis AM, Cooper RF, Dubra A, Carroll J. Repeatability of in vivo parafoveal cone density and spacing measurements. Optometry and Vision Science. 2012;89(5):632–643. doi: 10.1097/OPX.0b013e3182540562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scott IU, Moraczewski AL, Smiddy WE, Flynn HW, Jr, Feuer WJ. Long-term anatomic and visual acuity outcomes after initial anatomic success with macular hole surgery. Am J Ophthalmol. 2003;135:633–640. doi: 10.1016/s0002-9394(02)02240-7. [DOI] [PubMed] [Google Scholar]
- 10.Bottoni F, De Angelis S, Luccarelli S, Cigada M, Staurenghi G. The dynamic healing process of idiopathic macular holes after surgical repair: A spectral-domain optical coherence tomography study. Invest Ophthalmol Visual Sci. 2011;52(7):4439–4446. doi: 10.1167/iovs.10-6732. [DOI] [PubMed] [Google Scholar]