Abstract
The klotho gene has been identified as an aging suppressor that encodes a protein involved in cardiovascular disease (CVD). The inactivation of the klotho gene causes serious systemic disorders resembling human aging, such as atherosclerosis, diffuse vascular calcification and shortened life span. Klotho has been demonstrated to ameliorate vascular endothelial dysfunction and delay vascular calcification. Furthermore, klotho gene polymorphisms in the human are associated with various cardiovascular events. Recent experiments show that klotho may reduce transient receptor potential canonical6 (TRPC6) channels, resulting in protecting the heart from hypertrophy and systolic dysfunction. Fibroblast growth factor23 (FGF23) is a bone-derived hormone that plays an important role in the regulation of phosphate and vitamin D metabolism. FGF23 accelerates urinary phosphate excretion and suppresses 1,25-dihydroxy vitaminD3 (1,25(OH)2D3) synthesis in the presence of FGF receptor1 (FGFR1) and its co-receptor klotho, principally in the kidney. The hormonal affects of circulating klotho protein and FGF23 on vascular and heart have contributed to an understanding of their roles in the pathophysiology of arterial stiffness and left ventricular hypertrophy. Klotho and FGF23 appear to play a critical role in the pathogenesis of vascular disease, and may represent a novel potential therapeutic strategy for clinical intervention.
Keywords: Cardiac hypertrophy, Cardiovascular, Fibroblast growth factor23, Gene polymorphisms, Klotho, Vascular calcification
1. Introduction
Aging is a multifactorial process often characterized by a progressive decline in physiological functions. Advanced age is accompanied by a high prevalence of cardiovascular risk factors such as diabetes, hypertension and chronic kidney disease, all of which increase cardiovascular morbidity and mortality. The klotho (or more precisely alpha-klotho) has been identified as an aging suppressor protein, which is expressed predominantly in kidney tubular epithelium, and to a lesser extent in the parathyroid gland, epithelial cells of the choroid plexus and human vascular tissue.[1] The expression of the klotho gene is decreased during aging, which may contribute to age-related cardiovascular disease (CVD) in humans.
The human homolog of mouse klotho is composed of five exons and extends over 50 kb on chromosome 13q12, and the human klotho protein has 86% identity with the mouse counterpart.[2] The klotho gene was originally identified as an aging suppressor gene. Inactivation of klotho gene causes a syndrome resembling human aging, including osteoporosis, hyperphosphatemia, atherosclerosis, ectopic calcification, and shortened life span. It has been shown that transgenic mice with an over-expression of the klotho gene have a longer life-span.[3]
Klotho gene encodes a type I single-pass transmembrane protein that is related to β-glucuronidases. The full-length klotho protein has a large extracellular amino-terminal domain and a small intracellular carboxy-terminal domain. Klotho protein exists in at least two forms, the membrane form and the secreted form and each form has distinct functions.[4] Membrane klotho interacts with fibroblast growth factor (FGF) receptors (especially FGFR1) to form a high-affinity for FGF23, induces phosphate excretion into the urine and reduces the level of serum 1,25(OH)2D3, and inhibits secretion of parathyroid hormone. Secreted klotho protein functions as a humoral factor that modifies several ion channels and transporters, and other processes, including insulin and insulin-like growth factor-1 signaling. Soluble klotho also is involved in the regulation of nitric oxide (NO) production and the integrity and permeability of endothelium.[5],[6] The soluble form results from the cleavage of extracellular domain of full-length protein by secretases, which can be detected in blood, urine and cerebral spinal fluid.[7]
FGF23 is a mutated gene indentified in autosomal dominant hypophosphatemic rickets,[8] with 251-amino acid residuals in the protein. FGF23 is composed of an amino-terminal signal peptide (residues 1–24), a FGF-like sequence (residues 25–180), and a unique carboxyl-terminal extended sequence (residues 181–251).[9] The FGF family has 23 proteins that regulate cell proliferation, migration, differentiation and survival. Several known subfamilies of human FGFs have been defined. The FGF19 subfamily comprises FGF19, FGF21 and FGF23. FGF23 is synthesized by osteocytes, and regulates phosphate homeostasis via FGFR1 receptor signaling in the presence of klotho. In cooperation with klotho, FGF23 regulates blood calcium level by suppressing the synthesis of 1,25(OH)2D3 and reabsorption of phosphate in the proximal convoluted part of the nephron. FGF23 also can negatively regulate the secretion of parathyroid hormone.[10]
Increasingly, clinical and experimental studies have verified that an increased FGF23 level mediated adverse cardiovascular outcomes among patients with end-stage renal diseases. In the cross sectional studies, serum FGF-23 level is associated with high atherosclerotic burden,[11] endothelial dysfunction, arterial stiffness and vascular calcification.[12]
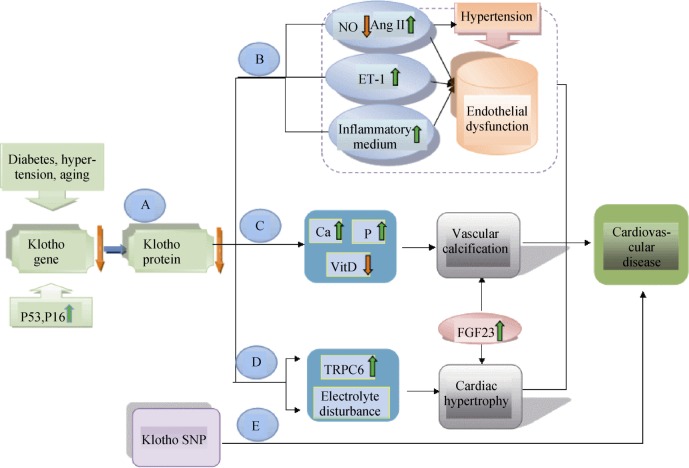
Klotho modulates the endothelial dysfunction via reducing the NO production and increasing oxidative stress, thereby promoting the progression of CVD. The klotho-FGF receptor complex binds to FGF23 with a higher affinity. Klotho and FGF23 may function in a common single transduction pathway to accelerate vascular calcification and cardiac hypertrophy. Indeed, klotho-null mice or FGF23-deficient mice shows early atherosclerosis, vascular calcifications, impaired angiogenesis, and vasculogenesis, suggesting the impact of this pairing on the pathophysiology of cardiovascular disorders.[13] In this paper, we will summarize the key areas of research on the significant roles of the anti-aging klotho and FGF23 in cardiovascular diseases (Figure 1).
Figure 1. Putative mechanisms by which Klotho and FGF23 result in cardiovascular diseases.
Main reasons for Klotho/FGF23 axis causing cardiovascular diseases are: vascular endothelial dysfunction, diffuse vascular calcification, and cardiac hypertrophy. Diabetes, hypertension, aging, and overexpression of P16 and P53 tumor suppressor proteins decrease klotho expression (A). Lower expression of klotho protein in humans reduces NO production and increases the level of ET-1 and inflammatory medium. These reactions can aggravate endothelial dysfunction (B). The klotho/FGF23 axis participates in vascular calcification, which is caused by deficiency of active vitamin D, hypercalcemia and hyperphosphatemia (C). Heart damage by lower expression of klotho is mediated by upregulation of TRPC6 and electrolyte disturbance. At the same time, higher level of FGF23 also separately affects cardiac structure and function (D). In addition, polymorphisms of klotho gene are associated with various cardiovascular events (E). Ang II: angiotensin II; ET-1: endothelin-1; FGF23: fibroblast growth factor23; NO: nitric oxide; SNP: single-nucleotide polymorphisms; TRPC6: transient receptor potential canonical6; VitD: vitamin D.
2. The role of klotho in CVD
The expression human klotho gene is under strict control of cis- and trans-acting factors. The expression in kidney is minimal in prenatal life, but increases after birth.[14] A reduction of klotho in kidney, serum, and urine has been observed in normal aging and in diseases characterized by premature vascular aging, such as renal diseases, diabetes and hypertension.[15] As demonstrated previously in humans, higher plasma klotho concentrations are independently related to a lower likelihood of cardiovascular events.[16]
Recent studies have suggested that the human functional variant of the klotho gene is associated with both reduced longevity and coronary-artery disease,[17] thus the klotho gene may play a role in the development of aging-related phenotypes in humans. Klotho-deficient gene mice exhibits a syndrome resembling accelerating human aging in conjunction with NO production and hyperphosphatemia, which causes serious disorders such as vascular endothelial dysfunction, atherosclerosis, diffuse vascular calcification, and cardiac hypertrophy.[18]
3. The effects of klotho on endothelial dysfunction
Endothelial dysfunction is a systemic pathological condition resulting from an imbalance between vasorelaxation and vasoconstriction. The imbalance is mainly caused by a reduced NO bioavailability and/or increased generation of reactive oxygen species. Inflammatory stimuli and hypertension accelerate the course of endothelial dysfunction. Klotho may induce NO production, or exhibit an anti-inflammatory action to protect the endothelium. In aging, the vasodilatory capacity is decreased with a reduced sensitivity to vasodilators (e.g., NO). On the other hand, vasoconstriction is increased with ligands as angiotensin II (AngII) and endothelin-1.[19] These disturbances are explained by an age-related endothelial dysfunction. Cumulative oxidative injury plays a major role in the process of cell aging. Oxidative stress and generation of free radicals increase with aging. Anti-aging soluble klotho has an important role in maintaining endothelial wall homeostasis and promoting the health of the vasculature. Klotho protein increases the NO availability and protects against endothelial dysfunction.[19],[20]
NO is an acetylcholine-induced vasodilator and is the main endogenous vasodilator. NO is synthesized from the precursor L-arginine by nitric oxide synthase (NOS) and can be inhibited by the superoxide anion and the NOS endogenous inhibitor, asymmetric dimethylarginine (ADMA).[20] NO not only produces vasodilatation but also prevents atherogenesis by suppressing smooth muscle cell proliferation, and inhibiting adhesion molecules and platelet aggregation. There is growing evidence that the klotho protein induces the expression of mitochondrial superoxide dismutase (MnSOD) and suppression of NADPH oxidases to protect against oxidative stress. Genetic mutation of klotho decreases the SOD expression while overexpression increases the expression, indicating that klotho may regulate SOD expression. Wang, et al.[21] showed that klotho not only down-regulated Nox2 protein expression and intracellular superoxide production, but also attenuated AngII-induced superoxide production, oxidative damage, and apoptosis. Klotho-induced suppression of Nox2 expression may be mediated by the cAMP/PKA pathway [Nox2 and its homologs are catalytic subunits of NAD(P)H oxidase].[21] Additionally, the circulatory FGF23 level independently correlates with endothelial dysfunction, possibly due to asymmetric dimethylarginine.[22]
In the meantime, Six, et al.[23] used three in vitro models (mouse aorta rings, human umbilical vein endothelial cells, and human vascular smooth muscle cells) to explore whether soluble klotho and FGF23 exert direct and rapid effects on the vessel wall. In conclusion, although phosphate, soluble klotho and FGF23 separately stimulate aorta contraction, klotho mitigates the effects of phosphate and FGF23 on contractility via increased NO production, thereby protecting the vessel to some extent against potentially noxious effects of high phosphate or FGF23 concentrations. Thus, klotho also mitigates the direct effects of combined phosphate and FGF23 on aortic contractility, thereby protecting the vessel wall.
According to a WHO (2012) report, hypertension causes approximately half the deaths in the CVD. The influence of hypertension in the progression of several pathologic conditions suggests hypertension playing a major etiologic role in the development of ischemic heart disease and cardiac and renal failure. Klotho plays a role in the regulation of vascular tone through homeostatic interplay between NO and the renin angiotensin system. Both klotho and FGF23 participate in the regulation of vascular tone.[24] This might explain the observed association of the klotho and/or FGF23 with high blood pressure.
The delivery of klotho gene enhances blood IL-10 level in spontaneous hypertensive rats (SHRs), suggesting that klotho may suppress inflammation to protect the vascular wall integrity.[23],[24] Soluble klotho suppresses tumor necrosis factor-α-induced expression of adhesion molecules, such as the intercellular adhesion molecule-1and vascular cell adhesion molecule-1(VCAM-1) in endothelium. In another clinical study, Malmqvist, et al.[25] showed that FGF-23 stimulated the production of cell adhesion molecules, E-selectin and VCAM. Higher levels of E-selectin and VCAM demonstrated the activation of vascular endothelium, which frequently occurs in essential hypertension patients with endothelial dysfunction.
Endothelin-1 (ET-1) plays an important role in the regulation of endothelial function. ET-1 increases vasoconstriction and promotes vascular remodeling with aging to aggravate endothelial injury.[26] The action of ET-1 is mediated by ETA receptors. Activation of ETA receptors increases NADPH oxidase activity and superoxide production which may contribute to aging-related kidney damage to down-regulation of renal klotho protein expression.[27] Because destroying the stability of the endothelial cell, aging-related changes in ET-1 and ET receptors are also reported in the heart.[28] Therefore, higher klotho may reduce the lower level of ET-1 to protect endothelium.
4. The effects of klotho/FGF23 on vascular calcification
A sensitive and specific assay has recently been developed for the measurement of soluble klotho in humans, and demonstrate a correlation between serum klotho and the prevalence of CVD, and we hypothesize that low serum klotho is associated with signs of vascular dysfunction, such as vascular calcification.[29],[30] Hypertension, diabetes and hyperlipidemia are risk factors for vascular calcification associated with cardiovascular events. Although condition-specific factors are likely to drive the calcification process, mineral accumulation in the vasculature is also equally important to calcification.
Indeed, in vascular smooth muscle cells (VSMCs) which are the predominant cell type involved in vascular calcification, these cells can undergo phenotypic transition to osteoblastic and osteocytic cells in a calcified environment.[31],[32] Furthermore, it has been demonstrated that phosphate accelerates this phenotypic differentiation. Soluble klotho ameliorates vascular calcification by enhancing phosphaturia, preserving glomerular filtration and directly inhibiting phosphate uptake by vascular smooth muscle. Phosphate reabsorption in the kidney is mediated by the sodium-phosphate co-transporters type 2 (Na/Pi-2a and Na/Pi-2c), which are expressed mainly in proximal tubular cells at their apical brush border membrane. Klotho inhibits Na/Pi-2a and Na/Pi-2c to diminish phosphate reabsorption, and increases urinary phosphate excretion to induce hypophosphatemia.[33] In addition, klotho proteins interact with the VEGFR-2/TRPC-1 complex on the surface of endothelial cells to maintain endothelial integrity.[34]
The serum level of klotho is an independent determinant of arterial stiffness, even after adjusting for age, gender, mean blood pressure, use of antihypertensive drugs, drinking and smoking.[35] Membrane klotho may inhibit type 2A Na-phosphate co-transporter by decreasing the numbers of cell-surface Na-phosphate co-transporter, thereby reducing cellular phosphate uptake in renal proximal tubular cells.[36] Unlike membrane klotho, serum klotho cannot efficiently support the FGF23-induced activation of FGF signaling. Instead, secreted klotho protein activates transient receptor potential vanilloid-5 (TRPV5), a calcium channel involved in calcium reabsorption in kidney,[37] which results in hypercalcemia to induce vascular calcification. Furthermore, circulating klotho functions as a hormone that prevents vascular calcification. A previous study by Lim, et al.[38] demonstrates klotho expressions in human arteries and aortic smooth muscle cells. Vessel-produced klotho has been shown to be an endogenous inhibitor of calcification. Klotho exerts direct cardiovascular-protective effects, which suggests that klotho may exhibit anti-aging effects in the arterial system.
The expression of klotho gene in kidney is confined to the distal tubule and is also the site for initial FGF23 binding and signaling. However, renal phosphate reabsorption mainly occurs in the proximal tubule. How FGF23 signaling in the distal tubule translates into decreased phosphate reabsorption in the proximal tubule remained unclear.
FGF23 is considered as a predictive marker of all-causes and cardiovascular mortality. Emerging evidence suggests that higher levels of serum FGF23 are associated with impaired vasoreactivity and increased arterial stiffness.[39] Serum FGF-23 is related to progression of coronary artery calcification score (CACS) independent of serum phosphorus levels.[40] Besides, FGF-23 may play a major role in progression of vascular calcification, especially at the early stages of calcification. In addition, the FGF23/klotho axis participates in vascular calcification, which may, in part, be caused by a deficiency of active vitamin D. Clinical studies have demonstrated that serum calcitriol levels are inversely correlated with coronary artery calcification.[41] Active vitamin D and its analog against vascular calcification is mediated by secreted klotho.[42] Vascular klotho deficiency potentiates the development of human artery calcification and mediates resistance to FGF23.[43]
Thus, based on current studies, we hypothesize that FGF23 predicts cardiovascular outcomes mainly by portraying vascular stress due to parallel changes in mineral metabolism and active vitamin D levels. In order to elucidate the potential mechanism through which FGF-23 may be exerting it's specific effect on VSMCs, Zhu, et al.[44] examined the PI3K/Akt and MAPK/ERK1/2 signaling pathways in VSMC calcification. This study suggested that the ERK1/2 signaling pathway is essential for FGF-23 to promote murine VSMC calcification in vitro. Furthermore, the study demonstrated that expression of FGFR1 and FGFR3 in human arteries from healthy individuals and CKD patients, is critical for vascular calcification, and that the physical association of Klotho, FGFR1 and FGFR3 is an essential mechanism to induce critical vascular calcification.[45]
The Klotho/FGF23 axis mediates vascular calcification by maintaining the mineral homeostasis and increasing the active vitamin D. The Erk1/2 signaling pathway may be essential for FGF23 to speed up murine VSMC calcification, and represent a novel therapeutic strategy for clinical interventions.
5. The effects of klotho and FGF23 on cardiac hypertrophy
We found that klotho attenuated the progression of hypertension and heart damage in spontaneous hypertensive rats,[46] but the mechanisms were unclear. However, a recent study provides compelling evidence indicating that soluble klotho protects the heart against stress-induced cardiac hypertrophy and remodeling. In the study, the authors conclude that cardioprotection by klotho is mediated by down-regulation of transient receptor potential canonical6 (TRPC6).[47]
The heart responds to injury and stress signals by pathological growth and remodeling that often progresses to heart failure and sudden death. One key regulatory step in the development of pathological cardiac growth and remodeling is activation of calmodulin-dependent, serine-threonine protein phosphatase calcineurin by abnormal calcium signaling. The TRPC family channels are Ca2+-permeable cation channels expressed in the plasma membrane of many tissues including the heart. The TRPC family includes seven members, and is divided into two groups based on structure and function. Evidence indicates that Ca2+ influx through cardiac TRPC channels is important in calcineurin signaling pathway and hypertrophic growth of hearts.[48] The expression of TRPC channels (such as TRPC6) are increased in hypertrophic hearts stimulated by various types or forms of stresses, and their down-regulation protects against cardiac hypertrophy. Soluble klotho inhibits cardiac TRPC6 channels and protects the heart against stress-induced pathological hypertrophy and remodeling.[49] Klotho-deficient mice demonstrate no cardiac dysfunction at baseline, but develop exaggerated cardiomyopathy in response to ISO treatment. The ISO treatment causes upregulation of TRPC6 mRNA in the heart, and also shows that IGFs (such as IGF1) provides a tonic stimulation for exocytosis of TRPC6 via phosphoinositide-3-kinase (PI3K). Activation of PI3K and the downstream Akt signaling cascade in the heart is important for physiological cardiac growth, but it can also lead to pathological cardiac hypertrophy.[50] Therefore, pharmacological TRPC antagonism is in development as a potential treatment of cardiac hypertrophy.
There are several possible mechanisms by which FGF-23 may affect cardiac structure and function, indirectly and directly.[51],[52] It remains controversial, however, as to whether FGF-23 has a direct effect on the cardiovascular system.
Recent studies have shown an association between circulating FGF23 levels and pathologic cardiovascular conditions, including left ventricular hypertrophy. Such associations have been investigated mainly in patients with chronic kidney disease.[53] Considering that cardiovascular events are increased in patients with a low glomerular filtration rate, the possibility exists that increased FGF23 levels mediate an adverse cardiovascular outcome among patients with end-stage renal disease. Circulating levels of calcium, phosphorus, and 1,25(OH)2D3 are reported to be associated with not only vascular calcification, but also ventricular hypertrophy, and elevated PTH levels are also associated with left ventricular mass and severity of heart failure.[54] The regulation exerted by FGF23 on calcitriol synthesis is clearly defined by the differential effects of FGF23 on the gene expression of the two enzymes that regulate the level of 1,25-(OH)2D3. FGF23 down-regulates the expression of Cyp27b1 gene that encodes 1-alpha-hydroxylase, the enzyme converting 25-(OH)D3 to 1,25-(OH)2D3. Meanwhile, it also stimulates the degradation pathway through the up-regulation of the Cyp24 gene that encodes 24-hydroxylase, the enzyme inactivating 1,25-OH)2D3,[55],[56] therefore, the level of 1,25(OH)2D3 is decreased. However, FGF23 is also able to inhibit the expression and production of PTH in parathyroid glands.
To sum up, FGF23 is involved into regulating the level of calcium, phosphorus, vitamin D, and PTH. Hence, FGF23 is related to development of cardiac dysfunction. Whether modulation of klotho concentration and FGF23 activity would improve cardiac outcome in such a high risk population awaits further investigation.
FGF23 is not only an essential component of the klotho-FGF23 receptor complex, but also has functions of its own. As such, fibroblast growth factors and their receptors are highly conserved signaling molecules that have been implicated in postnatal cardiac remodeling.
Interestingly, FGF23 and FGF2 have been shown to play pathophysiological roles in the heart. These FGFs are involved in cardiac remodeling via unique action mechanisms.[57] Faul, et al.[58] showed that intramyocardial and intravenous injections of FGF23 in mice resulted in left ventricular hypertrophy, which can be inhibited by an inhibitor of the FGF receptor. Cardiac hypertrophic effects of FGF23 are mediated by FGFR-dependent activation of the calcineurin-nuclear factor of activated T cells (NFAT) signaling cascade, but do not require klotho as a co-receptor.[59] FGF2 is the multifunctional protein synthesized as high- and low- molecular weight isoforms. It is expressed by many cell types, including cardiomyocytes and fibroblasts, which also express FGF receptors (FGFRs).[60] Although FGF23 and high-FGF2 induce a similar hypertrophic phenotype in isolated neonatal rat ventricle myocyte (NRVMs), they use different downstream signaling pathways. High-FGF2-induced hypertrophy depends primarily on activation of Erk, whereas FGF23-induced hypertrophy is partially Erk dependent, but primarily requires PLC-γ-calcineurin-NFAT activation.[61],[62] The PI3K-Akt pathway appears to contribute only modestly to high-FGF2 and not to FGF23 induced hypertrophy. These findings indicated that FGF23 and FGF2 cause pathological hypertrophy by activating different branches of canonical FGFR signaling in the heart.
6. The effects of polymorphisms of klotho on CVD
Polymorphisms in the human klotho gene are associated with longevity; various cardiovascular events like stroke; coronary artery disease as well as the cardiovascular risk factors like reduced high density lipoprotein-cholesterol levels; and elevated systolic blood pressure. In humans, single-nucleotide polymorphisms (SNP) in the klotho gene have been associated with arteriosclerotic diseases and metabolic syndrome, including hypertension.
Several studies have identified polymorphisms in klotho and association with a variety of phenotypes. Three SNPs, G-395A, rs564481 (C1818T) and rs9536314 (KL-VS), have been genotyped because they have been previously associated with a variety of phenotypes. KL-VS is a haplotype variant which consists of two amino acid substitutions (F352V and C370S) and can be defined by a single SNP, rs9536314. Several groups have associated KL-VS status with longevity, coronary artery disease and bone mineral density. [63] The C1818T (rs564481) variant is less well studied, but the T allele has been shown to be associated with a reduced risk of coronary artery disease in Korean women,[64] and with decreased bone density in Japanese women.[65] G-395A is a promoter SNP that has been shown to be associated with CVD in Korean women,[64] and decreased bone density in postmenopausal European-derived and Asian women.[65] In our previous study, we investigated the association of three single nucleotide polymorphism site distributions of klotho gene with diseases. AA gene type of G-395A polymorphism in the promotor region leads to hypertension and the GG gene type is probably a protective factor of coronary arteriosclerotic heart disease and diabetes. Heterozygosity of F352V and C370S in the coding region is protective for coronary arteriosclerotic heart disease.[66] But, as the associated SNPs are intronic, the genetic mechanism behind the increased expression is unknown.
The KL-VS variant of klotho consists of six sequence variants in perfect linkage disequilibrium, two of which result in the amino acid substitutions, F352V and C370S. Due to the presence of complete linkage disequilibrium across the SNPs, the single variant, F352V, has been used to tag the KL-VS haplotype. The presence of phenylalanine at the position 352 in the human klotho gene is highly conserved and its substitution by valine has been demonstrated to alter the in vitro excretion and activity of the protein.[67] Arking, et al.[68] reported a positive association between the functional KL-VS variant of klotho and early-onset occult CVD. In another study, Majumdar, et al.[69] reported a positive association of the KL-VS homozygosity of klotho with the incidence of early-onset ischemic stroke, particularly in the young (age ≤ 40 years).
The C1818T variant locates in the fourth exon. As a silent mutation, the variant is not likely to be functionally relevant. However, there is a report which demonstrates the association between coronary artery disease (CAD) and the C1818T variant in Koreans.[63] In the Asian population, the C1818T is associated with increased cardiovascular risk, blood pressure, lipid levels and so on. There have been two studies to research the relationship between C1818T polymorphism and CAD.[70],[71] The TT or CT gene type of the klotho gene participates in CAD. Therefore, our hypothesis is that the T allele may be harmful to humans.
The G395A in the promoter region of the klotho gene and is suggested to modify bone mineral density, which appears to be related to arterial atherosclerosis and calcification, both of which may potentially influence CAD. A study in Japan involving 197 patients demonstrates that the incidence of A allele carriers of the klotho gene is significantly higher in the CAD group than in the control group (29.9% vs. 19.0%), and A allele is an independent predictor of CAD.[72] Rhee, et al.[64] used multivariate analysis and identified the klotho gene G-395A mutant as an independent risk factor of CAD. They also implied that the klotho gene mutant exerts its potency in the young population rather than in elder CAD patient. Vascular access dysfunction occurs earlier and is more common in the A-allele carriers. In patients with essential hypertension, the frequency of A-allele carriers is increased in patients < 60 years of age compared to that found in patients > 60 years.[64] In fact, the 395A variant of the G-395A SNP may be protective against essential hypertension by up-regulating klotho expression.
7. Conclusions
Klotho is a novel humoral factor that confers resistance to oxidative stress and cardiac hypertrophy, and ameliorates mineral homeostasis. FGF23 and klotho may have independent actions on the cardiovascular systems, affecting vascular function, calcification and atherosclerosis as well as arteriolosclerosis. Their interactive activities may also have direct and indirect effects on interdependent cardiovascular pathophysiology. However, recent experimental studies have shown that overexpression of P16 and P53 tumor suppressor proteins decreases klotho expression. In contrast, increasing the concentration of calcium and phosphate ions stimulates klotho expression.[73] There is no doubt that we can suggest some interventions to increase or maintain serum klotho levels to prevent cardiovascular events and mortality.
Acknowledgments
This study was supported by National Key Clinical Specialties Construction Program of China (No. [2013]544).
References
- 1.Kuro-o M. Klotho. Pflugers Arch. 2010;459:333–343. doi: 10.1007/s00424-009-0722-7. [DOI] [PubMed] [Google Scholar]
- 2.Kuro-o M, Matsumura Y, Aizawa H, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 3.Devara J, Syed B, Chien A, et al. Validation of an immunoassay for soluble klotho protein: decreased levels in diabetes and increased levels in chronic kidney disease. Am J Clin Pathol. 2012;137:479–485. doi: 10.1309/AJCPGPMAF7SFRBO4. [DOI] [PubMed] [Google Scholar]
- 4.Matsumura Y, Aizawa H, Shiraki-Iida T, et al. Identification of the human klotho gene and its two transcripts encoding membrane and secreted klotho protein. Biochem Biophys Res Commun. 1998;242:626–630. doi: 10.1006/bbrc.1997.8019. [DOI] [PubMed] [Google Scholar]
- 5.Yamamoto M, Clark JD, Pastor JV, et al. Regulation of oxidative stress by the anti-aging hormone klotho. J Biol Chem. 2005;280:38029–38034. doi: 10.1074/jbc.M509039200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartke A. Long-lived Klotho mice: new insights into the roles of IGF-1 and insulin in aging. Trends Endocrinol Metab. 2006;17:33–35. doi: 10.1016/j.tem.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 7.Kurosu H, Yamamoto M, Clark JD, et al. Suppression of aging in mice by the hormone klotho. Science. 2005;309:1829–1833. doi: 10.1126/science.1112766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.White KE, Evans WE, Speer MC, et al. Autosomal dominant hypophosphateamic ricketsis associated with mutations in FGF23. Nat Genet. 2000;26:345–348. doi: 10.1038/81664. [DOI] [PubMed] [Google Scholar]
- 9.Fukumoto S, Yamashita T. FGF23 is a hormone-regulating phosphate metabolism-unique biological characteristics of FGF23. Bone. 2007;40:1190–1195. doi: 10.1016/j.bone.2006.12.062. [DOI] [PubMed] [Google Scholar]
- 10.Fukumoto S. Actions and mode of actions of FGF19 subfamily members. Endocr J. 2008;55:23–31. doi: 10.1507/endocrj.kr07e-002. [DOI] [PubMed] [Google Scholar]
- 11.Mirza MA, Hansen T, Johansson L, et al. Relationship between circulating FGF23 and total body atherosclerosis in the community. Nephrol Dial Transplant. 2009;24:3125–3131. doi: 10.1093/ndt/gfp205. [DOI] [PubMed] [Google Scholar]
- 12.Mirza MA, Larsson A, Lind L, et al. Circulating fibroblast growth factor-23 is associated with vascular dysfunction in the community. Atherosclerosis. 2009;205:385–390. doi: 10.1016/j.atherosclerosis.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Arking DE, Krebsova A, Macek M, Sr, et al. Association of human aging with a functional variant of klotho. Proc Natl Acad Sci USA. 2002;99:856–861. doi: 10.1073/pnas.022484299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohyama Y, Kurabayashi M, Masuda H, et al. Molecular cloning of rat Klotho cDNA: markedly decreased expression of Klotho by acute inflammatory stress. Biochem Biophys Res Commun. 1998;251:920–925. doi: 10.1006/bbrc.1998.9576. [DOI] [PubMed] [Google Scholar]
- 15.Ohata Y, Arahori H, Namba N, et al. Circulating levels of soluble alpha-klotho are markedly elevated in human umbilical cord blood. J Clin Endocrinol Metab. 2011;96:E943–E947. doi: 10.1210/jc.2010-2357. [DOI] [PubMed] [Google Scholar]
- 16.Richard DS, Anne RC, Stefania B, et al. Plasma klotho and cardiovascular disease in adults. J Am Geriatr Soc. 2011;59:1596–1601. doi: 10.1111/j.1532-5415.2011.03558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Navarro GJ, Donate CJ, Martínez SR, et al. Reduced Klotho is associated with the presence and severity of coronary artery disease. Heart. 2014;100:34–40. doi: 10.1136/heartjnl-2013-304746. [DOI] [PubMed] [Google Scholar]
- 18.Arking DE, Atzmon G, Arking A, et al. Association between a functional variant of the Klotho gene and high-density lipoprotein cholesterol, blood pressure, stroke, and longevity. Circ Res. 2005;96:412–418. doi: 10.1161/01.RES.0000157171.04054.30. [DOI] [PubMed] [Google Scholar]
- 19.Barton M. Ageing as a determinant of renal and vascular disease: role of endothelial factors. Nephrol Dial Transpl. 2005;20:485–490. doi: 10.1093/ndt/gfh689. [DOI] [PubMed] [Google Scholar]
- 20.Ignarro LJ. Physiology and pathophysiology of nitric oxide. Kidney Int. 1996;55:S2–S5. [PubMed] [Google Scholar]
- 21.Wang YH, Kuro-o M, Sun Z. Klotho gene delivery suppresses Nox2 expression and attenuates oxidative stress in rat aortic smooth muscle cells via the cAMP-PKA pathway. Aging Cell. 2012;11:410–417. doi: 10.1111/j.1474-9726.2012.00796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Donate-Correa J, Mora-Fernández C, Martínez-Sanz R, et al. Expression of FGF23/KLOTHO system in human vascular tissue. Int J Cardiol. 2013;165:179–183. doi: 10.1016/j.ijcard.2011.08.850. [DOI] [PubMed] [Google Scholar]
- 23.Six I, Okazaki H, Gross P, et al. Direct, acute effects of klotho and FGF23 on vascular smooth muscle and endothelium. PLoS One. 2014;9:e93423. doi: 10.1371/journal.pone.0093423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saito Y, Yamagishi T, Nakamura T, et al. Klotho protein protects against endothelial dysfunction. Biochem Biophys Res Commun. 1998;248:324–329. doi: 10.1006/bbrc.1998.8943. [DOI] [PubMed] [Google Scholar]
- 25.Malmqvist K, Wallen HN, Held C, et al. Soluble cell adhesion molecules in hypertensive concentric left ventricular hypertrophy. J Hypertens. 2002;20:1563–1569. doi: 10.1097/00004872-200208000-00019. [DOI] [PubMed] [Google Scholar]
- 26.Dhaun N, Goddard J, Kohan DE, et al. Role of endothelin-1 in clinical hypertension: 20 years on. Hypertension. 2008;52:452–459. doi: 10.1161/HYPERTENSIONAHA.108.117366. [DOI] [PubMed] [Google Scholar]
- 27.Zuo Z, Lei H, Wang X, et al. Aging-related kidney damage is associated with a decrease in klotho expression and an increase in superoxide production. Aging (Dordr) 2011;33:261–274. doi: 10.1007/s11357-010-9176-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang YH, Sun ZJ. Antiaging gene klotho regulates endothelin-1 levels and endothelin receptor subtype B expression in kidneys of spontaneously hypertensive rates. J Hypertens. 2014;32:1629–1636. doi: 10.1097/HJH.0000000000000233. [DOI] [PubMed] [Google Scholar]
- 29.Ix JH, Katz R, Kestenbaum BR, et al. Fibroblast growth factor-23 and death, heart failure, and cardiovascular events in community-living individuals: cardiovascular health study. J Am Coll Cardiol. 2012;60:200–207. doi: 10.1016/j.jacc.2012.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuro-o M. Klotho as a regulator of fibroblast growth factor signaling and phosphate/calcium metabolism. Curr Opin Nephrol Hypertens. 2006;15:437–441. doi: 10.1097/01.mnh.0000232885.81142.83. [DOI] [PubMed] [Google Scholar]
- 31.Speer MY, Chien YC, Quan M, et al. Smooth muscle cells deficient in osteopontin have enhanced susceptibility to calcification in vitro. Cardiovasc Res. 2005;66:324–333. doi: 10.1016/j.cardiores.2005.01.023. [DOI] [PubMed] [Google Scholar]
- 32.Zhu D, Mackenzie NCW, Millán JL, et al. The appearance and modulation of osteocyte marker expression during calcification of vascular smooth muscle cells. PLoS One. 2011;6:e19595. doi: 10.1371/journal.pone.0019595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fukumoto S, Yamashita T. FGF23 is a hormone-regulating phosphate metabolism—Unique biological characteristics of FGF23. Bone. 2007;40:1190–1195. doi: 10.1016/j.bone.2006.12.062. [DOI] [PubMed] [Google Scholar]
- 34.Kusaba T, Okigaki M, Matui A, et al. Klotho is associated with VEGF receptor-2 and the transient receptor potential canonical-1 Ca2+ channel to maintain endothelial integrity. Proc Natl Acad Sci USA. 2010;107:19308–193013. doi: 10.1073/pnas.1008544107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kitagawa M, Sugiyama H, Morinaga H, et al. A decreased level of serum soluble klotho is an independent biomarker associated with arterial stiffness in patients with chronic kidney disease. PLos One. 2013;8:e56695. doi: 10.1371/journal.pone.0056695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hu MC, Shi M, Zhang J, et al. Klotho: a novel phosphaturic substance acting as an autocrine enzyme in the renal proximal tubule. Faseb J. 2010;24:3438–3450. doi: 10.1096/fj.10-154765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cha SK, Ortega B, Kurosu H, et al. Removal of sialic acid involving Klotho causes cell-surface retention of TRPV5 channel via binding to galectin-1. Proc Natl Acad Sci USA. 2008;105:9805–9810. doi: 10.1073/pnas.0803223105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lim K, Lu TS, Molostvov G, et al. Vascular klotho deficiency potentiates the development of human artery calcification and mediates resistance to fibroblast growth factor 23. Circulation. 2012;125:2243–2255. doi: 10.1161/CIRCULATIONAHA.111.053405. [DOI] [PubMed] [Google Scholar]
- 39.Mirza MA, Larsson A, Lind L, et al. Circulating fibroblast growth factor-23 is associated with vascular dysfunction in the community. Atherosclerosis. 2009;205:385–390. doi: 10.1016/j.atherosclerosis.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 40.Ozkok A, Kelik C, Karahan GE, et al. FGF-23 associated with the progression of coronary artery calcification in hemodialysis patients. BMC Nephrology. 2013;14:241. doi: 10.1186/1471-2369-14-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Watson KE, Abrolat ML, Malone LL, et al. Active serum vitamin D levels are inversely correlated with coronary calcification. Circulation. 1997;96:1755–1760. doi: 10.1161/01.cir.96.6.1755. [DOI] [PubMed] [Google Scholar]
- 42.Lau WL, Leaf EM, Hu MC, et al. Vitamin D receptor agonists increase klotho and osteopontin while decreasing aortic calcification in mice with chronic kidney disease fed a high phosphate diet. Kidney Int. 2012;82:1261–1270. doi: 10.1038/ki.2012.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mark RH, Ichiro K, Ryan F, et al. The role of vitamin D in the FGF23, Klotho, and Phosphate Bone-Kidneyendocrine axis. Rev Endocr Metab Disord. 2012;13:57–69. doi: 10.1007/s11154-011-9199-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhu D, Mackenzie NC, Millan JL, et al. A protective role for FGF-23 in local defence against disrupted arterial wall integrity? Mol Cell Endocrinol. 2013;372:1–11. doi: 10.1016/j.mce.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu S, Tang W, Fang J, et al. Novel regulators of Fgf23 expression and mineralization in Hyp bone. Mol Endocrinol. 2009;23:1505–1518. doi: 10.1210/me.2009-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li BS, Ma HX, Wang YJ, et al. Klotho gene attenuates the progression of hypertension and heart damage in spontaneous hypertensive rats. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2012;29:662–668. doi: 10.3760/cma.j.issn.1003-9406.2012.06.008. [Article in Chinese] [DOI] [PubMed] [Google Scholar]
- 47.Xie J, Cha SK, An SW, et al. Cardioprotection by klotho through downregulation of TRPC6 channels in the mouse heart. Nat Commun. 2012;3:1238. doi: 10.1038/ncomms2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eder P, Molkentin JD. TRPC6 channels as effectors of cardiac hypertrophy. Circulation. 2011;108:265–272. doi: 10.1161/CIRCRESAHA.110.225888. [DOI] [PubMed] [Google Scholar]
- 49.Frey N, Katus HA, Olson EN, et al. Hypertrophy of the heart: a new therapeutic target? Circulation. 2004;109:1580–1589. doi: 10.1161/01.CIR.0000120390.68287.BB. [DOI] [PubMed] [Google Scholar]
- 50.Gutierrez OM, Januzzi JL, Isakova T, et al. Fibroblast growth factor 23 and left ventricular hypertrophy in chronic kidney disease. Circulation. 2009;119:2545–2552. doi: 10.1161/CIRCULATIONAHA.108.844506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Isha A, Noriko I, Joachim HI, et al. Fibroblast growth factor–23 and cardiac structure and function. J Am Heart Assoc. 2014;3:e000584. doi: 10.1161/JAHA.113.000584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee JH, Bell D, Hensrud DD, et al. Vitamin D deficiency an important, common, and easily treatable cardiovascular risk factor? J Am Coll Cardiol. 2008;52:1949–1956. doi: 10.1016/j.jacc.2008.08.050. [DOI] [PubMed] [Google Scholar]
- 53.Shibata K, Fujita SI, Morita H, et al. Association between circulating fibroblast growth factor 23, a-Klotho, and the left ventricular ejection fraction and left ventricular mass in cardiology inpatients. PLos One. 2013;8:e73184. doi: 10.1371/journal.pone.0073184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Visser M, Kestenbaum B, Siscovick DS, et al. Relation of vitamin D and parathyroid hormone to cardiac biomarkers and to left ventricular mass (from the Cardiovascular Health Study) Am J Cardiol. 2013;111:418–424. doi: 10.1016/j.amjcard.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Donate-Correa J, Muros-de-Fuentes M, Mora-Fernández C, et al. FGF23/Klotho axis: phosphorus, mineral metabolism and beyond. Cytokine Growth Factor Rev. 2012;23:37–46. doi: 10.1016/j.cytogfr.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 56.Liu S, Tang W, Zhou J, et al. Fibroblast growth factor 23 is a counter-regulatory phosphaturic hormone for vitamin D. J Am Soc Nephrol. 2006;17:1305–1315. doi: 10.1681/ASN.2005111185. [DOI] [PubMed] [Google Scholar]
- 57.Virag JA, Rolle ML, Reece J, et al. Fibroblast growth factor-2 regulates myocardial infarct repair: effects on cell and ventricular function. Am J Pathol. 2007;171:1431–1440. doi: 10.2353/ajpath.2007.070003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Faul C, Amaral AP, Oskouei B, et al. FGF23 induces left ventricular hypertrophy. J Clin Invest. 2011;121:4393–4408. doi: 10.1172/JCI46122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Itoh N, Ohta H. Pathophysiological roles of FGF signaling in the heart. Card Electr. 2013;4:1–4. doi: 10.3389/fphys.2013.00247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Eisemann A, Ahn JA, Graziani G, et al. Alternative splicing generates at least five different isoforms of the human basic-FGF receptor. Oncogene. 1991;6:1195–1202. [PubMed] [Google Scholar]
- 61.Santiago JJ, McNaughton LJ, Koleini N, et al. High molecular weight fibroblast growth factor-2 in the human heart is a potential target for prevention of cardiac remodeling. PLoS ONE. 2014;9:e97281. doi: 10.1371/journal.pone.0097281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wilkins BJ, Molkentin JD. Calcium-calcineurin signaling in the regulation of cardiac hypertrophy. Biochem Biophys Res Commun. 2004;322:1178–1191. doi: 10.1016/j.bbrc.2004.07.121. [DOI] [PubMed] [Google Scholar]
- 63.Choi YJ, Joo NR, Cho GY, et al. Klotho gene polymorphism is associated with coronary artery stenosis but not with coronary calcification in Korean population. Int Heart. 2009;50:23–32. doi: 10.1536/ihj.50.23. [DOI] [PubMed] [Google Scholar]
- 64.Rhee EJ, Oh KW, Yun EJ, et al. Relationship between polymorphisms G395A in promoter and C1818T in exon 4 of the klotho gene with glucose metabolism and cardiovascular risk factors in Korean women. J Endocrinol Invest. 2006;29:613–618. doi: 10.1007/BF03344160. [DOI] [PubMed] [Google Scholar]
- 65.Kawano K, Ogata N, Chiano M, et al. Klotho gene polymorphisms associated with bone density of aged postmenopausal women. J Bone Miner Res. 2002;17:1744–1751. doi: 10.1359/jbmr.2002.17.10.1744. [DOI] [PubMed] [Google Scholar]
- 66.Hua MJ, MA HX, Niu YN, et al. Association of three single nucleotide polymorphism site distribution of Klotho gene with diseases. China Journal of Modern Medicine. 2008;19:1174–1178. [Article in Chinese] [Google Scholar]
- 67.Majumdar V, Christopher R. Association of exonic variants of Klotho with metabolic syndrome in Asian Indians. Clinica Chimica Acta. 2011;412:1116–1121. doi: 10.1016/j.cca.2011.02.034. [DOI] [PubMed] [Google Scholar]
- 68.Arking DE, Becker DM, Yanek LR, et al. Klotho allele status and the risk of early-onset occult coronary artery disease. Am J Hum Genet. 2003;72:1154–1161. doi: 10.1086/375035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Majumdar V, Nagaraja D, Christopher R. Association of the functional KL-VS variant of Klotho gene with early-onset ischemic stroke. Biochem Biophys Res Commun. 2010;403:412–416. doi: 10.1016/j.bbrc.2010.11.045. [DOI] [PubMed] [Google Scholar]
- 70.He GP, Jin MF, Gao L, et al. Klotho gene C-1818T Polymorphism in elderly patients with acute coronary syndrome. Chinese Circulation Journal. 2008;23:203–206. [Google Scholar]
- 71.Jin MF, He GP, Gao lei, et al. Relationship between the Klotho gene C-1818T polymorphism and unstable angina pectoris. Journal of Jiang Su University. 2008;18:322–326. [Google Scholar]
- 72.Imamura A, Okumara K, Ogaway, et al. Klotho gene Polymorphism may be a genetic risk factor for atherosclerotic coronary artery disease but not for vasospstic angina in Japanese. Clin Chim Acta. 2006;371:66–70. doi: 10.1016/j.cca.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 73.Turan K, Ata P. Effects of intra- and extracellular factors on anti-aging klotho gene expression. Genet Mol Res. 2011;10:2009–2023. doi: 10.4238/vol10-3gmr1261. [DOI] [PubMed] [Google Scholar]