Abstract
Despite decades of advances in transplant immunology, tissue damage caused by acute allograft rejection remains the primary cause of morbidity and mortality in the transplant recipient. Moreover, the long-term sequelae of lifelong immunosuppression leaves patients at risk for developing a host of other deleterious conditions. Controlled drug delivery using micro- and nanoparticles (MNPs) is an effective way to deliver higher local doses of a given drug to specific tissues and cells while mitigating systemic effects. Herein, we review several descriptions of MNP immunotherapies aimed at prolonging allograft survival. We also discuss developments in the field of biomimetic drug delivery that use MNP constructs to induce and recruit our bodies' own suppressive immune cells. Finally, we comment on the regulatory pathway associated with these drug delivery systems. Collectively, it is our hope the studies described in this review will help to usher in a new era of immunotherapy in organ transplantation.
Keywords: Transplant Immunology, Microparticles, Nanoparticles, Biomaterials, Immunosuppression, Biomimetic, Controlled Release
1. Allotransplantation - From Mythology to Life Saving Medicine
The concept of replacing or transplanting damaged organs and tissues has likely existed as long as human disease and trauma [1, 2]. Initially only described as the medicine of mythology, it was not until the 1970s that effective combination immunosuppression was able to consistently yield successful long-term survival in early kidney transplant recipients. Further advances including the discovery of calcineurin inhibitor drugs (FK506 and Cyclosporine), the development of medical devices (such as the heart-lung machine), improvement in surgical techniques and a more mature understanding of the immune system has made the discipline of transplantation a ubiquitous and life saving therapeutic strategy [3].
The success of organ transplantation to date has hinged on the continued development (and improvement) of immunosuppression [3]. Though somewhat subjective, the development of immunosuppression (in the context of transplant immunology) can be divided into four distinct eras (Figure 1). The first era of immunosuppression employed rather rudimentary techniques including total body irradiation, splenectomies, thymectomies, and high dose corticosteroids [4]. The next era ushered in the development of more sophisticated pharmacological agents with more specific targets (Cyclosporine, FK506 and Mycophenolic Acid) [5–10]. Building upon these discoveries, research in the past twenty years focused the development of specific monoclonal antibodies and fusion proteins that target various lymphocyte ligands. The (future) fourth era of immunosuppression encompasses current experimental therapies being reported in small trials and preclinical studies in the literature. This includes the use of ex vivo cell therapy [11–14], organ engineering [15, 16], and smart immunosuppression using biomaterials such as microparticles and nanoparticles the later two will be the primary focus of this review.
Figure 1.
The progression of immunosuppression through four distinct eras.
2. Clinical Immunosuppression – Mechanism, Indications and Side Effects
Clinically, the process of allograft rejection is staved off by the prescription of immunosuppressive agents that are administered systemically. While effective at promoting allograft survival, the use of lifelong, high dose, multi-drug immunosuppression is associated with a well-established sequelae of global side effects [17, 18]. The following section will introduce some of the most commonly used immunosuppressants as well as their associated advantages and disadvantages.
2.1 Mycophenolic Acid (MMF)
Mycophenolic acid (MMF) is an anti-proliferative immunosuppression drug that is now generally used in most centers in lieu of the antimetabolite azathioprine due to its more favorable toxicity profile and specificity for lymphocytes. From a mechanistic standpoint, MMF is an inhibitor of inosine monophosphate dehydrogenase-2 (IMPDH2), which is the rate-limiting enzyme in de novo guanine synthesis. IMPDH2 activity is increased in highly proliferating cells as well as in tissues with rapidly proliferating cell populations (such as T cells and B cells). Complications with MMF include leukopenia, gastritis, esophagitis, and opportunistic CMV infection [19–21].
2.2 Calcineurin Inhibitors - FK506 and Cyclosporine
Cyclosporine (CsA), implemented by R.Y. Calne in 1979, dramatically changed the landscape of organ transplantation and is responsible for transplantation becoming the gold standard treatment for end-stage organ failure. Mechanistically, CsA is able to suppress T cell activity via inhibition of the intracellular enzyme calcineurin, resulting in reduced expression of pro-inflammatory cytokines necessary for activity of effector T cells. Though efficient in reducing the incidence of acute rejection, CsA has several severe side effects including end-organ toxicity, diabetes, hypertension and neurotoxicity [8].
FK506 (also known as tacrolimus) was developed as a next generation calcineurin inhibitor in the advent of the success of CsA. Described in 1987 by Kino and colleagues, FK506 is a macrolide antibiotic produced by Streptococcus tsukubaensis [6]. Of importance, FK506 has been shown to be 10–200 times more potent than CsA, allowing for FK506 to be a superior alternative for preventing episodes of acute rejection, and even positioning FK506 to be used as monotherapy in kidney transplantation [22–24]. Similar to CsA, FK506 unfortunately has numerous side effects including opportunistic infection, diabetes, nephrotoxicity and neurotoxicity [17, 25, 26].
2.3 Rapamycin
Rapamycin (also referred to as sirolimus or rapa) was initially discovered in the 1970s as a fermentation product of Streptomyces hygroscopicus with antifungal properties [27]. It was not until the discovery of the calcineurin inhibitor FK506 that researchers began to reevaluate rapamycin as a potential immunosuppressive agent [28]. Mechanistically, rapa inhibits the mammalian target of rapamycin (mTOR), a serine/threonine protein kinase. By inhibiting mTOR, rapamycin is able to regulate cell proliferation by preventing cell cycle progression from the G1 to the S phase and inhibit protein synthesis [28]. In addition to its ability to affect T cell proliferation, rapamycin is also able to influence dendritic cell (DC) function, driving DCs toward a more tolerogenic phenotype [29–31]. Furthermore, rapamycin has also been shown to synergize with cytokines such as TGF-β and IL-2 to expand naïve T cells toward a regulatory T cell (Treg) phenotype [32–36]. Because the anti-proliferative effects of rapa are not exclusive to cells of the immune system, it can interfere with fibroblast function resulting in impaired wound healing. Other adverse side effects observed with rapamycin include life threatening pneumonitis, proteinuria and in some instances diabetes [28, 37]. Despite these adverse effects, rapamycin has been shown to have negligible nephrotoxicity (a significant advantage over its calcineurin inhibitor counterparts) [28].
2.4 Limitations of Current Immunosuppressive Agents
While the current lineup of immunosuppressive agents that are administered to transplant patients allow for improved graft survival times, this is not without cost to the patient. As alluded to above, most forms of immunosuppression currently used are associated with significant toxicity and side effects including nephrotoxicity, opportunistic infections, diminished tumor immunosurveillance, serum sickness, anaphylaxis and neurotoxicity [17, 23, 25, 26, 28, 37]. Additionally, systemic delivery (either oral (P.O.) or intravenous (IV)) of these agents is often times associated with unpredictable blood levels of drug, leading to the presence of toxic (peak) or non-therapeutic (trough) concentrations. Taken together, organ transplantation is not technically a “curative” intervention, but rather one that forces patients to trade one chronic condition (end stage organ failure) for another chronic condition (becoming a transplant recipient patient).
Another barrier to improved patient outcomes after transplantation is the issue of patient compliance and non-adherence to the recommended treatment regimens. This is not surprising based on the laundry list of side effects listed above as well as the daunting dosing frequency of conventional immunosuppression protocols. While, there are multiple variables that can impact the degree of non-adherence (socioeconomic status, gender, education level), the incidence of non-adherence in some studies is as high as 68% [38–40]. Moreover, patient non-adherence is associated with poor outcomes, graft failure and increased costs to hospitals, patients and payers [38].
Collectively, these shortcomings (namely toxicity and non-adherence), underscore the importance of improving current therapies and developing future technologies to improve patient and graft outcomes in the context of allotransplantation. Such technologies would ideally be able to target delivery of therapeutic agents to the tissues/organs of interest (obviating systemic toxicity issues) and provide sustained release of factors (eliminating the need for regular and frequent consumption). To that end, the remainder of this review will focus on targeted and/or sustained drug delivery using nano- and microparticles.
3. Nano and Microparticle Based Immunosuppression
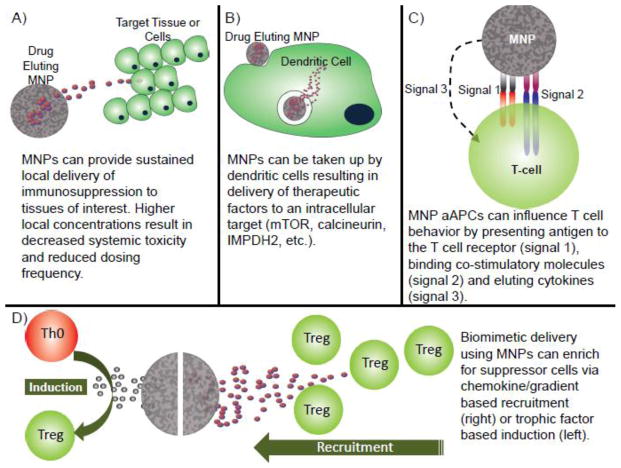
The future success of the field of transplant immunology centers upon the resolution of many of the current shortcomings with conventional immunosuppression. Decades of research in the fields of materials science and biomaterials have resulted in the development of numerous drug delivery devices that aid current immunosuppressants in eliciting improved results via sustained, targeted and controlled release of drugs [41]. Specifically, controlled drug delivery offers several key advantages over systemic therapy such as the ability to maintain therapeutic drug concentrations, reduce systemic toxicity by targeting the therapy to specific sites (tissues or cells), and protect therapeutic payloads [42]. In addition, recent advances in the field have provided engineers with the necessary tools to create, biocompatible and biodegradable delivery systems that can target various tissue types and even triggerably degrade in response to specific environmental cues. The most widely used drug delivery systems are polymeric micro- and nanoparticles (MNP). Properties of MNPs such as size, and release kinetics can be tuned by adjusting the chemical composition, fabrication method, degree of crosslinking and molecular weight [43–45]. MNP size and chemical composition can also be modified to allow for depot release or intracellular uptake. Chemical modification of the surface of MNPs can allow for covalent coupling of targeting molecules such as antibodies and receptors. Taken together these features improve the clinical efficacy of pharmacological therapies by limiting systemic exposure to a given drug, while concurrently providing controlled, local delivery to the desired tissue (Figure 2A).
Figure 2.
Delivery routes and mechanisms using MNP based drug delivery systems.
Mechanistically, the tissue and cellular distribution of the MNP systems described herein can be modified primarily via particle size. MNP size plays a critical role in the biodistribution of a given particle. Specifically, large microparticles (>10μm) can act as a depot at the site of injection as they are large enough to avoid phagocytic clearance and cannot pass thorough most biological barriers [43]. Smaller microparticles (approximately 2–3μm) are considered to be optimal for uptake by antigen presenting cells (APCs) such as macrophages and dendritic cells (DCs), but large enough non-specific pinocytotic cellular uptake [46]. Given the versatility of these drug delivery systems, there has been much interest in optimizing and improving current immunotherapies (described above) by incorporating them in MNP systems. Though there are numerous studies detailing MNPs in cancer therapy, vaccine development and infectious disease [41, 43, 47], we will focus on the use of MNPs in transplant immunology (Table 1).
Table 1.
Examples of currently developed MNP drug delivery systems for immunosuppression and regulation.
| Device Description | Key Findings | Ref. | |
|---|---|---|---|
| Small Molecule MNPs | FK506 Eluting PLGA MPs | Subcutaneous injection of FK MPs could maintain therapeutic blood levels of drug and prolong murine liver and islet allograft survival. | 58, 59 |
| FK506 Eluting PDLLA MPs | FK506 MPs accumulate in Peyer’s Patches after oral administration and prolong swine small bowl transplant survival. | 62 | |
| FK506 Loaded TGMS Hydrogel | A single injection of enzyme responsive FK506 TGMS hydrogel prolongs rat hind-limb transplant survival when compared to systemic FK506. | 66 | |
| Rapa Loaded PLA/Chitosan NPs | PLA/Chitosan NPs release rapa for over 8 days. NPs demonstrated excellent retention in procorneal area and prolong corneal allografts (rabbit) when compared to rapa eye drops. | 72 | |
| Rapa Eluting PLGA MNPs | Rapa MNPs can be taken up by DCs for intracellular rapa delivery. Treated DCs showed decreased immunostimulatory capacity and decreased expression of maturation markers. | 68–70 | |
| MMF Loaded PLGA NPs | Intraperitoneal injections of MMF NPs prolong murine skin allografts (across a full MHC barrier) and show a decreased toxicity profile when compared to systemic soluble MMF | 73 | |
| Antisense Oligonucleotide PEG/PVP MPs | Subcutaneous injection of MPs loaded with antisense CD40, CD80 and CD86 oligonucleotides reverse type 1 diabetes in NOD mice. | 80 | |
| Biomimetic MNPs | LIF Eluting Anti-CD4 Coated PLGA NPs | LIF NPs coated with anti-CD4 target naïve T cells, expand nonhuman primate Tregs (in vitro), expand murine Tregs in vivo and prolong murine heart allograft survival. | 87 |
| CCL22 Releasing PLGA MPs | CCL22 MPs set up a chemokine gradient to recruit adoptively Tregs in vivo and can prevent inflammatory effects of periodontal disease in both murine and canine models. | 92, 93 | |
| Rapa, IL-2, and TGFβ PLGA MPs | The combination of RapaMP/IL-2MP/TGFβ MP is able to induce naive T cells (mouse and human) toward FoxP3+ Treg phenotype in vitro. | 32 | |
| aAPC MNPs | Anti-CD3 and Anti-CD28 Affixed to IL-2 PLGA MP | IL-2 MPs with anti-CD3 and anti-CD28 coupled to the MP surface interact with CD8+ T cells in vitro and can induce robust proliferation. | 97, 99 |
| Anti-Fas and MHC Antigen Affixed to Polystyrene MPs | Killer aAPCs coated with apoptosis inducing anti-Fas can selectively delete antigen specific T cells and prolong skin allograft survival in a mouse model. | 102, 103 |
3.1 Calcineurin Inhibitor MNP Release Systems
As mentioned previously, CNIs currently remain the mainstay of most clinically employed immunosuppression protocols. Due to toxic side effects associated with systemic CNI therapy and poor oral bioavailability, there has been significant interest in developing CNI based MNP delivery systems [17, 48].
Sanchez et al. was among the first to develop, characterize and test CsA MNP formulations [49]. In these studies, three groups of poly(lactic-co-glycolic acid) (PLGA), MNPs (30 μm, 1 μm, and 0.2 μm) were fabricated. In vivo murine studies demonstrated that after subcutaneous injection, the 1 μm and 0.2 μm MNPs displayed a significant burst phenomena (i.e. high initial blood levels of CSA) whereas the 30 μm formulations lacked such such a large burst, providing steady blood levels of drug for over three weeks [49–51]. Building on this study, Urata et al. developed control release CsA poly(L-lactic acid) (PLA) microparticles containing fatty esters capable of releasing CsA in vivo for up to 4 weeks (without an initial burst). These formulations were effective at reducing symptoms of arthritis when administered in a rat model [52].
Targeted delivery of CsA MNPs (to both cells and lymphatics) offers all of the advantages of having a depot release of CsA while simultaneously providing a higher local concentration of drug to the tissues and cells of interest. Yoshikawa et al. were able to design PLGA nanoparticles (average diameter 260 nm) that released CsA for 30 days [53]. Moreover, intramuscular injection of CsA nanoparticles (femoral region) yielded undetectable plasma levels of CsA, but concentrations in the femoral lymph nodes were nearly 20 times that obtained when administering soluble systemic CsA [53].
Previous studies have revealed that polyethylene glycol (PEG) coated PLA particles (when compared to non PEG coated PLA particles) administered orally delivered significantly higher amounts of radioactive antigen to the lymphatic’s [54]. This effect was hypothesized to be a result of increased particle hydrophilicity after PEG coupling [45]. Building on this finding, Azzi et al. described a method for conjugating CsA to PLA/PEG nanoparticles (average size 84 nm). These CsA/PLA/PEG NPs were able to suppress T cell proliferation in mixed lymphocyte reaction (MLRs) in a comparable manner to soluble CsA. Moreover, they were able to show that DCs could effectively phagocytose and traffic NPs to the popliteal lymph nodes in a mouse model. Finally, NP-treated DCs were also able to suppress T cell proliferation (MLR) when compared to untreated DCs [55].
In addition to synthetic polymer-based MNPs, several groups have developed natural (biological material) based MNPs to release CsA. Natural materials can have advantages over their synthetic counterparts, including improved cytotoxicity, genotoxicity and hemocompatibility. Woo et al. described hyaluronic acid microparticles that exhibited superior dissolution, solubility and bioavailability characteristics when compared with soluble CsA [56]. Additional studies using chitosan and fibrin based nanoconstructs have been described, however applications to clinically relevant pathologies have not yet been explored [57, 58].
Controlled release formulations of FK506 have also been explored due to FK506’s potency and superiority to CsA [23, 24]. Sustained delivery of FK506 from PLGA-based MNPs is reported to be effective at preventing liver and islet allograft rejection and mitigating the effects of colitis in rodent models [59–62]. Investigators have also developed FK506 releasing Poly-D,L-lactic acid (PDLLA) releasing microparticles that (when administered orally) home to the Peyer’s patches and prolong small bowel transplant survival in a porcine model [63]. Significant research has also been conducted in the development of pH sensitive FK506 MNPs, which can deliver FK506 to the colon [64, 65]. These delivery systems have been used to treat murine models of inflammatory bowl disease (IBD); however, such technologies could be used in the context of small bowl transplantation [61, 62, 66].
Very recently Gajanayake et al. tested the ability of a triglycerol monostearate (TGMS) enzyme-responsive hydrogel loaded with FK506 to prevent hindlimb allograft transplant rejection in a rat model[67]. Specifically a one time injection of FK506 loaded TGFMS was shown to prolong hindlimb survival across a full MHC barrier for > 100 days. Furthermore, it was demonstrated that tissue levels of FK506 in treated animals were increased 10 fold over systemic levels [67]. While this study did not specifically utilize MNPs, it is possible to envision future applications of such a gel used in tandem (or as a carrier) with MNPs to provide dual delivery of immunosuppressive agents.
3.2 Rapamycin MNP Release Systems
Rapamycin based MNP systems are an especially attractive therapy as Rapa has been shown to not only inhibit T cell proliferation, but also inhibit DC maturation and function and enrich for suppressive FoxP3+ Regulatory T cells [28–31, 44]. As such, technologies taking advantage of both of these characteristics have been described in the literature. Forrest et al. described injectable rapamycin-loaded poly(ethylene glycol)-b-poly(epsilon-caprolactone) (PEG-PCL) micelle constructs that could provide over 6 days of in vitro release [68]. Due to the described effects that rapa can have on DCs, several studies have focused on targeted delivery of PLGA based MNPs to DCs [69]. Jhunjhunwala et al. described rapa microparticles (3.4 μm) that are phagocytosed by DCs and release their payload intracellularly for up to three weeks. DCs treated with these rapa microparticles had a decreased ability to induce T cell proliferation, and showed down regulation of surface co-stimulatory molecules [69]. Two other studies examined delivery of rapa to DCs (both human and mouse) with smaller PLGA nanoparticles (150–450 nm). These studies demonstrated that rapa nanoparticles are effectively taken up by DCs and decreased expression of all maturation markers (Figure 2B). Furthermore, when compared with free rapamycin, rapa nanoparticle-treated DCs showed decreased immunostimuatory capacity [70, 71].
In addition to PLGA based MNPs, there have been reports of alternative materials used in the development of rapamycin drug delivery systems. Kauffman and associates recently described rapamycin-loaded, acetylated dextran microparticles that have several advantages over PLGA microparticles such as more tunable release kinetics and differential release in acidic vs. neutral conditions [72]. Furthermore, Yuan et al. were able to develop rapamycin load chitosan/PLA nano particles to prevent rejection in a rabbit model of corneal transplantation. Chitosan/PLA rapa nanoparticles were able to show superior procorneal retention (as compared to free rapa) and were able to delay corneal allograft rejection [73].
3.3 MNP Delivery of Antimetabolites
In addition to intracellular delivery of rapamycin, there has been considerable interest in developing MNP based systems that can release the antimetabolite MMF. Shirali et al described an MMF PLGA based nanoparticle system that appears to prolong full thickness murine skin transplants across a full MHC mismatch (BALB/c → C57BL/6), when compared with daily systemic administration of MMF, via an apparent PD-L1 mediated mechanism. Notably, this therapeutic result was achieved despite MMF nanoparticles containing 1000 fold less MMF than is typically required with systemic administration of rapamycin. Moreover, treatment with MMF nanoparticles resulted in no detectable toxicity, whereas recipients receiving conventional MMF developed iatrogenic cytopenias [74]. In a similar study, MMF loaded in a nanoparticle containing cyclodextrins enclosed in a lipid bilayer (referred to as a nanogel), was capable of prolonging survival in a mouse model of lupus, when compared to free drug. The authors concluded that the enhanced ability of MMF nanogels to suppress inflammation could be attributed to nanoparticle uptake by DCs (Figure 2B) [75]. In yet another study from this group, researchers directly compared DC internalization ability of MMF PLGA nanoparticles to MMF nanogels [76]. The authors were able to show that while both MMF formulations were able reduce production of proinflammatory cytokines and stimulatory surface markers, the MMF nanogels were more effective, likely due to their enhanced uptake by DCs. As a caveat, while the MMF nanogels were more efficiently taken up by DCs, similar immunosuppressive effects could probably be seen with MMF PLGA nanoparticles at the expense of using a higher dose of nanoparticles [76].
3.4 MNP Delivery of Genetic Material for Immunomodulation
Given the boom of interest in of gene therapy, it should be of no surprise that this technology could be applied to immunomodulation and immunosuppression strategies. Specifically double stranded small interfering RNA (siRNA) or single stranded antisense oligonucleotides, can silence or down regulate expression of specific genes of interest in the inflammatory pathway. The fragile nature of these compounds in serum (due to the ubiquity of serum nucleases) makes direct infusion an inefficient delivery method [77, 78]. A potential solution to this problem would be to treat a patient’s own cells ex vivo with a given type of gene therapy, followed by reinfusion of the treated cells. While obviating issues concerning stability of said genetic material this approach is faced with the many cumbersome logistical and regulatory hurdles associated with cell therapy [79].
MNP systems represent effective carriers for genetic material as they can protect the payload and in some instances delivery the given material intracellularly allowing for efficient gene silencing. While gene therapy and delivery using MNPs has been described extensively in the literature for the treatment of a multitude of diseases, there are few studies examining the use of MNP-based gene delivery to suppress inflammation. Giannoukakis and Trucco have used DC’s treated with anti-sense CD40, CD80, and CD86 oligonucleotides for treatment of type 1 diabetes; this cell therapy approach is currently in clinical in trials [80]. Despite the success of this approach, Giannoukakis and Trucco have also described a microparticle based system for intracellular delivery of these same factors to DCs. Polyethylene glycol (PEG)/polyvinyl pyrrolidone (PVP) based microparticles loaded with the antisense oligonucleotides were prepared and injected subcutaneously to a site anatomically proximal to the pancreatic lymph nodes in non-obese diabetic mice [81]. These microparticles accumulated in both the pancreas and spleen after injection and could reverse type 1 diabetes in treated mice. Moreover, Tregs isolated from microparticle treated mice were able to prevent beta cell allorecognition when adoptively transferred to secondary recipients [81]. Given these convincing results, it is likely that this approach could be met with success if applied to organ transplantation as well.
4. Biomimetic Drug Delivery
Though controlled and targeted delivery of immunosuppressive drugs allows for more efficacious clinical outcomes with fewer side effects, advances in our understanding of the immunology of the immune system have allowed for the development of treatments that mimic the body’s own tolerogenic mechanisms. Indeed it is known that the cells of our own immune system have evolved to utilize a whole host of strategies to naturally maintain immunological homeostasis and promote tolerance, representing a level of sophistication that dwarfs clinically utilized immunosuppressive treatments. Notably, regulatory T cells (Treg) and tolerogenic dendritic cells (tDC) are two hallmark examples of cells with such homeostatic capabilities [13, 82]. The local presence (or absence) of these cells has been linked to the absence (or presence, respectively) of destructive inflammation in a number of diseases [14, 83, 84]. Indeed most therapeutic strategies to harness these cells have thus far focused on ex vivo expansion followed by in vivo re-administration. Though promising in experimental models and small trials, clinical implementation of these approaches faces numerous logistical and regulatory hurdles. These include GMP isolation and handling of cells, achieving ex vivo proliferation of isolated cells, and maintaining stability of adoptively-transferred cells [82]. As such, there is significant interest in the biomaterials community to develop synthetic (MNP based) cell constructs to mimic the effects of Tregs and tDCs as well as enrich these naturally occurring cell populations at a given site in the body (Figure 2D) [44].
4.1 MNP Delivery of Treg-Inducing and Recruiting Factors
It is well understood that the phenotypic fate of naïve T cells is determined by both soluble paracrine signals as well as T cell/APC contact in the immune synapse. Due to the potent immunoregulatory characteristics of Treg, strategies to induce naïve T cells to adopt a Treg phenotype have the potential to promote self tolerance in the context of autoimmunity, or acquired tolerance in the context of allotransplantation [33, 85–87]. MNP based systems that deliver cytokines and proregulatory factors to promote Treg induction and expansion have been described by several groups. For example, investigators have developed targeted anti-CD4 coated PLGA based nanoparticles that release leukemia inhibitory factor (LIF), an IL-6 like cytokine shown to induce FoxP3 expression in T cells [86, 88, 89]. These CD4 targeting LIF nanoparticles were capable of expanding FoxP3+ cells in vivo in treated mice and expanding nonhuman primate Tregs in vitro [88]. Additionally, these same nanoparticles prolonged vascularized heart allograft survival across a full MHC mismatch when compared with controls receiving no treatment[88]. In yet another study, investigators were able to prevent β-islets cell transplant rejection across a full MHC barrier and promote normoglycemia in diabetic mice by tethering LIF eluting PLGA nanoparticles to β-islets [90]. Jhunjhunwala et al. describe another mimetic MNP based system that can induce/expand Tregs. In this study, they demonstrated that the combination of TGFβ, IL-2 and rapamycin, (both soluble and in sustained release PLGA microparticle formulations) could convert both mouse and human naïve CD4+ T cells to FoxP3+ Tregs in vitro [32]. Specifically, after 4 days of co-culture with naïve T cells, this triple cocktail microparticle therapy efficiently induced FoxP3 expression in 80% of T cells. Importantly these microparticle-induced Tregs were capable of robust proliferation and expressed key canonical Treg cell surface markers (CD25, FR4 and GITR) [32]. Based on the results to date, it is possible that such Treg expanding and inducing microparticle formulations could serve as an off the shelf therapy for suppressing aberrant inflammation in the context of transplant immunology and autoimmunity.
While the idea of regulatory cell expansion and induction is an effective strategy to promote homeostasis, recruiting endogenous regulatory cells with particle-based “homing” systems is another viable strategy to enrich cells in a given location[44]. Indeed this concept is exploited often in nature by cells that secrete chemokines (chemoattractant cytokines) to recruit cells expressing the corresponding chemokine receptors to the source of the gradient. As a hallmark example, it has been shown that a variety of tumor cells secrete the chemokine CCL22, which appears to effectively recruit Tregs, which express the associated CCR4 receptor [91]. This local recruitment of Treg to the site of the malignancy is thought to enable tumor-specific immune evasion. Based on such phenomena, it is possible to envision strategies to recruit Tregs using CCL22 and induce therapeutically-relevant, local immunological hyporesponsiveness [92]. For instance, pancreatic islet cells have been transfected with an adenovirus designed to over express CCL22, as a means to recruit Tregs to the pancreas and prevent autoimmune diabetes in a mouse model [93].
Building on these findings, controlled release of CCL22 from PLGA microparticles was shown to promote site-specific recruitment of endogenous Tregs in vivo (Figure 2D) [94]. Specifically, CCL22 microparticles were able to recruit adoptively transferred Tregs in a mouse model to the site of microparticle injection and concurrently delay rejection of transplanted allogeneic cells (which were also injected at the site of microparticle administration) [94]. Moreover, Treg homing to a locally placed depot of CCL22 microparticles has been shown to obviate the inflammatory effects of periodontal disease in both murine and canine models [95].
4.2 MNP Systems to Induce Tolerogenic DCs
Building on the concept of multifactor microparticle based delivery, Lewis et al. recently demonstrated that combined (intracellular and extracellular) delivery of cytokines and small molecules to DCs could prevent DC maturation, thereby inducing a more tolerogenic phenotype [96]. Two classes of PLGA microparticles were designed; phagocytosable microparticles loaded with either rapamycin or retinoic acid (RA) and unphagocytosable microparticles loaded with either TGFβ or IL-10. Four distinct combinations of microparticles (Rapa/IL-10, Rapa/TGFβ, RA/IL-10 and RA/TGFβ) were tested, when cultured with DCs they were shown to decrease levels of surface expression of MHC II, CD80 and CD86 and were resistant to LPS stimulation. Furthermore, DCs treated with these dual microparticle systems were able to suppress T cell stimulation in mixed lymphocyte reactions and skew T cells toward a regulatory phenotype. Importantly, in all experiments it was shown that combinatorial parings of immunosuppressive microparticles were more effective than microparticles releasing any of the respective single factors[96].
4.3 Artificial Antigen Presenting Cells
It is known that DCs play a key role in the initial steps of T cell activation, namely via antigen presentation (signal 1) in the context of signal 2 (costimulation) and 3 (paracrine cytokine signaling). Accordingly, there is great interest in developing synthetic particle-based materials to mimic the function of DCs in this process, allowing for specific T cell targeting for drug delivery [97, 98]. Referred to as “artificial antigen presenting cells” (aAPCs), these MNP particles seek to engage the TCR receptor in a similar manner as a natural APC and elicit a given response (Figure 2C) [99]. In general, most aAPC constructs have a high density of surface bound anti-CD3 and anti-CD28 ligands for the T cell receptor (CD3) and the costimulatory receptors (CD28) (signals 1 and 2). Further, there are descriptions of aAPCs that (in addition to the aforementioned surface coatings) are also loaded with cytokine (signal 3). Though anti-CD3 can serve as a nonspecific activator for all T cell receptors, there are also descriptions of aAPCs that can present a given peptide in the context of an MHC class II complex (analogous to what is observed in nature), thus providing an antigen specific response [100].
The ideal aAPC should possess three characteristics, namely it should be 1.) Easily modifiable to allow for a variety of surface modifications, 2.) Capable of controlled release of cytokines when engaged with a T cell and 3.) Available in an off the shelf formulation [99, 101]. Steenblock et al. were the first to described such an all-inclusive aAPC [99]. Using PLGA as the base polymer, these aAPCs consisted of surface bound anti-CD3, and anti-CD28 as well as encapsulated soluble IL-2 (signals 1, 2, and 3). Surface modification was carried out by incorporating an avidin-palmitic acid conjugate into the PLGA emulsion (thus allowing the palmitate to interact with the hydrophobic PLGA core and the avidin residing on the surface) allowing for biotinylated anti-CD3/CD28 or peptide/MHC complex conjugation to the particle surface. This combination of antigen presentation, costimulation and paracrine delivery of IL-2 was able to enhance in vitro CD8+ T cell proliferation by a factor of 45 when compared with addition of exogenous IL-2 [99]. Building on this observation, further studies showed that the spatial and temporal characteristics of paracrine IL-2 release from an aAPC can have differential effects on CD8+ and CD4+ cells. Specifically, delivery of IL-2 upon aAPC contact with the TCR complex promotes IL-2 accumulation in the APC/T cell immune synapse, resulting in robust CD8+ proliferation, but activation induced apoptosis in CD4+ populations [101]. Interestingly, this result could not be replicated even with addition of 1000 fold exogenous IL-2, suggesting that a slow sustained release along with synaptic accumulation of IL-2 are responsible for this result [101].
Though the vast majority the applications of aAPCs in the literature focus on immunoustimulation and effector T cell proliferation, it is easy to envision strategies that employ the use of aAPCs for immunoregulatory applications. Indeed, Francisco et al. were able to show that surface bound PD-L1 on Dynabeads along with soluble TGFβ could enhance Treg induction when co-cultured with naïve T cells [102]. Schultz et al were the first to describe “killer aAPCs” that could target and delete antigen specific T cells [103, 104]. These killer aAPCs consisted of microparticles with the apoptosis inducing ligands (anti-Fas ligand (anti-FasL)) and HLA-A2 affixed to the particle surface [104]. When co-cultured with T cell populations, these aAPCs were able to promote antigen specific T cell depletion in a FasL dependent mechanism [104]. In another study, “killer aAPCs” were shown prolong skin allograft survival (in a mouse model) via the deletion of antigen specific, alloreactive T cells [105]. Given these promising results, it will be interesting to see how these therapies translate to solid organ transplantation.
The interactions between DCs and T cells in the immune synapse is a complex process, and the results from the aforementioned studies (while promising) underscore this complexity and the necessity of continued understanding of these interactions. Indeed, paracrine factor accumulation between cells and nanoparticles as well as the thermodynamic interactions between MNPs and cells has been described in the literature [106, 107]. Studies of this nature will continue to provide the necessary clues to developing an optimal artificial cell construct. Given this complexity, the continued success in the development of effective biomimetic aAPCs for the next generation immunotherapeutics will rely on the continued collaboration between engineers and immunologists.
5. Translational Considerations
Though the previously-described technologies are only in the in vitro development stages or in vivo preclinical studies, it should be noted that there are numerous MNP-based drug delivery systems, for other applications, that are both FDA approved and in clinical trials [108]. These systems utilize many of the materials such as PLGA, PLA and PEG and lipid based liposomes. Nonetheless, these systems all consist of an existing FDA-approved drug packed into a drug delivery system [109]. The advantages of controlled and sustained release have been described (namely decreased toxicity and improved patient adherence), however translation of nonconventional drug delivery systems (including MNP loaded with genetic material, biomimetic protein delivery, and aAPCS) will likely be more cumbersome as these technologies would be treated as a new drug [110]. For example, translation of a drug delivery system based on an existing FDA approved agent can cost between $20–50 million dollars and take 3–4 years [110]. In contrast, development of a new drug can cost upwards of $500 million dollars over 10 years [110]. Accordingly, it seems that in the immediate future, development of MNP systems that release FDA-approved immunosuppressive agents may have the easiest path to translation. This is not to say that the more complex delivery systems described are impractical, but a greater understanding of their toxicity profiles and clinical efficaciousness will need to be demonstrated before moving onto clinical trials.
While MNP systems that release FDA-approved immunosuppressives have an easier path to FDA approval, they also retain a host of issues that remain unresolved. The MNP systems currently approved by the FDA have a range of indications including delivery of chemotherapeutics, antipsychotics and antibiotics [109–111]. However there currently exist no MNP delivery system for immunosuppressive agents (for any application). The successful translation of such systems will hinge on demonstration of the safety of sustained release of highly toxic drugs such as FK506. Furthermore, delivery of immunosuppressive drugs using MNPs could present an issue if patient exposure to the delivered drug was to be discontinued (for example a transplant patient presenting with an active opportunistic infection). A potential solution to this problem would be to delivery MNPs in an injectable solution that could solidify after administration (such as a thermally responsive or shear responsive hydrogel)[112, 113]. The MNP gel complex would remain intact, provide sustained release of a given material and be easily removed in the event that therapy would need to be discontinued.
6. Concluding Remarks
The development of strategies for suppressing and modulating the immune responses following allotransplantation have improved considerably over the past 50 years. Rudimentary therapeutics such as corticosteroids and azathioprine that have global effects throughout the body have been replaced with more drugs that have T cell specific targets, with fewer (although still considerable) side effects. The development of both controlled, targeted delivery of these drugs as well as fabrication of biomimetic systems to modulate the immune system have the ability usher in a new era in transplantation in which allotransplantation may represent a true cure for end stage organ failure.
Highlights.
We describe developments in micro- and nanoparticle (MNP) based immunosuppression
MNP drug delivery can provide sustained, controlled and local delivery to the site of an allograft
Biomimetic MNP drug delivery can recruit, enrich and induce suppressor cells in vivo
Acknowledgments
The work of the authors was supported by the Arnold and Mabel Beckman Foundation (to S.R.L.), the Camille and Henry Dreyfus Foundation (to S.R.L.) and The National Institutes of Health (NIH) Award T32 AI 074490 (to J.D.F.).
Abbreviations
- MNP
Micro- and Nanoparticles
- MP
Microparticle
- NP
Nanoparticle
- aAPC
Artificial Antigen Presenting Cell
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kahan BD. Cosmas and Damien revisited. Transplantation proceedings. 1983;15:221–2216. [PubMed] [Google Scholar]
- 2.HC, Black KS, Fraser LA, Osborne JG, Achauer BM, Martin DC, Furnas DW. Cosmas and Damian in the laboratory. N Engl J Med. 1982;306:368–369. doi: 10.1056/NEJM198202113060620. [DOI] [PubMed] [Google Scholar]
- 3.Watson CJ, Dark JH. Organ transplantation: historical perspective and current practice. British journal of anaesthesia. 2012;108(Suppl 1):i29–42. doi: 10.1093/bja/aer384. [DOI] [PubMed] [Google Scholar]
- 4.DD, Fauci AS. The effect of in vivo hydrocortisone on subpopulations of human lymphocytes. J Clin Invest. 1974;53:240–246. doi: 10.1172/JCI107544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schafer DF, Sorrell MF. Optimising immunosuppression. The Lancet. 2002;360:1114–1115. doi: 10.1016/S0140-6736(02)11237-2. [DOI] [PubMed] [Google Scholar]
- 6.HH, Kino T, Hashimoto M, Nishiyama M, Goto T, Okuhara M, Kohsaka M, Aoki H, Imanaka H. FK-506, a novel immunosuppressant isolated from a Streptomyces. I. Fermentation, isolation, and physico-chemical and biological characteristics. The Journal of Antibiotics. 1987;40:1249–1255. doi: 10.7164/antibiotics.40.1249. [DOI] [PubMed] [Google Scholar]
- 7.DW, Warty V, Cadoff E, Todo S, Starzl T, Sanghvi A. FK506: a novel immunosuppressive agent. Characteristics of binding and uptake by human lymphocytes. Transplantation. 1988;46:453–455. doi: 10.1097/00007890-198809000-00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Italia JL, Bhardwaj V, Kumar MN. Disease, destination, dose and delivery aspects of ciclosporin: the state of the art. Drug discovery today. 2006;11:846–854. doi: 10.1016/j.drudis.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 9.Burke GW, Ciancio G. Show me the money—immunosuppression in kidney transplantation. The Lancet. 2004;364:481–483. doi: 10.1016/S0140-6736(04)16822-0. [DOI] [PubMed] [Google Scholar]
- 10.JC, Hariharan S, Bresnahan BA, Taranto SE, McIntosh MJ, Stablein D. Improved graft survival after renal transplantation in the United States, 1988 to 1996. N Engl J Med. 2000;342:605–612. doi: 10.1056/NEJM200003023420901. [DOI] [PubMed] [Google Scholar]
- 11.Raich-Regue D, Glancy M, Thomson AW. Regulatory dendritic cell therapy: from rodents to clinical application. Immunology letters. 2014;161:216–221. doi: 10.1016/j.imlet.2013.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Juvet SC, Whatcott AG, Bushell AR, Wood KJ. Harnessing regulatory T cells for clinical use in transplantation: the end of the beginning. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2014;14:750–763. doi: 10.1111/ajt.12647. [DOI] [PubMed] [Google Scholar]
- 13.Morelli AE, Thomson AW. Tolerogenic dendritic cells and the quest for transplant tolerance. Nature reviews Immunology. 2007;7:610–621. doi: 10.1038/nri2132. [DOI] [PubMed] [Google Scholar]
- 14.Trzonkowski P. First-in-man clinical results of the treatment of patients with graft versus host disease with human ex vivo expanded CD4+CD25+CD127- T regulatory cells. Clinical Immunology. 2009;133:22–26. doi: 10.1016/j.clim.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 15.Crapo PM, Gilbert TW, Badylak SF. An overview of tissue and whole organ decellularization processes. Biomaterials. 2011;32:3233–3243. doi: 10.1016/j.biomaterials.2011.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soto-Gutierrez A, Wertheim JA, Ott HC, Gilbert TW. Perspectives on whole-organ assembly: moving toward transplantation on demand. J Clin Invest. 2012;122:3817–3823. doi: 10.1172/JCI61974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Naesens M, Kuypers DR, Sarwal M. Calcineurin inhibitor nephrotoxicity. Clinical journal of the American Society of Nephrology : CJASN. 2009;4:481–508. doi: 10.2215/CJN.04800908. [DOI] [PubMed] [Google Scholar]
- 18.Errasti P, Izquierdo D, Martin P, Errasti M, Slon F, Romero A, Lavilla FJ. Pneumonitis associated with mammalian target of rapamycin inhibitors in renal transplant recipients: a single-center experience. Transplantation proceedings. 2010;42:3053–3054. doi: 10.1016/j.transproceed.2010.07.066. [DOI] [PubMed] [Google Scholar]
- 19.Remuzzi G, Lesti M, Gotti E, Ganeva M, Dimitrov BD, Ene-Iordache B, Gherardi G, Donati D, Salvadori M, Sandrini S, Valente U, Segoloni G, Mourad G, Federico S, Rigotti P, Sparacino V, Bosmans JL, Perico N, Ruggenenti P. Mycophenolate mofetil versus azathioprine for prevention of acute rejection in renal transplantation (MYSS): a randomised trial. The Lancet. 2004;364:503–512. doi: 10.1016/S0140-6736(04)16808-6. [DOI] [PubMed] [Google Scholar]
- 20.Knight SR, Russell NK, Barcena L, Morris PJ. Mycophenolate mofetil decreases acute rejection and may improve graft survival in renal transplant recipients when compared with azathioprine: a systematic review. Transplantation. 2009;87:785–794. doi: 10.1097/TP.0b013e3181952623. [DOI] [PubMed] [Google Scholar]
- 21.RJT Mechanism of action of mycophenolate mofetil. Ther Drug Monit. 1995;17:681–684. doi: 10.1097/00007691-199512000-00023. [DOI] [PubMed] [Google Scholar]
- 22.JDFJ, Liu Jun, Lane Willam S, Friedman Jeff, Weissman Irving, Schreiber Stuart L. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell. 1991;66:807–815. doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- 23.EM, Fung JJ, Todo S, Jain A, Demetris AJ, McMichael JP, Starzl TE, Meier P, Donner A. The Pittsburgh Randomized Trial of Tacrolimus Compared to Cyclosporine for Hepatic Transplantation. J Am Coll Surg. 1996;183:117–115. [PMC free article] [PubMed] [Google Scholar]
- 24.Abou-Jaoude MM, Najm R, Shaheen J, Nawfal N, Abboud S, Alhabash M, Darwish M, Mulhem A, Ojjeh A, Almawi WY. Tacrolimus (FK506) versus cyclosporine microemulsion (neoral) as maintenance immunosuppression therapy in kidney transplant recipients. Transplantation proceedings. 2005;37:3025–3028. doi: 10.1016/j.transproceed.2005.08.040. [DOI] [PubMed] [Google Scholar]
- 25.Ayres BDRCS, Wixon S, Buckels JAC, McMaster P, Mayer AD. Peripheral neurotoxicity with tacrolimus. The Lancet. 1994;343:862–863. doi: 10.1016/s0140-6736(94)92070-2. [DOI] [PubMed] [Google Scholar]
- 26.MA, Platz KP, Blumhardt G, Bachmann S, Bechstein WO, Kahl A, Neuhaus P. Nephrotoxicity following orthotopic liver transplantation. A comparison between cyclosporine and FK506. Transplantation. 1994;58:170–178. [PubMed] [Google Scholar]
- 27.KA, Vézina C, Sehgal SN. Rapamycin (AY-22,989), a new antifungal antibiotic. I. Taxonomy of the producing streptomycete and isolation of the active principle. J Antibiot. 1975;28:721–726. doi: 10.7164/antibiotics.28.721. [DOI] [PubMed] [Google Scholar]
- 28.MM, Saunders RN, Nicholson ML. Rapamycin in transplantation: A review of the evidence. Kidney Int. 2001;59:3–16. doi: 10.1046/j.1523-1755.2001.00460.x. [DOI] [PubMed] [Google Scholar]
- 29.Fischer THR, Taner T, Thomson AW. Use of Rapamycin in the Induction of Tolerogenic Dendritic Cells. Handbook of Experimental Pharmacology. 2009:215–232. doi: 10.1007/978-3-540-71029-5_10. [DOI] [PubMed] [Google Scholar]
- 30.Hackstein H, Taner T, Zahorchak AF, Morelli AE, Logar AJ, Gessner A, Thomson AW. Rapamycin inhibits IL-4--induced dendritic cell maturation in vitro and dendritic cell mobilization and function in vivo. Blood. 2003;101:4457–4463. doi: 10.1182/blood-2002-11-3370. [DOI] [PubMed] [Google Scholar]
- 31.Turnquist HR, Raimondi G, Zahorchak AF, Fischer RT, Wang Z, Thomson AW. Rapamycin-Conditioned Dendritic Cells Are Poor Stimulators of Allogeneic CD4+ T Cells, but Enrich for Antigen-Specific Foxp3+ T Regulatory Cells and Promote Organ Transplant Tolerance. The Journal of Immunology. 2007;178:7018–7031. doi: 10.4049/jimmunol.178.11.7018. [DOI] [PubMed] [Google Scholar]
- 32.Jhunjhunwala S, Balmert SC, Raimondi G, Dons E, Nichols EE, Thomson AW, Little SR. Controlled release formulations of IL-2, TGF-beta1 and rapamycin for the induction of regulatory T cells. Journal of controlled release : official journal of the Controlled Release Society. 2012;159:78–84. doi: 10.1016/j.jconrel.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.SA, Battaglia M, Draghici E, Gregori S, Mocchetti C, Bonifacio E. Rapamycin and interleukin-10 treatment induces T regulatory type 1 cells that mediate antigen-specific transplantation tolerance. Diabetes. 2006;55:40–49. [PubMed] [Google Scholar]
- 34.WT, Strauss L, Knights A, Bergmann C, Knuth A, Zippelius A. Selective survival of naturally occurring human CD4+CD25+Foxp3+ regulatory T cells cultured with rapamycin. Journal of Immunology. 2007;178:320–329. doi: 10.4049/jimmunol.178.1.320. [DOI] [PubMed] [Google Scholar]
- 35.SA, Battaglia M, Migliavacca B, Horejs-Hoeck J, Kaupper T, RMG Rapamycin promotes expansion of functional CD4+CD25+FOXP3+ regulatory T cells of both healthy subjects and type 1 diabetic patients. Journal of Immunology. 2006;177:8338–8347. doi: 10.4049/jimmunol.177.12.8338. [DOI] [PubMed] [Google Scholar]
- 36.SA, Battaglia M, Roncarolo MG. Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood. 2005;105:4743–4748. doi: 10.1182/blood-2004-10-3932. [DOI] [PubMed] [Google Scholar]
- 37.Lee HS, Huh KH, Kim YS, Kim MS, Kim HJ, Kim SI, Joo DJ. Sirolimus-induced pneumonitis after renal transplantation: a single-center experience. Transplantation proceedings. 2012;44:161–163. doi: 10.1016/j.transproceed.2011.11.059. [DOI] [PubMed] [Google Scholar]
- 38.Pinsky BW, Takemoto SK, Lentine KL, Burroughs TE, Schnitzler MA, Salvalaggio PR. Transplant outcomes and economic costs associated with patient noncompliance to immunosuppression. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2009;9:2597–2606. doi: 10.1111/j.1600-6143.2009.02798.x. [DOI] [PubMed] [Google Scholar]
- 39.Dew MA, DiMartini AF, De Vito Dabbs A, Myaskovsky L, Steel J, Unruh M, Switzer GE, Zomak R, Kormos RL, Greenhouse JB. Rates and risk factors for nonadherence to the medical regimen after adult solid organ transplantation. Transplantation. 2007;83:858–873. doi: 10.1097/01.tp.0000258599.65257.a6. [DOI] [PubMed] [Google Scholar]
- 40.Bosma OH, Vermeulen KM, Verschuuren EA, Erasmus ME, van der Bij W. Adherence to immunosuppression in adult lung transplant recipients: prevalence and risk factors. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2011;30:1275–1280. doi: 10.1016/j.healun.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 41.Hubbell JA, Thomas SN, Swartz MA. Materials engineering for immunomodulation. Nature. 2009;462:449–460. doi: 10.1038/nature08604. [DOI] [PubMed] [Google Scholar]
- 42.Danhier F, Ansorena E, Silva JM, Coco R, Le Breton A, Preat V. PLGA-based nanoparticles: an overview of biomedical applications. Journal of controlled release : official journal of the Controlled Release Society. 2012;161:505–522. doi: 10.1016/j.jconrel.2012.01.043. [DOI] [PubMed] [Google Scholar]
- 43.Kohane DS. Microparticles and nanoparticles for drug delivery. Biotechnology and bioengineering. 2007;96:203–209. doi: 10.1002/bit.21301. [DOI] [PubMed] [Google Scholar]
- 44.Glowacki AJ, Gottardi R, Yoshizawa S, Cavalla F, Garlet GP, Sfeir C, Little SR. Strategies to Direct the Enrichment, Expansion, and Recruitment of Regulatory Cells for the Treatment of Disease. Annals of biomedical engineering. 2014 doi: 10.1007/s10439-014-1125-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Balmert SC, Little SR. Biomimetic delivery with micro- and nanoparticles. Advanced materials. 2012;24:3757–3778. doi: 10.1002/adma.201200224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Champion JA, Walker A, Mitragotri S. Role of particle size in phagocytosis of polymeric microspheres. Pharm Res. 2008;25:1815–1821. doi: 10.1007/s11095-008-9562-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moon JJ, Huang B, Irvine DJ. Engineering nano- and microparticles to tune immunity. Advanced materials. 2012;24:3724–3746. doi: 10.1002/adma.201200446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.HMF Contributions of hepatic and intestinal metabolism and P-glycoprotein to cyclosporine and tacrolimus oral drug delivery. Advanced drug delivery reviews. 1997;15:201–214. doi: 10.1016/s0169-409x(97)00043-4. [DOI] [PubMed] [Google Scholar]
- 49.AJV-J, Sánchez, María J, Alonso Development of biodegradable microspheres and nanospheres for the controlled release of cyclosporin A. International journal of pharmaceutics. 1993;99:263–273. [Google Scholar]
- 50.Sánchez A, Seoane R, Quireza O, Alonso MJ. In vivo study of the tissue distribution and immunosuppressive response of cyclosporin a-loaded polyester micro- and nanospheres. Drug Delivery. 1995;2:21–28. [Google Scholar]
- 51.Sanchez A, Alonso MJ. Poly(D,L-lactide-co-glycolide) micro and nanospheres as a way to prolong blood/plasma levels of subcutaneously injected cyclosporin A. Journal of Pharmaceutics and Biopharmaceutics. 1995;41:31–37. [Google Scholar]
- 52.AK, Urata T, Nakano H. Modification of release rates of cyclosporin A from polyl(L-lactic acid) microspheres by fatty acid esters and in-vivo evaluation of the microspheres. Journal of Controlled Release. 1999;29:133–141. doi: 10.1016/s0168-3659(98)00146-1. [DOI] [PubMed] [Google Scholar]
- 53.SS, Yoshikawa H. Lymphotropic delivery of cyclosporin A by intramuscular injection of biodegradable microspheres in mice. Biol Pharm Bull. 1996;19:1527–1529. doi: 10.1248/bpb.19.1527. [DOI] [PubMed] [Google Scholar]
- 54.Tobíoa ASM, Vilaa A, Sorianob I, Evorab C, Vila-Jatoa JL, Alonsoa MJ. The role of PEG on the stability in digestive fluids and in vivo fate of PEG-PLA nanoparticles following oral administration. Colloids and Surfaces B: Biointerfaces. 2000;18:315–323. doi: 10.1016/s0927-7765(99)00157-5. [DOI] [PubMed] [Google Scholar]
- 55.Azzi J, Tang L, Moore R, Tong R, El Haddad N, Akiyoshi T, Mfarrej B, Yang S, Jurewicz M, Ichimura T, Lindeman N, Cheng J, Abdi R. Polylactide-cyclosporin A nanoparticles for targeted immunosuppression. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2010;24:3927–3938. doi: 10.1096/fj.10-154690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Woo JS, Piao MG, Li DX, Ryu DS, Choi JY, Kim JA, Kim JH, Jin SG, Kim DD, Lyoo WS, Yong CS, Choi HG. Development of cyclosporin A-loaded hyaluronic microsphere with enhanced oral bioavailability. International journal of pharmaceutics. 2007;345:134–141. doi: 10.1016/j.ijpharm.2007.08.050. [DOI] [PubMed] [Google Scholar]
- 57.Praveen G, Sreerekha PR, Menon D, Nair SV, Chennazhi KP. Fibrin nanoconstructs: a novel processing method and their use as controlled delivery agents. Nanotechnology. 2012;23:095102. doi: 10.1088/0957-4484/23/9/095102. [DOI] [PubMed] [Google Scholar]
- 58.SA, De Campos AM, Alonso MJ. Chitosan nanoparticles: a new vehicle for the improvement of the delivery of drugs to the ocular surface. Application to cyclosporin A. International journal of pharmaceutics. 2001;224:159–168. doi: 10.1016/s0378-5173(01)00760-8. [DOI] [PubMed] [Google Scholar]
- 59.Miyamoto Y, Uno T, Yamamoto H, Xiao-Kang L, Sakamoto K, Hashimoto H, Takenaka H, Kawashima Y, Kawarasaki H. Pharmacokinetic and immunosuppressive effects of tacrolimus-loaded biodegradable microspheres. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2004;10:392–396. doi: 10.1002/lt.20083. [DOI] [PubMed] [Google Scholar]
- 60.Wang Q, Uno T, Miyamoto Y, Hara Y, Kitazawa Y, Lu FZ, Funeshima N, Fujino M, Yamamoto H, Takenaka H, Kawashima Y, Li XK. Biodegradable microsphere-loaded tacrolimus enhanced the effect on mice islet allograft and reduced the adverse effect on insulin secretion. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2004;4:721–727. doi: 10.1111/j.1600-6143.2004.00423.x. [DOI] [PubMed] [Google Scholar]
- 61.Lamprecht A, Yamamoto H, Ubrich N, Takeuchi H, Maincent P, Kawashima Y. FK506 Microparticles Mitigate Experimental Colitis with Minor Renal Calcineurin Suppression. Pharmaceutical Research. 2005;22:193–199. doi: 10.1007/s11095-004-1186-2. [DOI] [PubMed] [Google Scholar]
- 62.Lamprecht A, Yamamoto H, Takeuchi H, Kawashima Y. Nanoparticles enhance therapeutic efficiency by selectively increased local drug dose in experimental colitis in rats. The Journal of pharmacology and experimental therapeutics. 2005;315:196–202. doi: 10.1124/jpet.105.088146. [DOI] [PubMed] [Google Scholar]
- 63.US, Eshita Y, Tabata Y, Sakamoto S, Egawa H, Hashida T, Inui K, Tanaka K. Drug delivery system using microspheres that contain tacrolimus in porcine small bowel transplantatio. Transpl Int. 2005;17:841–847. doi: 10.1007/s00147-004-0790-8. [DOI] [PubMed] [Google Scholar]
- 64.Nassar T, Rom A, Nyska A, Benita S. Novel double coated nanocapsules for intestinal delivery and enhanced oral bioavailability of tacrolimus, a P-gp substrate drug. Journal of controlled release : official journal of the Controlled Release Society. 2009;133:77–84. doi: 10.1016/j.jconrel.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 65.Meissner Y, Pellequer Y, Lamprecht A. Nanoparticles in inflammatory bowel disease: particle targeting versus pH-sensitive delivery. International journal of pharmaceutics. 2006;316:138–143. doi: 10.1016/j.ijpharm.2006.01.032. [DOI] [PubMed] [Google Scholar]
- 66.Lamprecht A, Yamamoto H, Takeuchi H, Kawashima Y. A pH-sensitive microsphere system for the colon delivery of tacrolimus containing nanoparticles. Journal of controlled release : official journal of the Controlled Release Society. 2005;104:337–346. doi: 10.1016/j.jconrel.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 67.Gajanayake ORT, Leclère FM, Dhayani A, Yang Z, Bongoni AK, Banz Y, Constantinescu MA, Karp JM, Vemula PK, Rieben R, Vögelin E. A single localized dose of enzyme-responsive hydrogel improves long-term survival of a vascularized composite allograft. Sci Transl Med. 2014;6 doi: 10.1126/scitranslmed.3008778. [DOI] [PubMed] [Google Scholar]
- 68.Forrest ML, Won CY, Malick AW, Kwon GS. In vitro release of the mTOR inhibitor rapamycin from poly(ethylene glycol)-b-poly(epsilon-caprolactone) micelles. Journal of controlled release : official journal of the Controlled Release Society. 2006;110:370–377. doi: 10.1016/j.jconrel.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 69.Jhunjhunwala S, Raimondi G, Thomson AW, Little SR. Delivery of rapamycin to dendritic cells using degradable microparticles. Journal of controlled release : official journal of the Controlled Release Society. 2009;133:191–197. doi: 10.1016/j.jconrel.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Haddadi A, Elamanchili P, Lavasanifar A, Das S, Shapiro J, Samuel J. Delivery of rapamycin by PLGA nanoparticles enhances its suppressive activity on dendritic cells. Journal of biomedical materials research Part A. 2008;84:885–898. doi: 10.1002/jbm.a.31373. [DOI] [PubMed] [Google Scholar]
- 71.Das S, Haddadi A, Veniamin S, Samuel J. Delivery of rapamycin-loaded nanoparticle down regulates ICAM-1 expression and maintains an immunosuppressive profile in human CD34+ progenitor-derived dendritic cells. Journal of biomedical materials research Part A. 2008;85:983–992. doi: 10.1002/jbm.a.31557. [DOI] [PubMed] [Google Scholar]
- 72.Kauffman KJ, Kanthamneni N, Meenach SA, Pierson BC, Bachelder EM, Ainslie KM. Optimization of rapamycin-loaded acetalated dextran microparticles for immunosuppression. International journal of pharmaceutics. 2012;422:356–363. doi: 10.1016/j.ijpharm.2011.10.034. [DOI] [PubMed] [Google Scholar]
- 73.Yuan XB, Yuan YB, Jiang W, Liu J, Tian EJ, Shun HM, Huang DH, Yuan XY, Li H, Sheng J. Preparation of rapamycin-loaded chitosan/PLA nanoparticles for immunosuppression in corneal transplantation. International journal of pharmaceutics. 2008;349:241–248. doi: 10.1016/j.ijpharm.2007.07.045. [DOI] [PubMed] [Google Scholar]
- 74.Shirali AC, Look M, Du W, Kassis E, Stout-Delgado HW, Fahmy TM, Goldstein DR. Nanoparticle delivery of mycophenolic acid upregulates PD-L1 on dendritic cells to prolong murine allograft survival. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2011;11:2582–2592. doi: 10.1111/j.1600-6143.2011.03725.x. [DOI] [PubMed] [Google Scholar]
- 75.Look M, Stern E, Wang QA, DiPlacido LD, Kashgarian M, Craft J, Fahmy TM. Nanogel-based delivery of mycophenolic acid ameliorates systemic lupus erythematosus in mice. J Clin Invest. 2013;123:1741–1749. doi: 10.1172/JCI65907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Look M, Saltzman WM, Craft J, Fahmy TM. The nanomaterial-dependent modulation of dendritic cells and its potential influence on therapeutic immunosuppression in lupus. Biomaterials. 2014;35:1089–1095. doi: 10.1016/j.biomaterials.2013.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Williford JM, Wu J, Ren Y, Archang MM, Leong KW, Mao HQ. Recent advances in nanoparticle-mediated siRNA delivery. Annual review of biomedical engineering. 2014;16:347–370. doi: 10.1146/annurev-bioeng-071813-105119. [DOI] [PubMed] [Google Scholar]
- 78.Singha K, Namgung R, Kim WJ. Polymers in small-interfering RNA delivery. Nucleic acid therapeutics. 2011;21:133–147. doi: 10.1089/nat.2011.0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Di Caro V, Giannoukakis N, Trucco M. In vivo delivery of nucleic acid-formulated microparticles as a potential tolerogenic vaccine for type 1 diabetes. The review of diabetic studies : RDS. 2012;9:348–356. doi: 10.1900/RDS.2012.9.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Machen J, Harnaha J, Lakomy R, Styche A, Trucco M, Giannoukakis N. Antisense Oligonucleotides Down-Regulating Costimulation Confer Diabetes-Preventive Properties to Nonobese Diabetic Mouse Dendritic Cells. The Journal of Immunology. 2004;173:4331–4341. doi: 10.4049/jimmunol.173.7.4331. [DOI] [PubMed] [Google Scholar]
- 81.Phillips B, Nylander K, Harnaha J, Machen J, Lakomy R, Styche A, Gillis K, Brown L, Lafreniere D, Gallo M, Knox J, Hogeland K, Trucco M, Giannoukakis N. A microsphere-based vaccine prevents and reverses new-onset autoimmune diabetes. Diabetes. 2008;57:1544–1555. doi: 10.2337/db07-0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Riley JL, June CH, Blazar BR. Human T regulatory cell therapy: take a billion or so and call me in the morning. Immunity. 2009;30:656–665. doi: 10.1016/j.immuni.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cobbold SP. Induction of foxP3+ regulatory T cells in the periphery of T cell receptor transgenic mice tolerized to transplants. Journal of Immunology. 2004;172:6003–6010. doi: 10.4049/jimmunol.172.10.6003. [DOI] [PubMed] [Google Scholar]
- 84.Wieckiewicz J, Goto R, Wood KJ. T regulatory cells and the control of alloimmunity: from characterisation to clinical application. Current Opinion in Immunology. 2010;22:662–668. doi: 10.1016/j.coi.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Long SA, Buckner JH. Combination of rapamycin and IL-2 increases de novo induction of human CD4(+)CD25(+)FOXP3(+) T cells. J Autoimmun. 2008;30:293–302. doi: 10.1016/j.jaut.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Metcalfe SM, Watson TJ, Shurey S, Adams E, Green CJ. Leukemia Inhibitory Factor Is Linked to Regulatory Transplantation Tolerance. Transplantation. 2005;79:726–730. doi: 10.1097/01.tp.0000149324.42994.38. [DOI] [PubMed] [Google Scholar]
- 87.Lu L. Characterization of protective human CD4CD25 FOXP3 regulatory T cells generated with IL-2, TGF-beta and retinoic acid. PLoS One. 2010;5 doi: 10.1371/journal.pone.0015150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.GW, Park J, Whiston R, Strom TB, Metcalfe S, Fahmy TM. Modulation of CD4+ T Lymphocyte Lineage Outcomes with Targeted, Nanoparticle-Mediated Cytokine Delivery. Mol Pharm. 2011;8:143–152. doi: 10.1021/mp100203a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gao W, Thompson L, Zhou Q, Putheti P, Fahmy TM, Strom TB, Metcalfe SM. Treg versus Th17 lymphocyte lineages are cross-regulated by LIF versus IL-6. Cell Cycle. 2014;8:1444–1450. doi: 10.4161/cc.8.9.8348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dong H, Fahmy TM, Metcalfe SM, Morton SL, Dong X, Inverardi L, Adams DB, Gao W, Wang H. Immuno-isolation of pancreatic islet allografts using pegylated nanotherapy leads to long-term normoglycemia in full MHC mismatch recipient mice. PloS one. 2012;7:e50265. doi: 10.1371/journal.pone.0050265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.CTJ Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nature Medicine. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 92.Lee I. Recruitment of Foxp3+ T regulatory cells mediating allograft tolerance depends on the CCR4 chemokine receptor. J Exp Med. 2005;201:1037–1044. doi: 10.1084/jem.20041709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Montane J, Soukhatcheva BLG, Dai DD, Hardenberg G, Levings M, Orban P, Kieffer T, Rusung T, Verchere C. Prevention of murine autoimmune diabetes by CCL22-mediated Treg recruitment to the pancreatic islets. The Journal of Clinical Investigation. 2011;121:3024–3028. doi: 10.1172/JCI43048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jhunjhunwala S, Raimondi G, Glowacki AJ, Hall SJ, Maskarinec D, Thorne SH, Thomson AW, Little SR. Bioinspired controlled release of CCL22 recruits regulatory T cells in vivo. Advanced materials. 2012;24:4735–4738. doi: 10.1002/adma.201202513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Glowacki AJ, Yoshizawa S, Jhunjhunwala S, Vieira AE, Garlet GP, Sfeir C, Little SR. Prevention of inflammation-mediated bone loss in murine and canine periodontal disease via recruitment of regulatory lymphocytes. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:18525–18530. doi: 10.1073/pnas.1302829110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lewis JS, Roche C, Zhang Y, Brusko TM, Wasserfall CH, Atkinson M, Clare-Salzler MJ, Keselowsky BG. Combinatorial delivery of immunosuppressive factors to dendritic cells using dual-sized microspheres. Journal of materials chemistry B, Materials for biology and medicine. 2014;2:2562–2574. doi: 10.1039/C3TB21460E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Stephan MT, Stephan SB, Bak P, Chen J, Irvine DJ. Synapse-directed delivery of immunomodulators using T-cell-conjugated nanoparticles. Biomaterials. 2012;33:5776–5787. doi: 10.1016/j.biomaterials.2012.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Stephan MT, Moon JJ, Um SH, Bershteyn A, Irvine DJ. Therapeutic cell engineering with surface-conjugated synthetic nanoparticles. Nature medicine. 2010;16:1035–1041. doi: 10.1038/nm.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Steenblock ER, Fahmy TM. A comprehensive platform for ex vivo T-cell expansion based on biodegradable polymeric artificial antigen-presenting cells. Molecular therapy : the journal of the American Society of Gene Therapy. 2008;16:765–772. doi: 10.1038/mt.2008.11. [DOI] [PubMed] [Google Scholar]
- 100.Oelke M, Maus MV, Didiano D, June CH, Mackensen A, Schneck JP. Ex vivo induction and expansion of antigen-specific cytotoxic T cells by HLA-Ig-coated artificial antigen-presenting cells. Nature medicine. 2003;9:619–624. doi: 10.1038/nm869. [DOI] [PubMed] [Google Scholar]
- 101.Steenblock ER, Fadel T, Labowsky M, Pober JS, Fahmy TM. An artificial antigen-presenting cell with paracrine delivery of IL-2 impacts the magnitude and direction of the T cell response. The Journal of biological chemistry. 2011;286:34883–34892. doi: 10.1074/jbc.M111.276329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Francisco LM, Salinas VH, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK, Sharpe AH. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. The Journal of experimental medicine. 2009;206:3015–3029. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Schütz OM, Schneck C, Mackensen JP, Fleck AM. Killer artificial antigen-presenting cells: the synthetic embodiment of a ‘guided missile’. Immunotherpy. 2010;2:39–550. doi: 10.2217/imt.10.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Schütz FM, Mackensen C, Zoso A, Halbritter AA, Schneck D, Oelke JPM. Killer artificial antigen-presenting cells: a novel strategy to delete specific T cells. Blood. 2008;111:3546–3552. doi: 10.1182/blood-2007-09-113522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shen C, He Y, Cheng K, Zhang D, Miao S, Zhang A, Meng F, Miao F, Zhang J. Killer artificial antigen-presenting cells deplete alloantigen-specific T cells in a murine model of alloskin transplantation. Immunology letters. 2011;138:144–155. doi: 10.1016/j.imlet.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 106.Labowsky M, Fahmy TM. Diffusive transfer between two intensely interacting cells with limited surface kinetics. Chemical engineering science. 2012;74:114–123. doi: 10.1016/j.ces.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Labowsky M, Fahmy TM. Effect of cell surface deformation on synaptic factor accumulation during the early stages of T cell activation. Chemical engineering science. 2013;90:275–283. [Google Scholar]
- 108.CP, Allen TM. Drug Delivery Systems: Entering the Mainstream. Science. 2004;303:18–22. doi: 10.1126/science.1095833. [DOI] [PubMed] [Google Scholar]
- 109.Zhang Y, Chan HF, Leong KW. Advanced materials and processing for drug delivery: the past and the future. Advanced drug delivery reviews. 2013;65:104–120. doi: 10.1016/j.addr.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Verma R, Garg S. Current Status of Drug Delivery Technologies and Future Directions. Pharm Technol On-Line. 2001;25:1–14. [Google Scholar]
- 111.Almeida AJ, Souto E. Solid lipid nanoparticles as a drug delivery system for peptides and proteins. Advanced drug delivery reviews. 2007;59:478–490. doi: 10.1016/j.addr.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 112.Guvendiren M, Hoang D, Burdick J. Shear-thinning hydrogels for biomedical applications. Soft Matter. 2012;8:260–272. [Google Scholar]
- 113.KS, Jeong B, Bae YH. Thermosensitive sol-gel reversible hydrogels. Advanced drug delivery reviews. 2002;54:37–51. doi: 10.1016/s0169-409x(01)00242-3. [DOI] [PubMed] [Google Scholar]