Abstract
Very high vaccination coverage is required to eliminate measles, but achieving high coverage can be constrained by the logistical challenges associated with subcutaneous injection. To simplify logistics of vaccine delivery, a patch containing micron-scale polymeric needles was formulated to encapsulate the standard dose of measles vaccine (1000 TCID50) and the immunogenicity of the microneedle patch was compared with subcutaneous injection in rhesus macaques. The microneedle patch was administered without reconstitution with diluent, dissolved in skin within 10 minutes, and caused only mild, transient skin erythema. Both groups of rhesus macaques generated neutralizing antibody responses to measles that were consistent with protection and the neutralizing antibody titers were equivalent. In addition, the microneedle patches maintained an acceptable level of potency after storage at elevated temperature suggesting improved thermostability compared to standard lyophilized vaccine. In conclusion, a measles microneedle patch vaccine was immunogenic in non-human primates, and this approach offers a promising delivery method that could help increase vaccination coverage.
Keywords: measles, vaccination, microneedle, stability, non-human primate
Introduction
Remarkable progress has been made in the global measles control program. From 2000 to 2012, annual reported measles incidence decreased 77%, from 146 to 33 cases per million population, and estimated measles deaths decreased 78%, from 562,400 to 122,000 [1], both historically low levels. This progress was due to widespread use of a safe, inexpensive, and effective vaccine which has been available since 1963, costs approximately $1.00 per delivered dose, and has a two-dose vaccine efficacy of ≥95% [2].
In 2012, the World Health Organization (WHO) and its global partners established the Global Vaccine Action Plan (GVAP) that recommended targets for vaccination coverage and measles elimination for 2015 and 2020, including a goal for measles elimination in five of the six WHO regions by 2020 [3]. By September 2013, all six WHO regions had set a goal for measles elimination by, or before 2020. Measles elimination has been achieved in the Region of the Americas, with the last endemic case reported in 2002, and the Western Pacific Region is approaching measles elimination [4, 5]. However, based on current performance trends, GVAP targets will not be achieved on time.
Due to the highly infectious nature of measles virus, population immunity of approximately 93–95% is needed to interrupt measles virus transmission [6]. To achieve this level of population immunity, high two-dose measles vaccination coverage is needed. GVAP targets for the coverage with the first dose of measles-containing vaccine (MCV1) through routine immunization services is ≥90% nationally, and ≥80% in every district [7]. Estimated MCV1 coverage among children aged 1 year increased from 73% to 84% during 2000–2009; however, coverage has remained stagnant at 84% through 2012 [8]. In 2012, the countries with the largest number of infants not receiving MCV1 were in the African, Eastern Mediterranean, and South-East Asian regions; these regions accounted for 98% of the estimated global measles mortality burden in 2012, and continue to experience large measles outbreaks, highlighting the need to strengthen immunization systems. To increase vaccination coverage, innovative vaccine delivery methods that overcome the logistical challenges associated with current vaccination delivery methods may be needed.
In 2012, the research priorities for global measles control and eradication, identified by a group of experts, included the need for improved vaccine delivery methods [9]. Currently available methods of vaccine delivery pose significant logistical drawbacks, particularly in resource-limited settings. For example, the currently used live-attenuated measles vaccine is typically supplied as a lyophilized pellet and packaged in multi-dose vials which must be kept in the cold chain, reconstituted with diluent prior to use, and discarded within 6 hours after reconstitution. Additionally, currently available measles vaccine formulations must be administered by subcutaneous injection requiring well-trained healthcare personnel to administer each vaccination and safely dispose of sharps waste.
We recently proposed the use of a microneedle patch for measles vaccination [10]. Microneedle patches contain micron-scale (<1000 µm), solid needles containing a dry formulation of vaccine that rapidly dissolves upon patch application and microneedle puncture into the upper layers of skin [11]. Use of microneedle patches offers the possibility of vaccination by minimally-trained personnel, no reconstitution with diluent, single-dose presentation, avoidance of sharps waste and reduced reliance on the cold chain, all of which could facilitate mass vaccination campaigns in developing countries.
Microneedle systems have been shown to be effective for delivering other vaccines in animals [12–17]. A prototype, metal microneedle patch evaluated for measles vaccination in cotton rats produced neutralizing antibody titers that were equivalent to titers obtained following subcutaneous injection [10]. In this study, rhesus macaques were used to study immunogenicity because they are the established animal model for studying the pathogenesis of measles and for evaluating the immunogenicity of measles vaccine [18–20]. Here, we report the formulation of a dissolving microneedle patch for measles vaccination and the evaluation of immune responses to microneedle patch vaccination in rhesus macaques.
Materials and Methods
Fabrication of microneedle patches for measles vaccination
The Edmonston-Zagreb measles vaccine strain was acquired from the U.S. Centers for Disease Control and Prevention (CDC). The viral stock was then propagated in Vero cells as previously described [10]. MRC-5 cells or primary chicken embryo fibroblasts are the standard substrates for production of commercial measles vaccine [21]; however Vero cells produce higher titers of measles which enabled preparation of the high-concentration casting solutions needed to fabricate the microneedles.
Molds consisting of a 10 × 10 array of 300 × 300 × 600 µm pyramidal microneedles in an area of 0.29 cm2 were fabricated out of polydimethylsiloxane (Ellsworth Adhesives Systems, Loganville, GA) as previously described [22, 23]. Approximately 20 µL of an antigen solution containing measles vaccine at a titer of 5.0 × 106 tissue culture infectious dose (TCID50) per mL was mixed in a 1:1 ratio with a solution consisting of 15% w/v sucrose (Sigma-Aldrich, St. Louis, MO), 300 mM threonine (Sigma-Aldrich) and 2% w/v carboxymethyl cellulose (Sigma-Aldrich). This combined solution was then applied to the microneedle mold, filled into the mold cavities and allowed to dry. Any dried vaccine on the surface of the mold was removed by tape stripping to localize all vaccine in the microneedles.
A microneedle matrix solution consisting of 8 g of sucrose (Sigma-Aldrich) and 8 g of poly-from the mold, thereby removing the microneedle array from the mold to form the complete microneedle patch. Microneedle patches were then lyophilized for 24 hours and stored at 22°C in a sealed pouch with desiccant and protected from light until use. Measles vaccine titer was determined from reconstituted microneedle patches by an endpoint dilution assay and the TCID50 was calculated by the Reed and Muench method, as described previously [10].
Characterization of microneedle patches for measles vaccination
Microneedle patches were characterized by measuring the force required for insertion into porcine cadaver skin, the kinetics of microneedle dissolution in skin, and the efficiency of vaccine delivery into skin. Microneedle insertion force was determined by applying microneedle patches to porcine cadaver skin using custom-made spring-loaded devices that applied known forces as measured by using a load cell (Mark-10, Copiague, NY). Successful skin penetration of the microneedles was determined by applying gentian violet to the skin, which selectively stains sites of skin puncture [24]. The minimum force that led to at least 90% of microneedles puncturing the skin was determined to be the insertion force.
Kinetics of microneedle dissolution in skin was determined by pressing microneedle patches into the skin at room temperature and removing them after varying amounts of time. Microneedle patches were then imaged by bright-field microscopy (Hirox KH-8700, Tokyo, Japan) to determine the fraction of microneedles that had dissolved away.
Efficiency of vaccine delivery into skin was determined by measuring measles vaccine dose (TCID50) in microneedle patches before use and then measuring the amount of measles vaccine remaining in the patches after application to rhesus macaque skin in the vaccination study described below.
Stability of measles vaccine in microneedle patches
Microneedle patches containing approximately 3 × 104 TCID50 infectivity units of measles vaccine were stored with desiccant in vinyl alcohol (Sigma-Aldrich) mixed into 15 mL of DI water was then spread in a thin layer over the microneedle mold using a spatula, filled into the mold cavities and allowed to dry. A circular disc of poly(methyl methacrylate) (McMaster-Carr, Atlanta, GA) coated with double-sided tape (MacTac, Stow, OH) was applied to the back of the mold. This disc was gently peeled away heat-sealed, opaque pouches (Oliver-Tolas Healthcare Packaging, Grand Rapids, MI) at 4°C, 22°C, and 40°C for up to 112 days. Commercial, lyophilized vaccine (Serum Institute of India, Pune, India) and Vero-derived, diluted liquid vaccine were stored at the same temperatures for up to 84 days. Vaccine stability was determined by measuring viral titer using the endpoint dilution assay.
Immunogenicity of measles vaccination
The immune response to measles vaccination was tested in rhesus macaques (Macaca mulatta), as approved by the Institutional Animal Care and Use Committees of the CDC and the Georgia Institute of Technology. Female, two-year-old rhesus macaques (Covance, Princeton, NJ) were verified to test negative for measles, influenza, polio and canine distemper virus antibodies. All procedures were carried out with animals under ketamine anesthesia (10 mg/kg).
For animals in the microneedle patch vaccination group (N = 4), a section of hair on the upper back was removed using electric shears followed by application of depilatory cream (Nair, Princeton, NJ) approximately 20 minutes before vaccination. Vaccination was carried out by manually pressing a microneedle patch onto the skin at the site of hair removal and leaving the patch on the skin for 10 minutes to facilitate dissolution of the microneedles in the skin. For the group receiving subcutaneous vaccination (N = 4), measles vaccine from the same stock used to formulate the microneedle patches was diluted using sterile saline and 0.5 ml was injected under the skin of the upper back using a 25-gauge hypodermic needle and syringe. The dose administered in all vaccinations was approximately 3100 TCID50. Following vaccination, animals were observed daily for adverse events and blood was drawn weekly from the femoral vein using Vacutainer® tubes (Becton Dickenson, Franklin Lakes, NJ).
Measles neutralizing antibody titers were measured by the plaque reduction neutralization (PRN) assay, as previously described [25]. Two-fold dilutions of serum were tested starting at a dilution of 1:4. An enzyme-linked immunosorbent IgM assay previously developed at the CDC [26] was used to detect serum IgM antibodies to measles virus. Results were determined by using the standard cut-off values as specified in the protocol [27].
Statistics
Statistics were calculated using Prism software version 6.02 (GraphPad, La Jolla, CA). Comparisons between individual samples were done using an unpaired t-test with a significance cut-off of p<0.05. Comparisons between multiple samples were done using a two182 way ANOVA with a Tukey post-test. The results from virus titrations are presented as geometric means of the TCID50 determined for each sample. All other data are presented as the arithmetic means of the samples. In this study, available resources permitted only four rhesus macaques per experimental group, which limited the statistical power to evaluate differences in the PRN titers.
Results
Formulation and stabilization of microneedle patches containing measles vaccine
Microneedle patches were designed for manual administration followed by rapid microneedle dissolution in the skin to release the vaccine and to provide vaccine thermostability during extended storage. Microneedle patches were produced by a two-step process in which stabilized measles vaccine was filled into micromolds, localizing the vaccine toward the microneedle tips. The microneedle matrix material solution was then cast onto the molds to form the remaining part of the microneedles and patch backing.
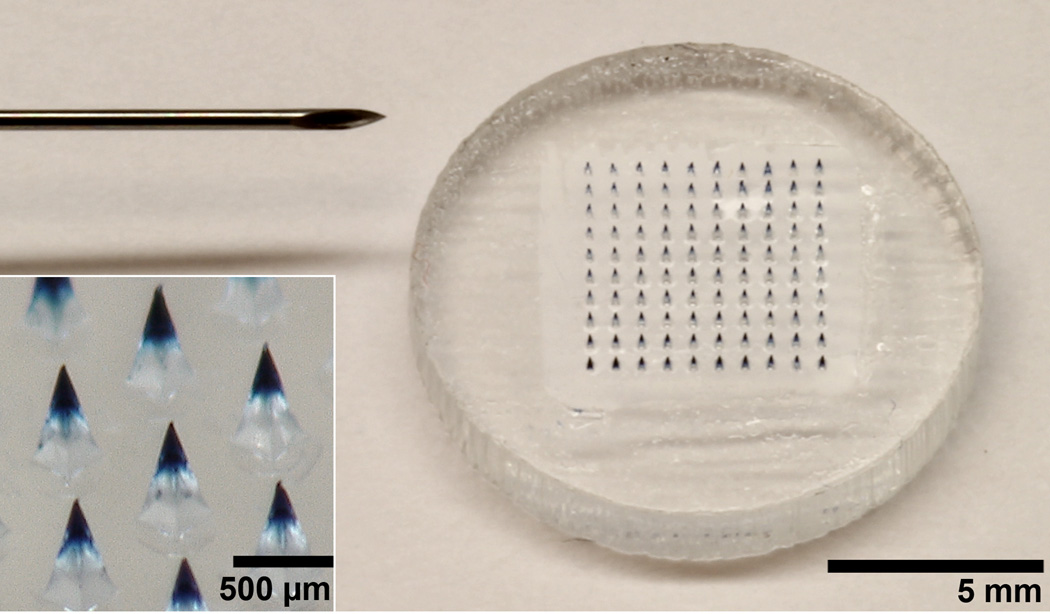
The microneedle patch contained 100 pyramidal microneedles, each measuring 600 µm tall, 300 µm wide at the base and tapering to a tip radius of less than 3 µm (Figure 1). The microneedles contained the standard dose of live-attenuated measles vaccine encapsulated in each patch. The microneedle patch could be pressed into the skin with a force of 28 N, which corresponds to 0.28 N per microneedle, in good agreement with previous findings [28]. This force can readily be applied by a human thumb [4, 29]. Microneedles dissolved rapidly upon insertion into porcine cadaver skin (Figure 2). Within one minute, the tips of the microneedles dissolved. Within 10 minutes, the microneedles were almost completely dissolved.
Figure 1.
Microneedle patch for measles vaccination. A microneedle patch is shown next to a 25-gauge hypodermic needle. The patch contains 100 solid microneedles made of water462 soluble excipients that encapsulate measles vaccine for delivery to the skin. The inset photo shows a magnified view of microneedles. To facilitate imaging, the microneedles encapsulated blue dye (Trypan blue) instead of vaccine.
Figure 2.
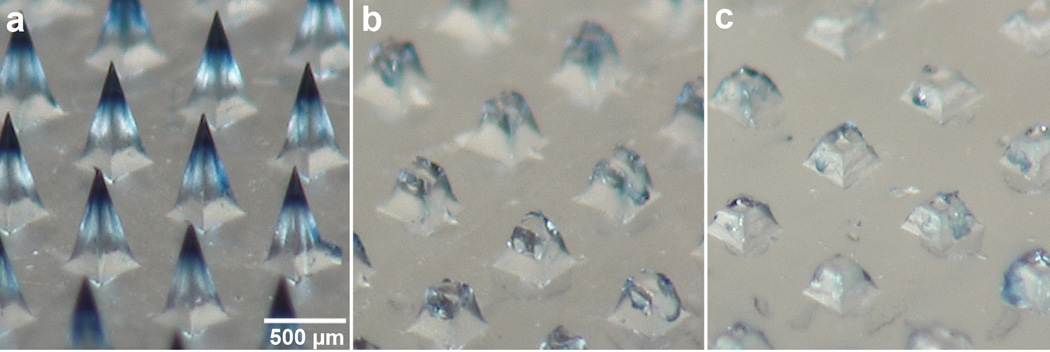
Dissolution of microneedles after insertion into porcine cadaver skin. A section of a microneedle patch is shown (a) before use, and (b) 1 min and (c) 10 min after insertion into porcine cadaver skin, and imaged by bright-field microscopy. This experiment was conducted at room temperature. To facilitate imaging, the microneedles encapsulated blue dye (Trypan blue) instead of vaccine.
To minimize vaccine wastage and provide accurate dosing, we confirmed that the measles vaccine was concentrated within the microneedles which enter the skin, rather than in the patch backing. Prior to use, each patch used for vaccination of monkeys contained approximately 3100 TCID50 of measles vaccine. Ten minutes after manual application to the skin of rhesus macaques, the microneedle patch backing and any remaining microneedle stubs contained 9.4 ± 2.2% of the original vaccine loaded into the microneedle patches, indicating that more than 90% of the vaccine dose was delivered to the skin.
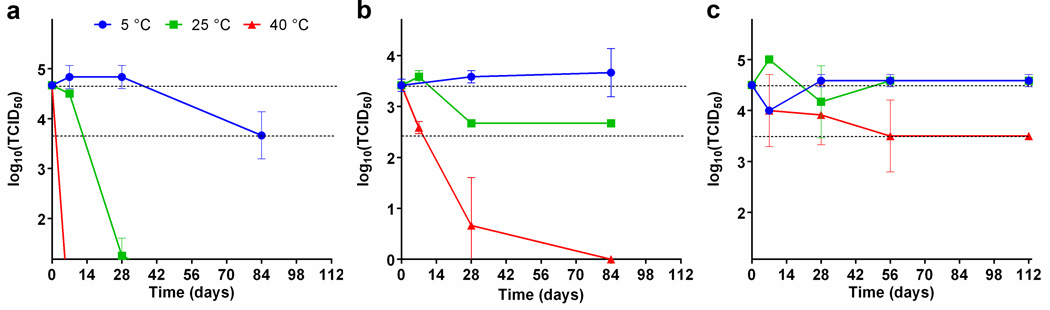
The stability of the measles microneedle vaccine was assessed by measuring the vaccine virus titer on microneedle patches stored at various temperatures for almost 4 months. Reconstituted liquid measles vaccine was unstable and lost essentially all potency within 28 days at 25°C and in less than one week at 40°C (Figure 3). Commercially available lyophilized vaccine also demonstrated instability at 40°C; potency was reduced by more than 100-fold after 28 days and more than 1000-fold within 3 months. In contrast, the microneedle patch maintained full potency for almost four months at 25°C, and had less than a 10-fold decrease in potency after almost 4 months at 40°C.
Figure 3.
Stability of measles vaccine in microneedle patches compared to standard measles vaccine. Microneedle patches containing measles vaccine were stored for up to four months at 4°C, 22°C, and 40°C, and then tested in duplicate for viral titer by endpoint dilution. These results were compared to standard lyophilized and reconstituted vaccine samples stored at the same temperatures for up to three months. The dashed line represents the range between 100% titer retention and a 10-fold loss of titer. Panel (a) shows results from reconstituted measles vaccine, panel (b) shows results for a commercial lyophilized measles vaccine, and panel (c) shows results for microneedle patches stored in desiccant pouches. Data points represent average titers (N = 2) and standards deviations (SD) are indicated.
Immunogenicity of measles vaccination using a microneedle patch
The immune response following measles vaccination using a microneedle patch was evaluated in rhesus macaques. The rhesus macaque is a well-established model for measles vaccination studies since the immune response in macaques shows a strong correlation to the human immune response [19, 20, 30]. In this study, one group of monkeys received a standard human dose of the live-attenuated measles vaccine via a microneedle patch and the positive control group received the same dose of vaccine via subcutaneous injection.
Serum samples were obtained weekly and tested for antibodies to measles virus. To monitor the progress of the immune response, serum samples were tested for the presence of measles-specific IgM by ELISA. Measles-specific IgM was detected for every animal in both groups as early as day 14 post vaccination (Figure S1). The presence of measles-specific IgM antibodies confirmed that all of the animals had generated a primary immune response to measles following vaccination.
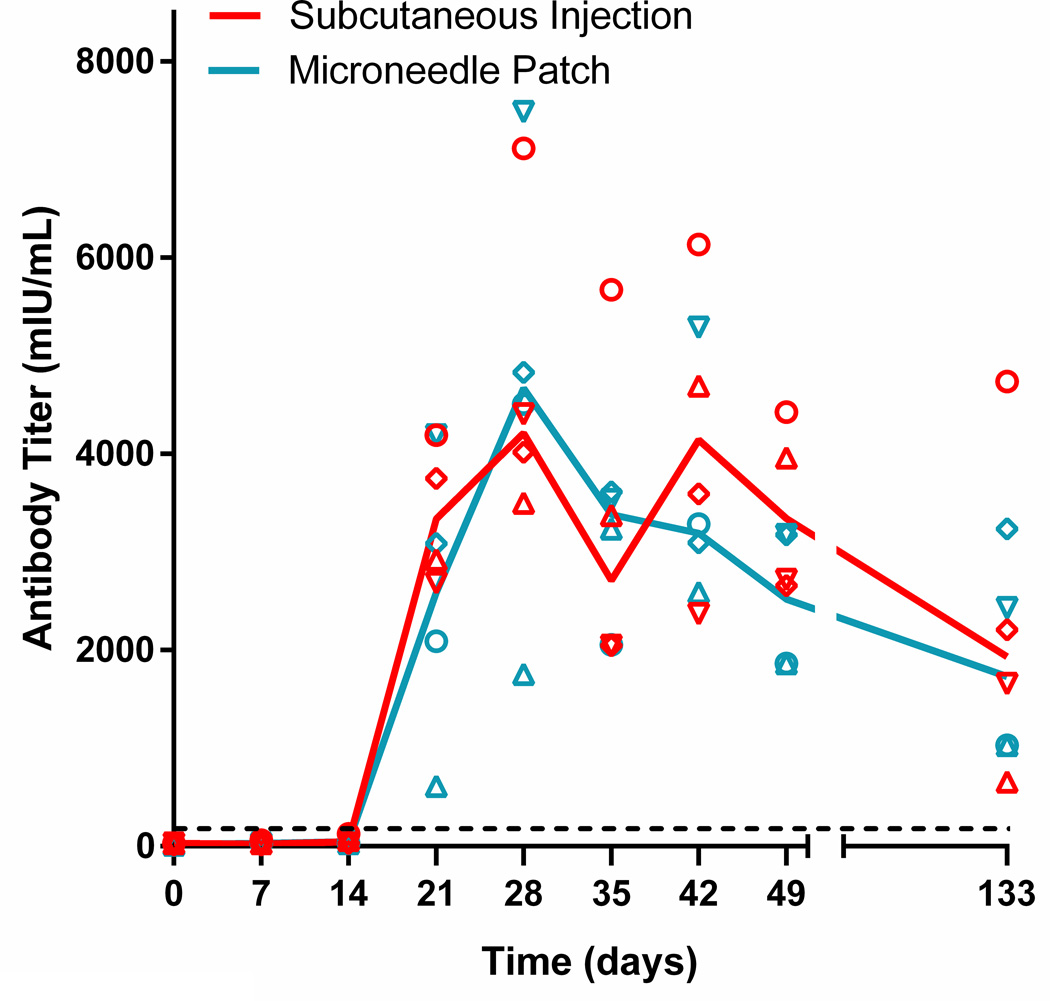
Neutralizing antibodies were first detected beginning 21 days post vaccination and increased to a peak on day 28 of approximately 4700 mIU/mL in both groups (range 3499 – 7115 mIU/ml for the subcutaneous injection group; 1753 – 7485 mIU/ml for the microneedle patch group) (Figure 4). The peak titers and the times to peak titer did not differ between the two groups. In both the microneedle patch and subcutaneous injection groups, all of the animals seroconverted and all of the animals had a titer of >120 mIU/mL, which is considered protective in humans [31, 32]. Neutralizing antibody titers were maintained for at least 133 days post vaccination. Overall, both vaccines generated a robust antibody response in the rhesus macaques and these data show that measles vaccination using a microneedle patch induced equivalent neutralizing antibody titers to vaccination by conventional subcutaneous injection.
Figure 4.
Measles neutralizing antibody titers following vaccination of rhesus macaques. Neutralizing titers were measured in serum samples obtained on a weekly basis after vaccination by microneedle patch or subcutaneous injection on day 0. Data points represent results from individual animals. The solid lines represent the average titer (N = 4) for all animals in that experimental group. The dashed line represents 120 mIU/ml which is considered the minimum titer required for protection [32].
Safety of measles vaccination using a microneedle patch
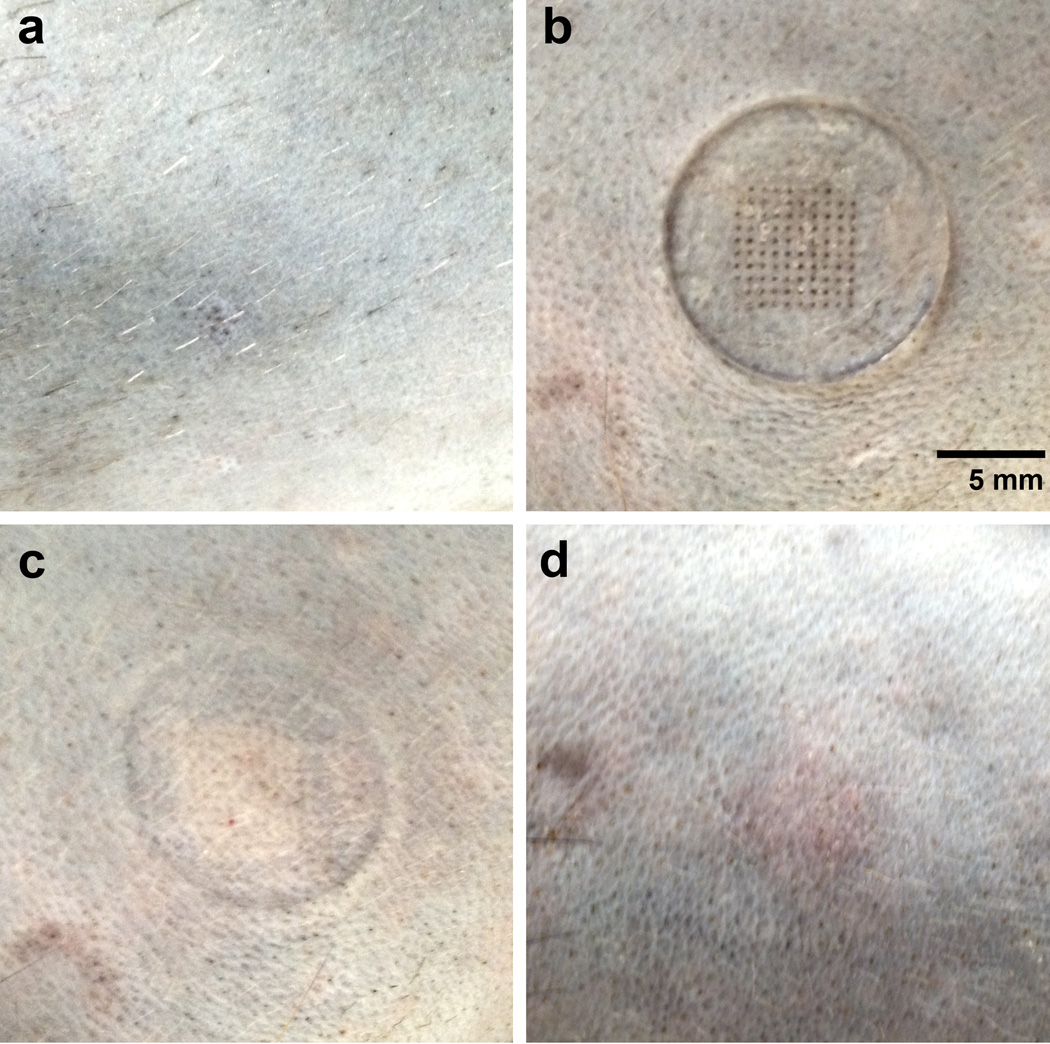
The microneedle patches caused no adverse effects that were observed by the investigators or veterinary staff. After microneedle patch removal, a small grid of puncture sites was faintly visible and very faint erythema could be seen where the edges of the patch had pressed against the skin (Figure 5). There was no evidence of bleeding. The microneedle patch application site was examined daily by veterinary staff and no signs of adverse effects were seen. The faint grid of puncture sites was no longer visible 2–3 days after patch removal and no swelling, discharge or other abnormalities were observed at any point during the study. One week after patch removal, the microneedle vaccination site was indistinguishable from other shaved portions of the animal (Figure 5). The veterinary staff reported no study-related health problems in the animals, and no irritation, reactogenicity, infection, or evidence of discomfort was observed in any animal at the microneedle patch vaccination sites.
Figure 5.
Rhesus macaque skin before, during, and after microneedle patch application. The representative images show the same area of skin on a rhesus macaque (a) after hair removal and immediately before patch application, (b) with the microneedle patch applied to the skin, (c) immediately after patch removal and (d) 1 week after patch removal.
Discussion
The use of subcutaneous injection to deliver measles vaccine is associated with logistic challenges that can make it difficult to achieve the high levels of vaccination coverage needed for measles elimination in many countries. To address some of the barriers to increased vaccination coverage, we developed and tested a microneedle patch for measles vaccination in a rhesus macaque animal model. The microneedle vaccine was prepared by a casting aqueous solutions containing the vaccine onto molds flowed by drying. This process can be scaled up for mass production using manufacturing equipment compatible with conventional pharmaceutical manufacturing processes [33].
The microneedle patch used in this study was administered by inserting the needles into the skin with a small amount of force, and we expect that a commercially produced microneedle patch vaccine would be as easy to administer as the application of an adhesive bandage. By removing the need for trained medical personnel to administer measles vaccine, microneedle patches could make house-to-house vaccination possible. Because microneedles dissolve in the skin, no biohazardous sharps waste is generated. The conventional lyophilized measles vaccine must be reconstituted with diluent immediately before use and handling errors can lead to unsafe injections [34, 35]. Microneedle patches do not need to be reconstituted which could significantly reduce the number of unsafe injections due to handling errors. Finally, current measles vaccine formulations must be discarded if not used within six hours after reconstitution of multi-dose vials, which can lead to wastage of up to 70% of vaccine doses [36]. Microneedle patches contain a single dose and could minimize vaccine wastage.
Currently available measles vaccine formulations must be refrigerated during storage and transport. Half of all measles vaccine doses in developing countries are damaged due to cold-chain failures [37]. The thermostability of microneedle patches observed in this study suggests that microneedle patches could be removed from the cold chain for at least a few days during the final stages of vaccine delivery to remote areas. Currently available measles vaccines are designed to have a loss in potency of up to 90% after one year of storage at 4–8°C and still deliver the minimum dose of 1000 TCID50 [38]. Our data suggest that microneedle patches could be removed from the cold chain for almost 4 months with titer losses that would be considered acceptable compared to currently available vaccine formulations.
Microneedle patches have previously been used to deliver other vaccines and biomolecules. Microneedle patch vaccines were immunogenic after delivering influenza virus, rotavirus, Bacillus Calmette-Guerin, human papilloma virus, anthrax, and malaria antigens [12–17, 39]. The ability to deliver a live-attenuated vaccine in this study and our prior work in cotton rats [10] represents a significant advance in microneedle technology, because the viability of the live vaccine virus must be maintained to generate a protective immune response. Vaccine delivery to the skin has been an area of active research because the skin layers that lie beneath the stratum corneum contain a high density of antigen-presenting cells, including Langerhans cells and dermal dendritic cells [11, 40].
This study showed that vaccination with a measles microneedle patch was highly immunogenic in non-human primates. All animals had detectable IgM responses to measles, seroconverted, and had protective levels of neutralizing antibodies. Neutralizing antibody titers were equivalent among monkeys vaccinated by microneedle patch and conventional subcutaneous injection and all titers were significantly higher than 120 mIU/ml, the level that is generally regarded as the minimum titer need for protection in humans [31, 32]. The neutralizing antibody levels measured in this experiment were comparable to the titers described in other studies of the immune response to live-attenuated measles vaccine in rhesus macaques [20, 41, 42]. In experimental challenge studies, only macaques with titers < 120 mIU/ml developed clinical signs of infection and had detectable viremia [20]. Vaccination via microneedle patches did not cause bleeding and only a slight and transient erythema was noted. There were no adverse events observed after microneedle patch vaccination of rhesus macaques.
Intradermal vaccination of humans with measles vaccine was previously evaluated in a number of clinical studies, but the results did not justify continuing to study this route of immunization [43]. Several of the studies reported suboptimal serologic responses following intradermal administration; however, the doses given were often much lower than the standard dose given by subcutaneous injection [44–47]. The studies also reported that intradermal injections were difficult to give because, at the time these studies were conducted, the available injection technology was limited. Our results with intradermal vaccination on non-human primates suggest that a standard dose of measles vaccine is immunogenic when given by the intradermal route. In addition, the dissolving microneedle represents a significant advancement in delivery technology.
While measles vaccination with a microneedle patch was shown to be immunogenic in rhesus macaques, additional studies will be required to assess safety and immunogenicity in humans. It will also be desirable to carry out further studies to improve the thermostability of the vaccine in microneedle patches and to include rubella vaccine in the microneedle patches. In addition, because cost is a critically important factor for vaccination programs, a detailed economic study will need to be conducted to evaluate the cost effectiveness of vaccination with microneedle patches and the costs associated with development of a new vaccine delivery method.
Conclusion
This study examined formulation and use of a novel microneedle patch to vaccinate rhesus macaques against measles. All animals seroconverted and had measles neutralizing antibody titers that correlated with protection. There were no significant differences between neutralizing antibody levels after vaccination by the microneedle patch and subcutaneous injection. There were no adverse reactions in the macaques that were associated with vaccination using the microneedle patch. Microneedle patches were produced by a manufacturing method expected to enable low-cost mass production. The microneedles were inserted easily into skin with thumb pressure and dissolved in the skin within 10 minutes. Measles vaccine in microneedle patches retained potency after extended storage at elevated temperature. These findings suggest that measles vaccination using a microneedle patch may facilitate increased vaccination coverage through simplified vaccine delivery and thereby contribute to measles elimination programs.
Supplementary Material
Detection of measles virus-specific IgM following vaccination of rhesus macaques. Measles-specific IgM was detected by ELISA following measles vaccination by microneedle patch or subcutaneous injection on day 0. Data points show ΔOD values serum samples collected at day 0 and up to 28 days post vaccination. The dashed line represents the cutoff for a positive result (ΔOD >0.10).
Acknowledgements
We thank Brianna Skinner, Robin Engel and Ryan Johnson for veterinary care and advice; Devin McAllister, Winston Pewin and Xin Dong Guo for helpful discussions of microneedle patch design and fabrication; Donna Bondy for administrative support; Susan Chu for help with obtaining financial support, Nobia Williams for help with the ELISAs, and NCHHSTP/CDC for assistance with rhesus macaques. This work was funded in part by the National Institutes of Health and the Global Immunization Division of the Centers for Disease Control and Prevention. Mark Prausnitz is an inventor of patents that have been licensed to companies developing microneedle-based products, is a paid advisor to companies developing microneedle-based products, and is a founder/shareholder of companies developing microneedle-based products.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
The resulting potential conflict of interest has been disclosed and is managed by Georgia Tech and Emory University.
References
- 1.Perry RT, Gacic-Dobo M, Dabbagh A, Mulders MN, Strebel PM, Okwo-Bele JM, et al. Global control and regional elimination of measles, 2000–2012. MMWR Morb Mortal Wkly Rep. 2014;63:103–107. [PMC free article] [PubMed] [Google Scholar]
- 2.Moss WJ, Strebel P. Biological feasibility of measles eradication. J Infect Dis. 2011;204(Suppl 1):S47–S53. doi: 10.1093/infdis/jir065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Global Vaccine Action Plan. Decade of vaccine collaboration. Vaccine. 2013;31(Suppl 2):B5–31. doi: 10.1016/j.vaccine.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 4.Norman JJ, Arya JM, McClain MA, Frew PM, Meltzer MI, Prausnitz MR. Microneedle patches: usability and acceptability for self-vaccination against influenza. Vaccine. 2014;32:1856–1862. doi: 10.1016/j.vaccine.2014.01.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strebel PM, Cochi SL, Hoekstra E, Rota PA, Featherstone D, Bellini WJ, et al. A world without measles. J Infect Dis. 2011;204(Suppl 1):S1–S3. doi: 10.1093/infdis/jir111. [DOI] [PubMed] [Google Scholar]
- 6.van Boven M, Kretzschmar M, Wallinga J, O'Neill PD, Wichmann O, Hahne S. Estimation of measles vaccine efficacy and critical vaccination coverage in a highly vaccinated population. J R Soc Interface. 2010;7:1537–1544. doi: 10.1098/rsif.2010.0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levin A, Burgess C, Garrison LP, Jr, Bauch C, Babigumira J, Simons E, et al. Global eradication of measles: an epidemiologic and economic evaluation. J Infect Dis. 2011;204(Suppl 1):S98–S106. doi: 10.1093/infdis/jir096. [DOI] [PubMed] [Google Scholar]
- 8.Monitoring progress towards measles elimination. Wkly Epidemiol Rec. 2010;85:490–494. [PubMed] [Google Scholar]
- 9.Goodson JL, Chu SY, Rota PA, Moss WJ, Featherstone DA, Vijayaraghavan M, et al. Research priorities for global measles and rubella control and eradication. Vaccine. 2012;30:4709–4716. doi: 10.1016/j.vaccine.2012.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edens C, Collins ML, Ayers J, Rota PA, Prausnitz MR. Measles vaccination using a microneedle patch. Vaccine. 2013;31:3403–3409. doi: 10.1016/j.vaccine.2012.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim YC, Park JH, Prausnitz MR. Microneedles for drug and vaccine delivery. Adv Drug Deliv Rev. 2012;64:1547–1568. doi: 10.1016/j.addr.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim YC, Quan FS, Compans RW, Kang SM, Prausnitz MR. Formulation and coating of microneedles with inactivated influenza virus to improve vaccine stability and immunogenicity. J Control Release. 2010;142:187–195. doi: 10.1016/j.jconrel.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raphael AP, Prow TW, Crichton ML, Chen X, Fernando GJ, Kendall MA. Targeted, needle-free vaccinations in skin using multilayered, densely-packed dissolving microprojection arrays. Small. 2010;6:1785–1793. doi: 10.1002/smll.201000326. [DOI] [PubMed] [Google Scholar]
- 14.Naito S, Ito Y, Kiyohara T, Kataoka M, Ochiai M, Takada K. Antigen-loaded dissolving microneedle array as a novel tool for percutaneous vaccination. Vaccine. 2012;30:1191–1197. doi: 10.1016/j.vaccine.2011.11.111. [DOI] [PubMed] [Google Scholar]
- 15.Matsuo K, Hirobe S, Yokota Y, Ayabe Y, Seto M, Quan YS, et al. Transcutaneous immunization using a dissolving microneedle array protects against tetanus, diphtheria, malaria, and influenza. J Control Release. 2012;160:495–501. doi: 10.1016/j.jconrel.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 16.Chandrasekhar S, Iyer LK, Panchal JP, Topp EM, Cannon JB, Ranade VV. Microarrays and microneedle arrays for delivery of peptides, proteins, vaccines and other applications. Expert Opin Drug Deliv. 2013;10:1155–170. doi: 10.1517/17425247.2013.797405. [DOI] [PubMed] [Google Scholar]
- 17.Sullivan SP, Koutsonanos DG, Del Pilar Martin M, Lee JW, Zarnitsyn V, Choi SO, et al. Dissolving polymer microneedle patches for influenza vaccination. Nat Med. 2010;16:915–920. doi: 10.1038/nm.2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Swart RL. Measles studies in the macaque model. Curr Top Microbiol Immunol. 2009;330:55–72. doi: 10.1007/978-3-540-70617-5_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu YD, Heath J, Collins J, Greene T, Antipa L, Rota P, et al. Experimental measles. II. Infection and immunity in the rhesus macaque. Virology. 1997;233:85–92. doi: 10.1006/viro.1997.8575. [DOI] [PubMed] [Google Scholar]
- 20.Lin WH, Griffin DE, Rota PA, Papania M, Cape SP, Bennett D, et al. Successful respiratory immunization with dry powder live-attenuated measles virus vaccine in rhesus macaques. Proc Natl Acad Sci U S A. 2011;108:2987–2992. doi: 10.1073/pnas.1017334108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aunins J. Encyclopedia of cell technology. New York: Wiley; 2000. Viral vaccine production in cell culture; pp. 1183–1207. [Google Scholar]
- 22.Park JH, Yoon YK, Choi SO, Prausnitz MR, Allen MG. Tapered conical polymer microneedles fabricated using an integrated lens technique for transdermal drug delivery. IEEE Trans Biomed Eng. 2007;54:903–913. doi: 10.1109/TBME.2006.889173. [DOI] [PubMed] [Google Scholar]
- 23.Chu LY, Choi SO, Prausnitz MR. Fabrication of dissolving polymer microneedles for controlled drug encapsulation and delivery: Bubble and pedestal microneedle designs. J Pharm Sci. 2010;99:4228–4238. doi: 10.1002/jps.22140. [DOI] [PubMed] [Google Scholar]
- 24.Lee JW, Park JH, Prausnitz MR. Dissolving microneedles for transdermal drug delivery. Biomaterials. 2008;29:2113–2124. doi: 10.1016/j.biomaterials.2007.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohen BJ, Audet S, Andrews N, Beeler J WHO. Plaque reduction neutralization test for measles antibodies: Description of a standardised laboratory method for use in immunogenicity studies of aerosol vaccination. Vaccine. 2007;26:59–66. doi: 10.1016/j.vaccine.2007.10.046. [DOI] [PubMed] [Google Scholar]
- 26.Erdman DD, Anderson LJ, Adams DR, Stewart JA, Markowitz LE, Bellini WJ. Evaluation of monoclonal antibody-based capture enzyme immunoassays for detection of specific antibodies to measles virus. J Clin Microbiol. 1991;29:1466–1471. doi: 10.1128/jcm.29.7.1466-1471.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Erdman DD, Heath JL, Watson JC, Markowitz LE, Bellini WJ. Immunoglobulin M antibody response to measles virus following primary and secondary vaccination and natural virus infection. J Med Virol. 1993;41:44–48. doi: 10.1002/jmv.1890410110. [DOI] [PubMed] [Google Scholar]
- 28.Park JH, Allen MG, Prausnitz MR. Biodegradable polymer microneedles: fabrication, mechanics and transdermal drug delivery. J Control Release. 2005;104:51–66. doi: 10.1016/j.jconrel.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 29.Li ZM, Goitz RJ. Biomechanical evaluation of the motor function of the thumb. Technology and health care : official journal of the European Society for Engineering and Medicine. 2003;11:233–243. [PubMed] [Google Scholar]
- 30.Auwaerter PG, Rota PA, Elkins WR, Adams RJ, DeLozier T, Shi Y, et al. Measles virus infection in rhesus macaques: altered immune responses and comparison of the virulence of six different virus strains. J Infect Dis. 1999;180:950–958. doi: 10.1086/314993. [DOI] [PubMed] [Google Scholar]
- 31.Chen RT, Markowitz LE, Albrecht P, Stewart JA, Mofenson LM, Preblud SR, et al. Measles antibody: reevaluation of protective titers. J Infect Dis. 1990;162:1036–1042. doi: 10.1093/infdis/162.5.1036. [DOI] [PubMed] [Google Scholar]
- 32.Dine MS, Hutchins SS, Thomas A, Williams I, Bellini WJ, Redd SC. Persistence of vaccine-induced antibody to measles 26–33 years after vaccination. J Infect Dis. 2004;189(Suppl 1):S123–S130. doi: 10.1086/380308. [DOI] [PubMed] [Google Scholar]
- 33.Pharmaceutical Manufacturing Handbook: Production and Processes. 1st ed: Wiley-Interscience; 2008. [Google Scholar]
- 34.Simonsen L, Kane A, Lloyd J, Zaffran M, Kane M. Unsafe injections in the developing world and transmission of bloodborne pathogens: a review. Bull World Health Organ. 1999;77:789–800. [PMC free article] [PubMed] [Google Scholar]
- 35.Kermode M. Unsafe injections in low-income country health settings: need for injection safety promotion to prevent the spread of blood-borne viruses. Health Promot Int. 2004;19:95–103. doi: 10.1093/heapro/dah110. [DOI] [PubMed] [Google Scholar]
- 36.Guichard S, Hymbaugh K, Burkholder B, Diorditsa S, Navarro C, Ahmed S, et al. Vaccine wastage in Bangladesh. Vaccine. 2010;28:858–863. doi: 10.1016/j.vaccine.2009.08.035. [DOI] [PubMed] [Google Scholar]
- 37.Davey S. Health: A key to prosperity: Success stories in developing countries. Health. 2000 [Google Scholar]
- 38.Expanded programme on immunization. Stability of vaccines. Wkly Epidemiol Rec. 1990;65:233–235. [PubMed] [Google Scholar]
- 39.Moon S, Wang Y, Edens C, Gentsch JR, Prausnitz MR, Jiang B. Dose sparing and enhanced immunogenicity of inactivated rotavirus vaccine administered by skin vaccination using a microneedle patch. Vaccine. 2013;31:3396–3402. doi: 10.1016/j.vaccine.2012.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Suh H, Shin J, Kim YC. Microneedle patches for vaccine delivery. Clin Exp Vaccine Res. 2014;3:42–49. doi: 10.7774/cevr.2014.3.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Christe KL, McChesney MB, Spinner A, Rosenthal AN, Allen PC, Valverde CR, et al. Comparative efficacy of a canine distemper-measles and a standard measles vaccine for immunization of rhesus macaques (Macaca mulatta) Comp Med. 2002;52:467–472. [PubMed] [Google Scholar]
- 42.Pasetti MF, Resendiz-Albor A, Ramirez K, Stout R, Papania M, Adams RJ, et al. Heterologous prime444 boost strategy to immunize very young infants against measles: pre-clinical studies in rhesus macaques. Clin Pharmacol Ther. 2007;82:672–685. doi: 10.1038/sj.clpt.6100420. [DOI] [PubMed] [Google Scholar]
- 43.Cutts FT, Clements CJ, Bennett JV. Alternative routes of measles immunization: a review. Biologicals. 1997;25:323–338. doi: 10.1006/biol.1997.0103. [DOI] [PubMed] [Google Scholar]
- 44.Comparative trial of live attenuated measles vaccine in Hong Kong by intramuscular and intradermal injection. Bull World Health Organ. 1967;36:375–384. [PMC free article] [PubMed] [Google Scholar]
- 45.Cooper C, Morley DC, Weeks MC, Beale AJ. Administration of measles vaccine by dermojet. Lancet. 1966;1:1076–1077. doi: 10.1016/s0140-6736(66)91015-4. [DOI] [PubMed] [Google Scholar]
- 46.Wood PB, Soheranda KS, Bracken PM, Houser NE. Measles vaccination in Zaire--when and how? Trans R Soc Trop Med Hyg. 1980;74:381–382. doi: 10.1016/0035-9203(80)90105-4. [DOI] [PubMed] [Google Scholar]
- 47.de Moraes JC, León ME, Souza VA, Pannuti C, Travisanello C, Halsey NA, et al. Intradermal administration of measles vaccines. Bull Pan Am Health Organization. 1994;28:250–255. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Detection of measles virus-specific IgM following vaccination of rhesus macaques. Measles-specific IgM was detected by ELISA following measles vaccination by microneedle patch or subcutaneous injection on day 0. Data points show ΔOD values serum samples collected at day 0 and up to 28 days post vaccination. The dashed line represents the cutoff for a positive result (ΔOD >0.10).