Abstract
Sphingosine 1-phosphate (S1P) receptor modulators possess a unique mechanism of action as disease modifying therapy for multiple sclerosis (MS). Subtype 1 S1P receptors are expressed on the surfaces of lymphocytes and are important in regulating egression from lymph nodes. The S1P receptor modulators indirectly antagonize the receptor’s function and sequester lymphocytes in lymph nodes. Fingolimod was the first S1P agent approved in the United States in 2010 for relapsing MS after two phase 3 trials (FREEDOMS and TRANSFORMS) demonstrated potent efficacy, and good safety and tolerability. Post-marketing experience as well as a third phase 3 trial (FREEDOMS II) also showed favorable results. More selective S1P receptor agents: ponesimod (ACT128800), siponimod (BAF312), ozanimod (RPC1063), ceralifimod (ONO-4641), GSK2018682, and MT-1303 are still in relatively early stages of development, but phase 1 and 2 trials showed promising efficacy and safety. However, these observations have yet to be reproduced in phase 3 clinical trials.
1. INTRODUCTION
Since the introduction of oral disease modifying therapies (DMTs) over the past several years, the treatment of patients with multiple sclerosis (MS) has changed vastly. Fingolimod was the first approved oral therapy in 2010. It has a unique mechanism of action being a sphingosine 1-phosphate (S1P) receptor modulator. Its clinical efficacy results from modulation of subtype 1 S1P receptors (S1P1), leading to lymphocyte sequestration in lymph nodes and presumably reduced migration to the central nervous system. However, interactions with other S1P receptor subtypes in other tissues and off-target pharmacologic effects led to interest in more selective S1P receptor agents, which are currently in various stages of development. This review will cover the mechanism of action of the S1P receptor modulators as well as their efficacy, safety, and tolerability in MS.
2. S1P RECEPTOR MODULATORS MECHANISM OF ACTION
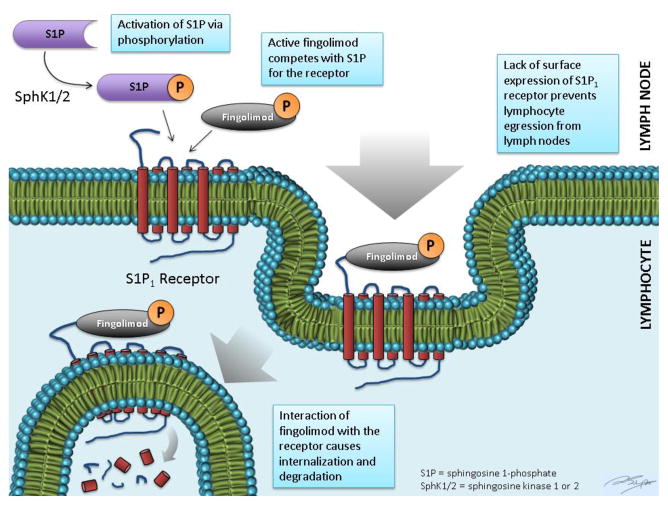
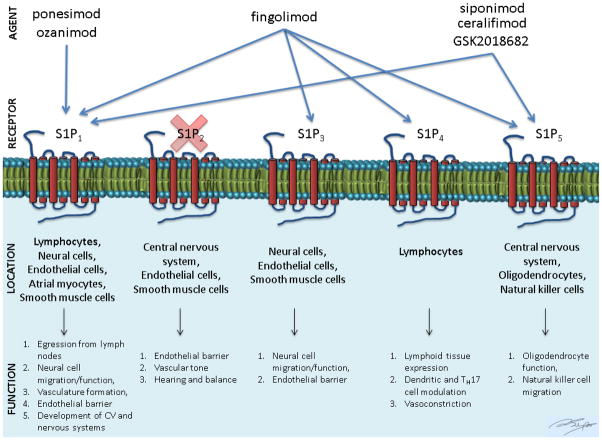
S1P is an active phospholipid that is the product of the phosphorylation of sphingosine by sphingosine kinase-1 or -2 (SphK1/2) (fig. 1). It regulates diverse cellular responses involved in immunity, heart rate, smooth muscle tone, and endothelial barrier function (fig. 2). It is abundant in erythrocytes, brain, spleen, and eyes [1]. S1P receptors have seven transmembrane segments and are coupled to G-proteins, which transduce their actions. There are five subtypes. Subtypes S1P1-3 are present ubiquitously whereas S1P4 is expressed in lymphoid tissue and S1P5 in the spleen and oligodendrocytes. B- and T-lymphocytes predominantly express S1P1 as well as S1P3 and S1P4 to a lower extent. The receptors are important in lymphocyte trafficking, particularly egression from lymph nodes.
Fig. 1.
Fig. 2.
In lymph nodes where S1P concentration is typically low, lymphocytes upregulate their S1P receptor expression. When S1P agonistically interacts with its receptor, the bound product is internalized, which leads to activation and transient retention of the T cell in the lymph node. The S1P receptor is then recycled back to the surface. The re-expression of the S1P receptor allows egression from the lymph nodes in response to the efferent lymph-lymph node chemotactic gradient.
3. FINGOLIMOD (FTY720, Gilenya®, Novartis Pharmaceuticals AG)
Fingolimod is a lipophilic sphingosine-like agent derived from the fungus Isaria sinclairii [2]. It has a structure similar to sphingosine and is phosphorylated by SphK1/2 to become fingolimod-P, an S1P analog. Similar to S1P, fingolimod-P binds to the S1P1 receptor and is then internalized. However, the S1P receptor is then degraded (fig. 1). This degradation prevents cell surface signaling. Hence, S1P receptor modulators such as fingolimod cause indirect antagonism of the S1P receptor’s function [3, 4]. The reduction in circulating lymphocytes is dose-dependent, with reduction of 20–30% within the first week of treatment and reaching a maximal response of approximately 70% [4, 5]. Fingolimod’s intended action is through binding of the S1P1 receptor on lymphocyte surfaces. However, its nonselective modulation of S1P3, S1P4, and S1P5 may lead to unwanted adverse-effects, which will be discussed further below [6, 7]. The elimination half-life of fingolimod is six to nine days, with about 81% of the dose excreted as inactive metabolites in urine [8].
a. Efficacy
In the FREEDOMS phase 3 trial, fingolimod was shown to decrease annualized relapse rate (ARR) by 54% and 60% respectively for 0.5 mg and 1.25 mg doses compared to placebo [5]. Results on time to first relapse and confirmed Expanded Disability Status Scale (EDSS) worsening also favored fingolimod. Fingolimod also significantly reduced gadolinium-enhancing (GdE) MRI lesions (~90%) and new/enlarged T2 lesions (~50%) at 24 months. Moreover, there was significant preservation of brain volume in participants on 0.5 mg than placebo (−0.84% versus −1.31%) from baseline to 24 months. These results were largely confirmed in a second placebo-controlled phase 3 trial, FREEDOMS II [9].
In the TRANSFORMS trial, both doses of fingolimod (0.5 mg and 1.25 mg) were demonstrated to be superior to interferon beta-1a (IFN-β1a) in decreasing the ARR by 52% and 38% respectively [10]. The proportion of relapse-free participants and time to confirmed relapse were greater in both fingolimod groups. On MRI, the numbers of GdE lesions and new/enlarged T2 lesions were significantly lower in the fingolimod groups compared to IFN-β1a. Brain volume reductions were significantly less with both fingolimod doses than with IFNβ-1a. Given the equivalent efficacy of the 2 doses, fingolimod 0.5 mg was approved by the US Food and Drug Administration (FDA) in 2010 as the first oral agent under the brand name Gilenya. In 2011 it was approved in Canada, Europe, and the rest of the world. In Japan, fingolimod is marketed as Imusera® and is developed by both Mitsubishi Tanabe and Novartis.
b. Safety, Tolerability, and Adverse Effects
Several adverse effects were noted in the three phase 3 trials (FREEDOMS, FREEDOMS II and TRANSFORMS), many of which were attributed to fingolimod’s nonselective modulation of the S1P receptors. Common adverse effects were first dose bradycardia or atrioventricular (AV) block, macular edema, headache, hypertension, cough, dyspnea, back pain, headache, influenza, and diarrhea.
Combined analysis from FREEDOMS and TRANSFORMS showed a mean decrease in heart rate of 8 beats per minute reaching a nadir 4–5 hours post first dose. The incidence of first degree AV block was 4.7%, Mobitz type 1 second degree AV block occurred in 0.2% of participants, and symptomatic bradycardia in 0.5% [11]. As a result, upon approval of fingolimod by the Food and Drug Administration (FDA), a strict monitoring protocol for the first 6 hours post-first dose administration was instituted. Discontinuation of fingolimod for greater than two weeks requires repeat first-dose monitoring due to the recurrence of bradycardia. The mechanism behind fingolimod’s effect on heart rhythm is via transient agonistic effects on S1P1 receptor in atrial myocytes followed by desensitization due to receptor down-modulation [12]. This is different from S1P receptor modulation in mice atrial myocytes, which is via S1P3 [13, 14]. Evidence to support this distinction is in the noted cardiac effects of selective S1P1 agents, as will be discussed later [15].
Several other adverse effects of fingolimod were noted, for which the underlying mechanism is less clear and may be due to off target effects via other S1P receptor subtypes. The incidence of macular edema from the pivotal trials was 0.3%, leading to the requirement for an ophthalmologic exam evaluating for macular edema prior to treatment and three months post initiation [16, 17]. The overall incidence of infections in the trials was similar in the fingolimod-treated participants and those treated with placebo (FREEDOMS, FREEDOMS II) and IFN-β1a (TRANSFORMS) [10, 9, 5]. However, herpes zoster, lower respiratory tract, and influenza had a somewhat higher in incidence in participants on fingolimod. In the clinical trials, two cases of fatal varicella zoster were reported [18]. The first patient was on fingolimod 1.25 mg for 10 months and developed primary disseminated zoster [10]. The second case was in a post-marketing observational study. The patient was previously on natalizumab and had positive varicella zoster titers; developed reactivation within six months of fingolimod 0.5 mg. Recently, a case of herpes simplex encephalitis in a patient on fingolimod was reported [19]. These cases highlight the relative increased risk of herpes infections in patients on fingolimod. Asymptomatic three-fold elevation of liver transaminases was reported in about 10% of fingolimod-treated participants in the combined analysis of FREEDOMS and TRANSFORMS. However the incidence of transaminase elevations was lower in the FREEDOMS II trial, and abnormalities normalized upon discontinuation of fingolimod without liver failure [9]. A few cases of lymphoma have been reported in animal trials and post-marketing data, but the relationship to fingolimod is uncertain [20].
Similar to other DMTs, fingolimod is pregnancy category C as preclinical studies have shown risk of fetal toxicity [21]. Pooled from nine phase II, III, and IV trials, 66 pregnancies with in utero exposure to fingolimod were reported. Of those, 24 underwent elective abortion, 9 had spontaneous abortion, 4 remained ongoing, and 1 was unknown. Two birth defects were seen, unilateral bowing of the tibia, and acrania. Three of the elective abortions were due to developmental defects, including a case of tetralogy of Fallot. The number of cases remains too small to draw proper conclusions. However, it is important to counsel patients on fingolimod for adequate contraception and allow a washout period of at least two months prior to conception.
To date, there are 11 known cases of progressive multifocal leukoencephalopathy (PML) in patients on fingolimod with prior exposure to natalizumab [22]. A case of PML on fingolimod was reported in 2012, but further investigation revealed the patient likely had neuromyelitis optica. In February 2015, Novartis reported a case PML in an MS patient treated with fingolimod for four years, without prior exposure to natalizumab [23]. A routine MRI scan showed two large non-enhancing T2 lesions in the cerebellum and temporal regions, one of which had high signal intensity on diffusion weighted imaging and apparent diffusion coefficient map. The diagnosis of PML was confirmed via positive John Cunningham virus (JCV) serology in blood and cerebrospinal fluid polymerase chain reaction. The patient had no known history of medications or comorbidities associated with PML, and absolute lymphocyte counts were 240–890 cells/μL. Despite this case report, the estimated risk of PML remains low based on the observed rate in more than 114,000 patients treated with fingolimod in clinical trials and clinical practice.
c. Fingolimod Administration and Monitoring
Fingolimod is approved by the FDA in the US as first-line therapy in relapsing forms of MS [8]. In Europe, it is approved for highly active multiple sclerosis, which failed at least one other DMT or because the disease is severe and getting worse rapidly. Fingolimod was not studied in the pediatric population nor is it approved for use in that age. A clinical trial assessing the safety and efficacy of fingolimod in pediatric patients with MS is underway (NCT01892722). At the Cleveland Clinic Mellen Center, fingolimod is offered to patients as first-line who are disinclined to injection therapy or those with highly active disease and are not appropriate candidates for natalizumab, i.e. JCV seropositive [24].
Prior to initiation of therapy, a complete blood count (CBC), alanine aminotransferase (ALT), aspartate transaminase (AST), pregnancy test, and varicella zoster IgG titer are checked (confirming exposure to chickenpox or shingles). Patients undergo ophthalmologic examination or optical coherence tomography (OCT) to assess for macular edema prior to treatment initiation. OCT of the macula can detect trace edema that might not be evident on dilated fundoscopy [25]. Baseline electrocardiogram (ECG) is done with consideration for a cardiology referral if the ECG is abnormal or there is a history of heart disease. Pulmonary referral is considered if there is a history of severe asthma or chronic obstructive pulmonary disease. While the patient is on therapy, ALT and AST are assessed at three and six months, ophthalmologic examination or OCT is repeated at 3–4 months, and pulmonary evaluation is considered if the patient develops dyspnea or persistent cough.
d. Long-term experience with fingolimod
The results of the extension phase of the FREEDOMS trial were presented at the 2012 American Academy of Neurology (AAN) meeting in New Orleans and were recently published [26, 27]. This extension phase included participants on treatment for up to four years. Participants who were on placebo were randomized to fingolimod 0.5 or 1.25 mg dose whereas those on fingolimod (0.5 or 1.25 mg) remained on the prior dose. Of the 81% who completed the 2-year core trial, 84% completed the extension phase (about two thirds of the original cohort). The ARR decreased from 0.4 to 0.2 in those who switched from placebo to fingolimod 0.5 mg. For participants who were on continuous fingolimod 0.5 mg throughout the trial, the overall reduction in relapse rate was 48% compared to placebo. 59% of participants who were maintained on 0.5 mg remained relapse-free compared to 37% of those who were switched. Similar improvements were noted on MRI measures (mean number of new/enlarging T2 and mean number of GdE). The risk of 6-month confirmed disability worsening was reduced in the continuous fingolimod 0.5 mg group by 31% compared to those switched.
LONGTERMS is an ongoing, multicenter, open-label, single-arm, long-term safety and tolerability study [28]. It includes participants from the phase 2 study, FREEDOMS, FREEDOMS II, TRANSFORMS, as well as some participants from phase 3b studies (e.g. FIRST, FIRST-LATAM, TOFINGO and VERIFY) who were on the approved 0.5 mg dose. The latest analysis includes annual assessments from 2010 to August 2013, although the study will continue until 2016. In the analysis, two cohorts were analyzed; those from the “core” phase 3 studies (Core Cohort or CC) who did not continue into the extension phase of their trial (n = 1212) and a “long-term” cohort (LC), which includes the CC in addition to participants from the aforementioned trials (n = 1655). LC also includes participants who were on placebo or IFN-β1a and were switched to fingolimod. The median duration of exposure to fingolimod was 3.7 years (up to 7.4 years) in the LC and 1.6 years (up to 2.4 years) in the CC. The incidence rate of adverse events was lower in the LC compared to CC, and included infections, cardiac events, skin cancer and other malignant neoplasms, thromboembolic events, hypertension, respiratory conditions, and macular edema. This decrease in incidence may be related to the loss of patients from the core studies (possibly due to adverse events). Importantly, no new adverse events were noted. There was a greater incidence rate of leucopenia and lymphopenia in the LC, but this did not appear to be associated with an increased risk of infection.
Hersh et al. reported 306 patients treated at a large academic center with 12-month follow up after starting fingolimod [29]. About 72.6% were switched directly from another DMT, most due to intolerance, risk, convenience, or breakthrough disease. Of those, 11.6% were switched from natalizumab. Most patients tolerated fingolimod well, but about 25% discontinued treatment, most often due to adverse effects (13%) or breakthrough disease (7%). There rates was comparable to the discontinuation rates in FREEDOMS (19%) and FREEDOMS II (32%). The range of adverse effects noted in this observational study was similar to that seen in the clinical trials.
e. Use of fingolimod following natalizumab
Fingolimod’s relatively strong efficacy in its phase 3 trials has led to its use as a second-line agent in patients with highly active disease who have antibodies to the JC virus. It has been a common agent to switch to in patients on natalizumab and who are at increased risk for developing PML due to JC virus seropositivity.
Rebound disease activity post natalizumab discontinuation is a potential complication. However, the ideal washout period between natalizumab and the next agent is uncertain [30, 31]. A French prospective study evaluated 333 patients switching from natalizumab to fingolimod with a mean inter-drug washout duration of 17 weeks [32]. During that period, 55% did not receive treatment and 39% received methylprednisolone. The risk of relapse was higher in patients who had a longer washout duration (20% for less than three months and 59% for greater than six months); although there was no difference in the risk of relapse with the use of methylprednisolone. Jokubaitis et al. retrospectively reviewed 536 patients on fingolimod from the MSBase Registry [33]. Of those, 89 patients were switched from natalizumab with a median inter-drug duration of 11.3 weeks. However, there was no significant increase in relapses in the first six months on fingolimod, as 85% of patients remained relapse-free. Despite the slight increase in relapse rate after the switch, the rate remained lower than pre-natalizumab and hence no evidence of rebound activity was seen. TOFINGO is an ongoing phase 4 trial that assesses immune function and MRI activity in patients switching from natalizumab to fingolimod (ClinicalTrials.gov identifier: NCT02325440). Preliminary MRI results were presented at the 2013 European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) meeting in Copenhagen, Denmark [34]. 142 participants were randomized to 8, 12, or 16 weeks of washout between the two drugs and MRI brain was performed at baseline and 8, 12, 16, 20, and 24 weeks. The mean number of active MRI lesions was significantly higher on the 16 week MRI (8.2 lesions) compared to the 8 and 12 week (2.1 and 1.7 lesions respectively). On the 24-week MRI, more participants were free of GdE with the shorter washout (75% for 8 week versus 47.5% for 16 week). Thus, several studies have shown that when switching from natalizumab to fingolimod, the risk of relapse increases with longer washout period [35, 36]. In most patients, fingolimod maintains good control of relapse activity.
f. Fingolimod in Progressive MS
INFORMS is a recently completed randomized, double-blind, placebo-controlled phase 3 trial of fingolimod in patients with primary progressive MS (ClinicalTrials.gov identifier: NCT00731692) [37]. In the trial, 970 patients were randomized to fingolimod 0.5 mg versus placebo. The primary end-point was reduction in risk of three-month confirmed disability worsening based on a composite measure combining EDSS, 9-hole peg test, and 25-foot walk. The trial was completed in September 2014 and has not yet been published. Preliminary results from the AAN 2015 annual meeting revealed neither the primary composite endpoint nor EDSS endpoint were met [38]. Although suppression of MRI inflammatory activity was noted, it did not improve the primary outcome or reduce brain volume loss.
4. SELECTIVE S1P RECEPTOR DRUGS
The favorable profile of fingolimod has led to an interest in developing small molecule S1P receptor modulators with shorter half-live, similar broad tissue distribution, preserved efficacy, more S1P receptor selectivity, and hopefully decreased adverse effects. Currently at least six potential S1P receptor drugs are in various stages of testing. Selectivity for S1P1 receptors theoretically is favored to preserve efficacy and minimize adverse events related to of S1P receptor subtypes [14].
a. Ponesimod (ACT-128800, Actelion Pharmaceuticals)
Ponesimod is a selective S1P1 receptor modulator that was investigated in a 24 week phase 2b double blinded, placebo-controlled trial, which aimed to evaluate the efficacy, safety, and tolerability of 3 doses (10 mg, 20 mg, and 40 mg) in relapsing-remitting MS (RRMS) [39]. The 20 mg and 40 mg arms included a titration schedule while the 10 mg and placebo groups had mock titrations. The primary endpoint was cumulative number of new GdE lesions from weeks 12–24. Secondary endpoints included ARR, and time to first confirmed relapse over 24 weeks, cumulative number of new or enlarging T2 lesions at 12–24 weeks, combined unique active lesions (CUALs – GdE and new or enlarging T2 lesions), and change in brain volume from baseline.
A total of 464 participants were randomized into four study arms, with about 79–90% of participants completing each arm. There was a significant reduction in new GdE lesions from week 12–24 with all three doses of ponesimod compared to placebo but more so in the 20 mg and 40 mg (83% and 77% reduction, respectively). Benefits on ARR reduction and time to first confirmed relapse were only significant in the 40 mg arm at 52% (p=0.0363) and 58% (p=0.0189), respectively. The 20 mg and 40 mg doses showed significant reduction in CUAL but not in new or enlarging T2 lesions; and all 3 doses showed preservation in brain volume at 24 weeks compared to placebo. Although there appears to be a dose-dependent effect, it does not continue above the 20 mg dose.
The 10 mg and 20 mg doses appeared to be well tolerated compared to the 40 mg dose. Only 2% of participants on ponesimod experienced bradycardia (about 15 beats per minute decrease); and 1.2% and 0.9% of the respective cohorts had first and second-degree heart block, all of which occurred only on the first day of the 10 mg dose, and within 2–3 hours of administration. Other adverse effects reported in the ponesimod cohorts include anxiety, dizziness, dyspnea, increased ALT (greater than three times the limit of normal), influenza, insomnia, and peripheral edema. Dyspnea and peripheral edema were dose-dependent, and seven participants prematurely discontinued due to dyspnea (six in the 40 mg group). The decrease in FEV1 was seen in 0.6–10% of participants on ponesimod, and returned to baseline within 1 week of discontinuation. Macular edema was seen in four participants, three of whom were in the 20 mg cohort and one was on placebo. The average reduction in lymphocyte count up to week 24 was 50%–69% for ponesimod as opposed to 3% for placebo. Most of the reduction occurred by day 8 and recovered within the first week of discontinuation. A five-year extension study is currently on-going (ClinicalTrials.gov Identifier: NCT01093326).
b. Siponimod (BAF312, Novartis Pharma AG)
Siponimod, currently under development by Novartis, binds to both S1P1 and S1P5. The phase 2 BOLD trial was a double blind, randomized trial completed in October 2010 comparing multiple doses of BAF312 (0.25 mg, 0.5 mg, 1.25 mg, 2 mg, and 10 mg) against placebo [40]. The 6-month study had a primary endpoint of CUAL (new GdE and new/enlarged T2 lesions without double counting) over the first three months. Secondary measures included total monthly new GdE lesions, total monthly new or enlarging T2 lesions, ARR, and proportion of participants who were relapse-free. Efficacy of the 2 and 10 mg doses was about the same, with a reduction in CUAL by 72% and 82% respectively, but was submaximal for the 0.5 mg dose. The ARR was significantly lower in the 2 mg and 10 mg doses (0.2 and 0.3 respectively) versus placebo (0.58). However, the ARR reduction was statistically significant for the 2 mg dose only.
5% of the higher dose (2 mg and 10 mg) cohorts experienced second degree AV block, and 17% had bradycardia. One participant with a history of coronary artery disease taking the 1.25 mg dose died 27 days after discontinuing the medication. This was suspected to be combination effect from the study drug as well as underlying coronary artery disease. Another participant taking the 10 mg dose had a nonfatal myocardial infarction 45 days post discontinuation. This was attributed to siponimod. Elevation in ALT (greater than three times the limit of normal) was more frequent in the higher doses. The magnitude of reduction in lymphocyte count at day 7 plateaued with the 2 mg, which implies maximal S1P modulation. Given the efficacy and safety data in this phase 2 trial, the 2 mg dose appeared to be the most appropriate dose for future trials, especially if titrated to mitigate its cardiac effects.
The phase 3 EXPAND trial is an ongoing placebo-controlled randomized trial in secondary progressive MS (SPMS) patients that began in December 2012 and is currently enrolling (ClinicalTrials.gov identifier: NCT01665144). The primary outcome measure is delay in time to confirmed disability worsening measured by EDSS with secondary measures including timed 25-foot walk, T2 lesion volume, ARR, time to first relapse, response rate on the 12-item MS walking scale (MSWS-12), number of patients with adverse events, and number of patients with abnormal lab tests.
c. Ozanimod (RPC1063, Receptos)
Ozanimod is a selective oral S1P1 modulator that is in clinical development by Receptos, Inc. It was previously tested as single and multiple doses in healthy volunteers [41].
RADIANCE is a phase 2/3 trial of ozanimod in adult patients with relapsing MS. The results of the phase 2 portion were presented at the 2014 ACTRIMS/ECTRIMS meeting in Boston [28]. In this 24-week placebo controlled trial, 258 participants were randomized to receive ozanimod 0.5 or 1 mg, or placebo. The primary efficacy endpoint was the cumulative number of GdE lesions on monthly scans from weeks 12 to 24. Secondary measures included the cumulative number of new or enlarging T2 lesions from weeks 12 to 24, the number of GdE lesions at week 24, ARR, and the safety and tolerability of ozanimod.
Overall, the study had a high rate of completion (>96%), similar across the treatment groups. The study met its primary endpoint with an 86% reduction in cumulative number of GdE lesions from weeks 12 to 24 for both doses compared to placebo. Secondary end-points favored ozanimod with 91% and 94% reduction in the total number of GdE lesions at week 24 (0.5 mg and 1 mg doses, respectively). The cumulative number of new or enlarging T2 lesions from weeks 12 to 24 was reduced by 84% and 91%. ARR was reduced by 31% and 53%, respectively, approaching significance for the higher dose.
In total, three serious adverse effects unrelated to therapy were reported in the 0.5 mg dose: optic neuritis, somatoform autonomic dysfunction, and uterine cervical squamous metaplasia. Other adverse reactions include nasophayngitis, headache, and urinary tract infection (no significant difference in incidence among the treatment groups). Rare elevation in ALT greater than three times the limit of normal was noted in both ozanimod groups (1–2%). The first-dose effects on cardiac conduction were minimal. Second degree atrioventricular block was seen in <3% of both ozanimod and placebo groups, and 74% of the ozanimod cohort maintained a heart rate above 60 beats-per-minute in the first 24-hours.
Based on the preliminary results of the phase 2 portion, the phase 3 portion of the RADIANCE study, comparing ozanimod against intramuscular IFN-β1a, was initiated. A second phase 3 trial, SUNBEAM (ClinicalTrials.gov identifier: NCT02294058), is in start-up.
d. Ceralifimod (ONO-4641, Ono Pharmaceutical)
ONO-4641 is an oral, selective S1P1 and S1P5 modulator. The phase 2 DreaMS trial, which was completed in 2012, 407 participants were randomized to 0.05 mg, 0.1 mg, or 0.15 mg of ONO-4641, or placebo. The primary outcome was total number of GdE lesions on monthly scans over 26 weeks. The results of the trial have not yet been published. Some data showing positive efficacy on MRI measures (82%, 92%, and 77% fewer GdE lesions for the 0.05 mg, 0.1 mg, and 0.15 mg doses (all p<0.0001), respectively) were reported at the 2012 and 2014 annual American Academy of Neurology meetings [42–44]. At this time, it is unclear whether further development of this drug will proceed.
e. GSK2018682 (GlaxoSmithKline)
GSK2018682 is a selective S1P1 receptor modulator with some activity at S1P5 that is currently under development by GlaxoSmithKline. Three phase 1 studies evaluating safety, tolerability, pharmacokinetics, and pharmacodynamics of GSK2018682 in humans were completed in 2010 and 2011 [45]. GSK2018682 has been reasonably tolerated following single oral doses of 0.6 mg to 24 mg (ClinicalTrials.gov Identifier: NCT01466322). However, the GlaxoSmithKline Product Development Pipeline from March 2015 does not list GSK2018682. Thus, it does not seem like the drug will be moving forward.
f. MT-1303 (Mitsubishi Tanabe Pharma)
MT-1303 is a selective S1P receptor (unknown subtype) modulator in development by Mitsubishi Tanabe. The drug was tested in a phase 2 dose-finding trial completed in October 2014 (ClinicalTrials.gov Identifier: NCT01742052). An extension of the phase 2 study is planned (ClinicalTrials.gov Identifier: NCT01890655).
5. SELECTIVE VS NONSELECTIVE S1P AGENTS
Based on the phase 2 data for the selective S1P receptor modulators, the effects on lymphocyte reduction, development of GdE lesions, and ARR are generally similar to those of fingolimod (table 1). The selective S1P agents, however, appear to have the advantage of a shorter half-life and more rapid lymphocyte recovery post discontinuation [46, 47, 4]. These differences would allow flexibility in retreatment with other agents, aid in washout to treat potential opportunistic infections, and address other treatment-related complications or eliminate the drug in unplanned pregnancy.
Table 1.
Summary of S1P receptor modulators
| Agent | fingolimod | ponesimod | siponimod | ozanimod | ||||
|---|---|---|---|---|---|---|---|---|
| Trial | Kappos, et al. 2006 [43] | Olsson, et al. 2014 [34] | BOLD [35] | RADIANCE [24] | ||||
| Trial type | phase 2 | phase 2 | phase 2 | phase 2 | ||||
| Trial duration | 6 months | 6 months | 6 months | 6 months | ||||
| Dose | 1.25 mg╪ | placebo | 40 mg | placebo | 10 mg | placebo | 1 mg | placebo |
| ARR | 0.35 | 0.77 | 0.25 | 0.53 | 0.30 | 0.58 | 0.24 | 0.5 |
| Percentage of patients relapse free at 6 months | 86% | 66% | 90.6% | 78.5% | 82% | 73% | NA | NA |
| Mean number of new or newly enlarged T2 lesions | NA | NA | 0.5 weeks 12–24 | 0.7 weeks 12–24 | 0.34 at 6 months | 2.09 at 6 months | 0.8 weeks 12–24 | 9 weeks 12–24 |
| Number of gadolinium-enhancing T1 lesions | 1.29± 5.8 at 6 months | 2.21 ± 4.3 at 6 months | 1.4* weeks 12 to 24 | 6.2* weeks 12 to 24 | 0.28* at 6 months | 2.94* at 6 months | 1.5* weeks 12–24 | 11.1* weeks 12–24 |
| Mean cumulative number of combined unique active lesions | NA | NA | 1.9 weeks 12 to 24 | 6.9 weeks 12 to 24 | 0.36 at 3 months | 1.39 at 3 months | NA | NA |
| Bradycardia | 3% at 5 mg dose | 0 | 2% at 10 mg dose | 0 | 28% | 2% | 0.6% at 0.25 mg dose | 0 |
| First-degree heart block | 1% at 1.25 mg dose | 0 | 1.2% at 10 mg dose | 0 | 6% | 0 | 0 | 0 |
| Second-degree heart block | 4% at 1.25 mg dose | 0 | 0.9% at 10 mg dose | 0 | 4% | 4% | 2.4% at 0.25 mg dose | 0 |
| Macular edema | NA | NA | 0 | 0.80% | 2% | 0 | 2.4% “retinal disorder” | 2.3% “retinal disorder” |
| Increase in ALT >3 times normal range | 10% | 0 | 4.20% | 0 | 4% | 0 | 1.20% | 0 |
| Herpes viral infections | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| SAE | NA | NA | 3.36% | 4.13% | 6% | 0% | 0 | 0 |
| Any adverse event leading to discontinuation of study drug | 5% | 4% | NA | NA | 20% | 4% | 0 | 0 |
| Mean decrease in FEV1 | 2.8% | 1.9% | 10.3% baseline to week 24 | NA | NA | NA | 0 | 0 |
Cumulative number of gadolinium enhancing T1 lesions
The approved 0.5 mg dose was not evaluated in the phase 2 trial
ARR = annualized relapse rate
CI = confidence interval
SD = standard deviation
NA = not available
ALT = alanine aminotransferase
SAE = serious adverse events
However, despite the selectivity of the new S1P receptor modulators, some side effects such as first-dose bradycardia and conduction block were still seen in some of the agents. This is because human S1P receptors mediating these effects in cardiac myocytes are S1P1 [13, 14]. In some instances, as in the case of ponesimod, respiratory adverse effects were also noted [39].
6. CONCLUSION
The development of S1P receptor modulators has opened the door for a novel and effective mechanism of reducing inflammatory lesion activity in MS patients. These agents are effective and have good safety and tolerability as evidenced by fingolimod’s post-marketing record. The newer selective agents also seem to show promise in maintaining that efficacy while adding the potential advantage improved pharmacodynamics and tolerability. However, these observations have yet to be reproduced in phase 3 clinical trials.
KEY POINTS.
S1P receptor modulators have a unique mechanism of action as treatment for patients with multiple sclerosis.
Fingolimod, a nonselective S1P agent, was effective in relapsing MS but not primary progressive MS.
Selective S1P agents may have comparable efficacy with an improved pharmacokinetic/pharmacodynamic profile and, possibly, better tolerability.
Acknowledgments
Funding: A Subei is supported by Clinician Care Fellowship Award CF 00104N-1 from the National Multiple Sclerosis Society, which funded the time allocated to developing the manuscript. Otherwise no direct funding was received.
Footnotes
Compliance with Ethical Standards:
Conflicts of interest: A Subei has nothing to disclose. JA Cohen is an Editor of Multiple Sclerosis Journal Experimental, Translational and Clinical and reports personal fees from EMD Serono, Genentech, Genzyme, Novartis, Receptos, Teva, and Vaccinex for consulting.
Contributor Information
Adnan M. Subei, Email: a_subei@yahoo.com, Mellen Center for MS Treatment and Research, Cleveland Clinic Foundation, 9500 Euclid Avenue/U10, Cleveland, OH 44195, Phone: 216-445-8110, Fax: 216-445-7013.
Jeffrey A. Cohen, Email: cohenj@ccf.org, Mellen Center for MS Treatment and Research, Cleveland Clinic Foundation, 9500 Euclid Avenue/U10, Cleveland, OH 44195, Phone: 216-445-8110, Fax: 216-445-7013.
References
- 1.Cohen JA, Chun J. Mechanisms of fingolimod’s efficacy and adverse effects in multiple sclerosis. Annals of neurology. 2011;69(5):759–77. doi: 10.1002/ana.22426. [DOI] [PubMed] [Google Scholar]
- 2.Chiba K, Adachi K. Sphingosine 1-phosphate receptor 1 as a useful target for treatment of multiple sclerosis. Pharmaceuticals (Basel) 2012;5(5):514–28. doi: 10.3390/ph5050514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chun J, Hartung HP. Mechanism of action of oral fingolimod (FTY720) in multiple sclerosis. Clinical neuropharmacology. 2010;33(2):91–101. doi: 10.1097/WNF.0b013e3181cbf825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mehling M, Johnson TA, Antel J, Kappos L, Bar-Or A. Clinical immunology of the sphingosine 1-phosphate receptor modulator fingolimod (FTY720) in multiple sclerosis. Neurology. 2011;76(8 Suppl 3):S20–7. doi: 10.1212/WNL.0b013e31820db341. [DOI] [PubMed] [Google Scholar]
- 5.Kappos L, Radue EW, O’Connor P, Polman C, Hohlfeld R, Calabresi P, et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. The New England journal of medicine. 2010;362(5):387–401. doi: 10.1056/NEJMoa0909494. [DOI] [PubMed] [Google Scholar]
- 6.Brinkmann V, Davis MD, Heise CE, Albert R, Cottens S, Hof R, et al. The immune modulator FTY720 targets sphingosine 1-phosphate receptors. The Journal of biological chemistry. 2002;277(24):21453–7. doi: 10.1074/jbc.C200176200. [DOI] [PubMed] [Google Scholar]
- 7.Paugh SW, Payne SG, Barbour SE, Milstien S, Spiegel S. The immunosuppressant FTY720 is phosphorylated by sphingosine kinase type 2. FEBS letters. 2003;554(1–2):189–93. doi: 10.1016/s0014-5793(03)01168-2. [DOI] [PubMed] [Google Scholar]
- 8.GILENYA® (fingolimod) Package Insert. Basel, Switzerland: Novartis Pharmaceuticals; 2014. [Google Scholar]
- 9.Calabresi PA, Radue EW, Goodin D, Jeffery D, Rammohan KW, Reder AT, et al. Safety and efficacy of fingolimod in patients with relapsing-remitting multiple sclerosis (FREEDOMS II): a double-blind, randomised, placebo-controlled, phase 3 trial. The Lancet Neurology. 2014;13(6):545–56. doi: 10.1016/S1474-4422(14)70049-3. [DOI] [PubMed] [Google Scholar]
- 10.Cohen JA, Barkhof F, Comi G, Hartung HP, Khatri BO, Montalban X, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. The New England journal of medicine. 2010;362(5):402–15. doi: 10.1056/NEJMoa0907839. [DOI] [PubMed] [Google Scholar]
- 11.DiMarco JP, O’Connor P, Cohen JA, Francis G, Collins W, Zhang-Auberson L, et al. First-dose effect of fingolimod: pooled safety data from two phase-3 studies (TRANSFORMS and FREEDOMS) Mult Scler. 2010;16(10 suppl):S290. doi: 10.1016/j.msard.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 12.Camm J, Hla T, Bakshi R, Brinkmann V. Cardiac and vascular effects of fingolimod: mechanistic basis and clinical implications. American heart journal. 2014;168(5):632–44. doi: 10.1016/j.ahj.2014.06.028. [DOI] [PubMed] [Google Scholar]
- 13.Forrest M, Sun S-Y, Hajdu R, Bergstrom J, Card D, Doherty G, et al. Immune Cell Regulation and Cardiovascular Effects of Sphingosine 1-Phosphate Receptor Agonists in Rodents Are Mediated via Distinct Receptor Subtypes. J Pharmacol Exp Ther. 2004;309(2):758–68. doi: 10.1124/jpet.103.062828. [DOI] [PubMed] [Google Scholar]
- 14.Sanna MG, Liao J, Jo E, Alfonso C, Ahn MY, Peterson MS, et al. Sphingosine 1-phosphate (S1P) receptor subtypes S1P1 and S1P3, respectively, regulate lymphocyte recirculation and heart rate. The Journal of biological chemistry. 2004;279(14):13839–48. doi: 10.1074/jbc.M311743200. [DOI] [PubMed] [Google Scholar]
- 15.Gergely P, Nuesslein-Hildesheim B, Guerini D, Brinkmann V, Traebert M, Bruns C, et al. The selective sphingosine 1-phosphate receptor modulator BAF312 redirects lymphocyte distribution and has species-specific effects on heart rate. British journal of pharmacology. 2012;167(5):1035–47. doi: 10.1111/j.1476-5381.2012.02061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Collins W, Cohen J, O’Connor P, de Vera A, Zhang-Auberson L, Jin FJ, et al. Long-term safety of oral fingolimod (FTY720) in relapsing multiple sclerosis: integrated analyses of phase 2 and 3 studies. Mult Scler. 2010;16(10 suppl):S295. [Google Scholar]
- 17.Zarbin MA, Jampol LM, Jager RD, Reder AT, Francis G, Collins W, et al. Ophthalmic evaluations in clinical studies of fingolimod (FTY720) in multiple sclerosis. Ophthalmology. 2013;120(7):1432–9. doi: 10.1016/j.ophtha.2012.12.040. [DOI] [PubMed] [Google Scholar]
- 18.Arvin AM, Wolinsky JS, Kappos L, Morris MI, Reder AT, Tornatore C, et al. Varicella-zoster virus infections in patients treated with fingolimod: risk assessment and consensus recommendations for management. JAMA Neurol. 2015;72(1):31–9. doi: 10.1001/jamaneurol.2014.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pfender N, Jelcic I, Linnebank M, Schwarz U, Martin R. Reactivation of herpesvirus under fingolimod: A case of severe herpes simplex encephalitis. Neurology. 2015;84(23):2377–8. doi: 10.1212/wnl.0000000000001659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.GILENYA® (fingolimod) Prescribing Information. Basel, Switzerland: Novartis Pharmaceuticals; 2014. [Google Scholar]
- 21.Karlsson G, Francis G, Koren G, Heining P, Zhang X, Cohen JA, et al. Pregnancy outcomes in the clinical development program of fingolimod in multiple sclerosis. Neurology. 2014;82(8):674–80. doi: 10.1212/wnl.0000000000000137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Subei A, editor. Pharmaceuticals N. Gilenya – Risk of Progressive Multifocal Leukoencephalopathy (PML) 2015. p. 12. [Google Scholar]
- 23.Pharmaceuticals N. [Accessed February 16, 2015];Gilenya Safety Update. 2015 http://www.novartis.com/newsroom/product-related-info-center/gilenya-safety-update.shtml.
- 24.Mellen Center Approaches: Use of fingolimod in MS (Gilenya, FTY720) 2010 http://my.clevelandclinic.org/services/neurological_institute/mellen-center-multiple-sclerosis/medical-professionals.
- 25.Brown JC, Solomon SD, Bressler SB, Schachat AP, DiBernardo C, Bressler NM. Detection of diabetic foveal edema: contact lens biomicroscopy compared with optical coherence tomography. Archives of ophthalmology. 2004;122(3):330–5. doi: 10.1001/archopht.122.3.330. [DOI] [PubMed] [Google Scholar]
- 26.Kappos L, Radue E-W, O’Connor P, Polman C, Hohlfeld R, Calabresi P, et al. Long-Term Efficacy and Safety of Fingolimod (FTY720) in Relapsing-Remitting Multiple Sclerosis (RRMS): Results from the Extension of the Phase III FREEDOMS Study (S41.004) Neurology. 2012;78:S41.004. Meeting Abstracts 1. [Google Scholar]
- 27.Kappos L, O’Connor P, Radue EW, Polman C, Hohlfeld R, Selmaj K, et al. Long-term effects of fingolimod in multiple sclerosis: The randomized FREEDOMS extension trial. Neurology. 2015;20(10):1462. doi: 10.1212/WNL.0000000000001462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cohen JA, Arnold DL, Comi G, Bar-Or A, Gujrathi S, Hartung JP, et al. Phase 2 results of the RADIANCE trial: a randomized, double-blind, placebo-controlled trial of oral RPC1063 in relapsing multiple sclerosis (LB1.1) Mult Scler. 2014;20(1 suppl):497. [Google Scholar]
- 29.Hersh CM, Hara-Cleaver C, Rudick RA, Cohen JA, Bermel RA, Ontaneda D. Experience with fingolimod in clinical practice. The International journal of neuroscience. 2014;29:29. doi: 10.3109/00207454.2014.969839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kerbrat A, Le Page E, Leray E, Anani T, Coustans M, Desormeaux C, et al. Natalizumab and drug holiday in clinical practice: an observational study in very active relapsing remitting multiple sclerosis patients. J Neurol Sci. 2011;308(1–2):98–102. doi: 10.1016/j.jns.2011.05.043. [DOI] [PubMed] [Google Scholar]
- 31.Miravalle A, Jensen R, Kinkel RP. Immune reconstitution inflammatory syndrome in patients with multiple sclerosis following cessation of natalizumab therapy. Arch Neurol. 2011;68(2):186–91. doi: 10.1001/archneurol.2010.257. [DOI] [PubMed] [Google Scholar]
- 32.Cohen M, Maillart E, Tourbah A, De Seze J, Vukusic S, Brassat D, et al. Switching from natalizumab to fingolimod in multiple sclerosis: a French prospective study. JAMA Neurol. 2014;71(4):436–41. doi: 10.1001/jamaneurol.2013.6240. [DOI] [PubMed] [Google Scholar]
- 33.Jokubaitis VG, Li V, Kalincik T, Izquierdo G, Hodgkinson S, Alroughani R, et al. Fingolimod after natalizumab and the risk of short-term relapse. Neurology. 2014;82(14):1204–11. doi: 10.1212/WNL.0000000000000283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kappos L, Radue E-W, Comi G, Montalban X, Butzkueven H, Wiendl H, et al. Oral presentation 167: Disease control and safety in relapsing remitting multiple sclerosis (RRMS) patients switching from natalizumab to fingolimod: a 32-week, rater- and patient-blind, randomized, parallel-group study (TOFINGO) Mult Scler. 2013;19(11 suppl):50. [Google Scholar]
- 35.Bianco A, Patanella AK, Nociti V, Marti A, Frisullo G, Plantone D, et al. Second-line therapy with fingolimod for relapsing-remitting multiple sclerosis in clinical practice: the effect of previous exposure to natalizumab. Eur Neurol. 2015;73(1–2):57–65. doi: 10.1159/000365968. [DOI] [PubMed] [Google Scholar]
- 36.Comi G, Gold R, Dahlke F, Sinha A, von Rosenstiel P, Tomic D, et al. Relapses in patients treated with fingolimod after previous exposure to natalizumab. Mult Scler. 2014:25. doi: 10.1177/1352458514549404. [DOI] [PubMed] [Google Scholar]
- 37.Miller D, Cree B, Dalton C, Freedman M, Hartung H, Kappos L, et al. Study Design and Baseline Characteristics of the INFORMS Study: Fingolimod in Patients with Primary Progressive Multiple Sclerosis (P07.116) Neurology. 2013;80:P07.116. Meeting Abstracts 1. [Google Scholar]
- 38.Lublin F, Miller D, Freedman M, Cree B, Wolinsky J, Weiner H, et al. Oral fingolimod versus placebo in patients with primary progressive multiple sclerosis (PPMS): results of the INFORMS phase III trial. Neurology. 2015;84(14 Supplement) [Google Scholar]
- 39.Olsson T, Boster A, Fernandez O, Freedman MS, Pozzilli C, Bach D, et al. Oral ponesimod in relapsing-remitting multiple sclerosis: a randomised phase II trial. Journal of neurology, neurosurgery, and psychiatry. 2014;85(11):1198–208. doi: 10.1136/jnnp-2013-307282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Selmaj K, Li DK, Hartung HP, Hemmer B, Kappos L, Freedman MS, et al. Siponimod for patients with relapsing-remitting multiple sclerosis (BOLD): an adaptive, dose-ranging, randomised, phase 2 study. The Lancet Neurology. 2013;12(8):756–67. doi: 10.1016/S1474-4422(13)70102-9. [DOI] [PubMed] [Google Scholar]
- 41.Olson A, Hartung J, Timony G, Peach R, Boehm M, Rosen H, et al. Safety and Tolerability of Orally Administered RPC1063, a Novel S1P1 Receptor Modulator, in Healthy Adult Volunteers, Results of a Phase 1 Study (P01.178) Neurology. 2013;80:P01.178. Meeting Abstracts 1. [Google Scholar]
- 42.Bar-Or A, Zipp F, Krzysztof S, Due B, Vollmer T. Effect of the Sphingosine 1-Phosphate Receptor Agonist ONO-4641 on Circulating Lymphocytes in Patients with Relapsing-Remitting Multiple Sclerosis: Results from the Phase 2 DreaMS Trial (P05.153) Neurology. 2013;80:P05.153. Meeting Abstracts 1. [Google Scholar]
- 43.Bar-Or A, Zipp F, Scaramozza M, Vollmer T, Due B, Thangavelu K, et al. Effect of Ceralifimod (ONO-4641), a Sphingosine-1-Phosphate Receptor-1 and -5 Agonist, on Magnetic Resonance Imaging Outcomes in Patients with Multiple Sclerosis: Interim Results from the Extension of the DreaMS Study (P3.161) Neurology. 2014;82(10 Supplement):P3.161. [Google Scholar]
- 44.Vollmer T, Selmaj K, Bar-Or A, Zipp F. A Double-Blind, Placebo-Controlled, Phase 2, 26-Week DreaMS Trial of a Selective S1P Receptor Agonist ONO-4641 in patients with Relapsing-Remitting Multiple Sclerosis (S31.005) Neurology. 2012;79(11):e90. [Google Scholar]
- 45.Xu J, Gray F, Henderson A, Hicks K, Yang J, Thompson P, et al. Safety, pharmacokinetics, pharmacodynamics, and bioavailability of GSK2018682, a sphingosine-1-phosphate receptor modulator, in healthy volunteers. Clinical Pharmacology in Drug Development. 2014;3(3):170–8. doi: 10.1002/cpdd.98. [DOI] [PubMed] [Google Scholar]
- 46.Gergely P, Nuesslein-Hildesheim B, Guerini D, Brinkmann V, Traebert M, Bruns C, et al. The selective sphingosine 1-phosphate receptor modulator BAF312 redirects lymphocyte distribution and has species-specific effects on heart rate. British journal of pharmacology. 2012;167(5):1035–47. doi: 10.1111/j.1476-5381.2012.02061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Krause A, Brossard P, D’Ambrosio D, Dingemanse J. Population pharmacokinetics and pharmacodynamics of ponesimod, a selective S1P1 receptor modulator. Journal of pharmacokinetics and pharmacodynamics. 2014;41(3):261–78. doi: 10.1007/s10928-014-9362-4. [DOI] [PubMed] [Google Scholar]