Abstract
Background
Despite the proven benefits of clopidogrel combined aspirin therapy for coronary artery disease (CAD), CAD patients with metabolic syndrome (MS) still tend to have coronary thrombotic events. We aimed to investigate the influence of metabolic risk factors on the efficacy of clopidogrel treatment in patients with CAD undergoing percutaneous coronary intervention (PCI).
Methods
Cohorts of 168 MS and 168 non-MS subjects with CAD identified by coronary angiography (CAG) were enrolled in our study. MS was defined by modified Adult Treatment Panel III criteria. All subjects had taken 100 mg aspirin and 75 mg clopidogrel daily for more than 1 month, and administered loading doses of 600 mg clopidogrel and 300 mg aspirin before PCI. Blood samples were taken 24 h after the loading doses of clopidogrel and aspirin. Platelet aggregation was measured using light transmittance aggregometry (LTA) and thrombelastography (TEG). Clopidogrel resistance was defined as more than 50% adenosine diphosphate (ADP) induced platelet aggregation as measured by TEG.
Results
Platelet aggregation inhibition rate by ADP was significantly lower in patients with MS as measured both by TEG (55% ± 31% vs. 68% ± 32%; P < 0.001) and LTA (29% ± 23% vs. 42% ± 29%; P < 0.001). In the multivariate analysis, elderly [OR (95% CI): 1.483 (1.047–6.248); P = 0.002], obesity [OR (95% CI): 3.608 (1.241–10.488); P = 0.018], high fasting plasma glucose level [OR (95% CI): 2.717 (1.176–6.277); P = 0.019] and hyperuricemia [OR (95% CI): 2.583 (1.095–6.094); P = 0.030] were all statistically risk factors for clopidogrel resistance. CAD patients with diabetes and obesity were more likely to have clopidogrel resistance than the CAD patients without diabetes and obesity [75% (61/81) vs. 43% (67/156); P < 0.001].
Conclusions
CAD patients with MS appeared to have poorer antiplatelet response to clopidogrel compared to those without MS. Obesity, diabetes and hyperuricemia were all significantly associated with clopidogrel resistance.
Keywords: Clopidogrel resistance, Coronary artery disease, Metabolic syndrome
1. Introduction
Metabolic syndrome (MS) patients are always in hypercoagulability,[1],[2] clinically resulting in coronary thrombosis and acute coronary syndrome (ACS).[3]–[5] Combining clopidogrel with aspirin therapy decreases the thrombosis events of patients with ACS.[6]–[9] However, a high individual variability in response to clopidogrel has been reported, and a previously published article showed the prevalence of poor response to clopidogrel varied from 4% to 30%,[10] depending on definition, study population and method. Poor response to clopidogrel is an independent predictor of major cardiac events in patients undergoing percutaneous coronary intervention (PCI).[11]–[13] Patients with diabetes tend to have clopidogrel resistance.[14]–[15] These findings have aroused queries of whether metabolic risk factors influence the antiplatelet effect of clopidogrel.
2. Methods
2.1. Study population
A total of 336 subjects with CAD (168 with MS and 168 non-MS) were enrolled from January to November 2013. MS was defined as the presence of any three of the five following components according to the modified Adult Treatment Panel III criteria: central obesity (defined as waist circumference ≥= 90 cm for men and ≥ 80 cm for women, according to the criteria for Asians); triglycerides ≥ 150 mg/dL (1.7 mmol/L); high-density lipoprotein (HDL) cholesterol < 40 mg/dL (1.03 mmol/L) in men or < 50 mg/dL (1.29 mmol/L) in women; fasting glucose ≥ 100 mg/dL (5.6 mmol/L) or previously diagnosed with type 2 diabetes; and blood pressure ≥ 130/85 mmHg or on antihypertensive medication. Excluded criteria: treating with any other drug with a known effect on platelet function or warfarin; acute myocardial infarction or cerebrovascular event within 3 month; platelet count < 100 × 109/L or > 300 × 109/L. All patients have received dual antiplatelet therapy (aspirin 100mg qd and clopidogrel 75 mg qd) for at least one month before PCI. Loading doses of clopidegrel (600 mg) and aspirin (300 mg) were administered to all enrolled patients, and blood samples were collected after PCI. All participants signed the written informed consents.
2.2. Laboratory investigations
Blood samples were analyzed using thrombelastography (TEG) mapping assay (Haemoscope Corp., USA). For the adenosine diphosphate (ADP) pathway, the method sensitivity was 80% and specificity was 86%. For the arachidonic acid (AA) pathway, the sensitivity was 100% and specificity was 92%. The cut off point for clopidogrel resistance was set at ≥ 50% ADP-induced platelet aggregation after stimulation with 2 µmol/L ADP at baseline as measured by TEG. All patients below the cut off points were defined as exhibiting normal on-treatment platelet reactivity. Aspirin resistance was defined as more than 50% platelet aggregation after stimulation by 1 mmol/L AA as measured by TEG.
2.3. Statistical analysis
All data were checked for normality and equality of variances. Continuous data were represented as mean ± SD if data were normally distributed. If not, data were presented as median and percentiles, or log-transformed to obtain normal distribution. For data with normal distribution, a two-sided t-test was used to test the differences between the two groups. For data that were not normally distributed, the Mann-Whitney test was used for the comparison of the two groups. To test differences in proportions between two or more groups, we used Fisher's exact test or the Chi-square test. Independent clopidogrel resistance risk factors were calculated using multivariable logistic regression models. A two-sided P < 0.05 was considered statistically significant. Graphs are presented with a mean bar if normally distributed or a median bar, if not. Statistical analyses were performed using SPSS 19.0 (SPSS Inc., Chicago, IL, USA).
3. Results
3.1. Clinical characteristics and prognosis of the subjects with MS
Clinical characteristics of the study subjects are presented in Table 1. As expected, body mass index (BMI), low-density lipoprotein (LDL), triglycerides, fasting plasma glucose (FPG) were all significantly greater in subjects with MS, while HDL cholesterol was significantly lower in the MS group. The incidence of metabolic risk factors in the MS group were obesity in 107 (64%) subjects, high triglyceride in 77 (46%) subjects, low HDL cholesterol in 32 (19%) subjects, hypertension in 101 (60%) subjects and diabetes in 91 (54%) subjects. Patients with MS had more triple-vessel disease (41% vs. 8.1%; P < 0.001), and associated with more stent implanted (2.1 ± 0.9 vs. 1.4 ± 1.1; P < 0.001). The MS group had more recurrent myocardial infarction events within 6 months (4.8% vs. 1.4%; P < 0.001).
Table 1. Clinical characteristics in CAD patients with and without MS.
| MS group (n = 168) | Control group (n = 168) | P | |
| Age (yr) | 59 ± 11 | 61 ± 11 | 0.116 |
| Men | 75.6% | 80.4% | 0.306 |
| BMI (kg/cm2) | 27.23 ± 3.21 | 24.08 ± 2.84 | < 0.001 |
| Waist girth (cm) | 96 ± 4 | 82 ± 7 | < 0.001 |
| SBP (mmHg) | 135 ± 18 | 130 ± 19 | 0.054 |
| DBP (mmHg) | 76 ± 10 | 74 ± 10 | 0.346 |
| Current smokers | 45.2% | 44.6% | 0.909 |
| Alcohol consumption | 24.4% | 20.9% | 0.464 |
| TC (mmol/L) | 4.29 ± 2.28 | 4.05 ± 1.96 | 0.36 |
| TG (mmol/L) | 2.46 ± 1.38 | 1.69 ± 1.21 | < 0.001 |
| HDL (mmol/L) | 1.48 ± 1.08 | 2.06 ± 1.52 | < 0.001 |
| LDL (mmol/L) | 2.14 ± 1.00 | 1.86 ± 0.92 | 0.014 |
| FPG (mmol/L) | 6.96 ± 2.19 | 6.22 ± 2.12 | 0.003 |
| Albumin (g/L) | 41 ± 4 | 40 ± 4 | 0.306 |
| Uric acid (µmol/L) | 355 ± 91 | 341 ± 95 | 0.322 |
| SCr (µmol/L) | 96.3 ± 109.3 | 77.4 ± 27.8 | 0.075 |
| BUN (mmol/L) | 7.65 ± 8.79 | 6.14 ± 2.95 | 0.09 |
| Triple vessel disease | 41% | 8.1% | < 0.001 |
| The number of stents | 2.1 ± 0.9 | 1.4 ± 1.1 | < 0.001 |
| MI patients discharged within 6 months | 4.8% | 1.4% | < 0.001 |
Data are presented as mean ± SD or percent unless other indicated. BMI: body mass index; BUN: blood urea nitrogen; CAD: coronary artery disease; DBP: diastolic blood pressure; FPG: fasting plasma glucose; HDL: high-density lipoprotein; LDL: low-density lipoprotein; MI: myocardial infarction; MS: metabolic syndrome; SBP: systolic blood pressure; TC: total cholesterol; TG: total triglycerides; SCr: serum creatinine.
3.2. The aggregation of platelet in MS patients treated with clopidogrel
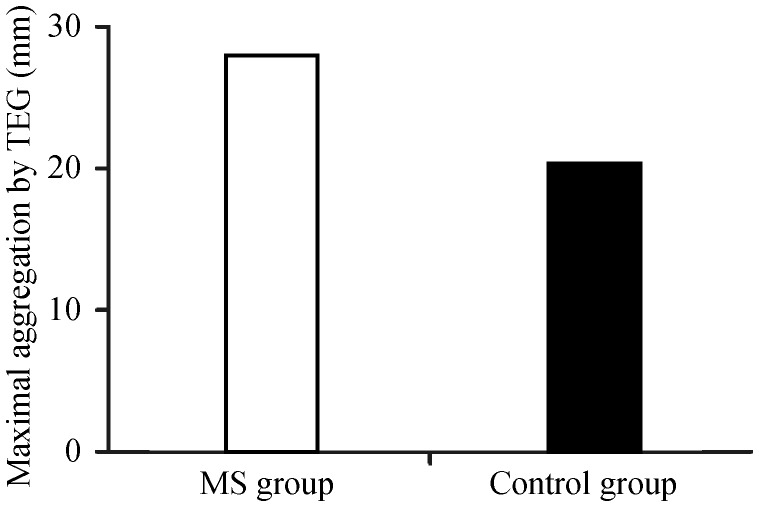
Clopidogrel aggregation inhibition rate induced by ADP was significantly lower in patients with MS than control patients as measured by both TEG (55% ± 31% vs. 68% ± 32%; P < 0.001) and LTA (29% ± 23% vs. 42% ± 29%; P < 0.001), which are shown in Table 2. The frequency of clopidogrel resistance in subjects with MS was 63% (106/168), significantly higher than 43% (73/168) in control subjects by the TEG (P = 0.001) test. The aggregation function (maximum amplitude) of platelet induced by ADP in MS patients was significantly higher than control patients (P < 0.001), which is show in Figure 1.
Table 2. Platelet aggregation and frequency of clopidogrel resistance in MS and non-MS patients.
| MS Subjects (n = 168) | Control Subjects (n = 168) | P | |
| TEG total MA | 63 ± 6 | 62 ± 7 | 0.018 |
| TEG inhibition rate by AA (%) | 77 ± 26 | 77 ± 21 | 0.759 |
| TEG inhibition rate by ADP (%) | 55 ± 31 | 68 ± 32 | < 0.001 |
| LTA inhibition rate by ADP (%) | 29 ± 23 | 42 ± 29 | < 0.001 |
| Clopidogrel resistance by TEG (%) | (106/168) 63% | (73/168) 43% | 0.001 |
Data are presented as mean ± SD unless other indicated. AA: arachidonic acid; ADP: adenosine diphosphate; LTA: light transmittance aggregometry; MA: maximum amplitude; MS: Metabolic syndrome; TEG: thrombelastography.
Figure 1. The aggregation function of platelet induced by ADP in MS patients and control group.
ADP: adenosine diphosphate; MS: Metabolic syndrome; TEG: thrombelastography.
3.3. The relationship between the clopidogrel resistance and risk factors of MS
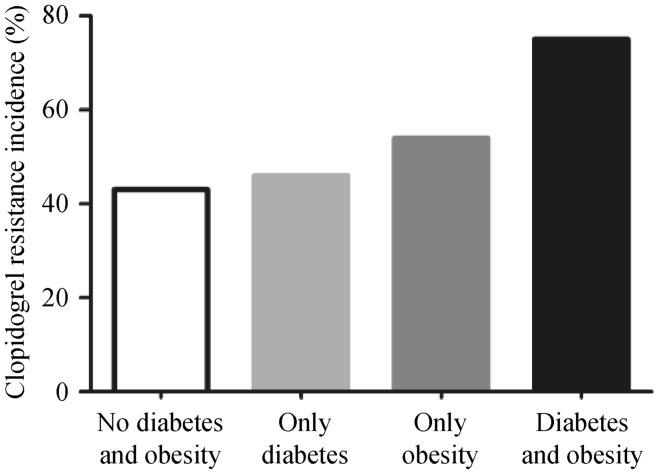
In the multivariate analysis, among the MS risk factors, elderly (> 65 year) [OR (95% CI): 1.483 (1.047–6.248), P = 0.002], obesity [OR (95% CI): 3.608 (1.241–10.488); P = 0.018], high FPG level [OR (95% CI): 2.717 (1.176–6.277); P = 0.019] and hyperuricemia (uric acid > 420 µmol/L) [OR (95% CI): 2.583 (1.095–6.094); P = 0.030] were all statistically significant risk factors for clopidogrel resistance, which is shown in Table 3. The CAD patients with diabetes and obesity were more likely to have clopidogrel resistance than the CAD patients without the diabetes and obesity [75% (61/81) vs. 43% (67/156), P < 0.001], which is shown in Figure 2.
Table 3. Independent risk factors of clopidogrel resistance in MS patients.
| Variables | β | P | OR | 95%CI |
| Sex (Gender) | 0.215 | 0.629 | 1.240 | 0.51–2.965 |
| Age (> 65 yr) | 1.113 | 0.002 | 1.483 | 1.047–6.248 |
| Hypertension | −0.368 | 0.532 | 0.692 | 0.21–2.197 |
| Obesity | 1.283 | 0.018 | 3.608 | 1.241–10.488 |
| Hypertriglyceridemia | −0.232 | 0.580 | 0.793 | 0.34–0.805 |
| Low HDL | 0.443 | 0.286 | 0.286 | 0.690–3.510 |
| High fasting plasma glucose | 0.999 | 0.019 | 2.717 | 1.176–6.277 |
| Hyperuricemia | 0.949 | 0.030 | 2.583 | 1.095–6.094 |
Low HDL: cholesterol < 40 mg/dL (1.03 mmol/L) in men or < 50 mg/dL (1.29 mmol/L) in women; high fasting glucose: fasting plasma glucose ≥ 100 mg/dL (5.6 mmol/L) or previously diagnosed with type 2 diabetes; hyperuricemia: uric acid > 420 µmol/L. HDL: high-density Lipoprotein; MS: metabolic syndrome.
Figure 2. Clopidogrel resistance in different metabolic disease.
4. Discussion
The clinical trial found that dual antiplatelet therapy with clopidogrel and aspirin in ACS reduced major cardiac vascular events by 20% when compared with aspirin monotherapy.[9] Our study demonstrated that the overall response to clopidogrel was significantly reduced in subjects with MS, and the frequency of clopidogrel resistance was significantly higher in patients with MS (63%). This finding suggested that 75 mg daily dose and 600 mg loading dose of clopidogrel might have not played its expected role in six out of every ten patients with MS. MS poor response of patients to clopidogrel has been associated with poor prognosis. Our results also may explain the reduced cardiovascular protection by clopidogrel in MS group, and poor short- and long-term outcomes for MS patients, which always have impaired myocardial perfusion after PCI.[16]
The mechanism of poor clopidogrel response in patients with MS is probably multifarious. Insulin could reduce platelet aggregation by inhibiting the P2Y12 pathway through insulin receptors. Insulin resistance and obesity could up-regulate the P2Y12 receptor, which is associated with clopidogrel resistance,[14],[15] and uric acid has a close relationship with other metabolic related factors, and is accompanied by arterial intima injury and increased the platelet aggregation effect.[17]
The findings of our study were in accordance with previous studies that had demonstrated higher platelet reactivity treated with clopidogrel in populations with single metabolic abnormalities, such as hyperglycemia,[18] insulin resistance,[19] or obesity,[20] However, the relation between clopidogrel resistance and hyperuricemia has not been previously reported. Uric acid is a risk factor for MS and plays an important role in insulin resistance and other metabolic factors during the development of MS.[21]–[23] A higher uric acid level, which is associated with high activity of platelet, might trigger thrombosis events. Large randomized trials have showed that patients with hyperuricemia had an increased risk of cardiovascular events.[24]
It is important for patients with MS to be tested with TEG, and MS patients with clopidogrel resistance should receive more positive antiplatelet treatment. Previous studies have found that the clopidogrel inhibition rate induced by ADP in patients with CAD was 68% when the dose was 75 mg, and 83.3% when the dose was 150 mg.[25] It has been suggested that increasing the current clopidogrel maintenance dose to 150 mg can further inhibit ADP-induced platelet aggregation for these MS patients. We can also prescribe ticagrelor rather than clopidogrel for MS patients with clopidogrel resistance. The PLATO trial compared ticagrelor with clopidogrel for the prevention of cardiac events in 18,624 ACS patients,[26] and found ACS patients with obesity or diabetes could benefit from the ticagrelor therapy. In brief, MS patients with clopidogrel resistance should received individual antiplatelet therapy.
Limitations of the study are as followed: (1) the limit number of enrolled patients resulted in a relatively low statistical power; (2) TEG itself is not the gold standard for platelet activity, and we have performed another method of LTA to test the platelet aggregation induced by ADP due to the lack of a gold standard for clopidogrel resistance; and (3) our study did not test the marker of insulin resistance, such as glycosylated hemoglobin and insulin levels.
References
- 1.Dentali F, Squizzato A, Ageno W. The metabolic syndrome as a risk factor for venous and arterial thrombosis. Semin Thromb Hemost. 2009;35:451–457. doi: 10.1055/s-0029-1234140. [DOI] [PubMed] [Google Scholar]
- 2.Nieuwdorp M, Stroes ES, Meijers JC, et al. Hypercoagulability in the metabolic syndrome. Curr Opin Pharmacol. 2005;5:155–159. doi: 10.1016/j.coph.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 3.Vaduganathan M, Alviar CL, Arikan ME, et al. Platelet reactivity and response to aspirin in subjects with the metabolic syndrome. Am Heart J. 2008;156:1002.e1–1002.e7. doi: 10.1016/j.ahj.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Kakafika AI, Liberopoulos EN, Karagiannis A, et al. Dyslipidaemia, hypercoagulability and the metabolic syndrome. Curr Vasc Pharmacol. 2006;4:175–183. doi: 10.2174/157016106777698432. [DOI] [PubMed] [Google Scholar]
- 5.Daly CA, Hildebrandt P, Bertrand M, et al. Adverse prognosis associated with the metabolic syndrome in established coronary artery disease: data from the EUROPA trial. Heart. 2007;93:1406–1411. doi: 10.1136/hrt.2006.113084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhatt DL, Bertrand ME, Berger PB, et al. Meta-analysis of randomized and registry comparisons of ticlopidine with clopidogrel after stenting. J Am Coll Cardiol. 2002;39:9–14. doi: 10.1016/s0735-1097(01)01713-2. [DOI] [PubMed] [Google Scholar]
- 7.Steinhubl SR, Berger PB, Mann JT, 3rd, et al. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA. 2002;288:2411–2420. doi: 10.1001/jama.288.19.2411. [DOI] [PubMed] [Google Scholar]
- 8.Yusuf S, Zhao F, Mehta SR, et al. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. 2001;345:494–502. doi: 10.1056/NEJMoa010746. [DOI] [PubMed] [Google Scholar]
- 9.Fox KA, Mehta SR, Peters R, et al. Benefits and risks of the combination of clopidogrel and aspirin in patients undergoing surgical revascularization for non-ST-elevation acute coronary syndrome: the Clopidogrel in Unstable angina to prevent Recurrent ischemic Events (CURE) Trial. Circulation. 2004;110:1202–1208. doi: 10.1161/01.CIR.0000140675.85342.1B. [DOI] [PubMed] [Google Scholar]
- 10.Buch AN, Singh S, Roy P, et al. Measuring aspirin resistance, clopidogrel responsiveness, and postprocedural markers of myonecrosis in patients undergoing percutaneous coronary intervention. Am J Cardiol. 2007;99:1518–1522. doi: 10.1016/j.amjcard.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 11.Campo G, Fileti L, de Cesare N, et al. Long-term clinical outcome based on aspirin and clopidogrel responsiveness status after elective percutaneous coronary intervention: a 3T/2R (tailoring treatment with tirofiban in patients showing resistance to aspirin and/or resistance to clopidogrel) trial substudy. J Am Coll Cardiol. 2010;56:1447–1455. doi: 10.1016/j.jacc.2010.03.103. [DOI] [PubMed] [Google Scholar]
- 12.Park KW, Jeon KH, Kang SH, et al. Clinical outcomes of high on-treatment platelet reactivity in Koreans receiving elective percutaneous coronary intervention (from results of the CROSS VERIFY study) Am J Cardiol. 2011;108:1556–1563. doi: 10.1016/j.amjcard.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 13.Wang L, Wang X, Chen F. Clopidogrel resistance is associated with long-term thrombotic events in patients implanted with drug-eluting stents. Drugs R D. 2010;10:219–224. doi: 10.2165/11539580-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Angiolillo DJ, Bernardo E, Ramirez C, et al. Insulin therapy is associated with platelet dysfunction in patients with type 2 diabetes mellitus on dual oral antiplatelet treatment. J Am Coll Cardiol. 2006;48:298–304. doi: 10.1016/j.jacc.2006.03.038. [DOI] [PubMed] [Google Scholar]
- 15.Angiolillo DJ, Fernandez-Ortiz A, Bernardo E, et al. Platelet function profiles in patients with type 2 diabetes and coronary artery disease on combined aspirin and clopidogrel treatment. Diabetes. 2005;54:2430–2450. doi: 10.2337/diabetes.54.8.2430. [DOI] [PubMed] [Google Scholar]
- 16.Celik T, Turhan H, Kursaklioglu H, et al. Impact of metabolic syndrome on myocardial perfusion grade after primary percutaneous coronary intervention in patients with acute ST elevation myocardial infarction. Coron Artery Dis. 2006;17:339–343. doi: 10.1097/00019501-200606000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Verdoia M, Barbieri L, Schaffer A, et al. Impact of diabetes on uric acid and its relationship with the extent of coronary artery disease and platelet aggregation: a single-centre cohort study. Metabolism. 2014;63:640–646. doi: 10.1016/j.metabol.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 18.Vaidyula VR, Boden G, Rao AK. Platelet and monocyte activation by hyperglycemia and hyperinsulinemia in healthy subjects. Platelets. 2006;17:577–585. doi: 10.1080/09537100600760814. [DOI] [PubMed] [Google Scholar]
- 19.Schneider DJ. Abnormalities of coagulation, platelet function, and fibrinolysis associated with syndromes of insulin resistance. Coron Artery Dis. 2005;16:473–476. doi: 10.1097/00019501-200512000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Sibbing D, von Beckerath O, Schomig A, et al. Impact of body mass index on platelet aggregation after administration of a high loading dose of 600 mg of clopidogrel before percutaneous coronary intervention. Am J Cardiol. 2007;100:203–205. doi: 10.1016/j.amjcard.2007.02.081. [DOI] [PubMed] [Google Scholar]
- 21.Gonçalves JP, Oliveira A, Severo M, et al. Cross-sectional and longitudinal associations between serum uric acid and metabolic syndrome. Endocrine. 2012;41:450–457. doi: 10.1007/s12020-012-9629-8. [DOI] [PubMed] [Google Scholar]
- 22.Santos RD. Elevated uric acid, the metabolic syndrome and cardiovascular disease: cause, consequence, or just a not so innocent bystander. Endocrine. 2012;41:350–352. doi: 10.1007/s12020-012-9657-4. [DOI] [PubMed] [Google Scholar]
- 23.Hsieh CH, Lin JD, Wu CZ, et al. Is lower uric acid level better? A combined cross-sectional and longitudinal study in the elderly. Endocrine. 2014;47:806–815. doi: 10.1007/s12020-014-0201-6. [DOI] [PubMed] [Google Scholar]
- 24.Gaffo AL, Roseman JM, Jacobs DR, Jr, et al. Serum urate and its relationship with alcoholic beverage intake in men and women: findings from the Coronary Artery Risk Development in Young Adults (CARDIA) cohort. Ann Rheum Dis. 2010;69:1965–1970. doi: 10.1136/ard.2010.129429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Madsen EH, Saw J, Kristensen SR, et al. Long-term aspirin and clopidogrel response evaluated by light transmission aggregometry, VerifyNow, and thrombelastography in patients undergoing percutaneous coronary intervention. Clin Chem. 2010;56:839–847. doi: 10.1373/clinchem.2009.137471. [DOI] [PubMed] [Google Scholar]
- 26.Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045–1057. doi: 10.1056/NEJMoa0904327. [DOI] [PubMed] [Google Scholar]