Abstract
Objective
To evaluate the epicardial fat tissue thickness (EFTT) as a diagnostic criterion for geriatric patients with metabolic syndrome (MetS).
Methods
Sixty geriatric patients over 65 years of age were recruited for the study. Patients were divided into two groups: Group 1 (n = 30) consisted of patients with MetS; Group 2 (n = 30) consisted of patients without MetS. Echocardiography was used to measure EFTT in all patients, and blood samples were analyzed for biochemical parameters.
Results
Compared to Group 2, EFTT levels of Group 1 were statistically higher (P < 0.05). In a binary logistic regression analysis, EFTT levels served as the independent factor for metabolic syndrome (B = 17.35, SE = 4.93, Wald = 12.36, P < 0.001). Receivers operating characteristic Curve (ROC-curve) analysis revealed that EFTT predicted MetS with 96.7% sensitivity and 86.7% specificity above the level of 7.3 mm [area under the curve = 0.969; 95% confidence interval (CI): 0.928–1.00].
Conclusions
The present study demonstrated that serum EFTT levels were higher in geriatric patients with MetS and can therefore be used as a diagnostic criterion for MetS.
Keywords: Epicardial fat tissue thickness, Geriatrics, Metabolic syndrome
1. Introduction
Metabolic syndrome (MetS) is a set of risks consisting of the following components: central obesity, increased systemic blood pressure, and impaired lipid and glucose metabolism. Compared to healthy individuals, individuals with MetS have three times the risk of myocardial infarction and ischemic stroke, twice the risk of death from these illnesses, and five times the risk of diabetes mellitus. A major component of MetS, visceral obesity, is also an important risk factor for cardiovascular diseases.[1],[2] Epicardial fat tissue thickness (EFTT) is also a visceral adipose tissue, and recent studies have observed increased EFTT levels in patients with cardiovascular diseases and MetS.[3],[4]
Waist circumference (WC) is a criterion for identifying MetS because this measurement shows general abdominal visceral adiposity; currently, however, WC is not considered to be a reliable indicator due to difficulties in distinguishing it from subcutaneous adipose tissue. According to recent studies, EFTT is considered to be an indicator for visceral adipose tissue. Although measurement of EFTT is easily obtained via high-speed CT and MRI, these methods may not be practical because of their associated costs and radiation exposure.[5],[6]
Although many studies have investigated the relationship between EFTT and MetS in the non-geriatric population, and waist circumference results for predicting the MetS have been shown to be unreliable, no research study has been performed to find the cut-off value of EFTT in geriatric patients with MetS. Therefore, in the current study, we aimed to determine the relationship between EFTT and the components of MetS in geriatric patients, as well as whether EFTT levels would be a suitable screening test and a criterion for diagnosing MetS in geriatric patients.
2. Methods
Patients admitted to the Harran University School of Medicine and who were over the age of 65 were included in this cross-sectional study, and those who participated in the study provided written consent. The ethics committee of the hospital granted its approval, thereby conforming to the principles of the 2nd Declaration of Helsinki.
A total of 60 geriatric patients were recruited for the study. All study subjects were divided into two groups: Group 1 (n = 30) consisted of patients with MetS, based on NCEP ATP III criteria,[7] and Group 2 (n = 30) consisted of healthy subjects. The exclusion criteria were as follows: recent acute infectious illness; any inflammatory or infiltrative disorder or autoimmune diseases; any evidence of liver, kidney, or respiratory disease; uncontrolled essential hypertension; heart failure; malignancy; regular alcohol use; hypothyroidism; hyperthyroidism; and an inadequate echocardiographic image. A detailed history of disease and demographic information was received from all patients, and physical examinations were performed on all patients upon admission.
2.1. Baseline definitions and measurements
Height and weight were measured according to standardized protocols. Body mass index was calculated as the weight in kilograms divided by the height in meters squared (kg/m2). Blood pressure was measured using a mercury manometer. Systolic blood pressure (SPB) and diastolic blood pressure (DBP) readings were recorded using the phase V Korotkoff sound, and the Korotkoff phase IV were recorded if phase V was not available. Body compositions were assessed with bioelectrical impedance analysis (BF 510, Omron Healthcare Co. Ltd., Kyoto, Japan). The WC was measured at the narrowest point of the waist while the abdomen was relaxed. A currently active smoking status was considered positive for smoking. A history of antihyperlipidemic drug use, a low-density lipoprotein (LDL) cholesterol > 160 mg/dL, or a triglyceride (TG) > 200 mg/dL were defined as hyperlipidemia. Diabetes was diagnosed according to the American Diabetes Association criteria.[8]
2.2. Evaluation of blood samples
Five-milliliter blood samples were taken from the forearm venous blood vessels of each subject and placed in Eppendorf tubes. The tubes were centrifuged at 1,500 r/min for 10 min to obtain serum samples for the measurements of biochemical parameters. After labeling in the biochemistry laboratory, all serum samples were stored at –80°C until the day of analysis. Serum urea, creatinine, fasting blood glucose (FBG), aspartate aminotransferase (AST), alanine aminotransferase (ALT), fT3, fT4, C-reactive protein (CRP), glycated hemoglobin (HbA1c), triglycerides (TG), total cholesterol (TC), and high-density and low-density lipoprotein cholesterol (HDL-C and LDL-C) levels were determined using commercially available assay kits (Roche, USA) with an auto-analyzer (Roche Cobas Integra 800 auto-analyzer).
2.3. Measurement of epicardial fat tissue thickness
Measurement was performed using a Vivid E9 (General Electric Medical Systems, Milwaukee, Wisconsin) echocardiography device and a 2.5 MHz echocardiography probe in the cardiology department. The EFTT measurement was performed using a two-dimensional echocardiographic method by transthoracic echocardiography, with subjects in the left lateral decubitus position. The EFTT was measured on the free wall of the right ventricle from both parasternal long- and short-axis views at the mid-ventricle during end diastole (marked by the R wave on the ECG recording). The maximum values at each site were measured, and the average value was considered. The measured value was expressed in cm.
2.4. Statistical analysis
Statistical Package for the Social Sciences 20.0 (SPSS, Chicago, Illinois) was used for all statistical analyses. The one-sample Kolmogorov-Smirnov test was used to verify the normality of data distributions. Results are expressed as mean ± SD. The Chi-square test was used for categorical variables. An independent sample t-test was used to analyze parametric numerical data, and the Mann–Whitney U-test was used to analyze non-parametric data. Pearson correlation coefficients were used to determine correlations between the components of MetS and EFTT in elderly patients with MetS. Binary logistic regression analysis was performed to find independent predictors of MetS in geriatric patients. ROC-curve analysis was performed to find the cut-off values of serum EFTT levels and the components of MetS in geriatric patients with MetS. Values of P < 0.05 were considered statistically significant for all results.
3. Results
Biochemical and demographic characteristics of all subjects are presented in Table 1. There was no statistical difference between the groups in terms of age or gender (P > 0.05). The EFTT was found to be higher in Group 1 than in the control group (P < 0.05). The Pearson correlation analysis demonstrated a positive correlation between EFTT values and triglycerides and waist circumferences (r = 0.412, P = 0.024, r = 0.364, and P = 0.048, respectively); however, there were no associations with the other MetS components (all values P > 0.05), (Table 2).
Table 1. Comparison of the demographic, laboratory and clinical characteristics of all subjects.
| Group 1 (n = 30) | Group 2 (n = 30) | P value | |
| Age (yr) | 72.37 ± 4.85 | 72.43 ± 5.29 | 0.960 |
| Gender (Female/Male) | 20/10 | 22/8 | 0.779 |
| Waist Circumference (cm) | 103.16 ± 9.64 | 95.36 ± 10.36 | 0.004 |
| Hip circumference (cm) | 107.10 ± 9.21 | 103.50 ± 8.12 | 0.114 |
| BMI (kg/m2) | 29.88 ± 3.05 | 28.31 ± 3.67 | 0.077 |
| Body fat (%) | 35.49 ± 10.18 | 34.95 ± 10.84 | 0.843 |
| Lean body mass percentage (%) | 28.44 ± 5.45 | 27.47 ± 4.56 | 0.480 |
| Visceral fat percentage (%) | 13.66 ± 2.9 | 11.60 ± 3.51 | 0.029 |
| Systolic blood pressure (mmHg) | 148.30 ± 17.75 | 125.50 ± 6.68 | < 0.001 |
| Diastolic blood pressure (mmHg) | 79.16 ± 7.58 | 75.76 ± 8.33 | 0.104 |
| FBG (mg/dL) | 151.9 ± 49.01 | 92.8 ± 8.01 | < 0.001 |
| Creatinine (mg/dL) | 0.84 ± 0.20 | 0.71 ± 0.15 | 0.009 |
| ALT (U/L) | 19.82 ± 7.42 | 19.90 ± 7.63 | 0.947 |
| AST (U/L) | 20.83 ± 7.93 | 21.90 ± 7.86 | 0.603 |
| LDL (mg/dL) | 108.46 ± 37.97 | 131.26 ± 33.81 | 0.017 |
| HDL (md/dL) | 46.06 ± 13.46 | 49.05 ± 13.10 | 0.388 |
| Total cholesterol (mg/dL) | 187.13 ± 31.63 | 212.94 ± 36.20 | 0.005 |
| Triglyceride (mg/dL) | 159.70 ± 67.94 | 139.31 ± 51.47 | 0.196 |
| Sedimentation (mm/saat) | 18.86 ± 15.34 | 11.86 ± 7.27 | 0.028 |
| C-reactive protein (mg/dL) | 0.69 ± 1.22 | 0.34 ± 0.28 | 0.127 |
| HbA1c (%) | 7.42 ± 1.85 | 5.52 ± 0.37 | < 0.001 |
| HOMA-IR | 5.00 ± 4.81 | 1.28 ± 0.59 | < 0.001 |
| EFTT (cm) | 1.00 ± 0.21 | 0.54 ± 0.15 | < 0.001 |
All measurable values were expressed as mean ± SD. ALT: Alanin transaminase; AST: Aspartate transaminase; BMI: body mass index; EFTT: epicardial fat tissue thickness; FBG: fasting blood glucose; HbA1c: glycosylated haemoglobin; HDL: high density lipoprotein; HOMA-IR: homeostasis model of assessment-insulin resistance; LDL: low density lipoprotein.
Table 2. Correlation analysis of epicardial fat tissue thickness and the components of metabolic syndrome.
| Epicardial fat tissue thickness |
||
| R | P | |
| FBG | 0.275 | 0.141 |
| HDL | –0.096 | 0.614 |
| Triglycerides | 0.412 | 0.024* |
| Systolic BP | 0.256 | 0.171 |
| Diastolic BP | –0.024 | 0.901 |
| Waist circumference | 0.364 | 0.048* |
BP: Blood pressure; FBG: Fasting blood glucose; HDL: High density lipoprotein cholesterol.
Binary logistic regression analysis revealed that EFTT was the independent factor for MetS in geriatric patients (B = 17.35, SE = 4.93, Wald = 12.36, P < 0.001). In ROC-curve analysis, EFTT levels above 7.3 mm showed 96.7% sensitivity and 86.7% specificity for predicting MetS in geriatric patients [area under the curve (AUC) = 0.969; 95% confidence interval (CI) = 0.928–1.00]. The other components of the Mets patients' AUC levels were determined as follows: glucose = 0.990; HDL-C = 0.411; TG = 0.619; SBP = 0.916; DBP = 0.603; and WC = 0.709 (Figure 1).
Figure 1. ROC chart analysis of epicardial fat tissue thickness and the components of metabolic syndrome.
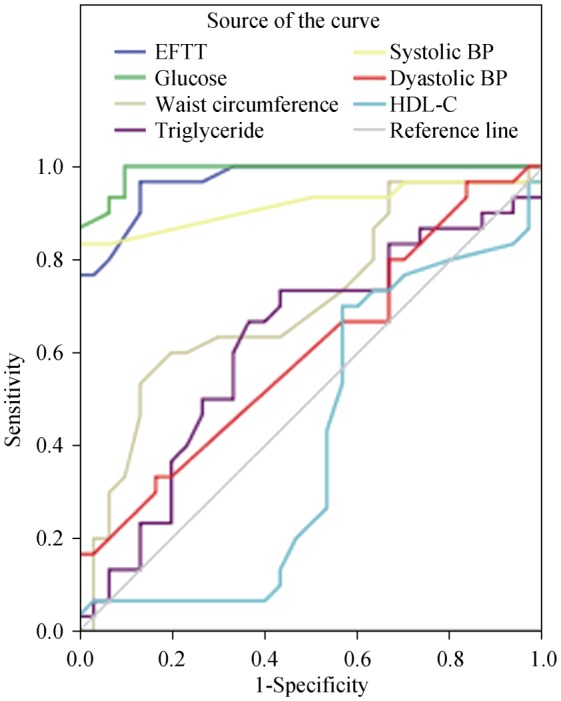
BP: Blood pressure; EFTT: epicardial fat tissue thickness; HDL: high density lipoprotein; ROC: receivers operating characteristic.
4. Discussion
To the best of our knowledge, this is the first study to evaluate the correlation between EFTT and the components of MetS in geriatric patients. The main findings of the present study are: (1) EFTT was higher in geriatric patients with MetS; and (2) EFTT was found to be a strong indicator of MetS in geriatric patients.
The literature describes many studies have demonstrated EFTT to be correlated with other cardiovascular risk factors, including carotid intima-media thickness, arterial stiffness, cardio-ankle vascular index, and systemic inflammation, sub-clinic hypothyroidism related, or not related, to MetS.[9]–[13] Iacobellis, et al.[5] first suggested the use of echocardiography to determine EFTT, and in subsequent studies, EFTT was found to be higher in patients with MetS.[6] Pierdomenico, et al.[14] found that EFTT was increased in patients with both hypertension and MetS even if body weight were normal with an acceptable WC. Stramaglia, et al.[15] measured EFTT by echocardiography and abdominal fat tissue by DEXA in elderly patients with MetS; the authors found that both abdominal fat thickness and EFTT were increased in the elderly patients with MetS, but only EFTT was associated with MetS. A strong correlation between EFTT and AFT was demonstrated; hepatosteatosis was also correlated with EFTT and AFT. Similarly, in the current study, EFTT was found to be higher, and weakly correlated with WC in geriatric patients with MetS.
Previous studies have investigated the correlation between EFTT and the components of MetS. The systematic review and meta-analysis by Rabkin, et al.[16] indicated that EFTT was 7.5 ± 0.1 mm in the MetS (n = 427) and 4.0 ± 0.1 mm in the control (n = 301). In this meta-analysis, EFTT was correlated significantly with SBP, TG, HDL-C, WC, and FBG. Yorgun, et al.[17] investigated increased EFTT levels using multidetector computed tomography in patients with MetS and found significant correlations between EFTT and the components of MetS. Consistent with the findings of Yorgun, Balcioğlu, et al.[18] found increased EFTT levels in patients with MetS, as well as a positive correlation between EFTT and body mass index, TG level, and WC, and a negative correlation with the HDL–C level. Karadag, et al.[19] demonstrated high levels of EFTT and WC, reflecting abdominal obesity in all age groups and both gender groups with MetS, but the correlation between EFTT and WC, although significant, was not high in the geriatric group. The authors suggested that this result might be related to the fact that using WC to determine the thickness of visceral adipose tissue was not entirely reliable in elderly persons, as demonstrated in previous studies.[19]–[21] In our study, EFTT was correlated only with TG and WC, but not with the other components of MetS.
In the literature, no research study has revealed an EFTT cut-off value to predict MetS in geriatric patients. Only Lima-Martínez, et al.[22] found that an EFTT level above 5 mm demonstrated high sensitivity and specificity in predicting MetS with an AUC of 0.852 in a Venezuelan population aged 20–65 years. In our study, EFTT was found to be an independent factor for MetS in geriatric patients; levels of EFTT above 7.3 mm predicted MetS with 96.7% sensitivity and 86.7% specificity in geriatric patients. The AUC level of the EFTT was found to be 0.969, which was higher than the AUC level (0.709) for WC.
In conclusion, our present study demonstrated EFTT was higher in geriatric patients with MetS and differed from the previous studies by providing important new insights and data revealing the cut off levels of the EFTT in geriatric patients with MetS. An EFTT level above 7.3 mm demonstrated high sensitivity and specificity for predicting MetS in geriatric patients and therefore, EFTT could be an indicator that can be used as a diagnostic criterion for MetS. The limitations of our study are the small number of subjects and the use of a cross-sectional design. Therefore, large-scale studies are needed in the future to clarify our results.
Acknowledgments
The authors declared no conflicts of interest.
References
- 1.Grundy SM, Brewer HB, Jr, Cleeman JI, et al. Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 2.Carr DB, Utzschneider KM, Hull RL, et al. Intra-abdominal fat is a major determinant of the National Cholesterol Education Program Adult Treatment Panel III criteria for the metabolic syndrome. Diabetes. 2004;53:2087–2094. doi: 10.2337/diabetes.53.8.2087. [DOI] [PubMed] [Google Scholar]
- 3.Mazurek T, Zhang L, Zalewski A, et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation. 2003;108:2460–2466. doi: 10.1161/01.CIR.0000099542.57313.C5. [DOI] [PubMed] [Google Scholar]
- 4.Iacobellis G, Corradi D, Sharma AM. Epicardial adipose tissue: anatomic, biomolecular and clinical relationships with the heart. Nat Clin Pract Cardiovasc Med. 2005;2:536–543. doi: 10.1038/ncpcardio0319. [DOI] [PubMed] [Google Scholar]
- 5.Iacobellis G, Leonetti F. Epicardial adipose tissue and insulin resistance in obese subjects. J Clin Endocrinol Metab. 2005;90:6300–6302. doi: 10.1210/jc.2005-1087. [DOI] [PubMed] [Google Scholar]
- 6.Sacks HS, Fain JN. Human epicardial adipose tissue: a review. Am Heart J. 2007;153:907–917. doi: 10.1016/j.ahj.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 7.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 8.American Diabetes Association. Diagnosis and cassification of diabetes mellitus. Diabetes Care. 2011;34(Suppl 1):S62–S69. doi: 10.2337/dc11-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sengul C, Cevik C, Ozveren O, et al. Echocardiographic epicardial fat thickness is associated with carotid intima-media thickness in patients with metabolic syndrome. Echocardiography. 2011;28:853–858. doi: 10.1111/j.1540-8175.2011.01471.x. [DOI] [PubMed] [Google Scholar]
- 10.Kim BJ, Kim BS, Kang JH. Echocardiographic epicardial fat thickness is associated with arterial stiffness. Int J Cardiol. 2013;167:2234–2238. doi: 10.1016/j.ijcard.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 11.Gökdeniz T, Turan T, Aykan AÇ, et al. Relation of epicardial fat thickness and cardio-ankle vascular index to complexity of coronary artery disease in nondiabetic patients. Cardiology. 2013;124:41–48. doi: 10.1159/000345298. [DOI] [PubMed] [Google Scholar]
- 12.Tok D, Kadife I, Turak O, et al. Increased epicardial fat thickness is associated with low grade systemic inflammation in metabolic syndrome. Turk Kardiyol Dern Ars. 2012;40:690–695. doi: 10.5543/tkda.2012.60207. [DOI] [PubMed] [Google Scholar]
- 13.Santos OC, Silva NA, Vaisman M, et al. Evaluation of epicardial fat tissue thickness as a marker of cardiovascular risk in patients with subclinical hypothyroidism. J Endocrinol Invest. 2015;38:421–427. doi: 10.1007/s40618-014-0199-x. [DOI] [PubMed] [Google Scholar]
- 14.Pierdomenico SD, Pierdomenico AM, Neri M, et al. Epicardial adipose tissue and metabolic syndrome in hypertensive patients with normal body weight and waist circumference. Am J Hypertens. 2011;24:1245–1249. doi: 10.1038/ajh.2011.134. [DOI] [PubMed] [Google Scholar]
- 15.Stramaglia G, Greco A, Guglielmi G, et al. Echocardiography and dual-energy x-ray absorptiometry in the elderly patients with metabolic syndrome: a comparison of two different tecniques to evaluate visceral fat distribution. J Nutr Health Aging. 2010;14:6–10. doi: 10.1007/s12603-010-0002-4. [DOI] [PubMed] [Google Scholar]
- 16.Rabkin SW. The relationship between epicardial fat and indices of obesity and the metabolic syndrome: a systematic review and meta-analysis. Metab Syndr Relat Disord. 2014;12:31–42. doi: 10.1089/met.2013.0107. [DOI] [PubMed] [Google Scholar]
- 17.Yorgun H, Canpolat U, Hazırolan T, et al. Increased epicardial fat tissue is a marker of metabolic syndrome in adult patients. Int J Cardiol. 2013;165:308–313. doi: 10.1016/j.ijcard.2011.08.067. [DOI] [PubMed] [Google Scholar]
- 18.Balcioğlu AS, Durakoğlugil ME, Ciçek D, et al. Epicardial adipose tissue thickness and plasma homocysteine in patients with metabolic syndrome and normal coronary arteries. Diabetol Metab Syndr. 2014;6:62. doi: 10.1186/1758-5996-6-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karadag B, Ozulu B, Ozturk FY, et al. Comparison of epicardial adipose tissue (EAT) thickness and anthropometric measurements in metabolic syndrome (MS) cases above and under the age of 65. Arch Gerontol Geriatr. 2011;52:e79–e84. doi: 10.1016/j.archger.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 20.Bonora E, Micciolo R, Ghiatas AA, et al. Is it possible to derive a reliable estimate of human visceral and subcutaneous abdominal adipose tissue from simple anthropometric measurements? Metabolism. 1995;44:1617–1625. doi: 10.1016/0026-0495(95)90084-5. [DOI] [PubMed] [Google Scholar]
- 21.Iwao S, Iwao N, Muller DC, et al. Does waist circumference add to the predictive power of the body mass index for coronary risk? Obes Res. 2001;9:685–695. doi: 10.1038/oby.2001.93. [DOI] [PubMed] [Google Scholar]
- 22.Lima-Martínez MM, Paoli M, Donis JH, et al. Cut-off point of epicardial adipose tissue thickness for predicting metabolic syndrome in Venezuelan population. Endocrinol Nutr. 2013;60:570–576. doi: 10.1016/j.endonu.2013.03.004. [DOI] [PubMed] [Google Scholar]


