Abstract
We sought to assess the performance of existing bleeding risk scores, such as HAS-BLED or OBRI, in patients with heart failure with reduced ejection fraction (HFrEF) in sinus rhythm (SR) treated with warfarin or aspirin. We calculated HAS-BLED and OBRI risk scores for 2,305 patients with HFrEF in SR enrolled in the Warfarin versus Aspirin in Reduced Cardiac Ejection Fraction (WARCEF) trial. Proportional hazards models were used to test whether each score predicted major bleeding, and comparison of different risk scores was performed using Harell’s c-statistic and net-reclassification improvement (NRI) index. For the warfarin arm, both scores predicted bleeding risk, with OBRI having significantly higher c-statistic (0.72 vs 0.61; p=0.03) compared to HAS-BLED, though the NRI for comparing OBRI to HAS-BLED was not significant (0.32, 95% CI - 0.18-0.37). Performance of the OBRI and HAS-BLED risk scores were similar for the aspirin arm. For participants with OBRI score of 0 to 1, warfarin compared with aspirin reduced ischemic stroke (HR 0.51, 95% CI 0.26-0.98, p=0.042) without significantly increasing major bleeding (HR 1.24, 95% CI 0.66-2.30, p=0.51). For those with OBRI score of ≥2, there was a trend for reduced ischemic stroke with warfarin compared to aspirin (HR 0.56, 95% CI 0.27-1.15, p=0.12), but major bleeding was increased (HR 4.04, 95% CI 1.99-8.22, p<0.001). In conclusion, existing bleeding risk scores can identify bleeding risk in HFrEF patients in SR, and could be tested for potentially identifying patients with a favorable risk / benefit profile for antithrombotic therapy with warfarin.
Keywords: Heart failure, sinus rhythm, warfarin, aspirin, bleeding
Introduction
Patients with heart failure with reduced ejection fraction (HFrEF) may be at increased risk for ischemic strokes due to left ventricular thrombus formation and subsequent embolism.1,2 Randomized clinical trials, such as the Warfarin and Antiplatelet Therapy in Chronic Heart Failure (WATCH) trial3 and the Warfarin versus Aspirin in Reduced Cardiac Ejection Fraction (WARCEF) trial,4 assessed whether warfarin therapy may benefit HFrEF patients who are in sinus rhythm (SR) and have no other indications for anticoagulation. Although warfarin therapy was found to reduce the risk of ischemic stroke in these trials, it also led to increased rates of major bleeding.3,4 A better characterization of bleeding risk in HFrEF patients who are in SR may therefore identify a subgroup of these patients who could benefit from anticoagulation. One potential approach is to consider existing bleeding risk scores, such as the HAS-BLED score and the Outpatient Bleeding Risk Index (OBRI), which has been used to predict risk of bleeding in patients receiving anticoagulation.5-9 The HAS-BLED score, in particular, has demonstrated superior performance compared to other bleeding risk scores in patients with atrial fibrillation,6,10 while the OBRI has been noted to be simple to calculate and has been validated in outpatients receiving warfarin therapy for any indication.7,11 However, it is unknown whether these risk scores can also apply to HFrEF patients who are in SR treated with warfarin or aspirin. We therefore undertook the present analysis of the patients enrolled in the WARCEF trial, to determine whether HAS-BLED and OBRI scores predicted bleeding risk in patients with HFrEF who are in SR, and to assess whether the effects of warfarin compared with aspirin varied depending on baseline risk of bleeding in this patient population.
Methods
The protocol for the randomized, double blinded WARCEF trial (http://www.ClinicalTrials.gov No. NCT00041938) has been described previously.4,12 Briefly, patients with left ventricular ejection fraction (LVEF) ≤35% and who were in SR were randomized to receive warfarin (target INR 2.75, with acceptable target range of 2.0 to 3.5) or aspirin (325mg daily). Additional eligibility criteria included being 18 years or older, having no contraindications to warfarin therapy, having a modified Rankin score of 4 or less (on a scale of 0 to 6, with higher scores indicating more severe disability), and treatment with a beta-blocker, an angiotensin-converting-enzyme (ACE) inhibitor or angiotensin-receptor blocker (ARB), or hydralazine and nitrates. Patients were excluded if they had a clear indication for warfarin or aspirin, or if they had a condition that conferred a high risk of cardiac embolism, such as atrial fibrillation, a mechanical cardiac valve, endocarditis, or an intracardiac mobile or pedunculated thrombus. Patients were also excluded if they were unable to follow an outpatient study protocol, or if they were unable to provide informed consent. Patients in any NYHA functional class were eligible, although patients in NYHA class I could account for no more than 20% of the total sample. A total of 2,305 participants were recruited from 168 centers in 11 countries from October 2002 to January 2010. The investigation conforms with the principles outlined in the Declaration of Helsinki, and the Institutional Review Boards at the coordinating centers for all sites approved the study. All subjects provided informed consent. The maximum follow-up time was 6 years, and the minimum was 1 year.
Major bleeding was defined as intracerebral, epidural, subdural, subarachnoid, spinal intramedullary, or retinal hemorrhage; any other bleeding causing a decline in the hemoglobin level of more than 2 g per deciliter in 48 hours; or bleeding requiring transfusion of 2 or more units of whole blood, hospitalization, or surgical intervention. This definition corresponds closely to the definition for major bleeding recommended by the Internal Society on Thrombosis and Haemostasis.13 Ischemic stroke was defined as a clinically relevant new lesion detected on computed tomography or magnetic resonance imaging (MRI) or, in the absence of a new lesion, clinical findings that were consistent with the occurrence of clinical stroke and that lasted for longer than 24 hours. An independent end-point adjudication committee, whose members were unaware of the treatment assignments, adjudicated all bleeding and stroke events.
Demographic characteristics including age, sex, race/ethnicity, and body mass index (BMI) were determined at the initial study visit. Health behaviors were assessed by self-report at time of enrollment, including smoking status and alcohol consumption. Clinical characteristics that were collected include medical comorbidities (atrial fibrillation, hypertension, diabetes mellitus, history of myocardial infarction, and history of ischemic heart disease), history of stroke or transient ischemic attacks (TIAs), presence of liver impairment (defined as having an aspartate aminotransferase [AST] level of 114 IU/L, which represents three times the upper limit of normal), renal impairment (defined as having a creatinine clearance level of <30 ml/min, estimated through the Cockcroft-Gault equation), anemia (defined as having a hematocrit of <30%), left ventricular ejection fraction (measured by quantitative echocardiography or radionuclide or contrast ventriculography), New York Heart Association (NYHA) functional class, and time-in-therapeutic range (calculated as the percentage of days when the INR is between 2.0 to 3.5). Additional details for these measures are as described previously.4
For each patient, the HAS-BLED risk score was calculated based on the approach described by Lip and colleagues,6 with modifications as described below. Because the HAS-BLED risk score was derived in patients on anticoagulation therapy, and because all patients in the WARCEF trial received either aspirin or warfarin but not both concurrently, the item for aspirin use in the HAS-BLED score was coded as 0 for all patients. Furthermore, for patients assigned to aspirin, the item for labile INR in the HAS-BLED score was coded as 0. For liver impairment, based on available data in WARCEF, we used a cut-off for AST level of >114 IU/L. The OBRI score was similarly calculated for each patient using the approach described by Beyth and colleagues.7 A brief summary of how HAS-BLED and OBRI risk scores were calculated is provided in Table 1.
Table 1.
Calculation of the HAS-BLED and OBRI risk scores. All risk factors are scored as 1 point each unless otherwise noted. Because the HASBLED risk score was derived in patients on anticoagulation therapy, and because no patient in warfarin received both aspirin and warfarin concurrently, aspirin use in the HAS-BLED score was coded as 0 in this analysis. Similarly, for patients assigned to aspirin, the item for labile INR in the HAS-BLED score was coded as 0 for these patients. For abnormal liver function, based on available data in WARCEF, a cut-off for AST level of >114 IU/L was used.
| HAS-BLED Risk Score | OBRI Risk Score |
|---|---|
| Hypertension (SBP >160 mmHg) | Age >65 years |
| Abnormal renal/ liver function (1 point for each) | History of stroke |
| Stroke | History of GIB |
| Bleeding | Recent MI, HCT <30%, Cr >1.5 mg/dL, or diabetes mellitus |
| Labile INRs (TTR <60%) | |
| Elderly (age >65 years) | |
|
Drugs (antiplatelet agents / NSAIDs) or excess alcohol (1 point for each) |
Abbreviations: SBP, systolic blood pressure; TTR, time in therapeutic range; NSAID, non-steroidal anti-inflammatory drugs; GIB, gastrointestinal tract bleeding; MI, myocardial infarction; HCT, hematocrit; Cr, creatinine.
Because bleeding risk is expected to differ for patients receiving warfarin and aspirin, we performed all analyses separately for the warfarin and aspirin arms of the WARCEF trial on an intent-to-treat (ITT) basis. For each arm, baseline demographic and clinical characteristics were compared between patients who had a major bleeding event during follow-up versus those who did not, using chi-squared tests for categorical variables and student’s t-tests for continuous variables. We further tabulated the proportion of participants with and without a major bleeding event by HAS-BLED and OBRI scores.
To assess whether HAS-BLED and OBRI scores were predictive of major bleeding, we constructed separate Cox proportional hazards models with the time to first major bleeding as the outcome and HAS-BLED or OBRI scores as ordinal predictor variables. We performed statistical testing for trend of increased risk of major bleeding associated with increased HAS-BLED or OBRI scores. We then calculated the c-statistics and the associated 95% confidence intervals (CI) for each proportional hazards model using the R software package “survcomp”. We also compared ability of HAS-BLED and OBRI scores to classify bleeding risk for each arm by calculating the net reclassification improvement (NRI) index and its associated 95% CIs, using the R software package “nricens” as per the methods described by Pencina and colleagues.14 Because the NRI is sensitive to the selection of cut-offs for defining risk categories,15 we used a two-category (i.e., high risk of bleeding versus low risk of bleeding) approach to calculate the NRI, based on the empirically observed rates of major bleeding across HAS-BLED and OBRI scores in the WARCEF trial.
We further constructed Cox proportional hazards models to assess the effect of warfarin versus aspirin on clinical outcomes, including death or ischemic stroke, ischemic stroke, and major bleeding, for subgroups of WARCEF participants defined by high versus low bleeding risk through HAS-BLED and OBRI scores. We also tested for interactions between treatment groups and bleeding risk for the above outcomes. P-values of <0.05 were considered statistically significant for all testing. All statistical analyses were conducted using SAS version 9.3 (SAS Institute, Cary, NC) or R version 3.0.1 (R Foundation for Statistical Computing, Vienna, Austria, 2013).
Results
The baseline characteristics of WARCEF participants by treatment group and bleeding risk are presented in Table 2. Of the 1,142 patients randomized to warfarin therapy, 66 (5.8%) experienced at least one major bleeding event. Those who experienced major bleeding were more likely to be 65 years or older, to be female, and to have renal impairment. For those randomized to aspirin, 31 (2.7%) of 1,163 patients had at least one major bleeding event, with those with major bleeding more likely to have lower BMI, to have had a prior stroke or TIA, to have renal impairment, and to have a previous history of alcohol use.
Table 2.
Characteristics of WARCEF participants by warfarin versus aspirin and by major bleeding status during follow-up.
| Variable | Warfarin | Aspirin | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Major bleeding (n=66) |
No major bleeding (n=1076) |
p-value | Major bleeding (n=31) |
No major bleeding (n=1132) |
p-value | |
| Age >65 yrs old | 36 (55%) | 358 (33%) | < 0.001 | 13 (42%) | 389 (34%) | 0.420 |
|
| ||||||
| Women | 21 (32%) | 215 (20%) | 0.021 | 8 (26%) | 216 (19%) | 0.349 |
|
| ||||||
| Non-Hispanic white | 45 (68%) | 814 (76%) | 0.182 | 24 (77%) | 855 (76%) | 0.866 |
| Non-Hispanic black | 9 (14%) | 157 (15%) | 5 (16%) | 161 (14%) | ||
| Hispanic | 9 (14%) | 76 (7%) | 1 (3%) | 80 (7%) | ||
| Other | 3 (6%) | 29 (3%) | 1 (3%) | 36 (3%) | ||
|
| ||||||
| Body mass index (kg/m2) | 0.228 | <0.001 | ||||
| < 25 | 22 (33%) | 279 (26%) | 16 (52%) | 263 (23%) | ||
| 25-30 | 26 (39%) | 400 (37%) | 4 (13%) | 452 (40%) | ||
| > 30 | 18 (27%) | 397 (37%) | 11 (35%) | 417 (37%) | ||
|
| ||||||
| Hypertension | 37 (59%) | 634 (61%) | 0.732 | 18 (62%) | 678 (62%) | 0.967 |
| Diabetes mellitus | 23 (35%) | 348 (32%) | 0.673 | 7 (23%) | 344 (30%) | 0.350 |
| Atrial fibrillation | 2 (3%) | 42 (4%) | 0.721 | 0 (0%) | 42 (4%) | 0.275 |
| Myocardial infarction | 33 (50%) | 516 (48%) | 0.747 | 12 (39%) | 551 (49%) | 0.273 |
| Ischemic heart disease | 33 (50%) | 455 (42%) | 0.219 | 9 (29%) | 494 (44%) | 0.105 |
|
| ||||||
| Prior stroke or TIA | 14 (21%) | 141 (13%) | 0.062 | 8 (26%) | 131 (12%) | 0.016 |
|
| ||||||
| Renal impairment | 4 (6%) | 22 (2%) | 0.033 | 2 (6%) | 16 (1%) | 0.026 |
|
| ||||||
| Liver impairment | 2 (3%) | 39 (4%) | 0.321 | 2 (6%) | 36 (3%) | 0.646 |
|
| ||||||
| Anemia, defined as hematocrit < 30% | 1 (2%) | 3 (0.3%) | 0.106 | 0 (0%) | 3 (0.3%) | 0.777 |
|
| ||||||
| Smoking status | 0.549 | 0.347 | ||||
| Current | 10 (15%) | 203 (19%) | 6 (19%) | 189 (17%) | ||
| Former | 38 (58%) | 547 (51%) | 19 (61%) | 585 (52%) | ||
| Never | 18 (27%) | 326 (30%) | 6 (19%) | 358 (32%) | ||
|
| ||||||
| Alcohol consumption | 0.240 | 0.025 | ||||
| Current, > 2oz/day | 17 (26%) | 262 (24%) | 6 (19%) | 287 (25%) | ||
| Previous, >2oz/day | 9 (14%) | 241 (22%) | 13 (42%) | 243 (21%) | ||
| Never | 40 (60%) | 573 (53%) | 12 (39%) | 602 (53%) | ||
|
| ||||||
| NYHA classification | 0.898 | 0.916 | ||||
| I | 8 (12%) | 146 (14%) | 5 (16%) | 163 (14%) | ||
| II | 35 (53%) | 596 (55%) | 18 (58%) | 643 (57%) | ||
| III | 22 (33%) | 324 (30%) | 8 (26%) | 311 (27%) | ||
| IV | 1 (2%) | 10 (1%) | 0 (0%) | 15 (1%) | ||
|
| ||||||
| LV ejection fraction (%) | 25.3±7.3 | 24.5±7.5 | 0.426 | 24.7±6.1 | 24.8±7.6 | 0.932 |
|
| ||||||
| TTR <60% | 33 (52%) | 465 (46%) | 0.349 | NA | NA | NA |
Renal impairment is defined as creatinine clearance of <30 ml/min; liver impairment is defined as AST level of >114 IU/L, which represents three times the upper limit of normal
Abbreviations: TIA, transient ischemic attack; NYHA, New York Heart Association; TTR, time in therapeutic range.
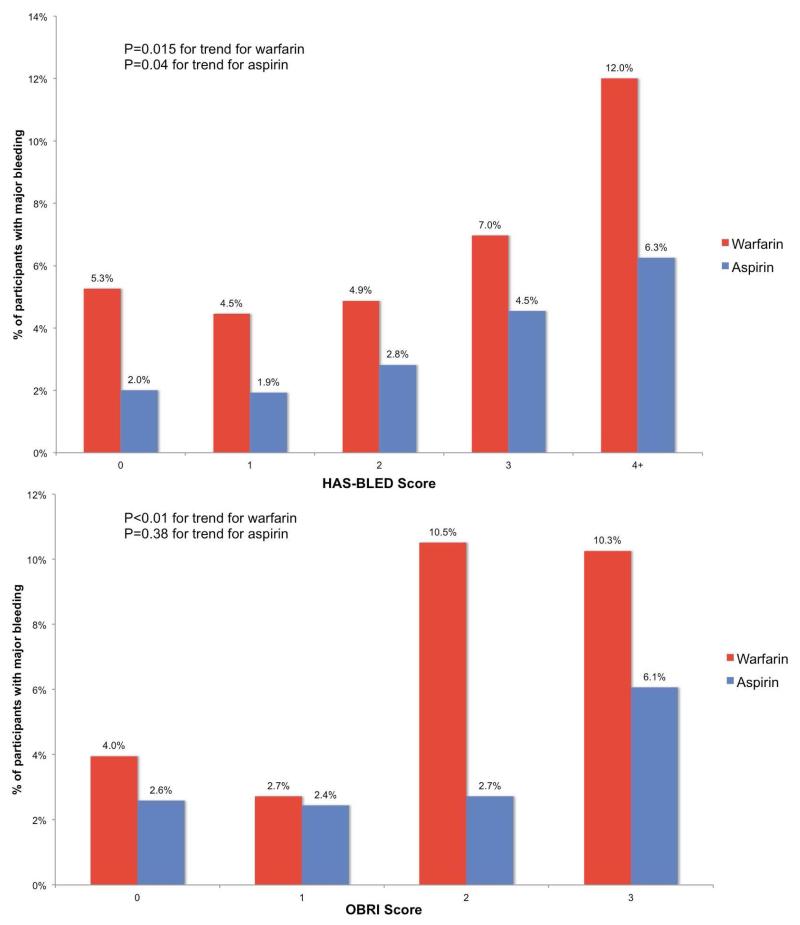
The distribution of HAS-BLED and OBRI scores for patients with and without major bleeding were calculated for each arm of the WARCEF trial (Table 3 and Figure 1). The proportion of patients who had any major bleeding on warfarin therapy was 5.3% for those with a HAS-BLED score of 0, increasing to 12.0% for those with a HAS-BLED score of 4 or above (p=0.015 for trend). For those randomized to aspirin, the proportion of patients who had any major bleeding was 2.0% for those with a HAS-BLED score of 0, increasing to 6.3% for those with a HAS-BLED score of 4 or above (p=0.04 for trend; Figure 1, top panel). Similarly, the proportion of patients who had any major bleeding on warfarin therapy was 4.0% for those with an OBRI score of 0, but was over 10% for those with an OBRI score of 2 or 3 (p=0.01 for trend; Figure 1, bottom panel). For patients randomized to aspirin, the proportion of those who had any major bleeding ranged from 2.6% for those with an OBRI score of 0 to 6.1% for those with an OBRI score of 3, but the increase was not statistically significant (p=0.38 for trend). Based on these results, for subsequent analyses, we defined a patient as having high risk of bleeding as having a score of 2 or above for OBRI and a score of 3 and above for HAS-BLED.
Table 3.
WARCEF participants by warfarin versus aspirin and by bleeding risk according to the HAS-BLED and OBRI scores. Numbers in parentheses represent column percents.
| Warfarin | Aspirin | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Risk scores | All patients (n=1142) |
Major bleeding (n=66) |
% with major bleeding |
All patients (n=1163) |
Major bleeding (n=31) |
% with major bleeding |
| HAS-BLED | ||||||
| 0 | 76 (7%) | 4 (6%) | 5.3% | 150 (13%) | 3 (10%) | 2.0% |
| 1 | 314 (28%) | 14 (21%) | 4.5% | 416 (36%) | 8 (26%) | 1.9% |
| 2 | 390 (34%) | 19 (29%) | 4.9% | 427 (37%) | 12 (39%) | 2.8% |
| 3 | 287 (25%) | 20 (30%) | 7.0% | 154 (13%) | 7 (23%) | 4.5% |
| 4 | 68 (6%) | 8 (12%) | 11.8% | 16 (1%) | 1 (3%) | 6.3% |
| 5 | 7 (1%) | 1 (2%) | 14.3% | 0 (0%) | 0 (0%) | 0 (0%) |
| Risk category | ||||||
| 0 to 2 | 780 (68%) | 37 (56%) | 4.7% | 993 (86%) | 23 (74%) | 2.3% |
| ≥3 | 362 (32%) | 29 (44%) | 8.0% | 170 (14%) | 8 (26%) | 4.7% |
|
| ||||||
| OBRI | ||||||
| 0 | 253 (22%) | 10 (15%) | 4.0% | 271 (23%) | 7 (23%) | 2.6% |
| 1 | 479 (42%) | 13 (20%) | 2.7% | 491 (42%) | 12 (39%) | 2.4% |
| 2 | 371 (32%) | 39 (59%) | 10.5% | 368 (32%) | 10 (32%) | 2.7% |
| 3 | 39 (3%) | 4 (6%) | 10.3% | 33 (3%) | 2 (6%) | 6.1% |
| Risk category | ||||||
| 0 to 1 | 732 (64%) | 23 (35%) | 3.1% | 762 (23%) | 19 (61%) | 2.5% |
| ≥2 | 410 (36%) | 43 (65%) | 10.5% | 403 (32%) | 12 (39%) | 3.0% |
Figure 1. Proportion of patients with major bleedings, by HAS-BLED and OBRI risk scores and by treatment arm.
P-values for trend were calculated using Cox proportional hazards models.
A comparison of the performance of HAS-BLED and OBRI scores for predicting time to the first major bleeding event is presented in Table 4. For the warfarin arm, the c-statistic for the OBRI score was 0.72 (95% CI, 0.62-0.81), which was significantly superior (p=0.003) to the c-statistic for the HAS-BLED score, although the NRI for comparing the OBRI to HAS-BLED was not significant (0.32, 95% CI −0.18-0.37). For patients randomized to aspirin, the c-statistics for HAS-BLED and OBRI scores were similar, and the NRI for the OBRI score compared to the HAS-BLED score was not significant.
Table 4.
Comparison of the HAS-BLED and OBRI risk scores for predicting major bleeding in WARCEF participants receiving warfarin or aspirin.
|
c-statistic for HAS-BLED |
c-statistic for OBRI |
p-value for difference in c-statistics |
NRI | |
|---|---|---|---|---|
| Warfarin | 0.61 (0.51-0.70) | 0.72 (0.62-0.81) | 0.03 | 0.32 (−0.18-0.37) |
| Aspirin | 0.63 (0.50-0.77) | 0.58 (0.43-0.71) | 0.50 | 0.13 (−0.19-0.15) |
Abbreviations: NRI, net reclassification index. For NRI, the directions of comparisons are for OBRI versus HAS-BLED.
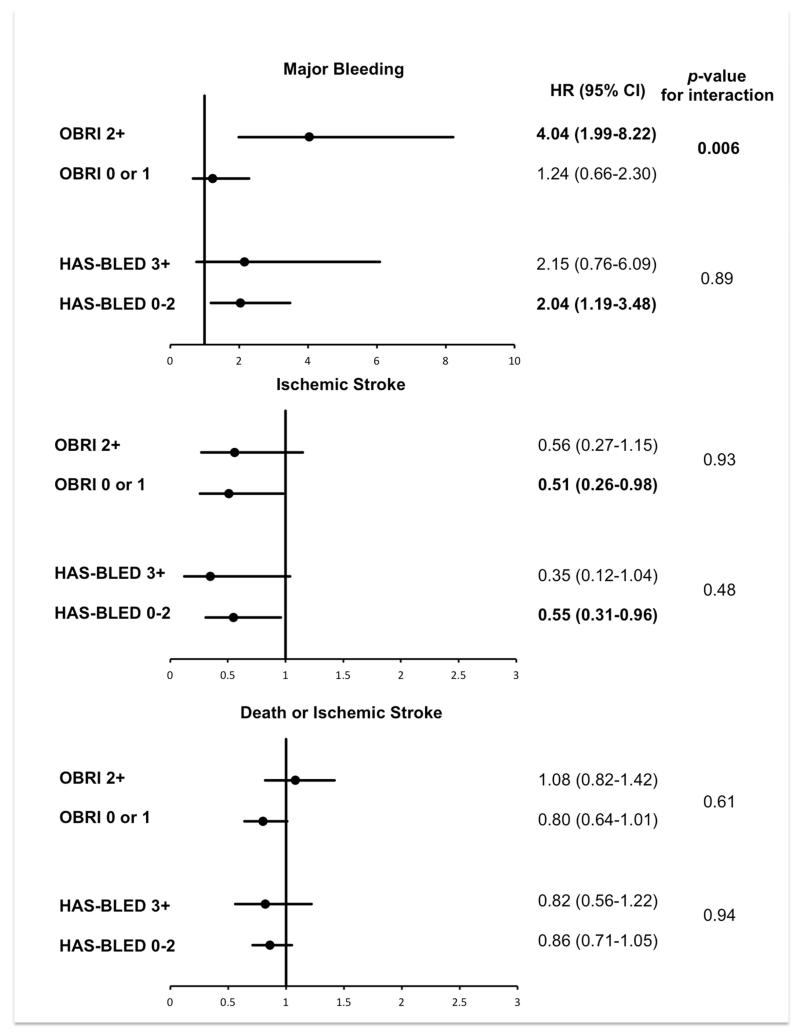
The treatment effect of warfarin versus aspirin on clinical outcomes and its interaction with major bleeding risk (categorized by OBRI and HAS-BLED scores) are presented in Figure 2. For the outcome of major bleeding, there was a significant interaction between the effect of warfarin versus aspirin and bleeding risk according to the OBRI score (p=0.006). Patients classified as high bleeding risk by an OBRI score of ≥2 had increased risk of major bleeding with warfarin compared with aspirin (hazard ratio [HR] 4.04, 95% CI 1.99-8.22, p<0.001), while bleeding risk was similar for warfarin versus aspirin in those classified as low bleeding risk by an OBRI score of 0 to 1 (HR 1.24, 95% CI 0.66-2.30; p=0.51). There was no significant interaction between warfarin versus aspirin and bleeding risk by the HAS-BLED score (p=0.89), though warfarin compared with aspirin significantly increased major bleeding in the subgroup with low bleeding risk identified a HAS-BLED score of 0 to 2 (HR 2.04, 95% CI 1.19-3.48; p=0.009).
Figure 2. The effect of warfarin versus aspirin on clinical outcomes for subgroups of WARCEF participants stratified by OBRI and HAS-BLED risk scores.
P-values represent results of tests of interaction between treatment assignment and bleeding risk subgroups identified by OBRI and HAS-BLED risk score.
For the outcome of ischemic stroke, there was no significant interaction between warfarin versus aspirin and bleeding risk defined by either bleeding risk scores (p=0.93 for OBRI and 0.48 for HAS-BLED). The effect of warfarin versus aspirin was similar across subgroups of bleeding risk, with warfarin significantly reducing ischemic strokes in patients classified as low bleeding risk by either score (Figure 2). For the composite outcome of death or ischemic stroke, the effect of warfarin versus aspirin was similar and non-significant across all bleeding risk subgroups, and there was no significant interaction between treatment assignment and bleeding risk subgroups identified with either score.
Discussion
In this retrospective analysis of patients enrolled in the WARCEF trial, we found that predictors of major bleeding differed between patients receiving warfarin and aspirin, suggesting that bleeding risk is affected by both anti-thrombotic choice and patient characteristics. Importantly, we confirmed that the HAS-BLED and OBRI bleeding risk scores can be used to predict major bleeding in HFrEF patients who are in SR. For patients assigned to warfarin, the OBRI score demonstrated superior discrimination to HAS-BLED, though the NRI for comparing the OBRI score to HAS-BLED was not significant, which may have been due to the relatively low number of bleeding events. For patients assigned to aspirin, the HAS-BLED risk score, but not OBRI, was predictive of major bleeding. Furthermore, we found that the increase in major bleeding for warfarin compared with aspirin varied by subgroups identified by the OBRI score, and that for WARCEF participants with an OBRI score of 0 or 1, warfarin reduced the risk of ischemic stroke but did not increase the risk of major bleeding.
Given the continued interest in identifying subgroups of heart failure patients that may benefit from anticoagulation,16 our analysis to assess bleeding risk in HFrEF patients who are in SR is a timely one. Previously published bleeding risk scores, such as the HAS-BLED and OBRI, were derived and validated in cohorts of patients with indications for anticoagulation such as atrial fibrillation or venous thromboembolism.6,7 Although the WARCEF trial excluded such patients, the performance of the HAS-BLED and OBRI scores are comparable to these earlier reports. For example, in an analysis of the Stroke Prevention Using an ORal Thrombin Inhibitor in Atrial Fibrillation (SPORTIF) III and V trials, Lip and colleagues reported that the c-statistics of previously reported bleeding risk scores, including the HAS-BLED and OBRI, ranged from 0.49 to 0.65 in a cohort of patients receiving warfarin or ximelagatran.6 Similarly, in another report by Burgess and colleagues, c-statistics for various published bleeding risk scores ranged from 0.61-0.74 for a real-world cohort of patients on warfarin therapy for diverse indications.17 The c-statistic of 0.72 for the OBRI risk score for WARCEF participants assigned to warfarin therapy is therefore near the upper range of what has been reported previously, and suggests that the OBRI score may be a useful prediction tool to characterize risk of major bleeding for this patient population.
We also assessed the effect of warfarin versus aspirin on clinical outcomes for subgroups of WARCEF patients stratified by predicted bleeding risk. We demonstrated that among patients having an OBRI risk score of 0 or 1, who accounted for more than half of all ischemic strokes observed during the WARCEF follow-up period, warfarin therapy reduced the risk of ischemic stroke compared with aspirin but did not significantly increase the risk of major bleeding. These results are broadly consistent with our previous subgroup analysis showing that younger patients (age <60 years) enrolled in the WARCEF trial had reduced risk of death, ischemic stroke, or intracranial hemorrhage with warfarin therapy compared to aspirin.18 In our current analysis, WARCEF participants with an OBRI risk score of 0 or 1 included most patients younger than 60 years, but also many patients who are older. Taken together, this subgroup consists of nearly two thirds of all patients enrolled in the WARCEF trial and accounts for more than half of all ischemic stroke events observed during the follow-up period. The findings from the present analysis therefore provide important insights for predicting the potential outcomes of antithrombotic therapies for a large number of patients with HFrEF who are in SR.
Our findings also raise the possibility of using bleeding risk scores to inform patient selection for subsequent trials of novel anticoagulants. Previous studies have often described a positive correlation between risk of stroke and risk of bleeding in patients who are candidates for oral anticoagulation therapy, as many risk factors, such as advanced age and hypertension, are shared by both of these adverse outcomes.19,20 This overlap is a major challenge for the use of bleeding risk scores as a part of a personalized approach for choosing anticoagulation strategy, as patients identified as having high risk of stroke also often have high risk of bleeding.21 Our results address this challenge by showing that predicted bleeding risk could play an important role in patient selection for consideration of antithrombotic therapy. Although warfarin compared with aspirin reduced ischemic strokes in WARCEF participants with both high and low predicted bleeding risk, the significant interaction between treatment assignment and OBRI risk categories for the outcome of major bleeding suggests that patients at low predicted bleeding risk may avoid increased bleeding with anticoagulation. Given the emergence of new non-vitamin K oral anticoagulants that have demonstrated favorable safety and efficacy profiles compared to warfarin,22 these findings may be useful for the design of future trials investigating the role of these agents for ischemic stroke prevention in HFrEF patients in SR.
There are a number of limitations to our study. Our retrospective analysis of the WARCEF trial had only a modest number of stroke and bleeding events, and our findings therefore are necessarily hypothesis generating and will require confirmation. Highlighting the challenges of applying existing risk scores to new populations and datasets, we made slight modifications to the HAS-BLED score to apply it to the WARCEF population. Our analysis of the performance of the HAS-BLED and OBRI bleeding risk scores will thus require further validation in independent cohorts of HFrEF patients who are in SR. However, while a new risk score derived from WARCEF data may better predict bleeding risk and estimate treatment effect sizes for this patient population, it is also likely that a well-performing, previously established risk score accepted by clinicians and practice guidelines will have broader impact.23 Finally, since the WARCEF trial only enrolled patients with HFrEF who are in SR, our findings may not be applicable to other subgroups of heart failure patients.
Relationships with Industry
Dr. Homma reports receiving payment from AGA Medical (now St. Jude Medical) for his work as a member of a data and safety monitoring board and consulting fees from Boehringer Ingelheim; Dr. Levin, receiving consulting fees from United Healthcare; Dr. Teerlink, receiving consulting fees/ grant support from Amgen, Corthera, Cytokinetics, NovaCardia/Merck and Novartis on behalf of himself and his institution; Dr. Graham, owning stock in March Pharmaceuticals, Medtronic, and Pfizer; Dr. Labovitz, receiving grant support from Boehringer Ingelheim on behalf of his institution, lecture fees from Boehringer Ingelheim, and fees for the development of educational presentations from the American College of Cardiology; Dr. Anker, receiving consulting fees from Amgen, Bosch Healthcare, GlaxoSmithKline, Helsinn, LoneStar Heart, Novartis, Professional Dietetics, PsiOxus, Relypsa, SHL Telemedicine, and Thermo Fisher, grant support from Vifor Pharma, and lecture fees from Novartis, holding patents with Brahms AG and Charité Berlin, and receiving royalties from Imperial College; Dr. Ponikowski, receiving consulting fees from Bayer, Boehringer Ingelheim, Coridea, Corthera, Johnson & Johnson, Pfizer, Respicardia, and Vifor Pharma, grant support from Vifor Pharma on behalf of himself and his institution, and lecture fees from Abbott, Boehringer Ingelheim, Merck Serono, Pfizer, Respicardia, Sanofi-Aventis, Servier, and Vifor Pharma; and Dr. Lip, receiving consulting fees from Astellas, AstraZeneca, Bayer, Biotronik, Boehringer Inhelheim, Bristol-Myers Squibb, Pfizer, Merck, Portola, and Sanofi-Aventis, speakers bureau fees from Bayer, Bristol-Myers Squibb, Pfizer, Boehringer Ingelheim, and Sanofi-Aventis, and payment for the development of educational presentations from Bayer, Boehringer Ingelheim, and Merck. No other potential conflict of interest relevant to this article was reported.
Supplementary Material
Acknowledgments
Funding:
The WARCEF trial was supported by grant #s U01-NS-043975 to S Homma, and U01-NS-039143 to JLP Thompson from the National Institute of Neurological Diseases and Stroke. Warfarin and warfarin placebo were provided by Taro Pharmaceuticals USA, and aspirin and aspirin placebo by Bayer HealthCare.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lip GY, Gibbs CR. Does heart failure confer a hypercoagulable state? Virchow’s triad revisited. J Am Coll Cardiol. 1999;33:1424–1426. doi: 10.1016/s0735-1097(99)00033-9. [DOI] [PubMed] [Google Scholar]
- 2.Kalaria VG, Passannante MR, Shah T, Modi K, Weisse AB. Effect of mitral regurgitation on left ventricular thrombus formation in dilated cardiomyopathy. Am Heart J. 1998;135:215–220. doi: 10.1016/s0002-8703(98)70084-5. [DOI] [PubMed] [Google Scholar]
- 3.Massie BM, Collins JF, Ammon SE, Armstrong PW, Cleland JGF, Ezekowitz M, Jafri SM, Krol WF, O’Connor CM, Schulman KA, Teo K, Warren SR, Investigators ftWT Randomized Trial of Warfarin, Aspirin, and Clopidogrel in Patients With Chronic Heart Failure: The Warfarin and Antiplatelet Therapy in Chronic Heart Failure (WATCH) Trial. Circulation. 2009;119:1616–1624. doi: 10.1161/CIRCULATIONAHA.108.801753. [DOI] [PubMed] [Google Scholar]
- 4.Homma S, Thompson JL, Pullicino PM, Levin B, Freudenberger RS, Teerlink JR, Ammon SE, Graham S, Sacco RL, Mann DL, Mohr JP, Massie BM, Labovitz AJ, Anker SD, Lok DJ, Ponikowski P, Estol CJ, Lip GY, Di Tullio MR, Sanford AR, Mejia V, Gabriel AP, del Valle ML, Buchsbaum R. Warfarin and aspirin in patients with heart failure and sinus rhythm. New Engl J Med. 2012;366:1859–1869. doi: 10.1056/NEJMoa1202299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lip GYH, Andreotti F, Fauchier L, Huber K, Hylek E, Knight E, Lane DA, Levi M, Marin F, Palareti G, Kirchhof P, reviewers D, Collet J-P, Rubboli A, Poli D, Camm J. Bleeding risk assessment and management in atrial fibrillation patients: a position document from the European Heart Rhythm Association, endorsed by the European Society of Cardiology Working Group on Thrombosis. Europace. 2011;13:723–746. doi: 10.1093/europace/eur126. [DOI] [PubMed] [Google Scholar]
- 6.Lip GYH, Frison L, Halperin JL, Lane DA. Comparative Validation of a Novel Risk Score for Predicting Bleeding Risk in Anticoagulated Patients With Atrial FibrillationThe HAS-BLED (Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly, Drugs/Alcohol Concomitantly) Score. J Am Coll Cardiol. 2011;57:173–180. doi: 10.1016/j.jacc.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 7.Beyth RJ, Quinn LM, Landefeld CS. Prospective evaluation of an index for predicting the risk of major bleeding in outpatients treated with warfarin. Am J Med. 1998;105:91–99. doi: 10.1016/s0002-9343(98)00198-3. [DOI] [PubMed] [Google Scholar]
- 8.Gage BF, Yan Y, Milligan PE, Waterman AD, Culverhouse R, Rich MW, Radford MJ. Clinical classification schemes for predicting hemorrhage: results from the National Registry of Atrial Fibrillation (NRAF) Am Heart J. 2006;151:713–719. doi: 10.1016/j.ahj.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 9.Fang MC, Go AS, Chang Y, Borowsky LH, Pomernacki NK, Udaltsova N, Singer DE. A New Risk Scheme to Predict Warfarin-Associated HemorrhageThe ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation) Study. J Am Coll Cardiol. 2011;58:395–401. doi: 10.1016/j.jacc.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Apostolakis S, Lane DA, Guo Y, Buller H, Lip GYH. Performance of the HEMORR2HAGES, ATRIA, and HAS-BLED Bleeding Risk–Prediction Scores in Patients With Atrial Fibrillation Undergoing AnticoagulationThe AMADEUS (Evaluating the Use of SR34006 Compared to Warfarin or Acenocoumarol in Patients With Atrial Fibrillation) Study. J Am Coll Cardiol. 2012;60:861–867. doi: 10.1016/j.jacc.2012.06.019. [DOI] [PubMed] [Google Scholar]
- 11.Aspinall SL, DeSanzo BE, Trilli LE, Good CB. Bleeding Risk Index in an anticoagulation clinic. Assessment by indication and implications for care. J Gen Intern Med. 2005;20:1008–1013. doi: 10.1111/j.1525-1497.2005.0229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pullicino P, Thompson JL, Barton B, Levin B, Graham S, Freudenberger RS. Warfarin versus aspirin in patients with reduced cardiac ejection fraction (WARCEF): rationale, objectives, and design. J Card Fail. 2006;12:39–46. doi: 10.1016/j.cardfail.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 13.Schulman S, Kearon C. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3:692–694. doi: 10.1111/j.1538-7836.2005.01204.x. [DOI] [PubMed] [Google Scholar]
- 14.Pencina MJ, D’Agostino RB, Sr., D’Agostino RB, Jr., Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172. doi: 10.1002/sim.2929. discussion 207-112. [DOI] [PubMed] [Google Scholar]
- 15.Pencina MJ, D’Agostino RB, Sr., Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30:11–21. doi: 10.1002/sim.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eikelboom JW, Connolly SJ. Warfarin in Heart Failure. New Engl J Med. 2012;366:1936–1938. doi: 10.1056/NEJMe1202504. [DOI] [PubMed] [Google Scholar]
- 17.Burgess S, Crown N, Louzada ML, Dresser G, Kim RB, Lazo-Langner A. Clinical performance of bleeding risk scores for predicting major and clinically relevant non-major bleeding events in patients receiving warfarin. J Thromb Haemost. 2013;11:1647–1654. doi: 10.1111/jth.12352. [DOI] [PubMed] [Google Scholar]
- 18.Homma S, Thompson JLP, Sanford AR, Mann DL, Sacco RL, Levin B, Pullicino PM, Freudenberger RS, Teerlink JR, Graham S, Mohr JP, Massie BM, Labovitz AJ, Di Tullio MR, Gabriel AP, Lip GYH, Estol CJ, Lok DJ, Ponikowski P, Anker SD. Benefit of Warfarin Compared With Aspirin in Patients With Heart Failure in Sinus Rhythm: A Subgroup Analysis of WARCEF, a Randomized Controlled Trial. Circ Heart Fail. 2013;6:988–997. doi: 10.1161/CIRCHEARTFAILURE.113.000372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roldán V, Marín F, Manzano-Fernández S, Gallego P, Vílchez JA, Valdés M, Vicente V, Lip GYH. The HAS-BLED Score Has Better Prediction Accuracy for Major Bleeding Than CHADS2 or CHA2DS2-VASc Scores in Anticoagulated Patients With Atrial Fibrillation. J Am Coll Cardiol. 2013;62:2199–2204. doi: 10.1016/j.jacc.2013.08.1623. [DOI] [PubMed] [Google Scholar]
- 20.Oldgren J, Alings M, Darius H, Diener H-C, Eikelboom J, Ezekowitz MD, Kamensky G, Reilly PA, Yang S, Yusuf S, Wallentin L, Connolly SJ. Risks for Stroke, Bleeding, and Death in Patients With Atrial Fibrillation Receiving Dabigatran or Warfarin in Relation to the CHADS2 Score: A Subgroup Analysis of the RE-LY Trial. Ann Intern Med. 2011;155:660–667. doi: 10.7326/0003-4819-155-10-201111150-00004. [DOI] [PubMed] [Google Scholar]
- 21.Lane DA, Lip GY. Use of the CHA(2)DS(2)-VASc and HAS-BLED scores to aid decision making for thromboprophylaxis in nonvalvular atrial fibrillation. Circulation. 2012;126:860–865. doi: 10.1161/CIRCULATIONAHA.111.060061. [DOI] [PubMed] [Google Scholar]
- 22.Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, Camm AJ, Weitz JI, Lewis BS, Parkhomenko A, Yamashita T, Antman EM. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383:955–62. doi: 10.1016/S0140-6736(13)62343-0. [DOI] [PubMed] [Google Scholar]
- 23.Camm AJ, Kirchhof P, Lip GY, Schotten U, Savelieva I, Ernst S, Van Gelder IC, Al-Attar N, Hindricks G, Prendergast B, Heidbuchel H, Alfieri O, Angelini A, Atar D, Colonna P, De Caterina R, De Sutter J, Goette A, Gorenek B, Heldal M, Hohloser SH, Kolh P, Le Heuzey JY, Ponikowski P, Rutten FH. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC) Eur Heart J. 2010;31:2369–2429. doi: 10.1093/eurheartj/ehq278. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.