Abstract
Working memory (WM) is one of the key constructs in understanding higher-level cognition. We examined whether patterns of activity in the resting state in individual subjects are correlated with their off-line working and short-term memory capabilities. Participants completed a resting-state fMRI scan and offline working and short-term memory (STM) tests with both verbal and visual materials. We calculated fractional amplitude of low frequency fluctuations (fALFF) from the resting state data, and also computed connectivity between seeds placed in frontal and parietal lobes. Correlating fALFF values with behavioral measures showed that the fALFF values in a widespread fronto-parietal network during rest were positively correlated with a combined memory measure. In addition, STM showed a significant correlation with fALFF within the right angular gyrus and left middle occipital gyrus, whereas WM was correlated with fALFF values within the right IPS and left dorsomedial cerebellar cortex. Furthermore, verbal and visuospatial memory capacities were associated with dissociable patterns of low-frequency fluctuations. Seed-based connectivity showed correlations with the verbal WM measure in the left hemisphere, and with the visual WM in the right hemisphere. These findings contribute to our understanding of how differences in spontaneous low-frequency fluctuations at rest are correlated with differences in cognitive performance.
Keywords: Short-term Memory, Working Memory, Resting state, fALFF, fMRI
Introduction
In the past decade a plethora of studies have lent weight to the idea that working memory (WM) is a construct crucial in the understanding of higher-level cognition (Daneman & Carpenter, 1980; Engle, Kane, & Tuholski, 1999; Jaeggi et al., 2008) and a strong predictor of academic achievement (Alloway & Alloway, 2010; Swanson & Siegel, 2001). Although several different WM theories exist (for a review see Baddeley, 2012), they all share the notion that WM is a system for temporary maintainance and manipulation of task-relevant information. One of the most widely recognized conceptualizations of WM is the model proposed by Baddeley and Hitch (1974), which proposes that WM consists of three separate components: a phonological loop, visuospatial sketchpad, and a central executive. Recently, the episodic buffer was proposed as a fourth component of WM, that allows for the binding of information across multiple modalities into integrated events (Baddeley, 2000). The Baddeley and Hitch model forms the basis of most contemporary frameworks, which all stress that WM includes verbal and visuospatial domains across different levels of processing demands.
Neuroimaging studies have investigated which brain regions play a role in WM and whether distinct neuronal substrates underlie the different components of WM that were proposed on the basis of behavioral studies. Given that all contemporary frameworks have embraced the multi-faceted nature of WM, different tasks investigate WM across different modalities and processing demands. Some of the most frequently employed WM tasks include n-back tasks, simple span tasks and complex span tasks. Across these different types of WM experiments a widespread bilateral fronto-parietal network has commonly been found active (Hampson, Driesen, Skudlarski, Gore & Constable, 2006; Owen, McMillan, Laird & Bullmore, 2005; Rottschy et al., 2011; Nee et al. 2013). In line with the idea of a fine-grained distinction between WM subprocesses, recent work has suggested that despite overlap in their underlying processes, behavioral performance on span and n-back tasks is only weakly correlated (Redick & Lindsey, 2013). This finding cautions against the use of these different tasks interchangeably as if reflecting a general WM measure, and stress the importance of investigating the distinct WM processes across different domains.
Several neuroimaging studies obtained evidence in support of the idea that distinct patterns of brain activity can be observed depending on the type of material stored in WM. For example, several researchers have argued that verbal WM is mainly left lateralized (Reuter-Lorenz et al., 2000; Smith & Jonides, 1999), memory for spatial information activates the dorsolateral prefrontal cortex (DLPFC) and memory for object information activates mid- and inferior frontal regions (Courtney, Petit, Haxby, & Ungerleider, 1998). These findings are consistent with the idea of separate dorsal and ventral processing streams for processing spatial and object information, respectively (Smith & Jonides, 1999; Wilson, Scalaidhe, & Goldman-Rakic, 1993). In addition, several researchers have argued that spatial WM is mainly right lateralized, whereas object WM predominantly activates regions in the left hemisphere (McCarthy et al., 1996; Reuter-Lorenz et al., 2000; Smith et al., 1995). A number of neuroimaging studies have provided evidence that a similar neuronal distinction can be found between tasks that differ in their processing demands. That is, ventral regions within the frontal cortex have shown to play a role in rehearsal during simple storage, whereas superior frontal regions seem to play a role in monitoring and manipulation of information (D’Esposito et al., 1998; Owen, 1997). These findings provide strong evidence for a widespread fronto-parietal network involved in WM, with the involvement of specific regions depending on the type of material and processes used in the task. Neuroimaging experiments, however, have mainly focused on the magnitude of metabolic activity associated with different WM processes during the performance of a task.
In order to provide a complete picture of inter-individual differences in memory functions, it’s crucial to also understand how these differences relate to changes in the regional intensity of spontaneous fluctuations in the BOLD signal. Several studies have provided evidence that low-frequency oscillations, occurring during rest, reveal wide-scale networks organized according to their sub-functions (Biswal, Van Kylen, & Hyde, 1997; Greicius, Krasnow, Reiss, & Menon, 2003). For example, studies have dissociated a sub-network encompassing primary visual regions from a network including extra-striate visual regions (Beckmann, DeLuca, Devlin, & Smith, 2005; Damoiseaux et al., 2006; Van den Heuvel et al., 2008). In a similar vein, somatotopically organized sub-networks have been revealed within the resting state network of the primary motor cortex (Van den Heuvel & Hulshoff Pol, 2010a). These findings lead to the speculation that connectivity between specific brain regions, occurring in the absence of an explicit task, may reflect performance in cognitive tasks that rely on the network’s underlying function (e.g., Van den Heuvel & Hulshoff Pol, 2010b). In support of this idea, Xiong and colleagues (2008) showed a significant increase in resting-state connectivity within primary motor areas as a function of long-term motor training.
Recent studies have investigated the relationship between resting state activity and working memory capacities. A study by Hampson and colleagues (2006) showed that individual differences in coupling strength between the posterior cingulate cortex (PCC) and medial superior frontal gyrus/ventral anterior cingulate cortex (MFG/vACC) both at rest and during a WM task predicted differences in behavioral performance. Similarly, Sala-Llonch and colleagues (2011) showed that resting-state connectivity of the precuneus/posterior cingulate predicted performance on their working memory task. In addition, they found a relationship between the degree to which the default mode network (DMN) and WM network were anti-correlated and behavioral performance. Together, these findings suggest that the functional coupling between specific brain regions, during a state of rest, can predict the effectiveness of cognitive processing in a task that relies on the interaction of those structures.
A recent study by Zou and colleagues (2013) investigated whether intrinsic fluctuations in the BOLD signal correlated with behavioral performance on a WM task. In their study they used the ALFF approach, a promising method for detecting regional intensity of spontaneous fluctuations in the BOLD signal. The amplitude of low-frequency fluctuations (ALFF) has shown to be a reliable measure of spontaneous activity, which is critical for a region’s corresponding cognitive processes (Zang et al., 2007; Hoptman et al., 2010). Zou et al. (2013) found a significant correlation between the amplitude of low-frequency fluctuations in the superior parietal lobule/precuneus and working memory performance. In addition, other studies have shown that ALFFs in the absence of a task correlate with task-evoked BOLD responses and behavioral measures (Mennes et al., 2011). Furthermore, low-frequency fluctuations (LFFs) have shown a high synchronization between areas that make up a neuroanatomical/functional network (Koyama et al., 2010; Biswal et al., 1995; Fox et al., 2005). These findings suggest that both intrinsic activity within and functional connectivity between specific brain regions during a resting-state, may aid processing in cognitive tasks that rely on those structures. Therefore, measures of regional activity amplitude and functional connectivity patterns provide a viable tool for investigating aspects of cognitive functioning. In the current study, we examined whether intrinsic brain activity and functional connectivity patterns are associated with general, domain-specific and demand-specific working memory performance.
To this end we measured resting state oscillations by calculating fractional ALFF (fALFF) in activity, occurring in the absence of a task, which we correlated with measures of general, domain-specific and demand-specific memory capacities. Domain-specific effects were investigated by administering both verbal (Digits) and visual-spatial (Dots Sequence) tests. Demand-specific effects were investigated by examining the difference between tests that only relied on the storage of information (Forward tests) versus tests that involved both storage and manipulation of information (Backward tests). We refer to the tests in which participants had to simply recall items in the order of presentation and therefore only relied on storage of information as short-term memory (STM) measure. On the other hand, tests in which participants had to repeat items in the reverse order of presentation, and therefore involved both storage and manipulation, as working memory (WM) measure. The sum of all memory measures (i.e., both STM and WM) will be referred to as general memory measure. In addition to ALFFs, we were interested if functional coupling during rest between frontal and parietal memory structures could predict inter-individual differences in memory scores. Therefore, we additionally conducted a connectivity analysis on the basis of the averaged time series extracted within a left dorsolateral prefrontal cortex (DLPFC), right DLPFC, left superior parietal lobule (SPL) and right SPL seed region. We hypothesized a positive relationship between both left and right fronto-parietal connectivity and participants’ general memory measure. Furthermore, we predicted that Left Hemisphere (LH) connectivity strength would be correlated with the verbal memory score (digits), whereas Right Hemisphere (RH) connectivity strength would be correlated with the visual-spatial memory score (dots).
METHODS
Participants
Eighteen right-handed individuals between 21 and 77 years of age (M = 55.11, SD = 18.28; 6 males) participated in the study as part of a larger project involving age-related changes in working memory. These participants were selected on the basis of having a complete set of behavioral data (both verbal and visual STM and WM tests) and agreed to undergo a resting state scan. All participants that met these criteria were used. In the resting state scan we obtained 158 volumes with a TR of 2 seconds, leading to a total acquisition time of approximately 5 minutes. The resting state scan was on average obtained about one week after the behavioral tests. All participants had normal or corrected-to-normal vision and no history of neurological disorders. Prior to the experiment, participants were informed about the experimental procedures and signed informed consent forms, according to a protocol approved by the Institutional Review Board of the University of South Carolina.
Experimental Procedures
Participants underwent a scanning session to obtain resting-state functional images. On a separate occasion they were administered the WOMBAT (Working Memory Battery; Englund, Decker, Woodlief, & DiStefano, 2014).
Behavioral Procedure
Participants completed four memory tasks, which are components of a larger Working Memory Battery that includes nine subtests (WOMBAT; Englund et al., 2014). The WOMBAT is a multicomponent test battery that measures separate Verbal, Static Visual-Spatial, and Dynamic Visual-Spatial domains across three levels of processing demands. Additionally, each subtest was calibrated using item response theory to create equal-interval scaling to measure working memory with generally high reliability estimates. For the present study, we were particularly interested in whether our short-term memory (STM) vs. working-memory (WM) measure would be associated with a distinct pattern of resting-state connectivity and whether this would differ for the verbal and dynamic visual-spatial domains. Therefore, we examined four subtests of the WOMBAT that represented these dimensions. Two of the tests only involved storage of information and can therefore be labeled as verbal and dynamic visual-spatial short-term memory tasks (i.e., Digits Forward and Dots Sequence Forward). For the other two tests the memory demands were higher given that they involved both storage and manipulation of information and can therefore be labeled as verbal and dynamic visual-spatial working memory tasks (i.e., Digits Backward and Dots Sequence Backward). An experimental session consisted of the four memory tests, administered on a standard computer in a self-paced manner. In the Digit tests, participants were auditorily presented with a string of digits (1–9) which they repeated by typing the respective digits either in the order of presentation (Digits Forward) or in the backwards order (Digits Backward).
In the Dots Sequence tests, participants saw dots presented in various locations on the computer screen (moving black dots in a square grid) for which they had to recall either the sequential location in the order of presentation (Dots Sequence Forward) or in the backwards order (Dots Sequence Backwards). Participants responded by moving their cursor and clicking boxes on the computer screen in a specific sequence. For the Digits tests the string length ranged between 2 and 11 items, whereas for the Dots Sequence tests the string length ranged between 1 and 10 items. In all tests, the number of items participants had to recall increased by one item following two correct responses and terminated after four consecutive error responses. Participants received an item score of 1 if their response was completely correct. Any type of error – commission, ommission, or sequencing – led to an item score of 0. After four consecutive errors on a particular subtest, the remaining items for that subtest received item scores of 0. The final score for each subtask reflected the total number of items correct (out of 20).
fMRI data acquisition
Participants were instructed to lie still and relax in the scanner with their eyes closed. Resting-state and anatomical images were acquired on a Siemens TRIO 3.0 T MRI system (Siemens, Erlangen, Germany) equipped with a 12-channel head coil. BOLD-sensitive resting-state functional images were collected using a single-shot gradient EPI sequence (echo time/repetition time = 37/2000 msec, 37 axial slices in ascending order, slice gap = 0.3 mm, field of view = 204 mm, flip angle = 90 degrees, voxel size = 3.0 × 3.0 × 3.3 mm3. High-resolution anatomical images were acquired using a MPRAGE sequence (echo time = 4.15 sec, voxel size = 1 × 1 × 1 mm3, 192 sagittal slices, field of view = 256 mm, flip angle of 90 degrees, TR = 2250 ms).
fMRI Data Analysis
Resting-state data were preprocessed and analyzed with the AFNI software package (Cox, 1996). A standardized preprocessing pipeline involved despiking of the data by fitting a smooth-ish curve to each voxel time series, time shifting, and registration of functional images to the anatomy. Subsequently, functional images were co-registered (Saad et al., 2009) and projected into standard stereotaxic space (Talairach and Tournoux, 1988). The normalized images were smoothed with an isotropic 5-mm FWHM Gaussian kernel, and the run mean of each voxel was scaled to 100. Nuisance signals and localized transient hardware artifacts were removed by regressing out local estimates of the white matter signal and the eroded large ventricle average, using ANATICOR (Jo, Saad, Simmons, Milbury, & Cox, 2010). In addition, we included six motion parameters and their derivatives as covariates of no interest.
Low frequency fluctuation analysis
The 3dRSFC program (Taylor & Saad, 2013) was used to calculate Amplitude of Low Frequency fluctuations (ALFFs; Zang et al., 2007). To calculate ALFFs, the program applies a bandpass filter (0.01 < f < 0.08 Hz) to reduce low-frequency drift and high-frequency respiratory artifacts (Biswal, Yetkin, Haughton, & Hyde, 1995; Lowe, Mock, & Sorenson, 1998). The square root of the power in this range provides ALFFs. Given that ALFF values are prone to noise from physiological sources (Zou et al., 2008), we used fALFF values instead, which were calculated by taking the ratio of ALFF values to the power spectrum of the initial time series (before a bandpass filter was applied). These fALFF values were subsequently transformed to Z-scores.
To examine how fALFF values were related to performance on the different memory tests, we correlated subjectwise scores for each test with each subject’s fALFF maps. Because the WOMBAT is based on a theoretical model that separates working memory task demands and processes, we are able to examine specific composite measures representing general, domain-specific and demand-specific memory capacities by creating covariates on the basis of the sum of their respective subtests. These were, general memory (all subtests), verbal domain (Digits Forward and Digits Backward), visual domain (Dots Sequence Forward and Dots Sequence Backward), short-term memory demand (Digits Forward and Dots Sequence Forward) and working memory demand (Digits Backward and Dots Sequence Backward). The group maps were thresholded at voxelwise p < 0.01 and corrected for multiple comparisons by removing clusters smaller than 1066 µl to achieve a mapwise corrected two-tailed p < 0.05.i Using the 3dClustSim program with 10,000 iterations, the cluster threshold was determined through Monte Carlo simulations that estimate the chance probability of spatially contiguous voxels exceeding the voxelwise p threshold. The analysis was restricted to a mask that excluded areas outside the brain, as well as deep white matter areas and the ventricles. Due to the correlation with age in multiple subtest scores and sums (see behavioral results below), age was used as a covariate in our analyses to partial out effects due to age.
Seed-based connectivity analysis
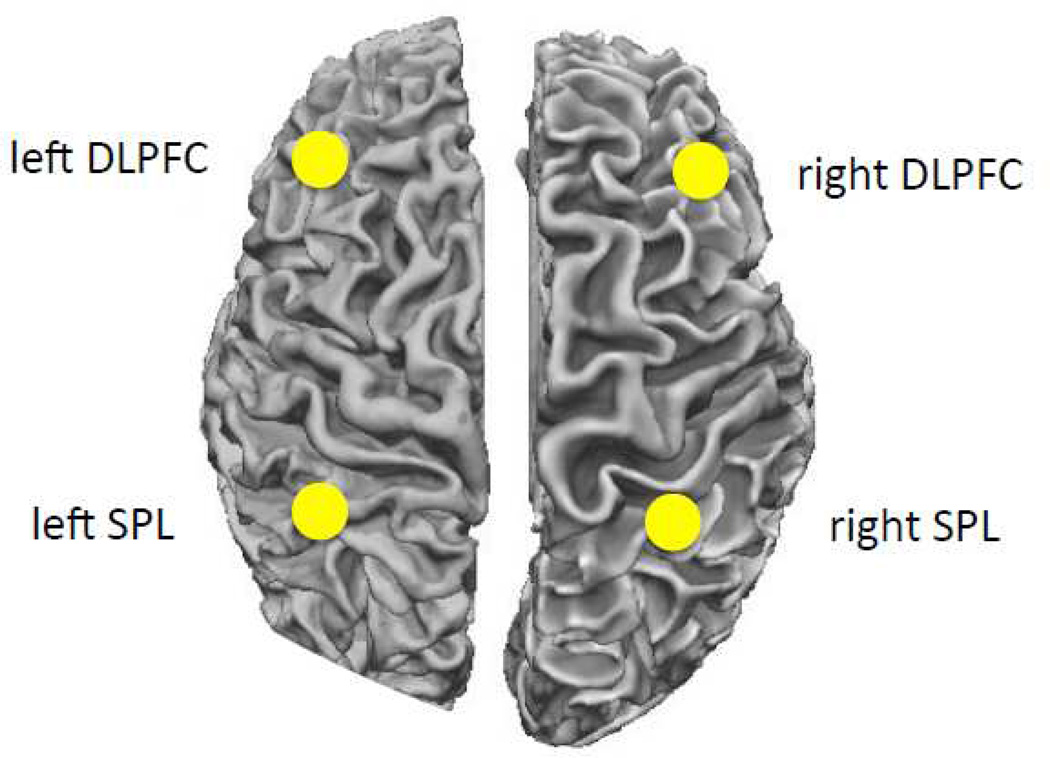
In addition to this whole-brain analysis based on fALFF values, we also examined resting state connectivity between seeds in two regions that are consistently associated with WM: the dorsolateral prefrontal cortex (DLPFC) and the superior parietal lobule (SPL; Rottschy et al., 2011; Owen et al., 2005; Hampson et al., 2006). We defined seeds on the basis of a meta-analysis by Rottschy and colleagues (2011). A sphere of 5 mm radius was used around each coordinate, after conversion to Talairach coordinates. For the DLPFC ROI we used the Talairach coordinates [43 35 31] falling in caudal lateral prefrontal cortex. For the SPL ROI we used a seed at [-34 −49 44], falling in superior parietal lobule/intraparietal sulcus. Each ROI was mirrored on the other hemisphere, resulting in two LH and two RH seeds. Average time series were extracted for each ROI and the signals from the ROIs within each hemisphere were subsequently correlated with each other. Six motion parameters, their derivatives, and third-degree polynomial regressors were added as regressors of no interest. The within-hemisphere correlation values were then correlated with the test scores and composite measures using Spearman’s rank correlation. Based on the literature, we hypothesized that both left and right hemisphere connectivity measures will be correlated with a participants’ overall memory score. Furthermore, we hypothesized a greater role of LH connectivity for verbal tests (digits) and RH connectivity for tests with visual material (dots).
RESULTS
Behavioral results
The scores for each subtest are summarized in Table 1 and reflect the total number of items correct (out of 20). In addition, a summary of the sum of different subtests that reflect general memory capacity (Grand Sum), storage only (STM Sum), storage + manipulation (WM Sum), verbal domain (Verbal Sum) and Visual-spatial domain (Visuospatial Sum) is provided.
Table 1.
Mean peformance rates (M) on the subtests of the WOMBAT with standard deviations (SD). Correlation with age and significance are given for each subtest.
| Measure |
M | SD | r | p |
|---|---|---|---|---|
| Test A (Digits Forward) | 11.22 | 1.35 | −0.30 | 0.12 |
| Test B (Digits Backward) | 7.67 | 1.81 | −0.58 | < 0.01** |
| Test I (Dots Sequence Forward) | 10.22 | 2.02 | −0.13 | 0.30 |
| Test J (Dots Sequence Backward) | 9.61 | 2.70 | −0.21 | 0.20 |
|
Sum |
||||
| Grand Sum (A + B + I + J) | 38.72 | 5.92 | −0.39 | 0.06 |
| STM Sum (A + I) | 21.44 | 2.38 | −0.28 | 0.13 |
| WM Sum (B + J) | 17.28 | 3.91 | −0.42 | 0.04* |
| Verbal Sum (A + B) | 18.89 | 2.63 | −0.55 | < 0.01** |
| Visuospatial Sum (I + J) | 19.83 | 4.37 | −0.19 | 0.22 |
P – values are one-sided due to expected one-way results in the correlations, as age typically influences working memory measures (Englund et al., 2014).
p < 0.05,
p < 0.01.
Given the high variance in our subject’s age, each subtest and sum was correlated with age. Digits Backward, WM Sum and Verbal Sum showed negative correlations with age (all ps < 0.05).
fMRI results
Grand Sum (A +B + I + J)
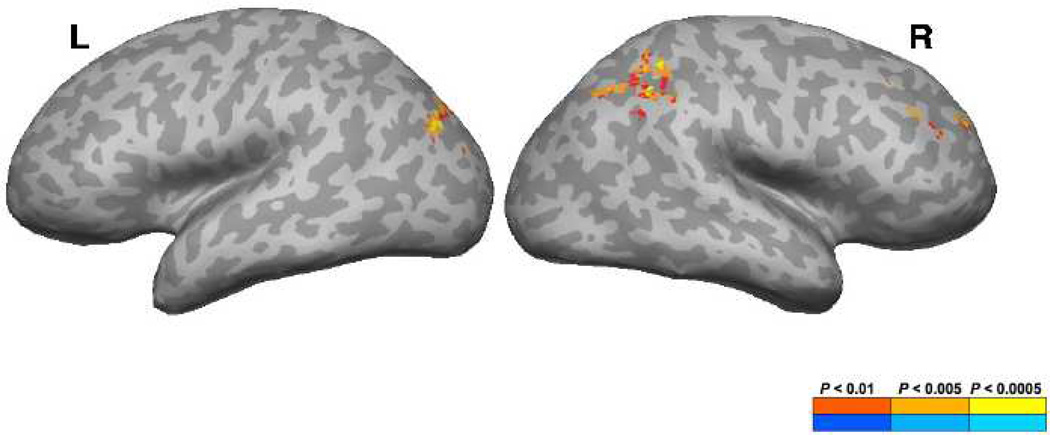
Areas that showed a significant positive correlation with the Grand Sum and fALFF values included the right supramarginal gyrus (SMG), right intraparietal sulcus (IPS), right middle frontal gyrus (MFG), as well as the left middle occipital gyrus (MOG), left superior transverse occipital sulcus and the bilateral angular gyrus (AnG) (Figure 1; Table 2).
Figure 1.
Brain regions showing a significant correlation between whole-brain fALFF values and general WM measures (A+B+I+J).
Table 2.
The volume of the cluster (µl), peak z-score, Talairach coordinates, and the anatomical structures that the clusters overlap are shown. L = left hemisphere, R = right hemisphere, g = gyrus, s = sulcus, ant = anterior, post = posterior, sup = superior, mid = middle, inf = inferior, AnG = angular gyrus, IPS = intraparietal sulcus, + = positive, - = negative.
| Volume | Max | x | y | z | Structure | Correlation |
|---|---|---|---|---|---|---|
| Grand Sum (A + B + I + J) | ||||||
| 2781 | 3.92 | 46 | −43 | 44 | R supramarginal g (AnG/IPS) | + |
| 1323 | 3.60 | −31 | −79 | 26 | L mid occipital g (AnG) | + |
| 1296 | 4.27 | 37 | 31 | 38 | R mid frontal g | + |
| Short-Term Memory (A + I) | ||||||
| 2646 | 3.92 | −34 | −85 | 26 | L mid occipital g (AnG) | + |
| 1593 | 3.99 | 61 | −52 | 32 | R AnG (supramarginal g) | + |
| Working Memory (B + J) | ||||||
| 1944 | 3.86 | 31 | −43 | 38 | R IPS (SPL) | + |
| 1080 | 3.09 | −7 | −52 | −6 | L/R dorsal cerebellum | + |
| Verbal Working Memory (A + B) | ||||||
| 2052 | 3.74 | −1 | −10 | 5 | L/R thalamus proper | + |
| 1809 | 3.73 | 34 | −46 | 29 | R IPS | + |
| 1269 | 3.03 | 4 | −52 | −6 | R dorsal cerebellum | + |
| 1080 | 4.14 | 10 | 4 | 8 | R caudate | + |
| 2214 | −3.19 | 40 | −43 | −48 | R ventral cerebellum | − |
| Visuospatial Working Memory (I + J) | ||||||
| 1809 | 3.46 | −19 | −61 | 14 | L parieto occipital s | + |
| 1647 | 4.53 | 31 | 55 | 32 | R mid frontal g | + |
| Test A (Digits Forward) | ||||||
| 1998 | 3.71 | 31 | 19 | −12 | R orbital g | + |
| 1458 | 3.41 | −43 | 19 | −12 | L orbital g | + |
| 1350 | 3.85 | −43 | −79 | 32 | L AnG/ mid occipital g | + |
| 1296 | 3.32 | −4 | −19 | −3 | L Thalamus proper | + |
| 11286 | −3.32 | −28 | −43 | −48 | L ventral cerebellum | − |
| Test J (Dots Sequence Backward) | ||||||
| 2538 | 3.87 | 4 | −46 | 44 | R precuneus | + |
| 2133 | 4.47 | 46 | −43 | 44 | R supramarginal g/ AnG | + |
| 1485 | 3.38 | 34 | −58 | 44 | R IPS / AnG | + |
| 1323 | 3.82 | 31 | 46 | 29 | R mid frontal g, s | + |
| 1215 | 3.55 | −22 | −76 | 29 | L IPS/ mid occipital g | + |
Short Term Memory (A + I)
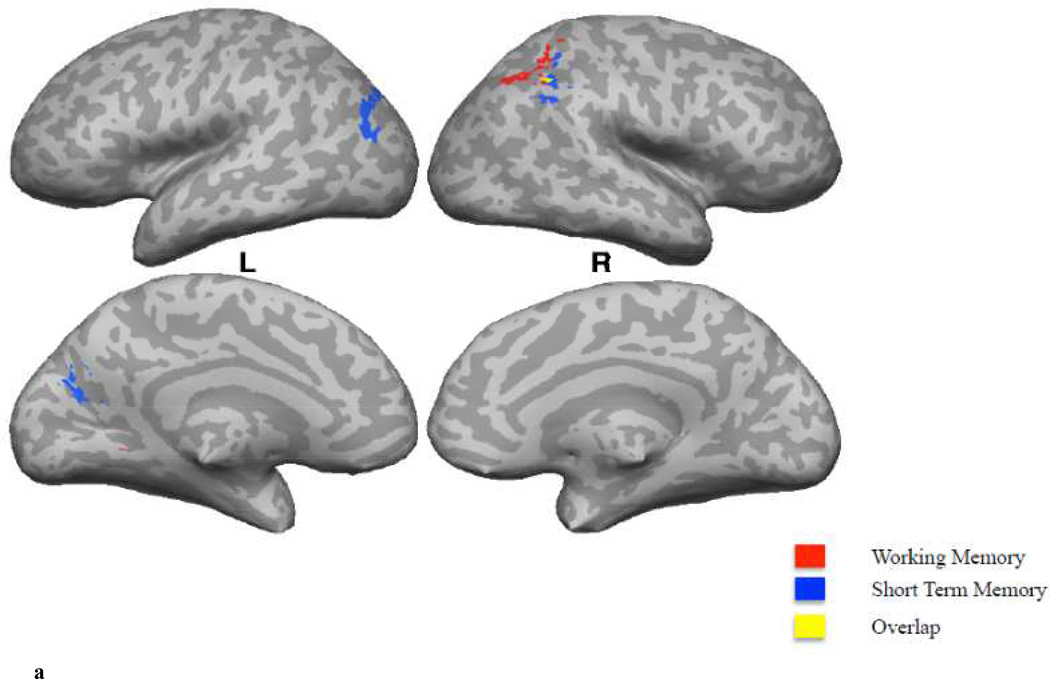
The STM Sum showed a significant positive correlation with fALFF values in the right AnG and the left MOG (spreading into AnG) (Figure 2A; Table 2).
Figure 2.
A. A comparison of correlations of whole-brain fALFF values with WM (B+J, in red) versus STM (A+I, in blue) measures. Regions in yellow represent the spatial intersection of the statistically thresholded maps corresponding to the correlation of whole-brain fALFF values with WM and STM measures.
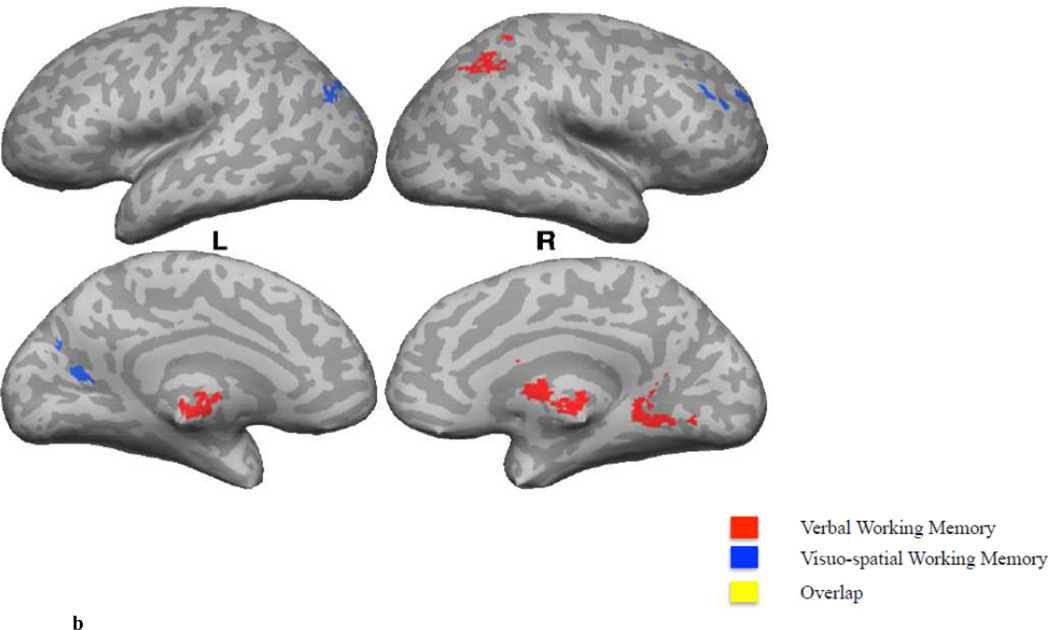
B. A comparison of correlations of whole-brain fALFF values with Verbal (A+B, in red) versus Visuospatial (I+J, in blue) measures. Regions in yellow represent the spatial intersection of the statistically thresholded maps corresponding to the correlation of whole-brain fALFF values with Verbal and Visuospatial measures.
Working Memory (B + J)
The WM Sum showed a significant positive correlation with fALFF values in the right IPS and left dorsomedial cerebellar cortex (Figure 2A; Table 2).
Verbal Memory (A + B)
Areas that showed a significant positive correlation between the Verbal Memory Sum and fALFF values included the right lingual gyrus, right caudate, right IPS and the bilateral thalamus proper. A negative correlation was observed in the right ventral anterior cerebellar cortex (Figure 2B; Table 2).
Visuospatial Memory (I + J)
Areas that showed a significant positive correlation between the Visuospatial Memory Sum and fALFF values included the left occipital-parietal sulcus and the right MFG (Figure 2B; Table 2).
Digits Forward (A)
Digits Forward showed a significant positive correlation with fALFF values in the bilateral orbital gyri, left AnG, left thalamus and the left ventral cerebellum.
Dots Sequence Backward (J)
Dots Sequence Backward showed a significant positive correlation with fALFF values in the right precuneus, right SMG, right IPS/AnG, right middle frontal gyrus and the left IPS/ middle occipital gyrus.
No significant activations were found for Digits Backward (B) and Dot Sequence Forward (I) tests.
Seed-based connectivity
Both left fronto-parietal connectivity (left DLPFC/left SPL) and right fronto-parietal connectivity (right DLPFC/right SPL) were significantly correlated with the Grand Sum measure (ρ = 0.47, p = 0.025, and ρ = 0.41, p = 0.044 for LH and RH respectively). Interestingly, left fronto-parietal connectivity was significantly correlated with the Verbal Memory Sum (ρ = 0.52, p = 0.013), whereas right fronto-parietal connectivity was significantly correlated with the Visual-spatial Memory Sum (ρ = 0.47, p = 0.024). We did not observe a significant correlation between right fronto-parietal connectivity and Verbal Memory Sum (ρ = 0.14, p = 0.296), as well as left fronto-parietal connectivity and Visual-spatial Memory Sum (ρ = 0.33, p = 0.093).
Given that our connectivity measures are likely to be highly correlated with age, we calculated partial correlations (with age as a covariate) between fronto-parietal connectivity and General, Verbal and Visuospatial memory measures. None of the partial correlations were significant.
Multiple regression analysis
The sum of the different subtests that reflect general memory capacity, short-term memory, working memory, verbal memory and visuospatial memory might share overlap in their variance. That is, some of the brain-behavior correlations that we observed in our study might be driven by variance that is shared between the different behavioral sum scores. A correlation analysis between the verbal and visuospatial memory sum scores showed a moderate correlation (ρ = 0.42, p = 0.04), whereas a high correlation was observed between the STM and WM sum scores (ρ = 0.72, p < 0.001). Given that the zero-order correlations were still lower than 0.80, we assumed that this degree of multicollinearity would not prevent the estimation of coefficients and standard errors (Kline, 1998).
Verbal memory (A +B)
After the verbal memory sum score was orthogonalized with respect to the visuospatial memory score, we obtained a positive correlation between the verbal sum score and fALFF values in the left caudate, right IPS, as well as the bilateral thalamus proper. A negative correlation was observed in the bilateral anterior cerebellar cortex (Table 3).
Table 3.
The volume of the cluster (µl), peak z-score, Talairach coordinates, and the anatomical structures that the clusters overlap are shown. L = left hemisphere, R = right hemisphere, g = gyrus, s = sulcus, ant = anterior, post = posterior, sup = superior, mid = middle, inf = inferior, AnG = angular gyrus, IPS = intraparietal sulcus, + = positive, - = negative.
| Volume | Max | x | y | z | Structure | Correlation |
|---|---|---|---|---|---|---|
| Grand Sum (A + B + I + J) | ||||||
| 2781 | 3.92 | 46 | −43 | 44 | R supramarginal g (AnG/IPS) | + |
| 1323 | 3.60 | −31 | −79 | 26 | L mid occipital g (AnG) | + |
| 1296 | 4.27 | 37 | 31 | 38 | R mid frontal g | + |
| Working Memory (B + J) | ||||||
| 1377 | 28 | 37 | 29 | R mid frontal g | + | |
| Verbal Working Memory (A + B) | ||||||
| 1404 | −4 | −13 | −3 | L/R thalamus proper | + | |
| 1080 | 22 | −52 | 35 | R IPS | + | |
| 1674 | −22 | −49 | −45 | L ventral cerebellum | − | |
| 1080 | −7 | −4 | 20 | L caudate | + | |
| 1701 | 40 | −43 | −48 | R ventral cerebellum | − | |
Visuospatial memory (I + J)
After the visualspatial memory sum score was orthogonalized with respect to the verbal memory score, we did not observe any regions for which the correlation between the visuospatial sum score and fALFF values survived the correction. However, the positive correlation we previously observed in the left parieto occipital sulcus and the right middle frontal gyrus bordered significance (p < 0.06).
Working memory
After the working memory sum score was orthogonalized with respect to the short-term memory score, we obtained a positive correlation between the working memory sum score and fALFF values in the right middle frontal gyrus (Table 3).
Discussion
The current study examined the relationship between individuals working memory ability and brain connectivity in default networks. Specifically, differences in spontaneous low frequency fluctuations during rest were used to predict differences in general, domain-specific and demand-specific WM performance.
General Memory capacity
In the current study, a significant positive correlation with the Grand Sum and fALFF values was observed in the right SMG (spreading into the IPS and AnG), right MFG, as well as the left MOG (spreading into the AnG). The SMG has been shown to play a role in phonological processing and verbal working memory (Sliwinska et al., 2012; Paulesu, Frith, & Frackowiak, 1993). The AnG, on the other hand, has shown to be activated in a variety of tasks including: semantics, attention and spatial cognition, memory retrieval, cross-modal integration and the default mode network (DMN) (for a review see Seghier, 2013; Binder et al. 2009). Its important role in memory might be facilitated by strong structural connectivity with the hippocampal system (Seghier, 2013). Our IPS activation spread into the superior parietal lobule (SPL). The superior parietal cortex has traditionally been strongly linked with visuospatial and attentional processing (Nachev & Husain, 2006; Sack, 2009). Studies, have reported consistent activation within our network of posterior parietal regions in a variety of working memory tasks (Hampson et al., 2006; Sandrini et al., 2012, for meta-analyses see Rottschy et al., 2011; Owen et al., 2005, Wager & Smith, 2003). The right MFG activation was located in the DLPFC (BA9/46), a region that has shown to play a role in sustained attention and working memory (Hampson et al., 2006; Rottschy et al., 2011; Owen et al., 2005; Brunoni & Vanderhasselt, 2014). These results show that in the absence of any task, spontaneous low frequency fluctuations in the BOLD signal within a widespread fronto-parietal network were correlated with general memory performance. These findings provide strong evidence for the idea that low-frequency fluctuations within fronto-parietal regions during resting state, affects performance in tasks that rely on general memory capacities.
Short Term Memory versus Working Memory
A significant positive correlation with STM sum and fALFF values was observed in the right AnG (spreading into the SMG) and the left MOG (spreading into AnG). That is, we mainly observed fALFF values in the bilateral inferior parietal cortex to be associated with STM. The inferior parietal cortex has shown to play an important role in episodic memory encoding and retrieval (Hutchinson, Uncapher, & Wagner, 2009; Ciaramelli, Grady, & Moscovitch, 2008; Vilberg & Rugg, 2008) and retrieval of verbal material (Jonides et al., 1998).
A significant positive correlation with the WM sum and fALFF values was observed in the right IPS (spreading into the SPL). The SPL is consistently found active during executive processes like updating, order and manipulation (Wager & Smith, 2003). Koenigs and colleagues (2009) compared a group of patients with SPL lesions to a group without lesions and a group with non-SPL lesions. They did not find any significant group differences for tests that involved simple retention and recall (Digit Span Forward and Spatial Span Forward), whereas patients with SPL lesions did significantly worse on tests that required rearrangement and manipulation of information in WM (Digit Span Backward and Spatial Span Backward). These results show a dissociation of STM and WM measures in the parietal cortex, with more inferior regions predicting STM performance and more superior regions reflective of the more demanding WM measure. It’s not surprising to find an overlap in the network of regions underlying what we label as “STM” and “WM”, given that they reflect overlapping measures of memory. That is, both our STM and WM tasks involve simple retention and recall of information, whereas the WM task requires an additional rearrangement or manipulation of information in WM.
Verbal Memory versus Visuospatial Memory
A significant positive correlation with the Verbal Working Memory Sum and fALFF values was observed in the right dorsal cerebellum, whereas a negative correlation was found in the right ventral cerebellum. The cerebellum has shown to contribute to verbal working memory (Durisko & Fiez, 2010), with certain findings suggesting an inferior-to-superior subdivision of the cerebellum. Desmond and colleagues (2007), for example, suggested that the superior cerebellum is associated with articulatory rehearsal, whereas the inferior cerebellum is associated with correction of errors within WM. In addition, we found a positive correlation in the right IPS and the bilateral thalamus. The right IPS activation is inline with an extensive body of research showing that verbal working memory is associated with activation in the SMG of the left IPL and the bilateral SPL (Awh et al., 1996; Smith, Jonides, & Koeppe, 1996; Jonides et al., 1997). In a meta-analysis, Owen and colleagues (2005) found activation within the thalamus when they contrasted n-back tasks that involved verbal identity-monitoring versus tasks that involved nonverbal identity-monitoring.
A significant positive correlation with the Visuospatial Working Memory Sum and fALFF values was observed in the right MFG (right DLPFC). The superior frontal sulcus in humans has shown to play a role in spatial working memory. These findings are supported by animal research, which have proposed a ventral-dorsal distinction within the prefrontal cortex in processing spatial and object information respectively (Courtney et al., 1998; Wilson, O’Scalaidhe, & Goldman-Rakic, 1993; O’Scalaidhe, Wilson, & Goldman-Rakic, 1997). The activation that we observed fell in the MFG/SFS. The left MOG activation spread into the IPS and the AnG. The posterior parietal cortex has shown to play a role in spatial working memory, however, activation is usually found in the right hemisphere (Van Asselen et al., 2006). These results show that during resting-state, low frequency fluctuations in two clearly distinct sets of brain regions were correlated with verbal and visuospatial WM measures. These findings are largely consistent with research on the neural correlates of verbal and visuospatial working memory. Similar to other studies that have failed to replicate the classic hemispheric effects (Clark et al., 2000; Courtney et al., 1996; Veltman, Rombouts & Dolan, 2003; Glabus et al., 2003; Tomlinson et al., 2014), the current resting state analyses did not show clear lateralization effects for verbal or visuospatial WM measures when using fALFF. A possibility is that no left lateralization effect was found for our verbal WM measure given that we looked at the combined effect of a task that mainly relied on maintenance (digits forward) and a task that relied additionally on manipulation of information (digits backward). Left lateralization was observed when solely looking at the effect of maintenance processes in verbal WM (i.e., digits forward). These findings are in line with the finding of a task (manipulation vs. maintenance) × load interaction in a predominantly right lateralized network, which was driven by the manipulation task (Veltman et al., 2003). Another possibility is that a lateralized pattern of activity might be present in regions previously reported to be involved in verbal and visuospatial working memory, however, those regions might not necessarily show a stable effect over subjects.
Seed-based Connectivity
The connectivity analysis showed that connectivity strength between the dorsolateral prefrontal cortex (DLPFC) and the superior parietal lobule (SPL) was correlated with several memory measures. That is, both left and right fronto-parietal connectivity strength was correlated with the general memory score. In contrast to the fALFF results, however, left fronto-parietal connectivity strength predicted the verbal memory score, whereas right fronto-parietal connectivity strength predicted the visuospatial memory score. These findings corroborate findings that memory relies on communication within a fronto-parietal network. The putative hemispheric specialization may depend on how hemispheric contribution is measured. While the intrinsic activity within each hemisphere does not show the left/right verbal/visual dissociation, the connectivity within each hemisphere does. Individual regions within a hemisphere may not favor verbal vs. visual material, these preferences likely emerge at a network level.
It could be argued that the correlation between fronto-parietal connectivity and the general memory measure reflects differences in the age of our participants. The fact that none of the partial correlations between fronto-parietal connectivity and memory measures were significant, after removing effects of age, supports this idea. Nonetheless, the results suggest that the strength of functional coupling between frontal and parietal memory structures, driven by age or other factors, can explain differences in general memory capacities. The dissociation in our analysis between left/right fronto-parietal connectivity strength and verbal/visuospatial working memory, argues against an unspecific deterioration of cerebral connectivity as explanation for a decline in general memory measures. That is, it illustrates the specificity of the relation between fronto-parietal connectivity strength and memory submeasures.
Multiple regression analyses
A multiple regression analysis in which STM and WM sum scores were orthogonalized with respect to each other, showed a positive correlation between the WM sum score and fALFF values within the Dorsal Lateral Prefrontal Cortex (DLPFC). No significant correlation was observed between the STM sum score and fALFF values, after orthogonalizing with respect to the WM sum score. These results are in line with the idea that there is a large overlap in the processes that underlie short-term memory and working memory constructs (Colom et al., 2006). STM is often operationalized as the process of maintenance of information, whereas WM is argued to involve the maintenance and manipulation of information (Davidson et al., 2006; Jensen et al., 2007). In this operationalization the STM component is part of both STM and WM tasks, whereas WM would tap into additional executive processes not shared with STM. This can explain the finding that no significant correlation was observed for the STM score after it was orthogonalized with respect to the WM score. In addition, the multiple regression analysis seems to suggest that the executive processes of WM, which are not shared with STM, might be located within the DLPFC. These findings mesh with evidence from both neuroimaging and patient studies, which point towards a critical role for the DLPFC in the active maintenance of information in working memory (Narayanan et al., 2005; Zarahn et al., 2005; Gazzaniga et al., 2009).
Furthermore, a multiple regression analysis in which verbal and visuospatial sum scores were orthogonalized with respect to each other did not change the results for the verbal memory component. The results for the visuospatial memory are weakened and become marginally significant. A number of studies have indeed reported high correlations between the scores on tests that measure verbal and visuospatial memory (Alloway, Gathercole & Pickering, 2006; Nadler & Archibald, 2014). In the case of our study, we acknowledge that part of the domain-specific effect that we observed might reflect variance that is shared between these two measures.
Conclusion
Our results show that spontaneous fluctuations in the BOLD signal during rest are associated with measures of general, domain-specific and demand-specific memory capacities. Low-frequency fluctuations in a wide-scale fronto-parietal network were associated with a measure of general memory performance. Distinct substructures within this network were associated with domain-specific and demand-specific memory capacities. These findings provide evidence that, spontaneous BOLD fluctuations in specific brain regions, occurring in the absence of an active task, affects performance on cognitive tasks that rely on those structures. A specialized WM system with distinct brain regions involved in domain-specific and demand-specific sub-processes was partially reflected in inter-individual differences in low-frequency fluctuations of the resting-state fMRI signal of our participants. In addition, the strength of functional coupling between frontal and parietal memory structures could explain inter-individual differences in memory capacities. These findings indicate that patterns of regional spontaneous brain activity and connectivity can be a valuable and powerful tool in revealing inter-individual differences in memory and other cognitive functions.
Figure 3.
Highlights.
Fractional amplitude of low frequency fluctuations (fALFFs) were calculated
Spontaneous fluctuations in the BOLD signal are associated with memory measures
Fronto-parietal connectivity strength explains differences in memory capacities
Acknowledgment
This research was supported by NIH/NIDCD grant R01 DC010783 (RHD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Woo et al. (2014) suggest using a voxelwise threshold of p < 0.001 or lower. This proposal was based on simulations on data from an experiment that involved physical application of painful heat at different levels of intensity. Such sensory tasks often cause strong activations that are large in extent, and hence the recommended more stringent threshold is appropriate. On the other hand, studies of higher cognitive functions often involve comparison of conditions that are closely matched in their sensory properties, and differ in more subtle ways. In our experience, such contrasts show activations are are lower in magnitude, and are much more variable between subjects. To balance false positives and false negatives in group maps, thresholds in the range of p < 0.025 – p < 0.005 are more appropriate in these cases. This is why a large number of cognitive experiments use voxelwise thresholds in the neighborhood of p < 0.01, and similar values are used as defaults in packages such as SPM. While Woo et al.’s (2014) recommendation remains valuable in many cases; our choice of threshold is based on these considerations.
References
- Alloway TP, Gathercole SE, Pickering SJ. Verbal and visuospatial short-term and working memory in children: are they separable? Child Development. 2006;77(6):1698–1716. doi: 10.1111/j.1467-8624.2006.00968.x. [DOI] [PubMed] [Google Scholar]
- Awh E, Jonides J, Smith E, Schumacher E, Koeppe R, Katz S. Dissociation of storage and rehearsal in verbal working memory: evidence from positron emission tomography. Psychological Science. 1996;7:25–31. [Google Scholar]
- Alloway TP, Alloway RG. Investigating the predictive roles of working memory and IQ in academic attainment. Journal of Experimental Child Psychology. 2010;106(1):20–29. doi: 10.1016/j.jecp.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Baddeley A. The episodic buffer: a new component of working memory? Trends in Cognitive Sciences. 2000;4(11):417–423. doi: 10.1016/s1364-6613(00)01538-2. [DOI] [PubMed] [Google Scholar]
- Baddeley A. Working memory: theories, models, and controversies. Annual Review of Psychology. 2012;63:1–29. doi: 10.1146/annurev-psych-120710-100422. [DOI] [PubMed] [Google Scholar]
- Baddeley A, Hitch G. The psychology of learning and motivation: advances in research and theory. New York: Academic Press; 1974. Working memory; pp. 47–89. [Google Scholar]
- Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philosophical Transactions of the Royal Society of Biological Sciences. 2005;360(1457):1001–1013. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Desai RH, Graves WW, Conant LL. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cerebral Cortex. 2009;19:2767–2796. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal BB, Van Kylen J, Hyde JS. Simultaneous assessment of flow and BOLD signals in resting-state functional connectivity maps. NMR in Biomedicine. 1997;10(4–5):165–170. doi: 10.1002/(sici)1099-1492(199706/08)10:4/5<165::aid-nbm454>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magnetic Resonance in Medicine. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Brunoni AR, Vanderhasselt M-A. Working memory improvement with non-invasive brain stimulation of the dorsolateral prefrontal cortex: A systematic review and meta-analysis. Brain and Cognition. 2014;86:1–9. doi: 10.1016/j.bandc.2014.01.008. [DOI] [PubMed] [Google Scholar]
- Ciaramelli E, Grady CL, Moscovitch M. Top-down and bottom-up attention to memory: a hypothesis (AtoM) on the role of the posterior parietal cortex in memory retrieval. Neuropsychologia. 2008;46(7):1828–1851. doi: 10.1016/j.neuropsychologia.2008.03.022. [DOI] [PubMed] [Google Scholar]
- Clark CR, Egan GF, McFarlane AC, Morris P, Weber D, Sonkkilla C, Tochon-Danguy HJ. Updating working memory for words: a PET activation study. Human Brain Mapping. 2000;9(1):42–54. doi: 10.1002/(SICI)1097-0193(2000)9:1<42::AID-HBM5>3.0.CO;2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colom R, Shih PC, Flores-Mendoza C, Quiroga MA. The real relationship between short-term memory and working memory. Memory. 2006;14:804–813. doi: 10.1080/09658210600680020. [DOI] [PubMed] [Google Scholar]
- Courtney SM, Petit L, Haxby JV, Ungerleider LG. The role of prefrontal cortex in working memory: Examining the contents of consciousness. Philosophical Transactions of the Royal Society of London: Series B. 1998;353:1819–1828. doi: 10.1098/rstb.1998.0334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney SM, Ungerleider LG, Keil K, Haxby JV. Object and spatial visual working memory activate separate neural systems in human cortex. Cerebral Cortex. 1996;6:39–49. doi: 10.1093/cercor/6.1.39. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Rombouts SA, Barkhof F, Scheltens P, Stam CJ, Smith SM, Beckmann CF. Consistent resting-state networks across healthy subjects. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(37):13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneman M, Carpenter PA. Individual differences in working memory and reading. Journal of Verbal Learning and Verbal Behaviour. 1980;19:450–466. [Google Scholar]
- Davidson MC, Amso D, Anderson LC, Diamond A. Development of cognitive control and executive functions from 4 to 13 years: evidence from manipulations of memory, inhibition, and task switching. Neuropsychologia. 2006;44:2037–2078. doi: 10.1016/j.neuropsychologia.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmond JE, Gabrieli JD, Wagner AD, Ginier BL, Glover GH. Lobular patterns of cerebellar activation in verbal working-memory and finger-tapping tasks as revealed by functional MRI. The Journal of Neuroscience. 1997;17(24):9675–9685. doi: 10.1523/JNEUROSCI.17-24-09675.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Esposito M, Aguirre GK, Zarahn E, Ballard D, Shin RK, Lease J. Functional MRI studies of spatial and nonspatial working memory. Cognitive Brain Research. 1998;7:1–13. doi: 10.1016/s0926-6410(98)00004-4. [DOI] [PubMed] [Google Scholar]
- Durisko C, Fiez JA. Functional activation in the cerebellum during working memory and simple speech tasks. Cortex. 2010;46(7):896–906. doi: 10.1016/j.cortex.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engle RW, Kane MJ, Tuholski SW. Individual differences in working memory capacity and what they tell us about controlled attention, general fluid intelligence, and functions of the prefrontal cortex. In: Miyake A, Shah P, editors. Models of working memory: Mechanisms of active maintenance and executive control. New York, NY: Cambridge University Press; 1999. pp. 102–134. [Google Scholar]
- Englund JA, Decker SL, Woodlief DT, DiStefano C. Development and evaluation of an online, multicomponent working memory battery. Assessment. 2014:1–19. doi: 10.1177/1073191114524016. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzaniga MS, Ivry RB, Mangun GR. Cognitive control – the lateral prefrontal cortex and working memory. In: Gazzaniga MS, Ivry RB, Mangun GR, editors. Cognitive Neuroscience: The Biology of the Mind. 3rd Edn. New York: W. W. Norton & Company, Inc.; 2009. pp. 558–564. [Google Scholar]
- Glabus MF, Horwitz B, Holt JL, Kohn PD, Gerton BK, Callicott JH, Berman KF. Interindividual differences in functional interactions among prefrontal, parietal and parahippocampal regions during working memory. Cerebral Cortex. 2003;13(12):1352–1361. doi: 10.1093/cercor/bhg082. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(1):253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M, Driesen NR, Skudlarski P, Gore JC, Constable RT. Brain connectivity related to working memory performance. The Journal of Neuroscience. 2006;26(51):13338–13343. doi: 10.1523/JNEUROSCI.3408-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoptman MJ, Zuo XN, Butler PD, Javitt DC, D’Angelo D, Mauro CJ, Milham MP. Amplitude of low-frequency oscillations in schizophrenia: a resting state fMRI study. Schizophrenia Research. 2010;117(1):13–20. doi: 10.1016/j.schres.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson JB, Uncapher MR, Wagner AD. Posterior parietal cortex and episodic retrieval: convergent and divergent effects of attention and memory. Learning and Memory. 2009;16(6):343–356. doi: 10.1101/lm.919109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeggi SM, Buschkuehl M, Jonides J, Perrig WJ. Improving fluid intelligence with training on working memory. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:6829–6833. doi: 10.1073/pnas.0801268105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen O, Kaiser J, Lachaux JP. Human gamma-frequency oscillations associatie with attention and memory. Trends in Neurosciences. 2007;30:317–324. doi: 10.1016/j.tins.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Jo HJ, Saad ZS, Simmons WK, Milbury LA, Cox RW. Mapping sources of correlation in resting state FMRI, with artifact detection removal. Neuroimage. 2010;52(2):571–582. doi: 10.1016/j.neuroimage.2010.04.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonides J, Schumacher EH, Smith EE, Koeppe RA, Awh E, Reuter-Lorenz PA, Marshuetz C, Willis CR. The role of parietal cortex in verbal working memory. Journal of Neuroscience. 1998;18(13):5026–5034. doi: 10.1523/JNEUROSCI.18-13-05026.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonides J, Schumacher EH, Smith EE, Lauber EJ, Awh E, Minoshima S, Koeppe RA. Verbal working memory load affects regional brain activation as measured by PET. Journal of Cognitive Neuroscience. 1997;9:462–475. doi: 10.1162/jocn.1997.9.4.462. [DOI] [PubMed] [Google Scholar]
- Kline RB. Principles and practice of structural equation modeling. 1st ed. New York: Guilford Press; 1998. [Google Scholar]
- Koenigs M, Barbey AK, Postle BR, Grafman J. Superior parietal cortex is critical for the manipulation of information in working memory. The Journal of Neuroscience. 2009;29(47):14980–14986. doi: 10.1523/JNEUROSCI.3706-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama MS, Kelly C, Shehzad Z, Penesetti D, Castellanos FX, Milham MP. Reading networks at rest. Cerebral Cortex. 2010;20:2549–2559. doi: 10.1093/cercor/bhq005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe MJ, Mock BJ, Sorenson JA. Functional connectivity in single and multislice echo-planar imaging using resting-state fluctuations. Neuroimage. 1998;7:119–132. doi: 10.1006/nimg.1997.0315. [DOI] [PubMed] [Google Scholar]
- McCarthy G, Puce A, Constable RT, Krystal JH, Gore JC, Goldman-Rakic P. Activation of human prefrontal cortex during spatial and nonspatial working memory tasks measured by functional MRI. Cerebral Cortex. 1996;6:600–611. doi: 10.1093/cercor/6.4.600. [DOI] [PubMed] [Google Scholar]
- Mennes M, Zuo XN, Kelly C, Di Martino A, Zang YF, Biswal B, Castellanos FX, Milham MP. Linking inter-individual differences in neural activation and behavior to intrinsic brain dynamics. Neuroimage. 2011;54:2950–2959. doi: 10.1016/j.neuroimage.2010.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachev P, Husain M. Disorders of visual attention and the posterior parietal cortex. Cortex. 2006;42:766–773. doi: 10.1016/s0010-9452(08)70415-5. [DOI] [PubMed] [Google Scholar]
- Nadler RT, Archibald LMD. The assessment of verbal and visuospatial working memory with school age Canadian children. Canadian Journal of Speech-Language Pathology and Audiology. 2014;38(3):262–279. [Google Scholar]
- Narayanan NS, Prabhakaran V, Bunge SA, Christoff K, Fine EM, Gabrieli JDE. The role of the prefrontal cortex in the maintenance of verbal working memory: an event-related fMRI analysis. Neuropsychologia. 2005;19:223–232. doi: 10.1037/0894-4105.19.2.223. [DOI] [PubMed] [Google Scholar]
- Nee DE, Brown JW, Askren MK, Berman MG, Demiralp E, Krawitz A, Jonides J. A meta-analysis of executive components of working memory. Cerebral Cortex. 2013;23:264–282. doi: 10.1093/cercor/bhs007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Scalaidhe SP, Wilson FAW, Goldman-Rakic PS. Areal segregation of face-processing neurons in prefontal cortex. Science. 1997;278:1135–1138. doi: 10.1126/science.278.5340.1135. [DOI] [PubMed] [Google Scholar]
- Owen AM. The functional organization of working memory processes within human lateral frontal cortex: The contribution of functional neuroimaging. European Journal of Neuroscience. 1997;9:1329–1339. doi: 10.1111/j.1460-9568.1997.tb01487.x. [DOI] [PubMed] [Google Scholar]
- Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Human Brain Mapping. 2005;25:46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulesu E, Frith CD, Frackowiak RSJ. The neural correlates of the verbal component of working memory. Nature. 1993;362:342–345. doi: 10.1038/362342a0. [DOI] [PubMed] [Google Scholar]
- Redick TS, Lindsey DRB. Complex span and n-back measures of working memory: A meta-analysis. Psychonomic Bulletin and Review. 2013;20:1102–1113. doi: 10.3758/s13423-013-0453-9. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Jonides J, Smith EE, Hartley A, Miller A, Marshuetz C, Koeppe RA. Age differences in the frontal lateralization of verbal and spatial working memory revealed by PET. Journal of Cognitive Neuroscience. 2000;12:174–187. doi: 10.1162/089892900561814. [DOI] [PubMed] [Google Scholar]
- Rottschy C, Lagner R, Dogan I, Reetz K, Laird AR, Schulz JB, Fox PT, Eickhoff SB. Modelling neural correlates of working memory: a coordinate-based meta-analysis. Neuroimage. 2011;60:830–846. doi: 10.1016/j.neuroimage.2011.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad ZS, Glen DR, Chen G, Beauchamp MS, Desai R, Cox RW. A new method for improving functional-to-structural MRI alignment using local Pearson correlation. Neuroimage. 2009;44:839–848. doi: 10.1016/j.neuroimage.2008.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack AT. Parietal cortex and spatial cognition. Behavioral Brain Research. 2009;202:153–161. doi: 10.1016/j.bbr.2009.03.012. [DOI] [PubMed] [Google Scholar]
- Sala-Llonch R, Peña-Gómez C, Arenaza-Urquijo Vidal-Piñeiro D, Bargalló N, Junqué C, Bartrés-Faz D. Brain connectivity during resting state and subsequent working memory task predicts behavioral performance. Cortex. 2012;48:1187–1196. doi: 10.1016/j.cortex.2011.07.006. [DOI] [PubMed] [Google Scholar]
- Sandrini M, Fertonani A, Cohen LG, Miniussi C. Double dissociation of working memory load effects induced by bilateral parietal modulation. Neuropsychologia. 2012;50:396–402. doi: 10.1016/j.neuropsychologia.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seghier ML. The angular gyrus: multiple functions and multiple subdivisions. The Neuroscientist. 2013;19(1):43–61. doi: 10.1177/1073858412440596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sliwinska MW, Khadilkar M, Campbell-Ratcliffe J, Quevenco F, Devlin JT. Early and sustained supramarginal gyrus contributions to phonological processing. Frontiers in Psychology. 2012;3(161):1–10. doi: 10.3389/fpsyg.2012.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EE, Jonides J. Storage and executive processes in the frontal lobes. Science. 1999;283:1657–1661. doi: 10.1126/science.283.5408.1657. [DOI] [PubMed] [Google Scholar]
- Smith E, Jonides J, Koeppe RA. Dissociating verbal and spatial working memory using PET. Cerebral Cortex. 1996;6:11–20. doi: 10.1093/cercor/6.1.11. [DOI] [PubMed] [Google Scholar]
- Smith EE, Jonides J, Koeppe RA, Awh E, Schumacher EH, Minoshima S. Spatial versus object working-memory: PET investigations. Journal of Cognitive Neuroscience. 1995;7:337–356. doi: 10.1162/jocn.1995.7.3.337. [DOI] [PubMed] [Google Scholar]
- Swanson HL, Siegel L. Learning disabilities as a working memory deficit. Issues in Education. 2001;7:1–48. [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. New York: Thieme Medical; 1988. [Google Scholar]
- Taylor PA, Saad ZS. FATCAT: (An efficient) functional and tractographic connectivity analysis toolbox. Brain Connectivity. 2013;3(5):523–535. doi: 10.1089/brain.2013.0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson SP, Davis NJ, Morgan HM, Bracewell RM. Cerebellar contributions to verbal working memory. Cerebellum. 2014;13(3):354–361. doi: 10.1007/s12311-013-0542-3. [DOI] [PubMed] [Google Scholar]
- Van Asselen M, Kessels RPC, Neggers SFW, Kapelle J, Frijns CJM, Postman A. Brain areas involved in spatial working memory. Neuropsychologia. 2006;44(7):1185–1194. doi: 10.1016/j.neuropsychologia.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Van den Heuvel MP, Hulshoff Pol HE. Specific somatotopic organization of functional connections of the primary motor network during resting-state. Human Brain Mapping. 2010a;31(4):631–644. doi: 10.1002/hbm.20893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Heuvel MP, Hulshoff Pol HE. Exploring the brain network: A review on resting-state fMRI functional connectivity. European Neuropsychopharmacology. 2010b;20(8):519–534. doi: 10.1016/j.euroneuro.2010.03.008. [DOI] [PubMed] [Google Scholar]
- Van den Heuvel MP, Mandl RC, Hulshoff Pol HE. Normalized group clustering of resting-state fMRI data. PloS ONE. 2008;3(4):e2001. doi: 10.1371/journal.pone.0002001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veltman DJ, Rombouts SARB, Dolan RJ. Maintenance versus manipulation in verbal working memory revisited: an fMRI study. Neuroimage. 2003;18(2):247–256. doi: 10.1016/s1053-8119(02)00049-6. [DOI] [PubMed] [Google Scholar]
- Vilberg KL, Rugg MD. Memory retrieval and the parietal cortex: a review of evidence from a dual-process perspective. Neuropsychologia. 2008;46(7):1787–1799. doi: 10.1016/j.neuropsychologia.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Smith EE. Neuroimaging studies of working memory: A meta-analysis. Cognitive, Affective, & Behavioral Neuroscience. 2003;3(4):255–274. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]
- Wilson FA, Scalaidhe SP, Goldman-Rakic PS. Dissociation of object and spatial processing domains in primate prefrontal cortex. Science. 1993;260:1955–1958. doi: 10.1126/science.8316836. [DOI] [PubMed] [Google Scholar]
- Woo CW, Krishnan A, Wager TD. Cluster-extent based thresholding in fMRI analyses: Pitfalls and recommendations. Neuroimage. 2014;91:412–419. doi: 10.1016/j.neuroimage.2013.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong J, Ma L, Wang B, Narayana S, Duff EP, Egan GF, Fox PT. Long-term motor training induced changes in regional cerebral blood flow in both task and resting states. Neuroimage. 2008;45(1):75–82. doi: 10.1016/j.neuroimage.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang YF, He Y, Zhu CZ, Cao QJ, Sui MQ, Liang M, Yu-Feng W. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain and Development. 2007;29(2):83–91. doi: 10.1016/j.braindev.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Zarahn E, Rakitin B, Abela D, Flynn J, Stern Y. Positive evidence against human hippocampal involvement in working memory maintenance of familiar stimuli. Cerebral Cortex. 2005;15:303–316. doi: 10.1093/cercor/bhh132. [DOI] [PubMed] [Google Scholar]
- Zou Q-H, Zhu C-Z, Yang Y, Zuo X-N, Long X-Y, Cao Q-J, Zang Y-F. An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: fractional ALFF. Journal of Neuroscience Methods. 2008;172:137–141. doi: 10.1016/j.jneumeth.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Q, Ross TJ, Gu H, Geng X, Xi-Nian Z, Hong LE, Yang Y. Intrinsic resting-state activity predicts working memory brain activation and behavioral performance. Human Brain Mapping. 2013;34:3204–3215. doi: 10.1002/hbm.22136. [DOI] [PMC free article] [PubMed] [Google Scholar]