Abstract
Cell culture studies show that the nanoscale lateral organization of surface receptors, their clustering or dispersion, can be altered by changing the lipid composition of the membrane bilayer. However, little is known about similar changes in vivo, which can be effected by changing dietary lipids. We describe the use of a newly developed method, k-space image correlation spectroscopy, kICS, for analysis of quantum dot fluorescence to show that a high fat diet can alter the nanometer-scale cluster of the murine T cell receptor, TCR on the surface of naïve CD4+ T cells. We found that diets enriched primarily in saturated fatty acids increased TCR nanoscale clustering to a level usually seen only on activated cells. Diets enriched in monounsaturated or n-3 polyunsaturated fatty acids had no effect on TCR clustering. Also none of the high fat diets affected TCR clustering on the micrometer scale. Furthermore, the effect of the diets was similar in young and middle aged mice. Our data establish proof-of-principle that TCR nanoscale clustering is sensitive to the composition of dietary fat.
Keywords: fatty acids, high fat diet, fluorescence, T cell receptor, quantum dots
Introduction
Lateral organization is a critical determinant of receptor function [1]. Studies in model membranes and in cell culture have shown that lipids through a variety of mechanisms including the formation of lipid microdomains, lipid-protein binding, and post-transcriptional fatty acylation of proteins, all regulate receptor clustering [2–5]. For example, several laboratories including our own, have demonstrated that the lateral organization of key immunological proteins can be disrupted in a variety of cell types upon cholesterol depletion, in vitro treatment with fatty acids, or knockdown of select lipid biosynthetic pathways [6–9].
There are several limitations in our understanding of how lipids target receptor clustering. One major limitation is the paucity of information on the nanoscale, the relevant length scale for receptor organization [10]. Studies demonstrating that lipids have a role in disrupting receptor organization have relied heavily on indirect methods ranging from experiments with biochemical detergent extraction to micrometer scale confocal imaging [11]. A second limitation is the lack of information at the animal level. No studies to date have addressed the impact of varying high fat diets on T cell receptor organization.
Here we address both of the limitations mentioned above by measuring the effects of high fat diets on the nanoscale organization of the T cell receptor for antigen, (TCR). Our approach relies on a newly developed method of quantitating nanoscale and micron scale receptor clustering, which entails imaging the stochastic blinking of quantum dots [12, 13]. This method grows out for an image analysis program for measuring diffusing and clustering on the micrometer scale [13]. We found that a high fat diet enriched in saturated fatty acids (SFA), but not diets enriched in monounsaturated (MUFA) fatty acids or in n-3 polyunsaturated fatty acids (PUFA), disrupts the nanoscale organization of the TCR.
Materials and Methods
Animals, diets, and cells
Male AND TCR transgenic mice (age 3 or 10 months) were fed either a control purified low-fat mouse chow (13% of total kcal from fat) or one of three experimental high fat diets (40% of total kcal from fat) (Harlan-Teklad). The high fat diets were enriched in SFAs, MUFAs, or n-3 PUFAs. The composition of the diets is shown in Table 1. Given the differences in age, data are presented as mice that are young (3 months old) and old (10 months old). The mice were fed for 2 weeks and diets were provide every other day to minimize oxidation. The rationale for selecting this time point was to avoid any differences in body weight, which can confound the results by altering the frequency of CD4+ T cells. Furthermore, we have previously demonstrated that 2 weeks of feeding is sufficient time for uptake of fatty acids into lymphocytes [14].
Table 1. Composition of experimental diets.
Values listed below are grams of ingredients per kilogram of diet for a low fat control diet and high fat diets enriched in saturated (SFA), monounsaturated (MUFA), and n-3 polyunsaturated (PUFA) fatty acids.
| Ingredient | Control | SFA | MUFA | n-3 PUFA |
|---|---|---|---|---|
| Casein | 185.0 | 220.0 | 220.0 | 220.0 |
| L-cystine | 2.5 | 3.0 | 3.0 | 3.0 |
| Corn Starch | 370.0 | 173.9 | 173.9 | 173.9 |
| Maltodextrin | 140.0 | 140.0 | 140.0 | 140.0 |
| Sucrose | 150.0 | 150.0 | 150.0 | 150.0 |
| Cellulose | 50.0 | 50.0 | 50.0 | 50.0 |
| Coconut oil | 0.0 | 185.0 | 0.0 | 0.0 |
| Soybean oil | 50.0 | 15.0 | 15.0 | 15.0 |
| Olive oil | 0.0 | 0.0 | 185.0 | 0.0 |
| Fish oil | 0.0 | 0.0 | 0.0 | 92.5 |
| Flaxseed oil | 0.0 | 0.0 | 0.0 | 92.5 |
| Mineral mix AIN-93M-MX | 35.0 | 42.0 | 42.0 | 42.0 |
| Vitamin mix AIN-93-VX | 15.0 | 18.0 | 18.0 | 18.0 |
| Choline Bitartrate | 2.5 | 3.0 | 3.0 | 3.0 |
| TBHQ | 0.02 | 0.06 | 0.06 | 0.06 |
Body weights, food intake, and the frequency of CD4+ T cells from the mice fed the differing diets were measured. The high fat diets had no impact on body weights (data not shown). Food intake was lower for mice on the high fat diets (p<0.05); however, on a kcal basis the food intake was equivalent. The frequency of CD4+ T cells did not change between the differing dietary groups (data not shown). CD4+ T cells were isolated from spleens using negative selection (Miltenyi Biotech) and purity (>90%) was verified using a BD LSRII flow cytometer. For select experiments, naïve 5C.C7 splenic T cells were stimulated in culture for 3 days with pigeon cytochrome C + anti-CD3 conjugated to nanoparticles [15].
Quantum dot imaging and k-space image correlation spectroscopy (kICS)
T cells were labeled with anti-CD3 antibody conjugated quantum dots and imaged as recently described [12]. Briefly, cells were imaged video imaged on a 3-I Marianas Live Cell Imaging Workstation. Each image in a series of 300–500 frames, taken 550 ms apart, was fast Fourier-transformed in two dimensions to convert to k-space followed by temporal correlation of the series [12]. Image analysis and extraction of DA, a measure of micron-scale clustering and theta ratio, a measure of the blinking frequency of the quantum fluorescence was done using custom MatLab routines available from the Wiseman Laboratory [13]. Theta ratio was interpreted in terms of clustering following the analysis in [12]. For image analyses, ~10 labeled cells were analyzed per treatment. Each cell image yielded information on 10’s of spots of fluorescence.
Statistics
All data are from multiple independent experiments. Data were analyzed using a one-way ANOVA followed by a post-hoc Bonferroni multiple comparisons t test or an unpaired two-tail t test. P<0.05 was considered statistically significant.
Results
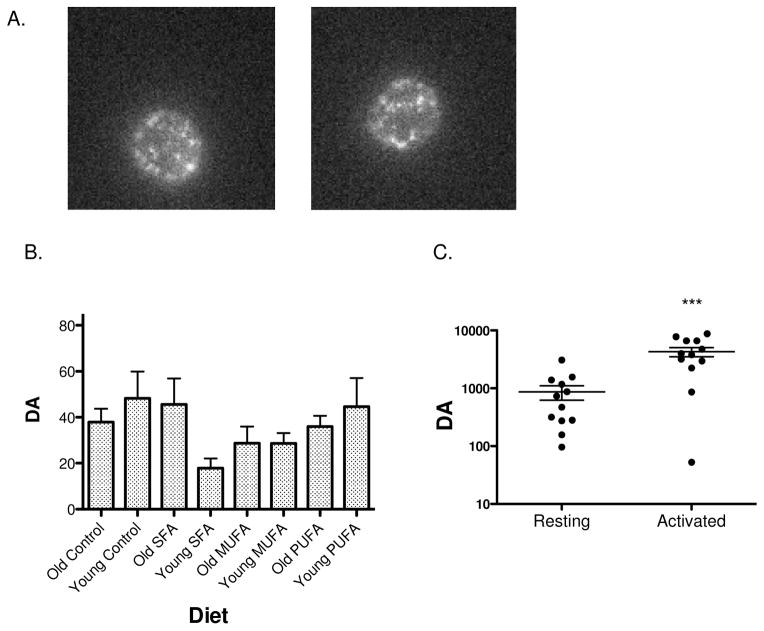
As seen in Figure 1A, T cells labeled on their TCR by antibody-conjugated quantum dots displayed large and small spots of fluorescence, labeling TCR. In movies of labeled cells it could be seen that some of these spots blinked on and off, a known property of quantum dot fluorescence (data not shown). Since the anti-CD3 quantum dots are ~60nm diameter, even small fluorescent spots (apparent diameter 200–300 nm) may contain several quantum dots. The problem then is to detect the spatial relationship of the dots within a single fluorescent spot. Rather than count and characterize individual spots, we used k-space image correlation spectroscopy, kICS, to characterize the photophysics (blinking) and distribution of fluorescence for all spots each cell [13]. This method proceeds through a time series image of a given cell calculating changes in correlation functions over space and time. Besides deriving parameters for micron-scale distribution of fluorescence, DA, these calculations characterize a blinking parameter, θ ratio, which is small when spots blink rapidly and large when they blink slowly or not at all. Correlative AFM and fluorescence microscopy has shown that slow blinking (long times for fluorescence on or off) is associated with nanometer proximity of quantum dots [16]. Together, θ ratio and DA, characterize clustering on scales of tens to hundreds of nanometers [12, 13].
Figure 1. kICS analyses reveal no influence of the type of dietary fat on CD4+ TCR micron scale clustering.
(A) Sample images demonstrating blinking of quantum dots on the surface of naïve CD4+ T cells isolated from mice fed differing diets. The approximate diameter of the cells is 7μm. (B) DA for CD4+ T cells isolated from mice fed a low fat or high fat diets enriched in SFAs, MUFAs, or n-3 PUFAs. Data are average ± S.E.M. from 30–44 cells analyzed from 3 independent experiments per diet per age group. Asterisks indicate significance relative to all other diets. (C) Control experiment demonstrating DA values are elevated in activated T cells relative to naïve resting T cells in culture. Note, in contrast to cells in A & B, here naïve cells were cultured for 3 days before analysis. Data are from 12 cells analyzed per condition. Asterisks indicate significance relative to the naïve condition: ***P<0.001.
We first measured the effects of the high fat diets, relative to a control low fat diet, on micrometer scale TCR organization in terms of DA. There was no significant effect on DA with the high fat diets compared to the control low fat diet (Figure 1B). The extent of clustering (magnitude of DA) was comparable to the low level, DA 10–200, measured for both naïve CD4+ (Figure 1C) and CD8+ T cells [12]. This contrasts with DA of activated T cells, which was 10–100-fold higher (Figure 1C) [12]. Thus, the high fat diets do not alter the large-scale distribution of labeled TCR.
We next analyzed images in terms of θ ratio (Figure 2A), a measure of clustering that is appropriate to detecting nanoscale changes in membrane organization, for example changes in lipid microdomains or in the organization of the membrane skeleton. Unlike DA which varies over orders of magnitude depending on T cell state, θ ratio has a limited range of values, in practice from ~0.2 to 1.0. kICS image analysis showed that TCR of cells from mice (both young and older) fed high fat diets enriched in SFAs were significantly more clustered than TCR on cells of mice fed either a low fat control diet or a high fat diet enriched in MUFAs or n-3 PUFAs (Figure 2A). Mean θ ratios for all cells except those fed SFA-enriched diet, were typical for naïve CD4+ cells, 0.4–0.5 [11]. In contrast, mean θ ratios for cells from animals fed SFA-enriched diet, ~0.6, were the same as those for CD4+ cells activated by antigen+anti-CD3 (Figure 2B).
Figure 2. kICS analyses reveal an increase in TCR nanoscale clustering with a diet enriched in saturated fatty acids.
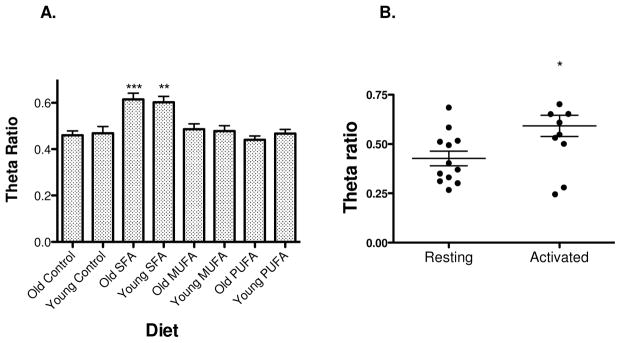
(A) Theta ratio for CD4+ T cells isolated from mice fed a low fat or high fat diets enriched in SFAs, MUFAs, or n-3 PUFAs. Data are average ± S.E.M. from 30–44 cells analyzed from 3 independent experiments per diet per age group. Asterisks indicate significance relative to all other diets: **P<0.01 and ***P<0.001. (B) Control experiment demonstrating theta ratio values are elevated in activated T cells relative to naïve resting T cells in culture after 3 days in culture. Data are from 12 cells analyzed per condition. Asterisk indicates significance relative to the naïve condition: *P<0.05.
Discussion
The present study advances the field by using in vivo dietary manipulation to modify cell plasma membrane organization. We show that a high fat diet enriched in SFAs enhances TCR clustering to the point that TCR of explanted naïve CD4+ T cells are distributed in a manner similar to their distribution on activated CD4+ T cells. Our approach is a significant step forward by relying on in vivo dietary manipulation rather than in vitro methods of disrupting the plasma membrane. The data raise the intriguing possibility that dietary fat composition is a regulator of nanoscale TCR clustering. This is relevant to the field of inflammation and metabolic disorders. There are numerous reports that diets high in select fatty acids, such as SFAs, are associated with chronic inflammation [17–20]. The enhanced nanoscale clustering of TCR in animals fed high SFA would be expected to enhance signaling upon antigen engagement and so increase and possibly prolong inflammatory responses. Several studies indeed show that adipose-tissue resident CD4+ T cells have a central role in the development of pro-inflammatory macrophages that contribute toward insulin resistance [21, 22].
If SFA affects clustering generally, then other membrane receptors provoking inflammation may be involved. For example, a report demonstrating that TLR4 activation is enhanced by SFA suggests that one mechanism is enhanced dimerization [23]. Furthermore, SFAs may be targeting the underlying lipid organization in many cell types. For instance, signaling lipid microdomains were shown to be disrupted with increased levels of saturated fatty acids in neurodegenerative disorders [24]. Gagon et al. demonstrated that stimulating the TCR with major histocompatibility complex loaded with peptide promoted the release of the CD3ε domain from the membrane, which was highly dependent on the local concentration of phosphatidylserine molecules [25]. It is conceivable that accumulation of saturated acyl chains in phosphatidylserines could have an impact on the release of the CD3ε domain.
Although SFAs had an impact on TCR nanoscale clustering, the unsaturated fatty acids had no effect. MUFAs generally do not disrupt lipid-protein spatial distribution and have limited efficacy in exerting changes in immune responses [14, 26]. However, several studies demonstrate that long chain n-3 PUFAs from fish oil disrupt protein localization and signaling in the immunological synapse accompanied by a reduction in downstream T cell cytokine secretion [27–29]. N-3 PUFAs generally have a role in influencing immune responses through a variety of mechanisms [30, 31]. We speculate that n-3 PUFAs may be exerting their effects on T cells by reorganizing other proteins (i.e. co-stimulatory molecules) that are essential for the formation of a mature immunological synapse. Alternatively, n-3 PUFAs may only exert their effects when the TCR is stimulated with cognate antigen presenting cells [32, 33]. Furthermore, given that eicosapentaenoic (EPA) and docosahexaenoic (DHA) acid in fish oils are not biologically equivalent [34–36], it is conceivable that EPA and DHA had opposite effects on TCR clustering, which could not be detected with the use of mixed n-3 PUFA enriched oils. Future studies with EPA and DHA alone will be essential to establish how TCR clustering is influenced.
Acknowledgments
This work was supported by NIH 1PO1AI072677 (J. Schneck PI) and NIH R01AT008375 (SRS). We thank Jonathan Powell and Avi Kupfer for providing T cells.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lee AG. How lipids and proteins interact in a membrane: a molecular approach. Mol BioSyst. 2005;1:203–212. doi: 10.1039/b504527d. [DOI] [PubMed] [Google Scholar]
- 2.Vrljic M, Nishimura SY, Moerner WE, McConnell HM. Cholesterol depletion suppresses the translational diffusion of class II major histocompatibility complex proteins in the plasma membrane. Biophys J. 2005;88:334–347. doi: 10.1529/biophysj.104.045989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lingwood D, Simons K. Lipid rafts as a membrane-organizing principle. Science. 2010;327:46–50. doi: 10.1126/science.1174621. [DOI] [PubMed] [Google Scholar]
- 4.Lavi Y, Gov N, Edidin M, Gheber LA. Lifetime of major histocompatibility complex class-I membrane clusters is controlled by the actin cytoskeleton. Biophys J. 2012;102:1543–1550. doi: 10.1016/j.bpj.2012.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glatz JFC. Lipids and lipid binding proteins: A perfect match. Prost Leukot Essent Fatty Acids. 2015;93:45–49. doi: 10.1016/j.plefa.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 6.Fessler MB, Parks JS. Intracellular lipid flux and membrane microdomains as organizing principles in inflammatory cell signaling. J Immunol. 2011;187:1529–1535. doi: 10.4049/jimmunol.1100253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fooksman DR, Gronvall GK, Tang Q, Edidin M. Clustering class I MHC modulates sensitivity of T cell recognition. J Immunol. 2006;176:6673–6680. doi: 10.4049/jimmunol.176.11.6673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kwik J, Boyle S, Fooksman D, Margolis L, Sheetz MP, Edidin M. Membrane cholesterol, lateral mobility, and the phosphatidylinositol 4,5-bisphosphate-dependent organization of cell actin. Proc Natl Acad Sci. 2003;100:13964–13969. doi: 10.1073/pnas.2336102100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shaikh SR, Rockett BD, Salameh M, Carraway K. Docosahexaenoic acid modifies the clustering and size of lipid rafts and the lateral organization and surface expression of MHC class I of EL4 cells. J Nutr. 2009;139:1632–1639. doi: 10.3945/jn.109.108720. [DOI] [PubMed] [Google Scholar]
- 10.Kusumi A, Fujiwara TK, Chadda R, Xie M, Tsunoyama TA, Kalay Z, Kasai RS, Suzuki KGN. Dynamic organizing principles of the plasma membrane that regulate signal transduction: Commemorating the fortieth anniversary of singer and nicolson’s fluid-mosaic model. Ann Rev Cell Dev Biol. 2012;28:215–250. doi: 10.1146/annurev-cellbio-100809-151736. [DOI] [PubMed] [Google Scholar]
- 11.Klammt C, Lillemeier BF. How membrane structures control T cell signaling. Front Immunol. 2012;3:291. doi: 10.3389/fimmu.2012.00291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boyle S, Kolin DL, Bieler JG, Schneck JP, Wiseman PW, Edidin M. Quantum dot fluorescence characterizes the nanoscale organization of T cell receptors for antigen. Biophys J. 2011;101:L57–59. doi: 10.1016/j.bpj.2011.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kolin DL, Ronis D, Wiseman PW. k-Space image correlation spectroscopy: A method for accurate transport measurements independent of fluorophore photophysics. Biophys J. 2006;91:3061–3075. doi: 10.1529/biophysj.106.082768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rockett BD, Salameh M, Carraway K, Morrison K, Shaikh SR. n-3 PUFA improves fatty acid composition, prevents palmitate-induced apoptosis, and differentially modifies B cell cytokine secretion in vitro and ex vivo. J Lipid Res. 2010;51:1284–1297. doi: 10.1194/jlr.M000851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lo Y-C, Edidin MA, Powell JD. Selective activation of antigen-experienced T cells by anti-CD3 constrained on nanoparticles. J Immunol. 2013;191:5107–5114. doi: 10.4049/jimmunol.1301433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu M, Van Orden A. Enhanced fluorescence intermittency of CdSe-ZnS quantum-dot clusters. Phy Rev Lett. 2006;97:237402. doi: 10.1103/PhysRevLett.97.237402. [DOI] [PubMed] [Google Scholar]
- 17.Eguchi K, Manabe I, Oishi-Tanaka Y, Ohsugi M, Kono N, Ogata F, Yagi N, Ohto U, Kimoto M, Miyake K, Tobe K, Arai H, Kadowaki T, Nagai R. Saturated fatty acid and TLR signaling link βcell dysfunction and islet inflammation. Cell Metab. 2012;15:518–533. doi: 10.1016/j.cmet.2012.01.023. [DOI] [PubMed] [Google Scholar]
- 18.Hommelberg PPH, Plat J, Langen RCJ, Schols AMWJ, Mensink RP. Fatty acid-induced NF-κB activation and insulin resistance in skeletal muscle are chain length dependent. Am J Phys Endo Metab. 2009;296:E114–E120. doi: 10.1152/ajpendo.00436.2007. [DOI] [PubMed] [Google Scholar]
- 19.Nakamura S, Takamura T, Matsuzawa-Nagata N, Takayama H, Misu H, Noda H, Nabemoto S, Kurita S, Ota T, Ando H, Miyamoto K-i, Kaneko S. Palmitate induces insulin resistance in H4IIEC3 hepatocytes through reactive oxygen species produced by mitochondria. J Biol Chem. 2009;284:14809–14818. doi: 10.1074/jbc.M901488200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murphy AM, Lyons CL, Finucane OM, Roche HM. Interactions between differential fatty acids and inflammatory stressors—impact on metabolic health. Prost Leukot Essent Fatty Acids. 2015;92:49–55. doi: 10.1016/j.plefa.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 21.Winer S, Chan Y, Paltser G, Truong D, Tsui H, Bahrami J, Dorfman R, Wang Y, Zielenski J, Mastronardi F, Maezawa Y, Drucker DJ, Engleman E, Winer D, Dosch HM. Normalization of obesity-associated insulin resistance through immunotherapy. Nat Med. 2009;15:921–929. doi: 10.1038/nm.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Nayer A, Lee J, Goldfine AB, Benoist C, Shoelson S, Mathis D. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med. 2009;15:930–939. doi: 10.1038/nm.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwartz EA, Zhang W-Y, Karnik SK, Borwege S, Anand VR, Laine PS, Su Y, Reaven PD. Nutrient modification of the innate immune response. Arterioscler Thromb Vasc Biol. 2010;30:802–808. doi: 10.1161/ATVBAHA.109.201681. [DOI] [PubMed] [Google Scholar]
- 24.Fabelo N, Martin V, Santpere G, Marin R, Torrent L, Ferrer I, Diaz M. Severe alterations in lipid composition of frontal cortex lipid rafts from Parkinson’s disease and incidental Parkinson’s disease. Mol Med. 2011;17:1107–1118. doi: 10.2119/molmed.2011.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gagnon E, Schubert DA, Gordo S, Chu HH, Wucherpfennig KW. Local changes in lipid environment of TCR microclusters regulate membrane binding by the CD3ε cytoplasmic domain. J Exp Med. 2012;209:2423–2439. doi: 10.1084/jem.20120790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Enns JE, Hanke D, Park A, Zahradka P, Taylor CG. Diets high in monounsaturated and polyunsaturated fatty acids decrease fatty acid synthase protein levels in adipose tissue but do not alter other markers of adipose function and inflammation in diet-induced obese rats. Prost Leukot Essent Fatty Acids. 2014;90:77–84. doi: 10.1016/j.plefa.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 27.Kim W, Fan YY, Barhoumi R, Smith R, McMurray DN, Chapkin RS. n-3 polyunsaturated fatty acids suppress the localization and activation of signaling proteins at the immunological synapse in murine CD4+ T cells by affecting lipid raft formation. J Immunol. 2008;181:6236–6243. doi: 10.4049/jimmunol.181.9.6236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yog R, Barhoumi R, McMurray DN, Chapkin RS. n-3 polyunsaturated fatty acids suppress mitochondrial translocation to the immunologic synapse and modulate calcium signaling in T cells. J Immunol. 2010;184:5865–5873. doi: 10.4049/jimmunol.0904102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fan YY, Ly LH, Barhoumi R, McMurray DN, Chapkin RS. Dietary docosahexaenoic acid suppresses T cell protein kinase C theta lipid raft recruitment and IL-2 production. J Immunol. 2004;173:6151–6160. doi: 10.4049/jimmunol.173.10.6151. [DOI] [PubMed] [Google Scholar]
- 30.Calder PC. Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochim Biophys Acta (BBA) 2015;1851:469–484. doi: 10.1016/j.bbalip.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 31.Shewchuk BM. Prostaglandins and n-3 polyunsaturated fatty acids in the regulation of the hypothalamic–pituitary axis. Prost Leukot Essent Fatty Acids. 2014;91:277–287. doi: 10.1016/j.plefa.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 32.Rockett BD, Melton M, Harris M, Bridges LC, Shaikh SR. Fish oil disrupts MHC class II lateral organization on the B-cell side of the immunological synapse independent of B-T cell adhesion. J Nutr Biochem. 2013;24:1810–1816. doi: 10.1016/j.jnutbio.2013.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rockett BD, Teague H, Harris M, Melton M, Williams J, Wassall SR, Shaikh SR. Fish oil increases raft size and membrane order of B cells accompanied by differential effects on function. J Lipid Res. 2012;53:674–685. doi: 10.1194/jlr.M021782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murali G, Desouza CV, Clevenger ME, Ramalingam R, Saraswathi V. Differential effects of eicosapentaenoic acid and docosahexaenoic acid in promoting the differentiation of 3T3-L1 preadipocytes. Prost Leukot Essent Fatty Acids. 2014;90:13–21. doi: 10.1016/j.plefa.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 35.Teague H, Harris M, Fenton J, Lallemand P, Shewchuk B, Shaikh SR. Eicosapentaenoic and docosahexaenoic acid ethyl esters differentially enhance B-cell activity in murine obesity. J Lipid Res. 2014;17:1420–1433. doi: 10.1194/jlr.M049809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Williams Justin A, Batten Shawn E, Harris M, Rockett Benjamin D, Shaikh Saame R, Stillwell W, Wassall Stephen R. Docosahexaenoic and eicosapentaenoic acids segregate differently between raft and nonraft domains. Biophys J. 2012;103:228–237. doi: 10.1016/j.bpj.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]