Abstract
Mounting evidence suggests that poorer motor skills may be related to more severe autism symptoms. This study investigated if atypical white matter microstructure in the brain mediated the relationship between motor skills and ASD symptom severity. Sixty-seven males with ASD and 42 males with typical development (5-33 years old) completed a diffusion tensor imaging scan and measures of grip strength, finger tapping, and autism symptom severity. Within the ASD group, weaker grip strength predicted more severe autism symptoms. Fractional anisotropy of the brainstem's corticospinal tract predicted both grip strength and autism symptom severity and mediated the relationship between the two. These findings suggest that brainstem white matter may contribute to autism symptoms and grip strength in ASD.
Keywords: autism, motor, diffusion tensor imaging, brainstem, grip strength, finger tapping
A growing body of evidence suggests impaired motor performance in individuals with autism spectrum disorder (ASD) across a number of motor domains (for a meta-analysis see Fournier et al. [2010]). These motor difficulties appear to be highly prevalent with more than half of children with ASD demonstrating motor deficits such as hypotonia (Ming et al. 2007). While motor delays in ASD may be similar to what is observed in other developmental disorders (Provost et al. 2007) and may be similar to motor difficulties observed in siblings of individuals with ASD (Libertus et al. 2014), perhaps one of the most intriguing findings to arise from this literature is the number of studies that have found that individual differences in motor impairments appear to be related to the severity of core ASD symptoms (i.e., social communication and repetitive behaviors). These findings hold across different ages and levels of functioning in ASD. For example, motor skills in infancy have been shown to predict communication skills at 18 months (Bhat et al. 2012), later speech fluency in middle childhood and adolescence (Gernsbacher et al. 2008), and current adaptive functioning and daily living skills in ASD (MacDonald et al. 2013). In middle childhood through adulthood, a diversity of motor skills (i.e., motor composites, postural stability, gestural praxis, oral motor skills, manual motor skills, and perceptual-motor integration) predicted the severity of ASD symptomatology (Dowell et al. 2009; Dziuk et al. 2007; Hilton et al. 2007; Kern et al. 2013; Linkenauger et al. 2012; Travers et al. 2013), emotional/behavioral disturbances (Papadopoulos et al. 2012), and repetitive behaviors/restricted interest (Radonovich et al. 2013; Travers et al. 2013). These studies provide converging evidence that a wide range of motor skills may be highly associated with a constellation of ASD traits across the life span.
Despite the relative consistency of these findings, we do not know the nature of the relationship between motor impairments and core ASD symptoms. Better understanding this relationship could lead to novel methods for intervention or insights into the interaction between motor ability and cognition in ASD and in other developmental disorders. Some researchers have pointed out how motor impairments early in life could contribute to social impairments in ASD (Bhat et al. 2011). For example, motor delays in infancy could hinder the development of a “like me” framework for social interaction (Meltzoff 2007), and motor clumsiness could prevent school-aged children from socializing with peers on the playground. It is also plausible that social impairments could impede motor learning in ASD, as many motor skills are learned by watching and engaging with other people. Yet a third possibility that has been suggested in the literature is that the motor-and-autism-symptom relation is driven by a third or mediating variable that simultaneously affects both motor skills and core ASD symptoms in this group (Dziuk et al. 2007). Because autism is a neurodevelopmental disorder, a possibility for a mediating variable affecting both motor ability and ASD severity would be a metric of brain structure or function.
In terms of different brain metrics that may contribute to both motor performance and autism symptom severity, a previous investigation by our group did not find that volume of different brain structures was strongly related to manual motor performance on tasks such as grip strength or finger tapping (Duffield et al. 2013). Similarly, Hardan and colleagues (2003) did not find that volume of the basal ganglia predicted manual motor performance in ASD. These results suggest that the volume of different brain structures may not be the best predictor of manual motor performance in this population. Therefore, a logical next step is to examine the effect of white matter microstructure on motor performance and ASD symptom severity. Myelinated axons that make up the white matter of the brain serve as the structural infrastructure for brain connectivity, and atypicalities in white matter microstructure have been observed in ASD across the literature (for a review see Travers et al. 2012). White matter microstructure may be particularly intriguing candidate for a third variable in this relation because motor function and social communication likely require an integration of information across the brain. Therefore, a disruption in white matter microstructure in ASD could theoretically affect both motor ability and core autism symptoms in ASD, and this disruption in white matter could either be widespread or localized. Given that manual motor skills may be particularly critical for early and later exploration of the world and for the completion of independent living skills tasks across the life span, the present study investigated both grip strength and finger tapping speed as our measures of motor performance.
The present study had three aims: 1) To examine group differences in manual motor performance (i.e., grip strength and finger tapping), 2) to examine the potential replication of a significant relation between motor performance and ASD symptom severity within the ASD group using our manual motor performance variables, and 3) to examine whether the relation between motor function and ASD symptoms could be mediated by white matter microstructural integrity, as measured by Diffusion Tensor Imaging (DTI). Based on the previous literature, we predicted that poorer manual motor performance would be observed in the ASD group and would be related to more severe ASD symptoms. Fractional anisotropy (FA) is a common DTI measure of circuit integrity, and we hypothesized that FA in at least one motor-related white matter tract would mediate the relationship between motor function and ASD symptom severity.
Methods
Participants
Sixty-seven males with ASD and 42 males with typical development between the ages of 5 and 33 years were included in this study. These participants were selected from Time 1 of a 10-year longitudinal neuroimaging study (109 ASD and 80 typically developing controls [TDC]) based on manual motor data completeness and age matches (three motor assessments were excluded in participants with ASD over 40 years of age because there were no typically developing matched controls in that age range).
Participants with ASD were diagnosed based on the Autism Diagnostic Interview- Revised (ADI-R) (Lord et al. 1994), the Autism Diagnostic Observation Scale-General (ADOS-G) (Lord et al. 2000), the DSM-IV (American Psychiatric Association 2000), and the ICD-10 criteria. Participants with ASD met criteria for a lifetime diagnosis of autistic disorder, Asperger's syndrome, or pervasive developmental disorder not otherwise specified. Participants with ASD were excluded from this study if they had a known medical cause of ASD (determined by patient history, physical exam, fragile-X testing, and karyotype), or if they had a history of severe head injury, hypoxia-ischemia, seizure disorder, or other neurological disorders. Participants with typical development were confirmed as having typical development through ADOS-G testing, history, IQ testing, neuropsychological assessment, and standardized psychiatric assessment. All participants were verbal at the time of testing and had English as their first language.
If an ASD or TD participant developed major depression or other severe psychiatric disorder during the course of the longitudinal study, they were retroactively not included in these Time 1 analyses in order to prevent possible confounds in the DTI effects. This was the case with two participants with typical development and three participants with ASD. In the broader longitudinal study, the retention rate (at least two scans and two assessments) was 85% for the group with ASD and 71% for the group with typical development. Motor and volumetric brain results from an age- and IQ-matched subset of these participants are reported in (Duffield et al. 2013). Participant groups were matched on age but significantly differed in full-scale IQ (Table 1). After complete description of the study to the participants, written informed consent was obtained by all participants.
Table 1.
Demographic characteristics of the group with autism spectrum disorder (ASD) and of the typically developing controls (TDC).
| ASD n | TDC n | ASD Mean(SD) | ASD Range | TDC Mean(SD) | TDC Range | t-value | p-value | |
|---|---|---|---|---|---|---|---|---|
| Age (Years) | 67 | 42 | 15.1 (6.96) | 5.02-33.25 | 15.99 (6.46) | 5.31-29.7 | 0.67 | 0.51 |
| IQ | 67 | 42 | 97.7 (18.65) | 58-137 | 119.43 (14.98) | 95-153 | 6.37 | <.001 |
| SRS Total | 62 | 36 | 95.41 (28.56) | 33.5-157 | 17.46 (12.33) | 1-60 | -15.53 | <.001 |
Materials
Grip strength
Following the guidelines of the Halstead-Reitan Battery (Reitan Laboratories: www.reitanlabs.com) (Heaton et al. 1991), grip strength was measured with a hand dynamometer and was recorded in kilograms. The participant was instructed to hold the upper part of the dynamometer in the palm of the hand and squeeze the stirrup with the fingers as tightly as possible. Participants first completed two trials. If the strength in kilograms of these two trials were within a five-point range, the mean was calculated from the two trials. Otherwise, a third trial was completed, and the average of those three trials was used. Scores for the preferred and non-preferred hands were averaged together to get an overall grip strength score. Grip strength from the dynamometer was recorded and reported in kilograms (as is the industry standard). However, it is important to note that newtons are the unit of measurement for force in the International System of Units. Kilograms can be converted to newtons by multiplying the kilograms by a factor of 9.8.
Finger tapping
As instructed by the Halstead-Reitan Battery (Reitan Laboratories: www.reitanlabs.com; (Heaton et al. 1991)), the number of taps performed over a period of 10 seconds was recorded for each trial, separately for each hand. The majority of participants completed the task on the manual finger tapping board, but the younger participants completed the task using the electronic finger tapping version. Participants completed a minimum of five trials, and these scores were averaged for each hand. The administration rule was that five trials needed to be within five finger taps of each other. If not, additional trials were administered until this administration rule was met or until 10 total trials were administered. Scores for the preferred and non-preferred hands were averaged together to get an overall finger tapping score.
Quantitative autistic traits/symptom severity
The Social Responsiveness Scale (SRS; Constantino 2002) is a 65-item parent report measure that examines ASD symptoms in the past six months across the ASD spectrum. Each item on the scale asks about an aspect of observed reciprocal social behavior and is rated on a scale from “0” (never true) to “3” (almost always true). Higher scores on the SRS indicate greater severity of social impairment. The test has been validated for clinical populations age four to 18 years. The test-retest reliability after three months for the SRS was very good, with a correlation of .88. After 27 months, the test-retest reliability for the SRS was still good with a correlation of .83. Because many participants in this study were outside of the normed age range for the SRS, raw scores rather than t-scores are reported. Sixty-two (of 67) participants with ASD and 36 (of 42) participants with typical development had completed SRS measures.
IQ
IQ was assessed in all participants using the Differential Abilities Scales (Elliott 1990), WISC-III (Wechsler 1991), or WAIS-III (Wechsler 1997), depending on the age and functioning level of the participant.
Procedure
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study. Participants completed all measures across multiple sessions. Grip strength and finger tapping were used as indices of manual motor performance. Quantitative autistic traits/symptom severity was measured via the Social Responsiveness Scale (SRS) total raw score (Constantino 2002).
Imaging parameters
MRI neuroimaging studies were performed at [blinded for review] on a Siemens Trio 3.0 Tesla scanner with an 8-channel head coil. The imaging protocol included collection of DTI data, which were acquired using a diffusion-weighted imaging (DWI) pulse sequence with diffusion-weighting of b=1000 s/mm2 applied in twelve diffusion encoding directions and a single b0 image (see (Alexander et al. 2007)). The acquisition was repeated 4 times and averaged prior to DTI analysis. Whole brain coverage was obtained using sixty contiguous axial slices (matrix = 128 × 128, FOV = 256 mm, resolution = 2 × 2 × 2.5 mm3, averages = 4, TR = 7000 ms, TE = 84 ms, pixel bandwidth = 1346 Hz).
DTI analysis
Diffusion weighted images were corrected for bulk head motion and eddy currents using the eddy_correct from the fMRIB software library (FSL) (Jenkinson et al. 2012) and gradient orientations were corrected for rotation (Leemans and Jones 2009). Then, tensors were fit using a robust estimation algorithm (RESTORE) (Chang et al. 2005) with Camino software (Cook et al. 2006). Fractional anisotropy (FA) and mean diffusivity (MD) maps were generated. Raw diffusion weighted images, computed FA, and computed MD maps were visually inspected for evidence of artifacts. One participant with typical development was found to have a DTI scan with inflated frontal DTI metrics and was subsequently excluded from the DTI analyses.
DTI maps were spatially normalized to a population-specific template using diffeomorphic registration implemented in DTI-TK (Zhang et al. 2006). Major white matter tracts were segmented using regions defined in the JHU ICBM-DTI-81 template (Mori et al. 2005) that was registered to our population FA template using diffeomorphic spatial normalization (ANTS) (Avants and Gee 2004). The template white matter ROIs were then inverse transformed to the native DTI space for each individual (Faria et al. 2011), visually inspected, and manually adjusted if necessary. The median FA and MD were extracted for each region. Measurements were obtained in eight bilateral tracts that have been associated with motor function in previous literature (i.e., the corticospinal tract of the brainstem, the middle, superior, and inferior cerebellar peduncles, the posterior limb of the internal capsule, and the genu, body, and splenium of the corpus callosum). The present corticospinal brainstem mask covered the deep white matter pyramidal tracts from the peduncles to the posterior limb of the internal capsule (See supplementary figure S1). Additionally, a whole brain average FA mask was calculated by taking the mean FA of the 48 JHU tracts. To account for the effects of potential group differences in head motion during scanning, we computed the total motion index (TMI) for each participant as described in (Yendiki et al. 2013).
Statistical analyses
ROI statistical analyses were performed in R version 2.15.1 (R. Core Team 2013). In our analyses, procedures were implemented to reduce the number of statistical comparisons. First, the left and right-handed motor measures were combined into a single average measure prior to analysis. Second, although SRS data were available in both groups, the motor-symptom correlations were only analyzed in the ASD group, which is in line with previous research in this domain. Third, we selected a single white matter tract (the bilateral corticospinal tract of the brainstem) to test as a candidate “third variable” in our path analysis of motor-symptom relations. This selection was based on preliminary partial correlations between our eight a priori selected white matter motor tracts and grip strength after accounting for age (see Table 2). The partial correlations were conducted with the ppcor package in R (Kim 2012). Structural equation model path analyses were conducted using the Latent Variable Analysis (lavaan) package in R (Rosseel 2012).
Table 2.
Partial Pearson R correlations between manual motor performance and fractional anisotropy (FA) and mean diflusivity (MD) of white matter tracts within the ASD group, controlling for age.
| Grip | Tap | |
|---|---|---|
| Inferior Corticospinal FA | +.39*** | +.36** |
| Inferior Corticospinal MD | −.37** | −.18* |
| Inferior Cerebellar Peduncle FA | +.16 | +.20 |
| Inferior Cerebellar Peduncle MD | −.09 | −.21 |
| Superior Cerebellar Peduncle FA | +.17 | +.37** |
| Superior Cerebellar Peduncle MD | −.06 | −.27* |
| Middle Cerebellar Peduncle FA | −.06 | +.07 |
| Middle Cerebellar Peduncle MD | −.18 | −.18 |
| Posterior Limb of Internal Capsule FA | +.08 | +.11 |
| Posterior Limb of Internal Capsule MD | −.09 | −.18 |
| Whole Brain Mean FA | +.20 | +.38** |
Note:
p < .05
p < .01
p < .001
After our hypothesis-driven statistical analyses were performed, we conducted differential analyses to determine the specificity of the inferior corticospinal tract white matter microstructure in predicting individual differences in autism symptom severity within the ASD group and to explore this pattern of results within the typically developing group. These differential analyses included 1) a follow-up voxel-based analyses (VBA) to examine the specificity of the brainstem white matter in predicting both grip strength and autism symptom severity, 2) three follow-up structural equation path models, examining finger tapping and symptom severity in ASD, and both finger tapping and grip strength in typically developing controls, and 3) correlations to ascertain that participant head motion (TMI) during the DTI scan was not a confounding variable in the relation between DTI metrics and symptom severity. The follow-up VBA analyses were conducted on normalized white matter masks in the ASD group, using Surfstat in Matlab. These two analyses examined 1) FA as a function of grip strength in the ASD group, controlling for age (FA~1+grip strength+age), and 2) FA as a function of symptom severity, controlling for age (FA~1+SRS+age). Given that the purpose of the VBA analyses was to corroborate the specificity of the brainstem FA in these relations, we used an alpha of .001, and no threshold for multiple comparisons was employed.
Results
Group differences in motor performance
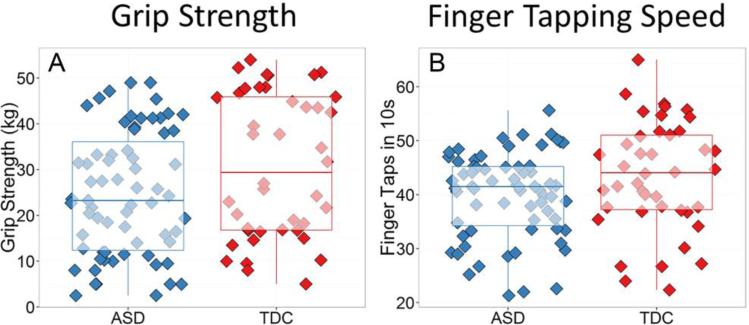
Initial analyses examined group differences in grip strength and finger tapping (see Figures 1a and 1b). Independent-samples t-tests indicated that participants with ASD demonstrated significantly weaker grip strength, t(107) = 2.21, p = .03, Cohen's d = 0.43, and slower finger tapping, t(107) = 2.03, p = .04, Cohen's d = 0.39, compared to participants with typical development. Each of these group differences corresponds to a small-to-medium effect size (Cohen, 1992). When conducting ANCOVA's that controlled for age and full scale IQ, group differences in grip strength remained significant (p = .02), but group differences in finger tapping speed became only marginally significant (p = .08).
Fig 1.
Bar-and-whisker plots with underlying raw scores depicting group differences between the ASD group and the typically developing controls (TDC) in (A) grip strength, and (B) finger tapping speed.
Motor performance and symptom severity correlations
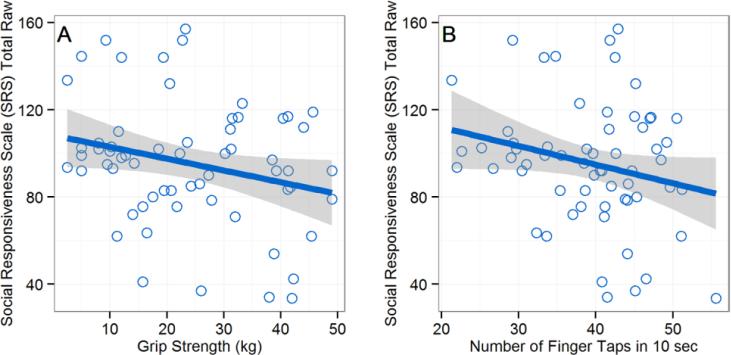
To investigate if poorer manual motor function was related to more severe autism symptoms in the ASD group, we conducted correlations between the manual motor performance measures (grip strength and finger tapping) and Social Responsiveness Scale (SRS) raw scores. We did not control for age or full-scale IQ in these analyses, as neither age nor full-scale IQ were significantly related to SRS scores in this group. Weaker grip strength was significantly correlated with more severe autism symptoms in the ASD group, r(60) = −.26, p = .045, and slower finger tapping was marginally correlated with more severe autistic traits in the ASD group, r(60) = −.23, p = .07. These relations corresponded to small-to-medium sized effects, and scatterplots of these relations can be seen in Figures 2a and 2b.
Fig 2.
Scatterplots depicting the relation between manual motor performance and symptom severity/adaptive functioning in the ASD group. (A) Stronger grip strength significantly predicts less severe autism symptoms on the Social Responsiveness Scale (SRS). (B) Faster tapping speed marginally predicts less severe autism symptoms on the Social Responsiveness Scale (SRS).
Motor performance and DTI metrics correlations
Given the relation between grip strength and autism symptom severity in our ASD sample, we wanted to examine if the white matter microstructural integrity of key motor tracts (as measured through DTI) may be a third variable in this correlation. Preliminary partial correlations (controlling for age) investigated which of the eight a priori selected motor tracts was most strongly related to grip strength in our sample with ASD. As can be seen in Table 2, the bilateral inferior corticospinal tract (both FA and MD) was the only significant predictor of grip strength. A follow-up statistical comparison of these correlations suggested that corticospinal FA was significantly more predictive of grip strength than whole brain FA composite (Z = −2.24, p = .03). For finger tapping speed, a more distributed network of white matter tracts appeared to contribute to performance in the ASD group, with whole brain white matter FA being the most robustly correlated with finger tapping speed.
Path analyses examining if inferior corticospinal tract accounts for motor and symptom severity relations
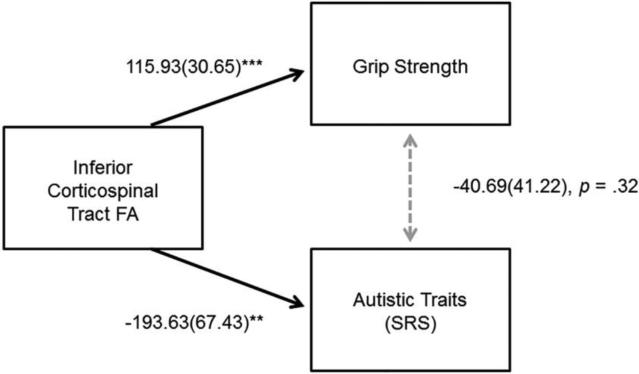
Structural equation model path analyses were conducted to examine if FA of the inferior corticospinal tract could account for the correlations observed between grip strength, symptom severity, and daily living skills in ASD. In our first path analysis, FA of the inferior corticospinal tract was entered as a variable affecting both grip strength and autism symptom severity in the ASD group (Figure 3). This path analyses demonstrated excellent model fit, determined according to values of root mean square error of approximation (RMSEA<.05), Standardized Root Mean Square Residual (SRMR < .05), Comparative Fit Index (CFI>.95), and a non-significant chi square statistic (Hooper et al. 2008). After accounting for the effects of corticospinal FA on both grip strength and SRS total raw scores, our first path analysis demonstrated that the relation between grip strength and autism symptom severity was no longer significant (p=.32). This pattern of results remained after accounting for age (see supplementary material figure S2).
Fig 3.
Results of structural equation model examining the effects of corticospinal FA on grip strength and autism symptom severity (SRS) in the ASD group: Unstandardized Coefficient (Standard Error). Comparative Fit Index = 1.00; Root mean square error of approximation < .001. *p < .05, **p < .01 , ***p < .001.
Differential analyses and results
To confirm that there were overlapping white matter regions only in the brainstem that predicted both symptom severity and grip strength, we conducted two separate VBA analyses: 1) examining which voxels in the white matter mask were significantly predicted by grip strength (controlling for age), and 2) examining which voxels in the white matter mask were significantly predicted by symptom severity (controlling for age). As can be seen in the results presented in Figure 4, both grip strength and symptom severity were significantly related to white matter (FA) in the brainstem region (p <.001, uncorrected), and in agreement with our ROI analyses, there were overlapping voxels that predicted both symptom severity and grip strength primarily in the right corticospinal tract of the brainstem.
Fig 4.
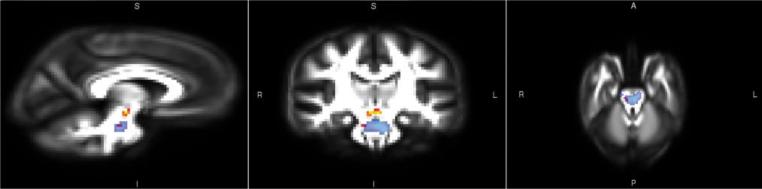
Voxel based analysis (VBA) results of grip strength predicting fractional anisotropy (FA) (Red/Yellow) and results of symptom severity predicting FA (Blue) in the ASD group, p < .001, uncorrected for multiple comparisons. Both analyses controlled for age.
To examine if FA of the inferior corticospinal tract additionally accounted for the marginally significant relation between finger tapping and autism symptom severity, a path analysis was performed replacing grip strength with finger tapping speed, and the same pattern of results within the ASD was found (supplementary material figure S3). Specifically, the marginally significant correlation between finger tapping speed and autism symptom severity diminished significantly after accounting for the upstream effects of FA of the inferior corticospinal tract.
To examine if this pattern of results in the ASD group held in the typically developing group, we performed the same SEM models in the typically developing group. The relations among grip strength, autism symptom severity, daily living skills, and age were different in the typically developing group compared to the ASD group, such that the inferior corticospinal tract did not predict grip strength or account for any relations between grip strength and autism symptom severity (see supplementary materials figures S4 and S5). Therefore, the contributions of the inferior corticospinal tract to grip strength may be unique to the ASD group after accounting for age.
To ascertain that movement during DTI scanning did not confound the results, we calculated the Total Motion Index (TMI) (as in Yendiki et al. [2013]), and we investigated possible correlations of TMI with inferior corticospinal FA and with the SRS raw scores within the ASD group. TMI did not correlate with inferior corticospinal FA, r = −.18, p = .15, SRS raw scores, r = +.12, p = .32, or grip strength, r = +.10, p = .43. Therefore, it was unlikely that head motion during scanning confounded these results.
To investigate whether there were group differences in the inferior corticospinal FA, we conducted an independent-samples t-test. We found that the inferior corticospinal FA in the ASD group (M = 0.44, SD = 0.05) was not significantly different from inferior corticospinal FA in the typically developing group (M = 0.46, SD = 0.04), t(106) = 1.86, p = .07, Cohen's d = 0.44.
Discussion
This study examined the interplay among motor symptoms, autism symptom severity, and white matter microstructure in ASD. At the group level, we found poorer manual motor performance in ASD. These group differences were similar to previous group differences observed in grip strength and grooved pegboard performance (Duffield et al. 2013; Hardan et al. 2003) and are broadly consistent with previous findings of motor impairments in ASD across various motor tasks (Fournier et al. 2010).
Within the ASD group, we found that weaker grip strength was associated with more severe autism symptoms. There was also a marginally significant relation between slower finger tapping and more severe autism symptoms. These findings are consistent with previous studies that have found poorer motor function across a number of motor domains to be related to a number of different aspects of autism symptomatology (Bhat et al. 2012; Gernsbacher et al. 2008; MacDonald et al. 2013; Dziuk et al. 2007; Linkenauger et al. 2012; Travers et al. 2013; Kern et al. 2013; Hilton et al. 2007; Dowell et al. 2009; Papadopoulos et al. 2012; Radonovich et al. 2013). In all, the collective body of research suggests that motor impairments may be intimately linked with other behavioral symptoms of ASD. An important aspect of our study was the ability to examine white matter microstructure of key motor tracts to probe the nature of these relations.
Our path analyses indicated that the white matter microstructure of the brainstem's inferior corticospinal tract not only predicted both grip strength and autism symptom severity within the group with ASD, but the white matter microstructure of this region also mediated the relation between grip strength and autism symptom severity. These results suggest that it is unlikely that there is a direct, causal relationship between motor ability (indexed by grip strength) and autism symptom severity. Rather, the microstructural integrity of the inferior corticospinal tract seems to be an upstream variable that affects both grip strength and autism symptom severity. Voxel-based analyses that did not pre-select white matter tracts of interest confirmed the specificity of brainstem corticospinal white matter microstructure in predicting both grip strength and autism symptom severity.
Given the corticospinal tract's well-known role in motor function, the present relation between grip strength and corticospinal FA was not surprising. Hand grip depends on the integrity of corticospinal tract fibers arising from the primary motor cortex (Schulz et al. 2012), and maximal force exertion requires the transmission of strong signals from the central nervous system (CNS) to activate large muscle motor units and synchronous signals for frequency summation. Both strength and synchrony of CNS signals to the muscles are required to achieve a strong grip, and thus performance on grip strength measures could be affected by atypical corticospinal tract microstructure. In addition, a larger number of corticospinal tract fibers arise from the primary somatosensory cortex, which suggests that the corticospinal tract could relate to the atypical sensory processing commonly reported in ASD. This is a key possibility that will need to be addressed by future research.
The present finding that the microstructure of the inferior corticospinal tract of the brainstem relates to severity of autism symptoms within the ASD group is relatively novel. However, this finding is consistent with other recent research suggesting brainstem atypicalities are related to central features of ASD. For example, Jou and colleagues (2009) found that gray matter volume of the brainstem was related to oral sensory sensitivity in ASD and that gray matter volume of the brainstem developed atypically in children and adolescents with ASD (Jou et al. 2013). In addition, Cohen and colleagues (I. L. Cohen et al. 2013) found that auditory brainstem responses (a behavioral proxy of brainstem connectivity) at four months of age predicted autism diagnosis and symptom severity at preschool age, and an early finding from Fein and colleagues (Fein et al. 1981) found that children with autism who had normal auditory brainstem responses had less severe symptoms than those with autism who had atypical auditory brainstem responses. Taken together, these results suggest that structural and functional aspects of the brainstem may be related to ASD symptomatology.
Although corticospinal tract forms a distinct tract as it courses downward through the brainstem, our corticospinal tract findings were in proximity to numerous other tracts coursing through the brainstem including the reticular formation. The reticular formation of the brainstem has a role in arousal, sleep, attention, alerting, sensory gating, and habituation (Brown et al. 2012; Hornung 2003; Hurley et al. 2010; Paus 2000), and in ASD, difficulties with attention (Dawson et al. 1998; Townsend et al. 1996), sleep (S. Cohen et al. 2014; Mazurek and Petroski 2014), and atypical sensory processing (Baranek et al. 2006; Ben-Sasson et al. 2009) have been well documented, and given the role of the reticular formation in attention and sleep, it is possible that these associated symptoms of ASD may be related to broader brainstem white matter. To investigate if components of the brainstem can predict other aspects of ASD symptomatology, future research will be needed to replicate and extend these findings using combined metrics of attentional arousal, sensory sensitivity, sleep/wakefulness, brainstem gray matter volume, white matter volume, and white matter microstructure in ASD.
In terms of brainstem group differences, it is important to note that only a limited number of previous studies have found group differences in the DTI metrics of the corticospinal tract/brainstem white matter in ASD compared to typically developing controls (Ben Bashat et al. 2007; Bloemen et al. 2010; Brito et al. 2009; Shukla et al. 2011). Even within the present study, there was only a marginally significant group difference in corticospinal FA in our study. Therefore, corticospinal microstructure may not be able to categorically define who presents with an ASD and who has typical development. However, our findings suggest that corticospinal microstructure may predict where along the continuum of symptom severity (both motor and core autism symptoms) an individual with ASD may fall. Being able to predict symptom severity within the autism spectrum would have major implications for individualizing the design and implementation of interventions. Furthermore, knowing that the inferior corticospinal microstructure may vary nearly as much as the behavioral profiles within the autism spectrum possibly explains why group differences in the microstructure of this tract have not been commonly detected in the literature. Future investigations will be needed to examine how the inferior corticospinal tract, motor performance, and autism symptoms longitudinally change with age in ASD compared to typical development.
There are several limitations of this study. First, our indices of motor function were limited to grip strength and finger tapping, and future research will benefit from including other measures of commonly reported motor difficulties in ASD. Second, the current study sample included a wide age and IQ range, which might have obscured the results. Nevertheless, it is important to note that we took measures to control for these effects when appropriate, and previous research suggests that motor and ASD symptom relations occur from infancy through adulthood, supporting the hypothesis that a wide age range would not likely alter these relations. Finally, prospective longitudinal studies will be necessary to determine the causal chain of events between variation in white matter microstructure, motor functioning, and autistic symptomatology across the lifespan in ASD.
Conclusions
Manual motor skills influence our fundamental ability to interact with, learn about, and react to the world around us. The present results suggest that individuals with ASD may struggle with weaker grip strength and slower finger tapping speeds. Our results support and extend the suggestion by MacDonald and colleagues (MacDonald et al. 2013) that impaired motor skills in ASD should be targeted through early intervention and should be continued as needed throughout the life span. Additionally, our motor findings across this wide age range raise questions about how to intervene to improve ASD symptoms. Interventions that target the inferior corticospinal tract or brainstem may be particularly efficacious, as this may have cascading beneficial effects on both motor performance and social skills in ASD, either directly or via connectivity with other networks in the brain.
Supplementary Material
Acknowledgments
This work was supported by the National Institute of Mental Health [RO1 MH080826 to JEL, ALA, NL, EDB; RO1 MH084795 to JEL, NL], the Eunice Kennedy Shriver National Institute of Child Health and Human Development [T32 HD07489 to BGT, and P30 HD003352 to the Waisman Center], the Hartwell Foundation [to BGT], the Poelman Foundation [to EDB]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health, the National Institute of Child Health & Development, or the National Institutes of Health. We thank Annahir Cariello, Celeste Knoles, Chad Ennis, Steven Hesse, Daniel Witt, Kristine McLaughlin, Emily Gerstein, and Joanna Mussey for their contributions to this project. We sincerely thank the children, adolescents, and adults with autism, the individuals with typical development, and all the families who participated in this study.
Footnotes
At the time of this study, Brittany G. Travers, Do P. M. Tromp, Nagesh Adluru, Dan Destiche, Danica Samsin, Andrew L. Alexander, and Janet E. Lainhart were at the Waisman Center at the University of Wisconsin-Madison (Madison, WI, USA). Erin D. Bigler and Tyler Duffield were at Brigham Young University (Provost, UT, USA). Nicholas Lange was at McLean Hospital, Harvard University (Boston, MA, USA). Alyson Froehlich and Molly D. B. Prigge were at the University of Utah (Salt Lake City, UT, USA).
References
- Alexander AL, Lee JE, Lazar M, Boudos R, DuBray MB, Oakes TR, et al. Diffusion tensor imaging of the corpus callosum in Autism. NeuroImage. 2007;34(1):61–73. doi: 10.1016/j.neuroimage.2006.08.032. doi:10.1016/j.neuroimage.2006.08.032. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR) 2000.
- Avants B, Gee JC. Geodesic estimation for large deformation anatomical shape averaging and interpolation. NeuroImage. 2004;23(Suppl 1):S139–50. doi: 10.1016/j.neuroimage.2004.07.010. doi:10.1016/j.neuroimage.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Baranek GT, David FJ, Poe MD, Stone WL, Watson LR. Sensory Experiences Questionnaire: discriminating sensory features in young children with autism, developmental delays, and typical development. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2006;47(6):591–601. doi: 10.1111/j.1469-7610.2005.01546.x. doi:10.1111/j.1469-7610.2005.01546.x. [DOI] [PubMed] [Google Scholar]
- Ben Bashat D, Kronfeld-Duenias V, Zachor DA, Ekstein PM, Hendler T, Tarrasch R, et al. Accelerated maturation of white matter in young children with autism: a high b value DWI study. NeuroImage. 2007;37(1):40–47. doi: 10.1016/j.neuroimage.2007.04.060. doi:10.1016/j.neuroimage.2007.04.060. [DOI] [PubMed] [Google Scholar]
- Ben-Sasson A, Hen L, Fluss R, Cermak SA, Engel-Yeger B, Gal E. A meta-analysis of sensory modulation symptoms in individuals with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2009;39(1):1–11. doi: 10.1007/s10803-008-0593-3. doi:10.1007/s10803-008-0593-3. [DOI] [PubMed] [Google Scholar]
- Bhat AN, Galloway JC, Landa RJ. Relation between early motor delay and later communication delay in infants at risk for autism. Infant Behavior & Development. 2012;35(4):838–846. doi: 10.1016/j.infbeh.2012.07.019. doi:10.1016/j.infbeh.2012.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat AN, Landa RJ, Galloway JC. Current perspectives on motor functioning in infants, children, and adults with autism spectrum disorders. Physical Therapy. 2011;91(7):1116–1129. doi: 10.2522/ptj.20100294. doi:10.2522/ptj.20100294. [DOI] [PubMed] [Google Scholar]
- Bloemen OJ, Deeley Q, Sundram F, Daly EM, Barker GJ, Jones DK, et al. White matter integrity in Asperger syndrome: a preliminary diffusion tensor magnetic resonance imaging study in adults. Autism Research. 2010;3(5):203–213. doi: 10.1002/aur.146. doi:10.1002/aur.146. [DOI] [PubMed] [Google Scholar]
- Brito AR, Vasconcelos MM, Domingues RC, Hygino da Cruz LC, Rodrigues Lde S, Gasparetto EL, Calcada CA. Diffusion tensor imaging findings in school-aged autistic children. Journal of Neuroimaging. 2009;19(4):337–343. doi: 10.1111/j.1552-6569.2009.00366.x. doi:10.1111/j.1552-6569.2009.00366.x. [DOI] [PubMed] [Google Scholar]
- Brown RE, Basheer R, McKenna JT, Strecker RE, McCarley RW. Control of sleep and wakefulness. Physiological Reviews. 2012;92(3):1087–1187. doi: 10.1152/physrev.00032.2011. doi:10.1152/physrev.00032.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang LC, Jones DK, Pierpaoli C. RESTORE: robust estimation of tensors by outlier rejection. Magnetic Resonance in Medicine. 2005;53(5):1088–1095. doi: 10.1002/mrm.20426. doi:10.1002/mrm.20426. [DOI] [PubMed] [Google Scholar]
- Cohen IL, Gardner JM, Karmel BZ, Phan HTT, Kittler P, Gomez TR, et al. Neonatal brainstem function and 4-month arousal-modulated attention are jointly associated with autism. Autism Research. 2013;6(1):11–22. doi: 10.1002/aur.1259. doi:10.1002/aur.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Conduit R, Lockley SW, Rajaratnam SM, Cornish KM. The relationship between sleep and behavior in autism spectrum disorder (ASD): a review. Journal of Neurodevelopmental Disorders. 2014;6(1):44. doi: 10.1186/1866-1955-6-44. doi:10.1186/1866-1955-6-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino J. The Social Responsiveness Scale. Western Psychological Services; Los Angeles, CA: 2002. [Google Scholar]
- Cook PA, Bai Y, Nedjati-Gilanio S, Seunarine KK, Hall MG, Parker GJ, Alexander DC. Camino: Open-Source Diffusion-MRI Reconstruction and Processing (Vol. Seattle, WA, p. 2759).. Presented at the International Society for Magnetic Resonance in Medicine.2006. [Google Scholar]
- Dawson G, Meltzoff AN, Osterling J, Rinaldi J, Brown E. Children with autism fail to orient to naturally occurring social stimuli. Journal of Autism and Developmental Disorders. 1998;28(6):479–485. doi: 10.1023/a:1026043926488. [DOI] [PubMed] [Google Scholar]
- Dowell LR, Mahone EM, Mostofsky SH. Associations of postural knowledge and basic motor skill with dyspraxia in autism: implication for abnormalities in distributed connectivity and motor learning. Neuropsychology. 2009;23(5):563–570. doi: 10.1037/a0015640. doi:10.1037/a0015640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffield TC, Trontel HG, Bigler ED, Froehlich A, Prigge MB, Travers B, et al. Neuropsychological investigation of motor impairments in autism. Journal of Clinical and Experimental Neuropsychology. 2013;35(8):867–881. doi: 10.1080/13803395.2013.827156. doi:10.1080/13803395.2013.827156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziuk MA, Gidley Larson JC, Apostu A, Mahone EM, Denckla MB, Mostofsky SH. Dyspraxia in autism: association with motor, social, and communicative deficits. Developmental medicine and child neurology. 2007;49(10):734–739. doi: 10.1111/j.1469-8749.2007.00734.x. doi:10.1111/j.1469-8749.2007.00734.x. [DOI] [PubMed] [Google Scholar]
- Elliott C. The Nature and Structure of Children's Abilities: Evidence from the Differential Ability Scales. Journal of Psychoeducational Assessment. 1990;8:376–390. [Google Scholar]
- Faria AV, Hoon A, Stashinko E, Li X, Jiang H, Mashayekh A, et al. Quantitative analysis of brain pathology based on MRI and brain atlases--applications for cerebral palsy. NeuroImage. 2011;54(3):1854–1861. doi: 10.1016/j.neuroimage.2010.09.061. doi:10.1016/j.neuroimage.2010.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein D, Skoff B, Mirsky AF. Clinical correlates of brainstem dysfunction in autistic children. Journal of Autism and Developmental Disorders. 1981;11(3):303–315. doi: 10.1007/BF01531513. [DOI] [PubMed] [Google Scholar]
- Fournier KA, Hass CJ, Naik SK, Lodha N, Cauraugh JH. Motor coordination in autism spectrum disorders: a synthesis and meta-analysis. Journal of Autism and Developmental Disorders. 2010;40(10):1227–1240. doi: 10.1007/s10803-010-0981-3. doi:10.1007/s10803-010-0981-3. [DOI] [PubMed] [Google Scholar]
- Gernsbacher MA, Sauer EA, Geye HM, Schweigert EK, Hill Goldsmith H. Infant and toddler oral- and manual-motor skills predict later speech fluency in autism. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2008;49(1):43–50. doi: 10.1111/j.1469-7610.2007.01820.x. doi:10.1111/j.1469-7610.2007.01820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardan AY, Kilpatrick M, Keshavan MS, Minshew NJ. Motor performance and anatomic magnetic resonance imaging (MRI) of the basal ganglia in autism. Journal of Child Neurology. 2003;18(5):317–324. doi: 10.1177/08830738030180050801. [DOI] [PubMed] [Google Scholar]
- Heaton R, Grant I, Matthews C. HRB Comprehensive Norms Computer Program. Psychological Assessment Resources, Inc; Odessa, Florida: 1991. [Google Scholar]
- Hilton C, Graver K, LaVesser P. Relationship between social competence and sensory processing in children with high functioning autism spectrum disorders. Research in Autism Spectrum Disorders. 2007;1(2):164–173. [Google Scholar]
- Hooper D, Coughlan J, Mullen M. Structural equation modelling: guidelines for determining model fit. Electronic Journal of Business Research Methods. 2008;6(1):53–60. [Google Scholar]
- Hornung J-P. The human raphe nuclei and the serotonergic system. Journal of Chemical Neuroanatomy. 2003;26(4):331–343. doi: 10.1016/j.jchemneu.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Hurley RA, Flashman LA, Chow TW, Taber KH. The brainstem: anatomy, assessment, and clinical syndromes. The Journal of Neuropsychiatry and Clinical Neurosciences. 2010;22(1):iv, 1–7. doi: 10.1176/jnp.2010.22.1.iv. doi:10.1176/appi.neuropsych.22.1.iv. [DOI] [PubMed] [Google Scholar]
- Jou RJ, Frazier TW, Keshavan MS, Minshew NJ, Hardan AY. A two-year longitudinal pilot MRI study of the brainstem in autism. Behavioural Brain Research. 2013;251:163–167. doi: 10.1016/j.bbr.2013.04.021. doi:10.1016/j.bbr.2013.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jou RJ, Minshew NJ, Melhem NM, Keshavan MS, Hardan AY. Brainstem volumetric alterations in children with autism. Psychological Medicine. 2009;39(8):1347–1354. doi: 10.1017/S0033291708004376. doi:10.1017/S0033291708004376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern JK, Geier DA, Adams JB, Troutman MR, Davis GA, King PG, Geier MR. Handgrip strength in autism spectrum disorder compared with controls. Journal of Strength and Conditioning Research. 2013;27(8):2277–2281. doi: 10.1519/JSC.0b013e31827de068. doi:10.1519/JSC.0b013e31827de068. [DOI] [PubMed] [Google Scholar]
- Kim S. ppcor: Partial and Semi-partial (Part) correlation. 2012 http://CRAN.R-project.org/package=ppcor.
- Leemans A, Jones DK. The B-matrix must be rotated when correcting for subject motion in DTI data. Magnetic Resonance in Medicine. 2009;61(6):1336–1349. doi: 10.1002/mrm.21890. doi:10.1002/mrm.21890. [DOI] [PubMed] [Google Scholar]
- Libertus K, Sheperd KA, Ross SW, Landa RJ. Limited fine motor and grasping skills in 6-month-old infants at high risk for autism. Child Development. 2014;85(6):2218–2231. doi: 10.1111/cdev.12262. doi:10.1111/cdev.12262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linkenauger SA, Lerner MD, Ramenzoni VC, Proffitt DR. A perceptual-motor deficit predicts social and communicative impairments in individuals with autism spectrum disorders. Autism Research. 2012;5(5):352–362. doi: 10.1002/aur.1248. doi:10.1002/aur.1248. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Leventhal BL, DiLavore PC, et al. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30(3):205–223. [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- MacDonald M, Lord C, Ulrich DA. The relationship of motor skills and social communicative skills in school-aged children with autism spectrum disorder. Adapted physical activity quarterly: APAQ. 2013;30(3):271–282. doi: 10.1123/apaq.30.3.271. [DOI] [PubMed] [Google Scholar]
- Mazurek MO, Petroski GF. Sleep problems in children with autism spectrum disorder: examining the contributions of sensory over-responsivity and anxiety. Sleep Medicine. 2014 doi: 10.1016/j.sleep.2014.11.006. doi:10.1016/j.sleep.2014.11.006. [DOI] [PubMed] [Google Scholar]
- Meltzoff AN. The “like me” framework for recognizing and becoming an intentional agent. Acta Psychologica. 2007;124(1):26–43. doi: 10.1016/j.actpsy.2006.09.005. doi:10.1016/j.actpsy.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming X, Brimacombe M, Wagner GC. Prevalence of motor impairment in autism spectrum disorders. Brain & Development. 2007;29(9):565–570. doi: 10.1016/j.braindev.2007.03.002. doi:10.1016/j.braindev.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Mori S, Wakana S, Nagae-Poetscher L, Van Zijl PC. MRI Atlas of Human White Matter. Elsevier; Amsterdam: 2005. [Google Scholar]
- Papadopoulos N, McGinley J, Tonge B, Bradshaw J, Saunders K, Murphy A, Rinehart N. Motor proficiency and emotional/behavioural disturbance in autism and Asperger's disorder: another piece of the neurological puzzle? Autism: the international journal of research and practice. 2012;16(6):627–640. doi: 10.1177/1362361311418692. doi:10.1177/1362361311418692. [DOI] [PubMed] [Google Scholar]
- Paus T. Functional anatomy of arousal and attention systems in the human brain. Progress in Brain Research. 2000;126:65–77. doi: 10.1016/S0079-6123(00)26007-X. doi:10.1016/S0079-6123(00)26007-X. [DOI] [PubMed] [Google Scholar]
- Provost B, Lopez BR, Heimerl S. A comparison of motor delays in young children: autism spectrum disorder, developmental delay, and developmental concerns. Journal of Autism and Developmental Disorders. 2007;37(2):321–328. doi: 10.1007/s10803-006-0170-6. doi:10.1007/s10803-006-0170-6. [DOI] [PubMed] [Google Scholar]
- Radonovich KJ, Fournier KA, Hass CJ. Relationship between postural control and restricted, repetitive behaviors in autism spectrum disorders. Frontiers in integrative neuroscience. 2013;7:28. doi: 10.3389/fnint.2013.00028. doi:10.3389/fnint.2013.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Core Team R. R: A Language and Environment for Statistical Computing. Vienna, Austria: 2013. http://www.R-project.org. [Google Scholar]
- Rosseel Y. lavaan: An R Package for Structural Equation Modeling. Journal of Statistical Software. 2012;48(2):1–36. [Google Scholar]
- Schulz R, Park C-H, Boudrias M-H, Gerloff C, Hummel FC, Ward NS. Assessing the integrity of corticospinal pathways from primary and secondary cortical motor areas after stroke. Stroke; a journal of cerebral circulation. 2012;43(8):2248–2251. doi: 10.1161/STROKEAHA.112.662619. doi:10.1161/STROKEAHA.112.662619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla DK, Keehn B, Muller RA. Tract-specific analyses of diffusion tensor imaging show widespread white matter compromise in autism spectrum disorder. Journal of child psychology and psychiatry, and allied disciplines. 2011;52(3):286–295. doi: 10.1111/j.1469-7610.2010.02342.x. doi:10.1111/j.1469-7610.2010.02342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend J, Harris NS, Courchesne E. Visual attention abnormalities in autism: delayed orienting to location. Journal of the International Neuropsychological Society: JINS. 1996;2(6):541–550. doi: 10.1017/s1355617700001715. [DOI] [PubMed] [Google Scholar]
- Travers BG, Adluru N, Ennis C, Tromp do PM, Destiche D, Doran S, et al. Diffusion tensor imaging in autism spectrum disorder: a review. Autism Research. 2012;5(5):289–313. doi: 10.1002/aur.1243. doi:10.1002/aur.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers BG, Powell PS, Klinger LG, Klinger MR. Motor difficulties in autism spectrum disorder: linking symptom severity and postural stability. Journal of autism and developmental disorders. 2013;43(7):1568–1583. doi: 10.1007/s10803-012-1702-x. doi:10.1007/s10803-012-1702-x. [DOI] [PubMed] [Google Scholar]
- Wechsler D. The Wechsler intelligence scale for children—third edition. The Psychological Corporation; San Antonio: 1991. [Google Scholar]
- Wechsler D. Wechsler Adult Iintelligence Scale-III (WAIS-III) Psychological Corporation; San Antonio: 1997. [Google Scholar]
- Yendiki A, Koldewyn K, Kakunoori S, Kanwisher N, Fischl B. Spurious group differences due to head motion in a diffusion MRI study. NeuroImage. 2013;88C:79–90. doi: 10.1016/j.neuroimage.2013.11.027. doi:10.1016/j.neuroimage.2013.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Yushkevich PA, Alexander DC, Gee JC. Deformable registration of diffusion tensor MR images with explicit orientation optimization. Medical image analysis. 2006;10(5):764–785. doi: 10.1016/j.media.2006.06.004. doi:10.1016/j.media.2006.06.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.