Abstract
A common feature of inflammatory bowel disease (IBD) is the loss of intestinal epithelial barrier function due to excessive apoptosis of intestinal epithelial cells (IECs). However, the molecular mechanism underlying increased IEC apoptosis remains unclear. Here, we investigated the role of PHLPP, a novel family of protein phosphatates, in regulating inflammation-induced IEC apoptosis in mouse models of colitis. Both Phlpp1 and Phlpp2 genes were deleted in mice. Compared with wild-type mice, PHLPP double knockout (DKO) mice were protected from colitis induced by DSS as demonstrated by lower histopathological scores, and this reduced susceptibility to colitis was associated with decreased apoptosis and increased Akt activity in IECs in vivo. In addition, epithelial organoids derived from PHLPP DKO mice were more resistant to inflammation-induced apoptosis while inhibition of Akt activity abolished the protective effect of PHLPP-loss. Furthermore, we found that PHLPP expression was significantly reduced in IECs following the induction of colitis by DSS and in human IBD patient samples. This inflammation-induced downregulation of PHLPP was partially blocked by treating cells with a proteasome inhibitor. Taken together, our results indicated that proteasome-mediated degradation of PHLPP at the onset of inflammation plays an important role in protecting IEC injury by inhibiting apoptosis.
Keywords: PHLPP, knockout mouse, intestine epithelial cells apoptosis, Akt, inflammatory bowel disease
1. INTRODUCTION
Inflammatory bowel disease (IBD) primarily includes ulcerative colitis and Crohn’s disease and is characterized by chronic or recurring inflammation of the gastrointestinal tract [1–3]. Progressive inflammation usually leads to irreversible damage to the gastrointestinal tract, and patients with IBD are at increased risk for developing colorectal cancer (CRC) [4, 5]. Although the underlying cause of IBD is still largely undefined, it is well known that intestinal epithelial cells (IECs), by forming a barrier to the luminal microbes, play a pivotal role in the pathogenesis of IBD. Apoptosis of IECs is a tightly regulated process in normal intestinal epithelium, and increased cell death has been detected at the inflammatory site in both IBD patients and mouse models of colitis [6–8]. However, the molecular mechanism underlying increased IECs apoptosis during intestinal inflammation remains unclear.
PHLPP (PH domain Leucine-rich-repeats Protein Phosphatase) belongs to a novel family of Ser/Thr protein phosphatases that play a critical role in controlling the balance of cell death and proliferation [9–12]. There are two isoforms, PHLPP1 and PHLPP2, identified in this family [9]. Previous studies have identified PHLPP as a protein phosphatase and negative regulator of Akt [13, 14]. It has been shown that PHLPP-mediated dephosphorylation inactivates Akt and leads to increased apoptosis [13, 14]. Numerous studies have shown that increased Akt activity suppresses apoptosis in both normal and cancer cells [15]. Interestingly, activation of Akt downstream of phosphoinositide 3-kinase (PI3K) has been shown to be required for inflammation-induced dysplasia in mouse models of IBD [16]. Given the role of PHLPP in negatively regulating Akt signaling [13, 14], it is of particular interest to determine the functional importance of PHLPP in the pathogenesis of IBD. In this study, we reported that genetic deletion of both PHLPP genes protects mice against colitis by inhibiting IEC apoptosis and upregulating Akt activity. In addition, the expression of PHLPP is significantly reduced in mouse IECs treated with DSS and in IBD patient samples. Our findings suggested that downregulation of PHLPP provides the initial protection against inflammation-induced cell death under colitis conditions. However, aberrant activation of Akt as a result of PHLPP-loss in IECs may contribute to the pathological progression to colon cancer.
2. MATERIALS AND METHODS
2.1. Animals
All animal procedures were performed by following protocols approved by the University of Kentucky Institutional Animal Care and Use Committee. The Phlpp1 null mouse stain on 129 Sv/C57BL6 background was generated as recently described [12, 17]. The Phlpp2 knockout mouse line was created using the Knockout-First (KF) strategy [18, 19], which allows both conventional and conditional knockout of Phlpp2 gene. Mouse ES cells containing the Phlpp2 KF allele were obtained from the European Mouse Mutant Cell Repository (EuMMCR). The Phlpp2 KF mouse strain was generated by using services provided by the University of California Davis Mouse Biology Program. The Phlpp2 knockout (Phlpp2−/−) mice are mice carrying the KF cassette which essentially ablates the expression of endogenous Phlpp2 gene. To produce animals used in this study, Phlpp1−/− were bred with Phlpp2−/− mice on C57BL6 background. These mice were then inter-crossed to sustain a heterogeneous mixed genetic background. The Phlpp1/2 DKO mice were fertile and born at the expected Mendelian ratio, and the normal physiological functions were generally not affected. Four cohorts of animals were produced, including wild-type (WT), Phlpp1−/−, Phlpp2−/−, and Phlpp1/2 double knockout (DKO). The following PCR primers were used for genotyping: for Phlpp1, 5′-TAGGAGAGACTAGTGACATC-3′, 5′-TGAGCTTATACGCTGTGATGC-3′, and 5′-AGCCGATTGTCTGTTGTGC-3′; and for Phlpp2, 5′-GATGCTCTGCTTCTGCTCCTGTGC-3′, 5′-GATATGAGGAACGAAGCAATATGGG-3′, and 5′-CAACGGGTTCTTCTGTTAGTCC-3′. The schematic of Phlpp2 knockout allele and a representative result of Phlpp2 genotyping are shown in Supplementary Fig. S1.
2.2. Reagents and cells
The following chemicals, including PI3K inhibitor LY290042 and proteasome inhibitor MG132, were purchased from EMD/CalBiochem. TNFα was obtained from Peprotech and lipopolysaccharide (LPS) was from Sigma-Aldrich. Human colon cancer Caco2 cells were cultured in DMEM supplemented with 10% fetal bovine serum (FBS, Sigma-Aldrich) and 1% penicillin/streptomycin.
2.3. Induction and assessment of colitis
The WT and PHLPP DKO mice, 10 to 12 week old including both male and female littermates, were randomized to administered 2% DSS (MW 36, 000–50,000, MP Biomedicals) in the drinking water for 7 days. The control groups were given regular drinking water. Daily changes in body weight and clinical signs of colitis, such as rectal bleeding, diarrhea, and bloody stool, were assessed and reported as a score from 0 to 4. Fecal Occult Blood Test (Sure-Vue, Fisher Healthcare, Houston, TX) was used to test fecal blood in stool samples. The disease activity index was determined based on weight loss, stool consistency, and bleeding, as described previously by Murthy et al. [20]. WT and Phlpp knockout mice used in the experiments were littermates that were raised in the same animal facility and treated at the same time.
For histological assessment of colonic damage, colons were opened longitudinally, flushed with PBS, and fixed in 10% buffered formalin. The colons were Swiss-rolled to examine entire length of the colon and processed in paraffin. Hematoxylin and eosin (H&E)-stained sections were prepared from fixed and paraffin embedded colon specimens by following standard techniques. Histological scores of colitis were determined based on the previously described morphological criteria [21].
For assessing apoptosis, TUNEL staining was performed using the In Situ Cell Death Detection Kit, Fluorescein (Roche Applied Science) according to manufacturer’s protocol. Tissue sections were counterstained with DAPI and analyzed using a Nikon TE2000 inverted microscope. The number TUNEL-positive cells per 100 enterocytes (termed apoptosis index) was quantified and analyzed using Nikon Element AR software.
2.4. Immunohistochemical (IHC) staining
For IHC staining, paraffin-embedded tissue sections were deparaffinized, rehydrated, and treated with hydrogen peroxide. Antigen retrieval was performed using Dako Target Retrieval Solution (DakoCytomation), and IHC staining was performed as previously described [12, 22]. The stained sections were visualized using a Leica DM750 microscope. The F4/80 antibody was purchased from Abcam and the β-catenin antibody was obtained from Bethyl Laboratory.
2.5. Culture of mouse intestine and colon organoids
Mouse intestine and colon crypt were isolated and cultured in 3D Matrigel as described previously [23] with minor modifications. Briefly, fresh mouse intestine or colon tissues were incubated in cold digestion buffer (2 mM EDTA and 0.5 mM DTT in PBS) for 30 minutes on ice, and then incubated in TrypLE Express (Life Technologies) at 37°C for 15 min. After removing TrypLE Express, crypts were dislodged in cold PBS via vigorous vortex, and the crypt suspension was passed through a cell strainer (70 μm) to remove un-disassociated tissue materials. Isolated crypts were collected after centrifugation at 200g for 3 minutes and subsequently re-suspended in 100 μl of 50% Martrigel [Matrigel mixed 1:1 (v:v) with basal medium (DMEM/F12 supplemented with penicillin/streptomycin, HEPES, 1 X N2, 1 X B27, and 1mM N-Acetylcysteine)] and seeded in each well of a 24-well plate. Once the Matrigel had set, organoid medium was added, and the organoids were cultured for 4–7 days as specified in the experiments. The organoid medium for mouse small intestine is basal medium supplemented with EGF (50 ng/ml), Noggin (100 ng/ml) and R-spondin-1 (1 μg/ml); and Wnt-3A condition medium was added to the small intestine organoid medium for culturing colon organoids [23].
To assess the effect of Akt activation on DSS-induced cell death in organoids, WT and DKO colon organoids grown in Matrigel were first recovered and resuspended in culture medium (DMEM + 10% FBS). 2% DSS or 2% DSS plus LY294002 (10 μM) dissolved in culture medium were added to the organoid suspension and incubated for 4 hours. At the end of the treatment, organoids were recovered via low speed centrifugation and analyzed using Western blot. The method of using short-term in vitro DSS treatment to induce apoptosis in primary intestinal epithelial cells and established epithelial cell lines has been used in a number of previously published studies [21, 24–26]. Disruption of barrier function and increased secretion of pro-inflammatory cytokines from epithelial cells have been implicated as potential mechanisms by which DSS induces cell death [24, 25].
To assess TNFα-induced cell death in organoids, WT and DKO organoids cultured in Matrigel for 4 days were treated with TNFα (0 or 20 ng/ml) plus cycloheximide (2.5 μg/ml) for 3 hours in the presence of propidium iodide (PI, 3 μM) as previously described [27]. At the end of treatment, cell death was visualized by incorporation of PI and analyzed using a Nikon TE2000 inverted microscope. The intensity of PI staining within each organoid was quantified and normalized to the size of the organoid using NIS-Elements AR software. This is defined as the PI positive area and used to represent the level of cell death.
To determine LPS-induced PHLPP degradation in organoids, colon organoids were embeded into Matrigel pre-mixed with LPS (50 μg/ml) in organoid maintaining medium as described above and allowed to grow for 24 hours. During the last 3 hours of LPS treatment, MG132 (20 μM) was added into organoid maintaining medium. Subsequently, organoids were recovered and lysed directly in lysis buffer.
2.6. Isolation and purification of intestinal epithelial cells
Intestinal epithelial cells were isolated from the colon of control and DSS-treated mice and purified using Percoll as described previously [28]. Briefly, mouse colon tissues were removed and cut into small pieces. The colon pieces were incubated in DMEM/F12 supplemented with 5% FBS and 2 mM DTT for 20 minutes at room temperature. After removing the supernatant, colon pieces were incubated in DMEM/F12 supplemented with 5% FBS and 10 mM EDTA for 30 minutes at room temperature. The dissociated intestine epithelial cells from colon were further purified in 30% Percoll solution after centrifugation at 1,300 rpm for 20 minutes at room temperature. The epithelial cells were collected from the top of Percoll layer and resuspended into cold PBS. The purified epithelial cells were then recovered after centrifugation at 2,000 rpm for 10 minutes. The viability of final isolated IECs from both untreated and DSS-treated mice was routinely around 80–90% as determined by FACS analysis using PI staining.
2.7. Western blot analysis
Human colon biopsies, purified colon epithelial cells, or cultured organoids were harvested and lysed in Lysis Buffer (50 mM Na2HPO4, 1 mM sodium pyrophosphate, 20 mM NaF, 2 mM EDTA, 2 mM EGTA, 1% Triton X-100, 1 mM DTT, 200 mM benzamidine, 40 mg ml-1 leupeptin, 200 mM PMSF) and the detergent-solublized cell lysates were obtained after centrifugation for 5 minutes at 16,000 g at 4°C. Equal amounts of cell lysates as determined by Bradford assays were resolved by SDS-PAGE and subjected to Western blot analysis. The density of ECL signals was obtained and quantified using a FluoChem digital imaging system (Alpha Innotech). The PHLPP1 antibody was purchased from Proteintech and the PHLPP2 antibody was from Bethyl Laboratory. The phospho-Akt (p-Akt for the Ser473 site), Akt and cleaved caspase 3 antibodies were obtained from Cell Signaling. The γ-tubulin antibody was from Sigma-Aldrich. The PHLPP1, PHLPP2, p-Akt and total Akt antibodies were used as 1:1000; the cleaved caspase 3 antibody was used at 1:500 and the γ-tubulin antibody at 1:2000.
2.8. Real-time PCR
Total RNA was isolated from purified colonic epithelial cells following the DSS treatment using RNeasy kit (Qiagen). Equal amounts of RNA were used as templates for the synthesis of cDNA using High Capacity cDNA Reverse Transcription kit (Applied Biosysems). Real-time PCR reaction was performed using PHLPP1- or PHLPP2-specific probes using StepOne Real-Time PCR system (Applied Biosysems). All values were normalized to the level of β-actin. The overall expression of β-actin mRNA remained unchanged in different treatment groups as determined by the Ct (threshold cycle) values for β-actin in each reaction.
2.9. Human colonic specimens
Biopsy specimens were obtained from human patients undergoing diagnostic or surveillance colonoscopies for UC or Crohn’s disease, or healthy individuals undergoing routine colon cancer surveillance. Average 2–3 biopsies were collected from each individual and tissue specimens were washed in PBS and further processed for Western blot analysis. The biopsy specimens from patients with confirmed UC and Crohn’s disease mainly consisted of inflamed tissues. No healthy control or patients were on colitis treatment at the time of biopsy. The Collection of all patient materials was approved by the University of Kentucky’s Office for the Protection of Human Subjects and performed at UK Good Samaritan Hospital.
2.10. Statistical analysis
Two-sample t-tests were used to compare statistical differences between two groups in Western blot analyses. Values were expressed as mean ± SEM. Comparison of colon length and histology score between WT versus DKO groups was performed using the two-sample t-test. The repeated measurements on disease index was analyzed using linear mixed effects model to compare trend over time with contrast statement generated in the model to compare slope between groups. The repeated body weight measurements were analyzed using linear mixed effects model with a quadratic adjustment for the follow-up time variable in the model and contrast generated to compare body weight measurements over time between the two groups.
3. RESULTS
3.1. DSS-induced colitis is suppressed in Phlpp1/2 DKO mice
We have previously shown that PHLPP-mediated dephosphorylation and inactivation of Akt results in an increase in apoptosis and a decrease in proliferation in colon cancer cells [13, 14, 22]. To determine if PHLPP plays a role in regulating normal IEC cell survival in colitis, WT and Phlpp1/2 DKO mice were subjected to DSS treatment. DSS-induced acute colitis is associated with body weight loss, presence of grossly bloody stool, crypt loss and damage of IECs [29]. After treatment with 2% DSS for 7 days, both WT and DKO mice lost weight. However, the rate of weight loss was significantly slower and the disease activity index was significantly lower in DKO mice compared to WT mice (Fig. 1A–B). Histological analysis of H&E stained colon tissues revealed less epithelial damage and disruption of crypt architecture in DKO mice than in WT mice (Fig. 1C); and the overall histology score was significantly lower in DKO mice indicating less severe injury to the epithelium (Fig. 1D). Moreover, DKO mice had relatively longer colon length compared with WT mice (Fig. 1E). Consistent with less damage to the colonic epithelium, macrophage infiltration was also reduced in DSS-treated DKO mice as indicated by immunohistochemistry staining of F4/80 (Fig. 1F). Taken together, our studies suggested that PHLPP deficiency provides protection against DSS-induced colitis in vivo.
Figure 1. Suppression of DSS-induced colitis in PHLPP-DKO mice.
Colitis was induced in wild-type (WT) and PHLPP1/2 double knockout (DKO) mice by giving 2% DSS in drinking water for 7 days. The clinical progression of the colitis was determined by the percentage change in body weight (A) and disease activity index (DAI) (B). The daily body weight after the administration of DSS was normalized to that of before the treatment. DAI of WT and DKO mice was calculated as described previously [20]. Data represent means ± SEM (n = 12 for WT group including 5 male and 7 females; and 13 animals for DKO group including 5 male and 8 females. *, P<0.0001 WT vs. DKO group by overall comparison of trend over time). Note that DKO mice of both genders were equally resistant to DSS-induced colitis compared to WT mice. (C) Colon tissues were collected from WT and DKO mice treated with 2% DSS for 7 days and analyzed using hematoxylin and eosin (H&E) staining. Scale bar for images on the left = 500μm; and for images on the right = 200μm. (D) Histopathological analysis of DSS-induced colitis. Histological damage after DSS treatment was scored based on H&E staining as shown in C. Data represent means ± SEM (n = 5 animals/group; *, P<0.005 by the two-sample t-test). (E) Colon length of WT and DKO mice exposed to DSS for 7 days. Data represent means ± SEM (n = 12 and 13 animals for WT and DKO group, respectively; *, P<0.0001 WT vs. DKO group by the two-sample t-test). (F) Analysis of the inflammatory cell infiltrate in DSS-induced colitis of WT and DKO mice. Colon from WT and DKO mice exposed to DSS for 7 days were analyzed using F4/80 staining. Scale bar for images on the left = 200μm; and for images on the right = 100μm.
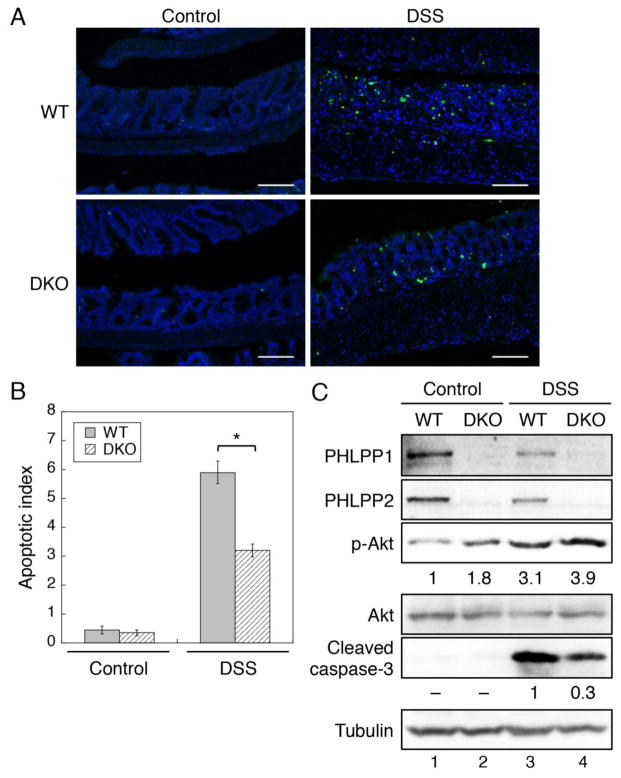
3.2. Suppression of DSS-induced IEC apoptosis in Phlpp1/2 DKO mice
To determine the molecular mechanism underlying the protective effect of PHLPP-loss on DSS-induced colitis, we determined the apoptotic index in WT and DKO mice after DSS treatment. TUNEL staining of mice colon showed that both WT and DKO mice had very low basal apoptosis rates before the DSS treatment. After the DSS treatment, the numbers of apoptotic IECs were markedly increased in WT mice, whereas DKO mice had significantly lower degree of apoptosis compared to WT mice (Fig. 2A–B). To further confirm the effect of PHLPP-loss on decreased apoptosis, the expression of cleaved caspase-3, another marker of apoptosis, was detected in isolated colon epithelial cells using Western blotting. Consistent with TUNEL staining results, the DSS treatment induced apoptosis of IECs as indicated by increased cleaved caspase-3 expression in both WT and DKO mice; however, the level of cleaved caspase-3 was largely reduced in DKO mice (Fig. 2C, compare lanes 3 and 4). Furthermore, the level of Akt phosphorylation was basally increased in DKO mice consistent with the phosphatase function of PHLPP in inactivating Akt, and DSS-induced activation of Akt was further potentiated in the absence of PHLPP expression (Fig. 2C). These results indicated that loss of PHLPP expression inhibits IEC apoptosis and activates Akt in DSS-induced colitis in vivo. Interestingly, we found that knockout of single PHLPP isoform was not sufficient to protect against DSS-induced colitis in mice as the body weight loss was similar in Phlpp1−/− and Phlpp2−/− in comparison with WT mice (Supplementary Fig. S2A–B). In addition, knockout of either Phlpp1 or Phlpp2 isoform resulted in an increase in the expression of the other PHLPP isoform in mouse intestine tissues indicating potential compensation of the two PHLPP isoforms. Indeed, although the basal phosphorylation of Akt was increased in Phlpp1 or Phlpp2 deficient mice, loss of single PHLPP isoform was not sufficient to potentiate Akt phosphorylation after DSS treatment in either Phlpp1 or Phlpp2 deficient mice (Supplementary Fig. S2C). Given our previous findings that two PHLPP isoforms function similarly at inactivating Akt, the results here suggested that loss of both PHLPP isoform is required to defense against colitis-induced apoptosis of intestinal epithelial cells.
Figure 2. PHLPP-loss inhibits DSS-induced IEC apoptosis in mice.
(A) TUNEL staining of colon tissues from DSS-treated WT and DKO mice. Scale bar, 100μm. (B) Apoptosis index was expressed as the number of TUNEL positive cells per 100 total cells counted. Colon tissues from 5 animals were analyzed in each group and cell counts were averaged from 4 randomly chosen areas. Data represent means ± SEM (n = 5; *, P<0.0001 by two-sample t-tests). (C) Western blot analysis of colonic epithelial cells isolated from control and DSS-treated WT and DKO mice. The relative levels of p-Akt and cleaved caspase-3 expression were quantified by normalizing ECL signals of p-Akt (p-S473) antibody to that of total Akt and the cleaved caspase-3 antibody to that of tubulin, respectively.
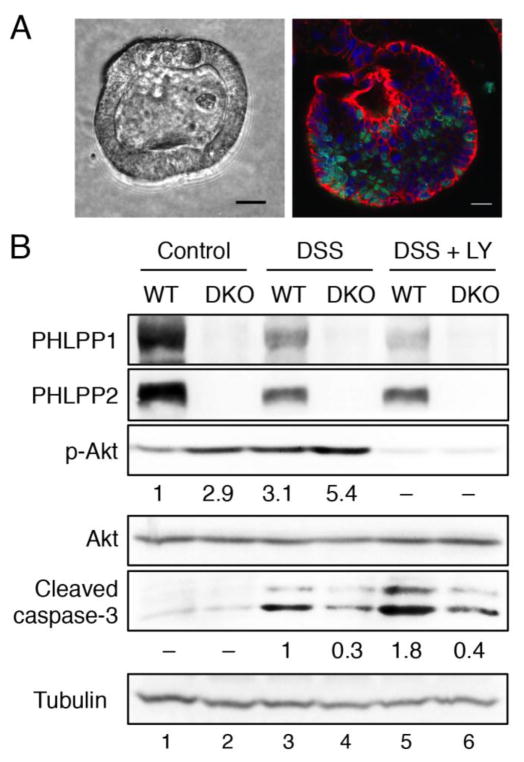
3.3. PHLPP-loss protects colonic organoids from DSS-induced apoptosis through Akt activation
Since PHLPP-loss resulted in increased Akt activation and decreased apoptosis of IECs in colitis (Fig. 2), we further tested the hypothesis that activation of Akt is required for the protective effect seen in Phlpp1/2 DKO mice. To this end, we first cultured colonic crypts isolated from WT and DKO mice in 3D Matrigel. It has been shown that the organoid model faithfully recapitulates in three dimensions the architecture of the intestinal epithelium [23, 30]. We found that the colonic crypts organized into 3D acinar structures as marked by membrane expression of β-catenin, and the actively proliferating cells were labeled with 5-ethynyl-2′-deoxyuridine (Edu) (Fig. 3A). The ability of colonic crypts to form organoids and the morphology and proliferation rates of the organoids were similar in WT and DKO mice (data not shown). This is likely due to the fact that organoids derived from crypt stem cells primarily rely on Wnt/β-catenin signaling for proliferation and differentiation [23]. To induce apoptosis, the organoids were treated with 2% DSS in the presence or absence of a PI3K inhibitor (LY294002) for 4 hours. Consistent with the results obtained in vivo, apoptosis was induced in both WT and DKO organoids treated with DSS as indicated by increased expression of cleaved caspase-3 (Fig. 3B). However, DKO organoids were more resistant to apoptosis compared with WT organoids as indicated by the reduced cleaved caspase-3 expression (Fig. 3B, compare lanes 3 and 4). Coincided with decreased apoptosis, the phosphorylation of Akt was increased in DKO organoids suggesting that activation of Akt likely protects IECs from DSS-induced apoptosis (Fig. 3B). Since Crohn’s disease can affect any part of the large intestine and small intestine, we determined the effect of PHLPP-loss in organoids derived from mouse small intestine as well. Similarly, DKO organoids of small intestine were more resistant to DSS-induced apoptosis, in that the level of cleaved caspase-3 expression was largely reduced upon DSS treatment when compared with WT organoids (Supplementary Fig. S3). Moreover, combined treatment with the PI3K inhibitor inactivated Akt in both WT and DKO organoids and the apoptotic effect induced by DSS was further exacerbated as indicated by the increased cleaved caspase-3 expression (Fig. 3B, lanes 5 and 6). The protective effect of PHLPP-loss against IEC apoptosis was largely attenuated in the presence of the PI3K inhibitor, suggesting that the decreased apoptosis seen in DKO organoids at least partially relies on increased activation of Akt (Fig. 3B). Taken together, these results indicated that PHLPP is capable of regulating DSS-induced apoptosis of IECs directly via controlling Akt activity.
Figure 3. PHLPP-loss protects colonic organoids from DSS-induced apoptosis.
Crypts isolated from WT and DKO colon tissues were seeded into Matrigel and cultured for 4 days to obtain organoids. (A) Phase contrast image of a representative colon organoid grown in 3D Matrigel and immunofluorescence staining of an organoid labeled with 5-ethynyl-2′-deoxyuridine (EdU) (green), β-catenin (red) and DAPI (blue). Scale bar, 50μm. (B) WT and DKO colon organoids grown in Matrigel were first recovered and resuspended in culture medium then treated with 2% DSS or 2% DSS plus LY294002 (10 μM) for 4 hours. Organoid lysates were analyzed for the expression of PHLPP1, PHLPP2, and cleaved caspase-3 as well as the phosphorylation status of Akt. The relative levels of p-Akt and cleaved caspase-3 expression were quantified by normalizing ECL signals of p-Akt (p-S473) antibody to that of total Akt and the cleaved caspase-3 antibody to that of tubulin, respectively.
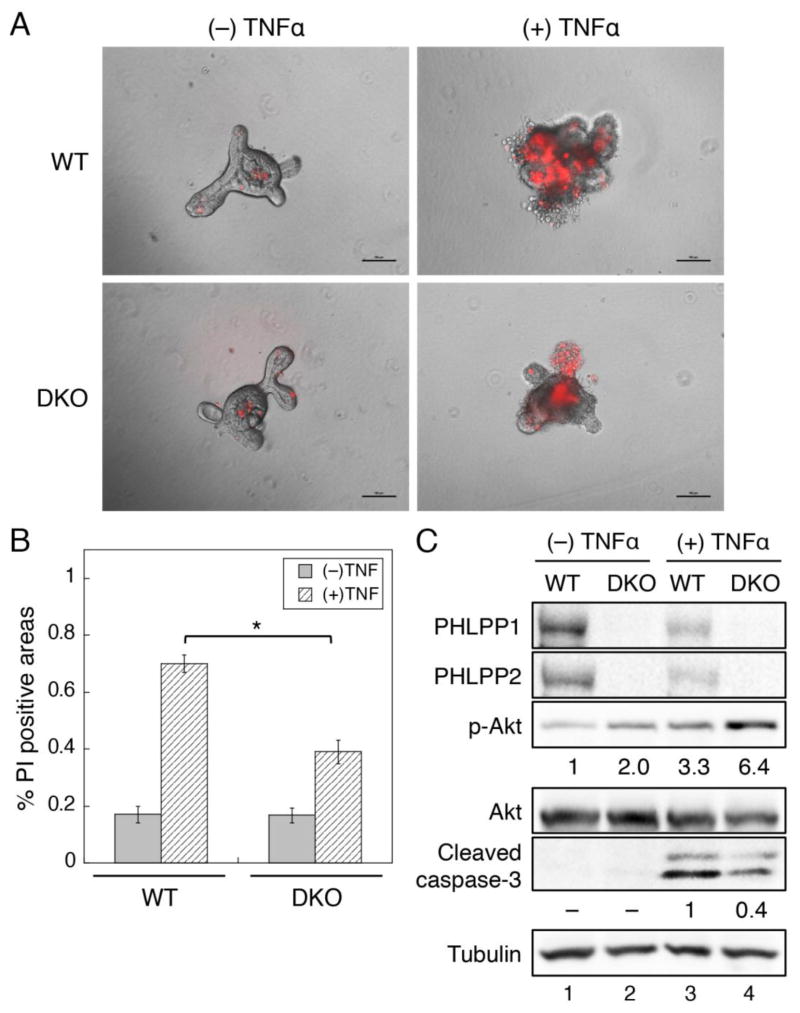
3.4. PHLPP-loss prevents TNFα-induced cell death in intestinal organoids
We next determine if PHLPP is involved in regulating the programmed cell death in IECs induced by TNFα, a major cytokine that promotes intestinal mucosal inflammation and damage in IBD. Numerous studies have shown that TNFα expression is increased in IBD patients, and TNFα-mediated signaling plays an important role in inducing apoptosis in small intestine and colon tissues [31, 32]. To examine TNFα-mediated apoptosis, intestinal crypts were isolated from the intestine of WT and DKO mice and cultured in 3D Matrigel for approximately 4 days to allow the development of organoids (Fig. 4A). Short-term treatment with TNFα induced marked apoptosis in WT organoids as indicated by increased penetration of propidium iodide into the organoids; however, this apoptosis was significantly inhibited in DKO organoids (Fig. 4A–B). Consistent with results obtained in DSS-treated organoids, TNFα-treatment resulted in an increase in cleaved caspase-3 expression indicating apoptosis induction (Fig. 4C). More importantly, PHLPP-loss decreased the level of cleaved caspase-3 in TNFα treated organoids, which coincided with increased Akt phosphorylation observed both basally and in TNFα treated DKO organoids (Fig. 4C). Collectively, these results demonstrated that loss of PHLPP expression protects IECs from inflammatory factor TNFα-induced apoptosis by upregulating Akt activity as well.
Figure 4. PHLPP-loss prevents TNFα-induced cell death in small intestinal organoids.
Crypts isolated from WT and DKO small intestine were seeded into Matrigel and cultured for 4 days to obtain organoids. (A) The WT and DKO organoids were treated with TNFα (0 or 20 ng/ml) plus cycloheximide (2.5 μg/ml) for 3 hours and stained with propidium iodide (PI). The appearance of PI penetrating into unfixed cells indicates apoptosis. Images shown are merged bright field and PI fluorescence images. Scale bar, 100μm. (B) The percentage of PI positive areas were quantified using Nikon Element AR software. Data represent means ± SEM (n = 5; *, P<0.001 by two-sample t-tests). (C) The WT and DKO organoids were treated with TNFα for 3 hours as described in (A). Organoid lysates were analyzed for the expression of PHLPP1, PHLPP2, and cleaved caspase-3 as well as the phosphorylation status of Akt. The relative levels of p-Akt and cleaved caspase-3 expression were quantified by normalizing ECL signals of p-Akt (p-S473) antibody to that of total Akt and the cleaved caspase-3 antibody to that of tubulin, respectively.
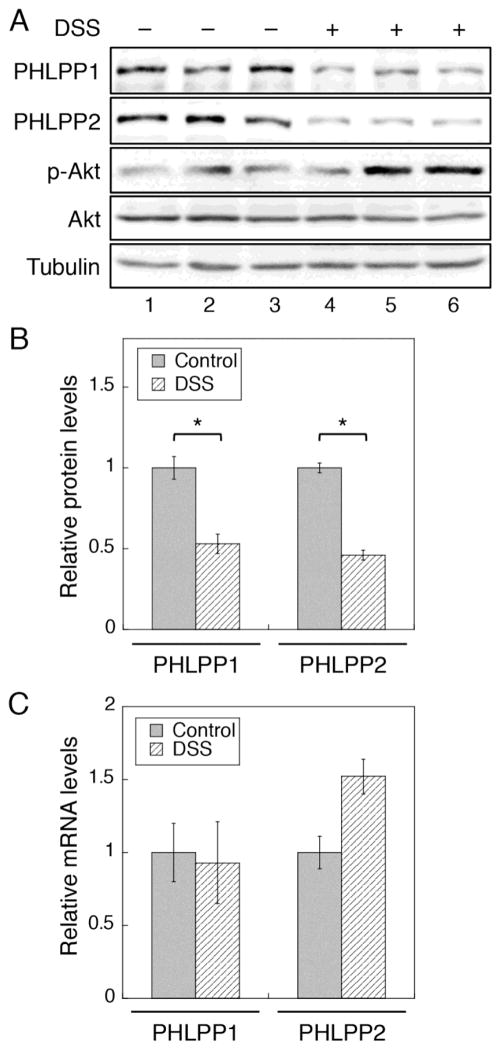
3.5. Inflammation promotes downregulation of PHLPP through proteasome-mediated degradation
As shown above, we have found that PHLPP-loss plays a protective role against DSS- and TNFα-induced apoptosis of IECs. To determine if the expression of PHLPP is altered in colitis, we analyzed the expression of PHLPP in IECs isolated from control and DSS-treated WT mice. The results showed that the expression of both PHLPP isoforms was significantly decreased in mice treated with DSS, and as a consequence, the level of Akt phosphorylation was increased (Fig. 5A–B). Similar decrease of PHLPP expression was observed in IL-10 knockout mice treated with piroxicam (Supplementary Fig. S4), another model of human IBD, which is consistent with previous reports that elevated Akt phosphorylation is commonly associated with this colitis model as well [16, 33]. We next addressed the question whether the DSS treatment alters the mRNA expression of PHLPP genes. Total RNAs were isolated from colon epithelial cells of control and DSS-treated mice, and the expression of Phlpp1 and Phlpp2 mRNA was analyzed using real-time RT-PCR. Interestingly, the mRNA levels of both PHLPP isoforms were not reduced by the DSS treatment (Fig. 5C), suggesting that colitis-induced PHLPP loss is likely controlled at the protein level.
Figure 5. PHLPP expression is downregulated in DSS-induced colitis in mice.
(A) WT mice were treated with 2% DSS for 4 days, and colonic epithelial cells were isolated from control and DSS-treated mice. Cell lysates were analyzed for the expression of PHLPP1, PHLPP2, and the phosphorylation level of Akt. (B) Western blots as shown in (A) were quantified and the relative levels of PHLPPs were obtained by normalizing ECL signals of PHLPP1 or PHLPP2 antibodies to that of tubulin. The expression level in untreated control mice was set to 1. Data represent the mean ± SEM (n=3, * p<0.01 by two-sample t-tests). (C) Total RNA was extracted from isolated colonic epithelial cells as described in (A). Real-time RT-PCR analysis was performed using probes specific for the mouse PHLPP1 or PHLPP2 gene. Data represent the mean ± SD (n=3).
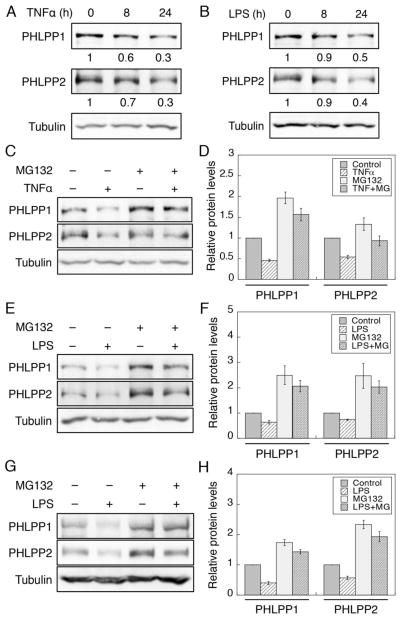
To further determine the molecular mechanism of inflammation-induced downregulation of PHLPP, we treated Caco2 cells with TNFα or LPS to activate the inflammatory response. Similar as in DSS-treated mice, both PHLPP isoforms were downregulated after 24 hours of TNFα or LPS treatment in Caco2 cells confirming that PHLPP expression is negatively regulated by inflammation (Fig. 6A–B). Furthermore, treating cells with MG132, a proteasome inhibitor, partially prevented the downregulation of PHLPP isoforms induced by TNFα or LPS (Fig. 6C–F). To confirm the results obtained in Caco2 cells, we treated colon organoids with LPS in the presence or absence of MG132. Similarly, the expression of both PHLPP isoforms was decreased upon LPS treatment and inhibition of proteasome activity reduced PHLPP downregulation (Fig. 6G–H). Taken together, our results here demonstrated that the proteasome-mediated degradation of PHLPP is upregulated under inflammation-induced stress.
Figure 6. Inflammatory stimuli decrease PHLPP expression by promoting protein degradation.
(A–B) Time course of TNFα- and LPS-induced PHLPP downregulation in Caco2 cells. Cells were treated with TNFα (20 ng/ml) (A) or LPS (50 μg/ml) (B) for the indicated time, and cell lysates were prepared and analyzed for the expression of PHLPP1, PHLPP2 and tubulin using Western blotting. The relative expression of PHLPP isoforms was quantified by normalizing ECL signals generated by the PHLPP antibodies to that of tubulin, and this number for the untreated cells was set to 1. (C–D) Caco2 cells were treated with TNFα for 24 hours as described above. Proteasome inhibitor MG132 (20 μM) or DMSO was added to the cells for the last 3 hours of the TNFα treatment. (C) Representative Western blots showing the expression of PHLPP1, PHLPP2 and tubulin in cell lysates after different treatments. (D) The relative expression of PHLPP isoforms was quantified by normalizing ECL signals generated by the PHLPP antibodies to that of tubulin. The expression level in the control cells was set to 1. Data represent the mean ± SEM (n=3). (E–F) Caco2 cells were treated with LPS for 24 hours as described above. Proteasome inhibitor MG132 (20 μM) or DMSO was added to the cells for the last 3 hours of the LPS treatment. (E) Representative Western blots showing the expression of PHLPP1, PHLPP2 and tubulin in cell lysates after different treatments. (F) The relative expression of PHLPP isoforms was quantified by normalizing ECL signals generated by the PHLPP antibodies to that of tubulin. The expression level in the control cells was set to 1. Data represent the mean ± SEM (n=3). (G) Colon organoids of WT mice grown in Matrigel were treated with LPS and MG132 as described above. Protein lysates were prepared from organoids after removing Matrigel. The expression of PHLPP1, PHLPP2 and tubulin was analyzed using Western blotting. (H) The relative expression of PHLPP isoforms was quantified by normalizing ECL signals generated by the PHLPP antibodies to that of tubulin. The expression level under control condition was set to 1. Data represent the mean ± SEM (n=3).
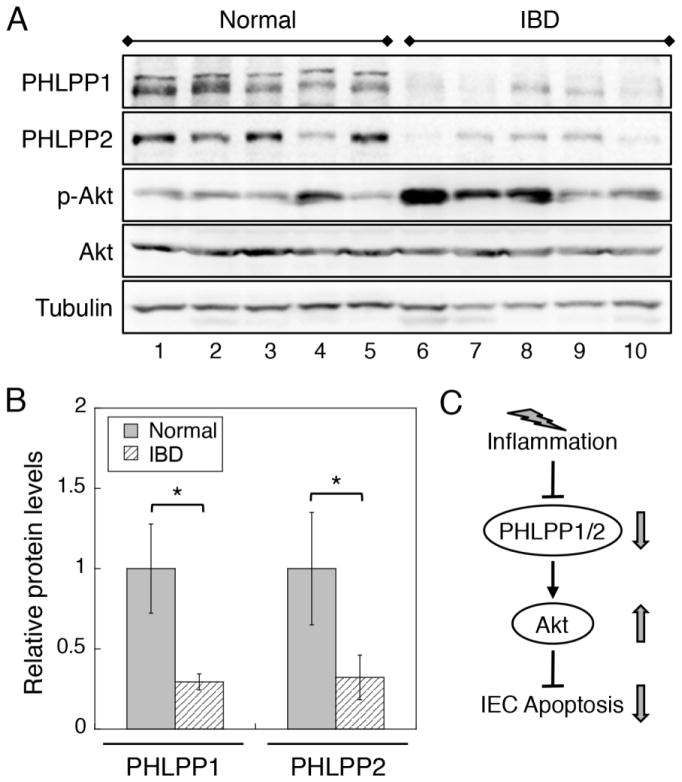
3.6. PHLPP is downregulated in human IBD patient samples
Furthermore, we determined the expression of PHLPP in human colitis specimens and uninvolved colonic tissues from patients with UC or Crohn’s disease. As shown in Fig. 7, the expression of both PHLPP1 and PHLPP2 isoform was significantly decreased in colonic biopsies collected from colitis tissues compared to normal controls. As a consequence, the level of Akt phosphorylation was increased in colitis tissues (Fig. 7A). Similar increase in Akt phosphorylation in colitis has been reported in mouse models of IBD and in patients with UC and active Crohn’s disease [16, 34].
Figure 7. The expression of PHLPP isoforms is downregulated in IBD patient samples.
(A) Biopsy specimens from normal controls or IBD patients were lysed and analyzed for the expression of PHLPP1, PHLPP2, and the phosphorylation level of Akt using Western blotting. (B) Western blots as shown in (A) were quantified and the relative levels of PHLPPs were obtained by normalizing ECL signals of PHLPP1 or PHLPP2 antibodies to that of tubulin. The expression level in control samples was set to 1. Data represent the mean ± SEM (n=5, * p<0.05 by two-sample t-tests). (C) Diagram showing the effect of inflammation-induced downregulation of PHLPP on intestinal epithelial cell (IEC) apoptosis. Our study demonstrates that PHLPP expression is downregulated by inflammatory factors that are associated with IBD through increased protein degradation. As a consequence, loss of PHLPP expression promotes the activation of Akt and protects IECs from inflammation-induced apoptosis.
In summary, we have identified PHLPP as a novel regulator of inflammation-induced apoptosis of IECs in colitis. Our results supported a model in which PHLPP expression in IECs is downregulated by proteasome-mediated degradation upon exposure to inflammatory factors. Subsequently, the activity of Akt is upregulated to provide a feedback protection against apoptosis (Fig. 7C). However, persistent activation of Akt signaling as the result of PHLPP downregulation under inflammation may drive early tumorigenesis in colitis-associated colon cancer.
4. DISCUSSION
Although the underlying cause of IBD is inflammation, aberrant apoptosis of IECs has been implicated as a major contributing factor in the pathogenesis of IBD [35–37]. Previous studies have shown that several known regulators of apoptosis, including p53, PUMA, and Bcl-2, are involved in regulating colitis-induced apoptosis of IECs [32, 36, 38]. In this study, we found that PHLPP deficiency abrogates DSS-induced acute colitis in mice by inhibiting IEC apoptosis. The resistance to apoptosis in PHLPP DKO mice is likely mediated by upregulation of Akt activity, as inhibition of PI3K/Akt attenuates the anti-apoptotic effect. In addition, the expression of PHLPP is significantly downregulated in DSS-treated mice as well as in human IBD patient specimens, which corresponds with elevated phosphorylation of Akt. Taken together, our data identified PHLPP as a critical mediator of IEC apoptosis and a novel regulator of IBD.
Previous studies have demonstrated that Akt activation downstream of various inflammatory stimuli is associated with enhanced cell survival and decreased epithelium damage in inflammation-induced mucosal injury [16, 39–41]. In addition, it has been suggested that maintaining Akt activity is required for promoting injury repair of intestinal epithelium. For example, colon-specific delivery of a probiotic-derived soluble protein has been shown to prevent IEC apoptosis and DSS-induced intestinal injury by activating EGFR and Akt signaling [42]. On the other hand, inhibition of PI3K/Akt signaling blocks the protective function of ErbB4 in inhibiting cytokine-induced epithelial apoptosis [41]. Here, we showed that both PHLPP1 and PHLPP2 isoforms, key negative regulators of Akt, could be readily downregulated in inflammation-induced colitis. This decreased expression of PHLPP provides a novel mechanism that allows rapid upregulation of Akt activity and subsequent protection against IEC apoptosis upon exposure to inflammatory insults. The short-term downregulation of PHLPP likely plays a positive role in protecting IECs from excessive death induced by acute colitis. However, prolonged activation of PI3K/Akt signaling has been implicated in the progression of chronic colitis to colitis-associated cancer [16]. Thus, inflammation-induced PHLPP-loss is potentially an important risk factor that predisposes IBD patients to colitis-associated cancer. Interestingly, increasing evidence has suggested that inflammation plays a key role in the initiation and progression of spontaneous colon cancers as well [43]. The prevalence of inflammatory tumor microenvironment suggests that inflammation may be a general mechanism leading to PHLPP-loss in cancer. It is intriguing that knockout of either PHLPP isoform by itself is insufficient to protect IECs from inflammation-induced apoptosis due to the compensative effect of the two PHLPP isoforms. This is consistent with previous findings that both PHLPP isoforms are commonly downregulated in colorectal cancer patients [12, 22].
In determining the molecular mechanism of PHLPP downregulation, we found that PHLPP expression is regulated at the post-translational level in which the PHLPP proteins are degraded by the proteasome in colitis. Similarly, we have shown previously that PHLPP is downregulated under hypoxia via the proteasome-mediated degradation in colon cancer cells [44]. A number of recent studies have implicated that tissue hypoxia, particularly in mucosal surfaces involving epithelial cells, coincides with acute and chronic intestinal inflammatory diseases [45–47]. Thus, it is likely that inflammation-induced hypoxia may lead to decreased expression of PHLPP by promoting proteasome-dependent PHLPP degradation, and downreguation of PHLPP contributes to the pro-survival effect activated by hypoxia. Although we have observed the effect of PHLPP-loss on IECs directly, our results do not rule out the contribution from other cell types since the PHLPP DKO mice used in this study are systemic knockout. It is possible that knockout of PHLPP expression in immune cells may affect IEC apoptosis triggered by inflammation. More studies are required to determine the involvement of PHLPP in different cell types in the development of colitis.
Taken together, our study identified PHLPP as a novel regulator of IEC apoptosis. Under acute colitis, PHLPP is downregulated as the result of activation of inflammatory factors, and loss of PHLPP expression inhibits IEC apoptosis by increasing Akt activity. It is attractive to consider PHLPP inhibition as a potential anti-IBD strategy to protect against IEC apoptosis. However, given the tumor suppressor role of PHLPP, prolonged inhibition of PHLPP may contribute to increased risk of colon cancer in IBD patients.
Supplementary Material
HIGHLIGHTS.
PHLPP knockout mouse models were generated to study colitis.
PHLPP-loss protects against inflammation-induced intestinal epithelium injury.
PHLPP inhibits Akt activation upon induction of colitis.
PHLPP is downregulated by inflammation in mouse and human colitis tissues.
Acknowledgments
This work was supported by NIH R01CA133429 (TG) and P20GM103527 (TG). The studies were supported by Biospecimen and Tissue Procurement and Biostatistics Shared Resources of the University of Kentucky Markey Cancer Center (P30CA177558). We thank Dana Napier at the Biospecimen and Tissue Procurement facility for her help with preparation and sectioning of paraffin-embedded mouse tissues. The Biostatistics Shared Resource Facility provided assistance with statistical analysis.
ABBREVIATIONS
- CRC
colorectal cancer
- DKO
double knockout
- IBD
inflammatory bowel disease
- IEC
intestine epithelial cell
- IHC
immunohistochemistry
- PHLPP
PH domain Leucine-rich-repeats Protein Phosphatase
- PI3K
phosphoinositide 3-kinase
- UC
ulcerative colitis
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Braus NA, Elliott DE. Advances in the pathogenesis and treatment of IBD. Clinical immunology. 2009;132:1–9. doi: 10.1016/j.clim.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 3.Uhlig HH. Monogenic diseases associated with intestinal inflammation: implications for the understanding of inflammatory bowel disease. Gut. 2013;62:1795–1805. doi: 10.1136/gutjnl-2012-303956. [DOI] [PubMed] [Google Scholar]
- 4.Grivennikov SI. Inflammation and colorectal cancer: colitis-associated neoplasia. Seminars in immunopathology. 2013;35:229–244. doi: 10.1007/s00281-012-0352-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dyson JK, Rutter MD. Colorectal cancer in inflammatory bowel disease: what is the real magnitude of the risk? World J Gastroenterol. 2012;18:3839–3848. doi: 10.3748/wjg.v18.i29.3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hagiwara C, Tanaka M, Kudo H. Increase in colorectal epithelial apoptotic cells in patients with ulcerative colitis ultimately requiring surgery. Journal of gastroenterology and hepatology. 2002;17:758–764. doi: 10.1046/j.1440-1746.2002.02791.x. [DOI] [PubMed] [Google Scholar]
- 7.Williams CS, Bradley AM, Chaturvedi R, Singh K, Piazuelo MB, Chen X, McDonough EM, Schwartz DA, Brown CT, Allaman MM, Coburn LA, Horst SN, Beaulieu DB, Choksi YA, Washington MK, Williams AD, Fisher MA, Zinkel SS, Peek RM, Jr, Wilson KT, Hiebert SW. MTG16 contributes to colonic epithelial integrity in experimental colitis. Gut. 2013;62:1446–1455. doi: 10.1136/gutjnl-2011-301439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gunther C, Neumann H, Neurath MF, Becker C. Apoptosis, necrosis and necroptosis: cell death regulation in the intestinal epithelium. Gut. 2013;62:1062–1071. doi: 10.1136/gutjnl-2011-301364. [DOI] [PubMed] [Google Scholar]
- 9.Brognard J, Newton AC. PHLiPPing the switch on Akt and protein kinase C signaling. Trends Endocrinol Metab. 2008;19:223–230. doi: 10.1016/j.tem.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qiao M, Wang Y, Xu X, Lu J, Dong Y, Tao W, Stein J, Stein GS, Iglehart JD, Shi Q, Pardee AB. Mst1 is an interacting protein that mediates PHLPPs’ induced apoptosis. Mol Cell. 2010;38:512–523. doi: 10.1016/j.molcel.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 11.Warfel NA, Newton AC. Pleckstrin homology domain leucine-rich repeat protein phosphatase (PHLPP): a new player in cell signaling. J Biol Chem. 2012;287:3610–3616. doi: 10.1074/jbc.R111.318675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li X, Stevens PD, Liu J, Yang H, Wang W, Wang C, Zeng Z, Schmidt MD, Yang M, Lee EY, Gao T. PHLPP is a negative regulator of RAF1, which reduces colorectal cancer cell motility and prevents tumor progression in mice. Gastroenterology. 2014;146:1301–1312. e1301–1310. doi: 10.1053/j.gastro.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao T, Furnari F, Newton AC. PHLPP: a phosphatase that directly dephosphorylates Akt, promotes apoptosis, and suppresses tumor growth. Mol Cell. 2005;18:13–24. doi: 10.1016/j.molcel.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 14.Brognard J, Sierecki E, Gao T, Newton AC. PHLPP and a second isoform, PHLPP2, differentially attenuate the amplitude of Akt signaling by regulating distinct Akt isoforms. Mol Cell. 2007;25:917–931. doi: 10.1016/j.molcel.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 15.Franke TF, Hornik CP, Segev L, Shostak GA, Sugimoto C. PI3K/Akt and apoptosis: size matters. Oncogene. 2003;22:8983–8998. doi: 10.1038/sj.onc.1207115. [DOI] [PubMed] [Google Scholar]
- 16.Lee G, Goretsky T, Managlia E, Dirisina R, Singh AP, Brown JB, May R, Yang GY, Ragheb JW, Evers BM, Weber CR, Turner JR, He XC, Katzman RB, Li L, Barrett TA. Phosphoinositide 3-kinase signaling mediates beta-catenin activation in intestinal epithelial stem and progenitor cells in colitis. Gastroenterology. 2010;139:869–881. 881 e861–869. doi: 10.1053/j.gastro.2010.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen M, Pratt CP, Zeeman ME, Schultz N, Taylor BS, O’Neill A, Castillo-Martin M, Nowak DG, Naguib A, Grace DM, Murn J, Navin N, Atwal GS, Sander C, Gerald WL, Cordon-Cardo C, Newton AC, Carver BS, Trotman LC. Identification of PHLPP1 as a Tumor Suppressor Reveals the Role of Feedback Activation in PTEN-Mutant Prostate Cancer Progression. Cancer Cell. 2011;20:173–186. doi: 10.1016/j.ccr.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Testa G, Schaft J, van der Hoeven F, Glaser S, Anastassiadis K, Zhang Y, Hermann T, Stremmel W, Stewart AF. A reliable lacZ expression reporter cassette for multipurpose, knockout-first alleles. Genesis. 2004;38:151–158. doi: 10.1002/gene.20012. [DOI] [PubMed] [Google Scholar]
- 19.Skarnes WC, Rosen B, West AP, Koutsourakis M, Bushell W, Iyer V, Mujica AO, Thomas M, Harrow J, Cox T, Jackson D, Severin J, Biggs P, Fu J, Nefedov M, de Jong PJ, Stewart AF, Bradley A. A conditional knockout resource for the genome-wide study of mouse gene function. Nature. 2011;474:337–342. doi: 10.1038/nature10163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murthy SN, Cooper HS, Shim H, Shah RS, Ibrahim SA, Sedergran DJ. Treatment of dextran sulfate sodium-induced murine colitis by intracolonic cyclosporin. Digestive diseases and sciences. 1993;38:1722–1734. doi: 10.1007/BF01303184. [DOI] [PubMed] [Google Scholar]
- 21.Tambuwala MM, Cummins EP, Lenihan CR, Kiss J, Stauch M, Scholz CC, Fraisl P, Lasitschka F, Mollenhauer M, Saunders SP, Maxwell PH, Carmeliet P, Fallon PG, Schneider M, Taylor CT. Loss of prolyl hydroxylase-1 protects against colitis through reduced epithelial cell apoptosis and increased barrier function. Gastroenterology. 2010;139:2093–2101. doi: 10.1053/j.gastro.2010.06.068. [DOI] [PubMed] [Google Scholar]
- 22.Liu J, Weiss HL, Rychahou P, Jackson LN, Evers BM, Gao T. Loss of PHLPP expression in colon cancer: role in proliferation and tumorigenesis. Oncogene. 2009;28:994–1004. doi: 10.1038/onc.2008.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sato T, Stange DE, Ferrante M, Vries RG, Van Es JH, Van den Brink S, Van Houdt WJ, Pronk A, Van Gorp J, Siersema PD, Clevers H. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology. 2011;141:1762–1772. doi: 10.1053/j.gastro.2011.07.050. [DOI] [PubMed] [Google Scholar]
- 24.Edelblum KL, Washington MK, Koyama T, Robine S, Baccarini M, Polk DB. Raf protects against colitis by promoting mouse colon epithelial cell survival through NF-kappaB. Gastroenterology. 2008;135:539–551. doi: 10.1053/j.gastro.2008.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Araki Y, Sugihara H, Hattori T. In vitro effects of dextran sulfate sodium on a Caco-2 cell line and plausible mechanisms for dextran sulfate sodium-induced colitis. Oncol Rep. 2006;16:1357–1362. [PubMed] [Google Scholar]
- 26.Koch S, Nava P, Addis C, Kim W, Denning TL, Li L, Parkos CA, Nusrat A. The Wnt antagonist Dkk1 regulates intestinal epithelial homeostasis and wound repair. Gastroenterology. 2011;141:259–268. 268 e251–258. doi: 10.1053/j.gastro.2011.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takahashi N, Vereecke L, Bertrand MJ, Duprez L, Berger SB, Divert T, Goncalves A, Sze M, Gilbert B, Kourula S, Goossens V, Lefebvre S, Gunther C, Becker C, Bertin J, Gough PJ, Declercq W, van Loo G, Vandenabeele P. RIPK1 ensures intestinal homeostasis by protecting the epithelium against apoptosis. Nature. 2014;513:95–99. doi: 10.1038/nature13706. [DOI] [PubMed] [Google Scholar]
- 28.Roche JK. Isolation of a purified epithelial cell population from human colon. Methods in molecular medicine. 2001;50:15–20. doi: 10.1385/1-59259-084-5:15. [DOI] [PubMed] [Google Scholar]
- 29.Cooper HS, Murthy SN, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993;69:238–249. [PubMed] [Google Scholar]
- 30.Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, Clevers H. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 31.Wang J, Fu YX. Tumor necrosis factor family members and inflammatory bowel disease. Immunol Rev. 2005;204:144–155. doi: 10.1111/j.0105-2896.2005.00218.x. [DOI] [PubMed] [Google Scholar]
- 32.Goretsky T, Dirisina R, Sinh P, Mittal N, Managlia E, Williams DB, Posca D, Ryu H, Katzman RB, Barrett TA. p53 mediates TNF-induced epithelial cell apoptosis in IBD. Am J Pathol. 2012;181:1306–1315. doi: 10.1016/j.ajpath.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khan MW, Keshavarzian A, Gounaris E, Melson JE, Cheon EC, Blatner NR, Chen ZE, Tsai FN, Lee G, Ryu H, Barrett TA, Bentrem DJ, Beckhove P, Khazaie K. PI3K/AKT signaling is essential for communication between tissue-infiltrating mast cells, macrophages, and epithelial cells in colitis-induced cancer. Clin Cancer Res. 2013;19:2342–2354. doi: 10.1158/1078-0432.CCR-12-2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dahan S, Roda G, Pinn D, Roth-Walter F, Kamalu O, Martin AP, Mayer L. Epithelial: lamina propria lymphocyte interactions promote epithelial cell differentiation. Gastroenterology. 2008;134:192–203. doi: 10.1053/j.gastro.2007.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Edelblum KL, Yan F, Yamaoka T, Polk DB. Regulation of apoptosis during homeostasis and disease in the intestinal epithelium. Inflammatory bowel diseases. 2006;12:413–424. doi: 10.1097/01.MIB.0000217334.30689.3e. [DOI] [PubMed] [Google Scholar]
- 36.Qiu W, Wu B, Wang X, Buchanan ME, Regueiro MD, Hartman DJ, Schoen RE, Yu J, Zhang L. PUMA-mediated intestinal epithelial apoptosis contributes to ulcerative colitis in humans and mice. J Clin Invest. 2011;121:1722–1732. doi: 10.1172/JCI42917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dirisina R, Katzman RB, Goretsky T, Managlia E, Mittal N, Williams DB, Qiu W, Yu J, Chandel NS, Zhang L, Barrett TA. p53 and PUMA independently regulate apoptosis of intestinal epithelial cells in patients and mice with colitis. Gastroenterology. 2011;141:1036–1045. doi: 10.1053/j.gastro.2011.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mizushima T, Arakawa S, Sanada Y, Yoshino I, Miyazaki D, Urushima H, Tsujimoto Y, Ito T, Shimizu S. Inhibition of epithelial cell death by Bcl-2 improved chronic colitis in IL-10 KO mice. Am J Pathol. 2013;183:1936–1944. doi: 10.1016/j.ajpath.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 39.Wang Y, Srinivasan K, Siddiqui MR, George SP, Tomar A, Khurana S. A novel role for villin in intestinal epithelial cell survival and homeostasis. J Biol Chem. 2008;283:9454–9464. doi: 10.1074/jbc.M707962200. [DOI] [PubMed] [Google Scholar]
- 40.Cario E, Gerken G, Podolsky DK. Toll-like receptor 2 controls mucosal inflammation by regulating epithelial barrier function. Gastroenterology. 2007;132:1359–1374. doi: 10.1053/j.gastro.2007.02.056. [DOI] [PubMed] [Google Scholar]
- 41.Frey MR, Edelblum KL, Mullane MT, Liang D, Polk DB. The ErbB4 growth factor receptor is required for colon epithelial cell survival in the presence of TNF. Gastroenterology. 2009;136:217–226. doi: 10.1053/j.gastro.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yan F, Cao H, Cover TL, Washington MK, Shi Y, Liu L, Chaturvedi R, Peek RM, Jr, Wilson KT, Polk DB. Colon-specific delivery of a probiotic-derived soluble protein ameliorates intestinal inflammation in mice through an EGFR-dependent mechanism. J Clin Invest. 2011;121:2242–2253. doi: 10.1172/JCI44031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klampfer L. Cytokines, inflammation and colon cancer. Curr Cancer Drug Targets. 2011;11:451–464. doi: 10.2174/156800911795538066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wen YA, Stevens PD, Gasser ML, Andrei R, Gao T. Downregulation of PHLPP expression contributes to hypoxia-induced resistance to chemotherapy in colon cancer cells. Mol Cell Biol. 2013;33:4594–4605. doi: 10.1128/MCB.00695-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Karhausen J, Furuta GT, Tomaszewski JE, Johnson RS, Colgan SP, Haase VH. Epithelial hypoxia-inducible factor-1 is protective in murine experimental colitis. J Clin Invest. 2004;114:1098–1106. doi: 10.1172/JCI21086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Robinson A, Keely S, Karhausen J, Gerich ME, Furuta GT, Colgan SP. Mucosal protection by hypoxia-inducible factor prolyl hydroxylase inhibition. Gastroenterology. 2008;134:145–155. doi: 10.1053/j.gastro.2007.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Colgan SP, Taylor CT. Hypoxia: an alarm signal during intestinal inflammation. Nature reviews Gastroenterology & hepatology. 2010;7:281–287. doi: 10.1038/nrgastro.2010.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.