Abstract
Amplitude modulation (AM) is an important temporal cue for precise speech and complex sound recognition. However, functional decline of the auditory periphery as well as degradation of central auditory processing due to aging can reduce the salience and resolution of temporal cues. Age-related deficits in central temporal processing have previously been observed at more rapid AM frequencies and various AM depths. These centrally observed changes result from cochlear changes compounded with changes along the ascending auditory pathway. In fact, a decrease in ability to detect temporally modulated sounds accurately could originate from changes in cochlear filtering properties and in cochlear mechanics due to aging. Nonetheless, few studies have examined cochlear mechanisms in AM detection. To assess integrity of the mechanical properties of the auditory periphery, distortion product otoacoustic emissions (DPOAEs) are a tool commonly used in clinics and in research. In this study, we measured DPOAEs to reveal age-related changes in peak f2/f1 ratio and degradation in AM detection by basilar membrane vibration. Two tones (f1 and f2, f2>f1) at various f2/f1 ratios and simultaneous presentation of one AM and one pure tone were used as stimuli to evoke DPOAEs. In addition of observing reduced DPOAE amplitudes and steeper slopes in the input-output DPOAE functions, higher peak f2/f1 ratios and broader f2/f1 tuning were also observed in aged animals. Aged animals generally had lower distortion product (DP) and first sideband (SB 1) responses evoked by an f1 pure tone and an f2 AM tone, regardless of whether the AM frequency was 45 Hz or 128 Hz. SB 1 thresholds, which corresponds to the smallest stimulus AM depth that can induce cochlear vibrations at the DP generator locus, were higher in aged animals as well. The results suggest that age-related changes in peak f2/f1 ratio and AM detection by basilar membrane vibration are consistent with a reduction in endocochlear potential and reduced prestin activity but with preserved hair cell bundle function. SB 1 responses evoked by f2 AM/f1 pure tone with various AM depths could serve as an estimate for cochlear AM detection. The sidebands of DP could also serve as additional physiological cues for detection of AM in the presence of other tone(s), even at typical conversational levels in speech.
Keywords: Amplitude modulation, Endocochlear potential, Hair cells, distortion product, Fischer 344 rat
1. Introduction
Speech, dynamic sounds, music and animal vocalizations are complex and contain rapid modulations in amplitude and frequency over time (Rosen 1992). Good resolution of these sounds is crucial for precise sound detection and recognition (Shannon, Zeng et al. 1995, Zeng, Nie et al. 2005). With aging, functional reduction of the auditory periphery and deficits of central auditory processing (Frisina, Frisina et al. 2001, Frisina 2010, Li-Korotky 2012) lead to a reduction of temporal resolution for speech and other complex sounds (Strouse, Ashmead et al. 1998). It has been reported that some older listeners with normal hearing thresholds have difficulties in understanding speech (Frisina and Frisina 1997). This problem is even more prominent in the presence of competing sounds or when the temporal cues for speech recognition are weak (Schneider, Daneman et al. 2005).
Amplitude modulations (AMs) contain important temporal cues that are extracted by the auditory system for speech recognition (Shannon, Zeng et al. 1995, Snell and Frisina 2001, Zeng, Nie et al. 2005). Many psychoacoustic and physiological studies have showed that older subjects performed worse in AM detection than younger subjects (Takahashi and Bacon 1992, Leigh-Paffenroth and Fowler 2006, He, Mills et al. 2008, Parthasarathy, Cunningham et al. 2010, Parthasarathy and Bartlett 2011, Parthasarathy and Bartlett 2012). Decreased ability in detecting temporally modulated sounds accurately, e.g. difficulties in encoding small AM depth, could originate from changes in cochlear filtering properties and in cochlear mechanics due to aging (Bian and Chen 2011). However, few studies have examined cochlear mechanisms in AM coding.
Distortion product otoacoustic emissions (DPOAEs) are commonly used as a tool in clinics and in research to assess the integrity of the mechanical properties of the auditory periphery (the cochlea), including outer hair cell (OHC) function (Shaffer, Withnell et al. 2003). DPOAEs at 2f1–f2 are generated when two tones at f1 (lower tone) and f2 (higher tone) are presented simultaneously to the cochlea. The production of DPOAEs in mammals results from the dual active processes of prestin-driven electromotility (Liberman, Zuo et al. 2004, Ashmore 2008) and mechanoelectrical transduction of the hair cell bundle (Kennedy, Crawford et al. 2005, Avan, Büki et al. 2013).. DPOAE amplitudes are dependent upon the stimulus-frequency ratio (f2/f1) of the two tones (Abdala 1996, Dhar, Long et al. 2005). It has been reported that, on average, an f2/f1 ratio of 1.22 evokes the largest DPOAEs in human adults (Abdala 1996) and in marmosets (Lasky, Snodgrass et al. 1995, Valero, Pasanen et al. 2008). “Peak-ratio” used in this paper is defined as the f2/f1 ratio that evokes the largest DPOAEs. The peak f2/f1 ratio was found to be slightly larger in rodents and rabbits (1.25–1.30) (Brown 1987, Whitehead, Lonsbury-Martin et al. 1992). It has been demonstrated that DPOAE amplitudes show a bandpass response as a function of f2/f1 ratio (Harris, Lonsbury-Martin et al. 1989). This indicates that there is one specific peak f2/f1 ratio which could elicit maximal DPOAEs in a tested subject.
Although two-tone stimuli and the evoked DPOAEs can provide much information about cochlear mechanics, sinusoidal AM tones can also be utilized to explore cochlear AM coding and motions for more complex sounds. Recently, Bian and Chen (2010) used simultaneous presentation of an AM tone and a pure tone as stimuli to study indirectly how OHCs respond to AM signals by measuring DPOAEs in normal-hearing human subjects. They systematically investigated the behavior of DPOAEs when either the f1 or the f2 was the AM tone. Their results showed that the dependence of DPOAE amplitudes on the AM stimulus was linear when the f1 was modulated but it was nonlinear and more complex when the f2 was an AM tone.
Although central deficits can also result in degradation of temporal processing during aging (Walton, Frisina et al. 1998, Walton, Simon et al. 2002, Simon, Frisina et al. 2004, Frisina and Walton 2006, Parthasarathy, Cunningham et al. 2010, Parthasarathy and Bartlett 2011), this paper focuses on how aging could alter peak-ratio and cochlear mechanics in detecting temporal modulated sounds, e.g. AM signals, particularly in the presence of another sound. According to some studies, cochlear responses may then be coded by central auditory neurons in some cases (Smoorenburg, Gibson et al. 1976, Fahey and Allen 1985, McAlpine 2004, Abel and Kössl 2009). To our knowledge, no study has been carried out to compare AM spectra and the potential for AM coding via a cochlear mechanism in aging using DPOAEs. In this study, we first examined if peak f2/f1 ratio would be different in young and aged animals, and then assessed cochlear AM coding using AM tone evoked DPOAEs. We hypothesize that there are age-related changes in peak-ratio and degradation in AM coding by basilar membrane vibration measured by DPOAEs. We aim to test the hypothesis by performing two experiments. In experiment 1, we investigated age-related differences of peak-ratio using two pure tones with various stimulus-frequency ratios to elicit DPOAEs (Harris, Lonsbury-Martin et al. 1989, Dhar, Long et al. 2005, Valero, Pasanen et al. 2008). In experiment 2, we assessed AM detectability of basilar membrane vibration in young and aged rats using a few combinations of one pure tone and one AM tone, in which the AM depth of the AM tone was varied systematically at different degrees.
2. Methods
2.1. Subjects
Depending on the protocol, 8 to 11 young (3–6 months, 250–300 g) and 8 to 14 aged (21–24 months, 400–450 g) male Fischer-344 rats (Taconic and Charles River laboratories) were used in each experiment in this study. All the animals were kept and raised in relatively quiet, standard laboratory conditions. All protocols were approved by the Purdue Animal Care and Use Committee (PACUC 1111000167).
2.2 Distortion Product Otoacoustic Emissions (DPOAEs)
All DPOAE measurements were performed in a 9′X9′, double walled acoustic chamber (Industrial Acoustics Corporation). Animals were anaesthetized initially by inhaling 4 % isoflurane in an induction chamber. They were then transferred to the manifold and maintained with 1.8 % isoflurane (for young rats) or 1.5 % isoflurane (for aged rats) on a water circulated warming blanket (Kent Scientific) set to 37°C during the whole recording session of 30–60 mins (Cederholm, Froud et al. 2012). Stimulus presentation and DPOAE recordings were performed using BioSig (Tucker Davis Technologies, TDT) in the acoustic chamber. An earpiece (Etymotic-10B), containing a miniature low noise microphone and two sound delivery tubes, was placed in the right ear canal of animals. Two multifunction closed field speakers (TDT), which delivered f1 and f2 tones to the ear canal, were connected to the earpiece via flexible tubes. While the speakers played sounds, the microphone recorded DPOAEs from ear canal simultaneously. The output of the microphone was delivered as an input to a TDT RZ 5 system which converted the responses from analog to digital. Each response is an averaged response of 100 stimulus sweeps presented continuously to the animals.
The DPOAE input/output (I/O) functions, with f1 and f2 centering at 8 kHz and f2/f1 ratio of 1.2, were tested in all animals. The intensity of f1 was varied from 50 dB to 75 dB SPL in 5-dB steps while the intensity of f2 was 10 dB lower than that of f1. This enabled us to estimate the integrity of OHCs around 8 kHz in each animal. Stimuli were set close to 8 kHz because the most sensitive hearing region of rats falls in the region of 6–16 kHz (Parthasarathy, Datta et al. 2014).
2.3. Auditory Brainstem Responses (ABRs)
Hearing threshold of 8 kHz was measured in each animal using ABRs. Similar to DPOAEs, ABRs were performed in an acoustic chamber. The experimental procedures were similar to those described in detail in Parthasarathy and Bartlett (2012 and 2014). Subdermal needle electrodes (Ambu) were positioned at two different configurations on the scalp. In the first configuration, i.e. channel 1, a positive electrode was placed along the midline of the head in the Fz to Cz position. Meanwhile, a negative electrode was placed under the mastoid of the ear ipsilateral to the inserted earpiece. A ground electrode was placed in the nape of the neck. In channel 2, another positive electrode was positioned horizontally along the interaural line and above the location of the inferior colliculus. Electrode impedances were ensured to be always less than 1 kHz using the low-impedance amplifier (RA4LI, TDT). After electrode placement, animals were sedated by intramascular injection of 0.1–0.2 mg/kg of dexmedetomidine (Domitor) and isoflurane was taken off to remove anesthetic effects on neural responses. ABR recording was performed 15 mins after cessation of isoflurane as the anesthetic effects take about 10 min to wear off (Parthasarathy and Bartlett 2012). Dexmedetomidine, an α-adrenergic agonist, acts as a sedative and an analgesic which decreases motivation but preserves behavioral as well as neural responses in rodents (Ter-Mikaelian, Sanes et al. 2007).
To obtain hearing threshold at 8 kHz, ABRs were recorded using brief tone stimuli of 2 ms duration (0.1-ms cos2 rise-fall time) with sound level decreasing from 95 to 5 dB SPL in 5-dB step. The tone at each sound level was repeated 1500 times to obtain an averaged and reliable ABR response. Tones were generated by SigGenRP (TDT) and presented by BioSig (TDT). The tones were presented to animals’ right ears via the same earpiece as described in DPOAEs.
2.4. Stimulus description, presentation and acquisition
Two main types of stimuli were used in this study: (1) pure tones at two different frequencies (f1 and f2 with f2>f1) presented simultaneously; and (2) a combination of one AM tone plus one pure tone presented simultaneously (either f1 or f2 can be the AM tone). Stimuli were set at or close to 8 kHz in both types because the most sensitive hearing region of rats falls in the region of 6–16 kHz (Parthasarathy, Datta et al. 2014). The duration of all stimuli were fixed at 200 ms.
In the first type of stimulus (type 1), f2 was fixed at 8 kHz while f1 varied from 5.5 to 7.95 kHz. These correspond to stimulus-frequency ratios (f2/f1 ratios) of 1.01–1.45. The f2/f1 range was chosen because it encompasses the commonly used ratio, which is 1.22, in most DPOAE studies (Harris, Lonsbury-Martin et al. 1989, Lasky, Snodgrass et al. 1995, Valero, Pasanen et al. 2008). The stimulus level of f1, L1, was fixed at both 75 dB SPL and 20 dB above animals’ hearing threshold at 8 kHz. For testing the peak-ratios, the intensity of f2, termed L2, was tested at the same level as that of f1 (L2=L1) and with L2 10 dB below L1.
In the second type of stimulus (type 2), f1/f2 ratios at 1.2 and the ratio that elicited the maximum DPOAE responses (peak-ratio, obtained from the results of type 1 stimuli) were used. The AM tone/pure tone combinations were either f1 AM/f2 pure tone or f1 pure tone/f2 AM. The pure tone was set at 8 kHz while the AM tone’s carrier frequency was determined by the value of f2/f1 ratio. AM frequencies of 45 Hz and 128 Hz were tested. The AM depth of the AM tone was manipulated from 3 to 100 % (0 to −30 dB) in order to examine how cochlear mechanics detect AM at different depths. L1 values of 65 and 75 dB SPL with L2=L1-10 dB were tested in all animals.
2.5. Data Analysis
2.5.1. Response analysis
2.5.1.1. Measurement of DPOAE peak and sideband amplitudes
DPOAE data were filtered from 80 Hz to 12 kHz using BioSig (TDT) and exported to MATLAB. For responses collected from type 1 stimuli, Fast Fourier Transforms (FFTs) were performed on time-domain waveforms with customized written programs in MATLAB. Only DPOAEs at 2f1–f2 frequencies were analyzed as distortion products (DPs) as these frequencies are the most prominent one among others. The maximum energy with in the window centered at 2f1–f2 ± three frequency bins (5.9 Hz/bin) was measured as the peak FFT amplitude. The noise floor was calculated as the average of eight frequency bins above and below 2f1–f2. DP amplitudes with a signal-to-noise (SNR) ratio of at least 6 dB (twice the noise floor) were treated as real peaks.
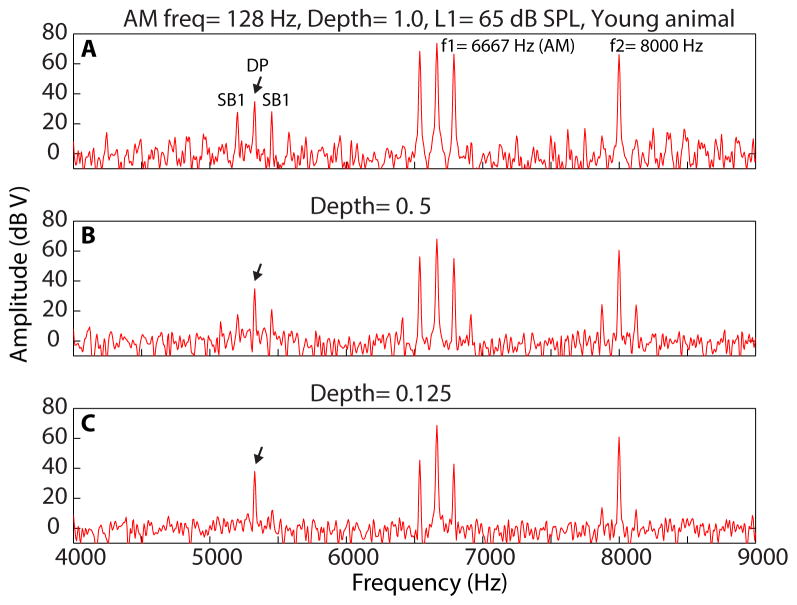
The analysis for DPOAEs using type 2 stimuli included amplitude measurement of the sidebands around 2f1–f2 (Figure 1, black arrow) as well. The method for estimation of the first sideband (2f1–f2 ± AM frequency) amplitude was similar to that of 2f1–f2. The calculation of noise floor, however, was moved further outside of the frequency range of 2f1–f2 ± 5 x AM frequency to avoid overestimation of the noise floor by other sidebands.
Figure 1.
Spectra of DPOAEs recorded using f1 AM/f2 pure tone (f1=6667 Hz modulated at 128 Hz, f2= 8000 Hz) at L1= 65 dB SPL (L2= L1-10) from a young animal. AM depth of f1 AM for each spectrum is indicated on top of each panel. Black arrow indicates the peak of a distortion product (DP) at 2f1–f2. The first sideband (SB 1) on each side of the DP is 128 Hz away from 2f1–f2.
2.5.2. Statistical Analysis
Statistical group differences in DPOAEs, including DP amplitudes, slopes of I/O functions, sideband amplitudes, and sideband thresholds, were analyzed using Wilcoxon’s rank-sum test with 5 % significance level for differences between young and aged animals. The ranks-sum test is a non-parametric test and the statistics were reported as W values and p values. Multiple comparisons among groups comparing peak-ratios for different age groups and different stimulus levels were analyzed using two-way ANOVA. The df and F values of two-way ANOVA were indicated in the results. Significant differences for pairwise comparisons were reported using repeated-measure ANOVA (rmANOVA) with 95 % confidence interval. For pairwise comparisons, df, t, and p values were reported in the results.
3. Results
3.1. Input-output (I/O) function of DPOAEs at 8 kHz
DPOAE I/O functions at 8 kHz were assessed in young and aged animals using two tones centered around 8 kHz and with a fixed f2/f1 ratio of 1.2. Figure 2A shows the averaged I/O functions of young and aged animals. In both young and aged groups, DPOAE amplitudes increased as stimulus intensity (L1) increased from 50 to 65 dB SPL. However, the I/O plots plateaued or decreased slightly when stimulus intensity increased beyond 65 dB SPL. At lower sound levels (50–65 dB SPL), DPOAE amplitudes of aged animals were significantly (p<0.05) smaller than young animals. In contrast, no significant difference in DPOAE amplitudes was observed between young and aged animals at higher sound levels (70 and 75 dB SPL).
Figure 2.
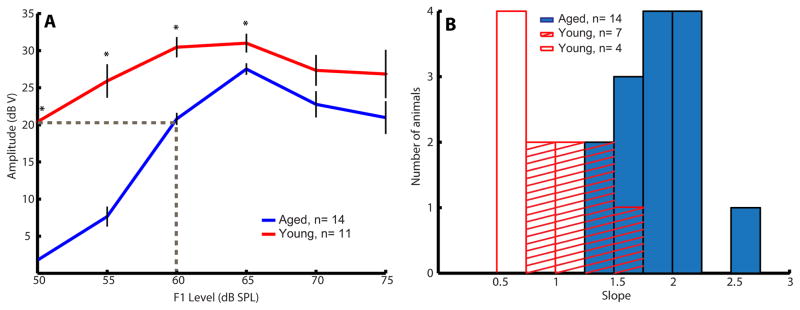
Aged animals had lower amplitudes and steeper low-level slopes in their DPOAE I/O functions. (A) Averaged DPOAE I/O functions of young (red) and aged (blue) animals. For each individual I/O function of an animal, a linear regression line was fitted onto the data points from 50 to 60 or 65 dB SPL (depends on the function’s breakpoint) to estimate the slope of the I/O function. Error bars indicate standard error, and asterisks represent statistically significant differences (p<0.05, rank-sum test). (B) Histogram of individual low-level slopes of young (red and red stripe bars) and aged (solid blue bars) animals. Four animals (red bars) with slopes < 0.75 dB/dB at 50–60 dB SPL were excluded from mean slope calculation. Most aged animals have slopes steeper than young animals.
To determine the slopes of individual I/O functions, we fitted a linear-regression equation to the linear portion of the functions (Valero, Pasanen et al. 2008) and termed it low-level slope. In most animals, the linear growth occurred between L1= 50 to 60 or 65 dB SPL, and the functions tended to saturate at 70–75 dB SPL. Four young animals have slopes < 0.75 dB/dB going from 50 to 60 dB SPL, for whom the DPOAE I/O functions may already have been in a compressed range even at 50 dB SPL. When these 4 animals were excluded from mean slope calculation, young animals have a mean slope of 1.19 ± 0.11 dB/dB. This value was close to the mean slope of 1 dB/dB, which was reported by Avan et al. (2003) and (Parham, Sun et al. 1999) in their studies derived from normal-hearing animals. In contrast, low-level slopes of aged animals were steeper as compared to young animals. The mean slope obtained from aged animals was 1.91 ± 0.09 dB/dB. This value was similar to the overall slope of 1.8 dB/dB obtained from animals with transient ischemic treatment (Avan, Bonfils et al. 2003). Transient ischemia is known to decrease the endocochlear potential (EP) drastically, and to impair the whole cochlear feedback loop and performance without OHC damage (Avan, Bonfils et al. 2003). Figure 2B shows the histogram of slopes of individual I/O functions from all young (including the 4 young animals) and aged animals. Most aged animals had I/O slopes larger than 1.5 dB/dB. Significant age-related differences in the means of I/O slope were observed (W=33, p<0.05).
3.2. Analysis of peak-ratio from DPOAE amplitudes vs. frequency ratio function
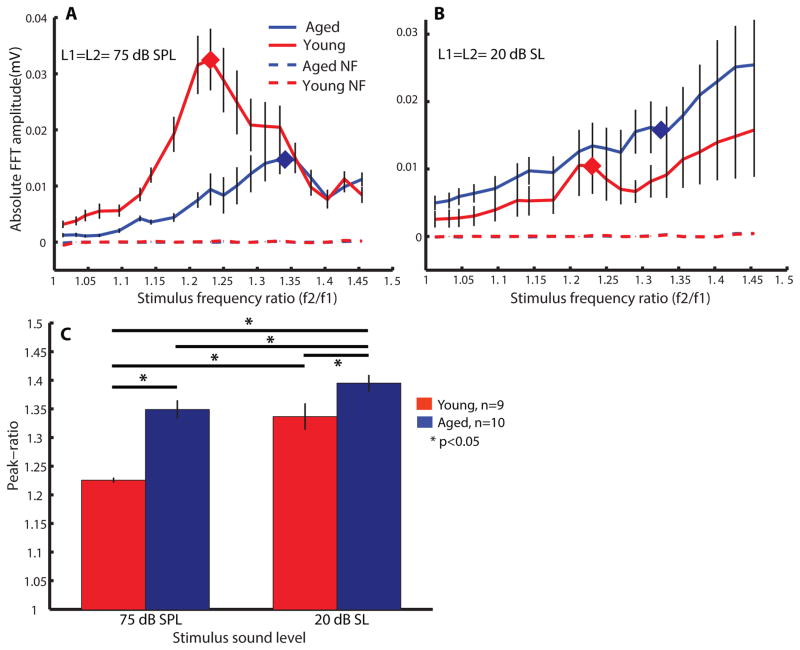
To estimate young and aged animals’ peak-ratio at regions around 8 kHz, two pure tones with frequency ratio ranging from 1.0–1.45 were used to obtain DPOAEs. At a stimulus intensity of L1= L2= 75 dB SPL, the frequency ratio function of young animal exhibited a sharp bandpass shape with a peak-ratio centered at 1.23 ± 0.01 (Figure 3A red curve). The averaged peak-ratio is closed to the peak f2/f1 ratio, 1.25, obtained from rodents (Brown 1987) and the peak in humans, which is 1.22 (Abdala 1996). The shape of the frequency ratio function of aged animals was different than young animals. The peak-ratio was shifted to a higher value of 1.34 ± 0.07. Statistical comparison of the young’s and the aged’s peak-ratios at 75 dB SPL was significant (df=17, t=−5.46, p<0.05). In addition, the same measurements were repeated at 20 dB above ABR threshold at 8 kHz (20 dB sensation level, SL). At 20 dB SL, the mean of stimulus levels presented to young animals was 49.4 ± 2.4 dB SPL while it was 62.5 ± 2 dB SPL for aged animals. At these sound intensities, the amplitudes of DPOAEs were similar but the peak-ratios of young and aged animals were significantly different (df=17, t=−2.58, p<0.05). The shapes of frequency ratio functions of both groups were more complex and multiple peaks could be observed (Figure 3B). A maximal peak-ratio of 1.35 ± 0.05 was obtained from young animals while a peak-ratio of 1.4 ± 0.05 was obtained from the frequency ratio function of aged animals at 20 dB SL. Peak-ratios at 75 dB SPL and 20 dB SL were also found to be different in aged animals (df=17, t=2.3, p<0.05). The peak-ratios were stimulus-intensity dependent in both young and aged animals (Figure 3C). According to a two-way rmANOVA analysis (age x stimulus intensity), age effect (F=25.77), stimulus-intensity effect (F=29.18) as well as their interactions (F=5.01) were all significant (df=17, p<0.05, rmANOVA). Interestingly, in most (about 6 out of 9) young animals, as can be seen in the population average, there was a peak between 1.2–1.25, suggesting that this f2/f1 ratio preference is present even at lower sound levels. We also performed the same recording procedure using L2 at 10 dB SPL lower than L1. Results were qualitatively similar when L2=L1-10 (data not shown).
Figure 3.
The peak-ratios of f2/f1 were shifted to a higher value at both 75 dB SPL and 20 dB SL in aged animals. Tuning of f2/f1 of young (red) and aged (blue) animals were estimated by plotting DPOAE amplitudes as a function of stimulus-frequency ratio at (A) L1= L2= 75 dB SPL and (B) L1= L2= 20 dB SL. Dashed lines indicate the averaged noise-floor (NF) of the measurements. Diamond markers in (A) and (B) mark out the peak-ratio obtained at 75 dB SPL. (C) Averaged peak-ratio obtained from young (red bars) and aged (blue bars) animals at 75 dB SPL and 20 dB SL. Repeated-measure ANOVA was used to perform multiple comparisons on all stimulus intensities and all age groups. Asterisks indicate statistically significant differences (df= 17, p<0.05).
3.3. Analysis of DPOAEs evoked by AM tone and pure tone combinations
3.3.1. Stimuli-frequency ratio fixed at 1.2
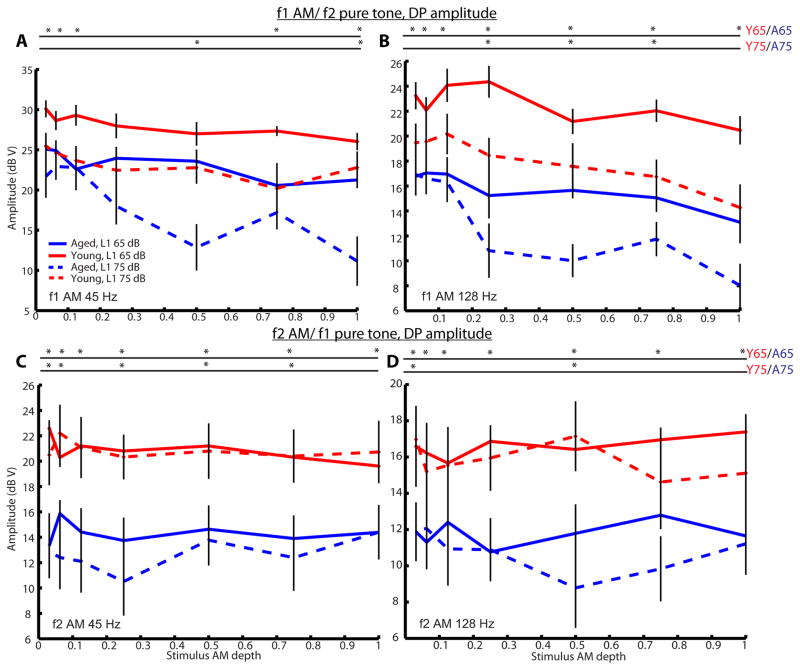
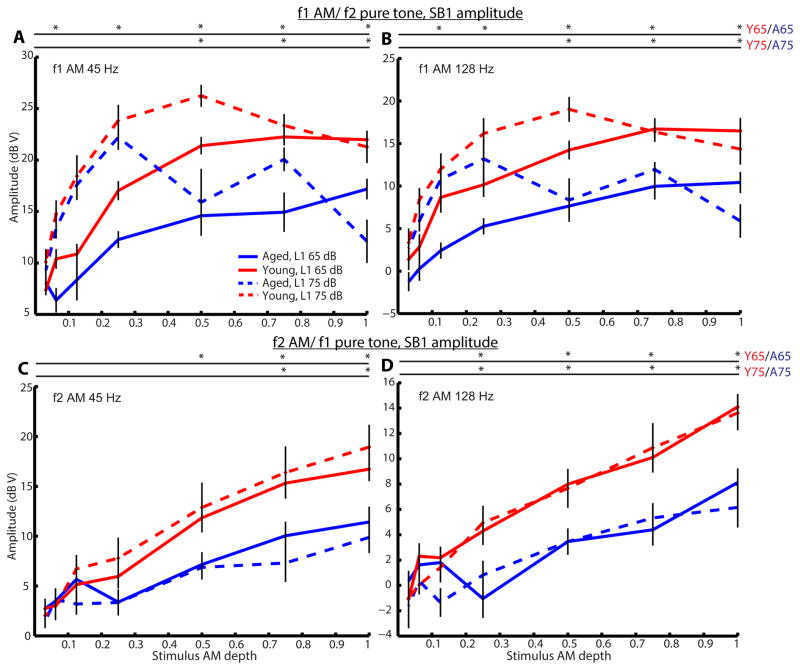
To investigate age effects on AM coding by cochlear mechanics, we measured DPOAEs to either f1 or f2 as an AM tone paired with f2 or f1 as a pure tone, respectively. DPOAE amplitudes at 2f1–f2 were largely insensitive to modulation depth, but there was approximately a 6-dB decrease in DPOAE amplitudes when changing from 45 to 128 Hz AM or when changing from modulation of f1 to modulation of f2 (Figure 4). In the frequency domain response to DPOAEs, multiple sidebands were observed at frequency differences corresponding to multiples of the modulation frequency when f1 was modulated. The first sidebands (SBs 1) on both the high- and low-frequency sides were the most prominent. In contrast, only one sideband was observed on each side of the 2f1–f2 peak when f2 was modulated. The appearance of sidebands was observed and their magnitude grew with increasing modulation depth of the AM tone (Figure 5). For f1 AM tone (Figure 5A and B), SB 1 amplitudes increased non-monotonically when L1 was 75 dB SPL (L2=L1-10), typically peaking at depths near 0.5 in young animals and 0.25 in aged animals. When L1 was 65 dB SPL, SB 1 amplitudes increased monotonically and tended to plateau as modulation depth approached 1. Younger rats had significantly larger SB 1 amplitudes at larger modulation depths (0.25–1.0) for L1=65 or 75 dB SPL. For f2 AM (Figure 5C and D), SB 1 magnitudes increased monotonically with increasing of f2 AM modulation depth. Similar results were obtained regardless L1 was 75 or 65 dB SPL (L2= L1-10). Younger rats also had significantly larger SB 1 amplitudes at larger modulation depths (0.5–1.0). In addition, age-related differences were still significant even when comparing SB 1 responses of aged animals at L1= 75 dB SPL to young animals at L1 = 65 dB SPL, making them more comparable with respect to sensation level (75 dB SPL in aged animals was about 32.5 dB SL in average while 65 dB SL in young animals was about 35.6 dB SL in average). Only when L1= 75 dB SPL and f1 was an AM tone, there was a slight decrease in 2f1–f2 magnitude when modulation depth approached 1. Overall, young animals had significantly larger 2f1–f2 magnitude than aged animals across all modulation depths. Similar results were obtained when modulation frequency of 128 Hz was used (Figure 4 and 5).
Figure 4.
DPOAE amplitudes were relatively stable across most AM depths when f2 was an AM tone but were reduced, especially in aged animals at L1= 75 dB SPL, at larger AM depths when f1 was an AM tone. DPOAE amplitudes of 2f1–f2 were plotted against stimulus AM depths for young (red) and aged (blue) animals at L1 of 65 dB (solid lines) or 75 dB SPL (dashed lines). DPOAEs were recorded using a combination of one AM tone plus one pure tone presented simultaneously. Either f1 (A and B) or f2 (C and D) was used as the AM tone. When f1 was modulated, the modulation was 45 (A) or 128 Hz (B) and f2 was a pure tone. When f2 was modulated, the modulation was 45 (C) or 128 Hz (D) and f1 was a pure tone. Error bars indicate standard error, and asterisks represent statistically significant differences (p<0.05, rank-sum test).
Figure 5.
As AM depth increased, SB 1 amplitude increased non-monotonically when f1 was an AM tone but elevated monotonically when f2 was an AM tone. Aged animals had lower SB 1 amplitude than young animals especially at larger AM depths. SB 1 amplitudes of 2f1–f2 were plotted against stimulus AM depths for young (red) and aged (blue) animals at L1 of 65 dB (solid lines) or 75 dB SPL (dashed lines). Stimulus conditions were the same as described in Figure 4. Error bars indicate standard error, and asterisks represent statistically significant differences (p<0.05, rank-sum test).
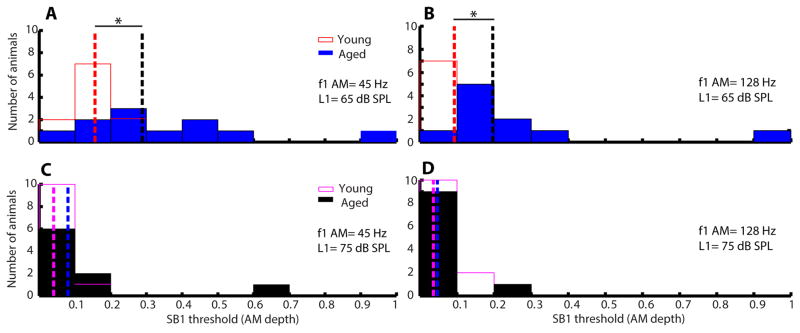
Figure 6 shows the histograms of SB 1 thresholds of young and aged animals for the condition of f1 AM. At lower L1 sound level (65 dB), SB 1 thresholds of young animals were significantly different from aged animals (45 Hz: W=157, p<0.05; 128 Hz: W=147, p<0.05). However, SB 1 thresholds have no significant difference (45 Hz: W= 116; 128 Hz: W=121.5) between young and aged animals at higher L1 (75 dB). The SB1 thresholds for L1=75 dB were uniformly low, often still detectible at 3% modulation depth (Figure 6C and D). Similar results were obtained when modulation frequency was either 45 or 128 Hz. The histograms of SB 1 thresholds for the condition of f2 AM are depicted in Figure 7. Thresholds were generally higher when f2 was modulated compared to when f1 was modulated (cf. Figures 6–7). When f2 AM = 45 Hz, SB 1 thresholds of young and aged animals were significantly different at both L1 = 65 and 75 dB (W=139, p<0.05). The difference between young and aged animals was significant at L1 = 75 dB when modulation frequency was 45 Hz but p= 0.0513 (W= 114) when modulation frequency was 128 Hz.
Figure 6.
Aged animals had significantly larger SB 1 thresholds than young animals when f1 was an AM tone at L1 = 65 dB SPL. Histograms of SB 1 thresholds in AM depth for young (opened bars) and aged (filled bars) animals were plotted. AM frequency of f1 was either 45 (A and C) or 128 Hz (B and D). Sound level of L1 was either 65 (A and B) or 75 dB SPL (C and D). Dashed lines indicate medians of the SB 1 thresholds for young (red or purple dashed lines) and aged (black and blue dashed lines), respectively. Asterisks indicate statistically significant differences in SB 1 thresholds of young and aged animals using rank-sum test.
Figure 7.
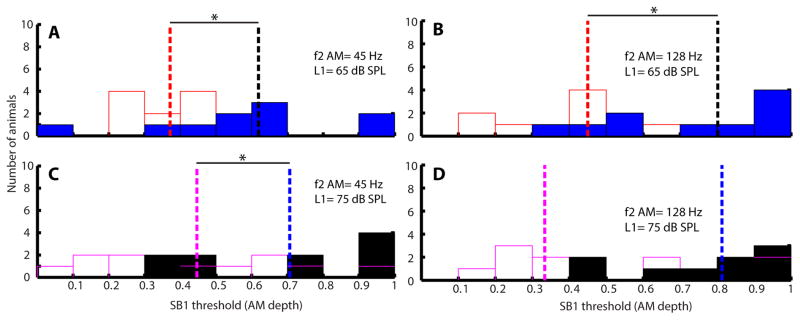
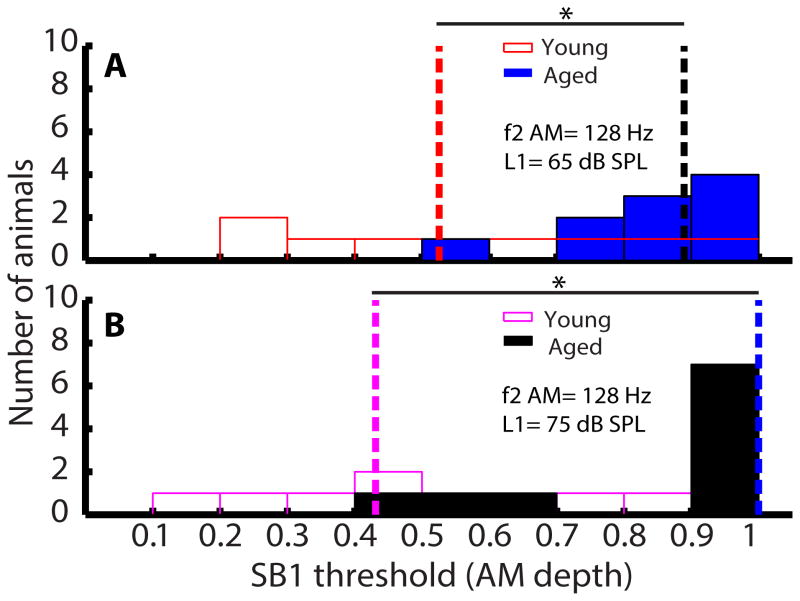
SB 1 thresholds of aged animals were significantly larger than young animals when f2 was an AM tone at L1 = 75 and 65 dB SPL. Histograms of SB 1 thresholds in AM depth for young (opened bars) and aged (filled bars) animals were plotted. AM frequency of f2 was either 45 (A and C) or 128 Hz (B and D). Sound level of L1 was either 65 (A and B) or 75 dB SPL (C and D). Dashed lines indicate medians of the SB 1 thresholds for young (red or purple dashed lines) and aged (black and blue dashed lines), respectively. Asterisks indicate statistically significant differences in SB 1 thresholds of young and aged animals using rank-sum test.
3.3.2. Stimuli-frequency ratio at a higher peak-ratio
Because an upward shift in peak-ratios was observed in the frequency ratio functions in aged animals and at 20 dB SL in young animals, another set of DPOAEs were recorded using f2/f1 at the respective peak-ratio (approximately 1.36) obtained from each individual’s frequency ratio function at 20 dB SL. In these sessions, the same conditions, including AM tone and pure tone combinations as well as manipulation of modulation depth, as the experiment mentioned above were used. These were performed to study whether a larger age-related difference would be observed in AM coding of cochlear mechanics at f2/f1= peak-ratio at 20 dB SL. Similar to when the f2/f1 ratio was 1.2, AM depth had little effect on 2f1–f2 amplitudes (Figure 8C) when f2 was modulated in both young and aged animals. Significant differences were observed between the 2f1–f2 responses of young and aged animals at L1= 75 dB SPL but not all depths were significant at L1= 65 dB SPL. When f1 was modulated, there was a slight suppression in DP amplitude as modulation depth increased in young animals (Figure 8A). Reduction in DP amplitudes became larger in aged animals especially at L1= 75 dB SPL and at higher modulation depths (0.5–1) at L1 = 65 dB SPL. For f1 AM and L1= 75 dB SPL, SB 1 magnitudes increased non-monotonically with modulation depth, typically peaking at about 0.25, and no significant differences between young and aged animals were obtained. SB 1 amplitudes increased monotonically when L1= 65 dB SPL with significant differences observed at higher stimulus depths (Figure 8B). When f2 was modulated, significant age-related differences were observed in SB 1 amplitudes at modulation depths above 0.25 at L1= 75 dB SPL (Figure 8D). Differences were not significant at L1= 65 dB SPL for most modulation depths except at modulation depth= 0.5 (W=103).
Figure 8.
Age-related differences in DPOAE and SB 1 amplitudes were observed in f2 AM/f1 pure tone when peak-ratios obtained at 20 dB SL were used. DP amplitudes (A and C) and SB 1 amplitudes (B and D) were plotted as a function stimulus AM depth. Stimuli that were used in (A) and (B) were f1 AM (modulated at 128 Hz) and f2 pure tone. An f2 AM and f1 pure tones were used in (C) and (D). Error bars indicate standard error, and asterisks represent statistically significant differences (p<0.05, rank-sum test).
When the higher peak-ratio was used as f2/f1 ratio in f1 AM, SB 1 threshold became harder to estimate because sidebands were either below SNR= 6 or were not observed in most animals although DPs were present. Therefore, only SB 1 thresholds of young and aged animals for the condition of f2 AM were analyzed and are presented in Figure 9. At both L1 = 65 (W=49) and 75 dB (W=43), SB 1 thresholds of aged animals were significantly higher than those of young animals (p<0.05). SB 1 thresholds of young animals were more widely spread than aged animals.
Figure 9.
SB 1 thresholds of aged animals were significantly larger than young animals at both 65 and 75 dB SPL when f2 as an AM tone and peak-ratio was used. AM frequency of f2 was 128 Hz and sound level of L1 was either 65 (A) or 75 dB SPL (B). Dashed lines indicate medians of the SB 1 thresholds for young (red or purple dashed lines) and aged (black and blue dashed lines), respectively. Asterisks indicate statistically significant differences in SB 1 thresholds of young and aged animals using rank-sum test.
4. Discussion
4.1. Reduced DPOAE amplitudes in I/O functions of aged animals
In this study, DPOAEs were used to assess peak-ratio and AM modulation detection in young and aged F344 rats. DPOAEs are sensitive to both temporary and permanent damage of the organ of Corti caused by aging, noise or other ototoxic factors (Varghese, Zhu et al. 2005, Buckiova, Popelar et al. 2007). DPOAEs are also sensitive in detecting changes in cochlear function in older humans even when their hearing thresholds are considered clinically normal (Lonsbury-Martin, Cutler et al. 1991, Kim, Frisina et al. 2002). Evidence has also shown that DPOAEs behave similarly across rodents and humans (Martin, Stagner et al. 2011). Based on the DPOAE I/O functions at 8 kHz (Figure 2A), aged animals had significantly lower responses than young animals, especially at lower sound levels (50–65 dB SPL). This is consistent with previous studies conducted in young and aged F344 rats (Buckiova, Popelar et al. 2007, Chen, Li et al. 2009). In human studies, older human subjects (50-year old) were shown to have smaller amplitude in DPOAE I/O functions and higher thresholds at high frequencies than younger human subjects (Lonsbury-Martin, Cutler et al. 1991).
4.2. Mechanism underlying reduction of DPOAEs
Previous studies have reported declines in DPOAE amplitudes with age correlated with OHC death or dysfunction at low and high frequencies (Chen, Li et al. 2009). There was little IHC loss in either study. Hair cell loss in aged F344 rats was small and comparable with that in aged Long Evan rats with normal DPOAEs (Popelar, Groh et al. 2006). This implies that the hair cells are present in the cochlea but their function may be impaired or there are additional factors.
One possibility for another factor is changes in the stria vascularis. Buckiova et al. (2007) discovered that there is a correlation between DPOAE reductions and changes in stria vascularis. They observed that collagen fibers were missing, and they also found many apoptotic fibrocytes and basal cells of the stria vascularis in the area connecting it with the spiral ligament. Stria vascularis, which is the highly vascular tissue in the cochlear lateral wall, was demonstrated to be the source that generates the EP (Tasaki and Spyropoulos 1959). Hence, changes in stria vascularis will impose an influence on the EP as well. The EP of aged F344/NHsd rats (24-month) was reported to be in the range of 75–85 mV while it was 90–100 mV in young rats (3-month) (Bielefeld, Coling et al. 2008). According to our previous study, the mean ABR audiogram of aged animals showed parallel upward shifts at low frequencies (1–8 kHz) but more drastic and steeper upward shifts at high frequencies (>8 kHz) (Parthasarathy, Datta et al. 2014). This kind of upward shift in hearing thresholds resembles the pattern of audiogram corresponding to a mixed metabolic and sensory audiometric phenotype of age-related hearing loss based on the classification published by Dubno et al. (2013). Metabolic hearing loss is consistent with EP reduction and results in an overall threshold shift even at low frequencies. Avan et al. (2003) showed that the slopes of DPOAE I/O functions at 12 kHz of CD1 mice (a strain with genetic cochlear impairment) with OHC loss and ABR threshold shift were near to 1 dB/dB and were parallel to the slopes of their positive controls (mice with intact OHC and normal ABR thresholds at 15 kHz). In their ischemic model, however, gerbils with transient ischemic treatment had steeper DPOAE I/O functions between 60 and 80 dB SPL. The mean slope that was derived from the gerbils with ischemic induction was 1.8 dB/dB. This value is similar to the mean slope of DPOAE I/O functions obtained from the aged rats in our study (Figure 2). EP is greatly reduced in the model of transient ischemia and this damages the whole cochlear feedback loop without damaging the OHCs (Avan, Bonfils et al. 2003). Moreover, a model of presbycusis in which the EP was reduced by chronic application of furosemide with no concomitant loss of hair cells, DPOAEs were quantitatively similar to those found in aged animals (Schmiedt, Lang et al. 2002). Taken together, this leads us to conclude that steeper DPOAE I/O functions in aged animals are primarily caused by an EP reduction and, to a lesser extent, by OHC dysfunction.
At high stimulus levels (>60 dB SPL, Figure 2), DPOAE amplitudes were similar between young and aged animals. Prior studies have also found preservation of DPOAE amplitudes at high stimulus levels in animals, even in the presence of furosemide or during aging, where the EP would be reduced (Ruggero and Rich 1991, Avan, Bonfils et al. 2003, Chen, Li et al. 2009). DPOAEs changes in amplitude versus sound level in humans was similar and consistent with the interpretation of a decline in EP (Gates, Mills et al. 2002).
4.3. Estimation of peak-ratio
We used DPOAEs to estimate whether peak f2/f1 ratio change due to aging. When DPOAE amplitudes are plotted as a function of f2/f1 ratio in normal-hearing subjects, it generates a bandpass shape (Harris, Lonsbury-Martin et al. 1989). To obtain two-tone stimuli with various f2/f1 ratios in experiment 1, we fixed f2 and varied f1 (fixed-f2 paradigm), which has been utilized to obtain peak f2/f1 ratio in rhesus monkeys (Lasky, Snodgrass et al. 1995, Park, Clark et al. 1995) and humans (Schmiedt 1986, Lasky 1998, Dreisbach and Siegel 2001). Since DPOAEs are first produced at regions on the basilar membrane closer to the f2 frequency, the place of DPOAEs generation is more constant using a fixed-f2 paradigm. By using this paradigm, we could ensure that changes in DPOAE levels are mainly due to the variation of f2/f1 ratio (Moulin 2000).
In human adults, it has been demonstrated that a peak f2/f1 ratio near 1.22 evokes the most robust and largest DPOAEs in humans (Harris, Lonsbury-Martin et al. 1989) and in non-human primates (Lasky, Snodgrass et al. 1995). In addition, it has been reported that the peak f2/f1 ratio for rodents and rabbits is 1.25–1.30 (Brown 1987, Whitehead, Lonsbury-Martin et al. 1992). In this study, a similar f2/f1 ratio of 1.25 was obtained as the peak-ratio for young rats at a higher stimulus level (75 dB SPL). There is no strong evidence indicating that the peak-ratio for animals is significantly different from human adults since the difference is merely 0.01 and the standard error obtained from this study is 0.01. Meanwhile, the peak-ratio increased to 1.34 in aged rats at L1= 75 dB SPL. As shown in Figure 3A, the bandpass shape of aged animals was broader and less specific than young animals. At an f2/f1 ratio of 1.2, aged animals would have a larger overlapped region of f1 and f2 on the basilar membrane compared to the young. Larger overlapped regions would result in suppression of DPOAEs while smaller overlapped region would decrease the interaction of f1 and f2. Hence, separation of f1 and f2 in aged animals needs to be wider in order to maintain the same area of overlapped region as in young animals. When f1 and f2 were further apart, f2/f1 ratio became larger. Hence, our data suggest that aged animals have broader f2/f1 tuning and so their peak f2/f1 was larger.
4.4. Estimation of AM coding by cochlear mechanics
In experiment 2, we were interested in using DPOAEs to compare differences of AM coding by DPOAE readouts of basilar membrane vibration in young and aged animals. Bian and Chen (2010) reported that temporal modulation of the DP amplitude, as reflected by the presence of sidebands, is clearly correlated with the modulation depth variation in the AM stimulus. Lee and Dhar (2013) also showed that AM perception is positively correlated to modulation depth of DP. These suggest that sidebands or temporal modulation of DP has a role in AM coding and perception. In this study, we used the stimulus paradigm of one AM tone and one pure tone in DPOAEs to investigate temporal AM coding of cochlear mechanics in aging.
When comparing Bian and Chen’s human data to our data obtained from young and aged animals using f2/f1= 1.2, the SB 1 behaviors are consistent with their observations (Figure 5). However, we observed a slight suppression of DP magnitudes at large stimulus AM depths for f1 AM, especially at L1= 75 dB SPL in aged animals, but DP magnitudes were relatively stable for f2 AM in young animals (Figure 4). The results of DP magnitudes are not completely similar to the results of Bian and Chen (2010) and Oswald (2006) where they observed a reduction in DP magnitude when f2, rather than f1, modulation increased. The AM frequencies that were tested by Bian and Chen (2010) were 8 and 32 Hz while 40 Hz was used by Oswald (2006). In fact, DP amplitudes of young animals for f1 AM/f2 pure tone at AM frequency of 45 Hz were more stable and consistent with their results (Figure 4A). At f1 AM frequency of 128 Hz, a slight reduction in DP magnitudes was observed at higher AM depths (Figure 4B). DP magnitudes were lower and fluctuated across different depths in aged animals at L1= 75 dB SPL. DP amplitude reduction is believed to resemble cochlear two-tone suppression (Oswald, Rosner et al. 2006), which originates from the saturating nonlinearity in OHC transduction (Geisler, Yates et al. 1990). The reduction is larger at 75 dB SPL because saturation normally occurs at higher levels (Bian and Chen 2011). As DP reduction is more apparent in aged animals than in the young, this indirectly suggests that aged animals have smaller OHC motile activities and OHC vibration becomes saturated relatively faster than young animals. Moreover, DP reduction in f1 AM/f2 pure tone is similar to the case in which FFRs to AM stimulus are decreased when the AM stimulus is presented simultaneously with a suppressor at a frequency higher than the carrier (Dolphin and Mountain 1993, John, Lins et al. 1998).
An upward shift in the peak f2/f1 ratio was observed at L1= 20 dB SL in young animals (experiment 1) compared to 75 dB SPL, so we used the obtained peak-ratio as f2/f1 ratio in experiment 2 as well. The behaviors of DP magnitude at peak-ratio were similar to the results when f2/f1= 1.2 was used. For the behavior of SB 1 magnitude, we did not see a significant difference between young and aged animals at f1 AM and L1= 75 dB SPL. This is related to Figure 3A where DPOAEs of young and aged animals were rather similar at higher f2/f1 ratios (>= 1.33) at L1= 75 dB SPL. In contrast, significant age-related differences and similar trends were observed in SB 1 magnitude for f2 AM at f2/f1 = 1.2 and at peak-ratio. These indicate that SB 1 magnitude has a linear relationship with f2 AM depth and is more sensitive to changes in f2 AM depth. Thus, we suggest that testing f2 AM/f1 pure tone with various AM depths could serve as an estimator for AM coding.
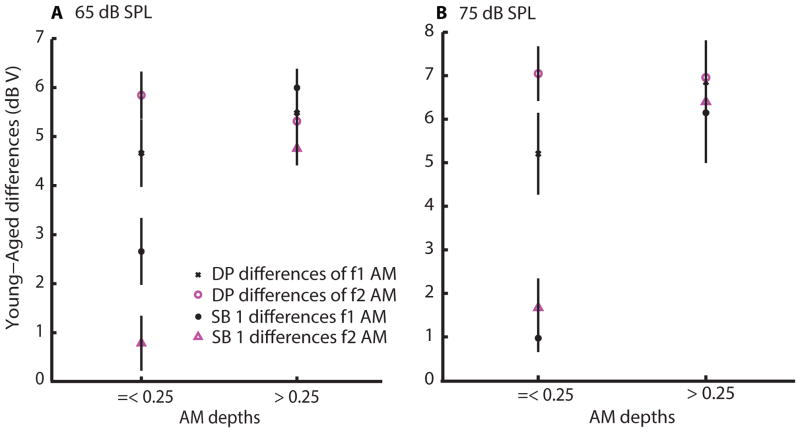
If the differences in DPs and the differences in SB 1 amplitudes are basically the same, it would suggest that there is no additional processing beyond DPOAE production for SB 1. That appeared to be the case for larger, but not smaller, but still perceptible, modulation depths. When comparing amplitude differences of DPs and SBs 1 between young and aged animals, we noticed that the SB 1 amplitude differences were small at lower AM depths (=< 0.25) but the differences became larger and comparable to DP amplitude differences at higher AM depths (> 0.25). There seemed to be a transition point in age-related SB 1 differences at 0.25, which can be observed in Figures 5 and 8, especially at L1= 75 dB SPL of f1 AM. This trend holds for different AM frequencies (45 and 128 Hz) and distinct f2/f1 ratios (1.2 and peak-ratio). Figure 10 summarizes the observations that are described above for sound levels of L1= 65 and 75 dB SPL, respectively. DP and SB 1 amplitude differences between young and aged animals were averaged separately for AM depths of =< 0.25 or > 0.25. At smaller AM depths (=< 0.25) of f2 AM, basilar membrane vibrations due to SBs 1 of both young and aged animals are probably operating near the limit of detectability, so SB 1 amplitudes and the differences between the two comparisons were small. With f1 AM, age-related SB 1 differences were small but SB 1 amplitudes of young and aged animals rose rapidly and similarly. At higher AM depths (> 0.25), however, age-related SB 1 differences behaved similarly to DP differences where larger differences were noticed between young and aged animals for both f1 and f2 AM.
Figure 10.
Comparison of age-related differences in DP amplitudes versus SB 1 amplitudes. Young-aged amplitude differences were averaged across AM frequency and peak-ratio for smaller AM depths (<= 0.25) and for larger AM depths (> 0.25). 65 dB and 75 dB SPL f1 sound levels were analyzed separately. Differences in DP amplitudes were fairly constant across conditions and depths for a given sound level. Differences in SB 1 amplitudes were comparable to DP amplitude differences for larger depths (> 0.25), but SB 1 differences were much smaller than DP differences for smaller depths.
Two main mechanisms have been used to explain the mechanism underlying the active process of the cochlear amplifier: one is the voltage-dependent somatic motility due to the activity of prestin motor protein in the OHCs and the other is hair-bundle (stereocilia) motility, which generates currents that alter hair cell membrane potential (Ashmore, Avan et al. 2010). Experiments using knockout mice have shown that functional stereocilia are required for DPOAE generation (Verpy, Weil et al. 2008) whereas prestin contributes to DPOAEs for lower amplitude sounds (Mills and Rubel 1994, Liberman, Zuo et al. 2004). Thus, at low stimulus levels (< 60–70 dB SPL), DPOAEs are nearly abolished in prestin-null mice (Liberman, Zuo et al. 2004), or with loop diuretics or transient hypoxia, where EP is decreased (Whitehead, Lonsbury-Martin et al. 1992, Mills and Rubel 1994). In contrast, high-level DPOAEs are not strongly affected in prestin knockouts (Liberman, Zuo et al. 2004)or furosemide and are probably driven by the stereocilia mechanical nonlinearity (Liberman, Zuo et al. 2004, Avan, Büki et al. 2013). Reduction of prestin immunoreactivity (Chen, Li et al. 2009) and EP (Bielefeld, Coling et al. 2008) has been reported in aged F344 rats while the stereocilia of aged OHCs were not detected to be different from young OHCs (Chen, Li et al. 2009). Taken together with our results, we infer that: (1) age-related differences in DP amplitudes and SB 1 amplitudes at AM depths > 0.25 were related to EP reduction in aging, which would affect the maximal currents generated by bundle movements; (2) at L1=75 dB SPL and f1 AM, SB 1 amplitudes were similar in young and aged animals at small AM depths (<= 0.25), suggesting that the louder sound levels were able to drive SB 1 responses primarily via mechanoelectrical transduction that was conserved in aged animals (3) at f2 AM for both L1= 75 and 65 dB SPL (L2= 65 and 55 dB SPL), SB 1 amplitudes at low AM depths of young and aged animals were both relatively small because there was diminished prestin-driven amplification and mechanoelectrical transduction was much weaker, such that cochlear motions and transmembrane currents were operating near the limit of detectability.
In a psychoacoustic study carried out by Lee and Dhar (2013), they tested AM perception to a 2-kHz carrier frequency modulated at 10 Hz and DPOAEs to f1 AM (10-Hz AM)/f2 pure tone in normal-hearing listeners. Their preliminary data showed that the modulation depth of DP (2f1–f2), which was defined as the relative amplitude of the DP and the sidebands, was positively correlated with AM perception. Listeners with stronger SB1 responses had better AM depth detection in their behavioral performance. However, they did not test DPOAEs to f2 AM/f1 pure tone in their study. From the results we observed in SB 1 magnitude and SB 1 threshold, f2 AM was more sensitive in distinguishing the abilities of cochlear mechanics in detecting AM sounds in young and aged animals. Hence, further study is required to correlate DPOAEs (evoked by either f1 AM or f2 AM) with behavioral AM coding in humans and animals, especially for those with hearing impairment.
It should be noted that, behaviorally and physiologically, AM detection in aging appears to differ from that observed for noise-induced hearing loss. According to some psychoacoustic findings, older subjects have poorer AM detection than young subjects (He, Mills et al. 2008, Kumar and A V 2011, Jin, Liu et al. 2014, Shen 2014). Auditory evoked potentials showed a decline (Parthasarathy and Bartlett 2011) or no change (Boettcher, Poth et al. 2001) in AM depth representation with age. This is in contrast to evoked potential (Zhong, Henry et al. 2014) and single-unit studies (Henry, Kale et al. 2014) showing enhanced envelope coding with noise-induced hearing loss. Furthermore, there are differences in cochlear anatomy of age-related hearing loss (metabolic type) and noise-induced hearing loss. Stereocilia of OHCs and/or IHCs can be damaged in noise-induced hearing loss and results in severe alteration of auditory nerve fiber tuning curves with upward shift of hearing thresholds and loss of frequency selectivity (Liberman and Dodds 1984, Henry, Snyder et al. 2013). Meanwhile, OHC stereocilia are intact but the EP is decreased in metabolic age-related loss (Schmiedt, Lang et al. 2002).
4.5. Neural sensitivity to DP in central auditory regions
There is evidence from psychoacoustic measurements showing that cochlear DP can be encoded neurally in listeners for perception (Zwicker 1981). Physiological studies demonstrated that cochlear DPs can elicit neural responses in the cochlear nucleus (Smoorenburg, Gibson et al. 1976, Fahey and Allen 1985, Faulstich and Kössl 1999), the inferior colliculus (McAlpine 2004, Abel and Kössl 2009), the medial geniculate body (Horner, de Ribaupierre et al. 1983) and the primary auditory cortex (Purcell, Ross et al. 2007). Studies of the neuronal response to cochlear DPs in the anteroventral cochlear nucleus of cats (Fahey and Allen 1985) and gerbils (Rübsamen, Mills et al. 1995, Faulstich and Kössl 1999) revealed that the magnitude of neural responses corresponded to the sound level of the DP. Another study performed by Smoorenburg et al. (1976) reported that the neurons in the anteroventral cochlear nucleus respond to cochlear DPs as if tones at the frequencies of DPs were actually delivered to the ear but with higher threshold than pure tone. All these findings suggest that cochlear DPs are fully encoded by neurons in the anteroventral cochlear nucleus, and DPOAEs measured in the ear canal reflect the output of nonlinear cochlear mechanics. In addition, McAlpine (2004) investigated neural sensitivity of the inferior colliculus to temporally modulated AM signals. He found that neurons in the inferior colliculus had their discharge rates modulated by the stimulated AM signals even though all spectral components of the signals lay outside the pure tone-evoked response areas of the neurons. This study also confirmed that cochlear DPs contribute to periodicity pitch detection, which is mapped in the midbrain. Abel and Kossl (2009) demonstrated neural representation of cochlear DPs in the auditory midbrain of gerbils using low to moderate stimulus levels. Their data showed that the neural response to the cochlear DPs were stronger than the response to a single tone at characteristic frequency in some cases. These data all support the notion that cochlear DPs may have large contributions to the neuronal activity during processing of complex sounds. These contributions will occur both at the CFs of the two tones as well as the DP place, with associated sidebands at each place if one or more of the tones are modulated. Our results lead us to further suggest that the sidebands of DP could serve as additional physiological cues for detection of AM in the presence of other tone(s), even at typical conversational levels in speech. Moreover, cochlear DPs and their sidebands will contribute to the population neurogram to discriminate between auditory “objects” and aid in grouping co-modulated spectral components that are likely to originate from the same object (Shamma, Elhilali et al. 2011).
In this study we have demonstrated steeper low-level slopes in I/O DPOAE functions, broadened in bandpass shape according to f2/f1 ratio tuning, an upward shift in peak f2/f1 ratio, lower amplitudes of distortion product SBs 1, and poorer SB 1 AM thresholds in aged animals. The overall results suggest that age-related changes in peak-ratio and AM coding by cochlear mechanics are correlated more with EP reduction and less with OHC dysfunction. It remains to be tested whether SB 1 magnitudes and thresholds can be used to predict the ability of AM coding and/or perception in aging as well as in other hearing impaired conditions, though there is some preliminary data to support this idea (Lee and Dhar 2013). The sidebands of DP could serve as additional physiological cues for AM representations in the presence of other tone(s). These results reveal a peripheral contribution of age-related deficits in AM processing and the mechanisms associated with age-related changes in peak-ratio and mechanics at supra-threshold sound levels. Thus, the findings also have implications in the studies of auditory diagnostics, AM perception and age-related changes in the auditory system.
Highlights.
DPOAEs of pure and amplitude-modulated tones were tested in young and old rats.
DPOAE amplitudes, peak-ratios and temporal detection were altered in old rats.
Age-related changes were correlated more with endocochlear potential reduction.
Distortion product sidebands may serve as additional cues for AM detection.
Acknowledgments
This study was supported by a grant from the National Institutes of Health (NIDCD R01DC011580). The authors would like to thank Dr. Aravindakshan Parthasarathy for his help in setting up equipment for DPOAE recordings, Dr. Robert Withnell for his helpful comments on an initial draft and Purdue statistical department for the help in statistical analysis.
Abbreviations
- AM
amplitude modulation
- DP
distortion product
- DPOAEs
distortion product otoacoustic emissions
- EP
endocochlear potential
- f2/f1
stimulus-frequency ratio
- I/O
input-output
- OHC
outer hair cell
- SB 1
first sideband
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdala C. Distortion product otoacoustic emission (2f1–f2) amplitude as a function of f2/f1 frequency ratio and primary tone level separation in human adults and neonates. J Acoust Soc Am. 1996;100(6):3726–3740. doi: 10.1121/1.417234. [DOI] [PubMed] [Google Scholar]
- Abdala C, Dhar S, Kalluri R. Level dependence of distortion product otoacoustic emission phase is attributed to component mixing. J Acoust Soc Am. 2011;129(5):3123–3133. doi: 10.1121/1.3573992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abel C, Kössl M. Sensitive response to low-frequency cochlear distortion products in the auditory midbrain. J Neurophysiol. 2009;101(3):1560–1574. doi: 10.1152/jn.90805.2008. [DOI] [PubMed] [Google Scholar]
- Allen JB, Fahey PF. A second cochlear-frequency map that correlates distortion product and neural tuning measurements. J Acoust Soc Am. 1993;94(2 Pt 1):809–816. doi: 10.1121/1.408182. [DOI] [PubMed] [Google Scholar]
- Ashmore J. Cochlear outer hair cell motility. Physiological Reviews. 2008;88(1):173–210. doi: 10.1152/physrev.00044.2006. [DOI] [PubMed] [Google Scholar]
- Ashmore J, Avan P, Brownell WE, Dallos P, Dierkes K, Fettiplace R, Grosh K, Hackney CM, Hudspeth AJ, Jülicher F, Lindner B, Martin P, Meaud J, Petit C, Santos-Sacchi J, Sacchi JR, Canlon B. The remarkable cochlear amplifier. Hear Res. 2010;266(1–2):1–17. doi: 10.1016/j.heares.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avan P, Bonfils P, Gilain L, Mom T. Physiopathological significance of distortion-product otoacoustic emissions at 2f1–f2 produced by high-versus low-level stimuli. J Acoust Soc Am. 2003;113(1):430–441. doi: 10.1121/1.1525285. [DOI] [PubMed] [Google Scholar]
- Avan P, Büki B, Petit C. Auditory distortions: origins and functions. Physiol Rev. 2013;93(4):1563–1619. doi: 10.1152/physrev.00029.2012. [DOI] [PubMed] [Google Scholar]
- Bian L, Chen SX. Behaviors of cubic distortion product otoacoustic emissions evoked by amplitude modulated tones. J Acoust Soc Am. 2011;129(2):828–839. doi: 10.1121/1.3531813. [DOI] [PubMed] [Google Scholar]
- Bielefeld EC, Coling D, Chen GD, Li M, Tanaka C, Hu BH, Henderson D. Age-related hearing loss in the Fischer 344/NHsd rat substrain. Hear Res. 2008;241(1–2):26–33. doi: 10.1016/j.heares.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettcher FA, Poth EA, Mills JH, Dubno JR. The amplitude-modulation following response in young and aged human subjects. Hear Res. 2001;153(1–2):32–42. doi: 10.1016/s0378-5955(00)00255-0. [DOI] [PubMed] [Google Scholar]
- Brown AM. Acoustic distortion from rodent ears: a comparison of responses from rats, guinea pigs and gerbils. Hear Res. 1987;31(1):25–37. doi: 10.1016/0378-5955(87)90211-5. [DOI] [PubMed] [Google Scholar]
- Brown AM, Gaskill SA, Carlyon RP, Williams DM. Acoustic distortion as a measure of frequency selectivity: relation to psychophysical equivalent rectangular bandwidth. J Acoust Soc Am. 1993;93(6):3291–3297. doi: 10.1121/1.405713. [DOI] [PubMed] [Google Scholar]
- Buckiova D, Popelar J, Syka J. Aging cochleas in the F344 rat: morphological and functional changes. Exp Gerontol. 2007;42(7):629–638. doi: 10.1016/j.exger.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Cederholm JM, Froud KE, Wong AC, Ko M, Ryan AF, Housley GD. Differential actions of isoflurane and ketamine-based anaesthetics on cochlear function in the mouse. Hear Res. 2012;292(1–2):71–79. doi: 10.1016/j.heares.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen GD, Li M, Tanaka C, Bielefeld EC, Hu BH, Kermany MH, Salvi R, Henderson D. Aging outer hair cells (OHCs) in the Fischer 344 rat cochlea: function and morphology. Hear Res. 2009;248(1–2):39–47. doi: 10.1016/j.heares.2008.11.010. [DOI] [PubMed] [Google Scholar]
- Dhar S, Long GR, Talmadge CL, Tubis A. The effect of stimulus-frequency ratio on distortion product otoacoustic emission components. J Acoust Soc Am. 2005;117(6):3766–3776. doi: 10.1121/1.1903846. [DOI] [PubMed] [Google Scholar]
- Dolphin WF, Mountain DC. The envelope following response (EFR) in the Mongolian gerbil to sinusoidally amplitude-modulated signals in the presence of simultaneously gated pure-tones. J Acoust Soc Am. 1993;94(6):3215–3230. doi: 10.1121/1.407227. [DOI] [PubMed] [Google Scholar]
- Dreisbach LE, Siegel JH. Distortion-product otoacoustic emissions measured at high frequencies in humans. J Acoust Soc Am. 2001;110(5 Pt 1):2456–2469. doi: 10.1121/1.1406497. [DOI] [PubMed] [Google Scholar]
- Dubno JR, Eckert MA, Lee FS, Matthews LJ, Schmiedt RA. Classifying human audiometric phenotypes of age-related hearing loss from animal models. J Assoc Res Otolaryngol. 2013;14(5):687–701. doi: 10.1007/s10162-013-0396-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahey PF, Allen JB. Nonlinear phenomena as observed in the ear canal and at the auditory nerve. J Acoust Soc Am. 1985;77(2):599–612. doi: 10.1121/1.391878. [DOI] [PubMed] [Google Scholar]
- Faulstich M, Kössl M. Neuronal response to cochlear distortion products in the anteroventral cochlear nucleus of the gerbil. J Acoust Soc Am. 1999;105(1):491–502. doi: 10.1121/1.424586. [DOI] [PubMed] [Google Scholar]
- Frisina DR, Frisina RD. Speech recognition in noise and presbycusis: relations to possible neural mechanisms. Hear Res. 1997;106(1–2):95–104. doi: 10.1016/s0378-5955(97)00006-3. [DOI] [PubMed] [Google Scholar]
- Frisina DR, Frisina RD, Snell KB, Burkard R, Walton JP, Ison JR. Auditory temporal processing during aging. San Diego: Functional Neurobiology of Aging; 2001. pp. 565–579. [Google Scholar]
- Frisina RD. Handbook of Auditory Science: The Auditory Brain. Oxford: 2010. Aging changes in the central auditory system; pp. 415–436. [Google Scholar]
- Frisina RD, Walton JP. Age-related structural and functional changes in the cochlear nucleus. Hear Res. 2006;216:216–223. doi: 10.1016/j.heares.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Gates GA, Mills D, Nam BH, D’Agostino R, Rubel EW. Effects of age on the distortion product otoacoustic emission growth functions. Hear Res. 2002;163(1–2):53–60. doi: 10.1016/s0378-5955(01)00377-x. [DOI] [PubMed] [Google Scholar]
- Geisler CD, Yates GK, Patuzzi RB, Johnstone BM. Saturation of Outer Hair Cell-Receptor Currents causes 2-tone Supression. Hear Res. 1990;44(2–3):241–256. doi: 10.1016/0378-5955(90)90084-3. [DOI] [PubMed] [Google Scholar]
- Harris FP, Lonsbury-Martin BL, Stagner BB, Coats AC, Martin GK. Acoustic distortion products in humans: systematic changes in amplitudes as a function of f2/f1 ratio. J Acoust Soc Am. 1989;85(1):220–229. doi: 10.1121/1.397728. [DOI] [PubMed] [Google Scholar]
- He NJ, Mills JH, Ahlstrom JB, Dubno JR. Age-related differences in the temporal modulation transfer function with pure-tone carriers. J Acoust Soc Am. 2008;124(6):3841–3849. doi: 10.1121/1.2998779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry K, Snyder S, Heinz M. Correlations between noninvasive and direct physiological metrics of auditory function in chinchillas with noise-induced hearing loss. Proceedings of Meetings on Acoustics. 2013;19:1–7. [Google Scholar]
- Henry KS, Kale S, Heinz MG. Noise-induced hearing loss increases the temporal precision of complex envelope coding by auditory-nerve fibers. Front Syst Neurosci. 2014;8:20. doi: 10.3389/fnsys.2014.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner K, de Ribaupierre Y, de Ribaupierre F. Neural correlates of cubic difference tones in the medial geniculate body of the cat. Hear Res. 1983;11(3):343–357. doi: 10.1016/0378-5955(83)90066-7. [DOI] [PubMed] [Google Scholar]
- Jin SH, Liu C, Sladen DP. The effects of aging on speech perception in noise: comparison between normal-hearing and cochlear-implant listeners. J Am Acad Audiol. 2014;25(7):656–665. doi: 10.3766/jaaa.25.7.4. [DOI] [PubMed] [Google Scholar]
- John MS, Lins OG, Boucher BL, Picton TW. Multiple auditory steady-state responses (MASTER): stimulus and recording parameters. Audiology. 1998;37(2):59–82. doi: 10.3109/00206099809072962. [DOI] [PubMed] [Google Scholar]
- Kemp DT. Stimulated acoustic emissions from within the human auditory system. J Acoust Soc Am. 1978;64:1386–1391. doi: 10.1121/1.382104. [DOI] [PubMed] [Google Scholar]
- Kennedy HJ, Crawford AC, Fettiplace R. Force generation by mammalian hair bundles supports a role in cochlear amplification. Nature. 2005;433(7028):880–883. doi: 10.1038/nature03367. [DOI] [PubMed] [Google Scholar]
- Kim SH, Frisina DR, Frisina RD. Effects of age on contralateral suppression of distortion product otoacoustic emissions in human listeners with normal hearing. Audiology and Neuro-Otology. 2002;7(6):348–357. doi: 10.1159/000066159. [DOI] [PubMed] [Google Scholar]
- Knight RD, Kemp DT. Indications of different distortion product otoacoustic emission mechanisms from a detailed f1, f2 area study. J Acoust Soc Am. 2000;107(1):457–473. doi: 10.1121/1.428351. [DOI] [PubMed] [Google Scholar]
- Kumar AU, SAV Temporal processing abilities across different age groups. J Am Acad Audiol. 2011;22(1):5–12. doi: 10.3766/jaaa.22.1.2. [DOI] [PubMed] [Google Scholar]
- Lasky RE. Distortion product otoacoustic emissions in human newborns and adults. I. Frequency effects. J Acoust Soc Am. 1998;103(2):981–991. doi: 10.1121/1.421215. [DOI] [PubMed] [Google Scholar]
- Lasky RE, Snodgrass EB, Laughlin NK, Hecox KE. Distortion product otoacoustic emissions in Macaca mulatta and humans. Hear Res. 1995;89(1–2):35–51. doi: 10.1016/0378-5955(95)00120-1. [DOI] [PubMed] [Google Scholar]
- Lee J, Dhar S. Can cochlear mechanics contribute to amplitude modulation perception?. Poster of the 21st International Congress on Acoustics.2013. [Google Scholar]
- Leigh-Paffenroth ED, Fowler CG. Amplitude-modulated auditory steady-state responses in younger and older listeners. J Am Acad Audiol. 2006;17(8):582–597. doi: 10.3766/jaaa.17.8.5. [DOI] [PubMed] [Google Scholar]
- Li-Korotky HS. Age-related hearing loss: quality of care for quality of life. Gerontologist. 2012;52(2):265–271. doi: 10.1093/geront/gnr159. [DOI] [PubMed] [Google Scholar]
- Liberman MC, Dodds LW. Single-neuron labeling and chronic cochlear pathology. III. Stereocilia damage and alterations of threshold tuning curves. Hear Res. 1984;16(1):55–74. doi: 10.1016/0378-5955(84)90025-x. [DOI] [PubMed] [Google Scholar]
- Liberman MC, Zuo J, Guinan JJ. Otoacoustic emissions without somatic motility: can stereocilia mechanics drive the mammalian cochlea? J Acoust Soc Am. 2004;116(3):1649–1655. doi: 10.1121/1.1775275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonsbury-Martin BL, Cutler WM, Martin GK. Evidence for the influence of aging on distortion-product otoacoustic emissions in humans. J Acoust Soc Am. 1991;89(4):1749–1759. doi: 10.1121/1.401009. [DOI] [PubMed] [Google Scholar]
- Martin GK, Stagner BB, Chung YS, Lonsbury-Martin BL. Characterizing distortion-product otoacoustic emission components across four species. J Acoust Soc Am. 2011;129(5):3090–3103. doi: 10.1121/1.3560123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlpine D. Neural sensitivity to periodicity in the inferior colliculus: evidence for the role of cochlear distortions. J Neurophysiol. 2004;92(3):1295–1311. doi: 10.1152/jn.00034.2004. [DOI] [PubMed] [Google Scholar]
- Mills DM, Rubel EW. Variation of distortion product otoacoustic emissions with furosemide injection. Hear Res. 1994;77(1–2):183–199. doi: 10.1016/0378-5955(94)90266-6. [DOI] [PubMed] [Google Scholar]
- Moulin A. Influence of primary frequencies ratio on distortion product otoacoustic emissions amplitude. I. Intersubject variability and consequences on the DPOAE-gram. J Acoust Soc Am. 2000;107(3):1460–1470. doi: 10.1121/1.428433. [DOI] [PubMed] [Google Scholar]
- Oswald JA, Rosner T, Janssen T. Hybrid measurement of auditory steady-state responses and distortion product otoacoustic emissions using an amplitude-modulated primary tone. J Acoust Soc Am. 2006;119(6):3886–3895. doi: 10.1121/1.2197789. [DOI] [PubMed] [Google Scholar]
- Parham K, Sun XM, Kim DO. Distortion product otoacoustic emissions in the CBA/J mouse model of presbycusis. Hear Res. 1999;134(1–2):29–38. doi: 10.1016/s0378-5955(99)00059-3. [DOI] [PubMed] [Google Scholar]
- Park JY, Clark WW, Coticchia JM, Esselman GH, Fredrickson JM. Distortion product otoacoustic emissions in rhesus (Macaca mulatta) monkey ears: normative findings. Hear Res. 1995;86(1–2):147–162. doi: 10.1016/0378-5955(95)00065-c. [DOI] [PubMed] [Google Scholar]
- Parthasarathy A, Bartlett E. Two-channel recording of auditory-evoked potentials to detect age-related deficits in temporal processing. Hear Res. 2012;289(1–2):52–62. doi: 10.1016/j.heares.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthasarathy A, Bartlett EL. Age-related auditory deficits in temporal processing in F-344 rats. Neurosci. 2011;192:619–630. doi: 10.1016/j.neuroscience.2011.06.042. [DOI] [PubMed] [Google Scholar]
- Parthasarathy A, Cunningham PA, Bartlett EL. Age-related differences in auditory processing as assessed by amplitude-modulation following responses in quiet and in noise. Front Aging Neurosci. 2010;2:152. doi: 10.3389/fnagi.2010.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthasarathy A, Datta J, Torres JA, Hopkins C, Bartlett EL. Age-related changes in the relationship between auditory brainstem responses and envelope-following responses. J Assoc Res Otolaryngol. 2014;15(4):649–661. doi: 10.1007/s10162-014-0460-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popelar J, Groh D, Pelánová J, Canlon B, Syka J. Age-related changes in cochlear and brainstem auditory functions in Fischer 344 rats. Neurobiol Aging. 2006;27(3):490–500. doi: 10.1016/j.neurobiolaging.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Purcell DW, Ross B, Picton TW, Pantev C. Cortical responses to the 2f1–f2 combination tone measured indirectly using magnetoencephalography. J Acoust Soc Am. 2007;122(2):992–1003. doi: 10.1121/1.2751250. [DOI] [PubMed] [Google Scholar]
- Rosen S. Temporal information in speech: acoustic, auditory and linguistic aspects. Philos Trans R Soc Lond B Biol Sci. 1992;336(1278):367–373. doi: 10.1098/rstb.1992.0070. [DOI] [PubMed] [Google Scholar]
- Ruggero MA, Rich NC. Furosemide alters organ of corti mechanics: evidence for feedback of outer hair cells upon the basilar membrane. J Neurosci. 1991;11(4):1057–1067. doi: 10.1523/JNEUROSCI.11-04-01057.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rübsamen R, Mills DM, Rubel EW. Effects of furosemide on distortion product otoacoustic emissions and on neuronal responses in the anteroventral cochlear nucleus. J Neurophysiol. 1995;74(4):1628–1638. doi: 10.1152/jn.1995.74.4.1628. [DOI] [PubMed] [Google Scholar]
- Schmiedt RA. Acoustic distortion in the ear canal. I. Cubic difference tones: effects of acute noise injury. J Acoust Soc Am. 1986;79(5):1481–1490. doi: 10.1121/1.393675. [DOI] [PubMed] [Google Scholar]
- Schmiedt RA, Lang H, Okamura HO, Schulte BA. Effects of furosemide applied chronically to the round window: a model of metabolic presbyacusis. J Neurosci. 2002;22(21):9643–9650. doi: 10.1523/JNEUROSCI.22-21-09643.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider BA, Daneman M, Murphy DR. Speech comprehension difficulties in older adults: cognitive slowing or age-related changes in hearing? Psychol Aging. 2005;20(2):261–271. doi: 10.1037/0882-7974.20.2.261. [DOI] [PubMed] [Google Scholar]
- Shaffer LA, Withnell RH, Dhar S, Lilly DJ, Goodman SS, Harmon KM. Sources and mechanisms of DPOAE generation: implications for the prediction of auditory sensitivity. Ear Hear. 2003;24(5):367–379. doi: 10.1097/01.AUD.0000090439.16438.9F. [DOI] [PubMed] [Google Scholar]
- Shamma SA, Elhilali M, Micheyl C. Temporal coherence and attention in auditory scene analysis. Trends Neurosci. 2011;34(3):114–123. doi: 10.1016/j.tins.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon RV, Zeng FG, Kamath V, Wygonski J, Ekelid M. Speech recognition with primarily temporal cues. Science. 1995;270(5234):303–304. doi: 10.1126/science.270.5234.303. [DOI] [PubMed] [Google Scholar]
- Shen Y. Gap detection and temporal modulation transfer function as behavioral estimates of auditory temporal acuity using band-limited stimuli in young and older adults. J Speech Lang Hear Res. 2014;57(6):2280–2292. doi: 10.1044/2014_JSLHR-H-13-0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon H, Frisina RD, Walton JP. Age reduces response latency of mouse inferior colliculus neurons to AM sounds. J Acoust Soc Am. 2004;116(1):469–477. doi: 10.1121/1.1760796. [DOI] [PubMed] [Google Scholar]
- Smoorenburg GF, Gibson MM, Kitzes LM, Rose JE, Hind JE. Correlates of combination tones observed in the response of neurons in the anteroventral cochlear nucleus of the cat. J Acoust Soc Am. 1976;59(4):945–962. doi: 10.1121/1.380954. [DOI] [PubMed] [Google Scholar]
- Snell KB, Frisina DR. Relationships among age-related differences in gap detection and word recognition. J Acoust Soc Am. 2001;107(3):1615–1626. doi: 10.1121/1.428446. [DOI] [PubMed] [Google Scholar]
- Strouse A, Ashmead DH, Ohde RN, Grantham DW. Temporal processing in the aging auditory system. J Acoust Soc Am. 1998;104(4):2385–2399. doi: 10.1121/1.423748. [DOI] [PubMed] [Google Scholar]
- Takahashi GA, Bacon SP. Modulation detection, modulation masking, and speech understanding in noise in the elderly. J Speech Hear Res. 1992;35(6):1410–1421. doi: 10.1044/jshr.3506.1410. [DOI] [PubMed] [Google Scholar]
- Tasaki I, Spyropoulos CS. Stria vascularis as source of endocochlear potential. J Neurophysiol. 1959;22(2):149–155. doi: 10.1152/jn.1959.22.2.149. [DOI] [PubMed] [Google Scholar]
- Ter-Mikaelian M, Sanes DH, Semple MN. Transformation of temporal properties between auditory midbrain and cortex in the awake Mongolian gerbil. J Neurosci. 2007;27(23):6091–6102. doi: 10.1523/JNEUROSCI.4848-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valero MD, Pasanen EG, McFadden D, Ratnam R. Distortion-product otoacoustic emissions in the common marmoset (Callithrix jacchus): parameter optimization. Hear Res. 2008;243(1–2):57–68. doi: 10.1016/j.heares.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varghese GI, Zhu XX, Frisina RD. Age-related declines in distortion product otoacoustic emissions utilizing pure tone contralateral stimulation in CBA/CaJ mice. Hear Res. 2005;209(1–2):60–67. doi: 10.1016/j.heares.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Verpy E, Weil D, Leibovici M, Goodyear RJ, Hamard G, Houdon C, Lefèvre GM, Hardelin JP, Richardson GP, Avan P, Petit C. Stereocilin-deficient mice reveal the origin of cochlear waveform distortions. Nature. 2008;456(7219):255–258. doi: 10.1038/nature07380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton JP, Frisina RD, O’Neill WE. Age-related alteration in processing of temporal sound features in the auditory midbrain of the CBA mouse. J Neurosci. 1998;18(7):2764–2776. doi: 10.1523/JNEUROSCI.18-07-02764.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton JP, Simon H, Frisina RD. Age-related alterations in the neural coding of envelope periodicities. J Neurophysiol. 2002;88(2):565–578. doi: 10.1152/jn.2002.88.2.565. [DOI] [PubMed] [Google Scholar]
- Whitehead ML, Lonsbury-Martin BL, Martin GK. Evidence for two discrete sources of 2f1–f2 distortion-product otoacoustic emission in rabbit: I. Differential dependence on stimulus parameters. J Acoust Soc Am. 1992;91(3):1587–1607. doi: 10.1121/1.402440. [DOI] [PubMed] [Google Scholar]
- Withnell RH, Shaffer LA, Talmadge CL. Generation of DPOAEs in the guinea pig. Hear Res. 2003;178(1–2):106–117. doi: 10.1016/s0378-5955(03)00064-9. [DOI] [PubMed] [Google Scholar]
- Zeng FG, Nie K, Stickney GS, Kong YY, Vongphoe M, Bhargave A, Wei C, Cao K. Speech recognition with amplitude and frequency modulations. Proc Natl Acad Sci U S A. 2005;102(7):2293–2298. doi: 10.1073/pnas.0406460102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Z, Henry KS, Heinz MG. Sensorineural hearing loss amplifies neural coding of envelope information in the central auditory system of chinchillas. Hear Res. 2014;309:55–62. doi: 10.1016/j.heares.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwicker E. Formulae for calculating the psychoacoustical excitation level of aural difference tones measured by the cancellation method. J Acoust Soc Am. 1981;69(5):1410–1413. doi: 10.1121/1.385823. [DOI] [PubMed] [Google Scholar]