Abstract
Purpose
The purpose of this instrument development project was to create a self-report tool to evaluate arm lymphedema and associated symptoms in breast cancer survivors.
Methods
The Lymphedema Symptom Intensity and Distress Survey-Arm (LSIDS-A) was developed and tested in three phases: Phase One--literature review and expert panel, Phase Two--preliminary validation, and Phase Three--final validation.
Results
Phase One: The most common symptoms experienced by breast cancer survivors with lymphedema were identified. A 52-item scale was developed. Phase Two: 128 community-dwelling breast cancer survivors (64 with lymphedema, 64 without lymphedema) completed the LSIDS-A. Feedback from the participants was that the format was “clear” and “made sense”; therefore, the response structure was left intact. Sixteen items were deleted leaving a 36-item revised instrument. Phase Three: Subsequent testing in a total sample of 236 breast cancer survivors with lymphedema was undertaken. The Cronbach's alpha reliability values for the overall intensity and distress scores were 0.93 and 0.94 respectively. The Kuder-Richardson values ranged from 0.66 to 0.92. Divergent validity evaluated against Marlowe-Crowne Social Desireability Scale overall was acceptable (intensity: rs = 0.08, distress: rs = -0.12). Convergent validity was acceptable as tested with multiple instruments (e.g., Functional Assessment of Cancer Therapy-Breast +4, overall intensity rs = -0.44, overall distress rs = -.48)
Conclusions
The 30-item LSIDS-A is a valid and reliable instrument that can be used to assess arm lymphedema and its associated symptoms.
Keywords: Lymphedema, instrument development, symptoms, distress, intensity, survey, breast cancer
INTRODUCTION
The growing body of literature describing symptoms associated with lymphedema has led to reconceptualization of the condition [1-4]. Previously defined as a collection of fluid in the tissues [5], lymphedema is now more accurately defined as a pathological ailment that occurs when fluid and proteins amass in the interstitial space that is associated with physical and psychological symptoms [6]. Once lymphedema occurs it is a chronic condition than can worsen over time [7]. Breast cancer survivors with lymphedema report a poorer quality of life (QOL) than those without lymphedema [8-10]. Individuals with lymphedema may view it as a reminder of their cancer, feel less sexually attractive, lose fine motor movement in the affected extremity, and often modify their wardrobes to accommodate their enlarged arms [11,12]. They may see affected limbs as being ugly and deformed [11,12]. They may have to change jobs because of their inability to lift, require assistance with dressing, experience psychological distress, and will often limit social and recreational activities. Pain and other altered sensations in the affected limb may also be associated with lymphedema [8,10]. There are few comprehensive, valid, and reliable instruments available for use by researchers or clinicians as lymphedema-related symptom assessment tools. Three instruments, the Lymphedema and Breast Cancer Questionnaire (LBCQ) [1,13], the Norman lymphedema survey (NLS) [14,15], and the Functional Assessment of Cancer Therapy plus 4 (FACT-B+4) [16], are often cited in the literature. Two, the LBCQ and the NLS, have predicted the presence of lymphedema, but none of the three comprehensively address psychological symptoms. Thus, many researchers and clinicians use multiple instruments to comprehensively capture symptoms in this patient population, creating burden for professionals and patients alike.
A single, self-report tool that is consistent with the evidence driven reconceptualization of lymphedema would identify physical and psychosocial lymphedema-related symptoms, as well as associated burden and serve several purposes. It would: 1) eliminate the need for clinicians to use multiple tools to assess physiological and psychological symptoms; 2) promote rapid identification of lymphedema-related symptoms; 3) identify areas where intervention or education are needed; 4) facilitate evaluation of treatment outcomes; and 5) promote communication between healthcare providers and patients. In this manuscript, the development and validation of the Lymphedema Symptom Intensity and Distress Survey-Arm (LSIDS-A), one of a battery of a tools we are developing to capture symptoms and associated symptom burden in individuals experiencing lymphedema, is reported [2].
METHODS
Development of the LSIDS-A
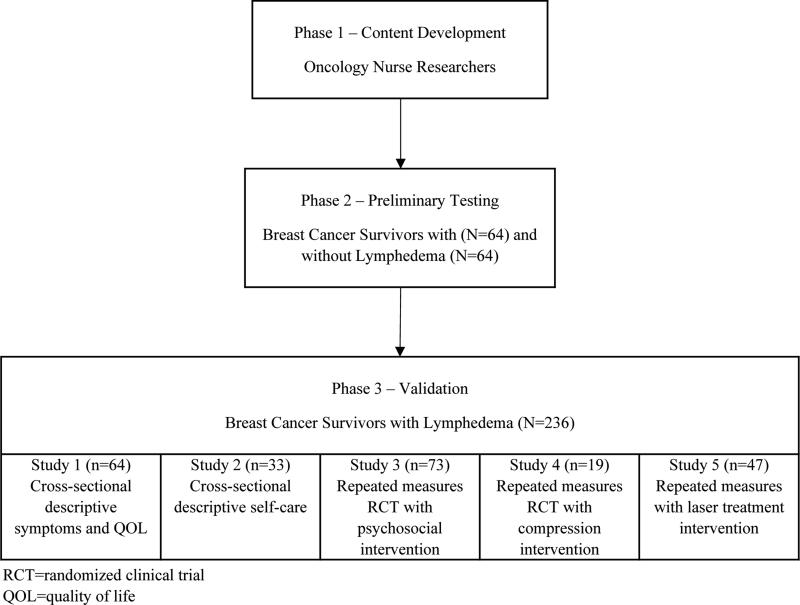
The LSIDS-A was developed in three phases (see Figure 1). In Phase One, preliminary content development was undertaken. Phase Two, preliminary testing, was completed in conjunction with a cross-sectional parent study [8]. Phase Three, validation, involved further testing across multiple studies that were comprised of breast cancer survivors with lymphedema. Studies had institutional review board approval and informed consent was obtained.
Figure 1.
Phases of Development
Phase One: Content development
The Lenz Theory of Unpleasant Symptoms was the conceptual framework for initial item development [17,18]. This theory suggests that situational, physiological, and psychological factors contribute to the development of symptom clusters. Thus, these categories guided a review of the literature. Medline and CINAHL searches were conducted using the following key words, either alone or in combination with lymphedema: lymphedema, symptoms, outcomes, instruments, depression, fatigue, body image, measurement, pain, treatment, QOL, breast cancer, arm function, range of motion, cellulitis, infection, manual lymphatic drainage, and complete decongestive physiotherapy. A list of proposed symptom content was then generated that included physical, psychological, and situational concerns.
An expert panel consisting of oncology nurse researchers reviewed this list. A 52-item scale with reflective period of one week was developed. Each symptom in the survey required a “yes/no” response. If “yes” was selected then a rating (1 slight to 10 severe) for both intensity (the actual severity of the symptom itself) and distress (the physical or emotional response to a symptom) was solicited.
Phase Two: Preliminary testing
Study 1: The focus of this study was to ensure that the instrument captured lymphedema-related symptoms, not symptoms related to breast cancer treatment. As part of a parent study, an adequately powered study of 128 community-dwelling breast cancer survivors (64 with lymphedema, 64 without lymphedema) was undertaken [8]. Convenience sampling was used and participants were age matched within three years. All participants completed the LSIDS-A in approximately 12 minutes. Participants were instructed to write down any lymphedema related problems not addressed and asked by the first author for comments about the tool. The tool was able to differentiate symptoms between the groups [8]. Participant feedback indicated that the tool was “clear” and “made sense”, and they endorsed the inclusion of both intensity and distress content. Therefore, the structure of the scale was left intact. Items were deleted if: 1) they were found to be redundant by participants’ report; 2) neither group endorsed an item; or, 3) items were only endorsed by those without lymphedema. For example, depression, loss of well-being, and inferiority were deleted based on redundancy, and sadness was retained as participants felt that best described their feeling. All items related to chest discomfort, except for burning, were deleted as they were either not endorsed by either group or only endorsed by those without lymphedema. Sixteen items in total were deleted. No new items were added. The revised instrument contained 36 items.
Phase Three: Validation
The revised 36-item tool was subsequently validated [3,8,11,12,19,20]. (Figure 1) Breast cancer survivors with previously diagnosed lymphedema completed the LSIDS-A using the 1-10 scale in addition to other measures used to evaluate validity and reliability. Figure 1 reflects the number of participants from each study that had initial exposure to the LSIDS-A who were included in the validation phase. (N=236)
Study 1: Responses to the revised 36-item LSIDS-A by the lymphedema participants in the parent study (N=64) were included in further validation efforts [8].
Study 2: Fifty-one individuals with a history of previous professionally administered therapy for lymphedema completed the 36-item LSIDS-A once in this cross-sectional study [3].
Study 3: There were 104 participants in this randomized clinical trial (RCT), 52 each in the control and experimental groups. All had Stage II lymphedema. Participants completed the 36-item LSIDS-A at baseline as well as 1 month, 3 months, and 6 months after completing the intervention [11,12].
Study 4: There were 42 participants in this pilot RCT, 21 each in the control and experimental groups. Participants completed the 36-item LSIDS-A at baseline and days 15 and 30 of treatment [19].
Study 5: Participants (N=46) in this RCT completed the 36-item LSIDS prior to an initial laser treatment and immediately after completion of the full intervention [20].
Data collection and measures
Study staff asked each patient to complete self-report instruments and answered any questions related to the forms. In addition to the LSIDS-A, the following instruments were completed in the various studies to assist with validation:
Demographic and Medical Questionnaires
In every study, demographic information (e. g., sex, age, years of education, employment status) and medical information (e.g., stage of breast cancer, onset of lymphedema, comorbid illnesses) was obtained during the first study visit.
FACT B and FACT B plus 4 [16,21]
The 36-item Functional Assessment of Cancer Therapy (FACT) consists of the FACT-G and a breast subscale and is frequently used to measure QOL in breast cancer patients and survivors. Phase Two and Phase Three participants in Studies 1, 2, 3, and 5 completed this instrument (Cronbach's alpha overall score=0.92, subscales=0.79-0.83). The Fact B plus 4 subscale, a lymphedema subscale, was completed by participants in Study 1 (Cronbach's alpha=0.70) [16,21].
Upper Limb Lymphedema 27 (ULL-27) [22]
The ULL-27 is a valid and reliable tool that examines QOL in individuals with upper limb lymphedema [22]. All Phase Two and Phase Three participants completed this instrument (Cronbach's alphas: overall score=0.93, subscales=0.80-0.92).
Functional Assessment Screening Questionnaire (FASQ) [23]
The FASQ is a 15-item instrument that captures functional impairment in adult ambulatory patients [23]. Validity of the FASQ has been supported in general family practice and chronic pain populations [23,24]. Phase Three participants in Studies 2, 3, and 4 completed this instrument (Cronbach's alpha=0.84).
Center for Epidemiologic Studies-Depression (CES-D) [25]
The CES-D Scale has 20 items that address presence and severity of depressive symptoms [25]. In a previous study by this team, Cronbach's alpha for the scores were 0.82 at baseline and 0.85 post intervention [20]. Phase Three participants in Studies 2 and 4 completed this instrument (Cronbach's alpha=0.85).
Profile of Mood States-Short Form (POMS-SF) [25,26]
The POMS-SF is an instrument that captures mood. The POMS-SF possesses reliability and validity equal to that of the full length POMS [25-27]. Cronbach's alphas for the POMS-SF total mood disturbance and subscale scores ranged from 0.82 to 0.94 in prior work by this team [20]. Phase Three participants in Studies 1, 2 and 4 completed this instrument (Cronbach's alpha: overall score=0.93, subscales=0.86-0.94).
Marlowe-Crowne Social Desireability Scale (MCSDS) [28,29]
This 33-item questionnaire assesses both the tendencies to deny common negative characteristics and ascribe to oneself positive characteristics that are believed to be rare in the general population. Higher scores reflect higher levels of social desirability response bias. Psychometric properties of the MCSDS have been well established [28,29]. Phase Two participants completed this instrument (Kuder-Richardson=0.82).
Statistical methods
Frequency distributions were used to summarize symptom prevalence, as well as the nominal and ordinal demographic characteristics. Due to the lack of normality of symptom characteristics, median and 25th-75th inter-quartile range (IQR) values were used to summarize responses to the intensity and distress experienced from the individual symptoms, as well as time since surgery and time since last treatment. SAS PROC VarClus was used to generate symptom clusters. While this approach borrows heavily from the conceptual underpinning of traditional factor analysis methods, statistically it derived from hierarchical clustering methods. This approach loosens many of the assumptions inherent in traditional factor analysis (e.g., normality of item response distributions), which makes it most suitable for symptom clustering. Clustering was conducted separately for the intensity and for the distress responses.
Once clusters of symptoms were identified, the internal consistency of the individual symptoms with their respective cluster was assessed using the Kuder-Richardson index for binary responses (prevalence) and the Cronbach's Alpha statistic for the Likert intensity and distress responses. Intensity and distress scores for the entire set of symptoms, as well as each cluster of symptoms, were generated by averaging the individual Likert item responses for those symptoms marked as present. Test-retest reliability of the overall and cluster scores was assessed using intra-class correlations among the repeated assessments of a subset of study participants (control group, study number). Convergent and divergent validity of the symptom clusters was assessed using Spearman correlations with the measures of other constructs collected at the same time.
RESULTS
Sample characteristics
Table 1 summarizes the demographic and clinical characteristics for the analysis sample (N=236). Most participants were middle aged (M=58.9 years), educated (M=14.7 years), Causasian (89%) women. Some (27.2%) lived in rural areas and many (35.2%) worked full time. Median lymphedema duration was 29.3 months, with a range of 0.0 months to 73.8 months. Most (84.5%) had Stage II lymphedema. None were undergoing cancer treatment.
Table 1.
Demographics (N=236)
| Characteristic | |
|---|---|
| Mean (SD) | |
| Age | 58.9 (11.0) |
| Education (Years) | 14.7 (2.6) |
| Race | N (%) |
| Caucasian | 210 (89.0) |
| African-American | 21 (8.9) |
| Other | 5 (2.1) |
| Marital Status | |
| Married | 160 (68.1) |
| Single | 41 (17.4) |
| Widowed | 25 (10.6) |
| Other | 8 (3.4) |
| Single-Live Partner | 1 (0.4) |
| Household Income | |
| <=$10.000 | 7 (3.3) |
| $10,001-20,000 | 19 (9.1) |
| $20,001-30,000 | 21 (10.0) |
| $30,001-40,000 | 26 (12.4) |
| $40,001-50,000 | 20 (9.6) |
| $50,001-60,000 | 12 (5.7) |
| >$60,000 | 104 (49.8) |
| Do not care to answer | 25 (10.6) |
| Work Status | |
| Full Time | 83 (35.2) |
| Part Time | 31 (13.1) |
| Homemaker | 31 (13.1) |
| Retired | 73 (30.9) |
| Unemployed | 16 (6.8) |
| Other | 2 (0.8) |
| Insurance | |
| Private | 72 (30.5) |
| Medicare | 54 (22.9) |
| None | 50 (21.2) |
| Other | 21 (8.9) |
| TennCare | 19 (8.1) |
| HMO | 14 (5.9) |
| Medicaid | 4 (1.7) |
| Area of Residence | |
| City | 153 (65.1) |
| Country | 64 (27.2) |
| Other | 18 (7.7) |
| School | |
| Grades 1-12 | 59 (25.0) |
| Grades 13-16 | 126 (53.4) |
| Grades >16 | 51 (21.6) |
| Median [IQR] (Min,Max) | |
| Time since surgery (years) | 4.8 [2,11] (0.1,50.8) |
| Time since last treatment (years) | 4.3 [1,10] (0.0,50.8) |
Note: IQR: 25th-75th inter-quartile range
Symptoms
With the exception of the items inquiring about sexual-related symptoms that had a no response option, no consistently missing individual item responses were apparent in this pool of data. Nine symptoms occurred in over 50% of the participants: swelling (90.2%), fatigue (75.7%), heavy arm (74.0%), tight arm (66.8%), difficulty sleeping (61.3%), ache arm (60.2%), appearance concerns (59.6%), decrease in physical activity (56.0%), and pain in arm (51.9%). Two very low frequency symptoms were identified, burning chest (14.9%) and flakey skin (14.4%). The intensity and distress distributions demonstrated variability in response with each of the item values ranging from the minimum of ‘0’ to a maximum value of ‘5’.
Clustering
Item clustering was conducted using both the original 1 to 10 response intensity and distress format, as well as a simplified reduced 1 to 5 format post data collection. The findings were essentially identical, thus results from the 1 to 5 format are subsequently reported ad the final LSIDS-A reflects a scoring range of 1 to 5. The findings from the clustering of the intensity responses and from the distress responses were also essentially identical. Therefore, the single solution is summarized in Table 2. Six items did not load (“Burning Arm,” “Burning Chest,” “Warm Arm,” “Cold Arm,” “Flaky Skin,” and “Increased Appetite”). Four were omitted from the final instrument (“Burning Chest,” “Cold Arm,” “Flaky Skin,” and “Increased Appetite”) because they occurred in less than 20% of the participants. The remaining 30 items fell into seven clusters. Six of the seven clusters clearly fit into three separate proposed domains: situational (resource cluster), psychological (sexuality cluster), and physiological (soft tissue sensations, neurologic sensations, function, and activity clusters). One cluster consisted of a compilation of generalized core symptoms that encompassed both physical and psychological domains (biobehavioral cluster).
Table 2.
LSIDS-A clustering face and content validity, internal consistency
| Soft Tissue Sensation | N | Prevalencea | Intensityb | Distressb |
|---|---|---|---|---|
| • Heavy Arm • Tight Arm • Swelling Arm • Hard Arm |
233 | 0.67 | 0.87 | 0.86 |
| Neurological Sensation | ||||
| • Stabbing Arm • Cramping Arm • Pain Arm • Numb Arm • Ache Arm • Tingle Arm • Pins/Needles Arm |
229 | 0.78 | 0.84 | 0.86 |
| Function | ||||
| • Side-to-Side Arm • Raise Arm Head |
233 | 0.70 | 0.80 | 0.86 |
| Biobehavioral | ||||
| • Sad • Anger • Lack Self-Confidence • Appearance Concerns • Misunderstood S/Oc • Less Sexually Attract • Loss Body Confidence • Fatigue • Loss Sleep |
206 | 0.77 | 0.88 | 0.87 |
| Resource | ||||
| • Lack Confidence Insurance • Frustration Insurance |
233 | 0.92 | 0.96 | 0.95 |
| Sexuality | ||||
| • Lack Sex Interest • Partner Lack Interest • Decrease Sex Active |
183 | 0.66 | 0.74 | 0.72 |
| Activity | ||||
| • Give Up Hobbies • Decrease Social Active • Decrease Physical Active |
233 | 0.66 | 0.79 | 0.78 |
Values in the cells are Kuder-Richardson statistics
Values in the cells are Cronbach's Alpha statistics
S/O refers to “significant other”
Scoring
The sum of symptoms reported within the set of 30 yields a total prevalence score. Cluster symptom scores are generated by summing the number of symptoms reported within each cluster. For the integrity of both the prevalence and cluster scores, a maximum of five missing items are allowed for generating overall LSIDS-A scores. For each of the six clusters, complete data are required. Overall and cluster symptom intensity and distress scores are derived by averaging the intensity and distress self-reports for the respective reported cluster symptoms. Detailed programming for scoring the instrument is available from the authors.
Reliability
The Kuder-Richardson reliability for overall sum of the dichotomous “presence” or “absence” of the symptoms was 0.88 (N=173). The Cronbach's alpha reliability for the overall intensity and distress scores were 0.93 and 0.94 respectively. As can be seen in Table 2, good internal consistency was also demonstrated for each of the symptom clusters. The Kuder-Richardson values ranged from 0.66 to 0.92. The reliabilities of the symptom cluster intensity and distress scores were 0.78 or higher for all the clusters except for the symptoms related to sexual relationships which were still acceptable at 0.74 and 0.72.
Validity
Descriptive summaries of the cluster scores are displayed in Table 3. While the complete range of possible symptom number, intensity, and distress values was used in the overall assessment and within each cluster, it is apparent from the median and IQR values that most of the values tended to be in the lower half of possible range of scores.
Table 3.
Descriptive summaries of number of symptoms within clusters, cluster intensity and distress scores
| Cluster | Max # Symptoms | Number of Cluster Symptoms Present | Average Intensity from Cluster Symptomsa | Average Distress from Cluster Symptomsa | |||
|---|---|---|---|---|---|---|---|
| Nb | Median [IQR] (Min,Max) | Nc | Median [IQR] (Min,Max) | Nc | Median [IQR] (Min,Max) | ||
| Overall | 30 | 233 | 12.0 [8-16] (0,30) | 231 | 2.3 [1-3] (1,5) | 231 | 1.8 [1-3] (0,5) |
| Soft Tissue Sensation | 4 | 233 | 3.0 [2-4] (0,4) | 219 | 2.0 [1-3] (0,5) | 219 | 1.5 [1-3] (0,5) |
| Neurologic Sensation | 7 | 229 | 2.0 [1-4] (0,7) | 189 | 2.0 [1-3] (0,5) | 189 | 1.5 [1-3] (0,5) |
| Function | 2 | 233 | 0.0 [0-1] (0,2) | 85 | 2.0 [1-4] (0,5) | 85 | 1.5 [1-3] (0,5) |
| Biobehavioral | 9 | 206 | 4.0 [2-6] (0,9) | 195 | 2.0 [1-3] (0,5) | 195 | 1.9 [1-3] (0,5) |
| Resource | 2 | 233 | 0.0 [0-0] (0,2) | 57 | 3.0 [2-5] (0,5) | 57 | 2.0 [1-5] (0,5) |
| Sexuality | 3 | 183 | 1.0 [0-2] (0,3) | 103 | 3.0 [2-5] (0,5) | 103 | 2.5 [1-4] (0,5) |
| Activity | 3 | 233 | 1.0 [0-2] (0,3) | 140 | 2.5 [1-4] (0,5) | 140 | 2.0 [1-4] (0,5) |
Note: IQR: 25th-75th inter-quartile range; “Max #” = maximum number
For cases reporting at least one symptom within the respective cluster of items.
Number of cases having the requisite number of item responses to generate a cluster score.
Number of cases reporting a symptom within this cluster and having the requisite number of item responses to generate a cluster score.
Each of the seven intensity and distress scores were included in the analysis of the convergent and divergent validity of the LSIDS-A symptom clusters (see Tables 4 and 5). The measures used for the validity analysis included multidimensional instruments of QOL (FACT-G, ULL-27) and measures of emotional states (CES-D, POMS-SF). The FASQ was also included as a function assessment tool; the MCSDS as a measure of social desirability of response.
Table 4.
Correlations of LSIDS-A cluster intensity-distress scores with the FACT-G, ULL-27, and FASQ scores
| FACT-G | ULL-27 | FASQ | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cluster | Scorea | Nb | Total | Breast +4 Scale | Funct | Emot | Social | Phys | N | Phys | Psych | Social | N | rs |
| Overall | I | 180-181 | −0.40 | −0.44 | −0.37 | −0.24 | −0.17 | −0.47 | 186-200 | −0.47 | −0.30 | −0.33 | 91 | 0.42 |
| D | −0.37 | −0.48 | −0.31 | −0.29 | −0.16 | −0.46 | −0.42 | −0.34 | −0.30 | 0.51 | ||||
| Soft Tissue Sensation | I | 168-169 | −0.27 | −0.41 | −0.28 | −0.03 | −0.16 | −0.31 | 175-190 | −0.52 | −0.11 | −019 | 86 | 0.31 |
| D | −0.33 | −0.50 | −0.29 | −0.19 | −0.16 | −0.39 | −0.45 | −0.26 | −0.26 | 0.40 | ||||
| Neurologic Sensation | I | 183-184 | −0.22 | −0.48 | −0.15 | −0.09 | −0.14 | −0.30 | 153-165 | −0.43 | −0.22 | −0.28 | 79 | 0.25 |
| D | −0.25 | −0.45 | −0.18 | −0.16 | −0.23 | −0.28 | −0.41 | −0.24 | −0.29 | 0.27 | ||||
| Function | I | 67 | −0.33 | −0.43 | −0.34 | −0.27 | −0.09 | −0.29 | 64-73 | −0.44 | −0.28 | −0.28 | 36 | 0.26 |
| D | −0.21 | −0.68 | −0.25 | −0.29 | −0.09 | −0.39 | −0.50 | −0.23 | −0.25 | 0.42 | ||||
| Biobehavioral | I | 155 | −0.48 | −0.40 | −0.43 | −0.36 | −0.19 | −0.50 | 165-174 | −0.36 | −0.38 | −0.39 | 84 | 0.49 |
| D | −0.40 | −0.44 | −0.35 | −0.30 | −0.16 | −0.46 | −0.33 | −0.32 | −0.28 | 0.60 | ||||
| Resource | I | 49-50 | −0.15 | 0.01 | −0.16 | −0.07 | −0.04 | −0.17 | 48-55 | −0.16 | −0.36 | −0.02 | 22 | −0.09 |
| D | −0.19 | −0.03 | −0.12 | −0.12 | −0.10 | −0.30 | −0.12 | −0.40 | −0.11 | −0.12 | ||||
| Sexuality | I | 82 | −0.33 | −0.14 | −0.26 | −0.25 | −0.24 | −0.22 | 87-95 | 0.15 | −0.41 | −0.24 | 48 | 0.32 |
| D | −0.19 | 0.12 | −0.17 | −0.18 | −0.10 | −0.17 | 0.01 | −0.19 | −0.05 | 0.43 | ||||
| Activity | I | 109 | −0.45 | −0.33 | −0.38 | −0.30 | −0.21 | −0.53 | 112-122 | −0.40 | −0.36 | −0.40 | 59 | 0.46 |
| D | −0.39 | −0.36 | −0.26 | −0.35 | −0.20 | −0.45 | −0.35 | −0.30 | −0.29 | 0.47 | ||||
I = Intensity Score; D = Distress Score; Funct=Funtction; Emot=Emotional; Phys=Physical
FACT-B+4 N=16-64
Note: Highlighted cells rs >= 0.40, p < .05
Table 5.
Correlations of LSIDS-A cluster intensity-distress scores with CESD, POMS-SF, and MCSDS scores
| CESD | POMS-SF | MCSDS | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cluster | Scorea | N | CESD | N | Tension | Depres | Anger | Vigor | Fatigue | Confus | N | MCSDS |
| Overall | I | 117 | 0.33 | 209-211 | 0.21 | 0.22 | 0.23 | −0.13 | 0.33 | 0.23 | 67 | −0.08 |
| D | 0.35 | 0.35 | 0.26 | 0.30 | −0.21 | 0.38 | 0.38 | −0.12 | ||||
| Soft Tissue Sensation | I | 111 | 0.12 | 195-199 | 0.01 | 0.06 | 0.08 | −0.12 | 0.19 | 0.06 | 62 | −0.17 |
| D | 0.21 | 0.26 | 0.18 | 0.26 | −0.12 | 0.30 | 0.30 | −0.25 | ||||
| Neurological Sensation | I | 99 | 0.12 | 169-171 | 0.20 | 0.22 | 0.23 | −0.04 | 0.36 | 0.26 | 57 | 0.02 |
| D | 0.17 | 0.31 | 0.24 | 0.30 | −0.01 | 0.31 | 0.31 | −0.09 | ||||
| Function | I | 52 | 0.35 | 74-77 | 0.29 | 0.24 | 0.23 | −0.07 | 0.33 | 0.26 | 27 | 0.21 |
| D | 0.25 | 0.41 | 0.19 | 0.32 | −0.14 | 0.47 | 0.47 | 0.24 | ||||
| Biobehavioral | I | 97 | 0.51 | 176-177 | 0.32 | 0.31 | 0.36 | −0.24 | 0.41 | 0.34 | 62 | −0.03 |
| D | 0.50 | 0.31 | 0.24 | 0.28 | −0.33 | 0.38 | 0.38 | 0.01 | ||||
| Resource | I | 34 | 0.29 | 54-56 | 0.33 | 0.27 | 0.16 | −0.11 | 0.07 | 0.27 | 18 | 0.08 |
| D | 0.31 | 0.47 | 0.36 | 0.23 | −0.15 | 0.27 | 0.27 | 0.07 | ||||
| Sexuality | I | 55 | 0.56 | 93-94 | 0.24 | 0.37 | 0.24 | 0.01 | 0.26 | 0.15 | 37 | 0.08 |
| D | 0.41 | 0.17 | 0.20 | −0.03 | −0.18 | 0.17 | 0.17 | 0.10 | ||||
| Activity | I | 72 | 0.42 | 122-124 | 0.38 | 0.33 | 0.35 | −0.19 | 0.31 | 0.43 | 41 | 0.11 |
| D | 0.32 | 0.40 | 0.34 | 0.33 | −0.17 | 0.37 | 0.37 | 0.22 | ||||
I = Intensity Score; D = Distress Score; Depress=Depression; Confus=Confusion
Note: Highlighted cells rs >= 0.40, p < .05
Convergent
Correlations of the LSIDS-A symptom intensity and distress scores with scores from other study measures are presented in Tables 4 and 5. All of the correlations reported here were of at least the magnitude 0.40 and were statistically significant (p < 0.05).
It was anticipated that intensity and distress scores associated with soft tissue and neurologic sensation would correlate strongest with the overall score of the FACT-G, the FACT-B+4 subscale, the physical subscales of both the FACT-G and the ULL-27, as well as the FASQ. Higher scores on the FACT-G and ULL-27 indicate better QOL, thus the observed inverse correlations supported most of these hypotheses (see Table 4). The strongest associations for the soft tissue cluster intensity and distress scores were with the FACT-B+4 (intensity: rs= -0.41, distress: rs= -0.50) and ULL-27 physical scores (intensity: rs= -0.51, distress: rs= -0.45), as well as with the FASQ (distress: rs = 0.40). The strongest associations for the neurologic sensation cluster were also with the FACT-B+4 (intensity: rs= -0.48, distress: rs= -0.45) and ULL-27 physical scores (intensity: rs= -0.43, distress: rs= -0.41). Consistent with the LSIDS-A soft tissue and neurologic sensations findings, the function cluster also correlated most strongly with the FACT-B+4 (intensity: rs= -0.43, distress: rs= -0.68) and ULL-27 physical scores (intensity: rs= -0.44, distress: rs= -0.50). The distress component of the function cluster scores also was associated with the tension, fatigue, and confusion scores on the POMS-SF measure (rs= 0.41, 0.47, and 0.47 respectively).
Symptoms comprising the LSIDS-A biobehavioral cluster were proposed to have wide-ranging associations. These were in fact observed (see Tables 4 and 5). The strongest of the associations were with the CESD (intensity: rs= 0.51, distress: rs= 0.50), FACT physical scale (intensity: rs= -0.50, distress: rs= -0.46) and FACT-G total scores (intensity: rs= -0.48, distress: rs= -0.40). The resource symptom cluster was expected to be most associated with the CES-D and POMS-SF scores. While the strongest correlation was for the associated distress of those symptoms with the POMS-SF tension scale (rs= 0.47), the only other associations of any note were with the ULL-27 psychological scores (intensity: rs= -0.36, distress: rs = -0.40). This cluster had the lowest prevalence rates of all the LSIDS-A clusters.
As anticipated, levels of intensity and distress for the LSIDS-A sexuality cluster were most strongly correlated with CES-D scores (intensity: rs= 0.56, distress: rs= 0.41). Finally, because activity curtailment is driven by physical health concerns but also creates personal loss and reduces social contact, the LSIDS-A activity cluster was expected to correlate strongly with the physical and social subscales of both the FACT-G and ULL-27, as well as the FASQ. The strongest associations of the activity cluster scores were with the FACT-G physical (intensity: rs= -0.53, distress: rs = -0.45) and FASQ scores (intensity: rs= 0.46, distress: rs= 0.47). (Tables 4 and 5)
Divergent
None of the clusters demonstrated meaningful or statistically significant correlations with the MCSDS (intensity: rs= -0.02-0.21, distress: rs = -0.01-0.25). Nor did they demonstrate statistically significant or meaningful associations with measures for which it could be reasonably assumed such correlations would not exist. For example, correlations with the FACT-G social scores ranged from absolute values of 0.04 to 0.24 (see Tables 4 and 5).
Test-retest
Intra-class correlations for repeated assessments (N=53) are shown in Table 6. The lowest values were for the function intensity and distress scores (0.69 and 0.75, respectively). The highest coefficients were for the sexuality and mood scores, as well as the overall LSIDS-A scores (all above 0.90).
Table 6.
Intra-class correlations of repeated assessments for LSIDS-A overall and cluster scores*
| LSIDS-A Score | ||
|---|---|---|
| Intensity | Distress | |
| Overall | 0.93 | 0.92 |
| Soft Tissue Sensation | 0.87 | 0.76 |
| Neurological Sensation | 0.86 | 0.82 |
| Function | 0.69 | 0.75 |
| Biobehavioral | 0.93 | 0.93 |
| Resource | 0.84 | 0.84 |
| Sexuality | 0.96 | 0.97 |
| Activity | 0.86 | 0.87 |
Study 3: N=53, 4 repeated assessments
DISCUSSION
The LSIDS-A was designed to serve as a single instrument to assess arm lymphedema and its multidimensional symptoms. Our results provide evidence that it can serve that intended purpose. During the course of instrument development, the LSIDS-A provided valuable information about lymphedema and associated symptoms that can be used to inform clinicians and enhance patient care. For example, we believe prevalent symptoms such as appearance concerns and difficulty sleeping represent new findings for this population. Additionally, symptom intensity was most problematic in nine symptoms: partner's lack of interest in sex, decrease in social activity, give up hobbies, cramping arm, increased appetite, decrease in sexual activity, lack of confidence in insurance company, frustration with insurance company, and lack of interest in sex. These symptoms are not traditionally addressed in other lymphedema assessment tools. Furthermore, when present, distress and intensity was most severe in two of those: partner's lack of interest in sex and decrease in sexual activity. Previously, sexuality has only been explored in a limited basis in this population [12]. Taken together, these findings suggest that for some breast cancer survivors with lymphedema, sexuality may create psychological or physical distress to the degree that psychological support or medical treatment may be needed.
Though low in prevalence, distress related to frustration with insurance was evident. This is not only problematic from a psychological perspective, but also has implications for potential limitations regarding access to resources needed to care for lymphedema. Unless access to lymphedema treatment and supplies becomes universal, this is likely to remain a serious issue for some patients.
The symptom-specific findings support that the LSIDS-A is a comprehensive symptom assessment tool. Thus, dependence on multiple other tools to evaluate patients with lymphedema can be reduced.
Findings from both internal consistency analyses and test-retest intra-class correlations show that the LSIDS-A is a reliable instrument. The LSIDS-A, in general, performed as expected with the other instruments used for validation purposes. Together, these results support that the instrument is valid and reliable. Across all phases of development, participants were able to complete the LSIDS-A without difficulty. The final 30-item tool can be completed in less than ten minutes. Thus, the LSIDS-A represents a tool that is feasible for use in both busy clinical settings and in research.
Findings should be considered in light of study limitations. Sample demographics reflect the known demographics in the breast cancer survivor population; however, most participants with lymphedema had Stage II lymphedema. Although this reflects the most common stage of the disease in patients who have been dealing with it over several years, a primary limitation is generalizability of these findings to Stages I and III. It is possible that those with Stage I lymphedema might have fewer symptoms, or symptoms that are less intense or distressing. Conversely, those with Stage III lymphedema might have more symptoms, or symptoms that are more severe. Median intensity and distress scores, though not identical, were close. Given the low median intensity and distress scores, the distribution is also somewhat skewed; however, the same distribution was found in the measures that were used for validation. This suggests that the LSIDS-A accurately captured symptom burden, and that, despite limitations, it represents a comprehensive symptom assessment tool for patients with lymphedema of the arm.
CONCLUSIONS
Breast cancer survivors with lymphedema experience multiple symptoms in addition to swelling. The LSIDS-A is reliable and valid. It can be used by clinicians and scientists to better understand this condition and to comprehensively evaluate response to treatment. Future research should involve data collection in patients with Stages I and II lymphedema and further evaluations determine if both intensity and distress assessments are warranted. Additionally, because of the lack of similar assessment tools for patients with lower limb, trunk, and head and neck lymphedema, further studies are being undertaken by this team to explore the development of the LSIDS as a tool for use in these patient populations. These studies will also facilitate further elucidation of conceptual and perceptual differences between intensity and distress.
Acknowledgments
Funding: Development of the LSIDS-A was supported by: 1) a grant from the American Cancer Society MRSG-07-012-01-CPPB; 2) a grant from the Oncology Nursing Society Foundation; 3) a grant from the National Center for Research Resources National Institutes of Health UL 1RR024975; 4) a National Research Service Award #1 F31 NR07854-02; 5) a Sigma Theta Tau Iota Chapter grant; 6) a Vanderbilt University Dissertation Enhancement Award; 7) a Vanderbilt School of Nursing Postdoctoral Award; 8) Tactile Systems Technology, Incorporated, 1331 Tyler Street NE, Suite 200, Minneapolis, MN 55413; and 9) The Martha Rivers Ingram Chair in Nursing at Vanderbilt University.
Footnotes
CONFLICT OF INTEREST DISCLOSURE
The authors have no other conflicts to disclose.
Contributor Information
Sheila H. Ridner, Vanderbilt University School of Nursing.
Mary S. Dietrich, Vanderbilt University School of Nursing.
REFERENCES
- 1.Bulley C, Coutts F, Blyth C, Jack W, Chetty U, Barber M, Tan CW. A Morbidity Screening Tool for identifying fatigue, pain, upper limb dysfunction and lymphedema after breast cancer treatment: a validity study. Eur J Oncol Nurs. 2014;18(2):218–227. doi: 10.1016/j.ejon.2013.10.006. doi:10.1016/j.ejon.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 2.Deng J, Ridner SH, Murphy BA, Dietrich MS. Preliminary development of a lymphedema symptom assessment scale for patients with head and neck cancer. Support Care Cancer. 2012;20(8):1911–1918. doi: 10.1007/s00520-011-1294-6. doi:10.1007/s00520-011-1294-6. [DOI] [PubMed] [Google Scholar]
- 3.Ridner SH, Dietrich MS, Kidd N. Breast cancer treatment-related lymphedema self-care: education, practices, symptoms, and quality of life. Support Care Cancer. 2011;19(5):631–637. doi: 10.1007/s00520-010-0870-5. doi:10.1007/s00520-010-0870-5. [DOI] [PubMed] [Google Scholar]
- 4.Weissleder H, Schuchhardt C. Lymphedema: Diagnosis and therapy. 4th edn. Viavital; Berlin: 2008. [Google Scholar]
- 5.Szuba A, Rockson SG. Lymphedema: Classification, diagnosis and therapy. Vasc Med. 1998;3(2):145–156. doi: 10.1177/1358836X9800300209. [DOI] [PubMed] [Google Scholar]
- 6.Ridner SH, Fu MR, Wanchai A, Stewart BR, Armer JM, Cormier JN. Self-management of Lymphedema: A systematic review of the literature from 2004 to 2011. Nurs Res. 2012;61(4):291–299. doi: 10.1097/NNR.0b013e31824f82b2. doi:10.1097/NNR.0b013e31824f82b2. [DOI] [PubMed] [Google Scholar]
- 7.International Society of Lymphology The Diagnosis and Treatment of Peripheral Lymphedema: Consensus document of the International Society of Lymphology. Lymphology. 2003;36:84–91. [PubMed] [Google Scholar]
- 8.Ridner SH. Quality of life and a symptom cluster associated with breast cancer treatment-related lymphedema. Support Care Cancer. 2005;13(11):904–911. doi: 10.1007/s00520-005-0810-y. doi:10.1007/s00520-005-0810-y. [DOI] [PubMed] [Google Scholar]
- 9.Ridner SH, Montgomery LD, Hepworth JT, Stewart BR, Armer JM. Comparison of upper limb volume measurement techniques and arm symptoms between healthy volunteers and individuals with known lymphedema. Lymphology. 2007;40(1):35–46. [PubMed] [Google Scholar]
- 10.Woods M. Patients' perceptions of breast-cancer-related lymphoedema. Eur J Cancer Care (Engl) 1993;2(3):125–128. doi: 10.1111/j.1365-2354.1993.tb00181.x. doi:10.1111/j.1365-2354.1993.tb00181.x. [DOI] [PubMed] [Google Scholar]
- 11.Ridner SH, Bonner CM, Deng J, Sinclair VG. Voices From the Shadows: Living with lymphedema. Cancer Nurs. 2012;35(1):E18–E26. doi: 10.1097/NCC.0b013e31821404c0. doi:10.1097/NCC.0b013e31821404c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ridner SH, Sinclair V, Deng J, Bonner CM, Kidd N, Dietrich MS. Breast Cancer Survivors with Lymphedema: Glimpses of their daily lives. Clin J Oncol Nurs. 2012;16(6):609–614. doi: 10.1188/12.CJON.609-614. doi:10.1188/12.CJON.609-614. [DOI] [PubMed] [Google Scholar]
- 13.Armer JM, Radina ME, Porock D, Culbertson SD. Predicting breast cancer-related lymphedema using self-reported symptoms. Nurs Res. 2003;52(6):370–379. doi: 10.1097/00006199-200311000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Brown JC, Kumar A, Cheville AL, Tchou JC, Troxel AB, Harris SR, Schmitz KH. Association between lymphedema self-care adherence and lymphedema outcomes among women with breast cancer-related lymphedema. Am J Phys Med Rehabil. 2014 doi: 10.1097/PHM.0000000000000178. doi:10.1097/PHM.0000000000000178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Norman SA, Miller LT, Erikson HB, Norman MF, McCorkle R. Development and validation of a telephone questionnaire to characterize lymphedema in women treated for breast cancer. Phys Ther. 2001;81(6):1192–1205. [PubMed] [Google Scholar]
- 16.Coster S, Poole K, Fallowfield LJ. The validation of a quality of life scale to assess the impact of arm morbidity in breast cancer patients post-operatively. Breast Cancer Res Treat. 2001;68(3):273–282. doi: 10.1023/a:1012278023233. [DOI] [PubMed] [Google Scholar]
- 17.Lenz ER, Pugh LC, Milligan RA, Gift A, Suppe F. The middle-range theory of unpleasant symptoms: an update. ANS Adv Nurs Sci. 1997;19(3):14–27. doi: 10.1097/00012272-199703000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Lenz ER, Suppe F, Gift AG, Pugh LC, Milligan RA. Collaborative development of middle-range nursing theories: toward a theory of unpleasant symptoms. ANS Adv Nurs Sci. 1995;17(3):1–13. doi: 10.1097/00012272-199503000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Ridner SH, Murphy B, Deng J, Kidd N, Galford E, Bonner C, Bond SM, Dietrich MS. A randomized clinical trial comparing advanced pneumatic truncal, chest, and arm treatment to arm treatment only in self-care of arm lymphedema. Breast Cancer Res Treat. 2012;131(1):147–158. doi: 10.1007/s10549-011-1795-5. doi:10.1007/s10549-011-1795-5. [DOI] [PubMed] [Google Scholar]
- 20.Ridner SH, Poage-Hooper E, Kanar C, Doersam JK, Bond SM, Dietrich MS. A pilot randomized trial evaluating low-level laser therapy as an alternative treatment to manual lymphatic drainage for breast cancer-related lymphedema. Oncol Nurs Forum. 2013;40(4):383–393. doi: 10.1188/13.ONF.383-393. doi:10.1188/13.ONF.383-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cella D. FACIT Manual: Manual of the Functional Assessment of Chronic Illness Therapy (FACIT) measurement system. Center on Outcomes, Research and Education; 1997. [Google Scholar]
- 22.Launois R, Mègnigbêto A, Le Lay K, Alliot F. CN5: A Specific Quality Of Life Scale In Upper Limb Lymphoedema: The Ull-27 Questionnaire. Value Health. 2001;4(6):407–408. [Google Scholar]
- 23.Seltzer GB, Granger CV, Wineberg DE. Functional Assessment: Bridge between family and rehabilitation medicine within an ambulatory practice. Arch Phys Med Rehabil. 1982;63(10):453. [PubMed] [Google Scholar]
- 24.Millard RW. The Functional Assessment Screening Questionnaire: Application for evaluating pain-related disability. Arch Phys Med Rehabil. 1989;70(4):303–307. [PubMed] [Google Scholar]
- 25.Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385. [Google Scholar]
- 26.Curran SL, Andrykowski MA, Studts JL. Short form of the Profile of Mood States (POMS-SF): Psychometric information. Psychol Assess. 1995;7(1):80–83. [Google Scholar]
- 27.Shacham S. A shortened version of the Profile of Mood States. J Pers Assess. 1983;47(3):305–306. doi: 10.1207/s15327752jpa4703_14. [DOI] [PubMed] [Google Scholar]
- 28.Crowne DP, Marlowe D. A new scale of social desirability independent of psychopathology. J Consult Psychol. 1960;24(4):349–354. doi: 10.1037/h0047358. [DOI] [PubMed] [Google Scholar]
- 29.Goldfried MR. A cross-validation of the Marlowe-Crowne Social Desirability Scale items. J Soc Psychol. 1964;64(1):137–145. doi: 10.1080/00224545.1964.9919551. [DOI] [PubMed] [Google Scholar]