Abstract
Long chain polyunsaturated fatty acids (LCPUFA) including docosahexaenoic acid (DHA) and arachidonic acid (ARA) are increasingly transferred from mother to fetus late in pregnancy. Infants born before this transfer is complete are at risk for deficiency. This study determines the relationship between gestational age (GA) and circulating LCPUFA levels to better understand the unique needs of premature infants born at various GAs. Whole blood was collected within the first 7 days of life from 60 preterm (≤34 weeks GA) and 30 term infants (≥38 weeks GA) and FA levels were analyzed. Since concurrent intravenous lipid emulsion can skew composition data, blood LCPUFA concentrations were also measured. Levels were compared among groups, and linear regression models were used to examine the association between FA composition and GA. Preterm infants had significantly lower DHA and ARA levels than term peers, and whether assessed as concentrations or compositions, both directly correlated with GA (p<0.0001). Moreover, FA comparisons suggest that premature infants have impaired synthesis of LCPUFAs from precursors and may require preformed DHA and ARA. This study confirms that essential FA status is strongly related to GA, and that those babies born the earliest are at the greatest risk of LCPUFA deficiency.
Keywords: Essential Fatty Acids, Docosahexaenoic Acid, Arachidonic Acid, Long Chain Polyunsaturated Acids, Prematurity, Neonatal Nutrition
1. Introduction
Premature birth is a leading cause of infant morbidity and mortality, and as survival has increased, there is renewed focus on improving both short- and long-term outcomes in this high-risk population. One way to do this is through tailored provision of key nutrients. Long chain polyunsaturated FAs (LCPUFAs), including arachidonic acid (ARA) and docosahexaenoic acid (DHA), are important for normal health and development. [1, 2] Increasing evidence demonstrates that DHA supplementation improves visual outcomes [3–5] and neurodevelopment [6–9] in premature infants and may decrease morbidity from bronchopulmonary dysplasia [10], necrotizing enterocolitis [11–14] and retinopathy of prematurity [15–17]. Although supplementation studies are promising, the optimal amount of individual fatty acids (FAs) at various gestational ages (GA) is still relatively unknown. [1]
There are two important families of essential LCPUFAs, the omega-6 FAs including ARA (20:4n-6) and its 18-carbon precursor linoleic acid (LA, 18:2n-6), and the omega-3 FAs including eicosapentaenoic acid (EPA, 20:5n-3), DHA (22:6n-3) and their 18-carbon precursor α-linolenic acid (ALA, 18:3n-3). These two families have different functions and are not interchangeable, making provision of both essential. The developing fetus is dependent on a maternal source during pregnancy. Most LCPUFA accumulation occurs during the third trimester [18, 19] and so, infants born before this process is complete are at risk of deficiency.
Previous studies have analyzed LCPUFA status in premature babies, most commonly as part of an intervention study [10, 20–27]. However, to our knowledge, only two studies have directly compared blood FAs between term and preterm infants across a range of GAs. [10, 20] Both studies analyzed a composition or weight percent of total blood FAs that could be influenced by the simultaneous infusion of lipid emulsions commonly administered within the first week of life to pre-term infants. This leaves some question regarding the exact relationship between LCPUFA status and GA. As part of a randomized trial of the use of enteral DHA supplementation in premature infants, we measured baseline blood FAs as both a percent composition and actual concentration and investigated the relationship of LCPUFAs with GA.
2. Patients and Methods
2.1 Study Population
Ninety infants (60 preterm and 30 term infants) who met study criteria were recruited from the Boekelheide NICU at Sanford Children’s Hospital between October 2012 and March 2014. Eligible preterm infants were less than or equal to seven days of age at enrollment and were further stratified into two groups: early preterm (GA of 24 0/7 to 27 6/7 weeks at birth) and late preterm (GA 28 0/7 to 33 6/7 weeks at birth). Preterm infants were excluded if they were considered non-viable by the medical team, or if they had multiple, severe congenital anomalies. Term infants (38 weeks GA or greater at birth) were excluded if they were born to diabetic mothers or were small for GA (SGA) which was defined by a birth weight less than the 10th% for adjusted GA on a World Health Organization (WHO) growth chart. These exclusion criteria for reference term infants were used because of previously reported differences in FA accretion in these groups. [20, 28–31] Infants were prospectively enrolled using an adaptive enrollment method to assure that early preterm infants were included in the study during the same time period as more commonly admitted late preterm and term infants. The study was approved by the Sanford Institutional Review Board, and all mothers (who had to be at least 18 years of age) provided written, informed consent after all procedures had been thoroughly explained and prior to their infant’s participation.
2.2 Clinical Data
Clinical data for enrolled infants and their mothers were collected from electronic medical records following HIPPA authorization. Maternal data included age, gravidity, parity, race, and obstetric estimate of GA at delivery. Maternal diabetes status was determined by the medical problem list and results of an oral glucose tolerance test if completed during the pregnancy. Baseline infant data included estimated GA at birth, sex, mode of delivery, reported race, day of life, birth weight, height, head circumference and growth chart percentile (Fenton Growth Curve, FGC, for preterm infants and WHO Growth Chart for term infants).
Per the standard of care in our NICU, premature infants begin receiving total parenteral nutrition on the first day of life which includes 10% soybean oil-based intravenous lipid emulsion (Intralipid®) titrated to a typical dose of 2–3 g/kg/d by the third day of life. Parenteral lipid emulsion is typically continued until they reach “full enteral feedings” (100 kcal/kg/day of breast milk or formula). In our study population, late preterm infants reached “full enteral feedings” at a mean (SD) of 15.1 (5.1) days, and early preterm infants at 29.5 (7.2) days. Thus, at the time of baseline analysis (average 6.3 days of life), all premature infants were receiving a significant amount of LA and ALA, but no ARA and DHA, from parenteral lipid administration. Emulsions containing fish oil (Omegaven®) were not used in this study.
2.3 Dried Blood Spot (DBS) Fatty Acid Analysis
Following enrollment, a single drop of scavenged whole blood was collected directly to a filter paper (Ahlstrom 226, PerkinElmer, Greenville, SC) that was pretreated with an antioxidant cocktail (Oxystop, OmegaQuant Analytics, LLC, Sioux Falls, SD) to protect unsaturated FAs from oxidation. Whole blood was chosen as the sample of choice because it is a global representation of circulating FA status (including both plasma and blood cells) and because analyses can be conducted on as little as a single drop. After collection, cards were stored in a re-sealable plastic bag at 4°C with batches delivered weekly to OmegaQuant Analytics for analysis by capillary gas chromatography as described previously [32]. Fatty acid levels are expressed both as a weight percent of total blood fatty acids (composition) and as a concentration (ug/mL blood). The latter was calculated based on the internal standard (22:3n-3) included in the assay and on the experimental determination that the average volume of dried blood contained in a 4-mm punch was 9.7 uL. The mean time between sample collection and analysis in this study was 6.8 days. The stability of FAs collected and stored in this manner has been previously evaluated by analyzing 5 blood samples (not from this study) weekly over a 4 week time period. There was no sample degradation detected. [33] Specifically, mean baseline and 4 week values were: ARA, 11.3% and 12.0%; and DHA, 4.5% and 4.3%. Coefficients of variation were 2.5% and 5.8%, respectively.
2.4 Statistical Analysis
For continuous variables, means and standard deviations were calculated. Categorical variables were described using frequencies and percents. Univariate analysis of all FAs was performed using the Kruskal-Wallis test. Linear mixed models were used to examine the association between fatty acid composition and gestational age. The mixed model accounts for possible correlation between twins and triplets present in the data set. The model covariance was assessed to determine if a mixed model was needed or if the assumption of independence could be made. For all models, the assumption of independence was justified, so linear regression models without a random effect were used. Continuous dependent variables were transformed as needed to meet model assumptions. Models were adjusted for maternal age, timing of blood collection and relative weight, and head circumference (growth curve percentiles) at birth. Primary outcome variables for this analysis were DHA and ARA as both compositions and concentrations. Model diagnostics were examined to ensure that models were not overly influenced by outliers. Additionally, two-sensitivity analyses were undertaken to examine the effect of inclusion criteria (preterm infants who were SGA or whose mother had diabetes during the pregnancy) and the day of sample collection. Results for both sensitivity analyses produced largely similar parameter estimates and therefore are not reported separately.
3. Results
3.1 Study Population
A total of 140 preterm and 176 term infants were screened and found to be eligible for the study. Thirty term infants and 60 preterm infants were enrolled. Of the latter, 47 were late preterm and 13 were early preterm (Table 1). For the preterm infants, the most common reason for not enrolling was parental refusal to participate in the DHA intervention study. For the term infants, the most common reasons were that the infant’s heel sticks were already done or that the target enrollment for term infants had already been met for that period (i.e., as noted above, the rate of preterm infant enrollment determined the rate of term infant enrollment using our adaptive enrollment method to ensure enrollment of infants from each GA over a similar time period).
Table 1.
Infant Characteristics
| Variable | Category | Early Preterm N = 13 |
Late Preterm N = 47 |
Term N = 30 |
|---|---|---|---|---|
| Gender N (%) | Male | 8 (61.5) | 22 (46.8) | 18 (60.0) |
| Female | 5 (38.5) | 25 (53.2) | 12 (40.0) | |
| Ethnicity N (%) | Non-Hispanic | 12 (92.3) | 42 (89.4) | 28 (93.3) |
| Unavailable/Unknown | 1 (7.7) | 4 (8.5) | 2 (6.7) | |
| Declined | 0 (0.0) | 1 (2.1) | 0 (0.0) | |
| Race N (%) | Caucasian/White | 10 (76.9) | 43 (91.5) | 28 (93.3) |
| Unavailable/Unknown | 1 (7.7) | 0 (0.0) | 0 (0.0) | |
| Native American | 2 (15.4) | 3 (6.4) | 2 (6.7) | |
| Native Hawaiian/Pacific Islander | 0 (0.0) | 1 (2.1) | 0 (0.0) | |
| Diabetic mother N (%) | Yes | 2 (15.4) | 3 (6.4) | 0 (0.0) |
| No | 11 (84.6) | 44 (93.6) | 30 (100.0) | |
| Type of Birth N (%) | Singleton | 11 (84.6) | 23 (48.9) | 30 (100.0) |
| Twins | 2 (15.4) | 18 (38.3) | 0 (0.0) | |
| Triplets | 0 (0.0) | 6 (12.8) | 0 (0.0) | |
| Birth Length (%) Mean (SD) | 34.25* (26.43) | 35.83* (26.89) | 60.87** (22.68) | |
| Birth Weight (%) Mean (SD) | 55.31* (26.70) | 42.60* (22.11) | 52.03** (16.66) | |
| Maternal Age Mean years (SD) | 27.00 (4.93) | 30.60 (5.51) | 26.79 (5.56) | |
| GA Range | 25–28 | 29–33 | 38–40 |
Percentile on Fenton Growth Curve for premature infants;
Percentile on WHO Growth Chart for term infants
3.2 Blood Fatty Acid Status
The average day of life at baseline collection was 6.3 days for the preterm infants and 2.9 days for term infants. Of the 30 term and 60 preterm infants enrolled, one preterm blood sample was damaged and could not be used, and one term infant was enrolled but discharged prior to sample collection. This left 29 term and 59 preterm participants with useable whole blood FA analyses.
When early and late preterm infants were compared with term infants, ARA levels were 27% and 12% lower when expressed as composition (percent of whole blood FAs) and were 27% and 15% lower when expressed as concentrations, respectively (Table 2). A similar pattern was found for DHA, where levels were 46% and 29% lower when expressed as a percent, and were 48% and 31% lower when expressed as concentrations. Therefore, whether expressed as composition or concentration, the gradients in FA levels were similar across the GA spectrum, with levels of both ARA and DHA being the lowest in the early preterms, intermediate in the late preterms and highest in the term infants. Interestingly, despite the infusion of intravenous lipid emulsion, circulating total FA concentrations did not vary across the GA spectrum. For the early-preterm, late-preterm and term infants the mean (SD) FA concentrations in ug/mL were 994 (420), 931 (222) and 952 (241), respectively). This may be why compositions and concentrations of individual FAs were similar.
Table 2.
Blood fatty acid levels (percent composition and concentration in early and late pre-term and term infants.
| Fatty Acids Expressed as Percent of Total Blood Fatty Acids | Percent Differences vs Term | Fatty Acids Expressed as a Concentration in Whole Blood (μg/mL) | Percent Differences vs Term | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Fatty Acid | Early Pre-term (n = 13) | Late Pre-term (n = 46) | Term (n=29) | p-value* | Early | Late | Early Pre-term (n = 13) | Late Pre-term (n = 46) | Term (n=29) | p-value* | Early | Late |
| C22:6n3 (DHA) | 2.66% (0.64) | 3.53% (0.54) | 4.97% (1.08) | <0.0001 | −46% | −29% | 24.99 (5.87) | 32.73 (7.41) | 47.66 (15.97) | <0.001 | −48% | −31% |
| C20:4n6 (ARA) | 12.19% (2.21) | 14.52% (1.97) | 16.59% (1.69) | <0.0001 | −27% | −12% | 115.73 (25.43) | 134.41 (28.26) | 158.75 (37.87) | <0.001 | −27% | −15% |
|
| ||||||||||||
| Other Fatty Acids | ||||||||||||
|
| ||||||||||||
| C14:0 | 0.39% (0.15) | 0.56% (0.45) | 0.66% (0.19) | <0.0001 | −41% | −15% | 3.76 (1.5) | 5.42 (5.84) | 6.28 (2.22) | <0.001 | −40% | −14% |
| C16:0 | 22.2% (1.70) | 23.78% (0.97) | 26.2% (1.44) | <0.0001 | −15% | −9% | 216.95 (68.52) | 221.11 (42.80) | 249.23 (53.08) | 0.003 | −13% | −11% |
| C16:1n7t | 0.07% (0.05) | 0.07% (0.04) | 0.08% (0.05) | 0.69 | −13% | −13% | 0.61 (0.29) | 0.68 (0.43) | 0.76 (0.39) | 0.46 | −20% | −11% |
| C16:1n7 | 0.43% (0.33) | 0.51% (0.16) | 1.25% (0.46) | <0.0001 | −66% | −59% | 4.13 (2.76) | 4.77 (2.02) | 11.81 (4.75) | <0.001 | −65% | −60% |
| C18:0 | 13.28% (1.70) | 14.44% (1.18) | 15.1% (0.69) | 0.0006 | −12% | −4% | 127.96 (32.458) | 133.92 (26.12) | 143.95 (30.75) | 0.05 | −11% | −7% |
| C18:1t | 0.25% (0.05) | 0.36% (0.18) | 0.33% (0.13) | 0.04 | −24% | 9% | 2.52 (1.06) | 3.42 (1.88) | 3.13 (1.28) | 0.2 | −19% | 9% |
| C18:1n9 | 17.88% (1.80) | 16.16% (1.29) | 15.83% (1.36) | 0.002 | 13% | 2% | 182.04 (88.98) | 151.06 (34.5) | 150.69 (33.03) | 0.42 | 21% | 0% |
| C18:2n6t | 0.35% (0.09) | 0.36% (0.10) | 0.38% (0.12) | 0.56 | −8% | −5% | 3.32 (1.09) | 3.37 (1.14) | 3.59 (1.97) | 0.56 | −8% | −6% |
| C18:2n6 | 21.66% (5.16) | 16.6% (3.08) | 8.42% (2.72) | <0.0001 | 157% | 97% | 229.37 (159.41) | 155.65 (45.22) | 79.67 (30.2) | <0.001 | 188% | 95% |
| C20:0 | 0.28% (0.05) | 0.28% (0.04) | 0.25% (0.05) | 0.0004 | 12% | 12% | 2.68 (0.67) | 2.55 (0.47) | 2.29 (0.41) | 0.03 | 17% | 11% |
| C18:3n6 | 0.16% (0.07) | 0.13% (0.04) | 0.08% (0.03) | <0.0001 | 100% | 63% | 1.61 (0.79) | 1.25 (0.48) | 0.8 (0.29) | <0.001 | 101% | 56% |
| C20:1n9 | 0.21% (0.03) | 0.22% (0.06) | 0.2% (0.07) | 0.12 | 5% | 10% | 2.07 (0.84) | 2.06 (0.60) | 1.89 (0.71) | 0.4 | 10% | 9% |
| C18:3n3 | 1.07% (0.49) | 0.53% (0.33) | 0.13% (0.08) | <0.0001 | 723% | 308% | 11.53 (9.63) | 5.04 (3.67) | 1.16 (0.80) | <0.001 | 894% | 334% |
| C20:2n6 | 0.39% (0.08) | 0.39% (0.11) | 0.23% (0.07) | <0.0001 | 70% | 70% | 3.76 (1.11) | 3.65 (1.12) | 2.13 (0.68) | <0.001 | 77% | 71% |
| C22:0 | 0.54% (0.10) | 0.51% 0.12) | 0.46% (0.13) | 0.02 | 17% | 11% | 5.11 (1.05) | 4.68 (0.93) | 4.18 (0.85) | 0.009 | 22% | 12% |
| C20:3n6 | 1.75% (0.56) | 2.1% (0.44) | 2.48% (0.46) | <0.0001 | −29% | −15% | 16.57 (5.83) | 19.43 (5.27) | 23.54 (6.30) | <0.001 | −30% | −17% |
| C24:0 | 0.37% (0.10) | 0.36% (0.09) | 0.37% (0.07) | 0.54 | 0% | −3% | 3.44 (0.69) | 3.32 (0.89) | 3.46 (0.74) | 0.54 | −1% | −4% |
| C20:5n3 | 0.35% (0.35) | 0.33% (0.35) | 0.35% (0.50) | 0.45 | 0% | −6% | 3.1 (3.04) | 2.99 (3.21) | 3.23 (4.63) | 0.33 | −4% | −7% |
| C24:1n9 | 0.28% (0.13) | 0.27% (0.08) | 0.24% (0.08) | 0.19 | 17% | 13% | 2.57 (.82) | 2.52 (0.79) | 2.27 (0.67) | 0.4 | 13% | 11% |
| C22:4n6 | 2.01% (0.42) | 2.54% (0.47) | 3.46% (0.52) | <0.0001 | −42% | −27% | 18.88 (4.11) | 23.33 (4.85) | 33.06 (8.34) | <0.001 | −43% | −29% |
| C22:5n6 | 0.73% (.20) | 0.96% (0.26) | 1.41% (0.33) | <0.0001 | −48% | −32% | 6.95 (2.26) | 8.82 (2.50) | 13.44 (3.92) | <0.001 | −48% | −34% |
| C22:5n3 | 0.5% (0.21) | 0.47%).13) | 0.51% (0.11) | 0.25 | −2% | −8% | 4.68 (1.77) | 4.36 (1.51) | 4.89 (1.57) | 0.33 | −4% | −11% |
These univariate trends between GA and ARA and DHA were also seen in the multivariable models using a natural logarithm transformation which included adjustments for maternal age, day of blood collection, and growth percentiles for weight and head circumference (Table 3). Indeed, DHA levels were 4.3% or 3.7% higher (composition or concentration) and ARA levels were 2.2% or 1.6% higher (composition or concentration) for each additional week of GA gained prior to birth. These relationships are illustrated in Figures 1 and 2.
Table 3.
Regression model estimates for DHA and ARA Concentration and Percent Composition
| DHA Blood Concentration (ug/mL) | ARA (ug/mL) | Blood | Concentration | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Parameter | Estimate | SE | p-value | Estimate | SE | p-value |
| Gestational Age | 0.03591 | 0.00739 | <.0001 | 0.01626 | 0.00621 | 0.0106 |
| Mother’s Age | −0.00444 | 0.00504 | 0.381 | −0.01359 | 0.00424 | 0.0019 |
| Weight % | 0.00097 | 0.00172 | 0.5739 | 0.00016 | 0.00144 | 0.9123 |
| H ead circumference % | 0.000282 | 0.00147 | 0.8485 | 3.9E-05 | 0.00124 | 0.9749 |
| Day blood collected | −0.01774 | 0.0146 | 0.228 | −0.00612 | 0.01228 | 0.6195 |
| DHA composition (wt:wt%) | ARA composition (wt:wt%) | |||||
| Parameter | Estimate | SE | p-value | Estimate | SE | p-value |
| Gestational Age | 0.0419 | 0.00527 | <.0001 | 0.02224 | 0.00382 | <.0001 |
| Mother’s Age | 0.00764 | 0.0036 | 0.037 | −0.00151 | 0.00261 | 0.5643 |
| Weight % | 0.00127 | 0.00123 | 0.3036 | 0.000459 | 0.000887 | 0.6061 |
| Head circumference % | 0.00151 | 0.00105 | 0.1544 | 0.00127 | 0.000759 | 0.0994 |
| Day blood collected | −0.01765 | 0.01043 | 0.0942 | −0.00604 | 0.00755 | 0.4261 |
Parameter estimates for data with a natural logarithm transformation
Figure 1.
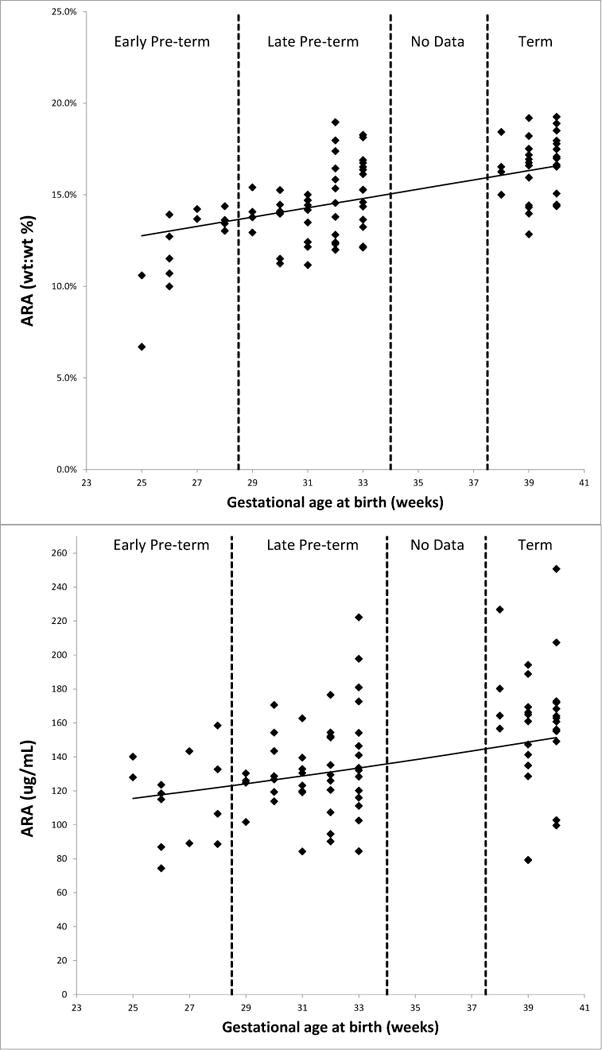
Relationships between gestational age and blood arachidonic acid (ARA) levels expressed as a composition or percent of total blood fatty acids (top) and as a concentration (bottom).
Figure 2.
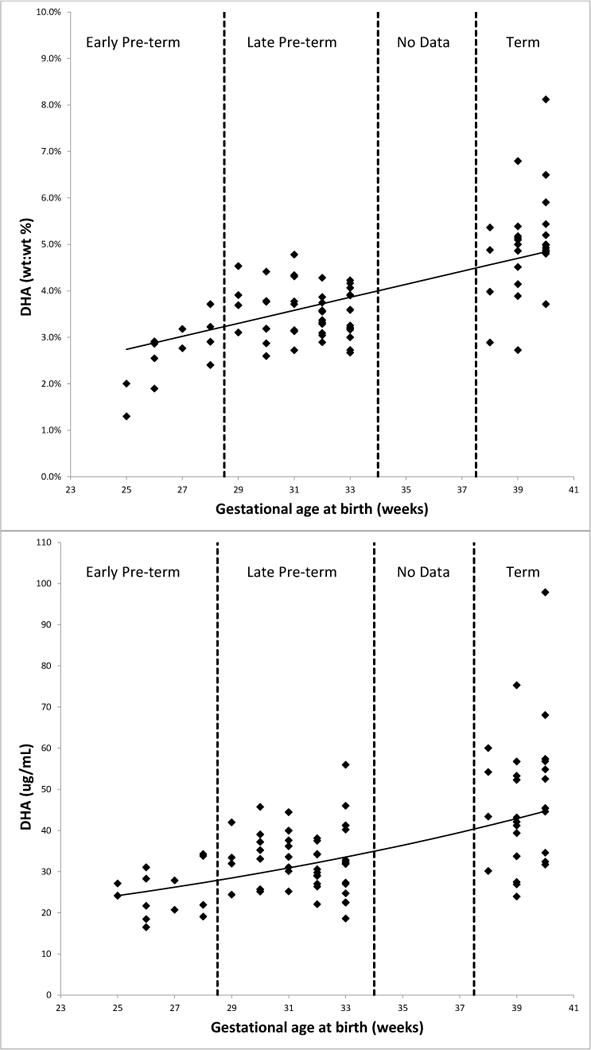
Relationships between gestational age and blood docosahexaenoic acid (DHA) levels expressed as a composition or percent of total blood fatty acids (top) and as a concentration (bottom).
Blood levels of the precursor essential FAs, LA (C18:2n6) and ALA (C18:3n3), varied markedly by GA (Table 2). Levels were much higher in early and late preterm infants compared with the term infants. LA concentrations were 188% and 95% higher, and ALA concentrations 894% and 334% higher, respectively. Similar patterns were also seen in percent composition. The elongation product of LA (C20:2n6) was elevated in the preterm infants compared with the term infants, but all of its desaturation/elongation metabolites (C20:3n6, ARA, C22:4n6, and C22:5n6) were depressed. This was observed for both percent composition and concentration.
4. Discussion and Conclusions
4.1 Discussion
In this cross sectional study comparing blood FA levels we observed lower ARA and DHA levels in preterm compared to term infants and demonstrated a direct correlation between these essential LCPUFAs and GA; thus very preterm infants have the highest risk of deficiency. Importantly, this was true whether expressed as a percent of total blood FAs (composition) or concentration which were feasibly measured using a dried blood spot methodology. Comparison of all FAs suggests that premature infants have impaired biosynthesis of LCPUFAs from precursor FAs compared to term infants and may require preformed provision of ARA and DHA.
Two previous studies have directly compared preterm to term LCPUFA status. First Agostoni, et al. reported reduced DHA, but not ARA, composition in late preterm (average of 35 weeks GA) vs. term (average of 38 weeks GA) infants who were studied at day 4 of life. [20] Infants who required intravenous lipid infusions (which could artificially alter FA status when reported as a percent of total blood FAs) were excluded from this study. DHA levels were 2.8% in preterm (mean 35 weeks GA) and 3.9% in term infants (mean 38 weeks GA; p<0.05). [20] Our findings, particularly those based on LCPUFA concentrations (which would be insensitive to dilution by intravenous lipids), support these findings from Italy. By design, Agostoni’s study [20] was limited to relatively healthy, older GA participants who did not require intravenous lipid emulsion and so conclusions about very premature infants could not be drawn.
Martin et al. included infants born at earlier GAs and found no difference in either ARA or DHA composition between premature (mean 27weeks) and term (>37 weeks) infants at birth. [10] However, by one week of age, DHA composition decreased 40% from 6.9% to 4.2% at the same time that LA levels increased from 7% to 19% of total FAs. [10] Because commercially available lipid emulsions contain precursor LA (50% of soy based emulsion) and ALA (9%) but do not contain preformed ARA or DHA, Martin’s findings could simply reflect a dilution of circulating DHA from other intravenously infused lipids or a decreased in vivo synthesis due to competition from ARA precursors (LA) through the same biosynthetic pathway.
Our present study sheds important light on these remaining questions by using two metrics to measure LCPUFA status: percent composition and concentration. The former has been the traditional means of expressing FA status, but in theory, composition may be markedly biased by artificial dilution with administration of intravenous lipid emulsions. Confirming lower LCPUFA concentrations (which would not be affected by the presence of infused precursor FAs) provides strong and independent confirmation that ARA and DHA levels are indeed lower in premature infants. Because concentrations of ARA were lower, it is also unlikely that competition (from infused LA) for the shared synthesis pathway is the cause of low DHA levels in premature infants.
Alternative explanations may include reasons that are unique to premature infants including very low adipose stores, an early catabolic energy state, increased tissue needs for rapid growth/development or decreased function of the shared enzymatic pathway of LCPUFA synthesis from precursor FAs. We demonstrated that total circulating FA concentrations were not higher in preterm compared to term infants. This suggests that premature infants rapidly clear intravenous lipids from circulation. This is consistent with clinical practice findings in our NICU where intravenous lipid infusions are not routinely advanced if serum triglyceride levels are significantly elevated (an uncommon event). This finding may also explain why the FA composition and concentration data were consistently associated. The observation that some or both of the immediate metabolic products of LA (elongation, C20:2n6 and Δ-6 desaturation, C18:2n6 and C18:3n6) are elevated in preterm babies, but all subsequent metabolites, (C20:3n6 through C22:5n6) are lower suggests a “blockade” in the production of C20:3n6 (dihomo-gamma-linolenic acid) is present in premature infants (Figure 3). If so, then provision of both preformed ARA and DHA may be necessary for very premature infants.
Figure 3.
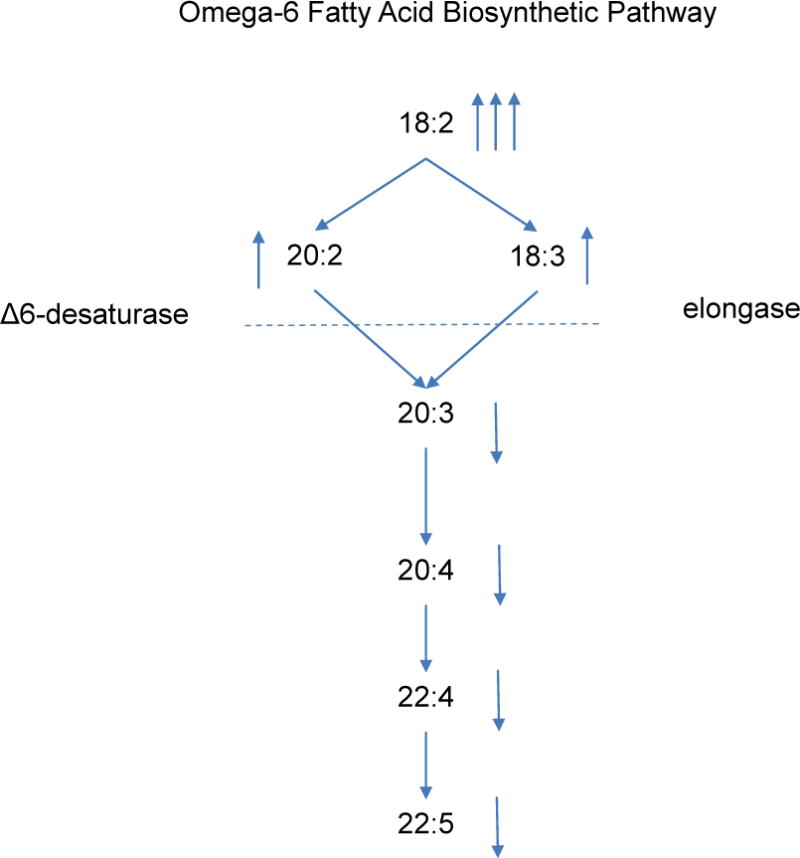
Apparent point of inhibition of omega-6 LCPUFA synthesis in premature infants (arrows indicate how FA levels in premature infants compare with those in term infants).
4.2 Strengths and Limitations
Significant strengths of this study include that it followed adaptive enrollment methods to assure that the study included infants in different gestational age groups over the same time period. The term reference group excluded both IDM and SGA neonates who reportedly have altered maternal-fetal LCPUFA transport. We used whole blood, which can be seen as a surrogate for all circulating FA fractions (plasma and cells), a validated dried blood spot method of collection for improved study logistics, and we reported FA status in both percent composition and concentration terms. In doing so, we demonstrated that FA profiles, both percent composition and concentration, can be obtained from one drop of scavenged blood, allowing the assessment of FA status in premature infants without additional painful procedures or blood collection. This is beneficial because neonates, especially very low birth weight infants, have a very limited blood volume with a higher risk of anemia, which can hinder serial blood sampling for research. Cord blood samples are generally easier to obtain without detrimental effects to the infant, but may not exclusively represent infant status. [34, 35]
Weaknesses include the lack of information on maternal dietary and supplementation habits. Clearly, higher maternal intake of DHA (whether from fish or supplements) can influence newborn blood DHA levels [36]. Also this was a single- center study that took place in the upper Midwest, where fish intake is typically minimal and mother’s milk DHA levels are very low [37]. This feature might actually be advantageous in exploring normal LCPUFA physiology relatively independent of diet.
Conclusion
This study sheds light on existing questions and advances the understanding of differences in essential FA status between preterm and term infants. We found that GA directly correlates with both ARA and DHA levels in the first week of life, so that infants born at the lowest GA are at the highest risk of deficiency. Additional findings suggest that due to impaired biosynthesis, premature infants have the need for preformed LCPUFAs rather than precursor essential FAs that are currently provided. Knowledge gained adds to a growing platform aimed at tailoring nutritional provision for the very premature infant with the hopes of improving both short- and long-term outcomes.
Summary.
Long chain polyunsaturated fatty acids (LCPUFA) including docosahexaenoic acid (DHA) and arachidonic acid (ARA) are increasingly transferred from mother to fetus late in pregnancy to support rapid growth. Infants born before this transfer is complete are at risk for deficiency. This study determines the relationship between gestational age (GA) and LCPUFA status to better understand the unique needs of premature infants. Whole blood was collected within the first week of life from 60 preterm (<34 weeks GA) and 30 term infants (>38 weeks GA). Both fatty acid (FA) composition and concentration were analyzed and compared, and the association between GA and LCPUFA status was examined. Preterm infants had significantly lower DHA and ARA levels than term peers and levels directly correlated with GA (p<0.0001). This study confirms that those babies born the earliest are at the greatest risk of LCPUFA deficiency.
Highlights.
Dried blood spot methodologies can facilitate the assessment of FA status in premature infants.
DHA and ARA levels directly correlate with GA; very preterm infants have a higher risk of deficiency.
Measuring FA composition and concentration expands the understanding of GA-related differences.
Impaired LCPUFA synthesis suggests that preformed provision is necessary for premature infants.
Acknowledgments
The study team gratefully acknowledges the many people who have made this study possible: Von “Mimi” Chau, Sanford Clinical Research Division (Dawn Hansen, Jessica Howard, Carmen Sandman, Melissa Hemenway), the Sanford IRB, NICU staff nurses, pharmacy staff (Linda Oyen), neonatologists, and especially our NICU babies and their families—it is such a privilege to care for you.
Source of Funding
Funding for this study was provided by a Sanford Health Seed Grant, a Gerber Foundation Pediatric Nutrition Grant (PN12-005-1372-3069), and Sanford Research. Dr. Baack also receives support from the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health (K08HD078504) and an NIH Center of Biomedical Research Excellence (COBRE) grant (P20 GM103620-01A1). Further information about this trial and ongoing efforts may be found at ClinicalTrials.gov (NCT01908907).
Abbreviations
- LCPUFA
long chain polyunsaturated fatty acids
- GA
gestational age
- FA
fatty acid
- DHA
docosahexaenoic acid
- ARA
arachidonic acid
- LA
linoleic acid
- ALA
α-linolenic acid
- SGA
small for gestational age
- WHO
World Health Organization
- FGC
Fenton Growth Curve
- NICU
neonatal intensive care unit
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
Dr. William S. Harris who serves as a senior mentor to Dr. Baack, is the Founder and President of OmegaQuant, LLC, but has no foreseen financial gain from publication of this work. Additional authors declare no conflict of interest.
References
- 1.Harris WS, Baack ML. Beyond building better brains: bridging the docosahexaenoic acid (DHA) gap of prematurity. Journal of perinatology : official journal of the California Perinatal Association. 2015;35:1–7. doi: 10.1038/jp.2014.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martin CR. Fatty Acid Requirements in Preterm Infants and Their Role in Health and Disease. Clinics in perinatology. 2014;41:363–82. doi: 10.1016/j.clp.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 3.O’Connor DL, Hall R, Adamkin D, Auestad N, Castillo M, Connor WE, et al. Growth and development in preterm infants fed long-chain polyunsaturated fatty acids: a prospective, randomized controlled trial. Pediatrics. 2001;108:359–71. doi: 10.1542/peds.108.2.359. [DOI] [PubMed] [Google Scholar]
- 4.Carlson SE, Werkman SH, Rhodes PG, Tolley EA. Visual-acuity development in healthy preterm infants: effect of marine-oil supplementation. The American journal of clinical nutrition. 1993;58:35–42. doi: 10.1093/ajcn/58.1.35. [DOI] [PubMed] [Google Scholar]
- 5.Birch EE, Birch DG, Hoffman DR, Uauy R. Dietary essential fatty acid supply and visual acuity development. Investigative ophthalmology & visual science. 1992;33:3242–53. [PubMed] [Google Scholar]
- 6.Clandinin MT, Van Aerde JE, Merkel KL, Harris CL, Springer MA, Hansen JW, et al. Growth and development of preterm infants fed infant formulas containing docosahexaenoic acid and arachidonic acid. The Journal of pediatrics. 2005;146:461–8. doi: 10.1016/j.jpeds.2004.11.030. [DOI] [PubMed] [Google Scholar]
- 7.Makrides M, Gibson RA, McPhee AJ, Collins CT, Davis PG, Doyle LW, et al. Neurodevelopmental outcomes of preterm infants fed high-dose docosahexaenoic acid: a randomized controlled trial. JAMA : the journal of the American Medical Association. 2009;301:175–82. doi: 10.1001/jama.2008.945. [DOI] [PubMed] [Google Scholar]
- 8.Henriksen C, Haugholt K, Lindgren M, Aurvag AK, Ronnestad A, Gronn M, et al. Improved cognitive development among preterm infants attributable to early supplementation of human milk with docosahexaenoic acid and arachidonic acid. Pediatrics. 2008;121:1137–45. doi: 10.1542/peds.2007-1511. [DOI] [PubMed] [Google Scholar]
- 9.Westerberg AC, Schei R, Henriksen C, Smith L, Veierod MB, Drevon CA, et al. Attention among very low birth weight infants following early supplementation with docosahexaenoic and arachidonic acid. Acta paediatrica. 2011;100:47–52. doi: 10.1111/j.1651-2227.2010.01946.x. [DOI] [PubMed] [Google Scholar]
- 10.Martin CR, Dasilva DA, Cluette-Brown JE, Dimonda C, Hamill A, Bhutta AQ, et al. Decreased postnatal docosahexaenoic and arachidonic acid blood levels in premature infants are associated with neonatal morbidities. The Journal of pediatrics. 2011;159:743–9. e1–2. doi: 10.1016/j.jpeds.2011.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caplan MS, Jilling T. The role of polyunsaturated fatty acid supplementation in intestinal inflammation and neonatal necrotizing enterocolitis. Lipids. 2001;36:1053–7. doi: 10.1007/s11745-001-0816-3. [DOI] [PubMed] [Google Scholar]
- 12.Lu J, Jilling T, Li D, Caplan MS. Polyunsaturated fatty acid supplementation alters proinflammatory gene expression and reduces the incidence of necrotizing enterocolitis in a neonatal rat model. Pediatr Res. 2007;61:427–32. doi: 10.1203/pdr.0b013e3180332ca5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohtsuka Y, Okada K, Yamakawa Y, Ikuse T, Baba Y, Inage E, et al. omega-3 fatty acids attenuate mucosal inflammation in premature rat pups. Journal of pediatric surgery. 2011;46:489–95. doi: 10.1016/j.jpedsurg.2010.07.032. [DOI] [PubMed] [Google Scholar]
- 14.Carlson SE, Montalto MB, Ponder DL, Werkman SH, Korones SB. Lower incidence of necrotizing enterocolitis in infants fed a preterm formula with egg phospholipids. Pediatr Res. 1998;44:491–8. doi: 10.1203/00006450-199810000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Connor KM, SanGiovanni JP, Lofqvist C, Aderman CM, Chen J, Higuchi A, et al. Increased dietary intake of omega-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nature medicine. 2007;13:868–73. doi: 10.1038/nm1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pawlik D, Lauterbach R, Walczak M, Hurkala J, Sherman MP. Fish-Oil Fat Emulsion Supplementation Reduces the Risk of Retinopathy in Very Low Birth Weight Infants: A Prospective, Randomized Study. JPEN Journal of parenteral and enteral nutrition. 2013;38:711–6. doi: 10.1177/0148607113499373. [DOI] [PubMed] [Google Scholar]
- 17.Beken S, Dilli D, Fettah ND, Kabatas EU, Zenciroglu A, Okumus N. The influence of fish-oil lipid emulsions on retinopathy of prematurity in very low birth weight infants: a randomized controlled trial. Early human development. 2014;90:27–31. doi: 10.1016/j.earlhumdev.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 18.Clandinin MT. Brain development and assessing the supply of polyunsaturated fatty acid. Lipids. 1999;34:131–7. doi: 10.1007/s11745-999-0347-y. [DOI] [PubMed] [Google Scholar]
- 19.Kuipers RS, Luxwolda MF, Offringa PJ, Boersma ER, Dijck-Brouwer DA, Muskiet FA. Fetal intrauterine whole body linoleic, arachidonic and docosahexaenoic acid contents and accretion rates. Prostaglandins, leukotrienes, and essential fatty acids. 2012;86:13–20. doi: 10.1016/j.plefa.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 20.Agostoni C, Marangoni F, Stival G, Gatelli I, Pinto F, Rise P, et al. Whole blood fatty acid composition differs in term versus mildly preterm infants: small versus matched appropriate for gestational age. Pediatr Res. 2008;64:298–302. doi: 10.1203/PDR.0b013e31817d9c23. [DOI] [PubMed] [Google Scholar]
- 21.Sabel KG, Lundqvist-Persson C, Bona E, Petzold M, Strandvik B. Fatty acid patterns early after premature birth, simultaneously analysed in mothers’ food, breast milk and serum phospholipids of mothers and infants. Lipids in health and disease. 2009;8:20. doi: 10.1186/1476-511X-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uauy R, Mena P, Wegher B, Nieto S, Salem N., Jr Long chain polyunsaturated fatty acid formation in neonates: effect of gestational age and intrauterine growth. Pediatr Res. 2000;47:127–35. doi: 10.1203/00006450-200001000-00022. [DOI] [PubMed] [Google Scholar]
- 23.Lapillonne A, J C. Reevaluation of the DHA requirement for the premature infant. Prostaglandins Leukot Essent Fatty Acids. 2009:143–50. doi: 10.1016/j.plefa.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 24.Lapillonne A, Picaud JC, Chirouze V, Goudable J, Reygrobellet B, Claris O, et al. The use of low-EPA fish oil for long-chain polyunsaturated fatty acid supplementation of preterm infants. Pediatr Res. 2000;48:835–41. doi: 10.1203/00006450-200012000-00022. [DOI] [PubMed] [Google Scholar]
- 25.Lapillonne A, Brossard N, Claris O, Reygrobellet B, Salle BL. Erythrocyte fatty acid composition in term infants fed human milk or a formula enriched with a low eicosapentanoic acid fish oil for 4 months. European journal of pediatrics. 2000;159:49–53. doi: 10.1007/s004310050009. [DOI] [PubMed] [Google Scholar]
- 26.Georgieff MK, Innis SM. Controversial nutrients that potentially affect preterm neurodevelopment: essential fatty acids and iron. Pediatr Res. 2005;57:99R–103R. doi: 10.1203/01.PDR.0000160542.69840.0F. [DOI] [PubMed] [Google Scholar]
- 27.Clandinin MT, Chappell JE, Heim T, Swyer PR, Chance GW. Fatty acid utilization in perinatal de novo synthesis of tissues. Early human development. 1981;5:355–66. doi: 10.1016/0378-3782(81)90016-5. [DOI] [PubMed] [Google Scholar]
- 28.Cetin I, Giovannini N, Alvino G, Agostoni C, Riva E, Giovannini M, et al. Intrauterine growth restriction is associated with changes in polyunsaturated fatty acid fetal-maternal relationships. Pediatr Res. 2002;52:750–5. doi: 10.1203/00006450-200211000-00023. [DOI] [PubMed] [Google Scholar]
- 29.Llanos A, Lin Y, Mena P, Salem N, Jr, Uauy R. Infants with intrauterine growth restriction have impaired formation of docosahexaenoic acid in early neonatal life: a stable isotope study. Pediatr Res. 2005;58:735–40. doi: 10.1203/01.PDR.0000180542.68526.A2. [DOI] [PubMed] [Google Scholar]
- 30.Thomas BA, Ghebremeskel K, Lowy C, Offley-Shore B, Crawford MA. Plasma fatty acids of neonates born to mothers with and without gestational diabetes. Prostaglandins, leukotrienes, and essential fatty acids. 2005;72:335–41. doi: 10.1016/j.plefa.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 31.Araujo JR, Correia-Branco A, Ramalho C, Keating E, Martel F. Gestational diabetes mellitus decreases placental uptake of long-chain polyunsaturated fatty acids: involvement of long-chain acyl-CoA synthetase. The Journal of nutritional biochemistry. 2013;24:1741–50. doi: 10.1016/j.jnutbio.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 32.Sarter B, Kelsey KS, Schwartz TA, Harris WS. Blood docosahexaenoic acid and eicosapentaenoic acid in vegans: Associations with age and gender and effects of an algal-derived omega-3 fatty acid supplement. Clinical nutrition (Edinburgh Scotland) 2014 doi: 10.1016/j.clnu.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 33.Johnston DT, Deuster PA, Harris WS, Macrae H, Dretsch MN. Red blood cell omega-3 fatty acid levels and neurocognitive performance in deployed U.S. Servicemembers. Nutritional neuroscience. 2013;16:30–8. doi: 10.1179/1476830512Y.0000000025. [DOI] [PubMed] [Google Scholar]
- 34.Hall JM, Lingenfelter P, Adams SL, Lasser D, Hansen JA, Bean MA. Detection of maternal cells in human umbilical cord blood using fluorescence in situ hybridization. Blood. 1995;86:2829–32. [PubMed] [Google Scholar]
- 35.Agostoni C, Galli C, Riva E, Rise P, Colombo C, Giovannini M, et al. Whole blood fatty acid composition at birth: from the maternal compartment to the infant. Clin Nutr. 2011;30:503–5. doi: 10.1016/j.clnu.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 36.Escolano-Margarit MV, Campoy C, Ramirez-Tortosa MC, Demmelmair H, Miranda MT, Gil A, et al. Effects of fish oil supplementation on the fatty acid profile in erythrocyte membrane and plasma phospholipids of pregnant women and their offspring: a randomised controlled trial. The British journal of nutrition. 2013;109:1647–56. doi: 10.1017/S0007114512003716. [DOI] [PubMed] [Google Scholar]
- 37.Baack ML, Norris AW, Yao J, Colaizy T. Long-chain polyunsaturated fatty acid levels in US donor human milk: meeting the needs of premature infants? Journal of perinatology : official journal of the California Perinatal Association. 2012;32:598–603. doi: 10.1038/jp.2011.152. [DOI] [PMC free article] [PubMed] [Google Scholar]


