Abstract
Social exclusion is a potent elicitor of distress. Previous studies have shown that medial frontal theta oscillations are modulated by the experience of social exclusion. Using the Cyberball paradigm, we examined event-related dynamics of theta power in the EEG at medial frontal sites while children aged 8–12 years were exposed to conditions of fair play and social exclusion. Using an event-related design, we found that medial frontal theta oscillations (4–8 Hz) increase during both early (i.e., 200–400 ms) and late (i.e., 400–800 ms) processing of rejection events during social exclusion relative to perceptually identical “not my turn” events during inclusion. Importantly, we show that only for the later time window (400–800 ms) slow-wave theta power tracks self-reported ostracism distress. Specifically, greater theta power at medial frontal sites to “rejection” events predicted higher levels of ostracism distress. Alpha and beta oscillations for rejection events were unrelated to ostracism distress at either 200–400 ms or 400–800 ms time windows. Our findings extend previous studies by showing that medial frontal theta oscillations for rejection events are a neural signature of social exclusion, linked to experienced distress in middle childhood.
Keywords: theta oscillations, social exclusion, ostracism, medial frontal cortex, children, event-related spectral perturbations
Introduction
Loss of social connections poses a threat to survival for many species (MacDonald and Leary, 2005). In humans, the potency of the experience is so strong that it triggers distress even when the excluders are fictitious (Crowley et al., 2009; Zadro et al., 2004) or from despised outgroups (Gonsalkorale and Williams, 2007). In real world situations, social exclusion negatively impacts physical and mental health (Cohen and Janicki-Deverts, 2009; Holt-Lunstad et al., 2010), reflected in lower levels of self-esteem (Deater-Deckard, 2001) and poorer academic self-confidence (Buhs, 2005), as well as higher levels of aggression, depression, and anxiety (Ladd, 2006). Within the neurosciences, the study of social pain has become a fruitful line of inquiry, creating situations in which the participant is left out of an interaction, evaluated poorly, or “voted off the island” (Eisenberger et al., 2003; Guyer et al., 2009; Kujawa et al., 2014).
One of the most widely used experimental paradigms for studying social exclusion, the Cyberball paradigm (Williams and Jarvis, 2006), reliably induces mild distress, offering a way to experimentally probe the neural correlates of being physically “left out” of an interaction. Ostensibly played over the Internet as a virtual ball toss-and-catch game, Cyberball requires a participant to make throws to, and receive throws from two or more cyber players. Unbeknownst to the participant, the players are in fact computer-generated. Seamlessly and without warning, an “exclusion” phase ensues—the cyber players exclude the participant, throwing only to one another. These seemingly simple bouts of ostracism negatively impact self-esteem and belonging (Ruggieri et al., 2013; Williams et al., 2000). For example, compared to those low in social anxiety, high socially anxious individuals are slower to recover from distress following ostracism (Oaten et al., 2008), and victims of bullying score lower on feelings of recognition by others after being socially excluded compared to non-victims (Ruggieri et al., 2013).
A number of studies have now used Cyberball to examine the neural correlates of social exclusion, and suggest that the medial frontal cortex plays a fundamental role in regulation of negative affect associated with exlclusion (Gunther-Moor et al., 2012; Rotge et al., 2015; Themanson et al., 2013). Functional magnetic resonance imaging (fMRI) and electroencephalogram (EEG) measures both show that various frontal responses are related to measures of distress, ostracism, mood, and attachment (Crowley et al., 2009; Crowley et al., 2010; Eisenberger et al., 2003; Masten et al., 2009; McPartland et al., 2011; Sreekrishnan et al., 2014; White et al., 2013; White et al., 2012). fMRI studies have identified a number of frontal regions engaged during social exclusion including the dorsal and ventral ACC, medial prefrontal cortex, ventro-lateral prefrontal cortex (Gunther-Moor et al., 2012), subgenual ACC (Masten et al., 2009; Masten et al., 2011), and right ventral prefrontal cortex (Eisenberger et al., 2003).
Several event-related potential (ERP) studies document activation patterns at frontal scalp sites that track experienced distressed during social exclusion. For example, rejection events have been shown to engage distinct slow-wave ERP activity at left/medial frontal sites, which is correlated with self-reported distress in adults (Crowley et al., 2009). Similarly, Crowley et al. (2010) found that in middle-childhood, children scoring higher in ostracism distress elicited larger (i.e., more negative) slow wave ERPs at medial frontal sites following rejection events in the Cyberball task. Similar results were reported by White et al. (2012) who found that larger negative slow-wave ERP activity in left/medial frontal sites to rejection events predicted decreased quality of attachment in children. More recently, the effects of kin exclusion were examined in children and their mothers. Slow-wave ERP activity at frontal sites was correlated with ostracism distress when linked to rejection events during exclusion by kin, but not strangers (Sreekrishnan et al., 2014).
Although the ERP approach has utility as a fixed-latency, average amplitude measure, it discards important information about task-relevant EEG oscillatory dynamics that could provide new insights into neural processing of social exclusion. Due to signal averaging, ERPs only reflect phase locked activity and typically do not distinguish among the EEG frequency spectra most strongly engaged for a particular event type (theta, 4–7 Hz; alpha, 8–15 Hz; beta, 16–30 Hz, etc.). Thus a useful complement to the ERP approach is the event-related spectral perturbation (ERSP), which reflects the event-locked spectral properties of induced power changes in the EEG signal (Makeig et al., 2004). Specifically, ERSP is a temporally sensitive measure of relative change in mean EEG power from baseline associated with stimulus presentation or response execution. Unlike ERPs, ERSPs capture changes in spontaneous EEG activity occurring across frequency spectra. They are sensitive to signal fluctuations that are temporally stable, but not coherent in phase angle (Makeig et al., 2004). A recent study by Cristofori et al. (2013) stronly supports the utility of examining EEG oscillations in response to social exclusion. They collected intracerebral EEG recordings while epileptic patients played Cyberball. Patients showed increased power in the theta band during overall exclusion versus inclusion experience, leading Cristofori et al. (2013) to propose theta as a “neural signature” of social exclusion.
While the theta-social exclusion connection is compelling, a large body of literature also links theta-band oscillatory dynamics to attentional processes and cognitive control (Basar et al., 2001; Cavanagh et al., 2010; Klimesch, 1999; Sauseng et al., 2006). Theta signatures in the EEG have been observed during error/conflict monitoring (Trujillo and Allen, 2007) and feedback processing (Cavanagh et al., 2010), behavioral inhibition and attentional control (Cavanagh et al., 2012), task switching (Sauseng et al., 2006), engagement of working memory (Sauseng et al., 2010), the regulation of affective responses (Knyazev, 2007; Luu et al., 2000b) and cognitive re-appraisal of emotions (Ertl et al., 2013). Many of these processes may be engaged during social exclusion, suggesting the nature by which theta oscillations can be considered a neural signature of social exclusion needs further elucidation.
Current Study
The goal of the current study is to understand how we might consider theta as a neural signature of social exclusion in Cyberball with a sample of typically developing children. This paper is a follow-up of a previous report on this sample (Crowley et al., 2010) in which we examined social exclusion and event-related potentials. Previous work implicated medial frontal ERP activity as a neural correlate social exclusion. Thus, we focus on this region for our event-related spectral analyses (see Supplemental Materials for posterior, lateral left and lateral right regional data). Building upon the established link between theta and an overall exclusion experience (Cristofori et al., 2013), we focus on the theta dynamics for the rejection events that comprise social exclusion. First we ask, do any aspects of a rejection event time course particularly engage theta dynamics versus alpha or beta EEG dynamics? Second, we contrast rejection events where participants do not receive the ball during exclusion with “not my turn” events where participants do not receive the ball during the course of an inclusion block (fair play). In this way, the perceptual characteristics of individual events compared (“rejection” vs. “not my turn”) are identical. Finally, we examine the association between theta during rejection events and the psychological experience of social exclusion referred to as ostracism distress.
Methods
Participants
Thirty-three children (17 male) 8–12 years of age participated in this study. Children’s ethnic backgrounds were as follows: 91% Caucasian, 9% African-American. Children played Cyberball while an EEG was acquired. Families were recruited via mass mailings with addresses provided by a credit and information agency. The parent of each child provided written parental informed consent while their child gave their written assent. The Yale University School of Medicine Human Investigation Committee approved this research. Children were compensated forty dollars for their participation
Procedure
Cyberball Social Exclusion Task
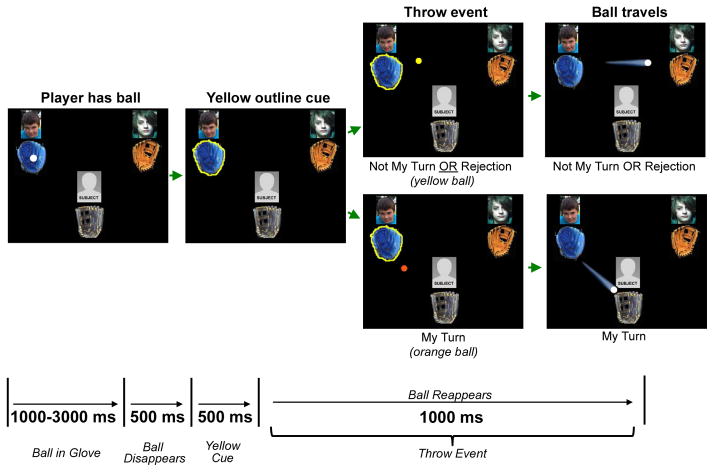
Cyberball is a computerized ball-toss game in which a participant ostensibly plays with two other players on over the internet. Players pass a white ball amongst themselves including the participant. The ball color changes with the ball position reflecting throws to the participant and the other players. Prior to each throw event, the ball disappears, then the glove is outlined in yellow as a cue signaling a throw will occur. The ball reappears on path to the subject or on a path to one of the other players (see Figure 1 for timing and more details). Abruptly, the other players exclude the participant, throwing only to one another. This exclusionary experience is distressing to participants, as per their self-reports of distress on a Need Threat Scale (described below).
Figure 1.
Example of the sequence of task events for a trial in Cyberball. The trial began with the ball being presented in the glove of one of the two players for a duration between 1000 and 3000 ms. Subsequently, the ball disappeared (500 ms) and the glove of the player throwing the ball was then outlined in yellow (500 ms). The throw event then took place in which the ball reappeared and, depending on the trial type, changed color to yellow or orange and travelled to the receiving player.
Each participant sat in a dimly lit (60w bulb), sound attenuated room, 60 cm from a 17 in. LCD monitor. Prior to beginning the experiment, the child’s gender and ethnicity were identified. Settings within the game ensured that the other players on the screen were of a similar age, ethnic appearance and gender (drawing on a bank of opponent pictures taken at the Child Study Center for use in research). At the outset of the game, the child saw an actual Google™ webpage, followed by a “Cyberball” web page, followed by a screen with a green status bar. Several other modifications were introduced to make the Cyberball game more engaging to children. The child chose from one of six different ball gloves to be his or her personal glove throughout the game. A female voice narrated instructions on the computer screen. From throw to throw, the ball traveled randomly along different paths (straight line, arc or sine wave); lifelike sound effects occurred as the ball traveled (swoosh) and landed in a glove. After the experiment, the child and parent were debriefed and informed that the other players were not real.
When the game began, the child’s glove was at the bottom center of the screen; the gloves of the other two players, chosen by the computer, were to the left and right of the screen center. Pictures of the other “players” appeared above their names and respective gloves. Participants used their left and right index fingers on a response pad to throw left or right to the other players. Each child was told that a picture was taken of them with a camera (focused on them) and that the other players would see this over the internet. Next, the child then overheard one experimenter telling a second experimenter s/he would knock on the door (closed) when the other players were ready to play on the internet. Three to five minutes elapsed before the knock occurred.
Our event-related version of Cyberball consisted of 155 trials over two blocks, a fair play and exclusion block. During the 108-trial fair play block, the cyber-players threw to the participant 36 times. Whether a ball was thrown to the participant during any one trial was pseudorandom and predetermined within a list such that the participant waited for either 0, 1, 2 or 3 throws by the other players before receiving the ball again (frequency 12, 12, 10 and 2, respectively). During fair play, cyber-players threw to one another and not to the participant 36 times, which comprised “not my turn” events. The participant threw back to the other “players” for the remaining 36 trials. Seamlessly, fair play folded into a 47-trial exclusion block. This block represented 96% exclusion. Of the 47 exclusion trials, the ball only came to the participant three times to maintain attention, once on trial fourteen, twenty-five and thirty-nine. Only 36 exclusion events from this block were used in ERP analyses. Eleven trials were not used. These included the first five trials of the exclusion block, the three throws to the participant during this block, and the three thrown back from the participant to the computer players.
Immediately after the game, children completed the Need Threat Scale, a reliable and valid 21-item ostracism distress measure which has been related to ERPs in this dataset (Crowley et al., 2010) and other studies (Crowley et al., 2009; White et al., 2012). In addition, rejection distress has been linked to fMRI BOLD signals in regions of the medial frontal cortex (Eisenberger et al., 2003; Gunther-Moor et al., 2012; Masten et al., 2009; Rotge et al., 2015). Children responded on the computer while still wearing the EEG cap. A female voice narrated each item and the child made his or her response to the item with a mouse. Once it was clear that the child understood how to use the mouse, the experimenter left the room so that the child could privately complete the need threat assessment. The Need Threat Scale gauges feelings of distress along four dimensions: belonging (“I felt rejected”), self-esteem (“I felt liked”), meaningful existence (“I felt invisible.”), control (“I felt powerful”), on a 5-point choice, from “Not at all” to “Extremely”. A majority of the research on the neural correlates of social exclusion relies on the sum of these four scales as an index of ostracism distress. For this scale, higher scores indicated greater distress. Items were summed for correlation analyses.
Electrophysiological Recording and Preprocessing
Using standard procedures, a high-density EEG was recorded from 128 Ag/AgCL electrodes (Electrical Geodesics Incorporated (EGI), Inc.) with Netstation v.4.2 software (EGI, Inc.) and high impedance amplifiers, sampled at 250hz (.1 Hz high pass, 100 Hz, low pass). All electrodes were referenced to Cz for recording. Before beginning, all impedances were at or under 40k ohms. The E-prime v.1.2 (Psychology Software Tools, Inc.) software package controlled the stimulus presentation.
Offline, task-related EEG data were submitted to a pre-processing procedure with custom in-house code created in MATLab 2010, executed in Octave 3.6.3 on the Shared Hierarchical Academic Research Computing Network (SHARCNet), to identify and remove artifacts. These pre-processing steps follow closely those that have been previously described (Desjardins and Segalowitz, 2013; van Noordt et al., 2015). After windowing the data in to 50% overlapping windows of 600 ms, the maximum correlation coefficient was calculated between each channel and the three nearest channels. Within each time window, channels that exceeded the 99% confidence interval were flagged as unreliable, and those that showed strong and invariable associations were flagged as bridged. In addition, time periods in which more than 10% of channels were unreliable were flagged for rejection. Once the identified channels and time periods were flagged, another iteration of the calculating the channel-neighbour correlation coefficient distribution was performed. In this distribution a channel was flagged if a coefficient was lower than the 99% confidence interval, and time containing more than 10% of flagged channels was also flagged. The data were then concatenated back into the continuous signal after flagged time windows and data segments of less than 2 seconds were removed. These remaining continuous data were de-trended and submitted to extended Infomax ICA with a PCA reduction of N-1 channels. A second ICA was run after following the same flagging and rejection procedure for channels, using the standard deviation of ICs, in order to indentify time courses which produce unreliable activations in at least 10% of the components. After ICA time pruning, IC weights from the second decomposition were applied to the raw continuous data, filtered DC-to-30 Hz.
From these data, eye blinks, saccades, electrocardiogram and electromyogram artifacts were rejected manually on the basis of IC topographical projections and the corresponding activation in their continuous signal. The scalp data were re-constructed using the non-artifactual ICs and interpolated back to a standard 90 channel montage following the 10–20 system. Segments were 3-seconds in length, 1000 ms pre-stimulus, and rejected if containing large voltage fluctuations exceeding +/− 75 μV. ERPs were derived only when the ball reappeared after leaving the glove of the cyber-players, but before traveling on the screen (see Figure 1 for events). The average number of trials included were similar across the “not my turn” (M = 33, SD = 3.84, minimum = 26) and “rejection” trial types (M = 29, SD = 4.32, minimum = 19). Analyses included 31 subjects due to the exclusion of two whose EEG was contaminated with artifact.
Time-frequency decomposition
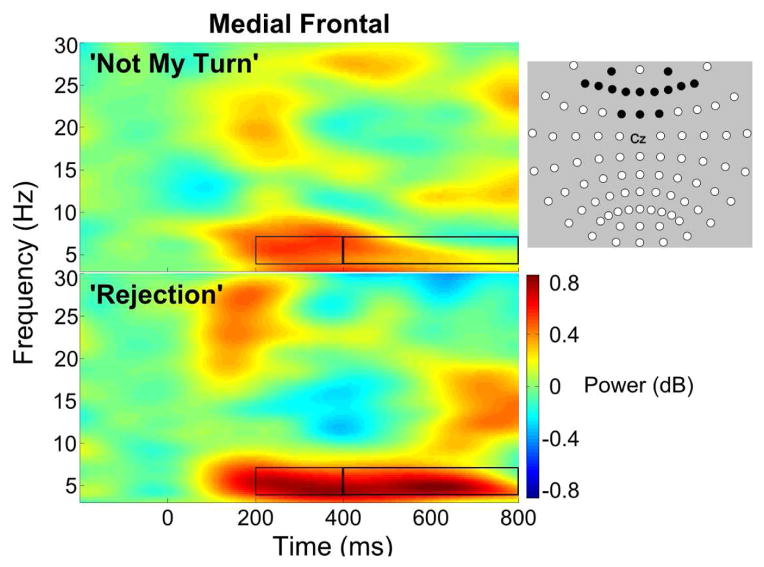
Changes in non-phase locked spectral power (ERSP) were extracted from time-frequency matrices that were created using the ‘newtimef’ function in EEGLab. The 3 second epochs were convolved using Morlet wavelets to yield a time x frequency spectrogram with frequencies ranging from 3 to 30 Hz and a time span of −444 to 1432 ms. The decomposition included 3 cycles at the lowest frequency and increased, by a factor of .5, to 15 at the highest frequency, with a sliding window length of 1116 ms. Peak event-related spectral power (event related spectral perturbation) was then extracted from the pre-computed matrices between 200–400 ms and 400–800 ms for theta (4–7 Hz), alpha (8–15 Hz), and beta (16–30 Hz) frequency bands. A cluster of medial frontal channels were averaged together to represent the region of interest (see Figure 2), approximating clusters that have been used in previous studies (Crowley et al., 2010; White et al., 2013).
Figure 2.
Time-frequency spectrograms show event-related changes in spectral power following ‘Not My Turn’ and ‘Rejection’ feedback (onset = time 0 ms) during Cyberball. Black rectangles highlight time ranges used for comparison of spectral power across feedback conditions, including early (200 – 400 ms) and slow-wave (400 – 800 ms) activity. The black channels in the montage represent the medial frontal cluster that was used to analyze event-related spectral perturbations.
Results
Need Threat Scale
For purposes of comparison with other Cyberball studies, we report ostracism distress as the mean across items on the Need Threat Scale. Following exclusion, mean distress across the whole sample was 3.15 (SD = .79). This level of ostracism following exclusion was similar to a recent adolescent and adult Cyberball study which found mean ostracism distress was 3.70 (SD = .87) (Sebastian et al., 2011). The scores we observed were somewhat greater than our previous study of children and adolescents 2.57 (SD = .77) (Bolling et al., 2011).
Medial frontal theta and rejection
The time-frequency spectrograms for the medial frontal cluster indicate an increase in theta power following feedback (see Figure 2). Particularly for rejection events it is also clear that the increases in theta power are relatively sustained bursts throughout the oscillation, with peaks during early (corresponding to the FRN latency range) and later (corresponding with slow-wave latency range) stages of processing.
A 2 (Condition: ‘Not My Turn’, ‘Rejection’) by 2 (Time: ‘200–400’, ‘400–800’) repeated measures ANOVA was performed to assess potential differences in peak theta power. As expected there was a main effect of condition indicating that peak theta power was greater during “rejection” events compared to “not my turn” (F(1,30) = 7.53, p = .01). There were no reliable differences in peak theta power across time windows, or the interaction between condition and time.
For comparison purposes, we also carried out the same 2 by 2 repeated measures ANOVA for the alpha and beta frequency bands. Peak alpha power showed main effects for condition (F(1,30) = 11.79, p = .002; greater for ‘rejection’) and time (F(1,30) = .63, p = .017; greater during ‘400–800’), but no significant interaction. Only a main effect of time was observed for peak beta power (F(1,30) = 13.57, p = .001; greater during ‘400–800’), with unreliable effects across conditions or the interaction between condition and time. See Figure 3 for a summary of the descriptive statistics used for the above ANOVAs.
Figure 3.
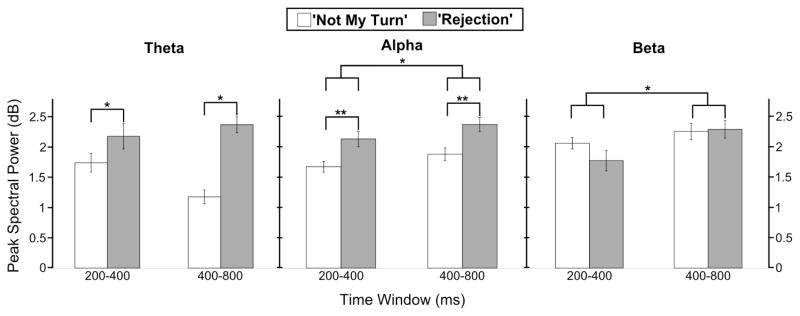
Bars represent peak changes in event-related spectral power following ‘Not My Turn’ and ‘Rejection’ feedback for theta, alpha, and beta frequency bands across early (200 – 400 ms) and late (400 – 800 ms) time windows. Theta power was greater for ‘Rejection’ compared to ‘Not My Turn’ events, irrespective of time window. Alpha power was greater for ‘Rejection’ compared to ‘Not My Turn’ events, and for the late compared to the early time window. Beta power was greater during the late compared to early time window, irrespective of feedback type.
* p <.05, ** p <.01
Medial frontal theta slow-wave during rejection and ostracism
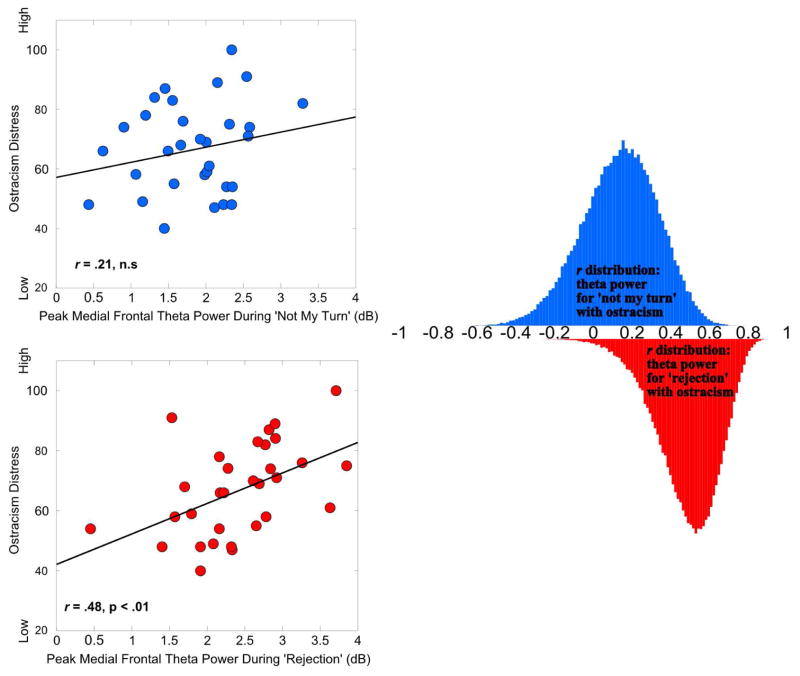
We then performed a series of Pearson r correlations, with False Discovery Rate (FDR) correction, to determine whether medial frontal theta slow-wave activity was associated with ostracism distress. This approach is extending our findings, given that “rejection” events elicit greater medial frontal theta power compared to “not my turn”, in conjunction with previous findings suggesting that slow-wave activity tracks ostracism distress. Supporting our hypothesis, and in line with previous results, we found that ostracism distress was related slow-wave theta band responses at medial frontal sites, specifically following social exclusion (see Figure 4). To provide discriminant validity we also tested the relationship between ostracism and peak activity across time for both alpha and beta frequency bands. None of these correlations were reliable even at the conventional .05 alpha level. See Table 1 for a summary of correlations.
Figure 4.
Scatterplots show bi-variate associations between self-reported Ostracism Distress and peak medial frontal theta power during slow-wave activity (400 – 800 ms) for ‘Not My Turn’ (blue) and ‘Rejection’ (red) feedback events. Histograms represent distribution of Pearson r coefficients obtained from bootstrap re-sampling using 100,000 iterations. The re-sampling procedure confirms that the relationship between slow-wave medial frontal theta elicited by ‘Rejection’ events and Ostracism Distress (Robustr = .56, p = .004, 95% CI [.19, .78]) is more robust than the correlation between medial frontal theta during ‘Not My Turn’ events (Robustr = .03, p = .72, 95% CI [−.32, .45]) and Ostracism Distress, which is unreliable.
Table 1.
Pearson R coefficients showing the relations between ostracism and peak spectral power for theta, alpha, and beta, across time and condition
| Theta | Alpha | Beta | ||||
|---|---|---|---|---|---|---|
| 200–400 | 400–800 | 200–400 | 400–800 | 200–400 | 400–800 | |
| ‘Not My Turn’ | .18 | .21 | .28 | .33 | .02 | .27 |
| ‘Rejection’ | .33 | .48** | .06 | .17 | .09 | .03 |
p < .01
Using a more in-depth analytical approach to verify the specificity of the correlation between medial frontal slow-wave frontal theta activity and ostracism, we implemented a robust estimation technique (Wilcox, 2012) for calculating Pearson r coefficients. Specifically, paired values were re-sampled with replacement to create surrogate distributions of the association between theta power and ostracism. Iterating this process 100,000 times and winsorizing 20% of the tails provides a robust measure of the correlation and confidence intervals around the coefficient. Although the there is some overlap in the distributions, the relationship between slow-wave medial frontal theta elicited by “rejection” events and ostracism (Robustr = .56, p = .004, 95% CI [.19, .78]) is robust, whereas the correlation between ostracism and medial frontal theta during “not my turn” events (Robustr = .03, p = .72, 95% CI [−.32, .45]). Moreover, the negative skew in the distribution of correlation coefficients for slow-wave “rejection” events suggests a stronger and more reliable relationship than the distribution for “not my turn” events, which approximates normality.
Discussion
This study solidly documents event-related theta oscillations as a neural signature of social exclusion. We observed that theta power at medial frontal scalp sites was greater for “rejection” events compared to “not my turn” events across both early (i.e., ‘200–400’ ms) and late (i.e., ‘400–800’ ms) portions of the event. Although condition and time effects emerged for the alpha frequency band, and a time effect emerged for the beta frequency band, only theta frequency responses (400–800 ms) elicited by “rejection” events significantly predicted self-reported ostracism distress. Moreover, robust estimation methods verified that the theta-ostracism association was unique to “rejection” events as compared to “not my turn” events. These data converge with evidence from intracerebral recordings (Cristofori et al., 2013) implicating theta oscillations during exclusion, and ERP studies implicating responses recorded at medial frontal sites during “rejection” events in social exclusion (Crowley et al., 2009; Crowley et al., 2010).
Cristofori et al. (2013) provided direct evidence for distinct theta-band activity that was linked to social exclusion, through the use of intra-cranial recordings in epileptic patients while they performed the Cyberball task. Their results indicated a general increase in theta activity during blocks of social exclusion as compared to inclusion blocks. By implementing an event-related design and measuring self-reported ostracism distress, our findings helped to clarify the associations between theta activity and social exclusion. At the level of the scalp, we showed that medial frontal theta activity was greatest when individuals were faced with social rejection events relative to perceptually matched control events (“not my turn”) also used in previous ERP (Crowley et al., 2010) and fMRI studies (Gunther-Moor et al., 2012). Importantly, the magnitude of theta activity only tracked experienced distress in the 400–800 ms time window.
Recent proposals suggest that medial frontal theta activity is a neural correlate of self-regulation (Luu, Tucker, Derryberry, Reed, & Poulsen, 2003), especially in contexts high in uncertainty and those demanding the dynamic control over behavior (Cavanagh and Frank, 2014). Indeed, based on ample data relating the P2-N2 time window to conflict processing and reward-prediction errors, theta acitivty in the 200–400 ms time range we examined may reflect behavioral conflict and/or violation of expectation (Cavanagh et al., 2013). It is therefore tempting to suggest that during this period, theta activity may tap into processes of expectancy violation, also associated with social exclusion (Somerville et al., 2006). By contrast, theta modulation at later processing stages is more closely linked to distress and anxiety, much like the frontal slow-wave activity that has been reported in several Cyberball studies (Crowley et al., 2010; Sreekrishnan et al., 2014; White et al., 2012). It is well established that increases in theta-band activity in humans are associated with experience of undesirable outcomes (Cohen et al., 2007), unpleasant experiences/events (Vecchiato et al., 2011), and/or negative affective states (Luu et al., 2000a). Thus, considering medial frontal theta as a bio-marker for the severity of experienced distress during social exclusion, particularly in the 400–800 ms time range, fits well with findings from other studies and current models focused on the neural correlates of anxiety, distress, and the adaptive control of behavior (Cavanagh and Shackman, 2014).
While our findings and those of Cristofori et al. (2013) point to enhanced theta as a signature of social exclusion, we recognize that theta activity has been studied in a variety of experimental contexts, with evidence linking this frequency band to a myriad of cognitive functions including memory, attention and other cognitive functions (Basar et al., 2001; Cavanagh et al., 2010; Klimesch, 1999; Sauseng et al., 2006). As noted earlier, Cyberball itself likely engages multiple cognitive processes. Attention may vary as a function of whether or not the participant will receive the ball in a given trial during fair play and across fair play and exclusion blocks. Memory processes, including working memory, may be tracking who has received the ball more or less over the course of the game (Gardner et al., 2000). Social-cognitive processes such as considering others intentions (e.g., mentalizing) are also likely to occur (Knowles, 2014).
A range of experimental approaches could be useful for considering the potential contribution of various cognitive processes to exclusion theta effects. In terms of attention, examining our “yellow ball throw event cue” might reflect differential attention during fair play and exclusion. Likewise, concurrent eye-tracking could be useful for considering differential neural response to exclusion and “not my turn events” and related gaze patterns (Silk et al., 2012). Using ERPs, innovative work by Themanson and colleagues has broken the Cyberball task down into event-related steps to consider attention and feedback as discrete processes (Themanson et al., 2015). A Cyberball paradigm building on their design and examining event-related oscillations is likely to provide new insights about theta and likely other relevant spectral frequencies.
In the broader domain of self-regulation, social exclusion has been linked to a host of negative outcomes in youth including lower levels of self-esteem (Deater-Deckard, 2001; Ruggieri et al., 2013) and compromised mood regulation as reflected in higher levels of depression and anxiety (Ladd, 2006). With respect to oscillatory dynamics, theta-band activity has been tied to higher and more stable levels of anxiety and neurochemical changes in dopaminergic systems (Mizuki et al., 1992). Theta band activity is enhanced in persons with a history of depression during the processing of negatively valenced outcomes (Cavanagh et al., 2011). Other studies have reported that changes in theta vary across individuals, such that loss-induced increases in theta correlate with measures of avoidance and neuroticism (Neo and McNaughton, 2011). Taken together, the results of several studies implicate late theta-band activity during engagement of negative affective states. Further research should attempt to clarify the developmental trends between theta activity, experienced distress, and mental health.
Systematic investigation into theta dynamics and ostracism distress is an important line of inquiry given the combination of frontal lobe development and the importance of increasing social interactions that characterize childhood and adolescence. We studied a relatively narrow age range, which did not allow for consideration of developmental factors in this study. Our other work with feedback monitoring indicates that developmental changes in frontal midline theta are likely (Crowley et al., 2014). Given that fMRI work in social exclusion indicates that adolescents are more vulnerable to the effects of social exclusion (Pharo et al., 2011; Sebastian et al., 2010) we might expect to see stronger engagement of frontal midline theta oscillations in adolescents.
Limitations
Our goal was to examine whether medial frontal theta responses in children were perturbed by social rejection events during exclusion, and whether these changes tracked experienced ostracism distress. Following from previous studies, we focused our analyses on medial frontal scalp sites and the dynamics of ERPs to social “rejection” events and “not my turn” events. Beyond statements about scalp location, we cannot affirm the location of cortical EEG sources. Approaches that use source modeling of oscillatory process such as beamformer could be informative here.
Application of ICA in the current study was employed to systematically remove known biological artifacts from the EEG, but our medial frontal cluster could still represent some degree of mixed signal projections at the scalp. it would be informative to apply other analytical techniques, such as analysis of independent components, that are capable of isolating spatially fixed patterns of activity that might be linked to social rejection. Deriving a latent factor from the EEG that is unique to the processing of rejection events could provide a richer picture of the relationship between theta and ostracism distress.
Investigating these relationships in a sample of healthy children limits generalization of these effects to other age groups or samples presenting with clinical levels of mood disorders (e.g., anxiety or depression). It will be important to consider the association between theta activity and ostracism distress across development, especially for linking theta and ostracism distress to individual differences in clinical levels of anxiety and depression. Establishing models of theta and social exclusion could be applicable in understanding rejection sensitivity and how individual differences early in development may later manifest as clinical presentations of anxiety (Zadro et al., 2006) and depression (Platt et al., 2013).
Supplementary Material
Acknowledgments
This work was supported by the Bial Fellowship Programme 169/08 and NIDA K01DA034125, awarded to Michael J. Crowley.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Basar E, Basar-Eroglu C, Karakas S, Schurmann M. Gamma, alpha, delta, and theta oscillations govern cognitive processes. International Journal of Psychophysiology. 2001;39:241–248. doi: 10.1016/s0167-8760(00)00145-8. [DOI] [PubMed] [Google Scholar]
- Bolling DZ, Pitskel NB, Deen B, Crowley MJ, Mayes LC, Pelphrey KA. Development of neural systems for processing social exclusion from childhood to adolescence. Developmental Science. 2011;14:1431–1444. doi: 10.1111/j.1467-7687.2011.01087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhs ES. Peer rejection, negative peer treatment, and school adjustment: Self-concept and classroom engagement as mediating processes. Journal of School Psychology. 2005;43:407–424. [Google Scholar]
- Cavanagh JF, Bismark AJ, Frank MJ, Allen JJB. Larger error signals in MDD are associated with better avoidance learning. Front Psychol. 2011;2:1–6. doi: 10.3389/fpsyg.2011.00331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JF, Frank MJ. Frontal theta as a mechanism for cognitive control. Trends Cogn Sci. 2014;18:414–421. doi: 10.1016/j.tics.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JF, Frank MJ, Klein TJ, Allen JJ. Frontal theta links prediction errors to behavioral adaptation in reinforcement learning. Neuroimage. 2010;49:3198–3209. doi: 10.1016/j.neuroimage.2009.11.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JF, Shackman AJ. Frontal midline theta reflects anxiety and cognitive control: Meta-analytic evidence. Journal of Physiology - Paris. 2014 doi: 10.1016/j.jphysparis.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JF, Zambrano-Vazquez L, Allen JJ. Theta lingua franca: a common mid-frontal substrate for action monitoring processes. Psychophysiology. 2012;49:220–238. doi: 10.1111/j.1469-8986.2011.01293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Janicki-Deverts D. Can we improve our physical health by altering our social networks. Perspectives on Psychological Science. 2009;4:375–378. doi: 10.1111/j.1745-6924.2009.01141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristofori I, Moretti L, Harquel S, Posada A, Deiana G, Isnard J, Mauguiere F, Sirigu A. Theta signal as the neural signature of social exclusion. Cerebral Cortex. 2013;23:2437–2447. doi: 10.1093/cercor/bhs236. [DOI] [PubMed] [Google Scholar]
- Crowley MJ, van Noordt SJ, Wu J, Hommer RE, South M, Fearon RM, Mayes LC. Reward feedback processing in children and adolescents: medial frontal theta oscillations. Brain and Cognition. 2014;89:79–89. doi: 10.1016/j.bandc.2013.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley MJ, Wu J, McCarty ER, David DH, Bailey CA, Mayes LC. Exclusion and micro-rejection: event-related potential response predicts mitigated distress. Neuroreport. 2009;20:1518–1522. doi: 10.1097/WNR.0b013e328330377a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley MJ, Wu J, Molfese PJ, Mayes LC. Social exclusion in middle childhood: rejection events, slow-wave neural activity, and ostracism distress. Social Neuroscience. 2010;5:483–495. doi: 10.1080/17470919.2010.500169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deater-Deckard K. Recent research examining the role of peer relations in development of psychopathology. Journal of Child Psychology and Psychiatry. 2001;42:565–579. [PubMed] [Google Scholar]
- Desjardins JA, Segalowitz SJ. Deconstructing the early visual electrocortical responses to face and house stimuli. J Vis. 2013;13 doi: 10.1167/13.5.22. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? An FMRI study of social exclusion. Science. 2003;302:290–292. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- Ertl M, Hildebrandt M, Ourina K, Leicht G, Mulert C. Emotion regulation by cognitive reappraisal - The role of frontal theta oscillations. Neuroimage. 2013;81:412–421. doi: 10.1016/j.neuroimage.2013.05.044. [DOI] [PubMed] [Google Scholar]
- Gardner WL, Pickett CL, Brewer MB. Social exclusion and selective memory: How the need to belong influences memory for social events. Personality and Social Psychology Bulletin. 2000;26:486–496. [Google Scholar]
- Gonsalkorale K, Williams KD. The KKK won’t let me play: Ostracism even by a despised outgroup hurts. European Journal of Social Psychology. 2007;37:1176–1186. [Google Scholar]
- Gunther-Moor B, Guroglu B, Op de Macks ZA, Rombouts SA, Van der Molen MW, Crone EA. Social exclusion and punishment of excluders: neural correlates and developmental trajectories. Neuroimage. 2012;59:708–717. doi: 10.1016/j.neuroimage.2011.07.028. [DOI] [PubMed] [Google Scholar]
- Guyer AE, McClure-Tone EB, Shiffrin ND, Pine DS, Nelson EE. Probing the neural correlates of anticipated peer evaluation in adolescence. Child Development. 2009;80:1000–1015. doi: 10.1111/j.1467-8624.2009.01313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt-Lunstad J, Smith TB, Layton JB. Social relationships and mortality risk: A meta-analytic review. PLoS Medicine. 2010;7:e1000316. doi: 10.1371/journal.pmed.1000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch W. EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Research: Brain Research Reviews. 1999;29:169–195. doi: 10.1016/s0165-0173(98)00056-3. [DOI] [PubMed] [Google Scholar]
- Knowles ML. Social rejection increases perspective taking. Journal of Experimental Social Psychology. 2014;55:126–132. [Google Scholar]
- Knyazev GG. Motivation, emotion, and their inhibitory control mirrored in brain oscillations. Neuroscience and Biobehavioral Reviews. 2007;31:377–395. doi: 10.1016/j.neubiorev.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Kujawa A, Arfer KB, Klein DN, Proudfit GH. Electrocortical reactivity to social feedback in youth: a pilot study of the Island Getaway task. Developmental Cognitive Neuroscience. 2014;10:140–147. doi: 10.1016/j.dcn.2014.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladd GW. Peer rejection, aggressive withdrawn behavior, and psychological maladjustment from ages 5 to 12 - An examination of four predictive models. Child Dev. 2006;77:822–846. doi: 10.1111/j.1467-8624.2006.00905.x. [DOI] [PubMed] [Google Scholar]
- Luu P, Collins P, Tucker DM. Mood, personality, and self-monitoring - Negative affect and emotionality in relation to frontal lobe mechanisms of error monitoring. Journal of Experimental Psychology: General. 2000a;129:43–60. doi: 10.1037//0096-3445.129.1.43. [DOI] [PubMed] [Google Scholar]
- Luu P, Flaisch T, Tucker DM. Medial frontal cortex in action monitoring. Journal of Neuroscience. 2000b;20:464–469. doi: 10.1523/JNEUROSCI.20-01-00464.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald G, Leary MR. Roles of Social Pain and Defense Mechanisms in Response to Social Exclusion: Reply to Panksepp (2005) and Corr (2005) Psychological Bulletin. 2005;131:237–240. doi: 10.1037/0033-2909.131.2.231. [DOI] [PubMed] [Google Scholar]
- Makeig S, Debener S, Onton J, Delorme A. Mining event-related brain dynamics. Trends in Cognitive Sciences. 2004;8:204–210. doi: 10.1016/j.tics.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Masten CL, Eisenberger NI, Borofsky LA, Pfeifer JH, McNealy K, Mazziotta JC, Dapretto M. Neural correlates of social exclusion during adolescence: understanding the distress of peer rejection. Social Cognitive and Affective Neuroscience. 2009;4:143–157. doi: 10.1093/scan/nsp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPartland JC, Crowley MJ, Perszyk DR, Naples A, Mukerji CE, Wu J, Molfese P, Bolling DZ, Pelphrey KA, Mayes LC. Temporal dynamics reveal atypical brain response to social exclusion in autism. Dev Cogn Neurosci. 2011;1:271–279. doi: 10.1016/j.dcn.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuki Y, Kajimura N, Kai S, Suetsugi M, Ushijima I, Yamada M. Differential responses to mental stress in high and low anxious normal humans assessed by frontal midline theta activity. International Journal of Psychophysiology. 1992;12:169–178. doi: 10.1016/0167-8760(92)90008-y. [DOI] [PubMed] [Google Scholar]
- Neo PSH, McNaughton N. Frontal theta power linked to neuroticism and avoidance. Cognitive, Affective, & Behavioral Neuroscience. 2011;11:396–403. doi: 10.3758/s13415-011-0038-x. [DOI] [PubMed] [Google Scholar]
- Oaten M, Williams KD, Jones A, Zadro L. The effects of ostracism on self-regulation in the socially anxious. Journal of Social and Clinical Psychology. 2008;27:471–504. [Google Scholar]
- Pharo H, Gross J, Richardson R, Hayne H. Age-related changes in the effect of ostracism. Social Influence. 2011;6:22–38. [Google Scholar]
- Platt B, Cohen Kadosh K, Lau JY. The role of peer rejection in adolescent depression. Depression and Anxiety. 2013;30:809–821. doi: 10.1002/da.22120. [DOI] [PubMed] [Google Scholar]
- Rotge JY, Lemogne C, Hinfray S, Huguet P, Grynszpan O, Tartour E, George N, Fossati P. A meta-analysis of the anterior cingulate contribution to social pain. Social Cognitive and Affective Neuroscience. 2015;10:19–27. doi: 10.1093/scan/nsu110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggieri S, Bendixen M, Gabriel U, Alsaker F. Cyberball: The impact of ostracism on the well-being of early adolescents. Swiss Journal of Psychology. 2013;72:103–109. [Google Scholar]
- Sauseng P, Griesmayr B, Freunberger R, Klimesch W. Control mechanisms in working memory: a possible function of EEG theta oscillations. Neuroscience and Biobehavioral Reviews. 2010;34:1015–1022. doi: 10.1016/j.neubiorev.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Sauseng P, Klimesch W, Freunberger R, Pecherstorfer T, Hanslmayr S, Doppelmayr M. Relevance of EEG alpha and theta oscillations during task switching. Experimental Brain Research. 2006;170:295–301. doi: 10.1007/s00221-005-0211-y. [DOI] [PubMed] [Google Scholar]
- Sebastian C, Viding E, Williams KD, Blakemore SJ. Social brain development and the affective consequences of ostracism in adolescence. Brain and Cognition. 2010;72:134–145. doi: 10.1016/j.bandc.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Sebastian CL, Tan GC, Roiser JP, Viding E, Dumontheil I, Blakemore SJ. Developmental influences on the neural bases of responses to social rejection: implications of social neuroscience for education. Neuroimage. 2011;57:686–694. doi: 10.1016/j.neuroimage.2010.09.063. [DOI] [PubMed] [Google Scholar]
- Silk JS, Stroud LR, Siegle GJ, Dahl RE, Lee KH, Nelson EE. Peer acceptance and rejection through the eyes of youth: pupillary, eyetracking and ecological data from the Chatroom Interact task. Social Cognitive and Affective Neuroscience. 2012;7:93–105. doi: 10.1093/scan/nsr044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Heatherton TF, Kelley WM. Anterior cingulate cortex responds differentially to expectancy violation and social rejection. Nat Neurosci. 2006;9:1007–1008. doi: 10.1038/nn1728. [DOI] [PubMed] [Google Scholar]
- Sreekrishnan A, Herrera TA, Wu J, Borelli JL, White LO, Rutherford HJ, Mayes LC, Crowley MJ. Kin rejection: social signals, neural response and perceived distress during social exclusion. Dev Sci. 2014;17:1029–1041. doi: 10.1111/desc.12191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Themanson JR, Khatcherian SM, Ball AB, Rosen PJ. An event-related examination of neural activity during social interactions. Soc Cogn Affect Neurosci. 2013;8:727–733. doi: 10.1093/scan/nss058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Themanson JR, Schreiber JA, Larsen AD, Dunn KR, Ball AB, Khatcherian SM. The ongoing cognitive processing of exclusionary social events: evidence from event-related potentials. Social Neuroscience. 2015;10:55–69. doi: 10.1080/17470919.2014.956899. [DOI] [PubMed] [Google Scholar]
- Trujillo LT, Allen JJ. Theta EEG dynamics of the error-related negativity. Clinical Neurophysiology. 2007;118:645–668. doi: 10.1016/j.clinph.2006.11.009. [DOI] [PubMed] [Google Scholar]
- van Noordt SJ, Desjardins JA, Segalowitz SJ. Watch out! Medial frontal cortex is activated by cues signaling potential changes in response demands. Neuroimage. 2015 doi: 10.1016/j.neuroimage.2015.04.021. [DOI] [PubMed] [Google Scholar]
- Vecchiato G, Toppi J, Astolfi L, De Vico Fallani F, Cincotti F, Mattia D, Bez F, Babiloni F. Spectral EEG frontal asymmetries correlate with the experienced pleasantness of TV commercial advertisements. Med Biol Eng Comput. 2011;49:579–583. doi: 10.1007/s11517-011-0747-x. [DOI] [PubMed] [Google Scholar]
- White LO, Wu J, Borelli JL, Mayes LC, Crowley MJ. Play it again - Neural responses to reunion with excluders predicted by attachment patterns. Dev Sci. 2013;16:850–863. doi: 10.1111/desc.12035. [DOI] [PubMed] [Google Scholar]
- White LO, Wu J, Borelli JL, Rutherford HJ, David DH, Kim-Cohen J, Mayes LC, Crowley MJ. Attachment dismissal predicts frontal slow-wave ERPs during rejection by unfamiliar peers. Emotion. 2012;12:690–700. doi: 10.1037/a0026750. [DOI] [PubMed] [Google Scholar]
- Wilcox RR. Statistical modeling and decision science. Academic Press; Amsterdam; Boston: 2012. Introduction to robust estimation and hypothesis testing; p. 1. online resource (xxi, 690 p.) [Google Scholar]
- Williams KD, Cheung CKT, Choi W. Cyberostracism: Effects of being ignored over the Internet. Journal of Personality and Social Psychology. 2000;79:748–762. doi: 10.1037//0022-3514.79.5.748. [DOI] [PubMed] [Google Scholar]
- Williams KD, Jarvis B. Cyberball - A program for use in research on interpersonal ostracism and acceptance. Behavior Research Methods. 2006;38:174–180. doi: 10.3758/bf03192765. [DOI] [PubMed] [Google Scholar]
- Zadro L, Boland C, Richardson R. How long does it last? The persistence of the effects of ostracism in the socially anxious. Journal of Experimental Social Psychology. 2006;42:692–697. [Google Scholar]
- Zadro L, Williams KD, Richardson R. How low can you go? Ostracism by a computer is sufficient to lower self-reported levels of belonging, control, self-esteem, and meaningful existence. Journal of Experimental Social Psychology. 2004;40:560–567. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.