Abstract
Background
Sleep-disordered breathing (SDB) is known to occur frequently in and may predict worsening progression of patients with congestive heart failure (CHF). SDB is also known to play an important role in the development of idiopathic pulmonary arterial hypertension (PAH) via inducing endothelial dysfunction and vascular remodeling, a pathological process that can be significantly influenced by factors such as osteoprotegerin (OPG) and endothelial progenitor cells (EPCs). The objective of this study is to determine if CHF with SDB is associated with changes in OPG, EPCs, and PAH.
Methods
EPCs were isolated, cultured, and quantified from CHF patients with SDB (n = 52), or without SDB (n = 68). OPG and N-terminal pro-brain natriuretic peptide (NT-proBNP) from each group was analyzed and correlated with EPCs and the mean pulmonary artery pressure (mPAP) measured by right heart catheterization.
Results
A significant decrease in circulating EPCs (29.30 ± 9.01 vs. 45.17 ± 10.51 EPCs/× 200 field; P < 0.05) was found in CHF patients with SDB compared to those without SDB. Both OPG (789.83 ± 89.38 vs. 551.29 ± 42.12 pg/mL; P < 0.05) and NT-proBNP (5946.50 ± 1434.50 vs. 3028.60 ± 811.90 ng/mL; P < 0.05) were also significantly elevated in SDB CHF patients who also had significantly elevated mPAP (50.2 ± 9.5 vs. 36.4 ± 4.1 mm Hg; P < 0.05). EPC numbers correlated inversely with the episodes of apnea and hypopnea per hour (RDI, r = –0.45, P = 0.037) and blood level of OPG (r = –0.53, P = 0.011). Although NT-proBNP was also increased significantly in patients with SDB, it had no correlation with either EPCs or RDI.
Conclusions
SDB due to hypoxemia from decompensated CHF is associated with (1) OPG elevation, (2) EPC depletion, and (3) mPAP elevation. The inverse relationship of circulating OPG with EPCs suggests a likely mechanism for hypoxemia and OPG in the development of pulmonary vascular dysfunction via depleting EPCs, thus worsening prognosis of CHF.
Keywords: Congestive heart failure, Endothelial progenitor cells, Osteoprotegerin, Sleep-disordered breathing
1. Introduction
Approximately 5.1 million people in the United States have clinically manifested congestive heart failure (CHF), and the prevalence continues to rise.[1] The lifetime risk of developing HF is 20% for Americans ≥ 40 years of age. In the United States the CHF incidence has largely remained stable over the past several decades, with > 650,000 new CHF cases diagnosed annually.[1],[2] Recently, some studies have shown that approximately 50% of CHF patients have sleep-disordered breathing (SDB), which consists of obstructive sleep apnea (OSA) and Cheyne-Stokes respiration with central sleep apnea (CSA).[3],[4] Some large studies have demonstrated that SDB is associated with adverse prognosis in CHF patients.[5],[6] In addition, it is well known that SDB is associated with the development of pulmonary arterial hypertension (PAH) because of nocturnal dyspnea. PAH also frequently develops during progression of CHF,[7],[8] and unfortunately, the development of PAH in patients with CHF is associated with a worsening prognosis and greater mortality.[1],[2]
In animal studies, pulmonary vascular remodeling is key to the pathogenesis of PAH.[5],[6] Although the specific mechanisms of pulmonary vascular remodeling remain not completely understood, experimental studies have implicated several molecules previously associated with idiopathic PAH that may play a central role. During chronic heart failure abnormal bone morphogenetic protein receptor type II (BMPR2) expression could secrete osteoprotegerin (OPG) from pulmonary artery endothelial cells.[9] OPG is a soluble member of the tumor necrosis factor receptor family, which regulates osteoclast differentiation.[9],[10] The BMPR2-OPG pathway activation may also result in damage for some types of cells, such as endothelial cells, by increasing apoptosis[10],[11] Endothelial injury and proliferation are fundamental processes leading to endothelial dysfunction, which plays an important part in the pathogenesis of pulmonary vascular remodeling and hypertension, an imbalance between the magnitude of injury and the capacity for repair.[11],[12]
A variety of experimental studies have shown that endothelial progenitor cells (EPCs) contribute to the repair process which occurs after vascular injury.[13],[14] EPCs can be mobilized from the bone marrow into the systemic circulation where they have the capacity to proliferate and differentiate into mature endothelial cells of the damaged vasculature. Such EPCs co-express hematopoietic stem/progenitor cell markers (CD34+ or AC133+) as well as endothelial markers [VE-cadherin or Vascular endothelial growth factor receptors (VEGFR-2)].[13],[14] Depletion and dysfunction of EPCs have been observed in patients with idiopathic PAH and coronary artery disease.[15]–[17] We therefore, in the current study, hypothesize that in patients with decompensated CHF and SDB, there is increased release of OPG and depletion of EPCs. Such changes may contribute to the development of pulmonary hypertension and worsening progression of CHF with SDB.
2. Methods
2.1. Patient selection
A total of 120 patients with decompensated CHF diagnosed at and admitted to Huaihe Hospital, College of Medicine of Henan University were enrolled from January 1, 2011 to December 31, 2013. All patients underwent standard care for acute CHF decompensation using intravenous diuretics guided by central venous pressure, and medications for chronic management derived from additional guidelines. The diagnosis of heart failure was made on patient history, symptomatic presentations and objective evidence of systolic dysfunction defined by left ventricular ejection fraction (LVEF) below 45% by echocardiography. CHF etiology was classified as ischemic (n = 44), or idiopathic dilated (n = 72), on the basis of patient's history and coronary angiography study with ischemic cardiomyopathy being defined as at least one major coronary artery (left anterior descending, circumflex, and right coronary) with > 70% stenosis. All patients underwent right heart catheterization via either the right internal jugular or right femoral vein approach, with PAH being defined as mean pulmonary arterial pressure (mPAP) ≥ 25 mm Hg.
All patients also underwent an overnight sleep study using a type 3 polygraph system (LS-300; Fukuda Denshi, Tokyo, Japan), with continuous monitoring of electrocardiograph (ECG), thoraco-abdominal motion, nasal airflow via airflow pressure transducer, and arterial oxyhemoglobin saturation (SpO2) via pulse oximetry. Apnea was defined as absence of airflow for >10 s. Hypopnea was defined as a > 30% reduction in monitored airflow accompanied by a decrease in SpO2 > 4%.[18] Standard definitions were used for obstructive or central sleep apnea (OSA and CSA) on the basis of the presence or absence of rib cage and abdominal excursions with an absence of airflow. Respiratory Disturbance Index (RDI) was defined as the number of apneas and hypopneas per hour during sleep time in bed. Based on the present study and previous reports,[19],[20] patients were further classified as CHF with SDB (RDI ≥ 20 events per hour; Group A, n = 52), or CHF without SDB (RDI < 20 events per hour; Group B, n = 68).
The main exclusion criteria were age < 18 or > 90 years old, active pulmonary disease, primary sleep-associated disorders, chronic thromboembolic disease, autoimmune or collagen vascular disease, HIV infection, liver disease and unwillingness to participate, or provide blood samples. All participants were also free of wounds, ulcers, retinopathy, inflammatory or malignant disease, or recent surgery that may influence EPC numbers.
2.2. Clinical characteristics and hemodynamics
Baseline demographic data and cardiovascular risk factors were documented for all patients. These included cigarette smoking status, diabetes mellitus, hypercholesterolemia, hypertension, atrial fibrillation. Hypertension was defined as systolic/diastolic blood pressure at rest > 140/90 mm Hg at two different clinic visits or the prescription of antihypertensive medication. Diabetes mellitus was defined as serum fasting glucose > 7.0 mmol/L or the use of hypoglycemic agents. Hypercholesterolemia was defined as fasting total plasma cholesterol > 4.9 mmol/L. Smoking status was recorded as smoker (current or former) or non-smoker. Patients were classified as having atrial fibrillation, or no atrial fibrillation, on the basis of current or previous disease history and by ECG confirmation. LVEF, left ventricular end-diastolic diameter (LVEDD), right ventricular end-diastolic diameter (RVEDD), and left atrial diameter (LAD) were measured using Doppler echocardiography imaging.
2.3. Isolation, culture, and characterization of circulating EPCs
EPCs were isolated, cultured and characterized according to previously described techniques.[15]–[19] Briefly, mononuclear cells were isolated from the peripheral blood of patients with CHF by Ficoll density gradient centrifugation and cultured on fibronectin (Chemicon)-coated dishes in Medium 199 (Sigma) supplemented with 10% fetal-calf serum and VEGF (50 ng/mL, Chemicon). After four days in culture, adherent cells were incubated with DiLDL (Molecular Probe) and stained with FITC-labeled Ulex europaeus agglutinin (UEA-1, Sigma). After the staining, samples were viewed with an inverted fluorescent microscope (Leica) and further demonstrated by laser scanning confocal microscope (LSCM, Leica). Cells exhibiting double-positive fluorescence were identified as differentiating EPCs.[18],[19] Two to three independent investigators evaluated the number of EPCs per well by counting 15 randomly selected high-power fields (×200) with an inverted fluorescent microscope.[17]–[20]
2.4. Biochemical measurements
Serum levels of OPG and N-terminal pro-brain natriuretic peptide (NT-proBNP) were quantified by ELISA with commercially available matched antibodies (BSD).
2.5. Statistical analysis
All values were expressed as mean ± SD. Student's unpaired t test was performed for comparison of data between two groups. Categorical variables were compared by means of the χ2 test. Correlations were tested by Pearson analysis. Values of P < 0.05 were considered significant. All statistical analyses were performed using SPSS 16.0.
3. Results
3.1. Clinical characteristics
The clinical characteristics of the study population are listed in Table 1. There were no significant differences in age, sex gender, hypertension, diabetes, hypercholesterolemia, and ischemic cardiomyopathy etiology between the two groups (all P values > 0.05). However, the percentage of atrial fibrillation was significantly higher in the group of patients with CHF and SDB than the group without SDB (all P values < 0.05).
Table 1. Patients clinical characteristics.
| CHF – SDB (n = 68) | CHF + SDB (n = 52) | |
| Age (yr) | 49.80 ± 11.45 | 52.45 ± 11.24 |
| Men (%) | 41 (60.00) | 34 (65.00) |
| BMI (kg/m2) | 24.8 ± 0.4 | 27.3 ± 0.3 |
| Blood pressure (mm Hg) | ||
| Systolic | 143.5 ± 1.9 | 142.9 ± 2.1 |
| Diastolic | 81.8 ± 0.8 | 83.1 ± 1.0 |
| Smoker (%) | 36 (52.94) | 26 (50.00) |
| Diabetes mellitus (%) | 12 (17.64) | 10 (19.23) |
| Hypercholesterolemia (%) | 14 (20.59) | 13 (25.00) |
| Hypertension (%) | 31 (45.58) | 26 (50.00) |
| Atrial fibrillation (%) | 14 (20.58) | 23 (44.20)* |
| Ischemic cardiomyopathy (%) | 26 (38.24) | 18 (34.61) |
Data are expressed as mean ± SD or as n (%). *P < 0.05 for atrial fibrillation. BMI: body mass index; CHF: congestive heart failure; SDB: sleep-disordered breathing.
3.2. Doppler echocardiography imaging parameters and mean pulmonary artery pressures
As listed in Table 2, Doppler echocardiography imaging showed that CHF patients with SDB had no significant differences in the LVEDD comparing with those without SDB (all P values > 0.05). However, the LAD and RVEDD were significantly larger in the SDB group (all P values < 0.05). Mean pulmonary artery blood pressures obtained by right heart catheterization were also significantly higher in CHF patients with SDB than CHF without SDB (all P values < 0.05), and the LVEF was significantly lower in the SDB group (P values < 0.05).
Table 2. Echocardiographic parameters in patients with or without SDB.
| CHF – SDB (n = 68) | CHF + SDB (n = 52) | |
| LVEDD (mm) | 67.90 ± 13.20 | 69.20 ± 9.60 |
| LVEF (%) | 42.40 ± 12.80 | 31.60 ± 11.40* |
| LAD (mm) | 42.20 ± 7.10 | 50.20 ± 9.30* |
| RVEDD (mm) | 21.80 ± 4.40 | 28.30 ± 7.00* |
| mPAP (mm Hg) | 36.40 ± 4.10 | 50.20 ± 9.50* |
*P < 0.05. Data are expressed as mean ± SD. CHF: congestive heart failure; LAD: left atrial diameter; LVEDD: left ventricular end diastolic diameter; LVEF: left ventricular ejection fraction; mPAP: mean pulmonary arterial pressure; RVEDD: right ventricular end diastolic diameter; SDB: sleep-disordered breathing.
3.3. Circulating levels of OPG, NT-proBNP, and EPCs
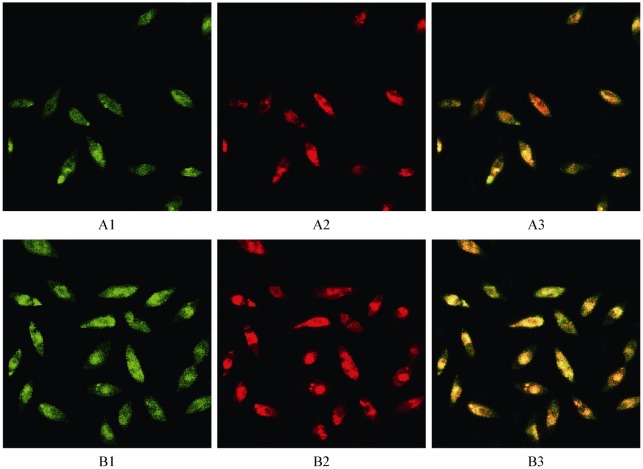
The levels of serum OPG and NT-proBNP from CHF patients with and without SDB are shown in Table 3. Significant differences exist between the two groups (all P values < 0.05). The numbers of EPCs were expanded from human blood in vitro and identified by DiLDL uptake and lectin staining as shown in Figure 1 (panels A1 to B3). EPC numbers decreased significantly in subjects with SDB patients compared with those without SDB (29.30 ± 9.01 vs. 45.17 ± 10.51 EPCs/ × 200 field; P < 0.05). Both OPG (789.83 ± 89.38 vs. 551.29 ± 42.12 pg/mL; P < 0.05) and NT-proBNP (5946.50 ± 1434.50 vs. 3028.60 ± 811.90 ng/mL; P < 0.05) were also significantly elevated in CHF patients with SDB. EPC numbers correlated inversely with RDI (r = –0.45, P = 0.037) and blood level of OPG (r = –0.53, P = 0.011). Blood levels of NT-proBNP were also increased significantly in patients with decompensated CHF and SDB, but had no significant correlation with either EPCs or RDI.
Table 3. Circulating levels of OPG, NT-proBNP and EPC numbers in patients with or without SDB.
| CHF – SDB (n = 68) | CHF + SDB (n = 52) | |
| OPG (pg/mL) | 551.29 ± 42.12 | 789.83 ± 89.38* |
| NT-proBNP (ng/mL) | 3028.60 ± 811.90 | 5946.50 ± 1434.50* |
| EPCs (number) | 45.17 ± 10.51 | 29.30 ± 9.01 * |
* P < 0.05. Data are expressed as mean ± SD. CHF: congestive heart failure; EPC: endothelial progenitor cell; NT-proBNP: N-terminal pro-brain natriuretic peptide; OPG: osteoprotegerin; SDB: sleep-disordered breathing.
Figure 1. Characteristics of CHF patient's with or without SDB peripheral blood mononuclear cells after seven days of culture.
Adherent cells lectin binding (green, exciting wave-length 477 nm) and DiLDL uptake (red, exciting wave-length 543 nm) were assessed under a laser scanning confocal microscope. Double positive cells appearing yellow in the overlay were identified as differentiating EPCs (× 200). Panels A1-A3: CHF with SDB. Panels B1-B3: CHF without PAH. CHF: congestive heart failure; EPCs: endothelial progenitor cells; PAH: pulmonary arterial hypertension; SDB: sleep-disordered breathing.
4. Discussion
The current study demonstrates that patients of decompensated heart failure with SDB have significantly decreased circulating EPCs and increased level of OPG from peripheral blood. These SDB patients also have significantly elevated mPAP. There is an inverse relationship between the changes in EPCs and OPG. SDB is known to cause hypoxemia. This likely contributes to the elevation in circulating OPG which in turn may cause depletion of EPCs and damage to the pulmonary vascular endothelial function resulting in adverse remodeling and development of pulmonary hypertension.
Heart failure is well recognized as a major cause of mortality and morbidity. The progressive nature of CHF symptoms lead to chronic disability and impaired quality of life. SDB is known to occur frequently in patients with CHF and may be a predictor of poor prognosis.[3],[4] In a study by Macdonald, et al.[21] SDB was found in 55% of patients with asymptomatic heart failure with LVEF less than 40%. Another study by Tremel, et al.[20] found 82% of the patients with heart failure also had SDB.In our study, 43% of patients with moderately depressed left ventricular systolic function were found to have SDB during index hospitalization. However, we also demonstrated that these patients had significantly elevated mPAP. This evidence lends to support the premise that hypoxemia may contribute to the pulmonary vascular endothelial dysfunction, remodeling, and development of pulmonary hypertension in heart failure patients with SDB. Although the detailed mechanisms are yet to be determined, SDB with resultant hypoxemia causing oxidative stress and pulmonary vasculature endothelial dysfunction remains elusive.
Also, well recognized is the development of pulmonary hypertension during progression in the majority of patients with chronic heart failure, which in turn results in worsening prognosis and complicating effective treatment.[11],[22] Experimental studies over the past decades have led to rapid advancement in the mechanisms of PAH during heart failure, which is now thought not to be solely due to a “passive” increase in pulmonary vascular pressures from elevated end diastolic pressure of the left ventricle, but rather a “reactive” contribution from the pulmonary vascular endothelial dysfunction and vascular remodeling.[1],[2],[22] This endothelial dysfunction ultimately represents an imbalance between the magnitude of vascular injury and its capacity for repair. There is strong evidence that circulating EPCs may play an important role in endothelium maintenance, being implicated in both re-endothelialization and neo-vascularization.[12],[22] Clinical studies have demonstrated that the number of circulating EPCs has an inverse relationship to the Framingham risk score and vascular endothelial function in healthy subjects.[22],[23] There is a decreased number of circulating EPCs in coronary artery disease patients who smoke.[16] In addition, experimental animals transplantation of EPCs has modestly attenuated monocrotaline-induced pulmonary hypertension (16% decrease in pulmonary vascular resistance). Intravenous infusion of autologous EPCs has beneficial effects on exercise capacity and pulmonary hemodynamics in adults with idiopathic PAH.[15] In this regard, our study demonstrated that circulating EPCs were significantly depleted in patients of systolic heart failure with SDB. This might suggest that the role of depletion of EPCs contributes to endothelial dysfunction and development of pulmonary hypertension in patients with SDB, which lead to worsening heart failure.[7],[8],[24] Therefore, supplementation of EPCs might reverse this disease process and improve outcome.
OPG is a soluble member of the tumor necrosis factor (TNF) receptor family. Numerous reports confirm the involvement of TNF-α in the pathogenesis of heart failure.[25],[26] Traditionally, OPG binds to the receptor activator of NF-κB ligand (RANKL) and competitively inhibits the interaction between RANKL and RANK, thus regulating osteoclast differentiation.[10],[11],[27] Recent experimental, clinical, and epidemiological data have also implicated a significant role of this signaling axis in the development and progression of cardiovascular diseases, such as aortic valve calcification, atherosclerosis, and diabetic macroangiopathy.[28]–[31] In both experimental animal models and clinical heart failure patients, elevated myocardial protein expression of OPG and dysregulated RANKL/OPG signaling axis have been demonstrated. These findings may suggest an important role of this axis in ventricular and vascular remodeling, which may lead to worsening myocardial function and clinical prognosis.[32]–[34]
A variety of pathological conditions are associated with elevations of circulating OPG.[34],[35] In experimental animals reduced BMPR2 expression stimulates OPG expression and secretion from pulmonary artery smooth muscle cells.[35],[36] Interestingly, elevated levels of OPG have also been observed in PAH associated with congenital heart disease.[37],[38] In our study circulating OPG is significantly increased in peripheral blood from patients with heart failure decompensated SDB compared to those patients without SDB. Our finding of a negative correlation between the OPG level and the number of EPCs supports the hypothesis that elevated OPG may down regulate circulating EPCs causing dysfunction of the endothelium, which plays an important part in the pathogenesis of pulmonary vascular remodeling and development of pulmonary hypertension in patients with SDB. Although the concentration of NT-proBNP, a well-established heart failure marker, was also increased significantly in peripheral blood from heart failure patients with SDB, the blood level of NT-proBNP had no correlation with circulating EPCs and RDI.[39]–[41] This is consistent with previous studies showing that although the NT-proBNP level correlated with the severity of heart failure symptoms, it had no correlation with pulmonary vascular remodeling.[39] Koyama, et al.[42] have investigated the potential benefit of an therapy such as short term use of adaptive servo ventilation in CHF measuring CHF parameters in 17 patients before and after nocturnal ventilation therapy using adaptive servo ventilation. They demonstrated an improvement during therapy for the following parameters: level of NT-proBNP, ejection fraction, and NYHA-class. The conclusion suggested adaptive servo ventilation therapy to be important contributors for decreasing NT-proBNP and improving cardiac function in patients with CHF accompanied by SDB.[42] However, whether this adequate treatment can decrease pulmonary artery pressure is still unknown.
This study has several limitations: first, the relatively small study population and a rather selected disease stage (i.e., systolic heart failure with at least moderately depressed left ventricular systolic function during decompensation) limit any possible generalization of our finding. However, effective intervention at this disease stage may render the most rewarding outcome including reversal of disease progression. Second, the direct functional study of circulating EPCs was not performed. Thus, the contribution of depleted EPCs to the development of pulmonary hypertension remains speculative. Thirdly, the molecular pathway of OPG responsible for impairment in circulating EPCs is yet to be determined, especially in this type of human study.
In conclusion, CHF patients with SDB had increased circulating OPG, depleted EPCs, and elevated mPAP. The inverse relationship of circulating OPG with EPCs suggests a likely mechanism for SDB and hypoxemia to stimulate OPG in the development of pulmonary vascular dysfunction, thus worsening prognosis for chronic CHF.
References
- 1.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;128:240–327. doi: 10.1161/CIR.0b013e31829e8776. [DOI] [PubMed] [Google Scholar]
- 2.Rosamond W, Flegal K, Furie K, et al. Heart disease and stroke statistics – 2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–e146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 3.Javaheri S, Parker TJ, Liming JD, et al. Sleep apnea in 81 ambulatory male patients with stable heart failure: types and their prevalences, consequences, and presentations. Circulation. 1998;97:2154–2159. doi: 10.1161/01.cir.97.21.2154. [DOI] [PubMed] [Google Scholar]
- 4.Javaheri S, Parker TJ, Wexler L, et al. Effect of theophylline on sleep-disordered breathing in heart failure. N Engl J Med. 1996;335:562–567. doi: 10.1056/NEJM199608223350805. [DOI] [PubMed] [Google Scholar]
- 5.Schulz R, Blau A, Börgel J, et al. Sleep apnoea in heart failure. Eur Respir J. 2007;29:1201–1205. doi: 10.1183/09031936.00037106. [DOI] [PubMed] [Google Scholar]
- 6.Wang HQ, Chen G, Li J, et al. Subjective sleepiness in heart failure patient with sleep-related breathing disorder. Chin Med J (Engl) 2009;122:1375–1379. [PubMed] [Google Scholar]
- 7.Lam CS, Roger VL, Rodeheffer RJ, et al. Pulmonary hypertension in heart failure with preserved ejection fraction: a community-based study. J Am Coll Cardiol. 2009;53:1119–1126. doi: 10.1016/j.jacc.2008.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yin J, Kukucka M, Hoffmann J, et al. Sildenafil preserves lung endothelial function and prevents pulmonary vascular remodeling in a rat model of diastolic heart failure. Circ Heart Fail. 2011;4:198–206. doi: 10.1161/CIRCHEARTFAILURE.110.957050. [DOI] [PubMed] [Google Scholar]
- 9.Abramowicz MJ, Van Haecke P, Demedts M, et al. Primary pulmonary hypertension after amfepramone (diethylpropion) with BMPR2 mutation. Eur Respir J. 2003;22:560–562. doi: 10.1183/09031936.03.00095303. [DOI] [PubMed] [Google Scholar]
- 10.Simonet WS, Lacey DL, Dunstan CR, et al. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89:309–319. doi: 10.1016/s0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- 11.Hofbauer LC, Khosla S, Dunstan CR, et al. The roles of osteoprotegerin and osteoprotegerin ligand in the paracrine regulation of bone resorption. J Bone Miner Res. 2000;15:2–12. doi: 10.1359/jbmr.2000.15.1.2. [DOI] [PubMed] [Google Scholar]
- 12.Lawrie A, Waterman E, Southwood M, et al. Evidence of a role for osteoprotegerin in the pathogenesis of pulmonary arterial hypertension. Am J Pathol. 2008;172:256–264. doi: 10.2353/ajpath.2008.070395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim JY, Park YJ, Kim KJ, et al. Osteoprotegerin causes apoptosis of endothelial progenitor cells by induction of oxidative stress. Arthritis Rheum. 2013;65:2172–2182. doi: 10.1002/art.37997. [DOI] [PubMed] [Google Scholar]
- 14.Hill JM, Zalos G, Halcox JP, et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348:593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 15.Gill M, Dias S, Hattori K, et al. Vascular trauma induces rapid but transient mobilization of VEGFR2+AC133+ endothelial precursor cells. Circ Res. 2001;88:167–174. doi: 10.1161/01.res.88.2.167. [DOI] [PubMed] [Google Scholar]
- 16.Walter DH, Rittig K, Bahlmann FH, et al. Statin therapy accelerate re-endothelialization: a novel effect involving mobilization and incorporation of bone marrow-derived endothelial progenitor cells. Circulation. 2002;105:3017–3024. doi: 10.1161/01.cir.0000018166.84319.55. [DOI] [PubMed] [Google Scholar]
- 17.Junhui Z, Xingxiang W, Guosheng F, et al. Reduced number and activity of circulating endothelial progenitor cells in patients with idiopathic pulmonary arterial hypertension. Respir Med. 2008;102:1073–1079. doi: 10.1016/j.rmed.2007.12.030. [DOI] [PubMed] [Google Scholar]
- 18.Silber MH, Ancoli-Israel S, Bonnet MH, et al. The visual scoring of sleep in adults. J Clin Sleep Med. 2007;15:121–131. [PubMed] [Google Scholar]
- 19.Takama N, Kurabayashi M. Effectiveness of adaptive servo-ventilation for treating heart failure regardless of the severity of sleep-disordered breathing. Circ J. 2011;75:1164–1169. doi: 10.1253/circj.cj-10-0831. [DOI] [PubMed] [Google Scholar]
- 20.Tremel F, Pépin JL, Veale D, et al. High prevalence and persistence of sleep apnoea in patients referred for acute left ventricular failure and medically treated over 2 months. Eur Heart J. 1999;20:1201–1209. doi: 10.1053/euhj.1999.1546. [DOI] [PubMed] [Google Scholar]
- 21.Macdonald M, Fang J, Pittman SD, et al. The current prevalence of sleep disordered breathing in congestive heart failure patients treated with beta-blockers. J Clin Sleep Med. 2008;4:38–42. [PMC free article] [PubMed] [Google Scholar]
- 22.Vasa M, Fichtlscherer S, Aicher A, et al. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001;89:e1–e7. doi: 10.1161/hh1301.093953. [DOI] [PubMed] [Google Scholar]
- 23.Kalka C, Masuda H, Takahashi T, et al. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc Natl Acad Sci USA. 2000;97:3422–3427. doi: 10.1073/pnas.070046397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen JZ, Zhu JH, Wang XX, et al. Effects of homocysteine on number and activity of endothelial progenitor cells from peripheral blood. J Mol Cell Cardiol. 2004;36:233–239. doi: 10.1016/j.yjmcc.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 25.Strehlow K, Werner N, Berweiler J, et al. Estrogen increases bone marrow-derived endothelial progenitor cell production and diminishes neointima formation. Circulation. 2003;107:3059–3065. doi: 10.1161/01.CIR.0000077911.81151.30. [DOI] [PubMed] [Google Scholar]
- 26.Ghio S, Gavazzi A, Campana C, et al. Independent and additive prognostic value of right ventricular systolic function and pulmonary artery pressure in patients with chronic heart failure. J Am Coll Cardiol. 2001;37:183–188. doi: 10.1016/s0735-1097(00)01102-5. [DOI] [PubMed] [Google Scholar]
- 27.Mann DL. Recent insights into the role of tumor necrosis factor in the failing heart. Heart Fail Rev. 2001;6:71–80. doi: 10.1023/a:1011449708842. [DOI] [PubMed] [Google Scholar]
- 28.Ueland T, Yndestad A, Oie E, et al. Dysregulated osteoprotegerin/RANK ligand/RANK axis in clinical and experimental heart failure. Circulation. 2005;111:2461–2468. doi: 10.1161/01.CIR.0000165119.62099.14. [DOI] [PubMed] [Google Scholar]
- 29.Lorenzo J, Horowitz M, Choi Y. Osteoimmunology: interactions of the bone and immune system. Endocr Rev. 2008;29:403–440. doi: 10.1210/er.2007-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kiechl S, Werner P, Knoflach M, et al. The osteoprotegerin/ RANK/RANKL system: a bone key to vascular disease. Expert Rev Cardiovasc Ther. 2006;4:801–811. doi: 10.1586/14779072.4.6.801. [DOI] [PubMed] [Google Scholar]
- 31.Andersen GØ, Knudsen EC, Aukrust P, et al. Elevated serum osteoprotegerin levels measured early after acute ST-elevation myocardial infarction predict final infarct size. Heart. 2011;97:460–465. doi: 10.1136/hrt.2010.206714. [DOI] [PubMed] [Google Scholar]
- 32.Steinmetz M, Skowasch D, Wernert N, et al. Differential profile of the OPG/RANKL/RANK system in degenerative aortic native and bioprosthetic valves. J Heart Valve Dis. 2008;17:187–193. [PubMed] [Google Scholar]
- 33.Ndip A, Williams A, Jude EB, et al. The RANKL/RANK/ OPG signaling pathway mediates medial arterial calcification in diabetic Charcot neuroarthropathy. Diabetes. 2011;60:2187–2196. doi: 10.2337/db10-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chang YH, Lin KD, He SR, et al. Serum osteoprotegerin and tumor necrosis factor related apoptosis inducingligand (TRAIL) are elevated in type 2 diabetic patients with albuminuria and serum osteoprotegerin is independently associated with the severity of diabetic nephropathy. Metabolism. 2011;60:1064–1069. doi: 10.1016/j.metabol.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 35.Røysland R, Masson S, Omland T, et al. Prognostic value of osteoprotegerin in chronic heart failure: the GISSI-HF trial. Am Heart J. 2010;160:286–293. doi: 10.1016/j.ahj.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 36.Ueland T, Dahl CP, Kjekshus J, et al. Osteoprotegerin predicts progression of chronic heart failure: results from CORONA. Circ Heart Fail. 2011;4:145–152. doi: 10.1161/CIRCHEARTFAILURE.110.957332. [DOI] [PubMed] [Google Scholar]
- 37.Ueland T, Yndestad A, Dahl CP, et al. TNF revisited: Osteoprotegerin and TNF-related molecules in heart failure. Curr Heart Fail Rep. 2012;9:92–100. doi: 10.1007/s11897-012-0088-6. [DOI] [PubMed] [Google Scholar]
- 38.Montagnana M, Lippi G, Danese E, et al. The role of osteoprotegerin in cardiovascular disease. Ann Med. 2013;45:254–264. doi: 10.3109/07853890.2012.727019. [DOI] [PubMed] [Google Scholar]
- 39.Condliffe R, Pickworth JA, Hopkinson K, et al. Serum osteoprotegerin is increased and predicts survival in idiopathic pulmonary arterial hypertension. Pulm Circ. 2012;2:21–27. doi: 10.4103/2045-8932.94819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cracowski JL, Yaici A, Sitbon O, et al. Biomarkers as prognostic factors in pulmonary arterial hypertension. Rationale and study design. Rev Mal Respir. 2004;21:1137–1143. doi: 10.1016/s0761-8425(04)71589-2. [DOI] [PubMed] [Google Scholar]
- 41.Brun H, Holmstrøm H, Thaulow E, et al. Patients with pulmonary hypertension related to congenital systemic-to-pulmonary shunts are characterized by inflammation involving endothelial cell activation and platelet-mediated inflammation. Congenit Heart Dis. 2009;4:153–159. doi: 10.1111/j.1747-0803.2009.00297.x. [DOI] [PubMed] [Google Scholar]
- 42.Koyama T, Watanabe H, Kobukai Y, et al. Beneficial effects of adaptive servo ventilation in patients with chronic heart failure. Circ J. 2010;74:2118–2124. doi: 10.1253/circj.cj-10-0082. [DOI] [PubMed] [Google Scholar]