Abstract
Motor overflow is a developmental phenomenon that typically disappears by late childhood. Abnormal persistence of motor overflow is often present in children with attention-deficit/hyperactivity disorder (ADHD). This study employed functional magnetic resonance imaging (fMRI) during a finger-sequencing task to examine whether excessive motor overflow in children with ADHD is associated with decreased extent of motor circuit activation. Thirty-four right-handed children (18 typically developing controls, 16 ADHD) completed fMRI while performing a finger-sequencing task. Motor overflow was evaluated during a finger-sequencing task and a motor examination (the PANESS) performed outside the scanner. Diagnostic differences in behavioral measures of overflow and extent of activation in the contralateral and ipsilateral motor network ROIs were examined, along with correlations between overflow and extent of activation. Children with ADHD demonstrated greater overflow and lesser extent of activation in left primary motor cortex (BA4) and bilateral premotor cortex (BA6) and supplementary motor area (SMA) during right-hand finger-sequencing compared to controls. Decreased extent of primary motor and premotor activation correlated with increased hand-related overflow movements in children with ADHD but not controls. These findings suggest that overflow movements in children with ADHD may reflect decreased recruitment of neural circuitry involved in active inhibition of homologous motor circuitry unnecessary to task execution.
Keywords: fMRI, PANESS, inhibition, finger-sequencing
1. Introduction
The phenomenon of motor overflow, or synkinesis, is defined as unintentional movement that accompanies voluntary task execution, with “mirror overflow” or “imitative synkinesis”, used to refer specifically to involuntary motor overflow movements that occur in homologous muscles on the opposite side of the body (Boissy et al., 1997). The frequency and severity of motor overflow decreases with age in typically developing children, and the phenomenon is largely absent by the time typically developing children reach adolescence (Lazarus and Todor, 1987; Lazarus and Whitall, 1999; Largo et al., 2003). In healthy adults, motor overflow is generally elicited only during periods of fatigue or particularly demanding motor activity (Zijdewind and Kernell, 2001). The abnormal persistence of motor overflow beyond the first decade of life is associated with attention-deficit/hyperactivity disorder (ADHD) and other developmental abnormalities (Szatmari and Taylor, 1984), and is characteristic of the delayed maturation of inhibitory networks associated with a number of neurodevelopmental disorders (Denckla, 1985; Jansiewicz et al., 2006). As such, the presence and extent of motor overflow has been explored as a physiological measure of motor network maturity and, for mirror overflow movements, interhemispheric inhibition.
While ADHD is commonly associated with cognitive impairments, children with ADHD also consistently demonstrate subtle motor abnormalities (Denckla and Rudel, 1978; Szatmari and Taylor, 1984; Mostofsky et al., 2003a). It is well established that children with ADHD perform motor tasks more slowly (Denckla and Rudel, 1978), with more variability (Rubia et al., 1999; Shiels Rosch et al., 2013), and show greater motor overflow (Denckla and Rudel, 1978; Szatmari and Taylor, 1984; Mostofsky et al., 2003a) compared with typically developing children. Abnormal persistence of motor overflow movements is a notable and clinically relevant aspect of ADHD, reflecting an impaired ability to inhibit unintentional movement. The presence of motor abnormalities, particularly motor overflow, has been shown to be correlated with impaired response inhibition on cognitive control tasks (Mostofsky et al., 2003a), an executive function widely recognized as a major contributor to the cognitive and behavioral abnormalities associated with ADHD (Denckla and Rudel, 1978; Barkley, 1997). Thus, intentional and unintentional inhibition of unwanted movement appears to be impaired in children with ADHD, suggesting generalized impairment in inhibitory control. Examining the neural basis of motor abnormities as potential clinically relevant indicators of ADHD may help deepen our understanding of the neural basis of the disorder.
Neuroimaging of inhibitory control in children with ADHD has largely focused on intentional inhibition of motor responses as assessed by cognitive control tasks, such as go/no-go and stop-signal. Differential activation during these tasks has been found in a number of regions, most notably in pre-supplementary motor area (pre-SMA), right inferior frontal gyrus (IFG) and in regions that make up a fronto-parietal network (Mostofsky et al., 2003b; Simmonds et al., 2007; Simmonds et al., 2008). There has been less research on the neural correlates of unintentional movements associated with execution of a motor response, reflecting another form of inhibitory control. To our knowledge, only two studies have used fMRI to examine differential neural activation during a motor task without explicit inhibitory demands in children with ADHD (Mostofsky et al., 2006a; Valera et al., 2010). Valera et al. (2010) used an externally-paced single finger tapping task as well as an unpaced task, finding differential patterns of activation during both tasks in brain regions that are classically associated with sensorimotor timing. In addition, findings from Mostofsky et al. (2006a) during a self-paced finger-sequencing task revealed that children with ADHD show a significantly reduced magnitude of neural activation in the right superior parietal lobe, as well as reduced extent of activation in the primary motor cortex (M1) contralateral to voluntary movement. It was hypothesized that the findings of lesser extent of M1 activation may reflect decreased recruitment of neural mechanisms necessary for inhibition of overflow movements. Neither of these previous studies examined associations among neural activation during motor tasks and the occurrence of motor overflow as indicated by behavioral measures.
The purpose of the current study is to replicate and extend our previous findings (Mostofsky et al., 2006a) using fMRI to evaluate the magnitude and extent of neural activation in premotor and motor regions during a simple finger-sequencing task in a larger and non-overlapping population of children who also completed a detailed motor examination standardized for children. Behavioral measures of mirror overflow were also obtained while participants performed a finger-sequencing task outside the scanner during which electrogoniometers were used to quantify mirror overflow (MacNeil et al., 2011). This allowed for examination of ADHD-dependent changes in neural activation during a simple motor task and associations with motor overflow and more general motor dysfunction, which has not yet been examined. We hypothesized that children with ADHD, relative to typically developing controls, would demonstrate (1) greater motor impairments, particularly increased overflow, and (2) reduced extent of neural activation during a finger-sequencing task in ipsilateral and contralateral primary motor and premotor regions of interest (BA4 and BA6). In addition, we expected increased overflow in children with ADHD to be associated with reduced extent of activation in motor network regions.
2. Methods
2.1. Participants
Thirty-four right-handed 8- to 12-year-old participants who successfully completed the finger-sequencing fMRI paradigm were included in this study, including 18 typically developing controls (7 female and 11 male) and 16 children with ADHD (5 female and 11 male). Groups did not differ in age or sex, although the Wechsler Intelligence Scale for Children-IV full-scale intelligence quotient (FSIQ) was higher in the control group (see Table 1). Recruitment for this study was accomplished through distribution of flyers at local schools, primary care pediatric clinics, and outpatient clinics at the Kennedy Krieger Institute. The study was also promoted through community-wide service groups, volunteer organizations and by word of mouth. After complete description of the study to the participants, written informed consent was obtained from a parent/guardian and assent was obtained from the participating child. This study was approved by the Johns Hopkins Medical Institutional Review Board.
Table 1.
Participant demographic information for ADHD and control groups. Children with ADHD had significantly lower FSIQ and GAI scores on the WISC-IV.
| Controls (n=18) | ADHD (n=16) | p-value | |
|---|---|---|---|
| Boys: Girls (n) | 11:7 | 11:5 | .729 |
| Age (years), mean (SD) | 10.5 (1.4) | 10.4 (1.4) | .770 |
| ADHD subtype (n) | n/a | 13:3 | n/a |
| Combined:inattentive | |||
| ODD (n) | n/a | 7 | n/a |
| SES, mean (SD) | 57.4 (9.9) | 46.9 (11.6) | .009 |
| WISC-IV FSIQ, mean (SD) | 112.6 (9.1) | 97.3 (12.0) | <.001 |
| WISC-IV GAI, mean (SD) | 114.2 (9.3) | 103.0 (15.3) | .016 |
| Handedness, mean (SD) | .85 (.15) | .89 (.1) | .408 |
Note: ODD = oppositional defiant disorder; SES = Hollingshead socioeconomic status; WISC-IV = Wechsler Intelligence Scale for Children, Fourth Edition; FSIQ = full scale IQ; GAI = General Ability Index. Handedness was assessed using the Edinburgh Handedness Inventory (Oldfield, 1971).
Parents and a selected teacher of potential participants completed the ADHD Rating Scale IV-Parent or Teacher Version (DuPaul et al., 1998) and the Conners’ Parent or Teacher Rating Scale-Revised (Conners et al., 1998). A child was determined to screen positive for ADHD if he or she met pre-established criteria on at least one rating scale completed by both the teacher and the parent. An ADHD diagnosis was confirmed using a structured interview (Diagnostic Interview for Children and Adolescents, Fourth Edition; DICA-IV)(Reich, 2000) administered to the parents by a licensed, master’s-level psychologist. A licensed child neurologist with decades of experience in evaluation and treatment of ADHD and other neuropsychiatric conditions confirmed all diagnoses.
The DICA-IV was also used to assess for additional comorbid psychiatric conditions. Subjects were excluded if they met criteria for conduct disorder, mood disorder, generalized anxiety disorder, separation anxiety disorder, or obsessive-compulsive disorder. Comorbid oppositional defiant disorder was permitted (see Table 1). Candidates were also excluded if their FSIQ was less than 80 on the Wechsler Intelligence Scale for Children, Fourth Edition (WISC-IV)(Wechsler, 2003) and for suspected reading disabilities, based on either parent report or discrepancy between full-scale IQ on the WISC-IV and the reading composite from the Wechsler Individual Achievement Test- Second Edition (WIAT-III) (Wechsler, 2002). Eleven of the children in the ADHD group were being treated with stimulant medication (all with a methylphenidate preparation), and their parents were requested to withhold the medication the day of and the day prior to testing, providing a 36-hour washout period. Children taking any other psychoactive medications were excluded from this study.
Participants in the control group did not meet diagnostic criteria for any psychiatric disorder based on responses from the DICA-IV. In addition, their scores had to be below clinical cutoff on both ADHD parent-report measures (ADHD Rating Scale IV-Parent Version and the Conners’ Parent Rating Scale-Revised) and on the teacher-report measures (when available). Control participants with a history of intellectual disability, seizures, traumatic brain injury or other neurological illnesses were excluded from this study.
2.2. Procedures
2.2.1. fMRI acquisition and finger-sequencing task
The fMRI data were acquired on a Philips 3T (Achieva, Philips Healthcare, Best, The Netherlands) using a single-shot, partially parallel (SENSE) gradient-recalled echoplanar imaging sequence (repetition time/echo time = 2500/30 ms, flip angle =70°, SENSE factor = 2, nominal voxel dimensions = 3 mm × 3 mm × 3 mm with no slice gap, 80 × 80 matrix and 46 slices; 216 dynamics). For the finger-sequencing task completed both inside and outside the scanner, subjects were instructed to raise one hand and tap each finger to their thumb in a set sequence (index-middle-ring-pinky) while resting their non-tapping hand on a pillow. Before starting the task subjects practiced finger-sequencing in the appropriate hand positions. Starting hand was counterbalanced across subjects and during the task blocks alternated between left-handed finger-sequencing (LHFS) and right-handed finger-sequencing (RHFS). Video recordings of participants while they performed the task in the scanner were used to identify when subjects started and ended each task block as well as the number of finger taps per block. Tapping speed was calculated as the number of taps per block, with more taps within the 45s block indicating faster tap speed.
The finger-sequencing task performed in the scanner consisted of eight blocks (four per hand) during which participants were instructed to rest their hands on the pillow for 20 s and then perform the finger-sequencing task for 45 s. Visual cues were presented through a projector using E-Prime software (Psychology Software Tools, Inc., Sharpsburg, PA) running on a Windows operating system. A large arrow accompanied by text directed the participants on which hand to tap or if they should rest (e.g., “Tap your Right Hand” or “Rest”). Participants were lying down with their hands extended on a pillow placed under their arms and across their waist. They were instructed to slightly raise their tapping hand off of the pillow to perform the task and to return their hand to the pillow during the rest period. Before the actual scan, participants practiced the in-scanner procedures in a “mock” MRI room with both audio and visual simulation of the fMRI scanner.
The finger-sequencing task performed outside of the scanner was very similar to the one performed inside the scanner except that it consisted of 10 blocks (5 per hand) which included a ten second baseline, during which the participant remained still, followed by finger-sequencing until they completed ~45 individual finger taps as determined by the examiner (MacNeil et al., 2011; Shiels Rosch et al., 2013). Participants sat in a chair with a pillow on their lap, which served as a rest position for their non-tapping hand and were instructed to bend the elbow of their tapping hand. In addition, participants did not look at a computer screen during the task performed outside the scanner. Instead, they were given instructions by the examiner. Subjects were video recorded, and angle-calibrated electrogoniometers coupled with AcqKnowledge v3.9.1 software (Biopac Systems Inc., Goleta, CA) recorded the angular deflection of the index and ring fingers. The video recordings were later used to identify the first 40 taps within each block for analysis of mirror overflow.
2.2.2. Quantification and analysis of mirror overflow during finger-sequencing
Video recordings of the finger-sequencing task performed inside and outside of the scanner were examined for each subject to count the number of finger taps and to verify that subjects performed the in-scanner task for the duration of the block. For the out-of-scanner task, blocks were extracted using subject specific start and end tap times that were visually identified from the video recordings. Goniometer tracings for the out-of-scanner task were imported into Matlab version 7.1 (The Mathworks, Inc., Natick, MA) for overflow quantification. Each block was filtered using a second order Butterworth filter with a cutoff frequency of 3.33 Hz. Total mirror overflow was measured as the cumulative angular deflection for each block on the non-tapping hand. Mirror overflow analysis was accomplished using IBM SPSS Statistics Version 20 (IBM, Chicago, IL). A univariate linear model was used to examine diagnostic group differences in total mirror overflow during LHFS and RHFS blocks combined with the between-subjects factors of diagnosis (Control, ADHD). Diagnostic group differences in finger-tapping speed during the task completed inside the scanner were also examined with a univariate linear model separately for LHFS and RHFS blocks and tap speed during each block was included as a covariate when examining diagnostic group differences in brain activation. To determine whether intellectual ability contributed to diagnostic differences in overflow, analyses were conducted with and without covarying for the WISC-IV General Ability Index (GAI). The WISC-IV GAI was included as a covariate in the analyses rather than FSIQ because FSIQ is influenced by difficulties in working memory and processing speed, which are often present in children with ADHD, with processing speed reflecting mechanisms of motor control being assessed in this study, whereas GAI is based on verbal and perceptual reasoning abilities and may therefore be a more appropriate measure of broad intellectual ability in children with ADHD.
2.2.3. Motor assessment and analysis
The Physical and Neurological Examination for Soft Signs (PANESS) is a structured, scripted assessment tool used to assess motor function that requires objective identification of motor findings (Denckla, 1985). An examiner documented the presence or absence of motor overflow and the time to complete different categories of motor tasks, including stressed gaits, balancing tasks, repetitive timed movements, and patterned timed movements, to produce a composite total PANESS score. The information most pertinent to this analysis was the total hand-related overflow score, generated by summing the number of hand movements during which overflow was present, with higher scores indicating worse motor control. Univariate linear models were used to examine diagnostic group differences in performance on the PANESS (total and hand-related overflow scores) with the between-subjects factor of diagnosis (Control, ADHD). These models were implemented with and without WISC-IV GAI included as a covariate to control for group differences in GAI.
2.2.4. MRI data analysis and processing
Pre-analysis image processing was carried out using MATLAB R2011b and SPM8 (Wellcome Department of Imaging Neuroscience, www.fil.ion.ucl.ac.uk/spm/software/8). The first 4 scans are discarded to account for signal stabilization. In order to adjust for time of acquisition differences inside of each volume, each volume was slice time-corrected. For each participant, the MPRAGE and functional volumes were spatially co-registered to the first functional volume. The MPRAGE was co-registered to the MNI 152 template (Montreal Neurological Institute) using unified segmentation-normalization in SPM (Ashburner and Friston, 2005), resulting in voxel dimensions =2 mm × 2 mm × 2 mm. The functional images were then smoothed (Calhoun et al., 2000) using a Gaussian kernel full width at half-maximum 4 × 4 × 4 mm. Head movement was measured using the differential (using one-back differences) of the three translational (x, y, z) and three rotational (roll, pitch, yaw) parameters estimated from spatial realignment. If subjects demonstrated greater than 3 mm of translational or 2 degrees of rotational head movement in any direction, that image was considered to be motion contaminated. Each contaminated image, along with the six subsequent images, was excluded to account for spin-history artifacts. These affected images were accounted for by using indicator functions as regressors in the general linear model (GLM) for four subjects (2 ADHD with 7 and 9 volumes regressed and 2 controls with 7 and 11 volumes regressed). Participants with more than one contiguous block of super-threshold motion during the scan were excluded from the analysis, resulting in the exclusion of four controls and four ADHD participants before conducting analyses with the final sample of 18 controls and 16 ADHD participants. The task-related regressors included the three main trials of interest (RHFS, LHFS and Rest) convolved with the canonical hemodynamic response function (HRF). Contrast maps were created for each subject, comparing brain activity during both LHFS and RHFS to the Rest condition.
Standard whole-brain, random effects analyses were performed in SPM8 to identify regions with significant amplitude of activation using an uncorrected threshold of p ≤ .001. The motor network regions of interest (ROI) were Brodmann areas BA4 (M1) and BA6 (premotor cortex), the supplementary motor area (SMA), and anterior cerebellum, defined using the Wake Forest Pickatlas (Maldjian et al., 2003). In order to verify that robust motor circuit activity was found within each group, one-sample t-tests were first performed for each finger-tap condition (LHFS>Rest and RHFS>Rest) in the ADHD and control groups separately. Group differences were then tested using two-sample t-tests for each finger-tap condition (Control>ADHD and ADHD>Control). To evaluate the extent of activation, as has been done in previous studies (e.g., Mostofsky et al., 2006b; Poulin-Lord et al., 2014), the first-level one-sample t-test maps were masked (LHFS>Rest and RHFS>Rest) and the total number of voxels within each mask with significant voxel-wise activation of p ≤ 0.001 was determined. Whole brain analyses to test for group differences were also performed with tap speed as a covariate because this may influence group-differences in activation.
Extent of activation analysis was accomplished using IBM SPSS Statistics Version 22 (IBM, Chicago). All reported findings for extent of activation are based on resampled 2mm isotropic voxel dimensions. For fMRI measures of extent of activation, two repeated measures analyses of variance (ANOVAs) were used to examine diagnostic group differences in extent of activation during the LHFS and RHFS blocks separately. The within-subjects factors were laterality of activation (ipsilateral motor circuit, contralateral motor circuit), and region of interest (ROI) (BA4, BA6, anterior cerebellum, and SMA) and the between-subjects factor of diagnosis (Control, ADHD). These analyses were conducted with and without controlling for GAI and tap speed, which differed between diagnostic groups (see Tables 1 and 2) and were correlated with some indices of neural activation in our preliminary analyses. Socioeconomic status (SES) also differed between diagnostic groups, but was not included as a covariate due to the lack of significant correlations between SES and neural activation in each of the ROIs. Finally, Pearson correlations were examined separately for each diagnostic group to evaluate the relationship among the behavioral measures of overflow and associations with fMRI measures of extent of activation for ROIs with significant diagnostic group differences.
Table 2.
Performance on motor tasks
Descriptive statistics for performance on the PANESS and the finger-sequencing task completed inside and outside of the scanner. Children with ADHD displayed increased hand-related overflow on the PANESS and greater total overflow during the finger sequencing tasks completed outside of the scanner. Typically developing controls demonstrated faster tap speed during the finger-sequencing task completed inside of the scanner as indicated by a greater number of finger taps during the 45-s blocks.
| Controls | ADHD | p-value | |
|---|---|---|---|
| PANESS total score | 19.8(6.8) | 30.6(11.2) | 0.002 |
| PANESS total hand-related overflow | 3.1(2.2) | 6.7(4.1) | 0.003 |
| Finger sequencing task (out of scanner) total overflow | 390 (243) | 726 (500) | 0.016 |
| Finger sequencing task (in scanner) | |||
| Total fingertaps RHFS | 514 (99) | 441 (76) | 0.028 |
| Total fingertaps LHFS | 504 (98) | 422 (77) | 0.019 |
Note: Values represent mean (SD). PANESS = Physiological and Neurological Examination for Subtle Signs; RHFS = right-hand finger sequencing; LHFS = left-hand finger sequencing.
3. Results
3.1. Behavioral measures of overflow
Consistent with prior studies (Mostofsky et al., 2006a), children with ADHD had significantly higher total PANESS scores, F(1,32)=11.9, p=0.002, indicating a poorer performance on this composite measure of subtle motor abnormalities. Furthermore, children with ADHD displayed significantly more motor overflow during timed hand-related tasks on the PANESS, F(1,32)=10.2, p=0.003 (Table 2). Effects of diagnosis remained significant when WISC-IV GAI was included in the analyses as a covariate, Total PANESS F(1,32)=5.0, p=0.032, PANESS Total Hand Related Overflow F(1,32)=4.6, p=0.040. For the finger-sequencing task performed outside of the scanner with goniometer measurement of overflow, children with ADHD demonstrated greater mirror overflow, F(1,32)=6.44, p=0.016. The inclusion of GAI as a covariate weakened these effects to trend level, F(1,31)=3.43, p=0.076.
3.2. Motor network extent of activation
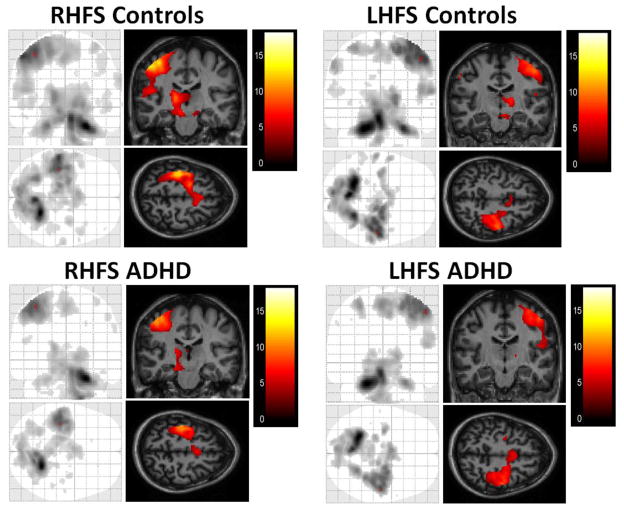
For both diagnostic groups, both right- and left-handed finger sequencing was associated with activation primarily in the anticipated contralateral motor networks as contrasted to activity during rest blocks (Fig. 1). Whole brain analyses using a standard GLM revealed no between-group differences in activation amplitude for any condition tested. In addition, there was no evidence of activation amplitude differences between the two groups when tap speed was included as a covariate.
Fig. 1.
Average activation in ADHD and control groups during the finger sequencing task, as compared with rest. For both right-handed finger sequencing (RHFS) and left-handed finger sequencing (LHFS), sectional images are shown at the peak of activation in the motor cortex.
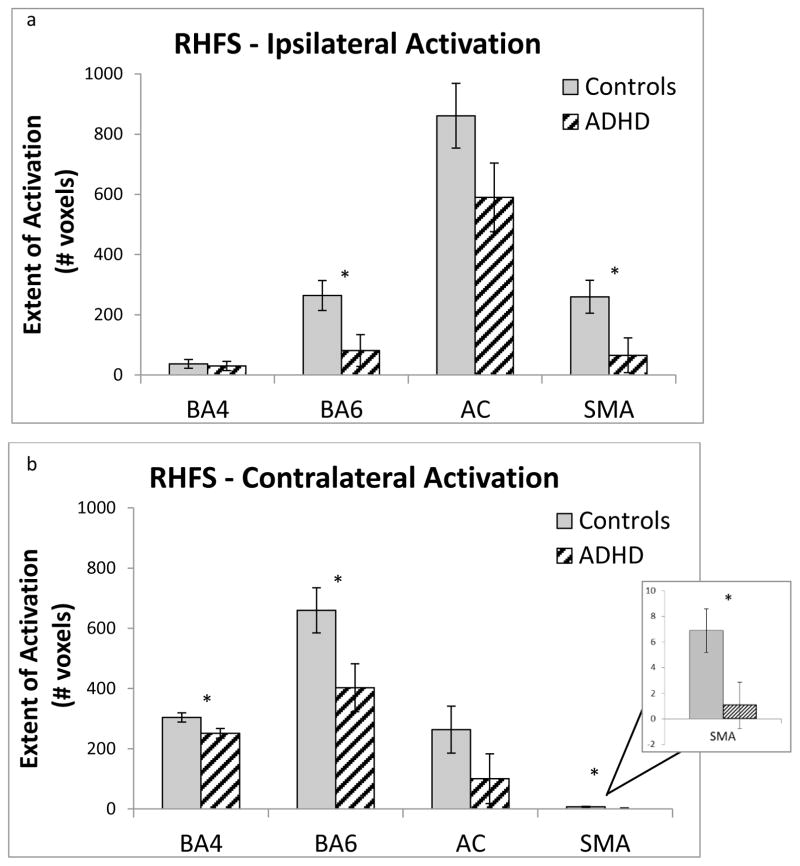
ROI analyses revealed overall reduced extent of activation in the motor circuit ROIs in children with ADHD during the RHFS condition, diagnosis F(1,32)=6.0, p=0.020 and a Diagnosis × ROI × Laterality interaction, F(3,32)=2.9, p=0.037. Post-hoc comparisons indicated significantly less extent of activation in the ADHD group compared to the TD group during RHFS in contralateral ROIs including left BA4, F(1,32)=5.5, p=0.025, left BA6, F(1,32)=5.6, p=0.025, and left SMA, F(1, 32)=5.5, p=0.025, but not left anterior cerebellum, F(1, 32)=2.2, p=0.161. Extent of activation was also smaller in the ADHD group compared to the TD group during RHFS in ipsilateral ROIs, including right BA6, F(1,32)=6.4, p=0.016, and right SMA, F(1,32)=5.9, p=0.021, but not in right BA4, F(1,32)=.113, p=0.739 or right anterior cerebellum, F(1,32)=2.1, p=0.161 (see Fig. 2). Thus, it appears that the three-way interaction is driven by the finding of less contralateral extent of activation during RHFS in left BA4 in children with ADHD compared to controls, whereas ipsilateral activation in right BA4 did not differ between diagnostic groups. In contrast, diagnostic groups differed in extent of activation during RHFS in both contralateral and ipsilateral BA6 and SMA. During LHFS, no significant effects of diagnosis were observed for extent of activation, diagnosis F(1,32)=3.4, p=0.076, Diagnosis × ROI × Laterality, F(1,32)=1.9, p=0.139.
Fig. 2.
Average number of voxels activated at a threshold of p < 0.001 during right-hand finger sequencing (RHFS) inside of (a) right (ipsilateral) and (b) left (contralateral) motor network regions of interest (BA4, BA6, supplementary motor area (SMA), and anterior cerebellum (AC)), for both typically developing controls and children with attention-deficit/hyperactivity disorder (ADHD). Error bars represent standard error of the mean.
As with the whole-brain analyses, the extent of activation analyses were also examined with tap speed during the finger-sequencing task performed in the scanner as a covariate and similar results were obtained for diagnostic effects on activation during RHFS. Although the main effect of diagnosis was no longer significant when tap speed was included as a covariate, F(1,30)= 2.2, p=0.151, the Diagnosis × ROI × Laterality interaction was stronger, F(3,30)=4.2, p=0.008. However, including GAI as a covariate eliminated the effects of diagnosis on extent of activation, diagnosis F(1,32)=0.7, p=0.425, Diagnosis × ROI F(3,31)=1.0, p=0.417.
3.3. Brain-behavior correlations
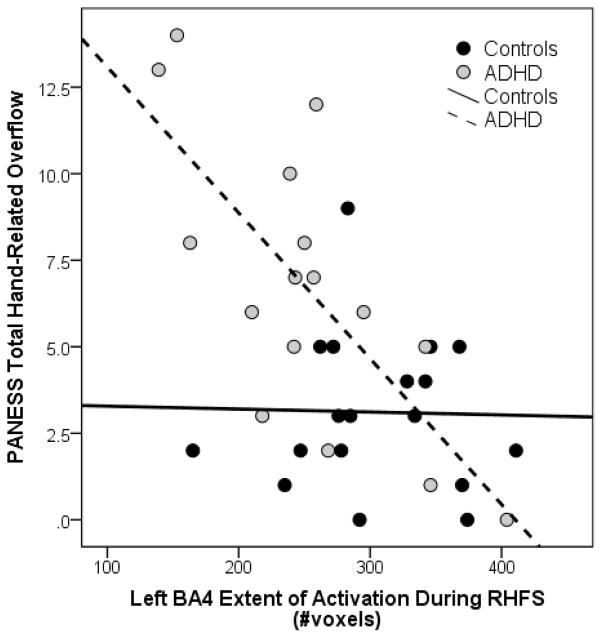
Associations among behavioral measures of overflow and extent of activation in motor network regions with significant diagnostic group differences (left BA4 and right and left BA6 and SMA during RHFS) were examined within diagnostic groups. Among children with ADHD, contralateral (left) BA4 extent of activation during RHFS was strongly correlated with hand-related overflow as measured by the PANESS (r = −.723, p = 0.002; Fig. 3) and the finger-sequencing task completed outside of the scanner (r = −0.635, p = 0.008). Neither measure of overflow was significantly correlated with contralateral (left) BA6 activation in children with ADHD, although similar associations were observed for both the PANESS (r = −0.442, p = 0.086) and the goniometer finger-sequencing task (r = −0.453, p = 0.078). These associations were weaker for ipsilateral (right) BA6 activation and overflow on the PANESS (r = −0.392, p = 0.133) and the goniometer finger-sequencing task (r = −0.384, p = 0.142). In addition, overflow was not significantly correlated with contralateral (left) SMA activation during RHFS in children with ADHD as measured by either the PANESS (r = −0.196, p = 0.466) or the goniometer finger-sequencing task (r = −0.182, p = 0.499) or with ipsilateral (right) SMA activation during RHFS on the PANESS (r = −0.406, p = 0.118) or goniometer task (r = −0.396, p = 0.129). In the control group, behavioral measures of overflow were unrelated to extent of activation in these regions (rs < 0.32, ps > 0.20).
Fig. 3.
Correlation between the total number of voxels activated at a threshold of p < 0.001 inside of left BA4 during right-handed finger sequencing (RHFS) and the PANESS Total Hand-Related Overflow score for typically developing controls and children with attention-deficit/hyperactivity disorder (ADHD).
4. Discussion
Consistent with previously published findings (Cole et al., 2008; MacNeil et al., 2011; Mostofsky et al., 2003a), increased motor overflow was observed in children with ADHD compared to their typically developing peers, providing further evidence that ADHD is associated with subtle motor abnormalities, or “subtle signs”. Also consistent with prior findings (Cole et al., 2008; MacNeil et al., 2011) children with ADHD in the 8- to 12-year-old age range showed increased hand-related overflow on the PANESS and on the finger-sequencing task completed outside of the scanner. The fMRI findings replicate those of our previous study using a similar finger-sequencing task (Mostofsky et al., 2006a), in which children with ADHD showed decreased activation in the contralateral primary motor cortex (left BA4) during finger sequencing. The current study expands upon our previous findings by examining additional regions included in the motor network (i.e., BA6, SMA, and anterior cerebellum) and brain-behavior correlations with an additional objective measure of overflow in a new sample of ADHD and control children. We had hypothesized that the nature of the decreased extent of neural activation in the primary motor cortex consistently demonstrated in ADHD children is likely to reflect insufficient recruitment of inhibitory networks, resulting in less effective suppression of motor overflow movements. Our hypothesis is supported by the finding that increased motor overflow was associated with decreased extent of activation in the contralateral primary motor cortex during finger sequencing with the dominant hand in children with ADHD.
In addition to replicating the previous fMRI findings, the current study found reduced extent of activation in premotor regions (BA6 and SMA) in children with ADHD during finger sequencing with the dominant hand. These findings suggest that altered recruitment is not restricted to M1 but occurs more generally in motor/premotor regions. This reduced recruitment may contribute to poor motor performance in children with ADHD. Interestingly, extent of activation was reduced in both the contralateral and ipsilateral premotor regions in children with ADHD whereas for the primary motor cortex, reduced extent of activation was only evident in the contralateral region. Thus, it may be that activation of bilateral premotor regions, involved in motor planning, contributes to successful motor task performance. Furthermore, the specificity of the diagnostic group differences in premotor and motor regions to dominant hand task performance might suggest that delayed development of the dominant motor control circuits more closely reflects a maturational delay or abnormality of the parallel higher-order systems that are necessary to control impulsive, hyperactive, and inattentive behavior.
Our findings revealed a diagnosis-dependent relationship between motor overflow observed during hand-related tasks and extent of premotor and motor cortex activation. For children with ADHD, decreased extent of activation in contralateral (left) M1 during RHFS was robustly correlated with the presence of increased motor overflow during timed repetitive and sequential movements of the hands/fingers as measured by the PANESS and with electrogoniometers during a finger-sequencing task. Similar, albeit weaker, associations were observed with activation in the contralateral premotor region. As such, our findings suggest that extent of activation in the contralateral primary motor and premotor cortices and, to a lesser extent, the ipsilateral premotor cortex is tied to motor control. Specifically, greater extent of activation represents recruitment of motor networks that are necessary for improved performance. As this relationship is uniquely observed in children affected by ADHD, it suggests a potential neurological basis for the motor impairments experienced by children with ADHD during simple motor tasks, particularly tasks involving the hands. The lack of association among controls could suggest alternative brain regions are involved in the extent to which typically developing children display motor overflow or it could be related to the smaller range of overflow scores in the control group.
These results are consistent with the hypothesis that overflow movements might originate from reduced inhibitory activity within the primary motor cortex in children with ADHD. Supportive evidence for this framework also comes from recent transcranial magnetic stimulation (TMS) studies revealing reduced M1 cortical inhibition in children with ADHD (Gilbert et al., 2011; Wu et al., 2012). Our finding that increased motor overflow is associated with decreased extent of neural activation in M1 contralateral to the voluntary movement likely reflects dendritic activity, as increased overflow movements would be expected to be associated with increased firing of pyramidal cells in M1. This outcome is consistent with the widely accepted understanding that the BOLD signal detected by fMRI methods primarily reflects dendritic, as opposed to neuronal body, activity (Logothetis et al., 2001; Viswanathan and Freeman, 2007; Freeman et al., 2009). As such, we suggest that the primarily dendritic activity associated with inhibitory neural network activation is driving the greater extent of M1 BOLD signal observed in typically developing children.
It is notable that the neural correlates of motor abnormalities found in the current study are not detectable using traditional tests examining amplitude of activation differences between groups. Rather, differences were found using an alternate method which examined the number of voxels in a region that were recruited for motor task performance. This latter method may reflect impairments in local coherence within prescribed motor regions rather than abnormalities in the structure of motor circuits. These results are consistent with previous findings of reduced regional homogeneity in other neural networks in ADHD examining resting state functional connectivity (Cao et al., 2006; Uddin et al., 2008; Wang et al., 2013). An interesting future direction would be to examine whether there was greater variability in the localization of the activation among children with ADHD than for controls, as was recently shown in a study comparing adults with autism to healthy controls (Poulin-Lord et al., 2014).
These findings should also be considered in relation to recently published TMS findings showing that children with ADHD show longer ipsilateral silent period (ISP) latencies than typically developing children and this longer ISP was correlated with increased overflow movements (Wu et al., 2012). ISP latency has been shown to reflect the efficiency of transcallosal inhibitory signaling between left and right M1. Our findings of an association between decreased extent of activation in M1 and increased mirror overflow provide additional support for the idea that increased overflow in ADHD reflects decreased recruitment of inhibitory mechanisms necessary to optimal control of actions. As a next step, it would be of interest to examine whether this decreased ISP latency is correlated with decreased extent of M1 activation.
In the current study, there was also evidence of decreased extent of activation during finger-sequencing with the dominant hand in premotor cortex (BA6 and SMA), which had not been previously reported (Mostofsky et al., 2006a). The finding of decreased extent of activation in premotor cortex in children with ADHD may be related to deficient motor planning while performing the finger-sequencing task with their dominant hand relative to their typically developing peers. Similar to the findings for M1, neural activation in the contralateral premotor cortex (BA6) was marginally correlated with the occurrence of overflow among the ADHD group, providing support for the relationship between extent of activation in premotor/motor regions and behavioral indicators of overflow.
The current study has some limitations, which should be acknowledged. While the number of participants in this study was adequate to detect statistically significant group differences in both neural activity and motor assessment, increasing our sample size might have allowed us to examine the effects of other important variables on this data such as age, gender, ADHD diagnostic subtype, and medication status. It will also be important in future research to examine whether this pattern of findings is specific to ADHD or also present in children with other developmental disorders. Group differences in IQ were also present in this sample. The decrement in IQ in the ADHD group is commonly reported (Frazier et al., 2004) and is considered to be an inherent group characteristic, suggesting that the inclusion of IQ as a covariate is inappropriate due to violations of the assumptions of ANCOVA (Miller and Chapman, 2001) and because this approach often produces overcorrected and counterintuitive findings about neurocognitive function (Dennis et al., 2009). Instead, the WISC-IV GAI was included as a covariate, which is less influenced by working memory and processing speed performance than FSIQ, but still a measure of general cognitive ability. However, GAI was significantly lower in our sample of children with ADHD, which is also not surprising given the broad range of cognitive deficits associated with ADHD. Therefore, controlling for diagnostic group differences in GAI may still account for variance associated with ADHD and has the potential to spuriously remove or weaken diagnostic group differences. The inclusion of WISC-IV GAI as a covariate eliminated diagnostic group differences in extent of activation during the finger-sequencing task, despite the minimal cognitive demands of the task, although the behavioral measures of overflow remained sensitive to diagnostic group after controlling for GAI. This finding might suggest similar biological mechanisms underlying the decrease in extent of activation during a motor task and reduced broad intellectual ability in children with ADHD. Alternatively, controlling for GAI likely eliminated variance attributable to ADHD and the broad cognitive deficits associated with this disorder, thereby weakening the diagnostic group difference.
Results from this study confirm earlier findings revealing that children with ADHD show both increased overflow and decreased extent of activation in contralateral motor cortex during repeated self-generated movements (Mostofsky et al., 2006a). Beyond this, our current findings reveal that this decreased extent of contralateral (dominant) M1 and premotor activation correlates with ADHD-associated impairments in basic motor control, particularly excessive overflow movements, which has not previously been shown. These findings, combined with those from recent TMS studies revealing reduced cortical inhibition in children with ADHD (Gilbert et al., 2011; Wu et al., 2012), provide insight into the neural basis of impaired motor control in ADHD, suggesting that this may, in part, be associated with impaired recruitment of cortical inhibitory mechanisms. Future research conducted with larger samples of children with ADHD and typically developing controls can build on these findings to examine the impact of age, gender, ADHD subtype, and medication history to better understand motor impairments associated with ADHD.
Highlights.
Children with ADHD demonstrated greater overflow.
Children with ADHD showed reduced activation in motor and premotor cortices.
Decreased neural activation correlated with increased overflow in the ADHD group.
Acknowledgments
This work was supported by the National Institute of Mental Health (grant numbers MH078160-06A1 and MH085328-08 to S.H.M. and MH101322 to K.S.R.), the Johns Hopkins General Clinical Research Center (M01 RR00052), the Kennedy Krieger Institute Intellectual and Developmental Disabilities Research Center (HD-24061), and the National Institute of Biomedical Imaging and Bioengineering (NIH P41 EB015909).
Footnotes
Conflict of interest
Dr. Pekar serves as Manager of the F.M. Kirby Research Center for Functional Brain Imaging, which receives research support from Philips HealthCare, which manufactures the MRI scanners used for this study. None of the other authors have any conflict of interests to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26 (3):839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Barkley RA. Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychological Bulletin. 1997;121 (1):65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Boissy P, Bourbonnais D, Kaegi C, Gravel D, Arsenault BA. Characterization of global synkineses during hand grip in hemiparetic patients. Archives of Physical Medicine and Rehabilitation. 1997;78 (10):1117–1124. doi: 10.1016/s0003-9993(97)90138-6. http://dx.doi.org/10.1016/S0003-9993(97)90138-6. [DOI] [PubMed] [Google Scholar]
- Calhoun V, Adali T, Kraut M, Pearlson G. A weighted least-squares algorithm for estimation and visualization of relative latencies in event-related functional MRI. Magnetic Resonance in Medicine. 2000;44 (6):947–954. doi: 10.1002/1522-2594(200012)44:6<947::aid-mrm17>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Cao Q, Zang Y, Sun L, Sui M, Long X, Zou Q, Wang Y. Abnormal neural activity in children with attention deficit hyperactivity disorder: a resting-state functional magnetic resonance imaging study. Neuroreport. 2006;17 (10):1033–1036. doi: 10.1097/01.wnr.0000224769.92454.5d. [DOI] [PubMed] [Google Scholar]
- Cole WR, Mostofsky SH, Larson JC, Denckla MB, Mahone EM. Age-related changes in motor subtle signs among girls and boys with ADHD. Neurology. 2008;71 (19):1514–1520. doi: 10.1212/01.wnl.0000334275.57734.5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conners CK, Sitarenios G, Parker JD, Epstein JN. The revised Conners’ Parent Rating Scale (CPRS-R): factor structure, reliability, and criterion validity. Journal of Abnormal Child Psychology. 1998;26 (4):257–268. doi: 10.1023/a:1022602400621. [DOI] [PubMed] [Google Scholar]
- Denckla MB. Revised Neurological Examination for Subtle Signs. Psychopharmacology Bulletin. 1985;21 (4):773–800. [PubMed] [Google Scholar]
- Denckla MB, Rudel RG. Anomalies of motor development in hyperactive boys. Annals of Neurology. 1978;3 (3):231–233. doi: 10.1002/ana.410030308. [DOI] [PubMed] [Google Scholar]
- Dennis M, Francis DJ, Cirino PT, Schachar R, Barnes MA, Fletcher JM. Why IQ is not a covariate in cognitive studies of neurodevelopmental disorders. Journal of the International Neuropsychological Society. 2009;15:331–343. doi: 10.1017/S1355617709090481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuPaul GJ, Power TJ, Anastopoulos AD, Reid R. ADHD Rating Scale—IV. Guilford Press; New York: 1998. [Google Scholar]
- Frazier TW, Demaree HA, Youngstrom EA. Meta-analysis of intellectual and neuropsychological test performance in attention-deficit/hyperactivity disorder. Neuropsychology. 2004;18 (3):543–555. doi: 10.1037/0894-4105.18.3.543. [DOI] [PubMed] [Google Scholar]
- Freeman WJ, Ahlfors SP, Menon V. Combining fMRI with EEG and MEG in order to relate patterns of brain activity to cognition. International Journal of Psychophysiology. 2009;73 (1):43–52. doi: 10.1016/j.ijpsycho.2008.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert DL, Isaacs KM, Augusta M, MacNeil LK, Mostofsky SH. Motor cortex inhibition: a marker of ADHD behavior and motor development in children. Neurology. 2011;76 (7):615–621. doi: 10.1212/WNL.0b013e31820c2ebd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansiewicz EM, Goldberg MC, Newschaffer CJ, Denckla MB, Landa R, Mostofsky SH. Motor signs distinguish children with high functioning autism and Asperger’s syndrome from controls. Journal of Autism and Developmental Disorders. 2006;36 (5):613–621. doi: 10.1007/s10803-006-0109-y. [DOI] [PubMed] [Google Scholar]
- Largo RH, Fischer JE, Rousson V. Neuromotor development from kindergarten age to adolescence: developmental course and variability. Swiss Med Wkly. 2003;133 (13–14):193–199. doi: 10.4414/smw.2003.09883. 2003/13/smw-09883. [DOI] [PubMed] [Google Scholar]
- Lazarus JA, Todor JI. Age differences in the magnitude of associated movement. Developmental Medicine and Child Neurology. 1987;29 (6):726–733. doi: 10.1111/j.1469-8749.1987.tb08817.x. [DOI] [PubMed] [Google Scholar]
- Lazarus JA, Whitall J. Motor overflow and children’s tracking performance: is there a link? Developmental Psychobiology. 1999;35 (3):178–187. [PubMed] [Google Scholar]
- Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412 (6843):150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- MacNeil LK, Xavier P, Garvey MA, Gilbert DL, Ranta ME, Denckla MB, Mostofsky SH. Quantifying excessive mirror overflow in children with attention-deficit/hyperactivity disorder. Neurology. 2011;76 (7):622–628. doi: 10.1212/WNL.0b013e31820c3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19 (3):1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Miller GA, Chapman JP. Misunderstanding analysis of covariance. Journal of Abnormal Psychology. 2001;110 (1):40–48. doi: 10.1037//0021-843x.110.1.40. [DOI] [PubMed] [Google Scholar]
- Mostofsky SH, Newschaffer CJ, Denckla MB. Overflow movements predict impaired response inhibition in children with ADHD. Perceptual and Motor Skills. 2003a;97 (3 Pt 2):1315–1331. doi: 10.2466/pms.2003.97.3f.1315. [DOI] [PubMed] [Google Scholar]
- Mostofsky SH, Rimrodt SL, Schafer JG, Boyce A, Goldberg MC, Pekar JJ, Denckla MB. Atypical motor and sensory cortex activation in attention-deficit/hyperactivity disorder: a functional magnetic resonance imaging study of simple sequential finger tapping. Biological Psychiatry. 2006a;59 (1):48–56. doi: 10.1016/j.biopsych.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Mostofsky SH, Rimrodt SL, Schafer JG, Boyce A, Goldberg MC, Pekar JJ, Denckla MB. Atypical motor and sensory cortex activation in attention-deficit/hyperactivity disorder: a functional magnetic resonance imaging study of simple sequential finger tapping. Biol Psychiatry. 2006b;59 (1):48–56. doi: 10.1016/j.biopsych.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Mostofsky SH, Schafer JG, Abrams MT, Goldberg MC, Flower AA, Boyce A, et al. fMRI evidence that the neural basis of response inhibition is task-dependent. Brain Research. 2003b;17 (2):419–430. doi: 10.1016/s0926-6410(03)00144-7. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh Inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Poulin-Lord MP, Barbeau EB, Soulieres I, Monchi O, Doyon J, Benali H, Mottron L. Increased topographical variability of task-related activation in perceptive and motor associative regions in adult autistics. Neuroimage Clin. 2014;4:444–453. doi: 10.1016/j.nicl.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich W. Diagnostic interview for children and adolescents (DICA) Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39 (1):59–66. doi: 10.1097/00004583-200001000-00017. [DOI] [PubMed] [Google Scholar]
- Rubia K, Taylor A, Taylor E, Sergeant JA. Synchronization, anticipation, and consistency in motor timing of children with dimensionally defined attention deficit hyperactivity behaviour. Perceptual and Motor Skills. 1999;89 (3 Pt 2):1237–1258. doi: 10.2466/pms.1999.89.3f.1237. [DOI] [PubMed] [Google Scholar]
- Shiels Rosch K, Dirlikov B, Mostofsky SH. Increased intrasubject variability in boys with ADHD across tests of motor and cognitive control. Journal of Abnormal Child Psychology. 2013;41 (3):485–495. doi: 10.1007/s10802-012-9690-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds DJ, Fotedar SG, Suskauer SJ, Pekar JJ, Denckla MB, Mostofsky SH. Functional brain correlates of response time variability in children. Neuropsychologia. 2007;45 (9):2147–2157. doi: 10.1016/j.neuropsychologia.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Simmonds DJ, Pekar JJ, Mostofsky SH. Meta-analysis of Go/No-go tasks demonstrating that fMRI activation associated with response inhibition is task-dependent. Neuropsychologia. 2008;46 (1):224–232. doi: 10.1016/j.neuropsychologia.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szatmari P, Taylor DC. Overflow movements and behavior problems: scoring and using a modification of Fogs’ test. Developmental Medicine and Child Neurology. 1984;26 (3):297–310. doi: 10.1111/j.1469-8749.1984.tb04446.x. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Kelly AM, Biswal BB, Margulies DS, Shehzad Z, Shaw D, et al. Network homogeneity reveals decreased integrity of default-mode network in ADHD. Journal of Neuroscience Methods. 2008;169 (1):249–254. doi: 10.1016/j.jneumeth.2007.11.031. [DOI] [PubMed] [Google Scholar]
- Valera EM, Spencer RM, Zeffiro TA, Makris N, Spencer TJ, Faraone SV, et al. Neural substrates of impaired sensorimotor timing in adult attention-deficit/hyperactivity disorder. Biological Psychiatry. 2010;68 (4):359–367. doi: 10.1016/j.biopsych.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanathan A, Freeman RD. Neurometabolic coupling in cerebral cortex reflects synaptic more than spiking activity. Nature Neuroscience. 2007;10 (10):1308–1312. doi: 10.1038/nn1977. [DOI] [PubMed] [Google Scholar]
- Wang X, Jiao Y, Tang T, Wang H, Lu Z. Altered regional homogeneity patterns in adults with attention-deficit hyperactivity disorder. European Journal of Radiology. 2013;82 (9):1552–1557. doi: 10.1016/j.ejrad.2013.04.009. [DOI] [PubMed] [Google Scholar]
- Wechsler DL. Wechsler Individual Achievement Test. 2. The Psychological Corporation; San Antonio, TX: 2002. (WIAT-II) [Google Scholar]
- Wechsler DL. Wechsler Intelligence Scale for Children. 4. The Psychological Corporation; San Antonio, TX: 2003. (WISC-IV) [Google Scholar]
- Wu SW, Gilbert DL, Shahana N, Huddleston DA, Mostofsky SH. Transcranial magnetic stimulation measures in attention-deficit/hyperactivity disorder. Pediatric Neurology. 2012;47 (3):177–185. doi: 10.1016/j.pediatrneurol.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zijdewind I, Kernell D. Bilateral interactions during contractions of intrinsic hand muscles. Journal of Neurophysiology. 2001;85 (5):1907–1913. doi: 10.1152/jn.2001.85.5.1907. [DOI] [PubMed] [Google Scholar]