Abstract
Sensitivity to the interoceptive effects of alcohol is blunted following a period of exposure to the stress hormone corticosterone (CORT), an effect that is suggested to be related, in part, to glutamatergic neuroadaptations. Group II metabotropic glutamate receptors (subtypes 2 and 3; mGluR2/3) modulate several drug- and alcohol-related behaviors, including the interoceptive (discriminative stimulus) effects of alcohol. Therefore, we sought to determine if manipulation of mGluR2/3 would restore sensitivity to the interoceptive effects of alcohol following CORT exposure. Using a two-lever drug discrimination task, male Long-Evans rats were trained to discriminate alcohol (1 g/kg, intragastric [IG]) vs. water. First, the effect of mGluR2/3 antagonism on the discriminative stimulus effects of alcohol was determined using LY341495 (0.3–3.0 mg/kg; intraperitoneal [IP]). Next, the effects of mGluR2/3 antagonism and activation were assessed in discrimination-trained animals exposed to CORT (300 μg/mL) in the home cage drinking water or water only, for 7 days. Following CORT exposure, decreased sensitivity to alcohol (1 g/kg) was observed. Pretreatment with the mGluR2/3 agonist LY379268 (1.0–3.0 mg/kg; IP), but not the mGluR2/3 antagonist (0.3–1.0 mg/kg; IP), restored sensitivity to alcohol. Additionally, in Water controls, mGluR2/3 antagonism and mGluR2/3 activation disrupted expression of the discriminative stimulus effects of alcohol. Together, these findings suggest that blunted sensitivity to the interoceptive effects of alcohol following an episode of heightened stress hormone levels may be due to adaptations in mGluR2/3-related systems. The ability of mGluR2/3 activation to restore sensitivity to alcohol under these conditions lends further support for the importance of these receptors under stress-related conditions.
Keywords: corticosterone, mGluR2/3, LY379268, LY341495, drinking, stress
Introduction
There is growing literature showing that Group II metabotropic glutamate receptors (subtypes 2 and 3 – mGluR2/3) may have therapeutic potential in reducing various drug-related behaviors. For example, pharmacological activation of mGluR2/3 has been shown to decrease alcohol, heroin, and psychostimulant self-administration and decrease reinstatement of drug-seeking behavior (Adewale, Platt, & Spealman, 2006; Bossert, Busch, & Gray, 2005; Kufahl et al., 2013; Martin-Fardon & Weiss, 2012), while having little to no effect (at higher doses) on non-drug reinforcers (Baptista, Martin-Fardon, & Weiss, 2004; Liechti, Lhuillier, Kaupmann, & Markou, 2007; Peters & Kalivas, 2006). Of particular interest to the present work are findings that pharmacological activation of mGluR2/3 is particularly effective at reducing stress-related behaviors, including relapse to alcohol/drug seeking and drug withdrawal (Griffin, Haun, Hazelbaker, Ramachandra, & Becker, 2014; Koltunowska, Gibula-Bruzda, & Kotlinska, 2013; Kufahl, Martin-Fardon, & Weiss, 2011; Martin-Fardon & Weiss, 2012; Sidhpura, Weiss, & Martin-Fardon, 2010; Zhao et al., 2006), an effect specific to drug reinforcers (Aujla, Martin-Fardon, & Weiss, 2008; Zhao et al., 2006), suggesting that mGluR2/3 may be prime pharmacological targets under stress-related conditions.
mGluR2/3 are perisynaptic Gi-coupled receptors, and their presence on glutamatergic and non-glutamatergic synapses suggests a circuit-dependent role for mGluR2/3 in modulating general excitation (Anwyl, 1999; Cartmell & Schoepp, 2000; Schoepp, 2001). Activation of the predominately pre-terminal mGluR2/3 occurs under conditions of enhanced glutamate release, thereby providing a regulatory role for these receptors in decreasing excess synaptic availability of glutamate and decreasing hyperexcitability (Cartmell & Schoepp, 2000; Conn & Pin, 1997; Forsythe & Barnes-Davies, 1997; Scanziani, Salin, Vogt, Malenka, & Nicoll, 1997; Schoepp, 2001). Therefore, because exposure to stress or exogenous administration of the stress hormone corticosterone (CORT) can enhance extracellular levels of glutamate (Karst et al., 2005; Venero & Borrell, 1999), and can enhance the demonstrated ability of mGluR2/3 agonists to decrease stress-induced behaviors, these findings suggest that agonist efficacy is likely related to dampening excitatory neurotransmission.
Interestingly, mGluR2/3 have been shown to modulate sensitivity to the interoceptive effects of alcohol (Cannady, Grondin, Fisher, Hodge, & Besheer, 2011) and some hallucinogens (Carbonaro et al., 2015; Winter, Eckler, & Rabin, 2004). This is highly relevant given that interoceptive drug cues can impact drug-taking, seeking, and relapse-like behaviors (Banks, Czoty, & Nader, 2007; Mihindou, Vouillac, Koob, & Ahmed, 2011; Paulus & Stewart, 2014; Schenk & Partridge, 1999; Stolerman, 1992; Verdejo-Garcia, Clark, & Dunn, 2012; Wise, Wang, & You, 2008). Discriminative stimulus (interoceptive) effects of drugs are commonly indexed using drug discrimination procedures in both humans and animals. Using these procedures, we have demonstrated that following a 7-day period of exposure to the stress hormone CORT, rats show decreased sensitivity to the discriminative stimulus effects of both experimenter- and self-administered alcohol (e.g., decreased alcohol-appropriate responses; Besheer, Fisher, Grondin, Cannady, & Hodge, 2012; Besheer, Fisher, Jaramillo, Frisbee, & Cannady, 2014). Additionally, these CORT-induced behavioral adaptations are suggested to be related, in part, to glutamatergic neuroadaptations, and functional involvement of the Group I metabotropic glutamate receptor subtype 5 (mGluR5) has been determined (Besheer et al., 2014). Together, these findings have led to the suggestion that decreased sensitivity to alcohol following CORT exposure may be related to an underlying hyperglutamatergic state, as observed in other studies (Joëls, Sarabdjitsingh, & Karst, 2012). Therefore, given that mGluR2/3 activation functionally reduces presynaptic glutamate release and dampens hyperglutamatergic tone (Anwyl, 1999; Mateo & Porter, 2007; Pinheiro & Mulle, 2008; Schoepp, 2001), we sought to investigate the functional role of mGluR2/3 in modulating sensitivity to the discriminative stimulus effects of alcohol following a period of CORT exposure using both an mGluR2/3 antagonist and agonist. Additionally, we sought to characterize the role of mGluR2/3 antagonism on modulating sensitivity to alcohol under control conditions, as this has not been previously determined. To address these goals, male Long-Evans rats were trained using a standard and well-characterized two-lever drug discrimination task to discriminate the effects of a moderate alcohol dose (1 g/kg, IG) vs. water. Previous work has shown that in the absence of CORT treatment, pharmacological activation of mGluR2/3 blunts the expression of the interoceptive effects of alcohol (Cannady et al., 2011). However, considering the ability of mGluR2/3 activation to reduce hyperglutamatergic conditions and that decreased sensitivity to alcohol following CORT exposure may be related to a hyperglutamatergic state, we hypothesized that following CORT exposure mGluR2/3 activation would restore sensitivity to alcohol. Conversely, we hypothesized that mGluR2/3 antagonism would exacerbate the CORT-induced decreased sensitivity to alcohol (i.e., potentiate a CORT exposure-induced hyperglutamatergic tone).
Methods
Animals
Individually housed male Long-Evans rats (Harlan Sprague-Dawley, Indianapolis, IN) were used in these studies. Food intake was restricted to maintain body weight between 325–340 g for all experiments. The colony room was maintained on a 12-h light/dark cycle and experiments were conducted during the light cycle. Animals were under continuous care and monitoring by veterinary staff from the Division of Laboratory Animal Medicine at UNC-Chapel Hill. All procedures were conducted in accordance with the NIH Guide to Care and Use of Laboratory Animals and institutional guidelines. Different rats were used for Experiments 1–4; however, as described below, several strategies were taken throughout this work, to reduce the number of animals needed for the conduct of the studies.
Apparatus
Behavioral chambers (Med Associates, Georgia, VT) measuring 31 × 32 × 24 cm were located in sound-attenuating cubicles equipped with an exhaust fan that provided both ventilation and masking of external sounds. The right wall of the chamber contained a liquid dipper receptacle, two retractable response levers, and stimulus lights (mounted above each lever). Lever press responses activated a dipper mechanism that presented 0.1 mL of a 10% sucrose solution for 4 sec. All chambers were interfaced (Med Associates) to a computer programmed to control sessions and record lever responses and locomotor data.
Alcohol Discrimination
Training
Daily training sessions (Monday–Friday) were conducted as we previously described (Besheer et al., 2012, 2014; Cannady et al., 2011). Briefly, alcohol (1 g/kg) or water was administered IG and immediately afterward, rats were placed in the chambers. Following a 10-min delay, both levers were introduced into the chamber and the house light was illuminated, signaling commencement of the 15-min session. Training days varied on a double alternation schedule (alcohol, alcohol, water, water, …). During an alcohol session, completion of a fixed ratio 10 (FR10) on the alcohol-appropriate lever (e.g., left lever) resulted in sucrose delivery. That is, after 10 total responses on that lever (not necessarily consecutive), sucrose was delivered. Alternatively, during a water session, completion of an FR10 on the water-appropriate lever (e.g., right lever) resulted in the delivery of sucrose. The following accuracy criteria were met prior to testing: the percentage of appropriate lever responses before the first reinforcer, and during the entire session was >80% for at least 8 out of 10 consecutive days.
Testing
Test sessions were similar to training sessions except that they were 2 min in duration (after 10-min delay), and an FR10 on either lever resulted in sucrose delivery. Sucrose reinforcement was delivered to examine the effects of treatments on overall response rates (measure of nonspecific motor effects). To confirm alcohol stimulus control, a baseline cumulative alcohol curve (0.1, 0.3, 1.0, 1.7 g/kg) was determined for all rats before testing of LY341495 (Experiment 1) and prior to CORT/Water exposure (Experiment 2, 3, and 4) as described in Besheer et al., 2012, 2014. For Experiment 1, test sessions were interspersed with training sessions and only occurred when performance during 3 of 4 previous training sessions met accuracy criteria and no more than two test sessions were conducted per week.
Home cage CORT / Water exposure
For 7 days (24-h daily access), rats were given one bottle (1-mL graduates) fitted with a ball-bearing stopper containing CORT (300 μg/mL) or water as the sole available fluid. CORT concentration was selected based on our previous work (Besheer et al., 2012, 2014; Besheer, Fisher, Lindsay, & Cannady, 2013). Fluid was measured and changed daily and rats were weighed daily. In our laboratory, this method of CORT exposure results in heightened plasma corticosterone levels (~400 ng/mL, which is approximately a two-fold increase relative to Water controls during the dark cycle; see characterization in Besheer et al., 2012). As a general comparison, while consideration must be made for duration of stressor exposure and time after stress exposure, typically, in male rats, CORT levels following restraint or swim-stress exposure can be within the 300–800 ng/mL range (Duncan, Knapp, Carson, & Breese, 1998; Kalil, Leite, Carvalho-Lima, & Anselmo-Franci, 2013; McKlveen et al., 2013). Given that we have previously characterized CORT levels using this method (Besheer et al., 2012, 2013) and in an effort not to disturb ongoing performance, plasma CORT analyses were not conducted in this work. Discrimination training was withheld during the CORT/Water exposure and rats remained in the home cage for the 7-day duration (Besheer et al., 2012, 2014).
Experimental Procedures
Experiment 1: Effect of mGluR2/3 antagonism on sensitivity to alcohol
Previous work from our laboratory has examined the effects of mGluR2/3 activation on sensitivity to the discriminative stimulus effects of alcohol (Cannady et al., 2011); however, the effects of mGluR2/3 antagonism on sensitivity to the discriminative stimulus effects of alcohol have not been previously assessed. Therefore, to characterize the effects of the mGluR2/3 antagonist and to determine an appropriate dose range for Experiment 3, rats were administered LY341495 (0.3, 1, 3 mg/kg, IP; n = 9/dose) 30 min before alcohol (1 g/kg, IG), after which rats underwent a test session.
Experiment 2: Sensitivity to alcohol following CORT
In previous studies, we have demonstrated decreased sensitivity to alcohol following CORT exposure using the same protocol as used in the present work (Besheer et al., 2012, 2014). Therefore, in an effort to reduce the number of rats required for the completion of these studies, the goal of this experiment was to generate the vehicle control groups that would be used for Experiments 3 and 4. These experiments were conducted alongside Experiments 3 and 4. As such, these vehicle controls would serve to replicate our previous findings of decreased sensitivity to alcohol following CORT exposure. Following the baseline cumulative alcohol curve (0.1, 0.3, 1.0, 1.7 g/kg, IG), all rats (n = 10) underwent 7 days of home-cage water exposure. Upon completion of Day 7, rats received a vehicle injection (saline, IP) 20 min before alcohol (1 g/kg, IG). Rats then underwent a test session, after which discrimination training resumed for at least 2 weeks. When testing criteria were met again, all rats underwent home cage CORT exposure (7 days). Upon completion of Day 7, rats received a vehicle injection prior to alcohol (1 g/kg, IG) and then underwent a test session.
Experiment 3: Effect of mGluR2/3 antagonism on sensitivity to alcohol following CORT exposure
Discrimination-trained rats began Water (n = 7–8) or CORT (n = 10) exposure after a baseline cumulative alcohol curve (0.1, 0.5, 1.0, 1.7 g/kg, IG) was determined. Upon completion of CORT/Water exposure (7 days), rats received the mGluR2/3 antagonist, LY341495 (0.3 or 1 mg/kg, IP), 20 min prior to alcohol (1 g/kg, IG) and were immediately placed in the chamber for the test session. In an effort to reduce the number of rats needed to complete this experiment and to test the efficacy of repeated testing for future studies, after the test, rats were returned to Water or CORT and on the following day, administered the other LY341495 dose, such that, rats in the Water and CORT groups were tested on both LY341495 doses. Therefore, half were tested on each dose each day.
Experiment 4: Effect of mGluR2/3 activation on sensitivity to alcohol following CORT
To determine the effects of mGluR2/3 activation on the discriminative stimulus effects of alcohol following CORT exposure, after the baseline cumulative alcohol curve (0.1, 0.5, 1.0, 1.7 g/kg, IG) was determined, rats underwent Water exposure (7 days). Upon completion of Day 7, rats received the mGluR2/3 agonist LY379268 (1 or 3 mg/kg, IP; n = 8/dose) 20 min prior to alcohol (1 g/kg, IG), after which rats were placed in the chamber for the test session. A similar within-subject design as used in Experiment 2 was used, such that after at least 2 weeks of intervening training sessions, all rats were exposed to CORT (7 days), the same testing protocol was used, and rats were administered the same dose of LY379268 that they had previously received.
Drugs
Alcohol (95%) was diluted in distilled water to a concentration of 20% (v/v) and administered IG, with volumes varied by weight to obtain the desired dose. Corticosterone hemisuccinate (4-pregnen-11β, 21-DIOL-3, 20-DIONE 21-hemisuccinate; Steraloids, Newport, RI) was dissolved in tap water by addition of NaOH and neutralized with HCl, to a final pH of 7.0–7.4 (Besheer et al., 2012, 2013; Gourley & Taylor, 2009). (1R,4R,5S,6R)-4-Amino-2-oxabicyclo[3.1.0]hexane-4,6-dicarboxylic acid disodium salt (LY379268; Tocris, Ellisville, Missouri), a highly selective (IC50~10 nM in the presence of LY341495; Imre, 2007) Group II (mGluR2/3) agonist, and (2S)-2-Amino-2-[(1S,2S)-2-carboxycycloprop-1-yl]-3-(xanth-9-yl) propanoic acid disodium salt (LY341495; Tocris, Ellisville, Missouri), a highly potent (Kd~0.8 nM) and selective Group II (mGluR2/3) antagonist, were dissolved in saline and injected at a volume of 1 mL/kg. The doses of the mGluR2/3 compounds were selected based on pilot studies in our lab and on previous work (Bäckstrom & Hyytiä, 2005; Cannady et al., 2011; Liechti et al., 2007; Monn et al., 1999; Sidhpura et al., 2010; Wright, Arnold, Wheeler, Ornstein, & Schoepp, 2001).
Data Analysis
Response accuracy was expressed as the percentage of alcohol-appropriate lever presses upon delivery of the first reinforcer. Response rate (responses/min) was analyzed for the entire session and served as an index of motor activity. If a rat did not complete an FR10 on the test, the rat was not included in the response accuracy measure, but was included in the response rate measure. In Experiment 1, a one-way repeated-measures ANOVA was used to analyze response accuracy and response rate. In Experiment 2, response accuracy and response rate were each compared by a paired t test. In Experiments 3 and 4, separate two-way repeated-measures ANOVAs (with one repeating factor – LY341495 dose in Experiment 3; exposure group in Experiment 4) were used to analyze response accuracy and response rate for the two doses of the mGluR2/3 compounds after CORT and Water exposure. Subsequently, to determine whether behavior differed from the vehicle controls, comparisons (t tests) were made between the drug dose and the respective (Water or CORT) vehicle condition. Tukey post hoc analyses were used to explore significant interactions. Complete expression of the discriminative stimulus effects of alcohol (i.e., full substitution) was defined as >80% choice of the alcohol lever upon completion of the first FR10 during test sessions. Significance was declared at p ≤ 0.05.
Results
For all Experiments (1–4), the baseline cumulative alcohol curve (prior to testing), and the average daily fluid consumption and CORT dose consumed for Experiments 2–4 are shown in Table 1.
Table 1.
Mean (± S.E.M.) baseline alcohol discrimination performance and daily consumption measures during CORT/Water exposure.
| Alcohol-appropriate Responses (%) Cumulative Alcohol Dose (g/kg, IG) |
Response Rate (resp/min) Cumulative Alcohol Dose (g/kg, IG) |
Daily fluid consumption (mL) |
Daily CORT dose (mg/kg) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||
| 0.1 | 0.3 | 1.0 | 1.7 | 0.1 | 0.3 | 1.0 | 1.7 | |||
| Exp 1 | ||||||||||
| 14.1±3.0 | 28.7±13.7 | 65.3±13.4 | 90.0±5.5 | 52.1±5.0 | 51.1±5.2 | 40.9±4.9 | 40.1±3.9 | n/a | n/a | |
| Exp 2 | ||||||||||
| Water | 8.0±2.4 | 29.2±9.9 | 79.5±5.9 | 97.3±1.4 | 72.8±7.2 | 64.1±4.3 | 58.6±3.5 | 46.4±4.3 | 20.4±1.8 | n/a |
| CORT | 7.7±2.7 | 23.0±9.0 | 62.3±12.3 | 96.2±1.4 | 68.1±5.6 | 57.6±5.5 | 58.9±5.9 | 53.6±4.2 | 23.4±2.4 | 21.5±2.3 |
| Exp 3 | ||||||||||
| Water | 21.0±10.0 | 47.6±14.7 | 77.0±12.0 | 93.1±4.5 | 55.2±6.6 | 53.9±6.0 | 51.1±4.0 | 42.3±2.3 | 15.1±0.7 | n/a |
| CORT | 21.9±7.3 | 37.4±14.4 | 75.9±12.4 | 98.3±1.7 | 53.1±3.7 | 45.8±2.3 | 83.8±12.9 | 43.6±3.2 | 16.4±0.7 | 15.3±0.6 |
| Exp 4 | ||||||||||
| Water | 6.0±3.0 | 13.8±7.4 | 75.6±9.0 | 93.8±6.3 | 51.8±4.0 | 53.3±3.5 | 52.0±1.6 | 48.3±1.5 | 25.2±1.0 | n/a |
| CORT | 10.8±5.7 | 8.4±7.7 | 74.9±11.9 | 91.6±7.7 | 49.5±3.8 | 47.1±3.5 | 52.2±2.1 | 48.0±2.5 | 23.4±0.8 | 20.7±0.7 |
|
|
||||||||||
Experiment 1: Effect of mGluR2/3 antagonist on sensitivity to alcohol
As shown in Fig. 1A, antagonism of mGluR2/3 by LY341495 dose-dependently reduced alcohol-appropriate responding. This was confirmed by a one-way repeated-measures ANOVA [F(3,21) = 5.16, p < 0.01]. Specifically, pretreatment with both the 1.0 and 3.0 mg/kg doses of LY341495 significantly decreased alcohol-appropriate responding relative to vehicle (p = 0.01; p = 0.03), suggesting decreased sensitivity to the discriminative stimulus effects of alcohol following mGluR2/3 antagonism. One rat did not complete an FR10 at the highest LY341495 dose and thus was not included in the response accuracy measure, but was included in the response-rate analysis. Additionally, a significant reduction in response rate was noted [F(3,22) = 3.16, p = 0.05; Fig. 1B]; however, Tukey post hoc analyses only showed a trend for a significant reduction at the highest LY341495 dose relative to vehicle (p = 0.06).
Fig. 1.
mGluR2/3 antagonism decreased sensitivity to the interoceptive effects of alcohol. (A) Pretreatment with the mGluR2/3 antagonist LY341495 prior to the alcohol-training dose (1 g/kg) decreased alcohol-appropriate responding. (B) Overall, LY341495 pretreatment significantly reduced response rate (responses/min), with a trend for a reduction at the highest dose (3 mg/kg, p = 0.06). Horizontal dashed line (>80%) represents full expression of the discriminative stimulus effects of alcohol. * signifies difference from vehicle (0). Values on graphs represent mean ± S.E.M.
Experiment 2: Sensitivity to alcohol following CORT
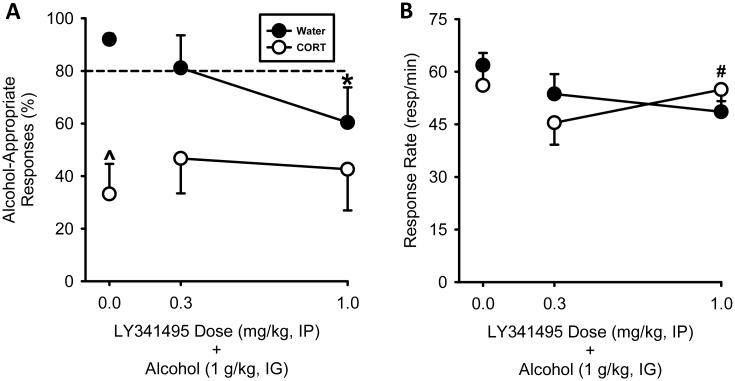
Following CORT exposure, a significant decrease in alcohol-appropriate responding was observed relative to Water exposure (t = 4.95, p = 0.001; illustrated to left of axis break in Fig. 2 & 3). There was no significant difference in response rate. This finding is consistent with our previous work and suggests decreased sensitivity to alcohol following CORT exposure (Besheer et al., 2012, 2014).
Fig. 2.
Following CORT exposure, decreased sensitivity to the interoceptive effects of alcohol emerges and is not altered by mGluR2/3 antagonism. (A) In the vehicle (0) controls (separate group of rats), CORT exposure (7 days) prevented the full expression of the discriminative stimulus effects of alcohol (1 g/kg). Pretreatment with the mGluR2/3 antagonist LY341495 (1.0 mg/kg) decreased the expression of the discriminative stimulus effects of alcohol in the Water group and did not exacerbate or restore sensitivity to alcohol in the CORT exposure (7 days). (B) In the vehicle (0) controls, CORT exposure (7 days) did not alter response rate. mGluR2/3 antagonism had mixed effects on response rate. A significant difference in response rate between the two LY341495 doses in the CORT group was evident. However, response rates did not differ from vehicle controls. Horizontal dashed line (>80%) represents full expression of the discriminative stimulus effects of alcohol. ^ signifies difference from Water in the Vehicle controls. * signifies difference from vehicle-Water group; # signifies difference from 0.3 mg/kg LY341495-CORT group. Values on graphs represent mean ± S.E.M.
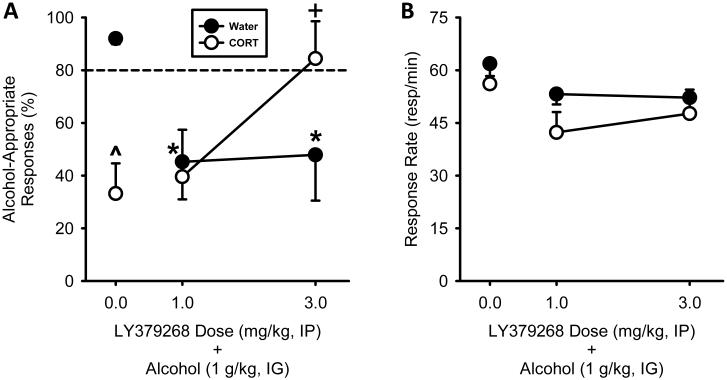
Fig. 3.
Following CORT exposure, sensitivity to the interoceptive effects of alcohol is restored by mGluR2/3 activation. (A) CORT exposure (7 days) prevented the full expression of the discriminative stimulus effects of alcohol (1 g/kg) following vehicle (0) pretreatment (separate group of rats). Pretreatment with the mGluR2/3 agonist, LY379268 (1 and 3 mg/kg, IP), decreased alcohol-appropriate responding in the Water group. Following CORT exposure, mGluR2/3 activation (3 mg/kg LY379268) restored expression of the discriminative stimulus effects of alcohol. (B) CORT exposure (7 days) did not alter response rate. LY379268 had no effect on response rate. Horizontal dashed line (>80%) represents full expression of the discriminative stimulus effects of alcohol. >^ signifies difference from Water in the Vehicle controls. * signifies difference from vehicle-Water group; + signifies difference from vehicle-CORT group. Values on graphs represent mean ± S.E.M.
Experiment 3: Effect of mGluR2/3 antagonism on sensitivity to alcohol following CORT exposure
Given that this experiment involved testing of the antagonist across two days/tests (i.e., half the rats tested at 0.3 mg/kg on the first day, and then tested at 1.0 mg/kg on the second day), we compared performance on the first and second test for the Water and CORT groups separately. No differences in alcohol-appropriate responses or response rate were observed, indicating the absence of testing order effects and supporting efficacy of the testing procedures.
Analysis of the effect of the mGluR2/3 antagonist on alcohol-appropriate responses did not show an exposure-group or treatment effect (Fig. 2A). However, when compared to the vehicle conditions, differences in discrimination performance were observed. In the Water group, LY341495 (1 mg/kg) significantly reduced alcohol-appropriate responding relative to vehicle-Water group (t = 2.62, p < 0.02), whereas 0.3 mg/kg did not differ from vehicle-Water group. This data pattern is consistent with the findings of Experiment 1, in which the 1 mg/kg, but not the 0.3 mg/kg, LY341495 dose significantly reduced alcohol-appropriate responses (Fig. 1). In the CORT group, both doses of LY341495 did not differ from vehicle-CORT group, indicating the persistence of decreased sensitivity to alcohol. In relation to response rate, a significant interaction between exposure group and LY341495 dose was observed [F(1,15) = 7.59, p < 0.02; Fig. 2B]. This interaction was driven by a significant difference in the CORT group between the two LY341495 doses (p < 0.02). While response rates did not differ significantly from vehicle, there was a trend for a reduction at the highest LY341495 dose (1 mg/kg) in the Water group (p = 0.06). Together, these findings show that mGluR2/3 antagonism did not restore sensitivity or exacerbate decreased sensitivity to the discriminative stimulus effects of alcohol following CORT exposure.
Experiment 4: Effect of mGluR2/3 activation on sensitivity to alcohol following CORT
The two-way ANOVA showed no significant main effect of LY379268 dose or exposure on discrimination performance (Fig. 3A). However, differences from the vehicle controls were observed. In the Water group, both LY379268 doses significantly reduced alcohol-appropriate responses relative to vehicle-Water group (1 mg/kg: t = 3.64, p < 0.01; 3 mg/kg: t = 2.83, p = 0.01). This data pattern is consistent with previous work showing decreased alcohol-appropriate responses following LY379268 pretreatment in the absence of CORT treatment (Cannady et al., 2011). In the CORT group, the lower dose (1 mg/kg) of LY379268 did not differ from the CORT-vehicle, indicating the persistence of blunted sensitivity to alcohol. However, pretreatment with the highest LY379268 dose (3 mg/kg) significantly increased alcohol-appropriate responding relative to vehicle-CORT group (t = 2.84, p = 0.01), indicating restored sensitivity to alcohol. No significant effects on response rates were observed (Fig. 3B); however, trends for a reduction at the 1 mg/kg LY379268 dose were observed relative to vehicle following both Water and CORT (p = 0.08). These findings show that mGluR2/3 activation can effectively restore sensitivity to alcohol following CORT exposure.
Discussion
The present study characterizes the functional role of mGluR2/3 antagonism and activation on sensitivity to the interoceptive effects of a moderate alcohol dose (1 g/kg) following a period of chronic exposure to the stress hormone CORT. Consistent with previous work, we show that sensitivity to the interoceptive effects of alcohol is blunted following a period of CORT exposure (Besheer et al., 2012, 2014). Importantly, we show that sensitivity to alcohol can be restored following pharmacological activation of mGluR2/3, whereas pharmacological antagonism of mGluR2/3 does not exacerbate the blunted response to alcohol or restore sensitivity to alcohol. Interestingly, under control conditions, pharmacological activation and antagonism of mGluR2/3 similarly reduced the discriminative stimulus function of the alcohol-training dose (1 g/kg; see later discussion). Together, these findings suggest that reduced sensitivity to the interoceptive effects of alcohol following an episode of heightened stress hormone levels may be related to adaptations in mGluR2/3-related systems.
Studies have identified various neuroadaptations in response to stress and/or CORT exposure. Within these studies, evidence for changes in dendritic and synaptic morphology and plasticity suggest a central role for glutamate (Elizalde et al., 2010; Joëls et al., 2012; McEwen, 1999, 2007; Popoli, Yan, McEwen, & Sanacora, 2012; Treccani et al., 2014). Further, the same CORT exposure protocol as used in the present study induces glutamatergic adaptations, as evidenced by loss of the predominantly postsynaptic mGluR5 in the ventral striatum (Besheer et al., 2014). Together, these findings have led to the suggestion that decreased sensitivity to alcohol following CORT exposure may be related to an underlying hyperglutamatergic state, as observed in other studies (Joëls et al., 2012). Therefore, the present findings showing that sensitivity to alcohol is restored following mGluR2/3 activation is consistent with this hypothesis, given that mGluR2/3 activation functionally reduces presynaptic glutamate release and dampens hyperglutamatergic tone (Anwyl, 1999; Mateo & Porter, 2007; Pinheiro & Mulle, 2008; Schoepp, 2001). Conversely, the mGluR2/3 antagonist would be predicted to exacerbate the CORT-induced decreased sensitivity to alcohol (i.e., potentiate a CORT exposure-induced hyperglutamatergic tone). This data pattern did not occur, as behavior in the CORT group was not altered following antagonist pretreatment (i.e., remained at approximately 40% – Fig. 2A). However, it may be difficult to observe potentiation of decreased sensitivity using a two-choice discrimination task (e.g., alcohol vs. water). This limitation may be addressed by the utilization of a three-choice (e.g., alcohol, water, lysergic acid diethylamide-LSD) drug discrimination procedure (Grant, 1999), that could behaviorally capture a qualitative shift from the stimulus being less alcohol-like, and more like another stimulus (LSD, for example). Additionally, given the complexity of the alcohol discriminative stimulus (e.g., contribution of various transmitter systems: Helms, McCracken, Heichman, & Moschak, 2013; Hodge & Cox, 1998; Kostowski & Bieńkowski, 1999; Platt & Bano, 2011) it is unlikely that manipulation of one system (glutamate) would completely block expression of the interoceptive effects of alcohol, as we had predicted.
Another critical aspect of the present work is the finding that the mGluR2/3 agonist had differential effects on discrimination behavior following Water and CORT exposure, whereas the mGluR2/3 antagonist did not. That is, following Water exposure, mGluR2/3 agonist pretreatment disrupted the expression of sensitivity to alcohol (i.e., decreased alcohol-appropriate responding; see later). However, following CORT exposure, mGluR2/3 agonist pretreatment restored sensitivity to alcohol (i.e., full substitution for the alcohol-training dose). This data pattern suggests that the receptor mechanisms that modulate the discriminative stimulus effects of alcohol change following CORT exposure. Consistent with this suggestion are our previous findings in which in the absence of CORT, the mGluR5-positive allosteric modulator CDPPB had little effect on alcohol discriminative stimulus effects; however, following CORT exposure, CDPPB pretreatment enhanced sensitivity to alcohol (Besheer et al., 2014). An important consideration is that a limited dose range of the mGluR2/3 agonist was tested. Even though a majority of the rats showed restored sensitivity to alcohol at the highest LY379268 dose tested (7 out of 8 showed >80% alcohol-appropriate responding), it will be important to examine whether this data pattern would also emerge with a higher dose range of the mGluR2/3 agonist. In contrast, following antagonist pretreatment, sensitivity to alcohol was not restored at any dose post-CORT exposure. To this end, an important consideration to address is that increasing the mGluR2/3 antagonist dose range may have resulted in restored sensitivity to alcohol. However, given the significant overall reduction in response rate in Experiment 1 following mGluR2/3 antagonist pretreatment (highest dose – 3 mg/kg), a lower dose range (0.3 – 1 mg/kg) was tested in Experiment 3 so that behavioral changes would not be confounded by antagonist-induced response rate reductions.
The findings of different effects of the mGluR2/3 agonist, depending on CORT exposure history, are consistent with literature showing differential effects of the mGluR2/3 agonist under basal/control conditions and conditions that challenge the glutamatergic system or induce an underlying hyperglutamatergic state (Aujla et al., 2008; Griffin et al., 2014; Hao, Martin-Fardon, & Weiss, 2010; Kufahl et al., 2011, 2013; Martin-Fardon & Weiss, 2012; Sidhpura et al., 2010; Zhao et al., 2006). For example, mGluR2/3 activation is more effective at reducing alcohol self-administration and stress-induced reinstatement in animals with a history of alcohol dependence relative to non-dependent animals (Griffin et al., 2014; Kufahl et al., 2013; Sidhpura et al., 2010). Additionally, this effect is accompanied by increased mGluR2/3 functional activity in brain regions associated with stress and drug reinforcement (Kufahl et al., 2011). It will be interesting for future studies to examine expression patterns of mGluR2/3 and assess glutamatergic synaptic activity to directly determine whether adaptations in this system are present following prolonged CORT exposure. Further, given the role of intra-amygdala mGluR2/3 in modulating the interoceptive effects of alcohol (Cannady et al., 2011), it will be of interest to investigate a possible role of this brain region in restoring sensitivity to alcohol following CORT. While future work will be needed, together with the existing literature, the present behavioral findings lend further support for the suggestion of a hyperglutamatergic system because of CORT exposure.
Interestingly, under Water control conditions, pretreatment with the mGluR2/3 agonist and antagonist prior to alcohol had similar behavioral consequences (i.e., decreased alcohol-appropriate responding), which could suggest that the mGluR2/3 compounds had non-specific effects on the discrimination behavior. The agonist effect (Water controls in Experiment 4) is consistent with previous work (Cannady et al., 2011); however, this is the first study to demonstrate that antagonism of mGluR2/3 by LY341495 also disrupts interoceptive sensitivity to alcohol (Experiment 1 and Water controls in Experiment 3). Importantly, when interpreting a decrease in alcohol-appropriate responding in a two-lever discrimination task, the change in accuracy performance can be interpreted as decreased sensitivity to alcohol, or alternatively that the compound is having unique interoceptive effects of its own that overshadow the discriminative control of alcohol (Green & Grant, 1998). Although no previous research to our knowledge has characterized the interoceptive effects of LY341495 or LY379268 alone, mGluR2/3 have been implicated in modulating the interoceptive effects of hallucinogens. For example, the discriminative stimulus effects of LSD, N,N-dimethyltryptamine (DMT), and N,N-diisopropyltryptamine (DiPT) are potentiated by mGluR2/3 antagonism (Carbonaro et al., 2015; Winter et al., 2004). On the other hand, mGluR2/3 activation disrupts the discrimination of LSD and phencyclidine (PCP; Carbonaro et al., 2015; Winter et al., 2004). Therefore, a possible explanation for the similar behavioral effects of the agonist and the antagonist (rather than general non-specific effects) is that the agonist, the antagonist, or both may produce interoceptive effects of their own that interfere with the expression of alcohol discriminative control. To this end, it is interesting that pretreatment with the mGluR2/3 agonist is able to restore sensitivity to alcohol following CORT exposure. One explanation is that the discriminative stimulus effects of the mGluR2/3 agonist become alcohol-like following CORT exposure. Indeed, it is plausible that under conditions of a hyperglutamatergic state (as proposed following CORT), the discriminative stimulus profile of an mGluR2/3 agonist (which functionally dampens the hyperglutamatergic state) may be different from those under non-CORT conditions. Indeed, future work could directly address this possibility by assessing the substitution profile of the mGluR2/3 agonist and antagonist following CORT exposure in the absence of alcohol.
Conclusion
Clearly, it is experimentally difficult to determine the direct and specific contribution of the interoceptive effects of alcohol on alcohol drinking in preclinical models. However, altered sensitivity to the subjective/interoceptive effects of alcohol may be a possible behavioral mechanism for escalated alcohol drinking during episodes of heightened elevations in glucocorticoid levels, such as stress (Besheer et al., 2012, 2013, 2014; Childs, O'Connor, & de Wit, 2011). That is, during these episodes, an individual may consume more alcohol to achieve the desired interoceptive effects. Therefore, the present findings demonstrating the ability of mGluR2/3 activation to restore sensitivity to alcohol and further replication of these findings could suggest that mGluR2/3 may have a functional behavioral role in reducing stress-induced drinking and also may have functional relevance for populations that show reduced sensitivity to alcohol (e.g., individuals with a family history of an alcohol-use disorder), who are at higher risk for developing alcohol-use disorders (Schuckit, 2009; Schuckit & Smith, 2000; Trim, Schuckit, & Smith, 2009). Further, the selective functionality of mGluR2/3 activation to restore sensitivity to alcohol following an episode of heightened stress hormone levels, lends further support for the role of these receptors under such conditions (e.g., stress).
Acknowledgments
This work was supported, in part, by funds from the National Institutes of Health AA019682 (JB) and the Bowles Center for Alcohol Studies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adewale AS, Platt DM, Spealman RD. Pharmacological stimulation of group ii metabotropic glutamate receptors reduces cocaine self-administration and cocaine-induced reinstatement of drug seeking in squirrel monkeys. The Journal of Pharmacology and Experimental Therapeutics. 2006;318:922–931. doi: 10.1124/jpet.106.105387. [DOI] [PubMed] [Google Scholar]
- Anwyl R. Metabotropic glutamate receptors: electrophysiological properties and role in plasticity. Brain Research. Brain Research Reviews. 1999;29:83–120. doi: 10.1016/s0165-0173(98)00050-2. [DOI] [PubMed] [Google Scholar]
- Aujla H, Martin-Fardon R, Weiss F. Rats with extended access to cocaine exhibit increased stress reactivity and sensitivity to the anxiolytic-like effects of the mGluR 2/3 agonist LY379268 during abstinence. Neuropsychopharmacology. 2008;33:1818–1826. doi: 10.1038/sj.npp.1301588. [DOI] [PubMed] [Google Scholar]
- Bäckström P, Hyytiä P. Suppression of alcohol self-administration and cue-induced reinstatement of alcohol seeking by the mGlu2/3 receptor agonist LY379268 and the mGlu8 receptor agonist (S)-3,4-DCPG. European Journal of Pharmacology. 2005;528:110–118. doi: 10.1016/j.ejphar.2005.10.051. [DOI] [PubMed] [Google Scholar]
- Banks ML, Czoty PW, Nader MA. The influence of reinforcing effects of cocaine on cocaine-induced increases in extinguished responding in cynomolgus monkeys. Psychopharmacology (Berl) 2007;192:449–456. doi: 10.1007/s00213-007-0732-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baptista MA, Martin-Fardon R, Weiss F. Preferential effects of the metabotropic glutamate 2/3 receptor agonist LY379268 on conditioned reinstatement versus primary reinforcement: comparison between cocaine and a potent conventional reinforcer. The Journal of Neuroscience. 2004;24:4723–4727. doi: 10.1523/JNEUROSCI.0176-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besheer J, Fisher KR, Grondin JJ, Cannady R, Hodge CW. The effects of repeated corticosterone exposure on the interoceptive effects of alcohol in rats. Psychopharmacology (Berl) 2012;220:809–822. doi: 10.1007/s00213-011-2533-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besheer J, Fisher KR, Jaramillo AA, Frisbee S, Cannady R. Stress hormone exposure reduces mGluR5 expression in the nucleus accumbens: functional implications for interoceptive sensitivity to alcohol. Neuropsychopharmacology. 2014;39:2376–2386. doi: 10.1038/npp.2014.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besheer J, Fisher KR, Lindsay TG, Cannady R. Transient increase in alcohol self-administration following a period of chronic exposure to corticosterone. Neuropharmacology. 2013;72:139–147. doi: 10.1016/j.neuropharm.2013.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Busch RF, Gray SM. The novel mGluR2/3 agonist LY379268 attenuates cue-induced reinstatement of heroin seeking. Neuroreport. 2005;16:1013–1016. doi: 10.1097/00001756-200506210-00026. [DOI] [PubMed] [Google Scholar]
- Cannady R, Grondin JJ, Fisher KR, Hodge CW, Besheer J. Activation of group II metabotropic glutamate receptors inhibits the discriminative stimulus effects of alcohol via selective activity within the amygdala. Neuropsychopharmacology. 2011;36:2328–2338. doi: 10.1038/npp.2011.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbonaro TM, Eshleman AJ, Forster MJ, Cheng K, Rice KC, Gatch MB. The role of 5-HT2A, 5-HT 2C and mGlu2 receptors in the behavioral effects of tryptamine hallucinogens N,N-dimethyltryptamine and N,N-diisopropyltryptamine in rats and mice. Psychopharmacology (Berl) 2015;232:275–284. doi: 10.1007/s00213-014-3658-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartmell J, Schoepp DD. Regulation of neurotransmitter release by metabotropic glutamate receptors. Journal of Neurochemistry. 2000;75:889–907. doi: 10.1046/j.1471-4159.2000.0750889.x. [DOI] [PubMed] [Google Scholar]
- Childs E, O'Connor S, de Wit H. Bidirectional interactions between acute psychosocial stress and acute intravenous alcohol in healthy men. Alcoholism: Clinical and Experimental Research. 2011;35:1794–1803. doi: 10.1111/j.1530-0277.2011.01522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annual Review of Pharmacology and Toxicology. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- Duncan GE, Knapp DJ, Carson SW, Breese GR. Differential effects of chronic antidepressant treatment on swim stress- and fluoxetine-induced secretion of corticosterone and progesterone. The Journal of Pharmacology and Experimental Therapeutics. 1998;285:579–587. [PMC free article] [PubMed] [Google Scholar]
- Elizalde N, Pastor PM, Garcia-García AL, Serres F, Venzala E, Huarte J, et al. Regulation of markers of synaptic function in mouse models of depression: chronic mild stress and decreased expression of VGLUT1. Journal of Neurochemistry. 2010;114:1302–1314. doi: 10.1111/j.1471-4159.2010.06854.x. [DOI] [PubMed] [Google Scholar]
- Forsythe ID, Barnes-Davies M. Synaptic transmission: well-placed modulators. Current Biology. 1997;7:R362–365. doi: 10.1016/s0960-9822(06)00175-8. [DOI] [PubMed] [Google Scholar]
- Gourley SL, Taylor JR. Recapitulation and reversal of a persistent depression-like syndrome in rodents. Current Protocols in Neuroscience. 2009:32. doi: 10.1002/0471142301.ns0932s49. Chapter 9, Unit 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant KA. Strategies for understanding the pharmacological effects of ethanol with drug discrimination procedures. Pharmacology, Biochemistry, and Behavior. 1999;64:261–267. doi: 10.1016/s0091-3057(99)00075-1. [DOI] [PubMed] [Google Scholar]
- Green KL, Grant KA. Evidence for overshadowing by components of the heterogeneous discriminative stimulus effects of ethanol. Drug and Alcohol Dependence. 1998;52:149–159. doi: 10.1016/s0376-8716(98)00086-6. [DOI] [PubMed] [Google Scholar]
- Griffin WC, 3rd, Haun HL, Hazelbaker CL, Ramachandra VS, Becker HC. Increased extracellular glutamate in the nucleus accumbens promotes excessive ethanol drinking in ethanol dependent mice. Neuropsychopharmacology. 2014;39:707–717. doi: 10.1038/npp.2013.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Y, Martin-Fardon R, Weiss F. Behavioral and functional evidence of metabotropic glutamate receptor 2/3 and metabotropic glutamate receptor 5 dysregulation in cocaine-escalated rats: factor in the transition to dependence. Biological Psychiatry. 2010;68:240–248. doi: 10.1016/j.biopsych.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms CM, McCracken AD, Heichman SL, Moschak TM. Ovarian hormones and the heterogeneous receptor mechanisms mediating the discriminative stimulus effects of ethanol in female rats. Behavioural Pharmacology. 2013;24:95–104. doi: 10.1097/FBP.0b013e32835efc5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge CW, Cox AA. The discriminative stimulus effects of ethanol are mediated by NMDA and GABA(A) receptors in specific limbic brain regions. Psychopharmacology (Berl) 1998;139:95–107. doi: 10.1007/s002130050694. [DOI] [PubMed] [Google Scholar]
- Imre G. The preclinical properties of a novel group II metabotropic glutamate receptor agonist LY379268. CNS Drug Reviews. 2007;13:444–464. doi: 10.1111/j.1527-3458.2007.00024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joëls M, Sarabdjitsingh RA, Karst H. Unraveling the time domains of corticosteroid hormone influences on brain activity: rapid, slow, and chronic modes. Pharmacological Reviews. 2012;64:901–938. doi: 10.1124/pr.112.005892. [DOI] [PubMed] [Google Scholar]
- Kalil B, Leite CM, Carvalho-Lima M, Anselmo-Franci JA. Role of sex steroids in progesterone and corticosterone response to acute restraint stress in rats: sex differences. Stress. 2013;16:452–460. doi: 10.3109/10253890.2013.777832. [DOI] [PubMed] [Google Scholar]
- Karst H, Berger S, Turiault M, Tronche F, Schütz G, Joëls M. Mineralocorticoid receptors are indispensable for nongenomic modulation of hippocampal glutamate transmission by corticosterone. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:19204–19207. doi: 10.1073/pnas.0507572102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koltunowska D, Gibula-Bruzda E, Kotlinska JH. The influence of ionotropic and metabotropic glutamate receptor ligands on anxiety-like effect of amphetamine withdrawal in rats. Progress in Neuro-psychopharmacology & Biological Psychiatry. 2013;45:242–249. doi: 10.1016/j.pnpbp.2013.04.013. [DOI] [PubMed] [Google Scholar]
- Kostowski W, Bieńkowski P. Discriminative stimulus effects of ethanol: neuropharmacological characterization. Alcohol. 1999;17:63–80. doi: 10.1016/s0741-8329(98)00035-4. [DOI] [PubMed] [Google Scholar]
- Kufahl PR, Martin-Fardon R, Weiss F. Enhanced sensitivity to attenuation of conditioned reinstatement by the mGluR 2/3 agonist LY379268 and increased functional activity of mGluR 2/3 in rats with a history of ethanol dependence. Neuropsychopharmacology. 2011;36:2762–2773. doi: 10.1038/npp.2011.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kufahl PR, Watterson LR, Nemirovsky NE, Hood LE, Villa A, Halstengard C, et al. Attenuation of methamphetamine seeking by the mGluR2/3 agonist LY379268 in rats with histories of restricted and escalated self-administration. Neuropharmacology. 2013;66:290–301. doi: 10.1016/j.neuropharm.2012.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liechti ME, Lhuillier L, Kaupmann K, Markou A. Metabotropic glutamate 2/3 receptors in the ventral tegmental area and the nucleus accumbens shell are involved in behaviors relating to nicotine dependence. The Journal of Neuroscience. 2007;27:9077–9085. doi: 10.1523/JNEUROSCI.1766-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Fardon R, Weiss F. (−)-2-oxa-4-aminobicylco[3.1.0]hexane-4,6-dicarboxylic acid (LY379268) and 3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]piperidine (MTEP) similarly attenuate stress-induced reinstatement of cocaine seeking. Addiction Biology. 2012;17:557–564. doi: 10.1111/j.1369-1600.2011.00345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateo Z, Porter JT. Group II metabotropic glutamate receptors inhibit glutamate release at thalamocortical synapses in the developing somatosensory cortex. Neuroscience. 2007;146:1062–1072. doi: 10.1016/j.neuroscience.2007.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Stress and the aging hippocampus. Frontiers in Neuroendocrinology. 1999;20:49–70. doi: 10.1006/frne.1998.0173. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiological Review. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- McKlveen JM, Myers B, Flak JN, Bundzikova J, Solomon MB, Seroogy KB, et al. Role of prefrontal cortex glucocorticoid receptors in stress and emotion. Biological Psychiatry. 2013;74:672–679. doi: 10.1016/j.biopsych.2013.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihindou C, Vouillac C, Koob GF, Ahmed SH. Preclinical validation of a novel cocaine exposure therapy for relapse prevention. Biological Psychiatry. 2011;70:593–598. doi: 10.1016/j.biopsych.2011.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monn JA, Valli MJ, Massey SM, Hansen MM, Kress TJ, Wepsiec JP, et al. Synthesis, pharmacological characterization, and molecular modeling of heterobicyclic amino acids related to (+)-2-aminobicyclo[3.1.0] hexane-2,6-dicarboxylic acid (LY354740): identification of two new potent, selective, and systemically active agonists for group II metabotropic glutamate receptors. Journal of Medicinal Chemistry. 1999;42:1027–1040. doi: 10.1021/jm980616n. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Stewart JL. Interoception and drug addiction. Neuropharmacology. 2014;76:342–350. doi: 10.1016/j.neuropharm.2013.07.002. Pt B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Kalivas PW. The group II metabotropic glutamate receptor agonist, LY379268, inhibits both cocaine- and food-seeking behavior in rats. Psychopharmacology (Berl) 2006;186:143–149. doi: 10.1007/s00213-006-0372-9. [DOI] [PubMed] [Google Scholar]
- Pinheiro PS, Mulle C. Presynaptic glutamate receptors: physiological functions and mechanisms of action. Nature Reviews. Neuroscience. 2008;9:423–436. doi: 10.1038/nrn2379. [DOI] [PubMed] [Google Scholar]
- Platt DM, Bano KM. Opioid receptors and the discriminative stimulus effects of ethanol in squirrel monkeys: Mu and delta opioid receptor mechanisms. European Journal of Pharmacology. 2011;650:233–239. doi: 10.1016/j.ejphar.2010.09.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popoli M, Yan Z, McEwen BS, Sanacora G. The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nature Reviews. Neuroscience. 2012;13:22–37. doi: 10.1038/nrn3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanziani M, Salin PA, Vogt KE, Malenka RC, Nicoll RA. Use-dependent increases in glutamate concentration activate presynaptic metabotropic glutamate receptors. Nature. 1997;385:630–634. doi: 10.1038/385630a0. [DOI] [PubMed] [Google Scholar]
- Schenk S, Partridge B. Cocaine-seeking produced by experimenter-administered drug injections: dose-effect relationships in rats. Psychopharmacology (Berl) 1999;147:285–290. doi: 10.1007/s002130051169. [DOI] [PubMed] [Google Scholar]
- Schoepp DD. Unveiling the functions of presynaptic metabotropic glutamate receptors in the central nervous system. The Journal of Pharmacology and Experimental Therapeutics. 2001;299:12–20. [PubMed] [Google Scholar]
- Schuckit MA. An overview of genetic influences in alcoholism. Journal of Substance Abuse Treatment. 2009;36:S5–14. [PubMed] [Google Scholar]
- Schuckit MA, Smith TL. The relationships of a family history of alcohol dependence, a low level of response to alcohol and six domains of life functioning to the development of alcohol use disorders. Journal of Studies on Alcohol. 2000;61:827–835. doi: 10.15288/jsa.2000.61.827. [DOI] [PubMed] [Google Scholar]
- Sidhpura N, Weiss F, Martin-Fardon R. Effects of the mGlu2/3 agonist LY379268 and the mGlu5 antagonist MTEP on ethanol seeking and reinforcement are differentially altered in rats with a history of ethanol dependence. Biological Psychiatry. 2010;67:804–811. doi: 10.1016/j.biopsych.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolerman I. Drugs of abuse: behavioural principles, methods and terms. Trends in Pharmacological Sciences. 1992;13:170–176. doi: 10.1016/0165-6147(92)90059-f. [DOI] [PubMed] [Google Scholar]
- Treccani G, Musazzi L, Perego C, Milanese M, Nava N, Bonifacino T, et al. Stress and corticosterone increase the readily releasable pool of glutamate vesicles in synaptic terminals of prefrontal and frontal cortex. Molecular Psychiatry. 2014;19:433–443. doi: 10.1038/mp.2014.5. [DOI] [PubMed] [Google Scholar]
- Trim RS, Schuckit MA, Smith TL. The relationships of the level of response to alcohol and additional characteristics to alcohol use disorders across adulthood: a discrete-time survival analysis. Alcoholism: Clinical and Experimental Research. 2009;33:1562–1570. doi: 10.1111/j.1530-0277.2009.00984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venero C, Borrell J. Rapid glucocorticoid effects on excitatory amino acid levels in the hippocampus: a microdialysis study in freely moving rats. The European Journal of Neuroscience. 1999;11:2465–2473. doi: 10.1046/j.1460-9568.1999.00668.x. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Clark L, Dunn BD. The role of interoception in addiction: a critical review. Neuroscience and Biobehavioral Reviews. 2012;36:1857–1869. doi: 10.1016/j.neubiorev.2012.05.007. [DOI] [PubMed] [Google Scholar]
- Winter JC, Eckler JR, Rabin RA. Serotonergic/glutamatergic interactions: the effects of mGlu2/3 receptor ligands in rats trained with LSD and PCP as discriminative stimuli. Psychopharmacology. 2004;172:233–240. doi: 10.1007/s00213-003-1636-2. [DOI] [PubMed] [Google Scholar]
- Wise RA, Wang B, You ZB. Cocaine serves as a peripheral interoceptive conditioned stimulus for central glutamate and dopamine release. PLoS One. 2008;3:e2846. doi: 10.1371/journal.pone.0002846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright RA, Arnold MB, Wheeler WJ, Ornstein PL, Schoepp DD. [3H]LY341495 binding to group II metabotropic glutamate receptors in rat brain. The Journal of Pharmacology and Experimental Therapeutics. 2001;298:453–460. [PubMed] [Google Scholar]
- Zhao Y, Dayas CV, Aujla H, Baptista MA, Martin-Fardon R, Weiss F. Activation of group II metabotropic glutamate receptors attenuates both stress and cue-induced ethanol-seeking and modulates c-fos expression in the hippocampus and amygdala. The Journal of Neuroscience. 2006;26:9967–9974. doi: 10.1523/JNEUROSCI.2384-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]