Abstract
Introduction
Falls and injuries resulting from falls in older adults represent a significant public health, personal and societal burden worldwide. Valuing wellbeing or quality of life more broadly may be a more appropriate method of measuring the full impact of falls prevention interventions. Our primary objective was to identify key factors relating to mobility and cognitive function explaining variation in wellbeing among community dwelling older fallers.
Methods
We conducted a longitudinal analysis of a 12-month prospective cohort study at the Vancouver Falls Prevention Clinic (Available Case Set: n=244/245). We constructed linear mixed models where assessment month (0, 6, 12) was entered as a within-subjects repeated measure, the intercept was specified as a random effect, and predictors and covariates were entered as between-subjects fixed effects. We included the predictors (i.e., Short Performance Physical Battery (SPPB) or Timed Up and Go (TUG) or Montreal Cognitive Assessment (MoCA)) to investigate sex differences over time in the relations between the predictor variable and the outcome variable, the ICECAP-O, a measure of wellbeing/quality of life.
Results
The SPPB and TUG were associated with wellbeing at baseline (p<0.05). Further a SPPB and TUG by time by sex interaction (p<0.05) was observed.
Conclusion
This study highlights a significant interaction of balance and mobility with wellbeing by time and sex. This study demonstrates that sex differences exist in the relationship between mobility and wellbeing with all men declining over time regardless of baseline mobility status and with women’s trajectories being dependent on their baseline function.
Introduction
Falls are a ‘geriatric giant’ and injuries resulting from falls in older adults represent a significant public health, personal and societal burden worldwide [1]. Approximately 30% of those aged 65 years and older who experience at least one fall each year with half of those falling recurrently [2]. Non-fatal fall injuries are associated with increased morbidity, decreased functioning and increased healthcare resource utilization. Falls and fall related injuries account for 10–15% of emergency department presentations and 6% of hospital admissions of those aged 65 years and older [3]. The most common diagnoses for fall related injuries are fractures and lacerations [3]. Older adults are at increased risk of falls and injurious falls. With populations across the globe ageing, the financial burden on our health care system is high and rising. Given the large financial burden imposed by falls and the scarcity of health care system resources, economic evaluations are essential to assist health care decision makers in allocating resources.
Economic evaluations are long established tools that are essential for guiding health policy decisions. Such evaluations aid decision makers in comparing the costs and consequences of various health interventions within and across sectors. A critical component in economic evaluations is how health outcomes are assessed. This is a particularly relevant challenge within falls research because there is a large degree of heterogeneity in the denominator of cost-effectiveness studies. Health outcomes (i.e., effectiveness) are valued in a number of ways (i.e., falls, Quality Adjusted Life Year (QALY)). The QALY is most often used to evaluate health related quality of life in economic evaluations because it provides the benefit of a common metric across conditions.
However, there is now increasing emphasis on wellbeing or quality of life more broadly as a critical outcome measure among specific populations such as older adults [4]. Of note, the terms wellbeing and quality of life are used interchangeable here and are distinct from health related quality of life because they are not focused on health alone. Many health care issues among older adults (i.e., falls, fracture, cognitive decline) [5] are accompanied by forms of care such as nursing homes, residential care, family member caregiving thus combining both health and social care. Social care encompasses the provision of social work, personal care, protection or social support services individuals (eg. older adults) in need (eg. at risk of decline). As such, it seems logical that an outcome measure for older fallers should aim to measure these potential gains or losses from a broader perspective than health alone [4]. Measuring individuals capabilities rather than focusing strictly on their functional ability to perform specific tasks is a promising approach to capture these broader benefits among older adults[6].
The ICECAP-O, an index of capability, is a preference-based outcome measure designed to provide a broader assessment of an individual’s quality of life or wellbeing [7,8]. This instrument was designed for use in economic evaluations across different sectors and intervention types. It is conceptually linked to Sen’s capability approach, which defines wellbeing in terms of what individuals are able to do (i.e., capabilities), not what individuals actually do (i.e., functionings). Specifically, capabilities reflect an individual’s ability to do a specific task; whereas, a functioning reflects whether or not the individual does a specific task or is in a specific state. Sen [9] emphasizes that an individual’s capabilities are most useful in assessing and comparing impact of interventions.
The ICECAP-O value system defines 1024 unique states valued using a best-worst scaling valuation method among older adults in England [7]. The value system provides a single summary index score, anchored at zero (‘no capability’) and 1.0 (‘full capability’). The ICECAP-O covers attributes of capability found to be important determinants of quality of life more broadly among older adults in the UK [7,10]. It includes the following five attributes: 1) attachment (love and friendship), 2) security (thinking about the future without concern), 3) role (doing things that make you feel valued), 4) enjoyment (enjoyment and pleasure) and 5) control (independence).
Key risk factors for falls include impaired physiological function [11] and cognition [2]. Falls are a common geriatric syndrome and are the third leading cause of chonic disability worldwide [12]. About 30% of community-dwellers over the age of 65 experience one or more falls every year [2]. Although not all falls lead to injury, about 20% require medical attention and 5% result in fracture, with one-third of those being hip fractures. In particular, of the 30% of community-dwelling seniors who fall, half fall recurrently and are at significant risk for hospitalization, institutionalization, and even death [13]. These events place these individuals at increased risk of health decline due to the morbidity deficits that are often incurred post trauma. Further, these deficits may impose functional [14], social or mood [15] related limitations on these older adults. As the proportion of the population over 65 continues to increase, falls will place an increasing burden on the public health system. As such, individuals who have sustained a fall (i.e., the Vancouver Falls Prevention Cohort) are at high risk of experiencing wellbeing decline and thus represents a critical population to intervene [4]. Prior to intervening, we must first ensure that we are appropriately capturing gains/losses in this population. Given that behavioural interventions aimed at promoting balance and mobility may have benefits that extend beyond health, it may be critical to explore such measures (i.e., the ICECAP-O) that aim to capture these gains or losses.
Previous cross-sectional studies have demonstrated an association between balance, mobility and cognition with wellbeing [16,17]. To date, the literature is relatively devoid of longitudinal data and understanding determinants of wellbeing or changes in wellbeing over time [18]. Hence, our primary objective was to determine the factors that predict change in wellbeing, as measured by the ICECAP-O, over time (i.e., at 6 and 12 months) among older men and women presenting to the Vancouver Falls Prevention Clinic. Further, one cross-sectional analysis demonstrated a significant association between sex and HRQoL among older adults who received a baseline assessment at the Vancouver Falls Prevention Clinic [16,17]. However, there was no significant association between sex and wellbeing. Predictors of any subtle differences between the sexes in wellbeing over time are not well established. However, given that men and women’s cognitive and mobility function progress and respond differently, we hypothesize that their wellbeing would be differentially affected as well. Therefore, our secondary objective was to determine whether sex moderated the relationship between the identified predictors and changes in wellbeing.
Materials and Methods
Study design
We conducted a longitudinal analysis of data from a 12-month prospective cohort study at the Vancouver Falls Prevention Clinic (www.fallclinic.com) from June 7, 2010 through October 24, 2013. Participants received a comprehensive assessment at the Vancouver Falls Prevention Clinic at baseline and 12-months.
Participants
Individuals presenting to the Vancouver Falls Prevention Clinic have all sustained a previous fall in the past 12 months and are at high risk of mobility impairments that may result from subsequent falls, fracture, and functional decline [16,19]. The sample consisted of 321 women and men referred by their general practitioner or emergency department physician to the Vancouver Falls Prevention Clinic. From June 2010 through October 2013, all patients presenting to the Vancouver Falls Prevention Clinic were invited to participate in a cohort study. Community dwelling women and men who lived in the lower mainland region of British Columbia were eligible for study entry if they:
were adults ≥ 70 years of age referred by a medical professional to the Falls Prevention Clinic as a result of seeking medical attention for a non-syncopal fall in the previous 12 months;
understood, spoke, and read English proficiently;
had a Mini Mental State Examination (MMSE) [20] score ≥ 24/30;
had a Physiological Profile Assessment (PPA) [21] score of at least 1.0 SD above age-normative value or Timed Up and Go Test (TUG) [22] performance of greater than 15 seconds or one additional non-syncopal fall in the previous 12 months;
were expected to live greater than 12 months (based on the geriatricians’ expert opinion);
were able to walk 3 metres with or without an assistive device; and
were able to provide written informed consent.
We excluded those with a neurodegenerative disease (e.g., Parkinson’s disease) or dementia, patients who recently had a stroke, those with clinically significant peripheral neuropathy or severe musculoskeletal or joint disease, and anyone with a history indicative of carotid sinus sensitivity (i.e., syncopal falls). We highlight that exclusions for this study were based on clinical grounds. The Falls Prevention Clinic is targeting treatment of older adults at risk of impaired mobility and functional decline specifically. Thus individual’s with neurodegenerative disease or dementia are referred to alternate clinics.
Ethical approval was obtained from the Vancouver Coastal Health Research Institute and the University of British Columbia’s Clinical Research Ethics Board (H09-02370). All participants provided written informed consent.
Vancouver Falls Prevention Clinic Measures at Baseline, 6 Months and 12 Months
A comprehensive set of measurements relating to mobility and cognitive function that were collected at baseline are described below.
Comorbidity, activities of daily living and depression
Functional comorbidity index (FCI) was calculated to estimate the degree of comorbidity associated with physical functioning [23]. This scale’s score is the total number of comorbidities. We used the 15-item Geriatric Depression Scale (GDS) [24,25] to indicate the presence of depression; a score of ≥ 5 indicates depression [26].
Balance and mobility
Mobility and balance were assessed using the Short Physical Performance Battery (SPPB) [27] and the Timed-Up-and-Go Test (TUG) [28]. For the Short Physical Performance Battery, participants were assessed on performances of standing balance, walking, and sit-to-stand. Each component is rated out of four points, for a maximum of 12 points; a score < 9/12 predicts subsequent disability [29]. For the TUG, participants rose from a standard chair, walked a distance of three meters, turned, walked back to the chair and sat down [28]. We recorded the time (s) to complete the TUG, based on the average of two separate trials. A TUG performance time of ≥ 13.5 seconds correctly classified persons as fallers in 90% of cases [28].
Physiological Falls Risk
Physiological falls risk was assessed using the short form of the Physiological Profile Assessment (PPA). The PPA is a valid and reliable [60] measure of falls risk. Based on a participant’s performance in five physiological domains – postural sway, reaction time, strength, proprioception, and vision – the PPA computes a falls risk score (standardized score) that has a 75% predictive accuracy for falls in older people [30,31]. A PPA Z-score of ≥ 0.60 indicates high physiological falls risk [32].
Cognitive Function
We assessed global cognition using the Mini Mental State Examination (MMSE) and the Montreal Cognitive Assessment (MoCA). The MMSE is a widely used and well-known questionnaire used to screen for cognitive impairment (i.e., MMSE <24) [20]. It is scored on a 30-point scale with a median score of 28 for healthy community dwelling octogenarians with more than 12 years of education [20]. The MMSE may underestimate cognitive impairment for frontal system disorders because it has no items specifically addressing executive function [20].
The Montreal Cognitive Assessment (MoCA), a brief screening tool for MCI [33] with high sensitivity and specificity, was used to categorize participants as with, or without, possible MCI. It is more sensitive than the MMSE in detecting mild cognitive impairment [33]. It is a 30-point test covering eight cognitive domains: 1) attention and concentration; 2) executive functions; 3) memory; 4) language; 5) visuo-constructional skills; 6) conceptual thinking; 7) calculations; and 8) orientation. Scores below 26 are considered to be indicative of possible MCI. A bonus point is given to individuals with less than 12 years of education.
Cognitive performance was also assessed using the Digit Symbol Substitution Test (DSST) [34]. The DSST mainly assesses psychomotor speed. It also assesses visual motor speed, sustained attention and working memory. The range of digits correctly substituted for symbols ranges from 0–133. The greater the number of correct digits the better the performance.
Primary Outcome Measure
The primary outcome was the ICECAP-O [35,36]. Patients completed the ICECAP-O using paper versions that were given to them upon their initial visit to the Vancouver Falls Prevention Clinic. Telephone interviews were used to complete the ICECAP-O at 6 and 12 months. No cards were used to aid interpretation.
ICECAP-O
We assessed quality of life/wellbeing using the ICECAP-O [7,10,37]. The ICECAP-O is a five item multiple choice questionnaire that measures an individual’s wellbeing and quality of life more broadly according to five attributes: attachment (love and friendship), security (thinking about the future without concern), role (doing things that make you feel valued), enjoyment (enjoyment and pleasure) and control (independence). Each domain has four possible response options. The ICECAP-O can be used to calculate a global capability index score on a zero to one scale where zero represents no capability and one represents full capability. QALYs can also be estimated from the ICECAP-O for use in economic evaluation [4,36].
Handling of Missing Data
Missing data were handled in three ways. First, using the restricted maximum likelihood estimator, all individuals with baseline data for the variables in the model (i.e., available case set) were included (ML analysis). Second, models were restricted to those individuals with ICECAP-O data at baseline and 12-month follow-up (i.e., complete case analysis). Third, multiple imputation using the ICE (Imputation by Chained Equations) procedure in STATA 10.0 was using to create five complete data sets (MI analysis). In the Vancouver Falls Prevention Cohort study, 52% of participants had complete data for all three time points. We followed recommendations by Oostenbrink [38,39] and Briggs [40,41] for multiple imputation of missing effectiveness data. We imputed missing ICECAP-O values at each time point (i.e., 6 and 12 months). For each missing value, we generated five possible values using multiple linear regression. Covariates included age, FCI, TUG, PPA and baseline ICECAP-O score, and the weight and value of the missing variable in the preceding period. The final imputed value was the mean value from the five data sets created.
Statistical Analyses
We report the available case set as our base case analysis. We report the complete case analysis and the imputed case analysis as part of our sensitivity analyses. Data were initially examined using visual analysis of histograms and computation of skew and kurtosis. The outcome variable, ICECAP-O, was not significantly skewed; therefore, analyses were conducted on the untransformed ICECAP-O.
For the main analyses, linear mixed models were constructed using the SPSS 22.0 MIXED procedure (IBM Corporation, 2013). Assessment month (0, 6, 12) was entered as a within-subjects repeated measure, the intercept was specified as a random effect, and predictors and covariates were entered as between-subjects fixed effects. A first-order auto-regressive covariance matrix provided superior model fit compared to an unstructured covariance matrix (based on the Bayesian Information Criterion) and allowed for model convergence across the models. Denominator degrees of freedom were calculated from the Satterthwaite approximation.[42]
A separate linear mixed model was constructed for each predictor variable examined. In addition to the specific predictor and its interaction with time, models include participant age and sex and their interactions with time. We also included the predictor X sex and predictor X sex X time interaction terms in order to investigate sex differences in the relations between the predictor variable and the outcome variable, ICECAP-O score. If not statistically significant, these terms were dropped. Additionally, in the examination of SPPB and TUG as key mobility related predictors, the use of armrest was included as a covariate, along with its interaction with time. (Note: the use of armrest did not interact with the main variables of interest the model, and therefore these interaction terms were excluded). In the text, we report the unstandardized beta estimate (B), its 95% confidence interval, and its significance value. Given a significant interaction with sex, we stratified the data and ran the models separately for males and females. To visualize significant interaction effects, we used model-based estimated marginal means at low (−1 SD), average (0 SD), and high (+1 SD) levels of the predictor.[43] When a higher-order interaction was significant (e.g., 3-way interaction), we do not report significant lower-order interactions (2-way interactions) or main effects.
RESULTS
Two-hundred and forty five participants are included in our base case analysis (available case analysis) for the SPPB and 244 participants are included in our base case analysis for the TUG. For our two sensitivity analyses, 144 participants are included in the complete case analysis and 286 participants are included in the imputed data set.
Participants
Table 1 reports descriptive statistics of the base case analysis (all available cases) at baseline for our variables of interest for this cohort. At baseline, this cohort of community-dwelling senior women has a mean (SD) ICECAP-O of 0.805 (0.137), a mean SPPB of 7.3 (2.5) and a mean TUG of 19.7 (10.5). On average, participants had at least two existing co-morbidities and were 82.5 ± 6.5 years of age. Participants were classified as having high falls risk with a mean PPA score of 1.7 ± 1.1. Further, the mean MMSE score was 26.4 (3.2) and the mean MoCA score was 22.1 ± 4.6. A cut-off of 26 or lower is used to classify individuals with mild cognitive impairment.
Table 1.
Baseline Characteristics of the Vancouver Falls Prevention Cohort (Available Case Analysis)
| Variables at Baseline | Mean (SD) or Number (%) |
|---|---|
| Age (years) (n=315) | 82.5 (6.5) |
| Sex (Male/Female) (n=308) | 112 (36.4)/196 (63.4) |
| Living status (n=253) | |
| Lives alone | 100 (39.5) |
| Lives with others | 122 (48.2) |
| Assisted living | 31 (12.3) |
| Education (n=299) | |
| < Grade 9 | 33 (11.0) |
| Grades 9–13, no diploma | 59 (19.7) |
| High school with diploma | 58 (19.4) |
| Trades school | 23 (7.7) |
| Some university | 36 (12.0) |
| University | 90 (30.1) |
| FCI (n=320) | 2.5 (1.9) |
| GDS (n=315) | 3.1 (2.6) |
| ICECAP-O (n=248) | 0.805 (0.137) |
| SPPB (n=303) | 7.3 (2.5) |
| TUG (n=296) | 19.7 (10.5) |
| PPA (n=311) | 1.7 (1.1) |
| MMSE (n=315) | 26.4 (3.2) |
| MoCA (n=303) | 22.1 (4.6) |
Base Case Analysis
The base case (i.e., available case) analysis is presented in Table 2.
Table 2.
The Maximum Likelihood Model for the available case analyses for the SPPB and TUG
| Maximum Likelihood | ||
|---|---|---|
|
| ||
| Predictor | B (95%CI) | P value |
| SPPB, N=245 | ||
| SPPB | .0194 (.010, .0286)* | <.001 |
| SPPB X time | −.0036 (−.0162, .0090) | .576 |
| SPPB X sex | −.0097 (−.0227, .00326) | .142 |
| SPPB X sex X time | .0196 (.0015, .0377)* | .034 |
|
| ||
| TUG, N=244 | ||
| TUG | −.250 (−.361, −.138)** | <.001 |
| TUG X time | .165 (.021, .309)* | .025 |
| TUG X sex | .182 (−.012, .377) | .066 |
| TUG X sex X time | −.480 (−.733, −.226)** | <.001 |
p<0.05
p<0.01
Mobility
Short Performance Physical Battery
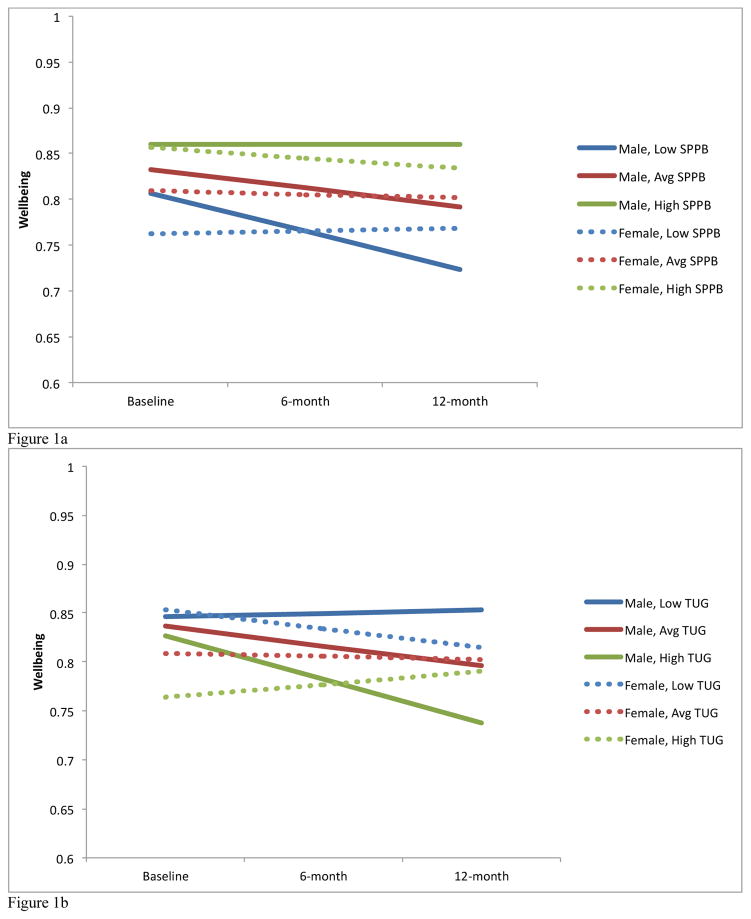
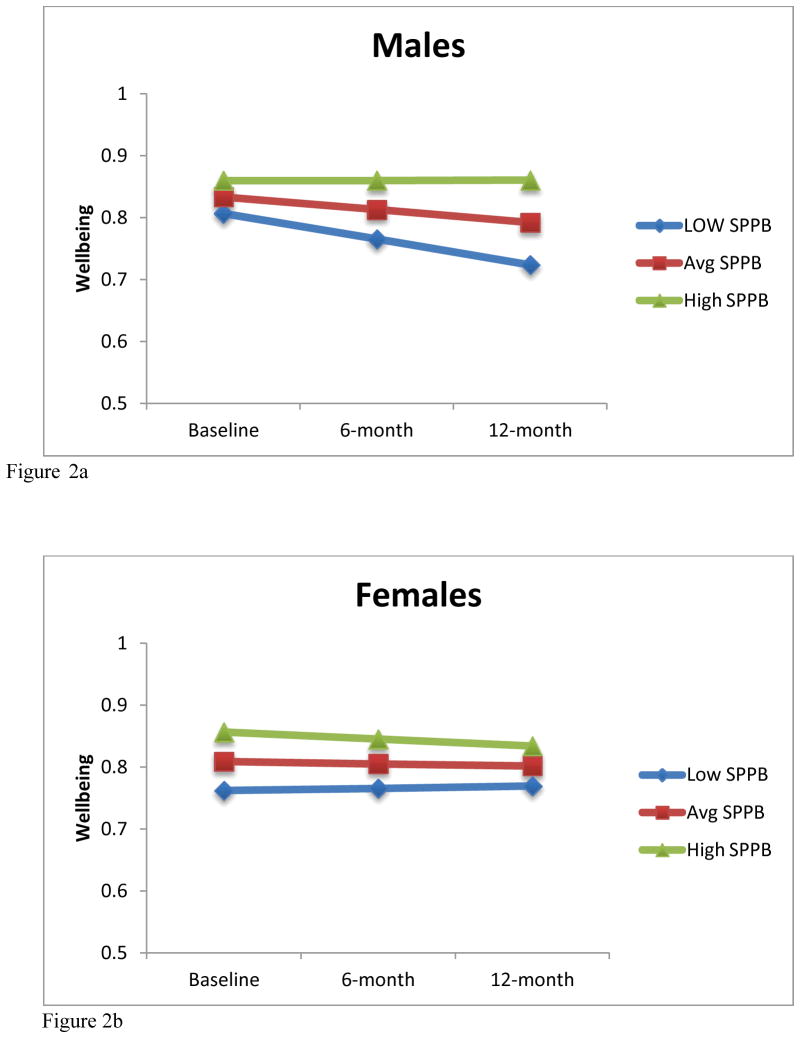
The Maximum Likelihood Model for the available case analysis (n=245) demonstrated that the SPPB at baseline is associated with wellbeing at baseline (p<0.001). Further, a SPPB by time by sex (p=0.034) interaction was observed (Figure 1a and 1b). When the analyses were run separately for males and females using the available case set (n=245), we found that for males (n = 80), there were significant effects demonstrating baseline SPPB was associated with baseline ICECAP-O (B = .01, p = 0.001) and there was a trend for SPPB to predict change in ICECAP-O over time (B = .02, p = .077). Alternatively, for females (n = 165), SPPB was associated with baseline ICECAP-O (B = .02, p < 0.001) but did not predict change in ICECAP-O over time (B = −.01, p = .417). These effects among men and women are graphed in Figures 2a and 2b, using model-based estimated marginal means for average, low (−1 SD) and high (+1 SD) SPPB scores.
Figure 1.
Figure 1a: SPPB by time by sex interaction over 12 months
Figure 1b: TUG by time by sex interaction over 12 months
Figure 2.
Figure 2a: Model-based estimated marginal means for low (−1 SD), average and high (+1 SD) SPPB scores for males.
Figure 2b: Model-based estimated marginal means for low (−1 SD), average and high (+1 SD) SPPB scores for females.
Timed Up and Go
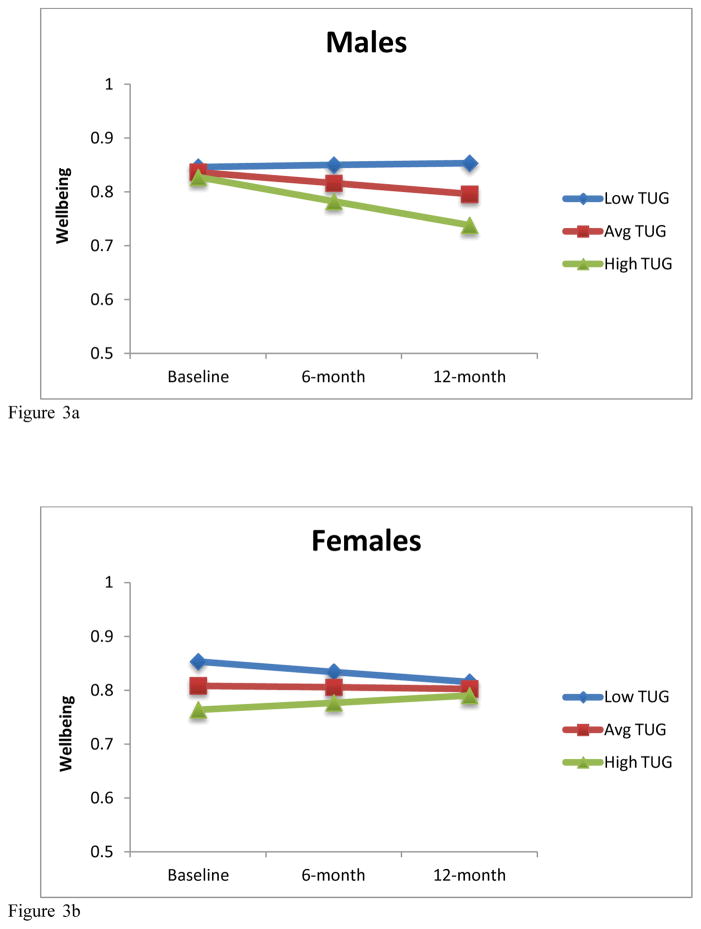
The Maximum Likelihood Model for the available case analysis (n=244) demonstrated that the TUG at baseline predicts baseline ICECAP-O. Further, a significant TUG by time interaction (p=0.025) and TUG by time by sex interaction (p<0.001) were also observed. When the analyses were run separately for males and females using the available case set, we found that for males (n = 81), the TUG at baseline was not associated with baseline ICECAP-O (B = −0.06, p = .475) but did predict change in ICECAP-O over time such that higher baseline TUG scores predict decreases in ICECAP-O scores (B= −0.32, p=0.011). For females (n = 165), the baseline TUG was associated with baseline ICECAP-O (B = −0.25, p <0.001) and change in ICECAP-O over time (B = 0.18, p = .022). These effects among men and women are graphed in Figures 3a and 3b, using model-based estimated marginal means for average, low (−1 SD) and high (+1 SD) TUG scores.
Figure 3.
Figure 3a: Model-based estimated marginal means for low (−1 SD), average and high (+1 SD) TUG scores for males.
Figure 3b: Model-based estimated marginal means for low (−1 SD), average and high (+1 SD) TUG scores for females.
Cognitive Function
The MoCA (p=0.027 for the available case analysis, n=255 and p=0.009 for the complete case analysis, n=149) and DSST (p=0.056 for the available case analysis, n=244 and p=0.027 for the complete case analysis, n=143) at baseline predicted ICECAP-O score at baseline. No statistically significant MoCA by time or DSST by time interactions were observed.
Sensitivity Analysis
All sensitivity analyses were conducted on the transformed and non-transformed EQ-5D data are presented in Table 3.
Table 3.
Mixed Linear Models for the multiply imputed and complete case sets for the SPPB and TUG
| Multiple Imputation N = 290 |
Complete Case N = 144 |
|||
|---|---|---|---|---|
|
| ||||
| Predictor | B (95% CI) | p value | B (95% CI) | p value |
| SPPB | B (p value) | B (p value) | ||
| SPPB | .0183 (.0083, .028) | .001** | .0240 (.0114, .0366) | <.001** |
| SPPB X time | −.0019 (−.0081, .0043) | .529 | −.0054 (−.0189, .0081) | .431 |
| SPPB X sex | −.0084 (−.0198, .0028) | .142 | −.0273 (−.0456, −.0091) | .004** |
| SPPB X sex X time | .0034 (−.0063, .013) | .471 | .0345 (.0149, .0540) | .001** |
|
| ||||
| TUG | N=290 | N = 144 | ||
|
| ||||
| TUG | −.235 (−.354, −.116) | <.001** | −.301 (−.450, −.153) | <.001** |
| TUG X time | .0634 (−.0040, .131) | .065 | .174 (.0203, .327) | .027* |
| TUG X sex | .139 (−.0334, .311) | .114 | .357 (.0969, .616) | .007** |
| TUG X sex X time | −.115 (−.218, −.012) | .029* | −.599 (−.869, −.328) | <.001** |
p<0.05
p<0.01
Complete Case Analysis
Short Performance Physical Battery
The Mixed Linear Model for the complete case analysis (n=144) demonstrated that baseline SPPB is associated with baseline ICECAP-O (p<0.001). Further, a SPPB by sex interaction (p=0.004) and a SPPB by time by sex interaction (p=0.001) were also observed. When the analyses were run separately for males and females using the available case set (n=144), we found that for males (n = 49), baseline SPPB was not associated with baseline ICECAP-O (B = −0.003, p = 0.727); however, baseline SPPB did predict change in ICECAP-O over time (B = .03, p = .001). Alternatively, for females (n = 95), baseline SPPB was associated with baseline ICECAP-O (B = −.02, p < 0.002) but did not predict change in ICECAP-O over time (B = −.02, p = .264).
Timed Up and Go
The Mixed Linear Model for the complete case analysis (n=144) demonstrated that the TUG at baseline predicts baseline ICECAP-O (p<0.001). Further, a significant TUG by time interaction (p=0.027), TUG by sex interaction (p=0.007) and TUG by time by sex interaction (p<0.001) were observed. When the analyses were run separately for males and females using the complete case set, we found that for males (n = 49), baseline TUG was not associated with baseline ICECAP-O (B = 0.04, p = .759) but did predict change in ICECAP-O over time such that higher baseline TUG scores predict decreases in ICECAP-O scores (B= −0.42, p=0.002). For females (n = 95), baseline TUG was associated with baseline ICECAP-O (B = −0.30, p <0.001) and change in ICECAP-O over time (B = 0.19, p = .026).
Imputed Case Analysis
The Mixed Linear Model for the complete case analysis (n=148) demonstrated that the TUG at baseline predicts baseline EQ-5D HSUVs. Further, a significant TUG by time by sex interaction (p<0.05) was also observed for the transformed and non-transformed EQ-5D data.
Short Performance Physical Battery
The Mixed Linear Model for the imputed case analysis (n=286) demonstrated that the SPPB at baseline predicts baseline ICECAP-O. No significant SPPB by time interaction (p>0.05) and SPPB by time by sex ineraction (p>0.05) were observed.
Timed Up and Go
The Mixed Linear Model for the imputed case analysis (n=290) demonstrated that the TUG at baseline predicts baseline ICECAP-O (p<0.001). Further, a significant TUG by time by sex interaction (p=0.029) was observed. When the analyses were run separately for males and females using the imputed case set, we found that for males (n = 101), baseline TUG was not associated with baseline ICECAP-O (B = −0.09, p = .284) nor change in ICECAP-O over time (B= −0.09, p=0.261). For females (n = 189), the baseline TUG was associated with baseline ICECAP-O (B = −0.23, p =0.001) and there was a non-significant trend demonstrating change in ICECAP-O over time (B = 0.07, p = .071).
Discussion
This is the first study to examine the key factors that explain variation in wellbeing over time among older fallers. This is a critical research area to develop in order to appropriately tailor future intervention strategies targeting wellbeing among older fallers – a population at high risk of both functional and cognitive decline. We found that two valid and reliable measures of balance and mobility explained significant variation in wellbeing over time. Specifically, the SPPB demonstrated a significant interaction with time by sex and the TUG demonstrated a significant interaction with time and with time and sex. Interestingly, cognition and specifically executive function explained variation in wellbeing at baseline only, not over time.
There is limited longitudinal data that examines factors that explain variation in wellbeing among older adults [18]. Previously, cross-sectional data demonstrated that the ICECAP-O described between measures of depression, instrumental activities of daily living and the presence or absence of social activity limitations. The ICECAP-O has also demonstrated discriminative ability between multi-morbid elderly and those with high or low health related quality of life scores (measured using the EQ-5D). Mobility is affected by all of the above items (depression, instrumental activities of daily living, mood, social activities and health related quality of life). As such, our findings that mobility is a key factor accounting for variation in wellbeing over time builds on existing literature in this field. Interestingly, the ICECAP-O does not have a specific physical dimension; however, previous research has demonstrated its capacity to capture the effects of decreased physical function on wellbeing through the control and role dimensions [44,45].
We did observe sex specific differences over time and these observations were different between the SPPB and the TUG. In summary for the SPPB, although the trends for males and females were different in explaining wellbeing over time, these trends were non-significant. In contrast, the TUG was significant for males and females in explaining variation in wellbeing over time. This is a new finding compared with previous cross-sectional research that did not demonstrate any significant associations between ICECAP-O and sex [45]. Because these analyses are exploratory, it is too early to draw strong conclusions regarding the effect of sex on wellbeing over time. Of note, these findings do highlight that future intervention strategies aimed at improving wellbeing among older adults may need to consider different mobility related interventions for males and females.
We note the following limitations of our study. First, there was considerable missing data could influence the interpretation of the results. It is possible that individuals who did not complete the cohort study were different that those who did. To investigate the impact of the missing data on our conclusions, we conducted two sensitivity analyses with the multiply imputed case set and the complete case set and report the results both ways. Importantly, there are also limited longitudinal ICECAP-O data published to date. As such, we had limited data to compare our findings with. Additional longitudinal studies are needed to understand the sensitivity of the ICECAP-O to change among older adults at risk of mobility impairment. Further, future research should explore whether these overall findings are restricted to those with impaired mobility or if these findings can be extended to a general population. Lastly, we also used the UK ICECAP-O valuations because there are no Canadian valuations published to date.
Conclusions & future directions
This study is the first to investigate predictors of wellbeing over time. This study highlighted that mobility is a critical factor in explaining wellbeing at baseline and over time. Cognition at baseline did not explain wellbeing over time. Further, this study highlights the unique contribution of mobility to wellbeing over time between men and women. Specifically, men in the average or lower functioning mobility tertiles demonstrated decline over time regardless; whereas women regardless of their baseline status demonstrated a regression to the mean trend. This study provides an initial benchmark that mobility is an important factor contributing to older adults wellbeing. As such, future intervention strategies aimed at improving wellbeing should consider mobility as a primary target.
Acknowledgments
We thank the Vancouver Falls Prevention Cohort study participants. The Canadian Institute for Health Research Emerging Team Grant (CIHR, MOB-93373 to Karim Khan, TLA, LL) provided funding for this study. TLA is a Canada Research Chair in Physical Activity, Mobility, and Cognitive Neuroscience, a Michael Smith Foundation for Health Research (MSFHR) Scholar, a Canadian Institutes of Health Research (CIHR) New Investigator, and a Heart and Stroke Foundation of Canada’s Henry JM Barnett’s Scholarship recipient. JCD and JB are funded by a CIHR and MSFHR Postdoctoral Fellowship. LL is a MSFHR Scholar and a Canada Research Chair. CLS is a CIHR Doctoral Trainee. These funding agencies did not play a role in study design. We obtained approval for the Vancouver Falls Prevention Clinic Cohort study from UBC Clinical Ethics Review Board.
Footnotes
Conflict of Interest
The authors declare that they have no competing interests.
Author’s Contributions
TLA was principal investigator for the Vancouver Falls Prevention Clinic Cohort study. TLA and JCD were responsible for study concept and design, acquisition of data, data analysis and interpretation, writing and reviewing of the manuscript. JCD and JB were responsible for data analysis. JCD, TLA, JB, SB, CLH, LL, CG, and KV drafted and revised the manuscript. JCD, TLA and SB acquired and interpreted the data.
References
- 1.Tinetti ME, Williams CS. Falls, injuries due to falls, and the risk of admission to a nursing home. N Engl J Med. 1997;337(18):1279–1284. doi: 10.1056/NEJM199710303371806. [DOI] [PubMed] [Google Scholar]
- 2.Tinetti ME, Speechley M, Ginter SF. Risk factors for falls among elderly persons living in the community. N Engl J Med. 1988;319(26):1701–1707. doi: 10.1056/NEJM198812293192604. [DOI] [PubMed] [Google Scholar]
- 3.Woolcott JC, Khan KM, Mitrovic S, Anis AH, Marra CA. The cost of fall related presentations to the ED: a prospective, in-person, patient-tracking analysis of health resource utilization. Osteoporos Int. 2012;23(5):1513–1519. doi: 10.1007/s00198-011-1764-1. [DOI] [PubMed] [Google Scholar]
- 4.Makai P, Looman W, Adang E, Melis R, Stolk E, Fabbricotti I. Cost-effectiveness of integrated care in frail elderly using the ICECAP-O and EQ-5D: does choice of instrument matter? Eur J Health Econ. 2014 doi: 10.1007/s10198-014-0583-7. [DOI] [PubMed] [Google Scholar]
- 5.Liu-Ambrose T, Nagamatsu LS, Graf P, Beattie BL, Ashe MC, Handy TC. Resistance training and executive functions: a 12-month randomized controlled trial. Arch Intern Med. 2010;170(2):170–178. doi: 10.1001/archinternmed.2009.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Janabi H, Flynn TN, Coast J. Development of a self-report measure of capability wellbeing for adults: the ICECAP-A. Qual Life Res. 2012;21(1):167–176. doi: 10.1007/s11136-011-9927-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coast J, Flynn TN, Natarajan L, Sproston K, Lewis J, Louviere JJ, Peters TJ. Valuing the ICECAP capability index for older people. Soc Sci Med. 2008;67(5):874–882. doi: 10.1016/j.socscimed.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 8.Rowen D, Brazier J, Tsuchiya A, Alava MH. Valuing states from multiple measures on the same visual analogue sale: a feasibility study. Health Econ. 2011 doi: 10.1002/hec.1740. [DOI] [PubMed] [Google Scholar]
- 9.Sen A, Nussbaum M. Capability and well-being. Oxford: Oxford University Press; 1993. [Google Scholar]
- 10.Coast J, Peters TJ, Natarajan L, Sproston K, Flynn T. An assessment of the construct validity of the descriptive system for the ICECAP capability measure for older people. Qual Life Res. 2008;17(7):967–976. doi: 10.1007/s11136-008-9372-z. [DOI] [PubMed] [Google Scholar]
- 11.Lord SR, Clark RD, Webster IW. Physiological factors associated with falls in an elderly population. J Am Geriatr Soc. 1991;39(12):1194–1200. doi: 10.1111/j.1532-5415.1991.tb03574.x. [DOI] [PubMed] [Google Scholar]
- 12.Murray C, Lopez A. Global and regional descriptive epidemiology of disability: incidence, prevalence, health expectancies, and years lived with disability. In: Murray C, Lopez A, editors. The global burden of disease. Boston: The Harvard School of Public Health; 1996. pp. 201–246. [Google Scholar]
- 13.Pluijm SM, Smit JH, Tromp EA, Stel VS, Deeg DJ, Bouter LM, Lips P. A risk profile for identifying community-dwelling elderly with a high risk of recurrent falling: results of a 3-year prospective study. Osteoporos Int. 2006;17(3):417–425. doi: 10.1007/s00198-005-0002-0. [DOI] [PubMed] [Google Scholar]
- 14.Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, Mitnitski A. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173(5):489–495. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Djernes JK. Prevalence and predictors of depression in populations of elderly: a review. Acta Psychiatr Scand. 2006;113(5):372–387. doi: 10.1111/j.1600-0447.2006.00770.x. [DOI] [PubMed] [Google Scholar]
- 16.Davis JC, Bryan S, McLeod R, Rogers J, Khan K, Liu-Ambrose T. Exploration of the association between quality of life, assessed by the EQ-5D and ICECAP-O, and falls risk, cognitive function and daily function, in older adults with mobility impairments. BMC Geriatr. 2012;12:65. doi: 10.1186/1471-2318-12-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davis JC, Liu-Ambrose T, Richardson CG, Bryan S. A comparison of the ICECAP-O with EQ-5D in a falls prevention clinical setting: are they complements or substitutes? Qual Life Res. 2012 doi: 10.1007/s11136-012-0225-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Comans TA, Peel NM, Gray LC, Scuffham PA. Quality of life of older frail persons receiving a post-discharge program. Health Qual Life Outcomes. 2013;11:58. doi: 10.1186/1477-7525-11-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davis JC, Liu-Ambrose T, Richardson CG, Bryan S. A comparison of the ICECAP-O with EQ-5D in a falls prevention clinical setting: are they complements or substitutes? Qual Life Res. 2013;22(5):969–977. doi: 10.1007/s11136-012-0225-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 21.Lord S, Sherrington C, Menz H. Falls in older people. Risk factors and strategies for prevention. Cambridge: Cambridge University Press; 2001. A physiological profile approach for falls prevention; pp. 221–238. [Google Scholar]
- 22.Whitney JC, Lord SR, Close JC. Streamlining assessment and intervention in a falls clinic using the Timed Up and Go Test and Physiological Profile Assessments. Age Ageing. 2005;34(6):567–571. doi: 10.1093/ageing/afi178. [DOI] [PubMed] [Google Scholar]
- 23.Groll DL, To T, Bombardier C, Wright JG. The development of a comorbidity index with physical function as the outcome. J Clin Epidemiol. 2005;58(6):595–602. doi: 10.1016/j.jclinepi.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 24.Yesavage JA. Geriatric Depression Scale. Psychopharmacol Bull. 1988;24(4):709–711. [PubMed] [Google Scholar]
- 25.Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17(1):37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 26.van Marwijk HW, Wallace P, de Bock GH, Hermans J, Kaptein AA, Mulder JD. Evaluation of the feasibility, reliability and diagnostic value of shortened versions of the geriatric depression scale. Br J Gen Pract. 1995;45(393):195–199. [PMC free article] [PubMed] [Google Scholar]
- 27.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332(9):556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shumway-Cook A, Brauer S, Woollacott M. Predicting the probability for falls in community-dwelling older adults using the Timed Up & Go Test. Phys Ther. 2000;80(9):896–903. [PubMed] [Google Scholar]
- 29.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-Extremity Function in Persons over the Age of 70 Years as a Predictor of Subsequent Disability. N Engl J Med. 1995;332(9):556–562. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lord S, Clark R, Webster I. Physiological factors associated with falls in an elderly population. Journal of American Geriatrics Society. 1991;39:1194–1200. doi: 10.1111/j.1532-5415.1991.tb03574.x. [DOI] [PubMed] [Google Scholar]
- 31.Lord S, Ward J, Williams P, Anstey K. Physiological factors associated with falls in older community-dwelling women. Journal of American Geriatrics Society. 1994;42:1110–1117. doi: 10.1111/j.1532-5415.1994.tb06218.x. [DOI] [PubMed] [Google Scholar]
- 32.Delbaere K, Close JCT, Brodaty H, Sachdev P, Lord SR. Determinants of disparities between perceived and physiological risk o falling among elderly people: cohort study. British Medical Journal. 2010 doi: 10.1136/bmj.c4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 34.Sevick MA, Bradham DD, Muender M, Chen GJ, Enarson C, Dailey M, Ettinger WH., Jr Cost-effectiveness of aerobic and resistance exercise in seniors with knee osteoarthritis. Med Sci Sports Exerc. 2000;32(9):1534–1540. doi: 10.1097/00005768-200009000-00002. [DOI] [PubMed] [Google Scholar]
- 35.Flynn TN, Chan P, Coast J, Peters TJ. Assessing quality of life among British older people using the ICEPOP CAPability (ICECAP-O) measure. Appl Health Econ Health Policy. 2011;9(5):317–329. doi: 10.2165/11594150-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 36.Flynn TN, Louviere JJ, Peters TJ, Coast J. Using discrete choice experiments to understand preferences for quality of life. Variance-scale heterogeneity matters. Soc Sci Med. 2010;70(12):1957–1965. doi: 10.1016/j.socscimed.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 37.Coast J, Al-Janabi H. 2008 http://www.icecap.bham.ac.uk/
- 38.Oostenbrink JB, Al MJ. The analysis of incomplete cost data due to dropout. Health Econ. 2005;14(8):763–776. doi: 10.1002/hec.966. [DOI] [PubMed] [Google Scholar]
- 39.Oostenbrink JB, Al MJ, Rutten-van Molken MP. Methods to analyse cost data of patients who withdraw in a clinical trial setting. Pharmacoeconomics. 2003;21(15):1103–1112. doi: 10.2165/00019053-200321150-00004. [DOI] [PubMed] [Google Scholar]
- 40.Briggs A, Clark T, Wolstenholme J, Clarke P. Missing… presumed at random: cost-analysis of incomplete data. Health Econ. 2003;12(5):377–392. doi: 10.1002/hec.766. [DOI] [PubMed] [Google Scholar]
- 41.Briggs AH, Gray AM. Handling uncertainty when performing economic evaluation of healthcare interventions. Health Technol Assess. 1999;3(2):1–134. [PubMed] [Google Scholar]
- 42.Satterthwaite FE. An Approximate Distribution of Estimates of Variance Components. Biometrics Bulletin. 1946;2(6):110–114. [PubMed] [Google Scholar]
- 43.Wolitzky-Taylor KB, Arch JJ, Rosenfield D, Craske MG. Moderators and Non-Specific Predictors of Treatment Outcome for Anxiety Disorders: A Comparison of Cognitive Behavioral Therapy to Acceptance and Commitment Therapy. Journal of Consulting and Clinical Psychology. 2012 doi: 10.1037/a0029418. [DOI] [PubMed] [Google Scholar]
- 44.Makai P, Beckebans F, van Exel J, Brouwer WB. Quality of life of nursing home residents with dementia: validation of the German version of the ICECAP-O. PLOS ONE. 2014;9(3):e92016. doi: 10.1371/journal.pone.0092016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Makai P, Koopmanschap MA, Brouwer WB, Nieboer AA. A validation of the ICECAP-O in a population of post-hospitalized older people in the Netherlands. Health Qual Life Outcomes. 2013;11:57. doi: 10.1186/1477-7525-11-57. [DOI] [PMC free article] [PubMed] [Google Scholar]