Abstract
Objective
Doppler derived mitral peak early diastolic filling velocity to deceleration time ratio (E/DT) has been proposed as parameter for predicting prognosis in general population. This study prospectively investigates the incremental prognostic value of E/DT over clinical, conventional echocardiographic and mitral-Doppler variables in patients hospitalized for decompensated heart failure (HF).
Methods
We analyzed 95 HF patients (mean age 64.8 ± 12.2 years) hospitalized at our institution from January 2010 to March 2012. The primary end-point was cardiac death or hospitalization, whichever occurred first. Cox regression analysis was performed to identify significant predictors of outcomes.
Results
During follow-up (median 37.7 months) 13 patients died and 44 were hospitalized for a cardiac event. At univariable analysis, New York Heart Association (NYHA) functional class, furosemide dosage, lateral tricuspidal annular plane systolic excursion, deceleration time and E/DT were predictive of outcome. At multivariable analysis, E/DT was the only predictor of prognosis (hazard ratio = 1.02, P = 0.018), giving incremental prognostic information to clinical and other echocardio-graphic measures (global chi-square from 15.4 to 25.2; P = 0.032).
Conclusions
E/DT gives independent and incremental prognostic information in HF patients.
Keywords: Echocardiographic evaluation, Left ventricular function, Risk stratification
1. Introduction
Predictors of poor outcome in patients with chronic heart failure (HF) help physicians in medical decision making. Previous studies have identified a number of epidemiological, clinical and laboratory variables predictive of poor outcome.[1]–[3] Multivariable models that incorporate clinical and laboratory variables, medications and devices have been developed to estimate survival of HF patients, in order to create a valid assessment of prognosis in patients with HF.[4]–[6] Several risk scores are available as an interactive application to calculate the risk of mortality in ambulatory patients with HF.[7]
Although a complete history and physical examination are important first steps in HF patients, a two-dimensional and Doppler echocardiography, is a very useful test in the evaluation of these patients. The addition of standard echocardiographic variables to multivariable risk score models results in a significant improvement in risk prediction.[8] Many echocardiographic variables correlate with prognosis in HF, including left ventricular (LV) volumes and function, pulmonary hypertension, right ventricular systolic function and Doppler derived diastolic filling variables.[9]–[12] Recently, the ratio of mitral peak early diastolic filling velocity to deceleration time (E/DT) has been demonstrated to give important prognostic information in a middle aged to elderly population with a high prevalence of diabetes and hypertension.[13] Our purpose was to evaluate the prognostic value of this parameter, after adjustment for conventional data, in HF patients either with reduced ejection fraction (HFrEF) or preserved ejection fraction (HFpEF).
2. Methods
2.1. Study population
From January 2010 to March 2012, we prospectively enrolled 120 patients admitted for acute decompensated heart failure to the Internal Medicine Section of our Department. All patients, after structured history and physical examination, fulfilled the Framingham criteria for diagnosis of HF.[14] Demographic, clinical and laboratory data were collected in all patients before discharge, and after clinical stabilization. Baseline characteristics included patients demographics (age, gender), clinical data (height, weight, NYHA functional class, cardiac rhythm, blood pressure, history of coronary artery disease, smoking habits, diabetes, presence of implantable cardioverter-defibrillator), and laboratory data (serum hemoglobin, lymphocytes, cholesterol, urea nitrogen, sodium). Patients were considered to have idiopathic dilated cardiomyopathy when no obvious underlying cause of HF could be discovered during routine evaluation (electrocardiogram, two-dimensional echocardiography, stress test, perfusion imaging). The diagnosis of ischemic heart disease was based on clinical history, or evidence of previous Q-waves myocardial infarction. When after routine evaluation the diagnosis was still uncertain, coronary angiography was performed. Patients with hemodynamically significant valvular disease, myocarditis, hypertrophic obstructive cardiomyopathy, angina at rest, myocardial infarction within six months before enrollment, uncontrolled hypertension and patients undergoing biventricular pacing for resynchronization therapy were excluded. We also excluded patients in chronic dialytic treatment and patients with major cerebrovascular diseases or other severe illness. Therapy for HF consisted of furosemide in all patients, anti-aldosteronic agents in 15%, angiotensin-converting enzyme inhibitors in 42%, β-blockers in 70%, angiotensin AT1 receptor blockers in 31% of patients.
The investigation conformed to the principles outlined in the Declaration of Helsinki, and the study protocol was approved by our institutional committee on human research. All patients provided their written informed consent.
2.2. Two-dimensional and Doppler echocardiography
Standard M-mode, two-dimensional and Doppler examinations were performed during hospitalization after clinical stabilization. In all patients left and right ventricular systolic and diastolic function were evaluated by M-mode, two-dimensional and Doppler echocardiography using a Philips digital ultrasound machine, Model i-E-33. Left ventricular ejection fraction (LVEF) was calculated by Simpson's methods. We categorized individuals with a LVEF of ≤ 40% as having HFrEF, and those with ejection fraction of > 40% as having HFpEF. For the study of diastolic function, the following mitral wave Doppler and tissue Doppler parameters were measured: peak early (E) and late (A) diastolic filling velocities, E/A ratio, deceleration time of E wave (DT), septal early diastolic mitral annular tissue velocity (septal-e'), lateral early diastolic mitral annular tissue velocity (lateral-e'). We also calculated E/e' ratio by dividing transmitral E peak by e', this latter averaged from the two acquisition sites. Right ventricular (RV) diameters were measured at end-diastole from a right ventricle focused apical 4-chamber view, as recommended by the American Society of Echocardiography. Using M-mode echocardiography, lateral tricuspidal annular plane systolic excursion (TAPSE) was measured as the distance between the basal, end-diastolic position of the tricuspid annulus taken at the beginning of the electrocardiographic QRS complex and its greatest apical long-axis movement. Two-dimensional echocardiography-derived RV area measurements were taken from the apical four-chamber view at end-diastole and end-systole, and the fractional area change (FAC) was calculated (difference between end-diastolic and end-systolic areas divided by end-diastole area × 100).[15] A TAPSE value < 16 mm was considered abnormal, while a DT < 140 ms was considered to predict an elevated LV filling pressure. Pulmonary artery systolic pressure (PAPs) was estimated using Doppler echocardiography by calculating the RV to right atrial pressure gradient during systole, approximated by the modified Bernoulli equation as 4v2, where v is the velocity of the tricuspid regurgitation jet in m/s. The tricuspid regurgitant signal was recorded by continuous wave Doppler echocardiography, and its maximal velocity was measured. Right atrial pressure, estimated on the basis of echocardiographic characteristics of the inferior vena cava and assigned a standardized value, was added to the calculated gradient to give PAPs. All mean values were derived from ≥ 3 different measurements, and five if in presence of arrhythmia. All recordings were analyzed by two operators and the results were averaged.
2.3. Follow-up
After discharge, patients periodically attended our institution outpatient unit for at least 24 months. For patients who died or were hospitalized during the follow-up, information was obtained from the attending physicians, or by phone contact and by review of hospital, or physicians' records by individuals blinded to the patient's echocardiographic data. The primary end-point was the occurrence of cardiac death or hospitalization due to cardiac events, whichever occurred first. Cardiac death, defined as due to acute myocardial infarction, ventricular arrhythmias, refractory HF, or cardiogenic shock, was confirmed by review of death certificates, hospital charts, or physician's records. Acute coronary syndrome was defined based on the criteria of typical chest pain, enzyme levels, and ECG alterations. The date of the last examination, or consultation, was used to determine the length of follow-up.
2.4. Statistical analysis
Continuous variables were expressed as mean ± SD and categorical data as percentages. Differences between groups were analyzed by t-test and chi-square test, as appropriate. A P value < 0.05 was considered statistically significant. Event-free survival curves were estimated with Kaplan-Meier analysis and compared by the log-rank test. Univariable associations with cardiac events were determined by Cox proportional hazards regression. For this purpose, clinical, laboratory and echocardiographic variables were considered. Before performing the regression analysis, the proportional hazard assumption was validated by visual inspection of the log [−log(survivor function)] for categorical variables and with statistical tests based on Schoenfeld residuals for continuous variables. The proportional hazard assumption was not rejected for any covariate included in the Cox model. Finally, a stepwise multivariable Cox regression analysis was performed (P < 0.10 for model entry and P < 0.15 for model retention), considering all variables showing a P value < 0.10 at univariable analysis.
3. Results
The study group consisted of 120 HF patients, 13 of which were excluded because the diastolic function could not be reliably determined, whereas 12 were lost at follow-up. Thus, the outcome cohort included 95 patients (69 men and 26 women, mean age 64.8 ± 12.2 years). The echocardiographic parameters of patients with HFrEF (n = 51) and patients with HFpEF (n = 44) are reported in Table 1.
Table 1. Echocardiographic parameters of patients with HFrEF and with HFpEF.
| HFpEF (n = 51) | HFrEF (n = 44) | P value | |
| Left ventricle parameters | |||
| LVEF (%) | 47 ± 5 | 32 ± 5 | 0.000* |
| LVIDd (mm) | 55 ± 6.3 | 60 ± 5.9 | 0.000* |
| LVIDs (mm) | 41 ± 6.2 | 49 ± 7.5 | 0.000* |
| LVEDV (mL) | 138 ± 42 | 169 ± 42 | 0.001* |
| LVESV (mL) | 73 ± 26 | 116 ± 35 | 0.000* |
| Mitral E-wave (cm/s) | 73.6 ± 29.5 | 81.4 ± 31.6 | 0.21 |
| Mitral DT (ms) | 195 ± 62 | 212 ± 70 | 0.22 |
| E/e' | 16.2 ± 7.5 | 15.7 ± 6.7 | 0.68 |
| E/DT (m/s2) | 50.5 ± 34.4 | 41.4 ± 27.1 | 0.15 |
| Right ventricle parameters | |||
| TAPSE | 19 ± 4 | 19 ± 4 | 0.96 |
Data are presented as mean ± SD. *P < 0.05 compared with HFpEF and HFrEF groups. E/DT: mitral peak early diastolic filling velocity to deceleration time ratio; E/e': mitral peak early diastolic filling velocity and early diastolic velocity by Doppler tissue imaging ratio; HFpEF: heart failure with preserved ejection fraction; HFrEF: heart failure with reduced ejection fraction; LVEDV: left ventricular end diastolic volume; LVEF: left ventricular ejection fraction; LVESV: left ventricular end systolic volume; LVIDd: diastolic left ventricular internal diameter; LVIDs: systolic left ventricular internal diameter; Mitral E wave: peak early diastolic filling velocity; TAPSE: tricuspid annular plane systolic excursion.
During a median follow-up of 37.7 months (5th to 95th percentile, 24.7 to 49.5 months) we observed 57 combined events (death or cardiovascular hospitalization). A fatal cardiac event occurred in 13 patients, five sudden cardiac death and eight worsening of cardiac disease. Furthermore, 44 patients were hospitalized, 41 for decompensated HF, two for occurrence of acute coronary syndrome and one for arrhythmic episode. Baseline characteristics of patients with or without events at follow-up are reported in Table 2. The two groups were comparable for demographic, clinical and laboratory data. Also there was no significant difference for medications between the two groups. The echocardiographic parameters in patients with, or without events, are reported in Table 3 in patients with cardiac events a significantly lower TAPSE (18.3 ± 3.4 mm vs. 20 ± 3.8 mm; P = 0.03) compared to patients without events was observed.
Table 2. Baseline characteristics of patients with and without events at follow-up.
| Clinical and laboratory characteristics | Events (n = 57) | No events (n = 38) | P value |
| Age (yr) | 65.9 ± 11.8 | 63.3 ± 12.6 | 0.3 |
| Gender (M/F) | 42/15 | 27/11 | 0.8 |
| Weight (kg) | 78.9 ± 17.1 | 74.5 ± 15.6 | 0.2 |
| NYHA class | I = 1 (1.7) | I = 3 (7.8) | 0.1 |
| II = 29 (50.8) | II = 24 (63.2) | ||
| III = 27 (47.3) | III = 11 (29) | ||
| CAD | 44 (57) | 28 (74) | 0.7 |
| Smoking | 20 (35) | 12 (32) | 0.7 |
| Diabetes | 19 (33) | 12 (32) | 0.9 |
| SBP (mmHg) | 125 ± 17 | 124 ± 13 | 0.7 |
| Haemoglobin (g/dL) | 12.7 ± 2.1 | 13.2 ± 1.7 | 0.2 |
| Lymphocytes (%) | 22.7 ± 7.5 | 22.2 ± 7.3 | 0.7 |
| Total cholesterol (mg/dL) | 163.9 ± 50.8 | 179.7 ± 36.3 | 0.2 |
| Serum uric acid (mg/dL) | 5.7 ± 1.9 | 6.2 ± 1.9 | 0.3 |
| Serum sodium (mEq/L) | 140.4 ± 3.6 | 140.4 ± 2.8 | 0.9 |
| Sinus rhythm | 41 (72) | 29 (76) | 0.6 |
| ICD | 7 (12) | 4 (10.5) | 0.8 |
| Medications | |||
| β-blockers | 42 (74) | 24 (63) | 0.3 |
| ACE-I | 22 (39) | 18 (47) | 0.4 |
| ARB | 19 (33) | 11 (29) | 0.6 |
| Antialdosteronic agents | 9 (16) | 5 (13) | 0.7 |
| Statins | 30 (53) | 23 (61) | 0.4 |
| Allopurinol | 7 (12) | 6 (16) | 0.6 |
| Furosemide (mg/day) | 48.6 ± 94.1 | 29.8 ± 48.4 | 0.3 |
Data are presented as mean ± SD or n (%). ACE-I: Angiotensin converting enzyme-inhibitors; ARB: angiotensin receptor blockers; CAD: coronary artery disease; ICD: implantable cardioverter defibrillator; NYHA: New York Heart Association; SBP: systolic blood pressure; SD: standard deviation.
Table 3. Echocardiographic measures of patients with and without events at follow-up.
| Events | No-events | P value | |
| Left ventricular parameters | |||
| LVEF (%) | 40 ± 10 | 39 ± 9 | 0.53 |
| LVIDd (mm) | 57.6 ± 6.7 | 57.8 ± 6.9 | 0.91 |
| LVIDs (mm) | 45.5 ± 7.9 | 46.2 ± 8 | 0.67 |
| LVEDV (mL) | 149.5 ± 45.5 | 162.9 ± 42.7 | 0.15 |
| LVESV (mL) | 91.6 ± 36.6 | 102.7 ± 40.1 | 0.17 |
| LVM (g/m2) | 248.6 ± 65.3 | 246.6 ± 62.4 | 0.88 |
| Mitral E-wave (cm/s) | 79.2 ± 31.2 | 74.1 ± 30 | 0.43 |
| Mitral DT (ms) | 197.2 ± 66.4 | 215.4± 65.9 | 0.2 |
| e' (cm/s) | 5.1 ± 1.2 | 5 ± 1.5 | 0.8 |
| E/e' | 16.4 ± 7.8 | 15.3 ± 5.6 | 0.5 |
| E/DT (m/s2) | 49.16 ± 33.94 | 40.23 ± 25.11 | 0.17 |
| Right ventricular parameters | |||
| TAPSE (mm) | 18.3 ± 3.4 | 20 ± 3.8 | 0.03* |
| FAC (%) | 35.4 ± 13.2 | 32.1 ± 11.8 | 0.2 |
| PAPs (mmHg) | 35.4 ± 13.2 | 32.0 ± 11.7 | 0.2 |
| RVD basal (mm) | 38 ± 5.5 | 36.5 ± 4.5 | 0.2 |
* P < 0.05. DT: deceleration time; E: mitral peak early diastolic filling velocity and early diastolic velocity by Doppler tissue imaging ratio; e': early diastolic velocity by Doppler tissue imaging; FAC: fractional area change; LVEDV: left ventricular end diastolic volume; LVEF: left ventricular ejection fraction; LVESV: left ventricular end systolic volume; LVIDd: diastolic left ventricular internal diameter; LVIDs: systolic left ventricular internal diameter; LVM: left ventricular mass; Mitral E wave: peak early diastolic filling velocity; PAPs: pulmonary artery systolic pressure; RVD: right ventricular diameter; TAPSE: tricuspid annular plane systolic excursion.
At univariable analysis we found that NYHA class (HR = 1.76; P = 0.02), furosemide dosage (HR = 1.01; P = 0.04), TAPSE (HR = 0.93; P = 0.04), DT (HR = 0.99; P = 0.03) and E/DT (HR = 1.01; P = 0.017) were predictive for occurrence of events. Kaplan-Meyer survival estimates for TAPSE or DT are reported in Figure 1. As shown, patients with TAPSE < 16 mm had lower event free survival than patients with TAPSE ≥ 16 mm (P = 0.02). Similarly, the outcome was poorer in patients with DT < 140 ms than in patients with DT ≥ 140 ms (P = 0.04).
Figure 1. Kaplan-Meier event-free survival estimates.
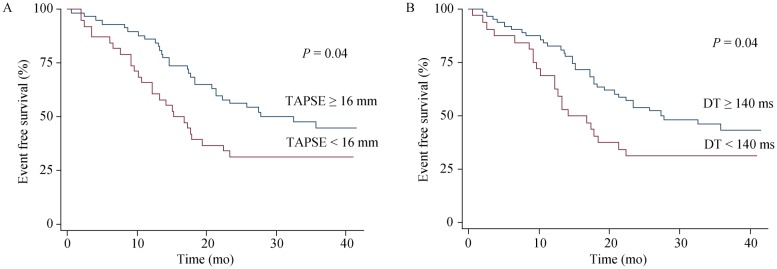
Kaplan-Meier plots showed the time of the combined end point of death and cardiac hospitalization for patients with (A) TAPSE < 16 mm vs. ≥ 16 mm and (B) DT < 140 ms vs. ≥ 140 ms. DT: deceleration time; TAPSE: tricuspid annular plane systolic excursion.
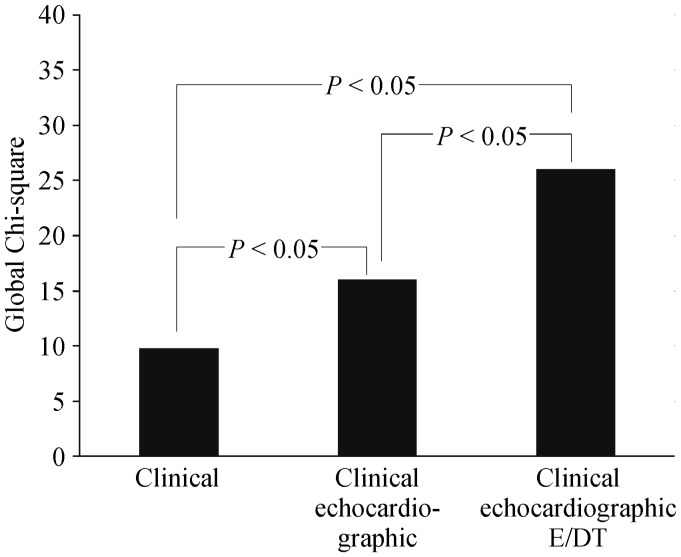
At multivariable analysis E/DT (HR = 1.02; P = 0.017) was the only independent predictor of poor prognosis after adjustment for NYHA class, furosemide dosage, TAPSE, and DT (Table 4). Values for the chi-square statistics as an index of the incremental predictive power of clinical variables, echocardiographic data, and E/DT are reported in Figure 2. As shown, E/DT increased the prognostic power of the model after considering clinical and echocardiographic parameters (global chi-square from 15.4 to 25.2; P = 0.032).
Table 4. Predictors of events at Cox univariable and multivariable analysis.
| Univariable analysis |
|||
| Hazard ratio | 95% CI | P value | |
| NYHA class I, II or III | 1.76 | 1.09–2.84 | 0.02 |
| Furosemide dosage (mg/day) | 1.01 | 1.001–1.006 | 0.04 |
| DT (ms) | 0.99 | 0.991–0.910 | 0.03 |
| TAPSE (mm) | 0.93 | 0.88–0.99 | 0.04 |
| E/DT (m/s2) | 1.01 | 1.001–1.018 | 0.017 |
DT: deceleration time; E/DT: mitral peak early diastolic filling velocity and deceleration time ratio; NYHA: New York Heart Association; TAPSE: tricuspid annular plane systolic excursion.
Figure 2. Incremental prognostic value of clinical, echocardiographic data and E/DT added sequentially.
Global Chi-square on Y axis indicates incremental prognostic value of clinical, echocardiographic data and E/DT added sequentially. E/DT: mitral peak early diastolic filling velocity and deceleration time ratio.
4. Discussion
This study demonstrates that E/DT gives independent and incremental prognostic information in a cohort of patients hospitalized for HF after adjustment for clinical, laboratory and conventional echocardiographic parameters. Of note, we considered consecutive patients with reduced, or preserved LVEF, so our data may be applied to a population of HF patients with different LV functional abnormalities.
In our study, among clinical variables, NYHA class and furosemide dosage were predictive of the outcome at univariable analysis. The NYHA functional class and furosemide dosage have long been recognized in HF patients as important indicators of the severity of cardiovascular impairment and of survival.[16],[17] Our data also confirm the prognostic utility of TAPSE (Figure 1A), an echocardiographic parameter of RV function, easy to obtain in all patients irrespective of heart rate and rhythm. Previous studies reported that TAPSE adds significant prognostic information to the NYHA functional class, echocardiographic evaluation of LV function, and mitral Doppler variables.[18],[19]
In our two-dimensional Doppler echocardiographic study, DT (a measure of LV relaxation) was shorter in patients with events at follow-up compared to those without events and was, therefore, a predictor of the outcome at follow-up either in HFrEF or in HFpEF patients (Figure. 1B). Several studies have shown that noninvasive Doppler measurements of LV relaxation and filling pressures are predictors of outcome in patients with HFrEF and HFpEF.[20],[21] In particular, Giannuzzi, et al.[22] found that in patients with LV systolic dysfunction a short DT adds important prognostic information to the other clinical, functional and echocardiographic variables.
Interestingly, our results do not confirm the prognostic value of tissue Doppler echocardiography. Hirata, et al.[23] found that E/e' ratio > 15 was an excellent predictor of adverse outcome in HF patients and demonstrated the usefulness of a combination of systolic function by LVEF and diastolic function by E/e'. Nevertheless, it must be outlined that the value of E/e' has been recently questioned.[24],[25] In particular Mullens, et al.[25] demonstrated that E/e' ratio may not be reliable in predicting intra-cardiac filling pressures in patients with severe HF and extensive reverse remodeling.
Instead, our study shows that an increase in E/DT is associated with a poor prognosis. Nguyen, et al.[26] in an animal model of pressure overload induced HF, found that the mitral Doppler parameter E/DT reliably predicts the presence of lung remodeling better than E/e', suggesting that this parameter could be useful in other models of HF and in humans.
Opposite to our findings, Mishra, et al.[13] showed that lower E/DT predicted cardiovascular events, whereas peak E velocity and DT alone did not in a population of 3,102 subjects free of prevalent cardiovascular disease and after adjustment for traditional clinical cardiovascular risk factors. These different results may be explained by taking into account the study population composition. Mishra, et al.[13] studied middle aged to elderly patients with high prevalence of diabetes or hypertension without prevalent cardiovascular disease. In these patients active relaxation of LV is attenuated, resulting in a reduced atrio-ventricular pressure gradient and in a decreased mitral peak early diastolic filling velocity.[27] The slow ventricular filling also reduces the atrio-ventricular pressure gradient that, after peak E velocity, is a determinant of DT; so this latter parameter is prolonged. On the other hand, in our HF population, atrial pressure is increased in account of loading conditions, atrio-ventricular gradient is higher irrespective of LV relaxation, and as a consequence, peak E velocity increases. Under these circumstances rapid ventricular filling time (DT) shortens. Therefore, in such conditions of increased loading, E/DT is increased. Of note, in our study population, DT was < 140 ms in 32 out of 95 HF patients, a value that is a commonly observed in patients with elevated filling pressures.[28]
There are limitations to this study. First, this is a single centered study, with a small study population. For this reason, we did not perform separate analyses for patients with HFrEF or HFpEF. Second, pseudo-normalization could be a problem in evaluating the prognostic value of E/DT in patients with severe HF. Finally, the predictive value of echocardiographic parameters was not compared with other prognostic parameters, such as brain natriuretic peptide. However, at our Institution, brain natriuretic peptide levels are not routinely evaluated, and thus, we cannot perform this analysis.
In conclusion, our results demonstrate that E/DT gives independent and incremental prognostic information at follow-up after considering clinical and echocardiographic parameters in HF patients.
References
- 1.Lee DS, Austin PC, JL, et al. Predicting mortality among patients hospitalized for heart failure: derivation and validation of a clinical model. JAMA. 2003;290:2581–2587. doi: 10.1001/jama.290.19.2581. [DOI] [PubMed] [Google Scholar]
- 2.Bello NA, Claggett B, Desai AS, et al. Influence of previous heart failure hospitalization on cardiovascular events in patients with reduced and preserved ejection fraction. Circ Heart Fail. 2014;7:590–595. doi: 10.1161/CIRCHEARTFAILURE.113.001281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zafrir B, Goren Y, Paz H, et al. Risk score model for predicting mortality in advanced heart failure patients followed in a heart failure clinic. Congest Heart Fail. 2012;18:254–261. doi: 10.1111/j.1751-7133.2012.00286.x. [DOI] [PubMed] [Google Scholar]
- 4.Peterson PN, Rumsfeld JS, Liang L, et al. A validated risk score for in-hospital mortality in patients with heart failure from the American Heart Association get with the guidelines program. Circ Cardiovasc Qual Outcomes. 2010;3:25–32. doi: 10.1161/CIRCOUTCOMES.109.854877. [DOI] [PubMed] [Google Scholar]
- 5.O'Connor CM, Abraham WT, Albert NM, et al. Predictors of mortality after discharge in patients hospitalized with heart failure: an analysis from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF) Am Heart J. 2008;156:662–673. doi: 10.1016/j.ahj.2008.04.030. [DOI] [PubMed] [Google Scholar]
- 6.Pocock SJ, Wang D, Pfeffer MA, et al. Predictors of mortality and morbidity in patients with chronic heart failure. Eur Heart J. 2006;27:65–75. doi: 10.1093/eurheartj/ehi555. [DOI] [PubMed] [Google Scholar]
- 7.Alba AC, Agoritsas T, Jankowski M, et al. Risk prediction models for mortality in ambulatory patients with heart failure: a systematic review. Circ Heart Failure. 2013;6:881–889. doi: 10.1161/CIRCHEARTFAILURE.112.000043. [DOI] [PubMed] [Google Scholar]
- 8.Agha SA, Kalogeropoulos AP, Shih J, et al. Echocardiography and risk prediction in advanced heart failure: incremental value over clinical markers. J Card Fail. 2009;15:586–592. doi: 10.1016/j.cardfail.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 9.Wong M, Staszewsky L, Latini R, et al. Severity of left ventricular remodeling defines outcomes and response to therapy in heart failure: Valsartan heart failure trial (Val-HeFT) echocardiographic data. J Am Coll Cardiol. 2004;43:2022–2027. doi: 10.1016/j.jacc.2003.12.053. [DOI] [PubMed] [Google Scholar]
- 10.Bursi F, McNallan SM, Redfield MM, et al. Pulmonary pressures and death in heart failure: a community study. J Am Coll Cardiol. 2012;59:222–231. doi: 10.1016/j.jacc.2011.06.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kjaergaard J, Akkan D, Iversen KK, et al. Right ventricular dysfunction as an independent predictor of short- and long-term mortality in patients with heart failure. Eur J Heart Fail. 2007;9:610–616. doi: 10.1016/j.ejheart.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 12.Xie GY, Berk MR, Smith MD, et al. Prognostic value of Doppler transmitral flow patterns in patients with congestive heart failure. J Am Coll Cardiol. 1994;24:132–139. doi: 10.1016/0735-1097(94)90553-3. [DOI] [PubMed] [Google Scholar]
- 13.Mishra RK, Galloway JM, Lee ET, et al. The ratio of mitral deceleration time to E-wave velocity and mitral deceleration slope outperform deceleration time alone in predicting cardiovascular outcomes: the Strong Heart Study. J Am Soc Echocardiogr. 2007;20:1300–1306. doi: 10.1016/j.echo.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 14.Ho KK, Pinsky JL, Kannel WB, et al. The epidemiology of heart failure: the Framingham Study. J Am Coll Cardiol. 1993;22:6A–13A. doi: 10.1016/0735-1097(93)90455-a. [DOI] [PubMed] [Google Scholar]
- 15.Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685–713. doi: 10.1016/j.echo.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 16.Madsen BK, Hansen JF, Stokholm KH, et al. Chronic congestive heart failure. Description and survival of 190 consecutive patients with a diagnosis of chronic congestive heart failure based on clinical signs and symptoms. Eur Heart J. 1994;15:303–310. doi: 10.1093/oxfordjournals.eurheartj.a060495. [DOI] [PubMed] [Google Scholar]
- 17.Cody RJ, Kubo SH, Pickworth KK. Diuretic treatment for the sodium retention of congestive heart failure. Arch Intern Med. 1994;154:1905–1914. [PubMed] [Google Scholar]
- 18.Damy T, Kallvikbacka-Bennett A, Goode K, et al. Prevalence of, associations with, and prognostic value of tricuspid annular plane systolic excursion (TAPSE) among out-patients referred for the evaluation of heart failure. J Card Fail. 2012;18:216–225. doi: 10.1016/j.cardfail.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 19.Ghio S, Recusani F, Klersy C, et al. Prognostic usefulness of the tricuspid annular plane systolic excursion in patients with congestive heart failure secondary to idiopathic or ischemic dilated cardiomyopathy. Am J Cardiol. 2000;85:837–842. doi: 10.1016/s0002-9149(99)00877-2. [DOI] [PubMed] [Google Scholar]
- 20.Ohtani T, Mohammed SF, Yamamoto K, et al. Diastolic stiffness as assessed by diastolic wall strain is associated with adverse remodelling and poor outcomes in heart failure with preserved ejection fraction. Eur Heart J. 2012;33:1742–1749. doi: 10.1093/eurheartj/ehs135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Werner GS, Schaefer C, Dirks R, et al. Prognostic value of Doppler echocardiographic assessment of left ventricular filling in idiopathic dilated cardiomyopathy. Am J Cardiol. 1994;73:792–798. doi: 10.1016/0002-9149(94)90883-4. [DOI] [PubMed] [Google Scholar]
- 22.Giannuzzi P, Temporelli PL, Bosimini E, et al. Independent and incremental prognostic value of Doppler-derived mitral deceleration time of early filling in both symptomatic and asymptomatic patients with left ventricular dysfunction. J Am Coll Cardiol. 1996;28:383–390. doi: 10.1016/0735-1097(96)00163-5. [DOI] [PubMed] [Google Scholar]
- 23.Hirata K, Hyodo E, Hozumi T, et al. Usefulness of a combination of systolic function by left ventricular ejection fraction and diastolic function by E/E' to predict prognosis in patients with heart failure. Am J Cardiol. 2009;103:1275–1279. doi: 10.1016/j.amjcard.2009.01.024. [DOI] [PubMed] [Google Scholar]
- 24.Dokainish H, Nguyen JS, Bobek J, et al. Assessment of the American Society of Echocardiography-European Association of Echocardiography guidelines for diastolic function in patients with depressed ejection fraction: an echocardiographic and invasive haemodynamic study. Eur J Echocardiogr. 2011;12:857–864. doi: 10.1093/ejechocard/jer157. [DOI] [PubMed] [Google Scholar]
- 25.Mullens W, Borowski AG, Curtin RJ, et al. Tissue Doppler imaging in the estimation of intracardiac filling pressure in decompensated patients with advanced systolic heart failure. Circulation. 2009;119:62–70. doi: 10.1161/CIRCULATIONAHA.108.779223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nguyen TD, Shingu Y, Schwarzer M, et al. The E-Wave deceleration rate E/DT outperforms the tissue doppler-derived index E/e' in characterizing lung remodeling in heart failure with preserved ejection fraction. PLoS One. 2013;8:e82077. doi: 10.1371/journal.pone.0082077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lester SJ, Tajik AJ, Nishimura RA, et al. Unlocking the mysteries of diastolic function: deciphering the Rosetta Stone 10 years later. J Am Coll Cardiol. 2008;51:679–689. doi: 10.1016/j.jacc.2007.09.061. [DOI] [PubMed] [Google Scholar]
- 28.Stoddard MF, Pearson AC, Kern MJ, et al. Left ventricular diastolic function: comparison of pulsed Doppler echocardiographic and hemodynamic indexes in subjects with and without coronary artery disease. J Am Coll Cardiol. 1989;13:327–336. doi: 10.1016/0735-1097(89)90507-x. [DOI] [PubMed] [Google Scholar]