Abstract
Non-obstructive hypertrophic cardiomyopathy (HC) patients are considered low-risk, generally not requiring aggressive intervention. However, non- and labile-obstructive HC have been traditionally classified together and it is unknown if these 2 sub-groups have distinct risk profiles. We compared cardiovascular outcomes in 293 HC patients (96 non-obstructive, 114 labile-obstructive and 83 obstructive) referred for exercise echocardiography and magnetic resonance imaging and followed for 3.3±3.6 years. A sub-group (34 non-obstructive, 28 labile-obstructive, 21 obstructive) underwent positron emission tomography (PET). The mean number of sudden cardiac death risk factors was similar among groups (non-obstructive: 1.4 vs. labile-obstructive: 1.2 vs. obstructive: 1.4 risk factors, p=0.2). Prevalence of late gadolinium enhancement (LGE) was similar across groups but more non-obstructive patients had LGE≥20% of myocardial mass [23(30%) vs. 19(18%) labile-obstructive and 8(11%) obstructive, p=0.01]. Fewer labile-obstructive patients had regional PET perfusion abnormalities [12(46%) vs. non-obstructive 30(81%) and obstructive 17(85%), p=0.003]. During follow-up, 60 events were recorded (36 VT/VF, including 30 defibrillator discharges, 12 heart failure worsening and 2 deaths). Non-obstructive patients were at higher risk of VT/VF at follow-up, when compared to labile-obstructive (HR 0.18, 95%CI 0.04–0.84, p=0.03) and the risk persisted after adjusting for age, gender, syncope, family history of sudden cardiac death, abnormal blood pressure response and septum≥3cm (p=0.04). Appropriate defibrillator discharges were more frequent in non-obstructive [8(18%)] compared to labile-obstructive [0(0%), p=0.02] patients. In conclusion, non-obstructive hemodynamics is associated with more pronounced fibrosis and ischemia than labile-obstructive and is an independent predictor of VT/VF in HC.
Keywords: hypertrophic cardiomyopathy, arrhythmia, defibrillation
Novel imaging technologies have indicated that characteristics unrelated to outflow hemodynamics but related to the primary myopathy, such as fibrosis by imaging,1 microvascular ischemia2, 3 and abnormal myocardial mechanics,4, 5 are highly prevalent in Hypertrophic Cardiomyopathy (HC) and may be important arbiters of outcomes.1, 6, 7 Therefore, non-obstructive hemodynamics alone may not always confer low risk, a viewpoint corroborated by several anecdotal examples in our large-volume practice. Moreover, previously published outcome studies did not separate non-obstructive (resting and provoked gradients <30 mmHg) and labile-obstructive (resting <30 mmHg; provoked ≥30 mmHg) variants,8–10 as is the current clinical practice.11 Therefore, it is additionally unclear if there are differences in outcomes between non-obstructive versus labile-obstructive HC phenotypes not evident in existing published literature since both these groups were combined.
METHODS
This study was approved by the Institutional Review Board. A total of 344 patients were recruited at their first visit to the Johns Hopkins Hypertrophic Cardiomyopathy Center from 2005 to 2013 if they fulfilled previously used diagnostic criteria for HC, which primarily was a maximal septal wall thickness ≥15mm in the absence of other cardiac or systemic disease that may produce a similar degree of left ventricular hypertrophy8, 11, 12 and 293 of them were followed for a mean of 3.3±3.6 years. Patients with a previous myectomy or alcohol septal ablation were excluded. Clinical information was collected as previously described.13 We compared clinical features and outcomes within the 3 HC sub-groups.
Sustained ventricular tachycardia (VT), ventricular fibrillation (VF), appropriate implantable cardioverter defibrillator (ICD) discharge, heart failure worsening (defined as NYHA class worsening to class III or IV) and death were recorded by reviewing Holter and exercise ECG tracings, ICD interrogation reports and clinical visit notes. Appropriate ICD discharges were defined as documented ventricular tachycardia or fibrillation events at heart rate ≥180bpm.14, 15 Sudden cardiac death (SCD) risk was assessed by noting non-sustained ventricular tachycardia (NSVT), unexplained syncope of non-neurocardiogenic origin, previous VT/VF, family history of SCD, septum≥3cm and abnormal blood pressure response.11
Echocardiography was performed using a GE Vivid 7 ultrasound machine (GE Ultrasound, Milwaukee, WI) using a standard clinical protocol. Conventional measurements were performed as previously published.16, 17 Systolic anterior movement of the mitral valve was defined as absent, incomplete (no contact with the septum) and complete (contact between leaflet and septum).18 Left ventricular outflow tract (LVOT) gradients were measured pre and immediately post a symptom-limited exercise test19, 20 and patients were classified into non-obstructive (<30mmHg at rest and exercise), labile-obstructive (<30mmHg at rest and ≥30mmHg with exercise) and obstructive (≥30mmHg at rest).11
Cardiac magnetic resonance imaging (CMR) was performed on a 1.5-Tesla system (MAGNETOM Avanto, Siemens Healthcare, Erlangen, Germany), as described previously,21 with contrast, gadopentetate dimeglumine at 0.2 mmol/kg (Magnevist; Bayer Schering, Berlin, Germany). Late gadolinium enhancement (LGE) images were assessed in short axis view with validated software (QMASS 7.4, Medis) by an experienced reader (C.C.V). Endocardial and epicardial borders were manually traced in each slice and the myocardium was divided into 16 segments starting from the anterior insertion point of the right ventricle. A region of interest was placed in an area of normal appearing nulled myocardium, typically the basal lateral wall. Pixels with signal intensity greater than 6 standard deviations higher than the mean of normal myocardium were considered abnormal.22 The extent of LGE was expressed as a percentage of total left ventricular (LV) myocardial mass.
Patients with angina ≥3 months despite optimal medical therapy were referred for PET scanning and were imaged using a GE Discovery VCT PET/CT system. Regional myocardial perfusion was assessed using a same day rest/stress protocol as described previously.3, 21, 23, 24 Attenuation-corrected PET images were reconstructed by an iterative algorithm with post-processing filtering and static datasets analyzed using CardIQ Physio (GE Healthcare). Regional myocardial perfusion was semi-quantitatively assessed from the re-oriented images on different cardiac planes (short, horizontal, and vertical long axes) using the standard 17 American Heart Association segmentation, 5-point visual score method.3 The summed stress score (SSS) and summed rest score (SRS) consisted of the summation score of the 17 LV segments during vasodilator-stress and rest perfusion imaging. The summed difference score (SDS) consisted of the difference between SSS and SRS. An SDS ≥2 was considered abnormal in this study.
Data were analyzed using STATA software version 13 (StataCorp LP, College Station, Texas). Continuous variables are presented as mean ± standard deviation and categorical variables as the total number and percentage. Comparison of variables across groups was performed using ANOVA and Chi-square or Fisher’s exact test as appropriate. Statistical significance was set at p<0.05. We used Kaplan-Meier procedure to estimate the survival function for each category of HC. We then used a log-rank test to determine whether there was a significant difference in the 3 survival functions. A Cox proportional multivariate hazard model was built to control for potential confounders.
RESULTS
Clinical and echocardiographic characteristics of the study population, which included 96 non-obstructive (33%), 114 labile-obstructive (39%) and 83 obstructive patients (28%), are summarized in Tables 1–2. Obstructive patients were older and had more dyspnea at presentation, while gender distribution, co-morbidity profiles and body mass index did not differ among groups. Family history of HC, history of VT/VF, NSVT and ICD in place were more common in non-obstructive patients (Table 1). Maximum septal wall thickness and left ventricular ejection fraction were similar among groups. Obstructive patients had a higher E/e’ ratio and a larger left atrial diameter (Table 2).
Table 1.
Baseline characteristics
| Variable | Non- Obstructive (n=96) |
Labile- Obstructive (n=114) |
Obstructive (n=83) |
Total (n=293) |
p-value | |
|---|---|---|---|---|---|---|
| Length of follow-up (years) | 3.5±3.8 | 3.2±3.5 | 2.8±3.2 | 3.3±3.6 | 0.5 | |
| Age (years) | 49±15 | 50±15 | 55±13 | 51±15 | 0.01 | |
| Male | 60 (63%) | 83 (73%) | 51 (61%) | 194 (66%) | 0.2 | |
| Body Mass Index (kg/m2) | 29.4±5.7 | 29.7±5.3 | 30.4±6.0 | 29.8±5.7 | 0.5 | |
| NYHA | I | 64 (67%) | 71 (62%) | 30 (36%) | 165 (56%) | |
| II | 24 (25%) | 24 (21%) | 37 (45%) | 85 (29%) | <0.001 | |
| III | 8 (8%) | 19 (17%) | 16 (19%) | 43 (15%) | ||
| Angina pectoris | 33 (34%) | 46 (40.4) | 37 (44.6) | 116 (39.6) | 0.4 | |
| Syncope | 17 (18%) | 25 (21.9) | 14 (17.1) | 56 (19.2) | 0.6 | |
| Ventricular tachycardia/fibrillation | 10 (10%) | 0 (0%) | 1 (1%) | 11 (4%) | <0.001 | |
| Non-sustained ventricular tachycardia | 18 (19%) | 9 (8%) | 8 (10%) | 35 (12%) | 0.047 | |
| Atrial Fibrillation | 17 (18%) | 9 (8%) | 8 (10%) | 34 (12%) | 0.08 | |
| Implantable Cardioverter Defibrillator | 18 (19%) | 6 (5%) | 9 (11%) | 33 (11%) | 0.009 | |
| Family History | ||||||
| Hypertrophic Cardiomyopathy | 29 (31%) | 16 (15%) | 10 (12%) | 55 (19%) | 0.003 | |
| Sudden cardiac death | 26 (27%) | 28 (26%) | 19 (23%) | 73 (25%) | 0.8 | |
| Sudden cardiac death risk factors | 1.4±1.1 | 1.2±1.0 | 1.4±1.0 | 1.3±1.0 | 0.2 | |
| Medications | ||||||
| β-blocker | 68 (71%) | 77 (68%) | 67 (81%) | 212 (73%) | 0.1 | |
| Disopyramide | 0 (0%) | 6 (5%) | 5 (6%) | 11 (4%) | 0.06 | |
| Ca-blocker | 13 (14%) | 28 (25%) | 25 (30%) | 66 (23%) | 0.02 | |
Table 2.
Exercise Echocardiography
| Variables | Non- Obstructive |
Labile- Obstructive |
Obstructive | Total | p-value | |
|---|---|---|---|---|---|---|
| Max wall thickness (cm) | 2.0±0.5 | 2.0±0.5 | 2.2±0.5 | 2.1±0.5 | 0.1 | |
| Left atrium (cm) | 4.0±0.7 | 4.1±0.7 | 4.5±0.7 | 4.2±0.7 | <0.001 | |
| Ejection fraction (%) | 63±10 | 64±8 | 65±9 | 64±9 | 0.2 | |
| E/A | 1.4±0.8 | 1.3±0.5 | 1.3±0.7 | 1.3±0.6 | 0.4 | |
| E/e' | 16±9 | 17±9 | 24±13 | 19±11 | <0.001 | |
| Left ventricular outflow tract gradient at rest (mmHg) | 8±4 | 15±7 | 58±25 | 25±26 | <0.001 | |
| Left ventricular outflow tract gradient at stress (mmHg) | 18±6 | 69±42 | 111±40 | 64±50 | <0.001 | |
| Systolic anterior motion of mitral valve | no | 41 (43%) | 31 (27%) | 2 (2%) | 74 (25%) | <0.001 |
| incomplete | 54 (57%) | 68 (60%) | 44 (53%) | 166 (57%) | ||
| complete | 0 (0%) | 15 (13%) | 37 (45%) | 52 (18%) | ||
| Exercise time (seconds) | 552±195 | 573±224 | 486±184 | 545±206 | 0.04 | |
| Exercise capacity (METs) | 10.7±3.6 | 10.6±4.4 | 8.0±3.5 | 9.9±4.1 | <0.001 | |
| Heart rate at rest (beats/min) | 66±13 | 63±14 | 65±12 | 65±13 | 0.2 | |
| Systolic blood pressure at rest (mmHg) | 128±19 | 134±17 | 131±18 | 131±18 | 0.05 | |
| Diastolic blood pressure at rest (mmHg) | 77±12 | 78±11 | 74±11 | 76±11 | 0.05 | |
| Heart rate at stress (beats/min) | 147±27 | 147±28 | 135±26 | 143±27 | 0.004 | |
| Systolic blood pressure at stress (mmHg) | 166±30 | 167±34 | 147±34 | 161±34 | <0.001 | |
| Diastolic blood pressure at stress (mmHg) | 82±17 | 84±17 | 75±17 | 81±18 | 0.002 | |
| Age-predicted heart rate (%) | 86.1±14.5 | 86.7±14.3 | 81.2±14.1 | 84.9±14.5 | 0.02 | |
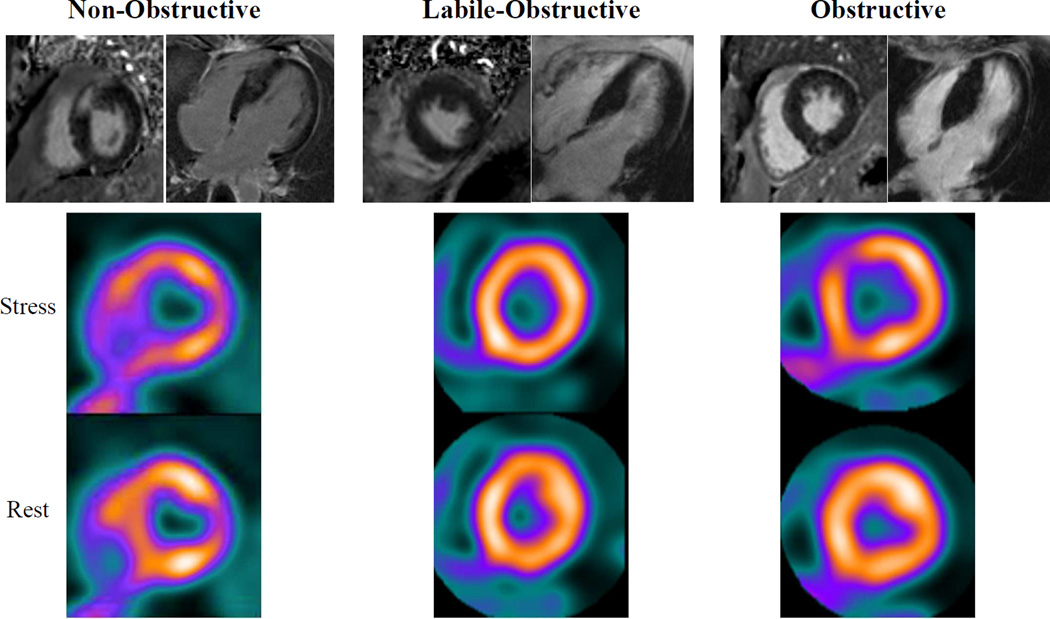
LGE images were available in 77 non-obstructive, 105 labile-obstructive and 72 obstructive patients (87% of the original sample), with clinical and echocardiographic characteristics comparable to those of the original groups. Presence of LGE was similar between groups [non-obstructive: 51(66%) vs. labile-obstructive: 64(61%) vs. obstructive: 49(68%), p=0.6]. However, extent of LGE was greater in non-obstructive (non-obstructive: 21±16 vs. labile-obstructive: 11±12 vs. obstructive: 12±10 %, p=0.002) and a larger proportion of non-obstructive patients carried a high LGE burden (≥20% myocardial mass) [non-obstructive: 23(30%) vs. labile-obstructive: 19(18%) vs. obstructive: 8(11%), p=0.01] (Figure 1).
Figure 1. Representative magnetic resonance and PET images of the 3 HC groups.
Patients without obstruction demonstrate higher late gadolinium enhancement (arrows) in magnetic resonance images. Non-obstructive and obstructive patients demonstrate perfusion abnormalities in PET images (arrows) compared to labile-obstructive, who demonstrate no clear perfusion abnormalities.
A sub-set of 83 patients (34 non-obstructive, 28 labile-obstructive, and 21 obstructive) underwent ammonia PET scanning. Fewer labile-obstructive patients had SDS≥2, indicating a lower extent of regional perfusion abnormalities [non-obstructive: 28(82%) vs. labile-obstructive: 15(54%) vs. obstructive: 16(76%), p=0.04] (Figure 1).
We noted 60 events (36 VT/VF including 30 ICD discharges, 12 heart failure worsening and 2 deaths) in the 293 patients during follow-up. Follow-up time and the mean number of SCD risk factors did not differ among groups (Table 1).
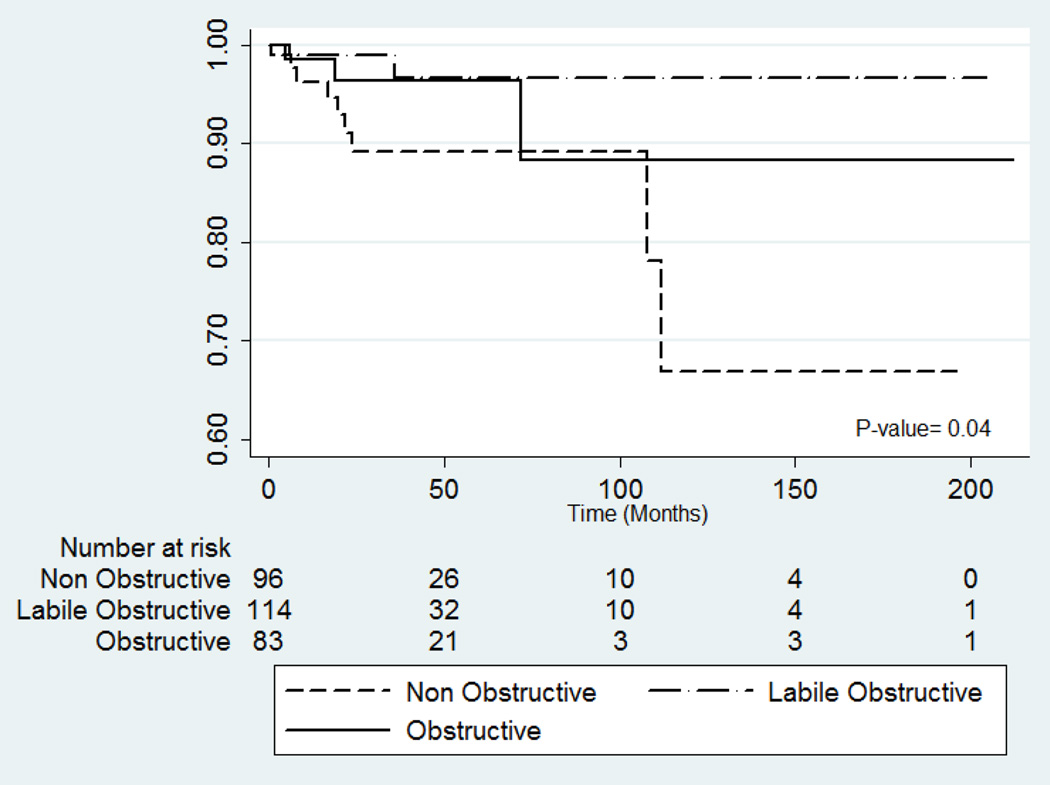
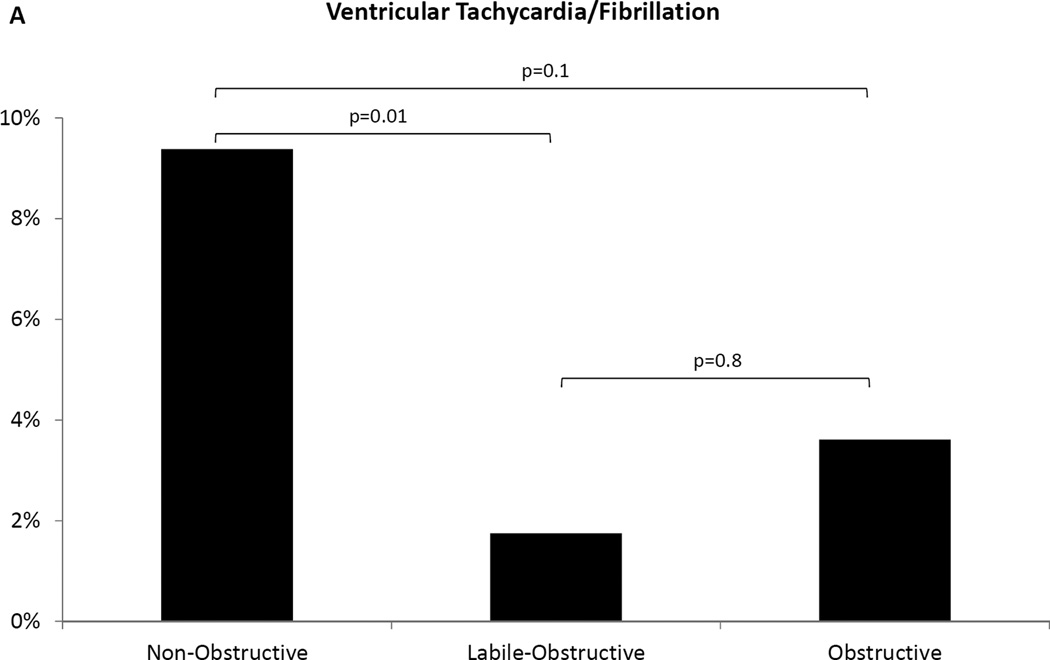
Kaplan Meier (Figure 2) and univariable Cox regression analysis indicated non-obstructive patients were at significantly higher risk of VT/VF during follow-up when compared to labile-obstructive (p=0.03, Table 3). Adjusting for age, gender and the established SCD risk factors (syncope, family history of SCD, abnormal blood pressure response, septal thickness ≥3cm)11, non-obstructive patients remained significantly at higher risk than labile-obstructive (p=0.04; Table 3). History of NSVT, family history of HC, NYHA functional class, presence or extent of LGE, CFR and SDS by PET, ejection fraction, left atrial diameter, septal thickness, E/e’ ratio and LVOT gradients at rest or exercise were not associated with a higher risk of VT/VF in univariate analysis. A higher proportion of non-obstructive patients experienced a VT/VF at follow-up when compared to labile-obstructive (9.4% vs. 1.8%, p=0.01). A similar trend was noted when compared to the obstructive group (9.4% vs. 3.6%, p=0.1; Figure 3A).
Figure 2. Kaplan-Meier curve for ventricular tachycardia/fibrillation events.
Non-obstructive patients had a higher rate for ventricular tachycardia/fibrillation events.
Table 3.
Cox proportional hazard ratios (95% confidence interval) for the prediction of ventricular tachycardia/fibrillation
| Unadjusted Hazard Ratio (95% CI) |
p-value | Adjusted Hazard Ratio* (95% CI) |
p-value | |
|---|---|---|---|---|
| HC group | ||||
| Non-Obstructive | 1.0 | - | 1.0 | - |
| Labile-Obstructive | 0.18 (0.04–0.84) | 0.03 | 0.2 (0.04–0.98) | 0.04 |
| Obstructive | 0.45 (0.12–1.66) | 0.23 | 0.5 (0.11–2.02) | 0.31 |
Adjusted for age, gender, syncope, family history of sudden cardiac death, max wall thickness ≥3cm and abnormal blood pressure response.
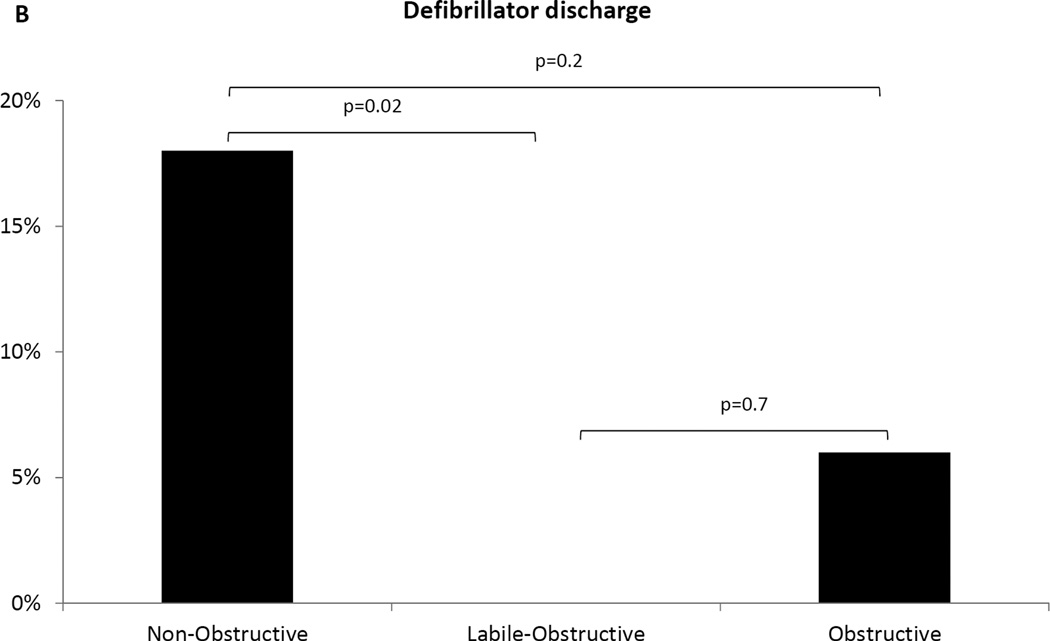
Figure 3. Ventricular tachycardia events and ICD discharges at follow-up.
(A) Non-obstructive patients had the highest prevalence of ventricular tachycardia/fibrillation events among the three groups. (B) More non-obstructive patients experienced appropriate ICD discharges compared to the other groups, while no events were recorded in the labile-obstructive group.
There were no inter-group differences in the rates of heart failure worsening [non-obstructive: 6 (6.3%) vs. labile-obstructive: 2 (1.8%) vs. obstructive: 4 (4.8%), p=0.2) and death - 1 non-obstructive patient died of sepsis and 1 labile-obstructive patient of cardiac arrest (p=0.7).
Sensitivity analysis was performed to assess for the potential bias introduced by differences in ICD prevalence and history of VT/VF among groups. When considering only those patients with an ICD in place at baseline or implanted at follow up [45(47%) in non-obstructive vs. 33(29%) in labile-obstructive vs. 33(40%) in obstructive, p=0.03], we found that more non-obstructive patients had at least one ICD discharge (n=8, 14%) as compared to labile-obstructive (n=0, 0%; p=0.02) and obstructive (n=2, 6%; p=0.2) (Figure 3B). In addition, the total number of appropriate ICD discharges was significantly higher in the non-obstructive (n=28) as compared to the labile-obstructive group (n=0, p=0.02) and similar in the non-obstructive and obstructive groups (n=2, p=0.2).
After excluding patients with a history of VT/VF, a similar trend was noticed, with more non-obstructive patients experiencing VT/VF at follow up (6.9%) compared to labile-obstructive (1.8%, p=0.065) and obstructive (2.5%, p=0.2). For those with an ICD but without a history of VT/VF, more non-obstructive patients had discharges (14%) compared to labile-obstructive (0%, p=0.03). A similar trend was noted when compared to obstructive patients (3%, p=0.1).
Finally, in order to examine whether patients with end-stage HC were contributing to the higher prevalence of adverse events in the non-obstructive group, we specifically examined patients with ejection fraction <50% (11 non-obstructive, 5 labile-obstructive, 4 obstructive patients). Mean EF was similar among sub-groups (non-obstructive: 44±5% vs. labile-obstructive: 44±6% vs. obstructive: 44±7%, p=0.99). None of these patients had a VT/VF episode and heart failure worsening from NYHA class II to III was recorded in 1 patient in the non-obstructive group.
DISCUSSION
Our study presents novel results with important clinical implications. 1) Non-obstructive HC is associated with significantly higher rates of ventricular arrhythmias compared to labile-HC, and similar to obstructive HC despite a similar mean number of currently used clinical SCD risk factors across the 3 HC sub-groups. These findings are in contrast with previously held concepts that non-obstructive HC patients experience a stable clinical course without significant symptoms or a high-risk profile. 2) Hemodynamic sub-types of HC have characteristic myopathic profiles. Non-obstructive patients have higher prevalence of large LGE burden on magnetic resonance and microvascular ischemia by PET. On the other hand, labile-obstructive HC is characterized by the least myopathic profile and the most favorable outcomes.
Previous clinical outcome studies8, 10 classified HC into obstructive and non-obstructive groups, the latter including those with labile obstruction. Since the emergence of the concept of labile obstruction,25 non-obstructive HC is currently parsed into the true non-obstructive (resting and provoked gradients <30 mmHg) and labile-obstructive (provoked gradients ≥30 mmHg).11 Our experience and review of HC literature8, 26–30 led us to question whether there were wider clinical differences between non-obstructive and the other HC groups. Our study confirmed that non-obstructive patients had higher rates of arrhythmias and more frequent ICD discharges. Higher rates of ventricular arrhythmias historically (Table 1) further support our prospective findings. After excluding those with previous VT/VF we found non-obstructive patients having 4 times the VT/VF episodes compared to labile-obstructive and 3 times that of obstructive. ICD discharges were also more frequent in this group.
Ventricular arrhythmias and ICD discharges were not related to systolic or diastolic function, or outflow tract gradients. In patients with EF<50% we found none with VT/VF.
Similarly, fewer arrhythmias were noted in labile-obstructive compared to non-obstructive HC in the only other study comparing arrhythmia events between these 2 HC groups.26 A study that indicated worse outcomes in obstructive HC, also noted that the annual rate of SCD events was only marginally higher in obstructive compared to patients without obstruction at rest (1.5 vs. 0.9%).8 Yet another study revealed that only 30% of HC-related deaths were associated with obstructive hemodynamics.27 In HC patients with a benign presentation and without risk factors, only 29% with SCD had obstruction and the rates (4.2%) were similar to those in our study (3.6%).28 A recent study examining the utility of extent of scar on SCD risk stratification found that most patients experiencing an arrhythmia had low outflow gradients.29 Data presented in these studies is highly concordant with ours. Our current findings imply that the favorable outcomes in the labile-obstructive group may drive the overall positive prognosis in the combined non-obstructive/labile-obstructive group presented in previous publications. Furthermore, previous studies that have shown no association between LVOT gradient and ventricular arrhythmias8, 11, 30 indirectly validate our results.
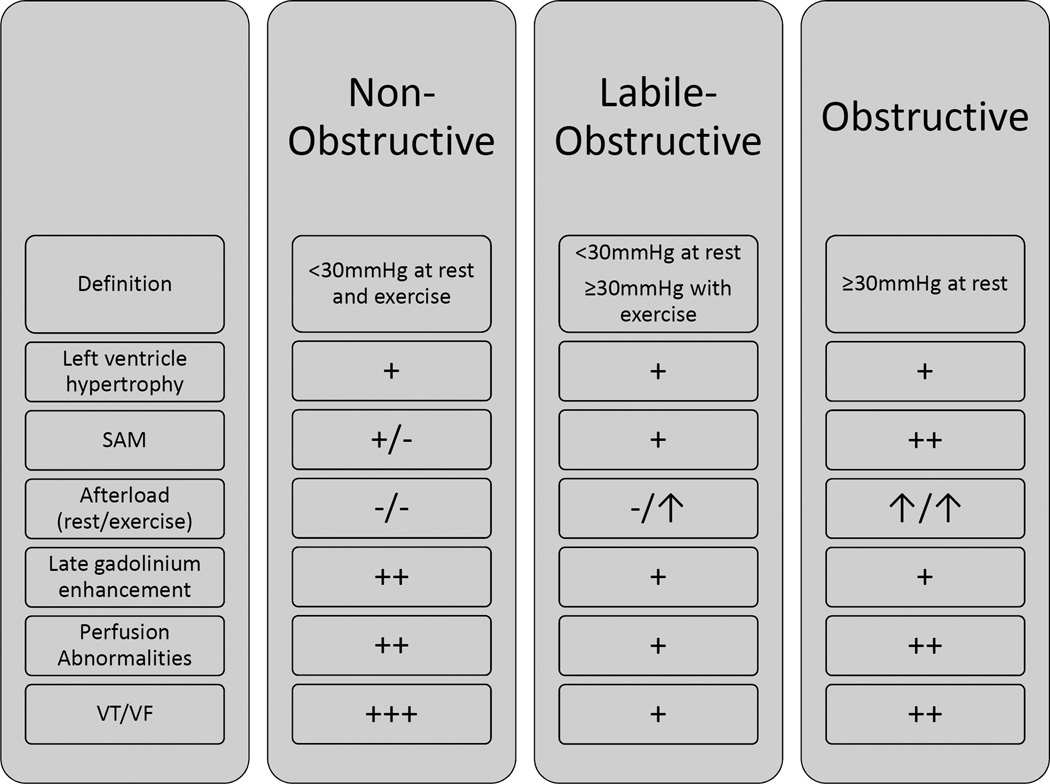
Additional characterization using novel imaging techniques helps validate our clinical outcomes results. Non-hemodynamic pathologic features of HC, which we for the purposes of this paper are labeling as the myopathic features, were more pronounced in the non-obstructive group. Conversely, the labile-obstructive patients were found to have the least myopathic profile and, interestingly, have the most favorable outcomes. Our data therefore demonstrate that the three hemodynamic subtypes of HC are associated with distinct myopathic profiles. Figure 4 summarizes the clinical and morphological characteristics of the 3 HC groups. Overall, we propose that there is a conglomeration of adverse factors in non-obstructive HC leading to unfavorable outcomes in this group. Notwithstanding these results, our data indicate that the relationship between these myopathic features and clinical outcomes, particularly ventricular arrhythmias, is not straightforward. This suggests the need for a wider examination of the relative importance of microvascular ischemia versus scar burden as a trigger for arrhythmias.3, 21 Moreover, arrhythmias were noted in HC patients with neither LGE nor ischemia, thus other factors may be operative. Notwithstanding the statistical results, the significantly higher proportion of non-obstructive patients with high LGE burden and PET-based ischemia suggests a role for fibrosis and ischemia in driving this risk. In our study, non-obstructive patients experienced almost 3 times as many VT/VF episodes compared to obstructive and 5 times as many compared to labile-obstructive patients. Consequently, our finding that non-obstructive patients are at high risk for adverse events revises a long-held concept in HC management.
Figure 4. Summary of anatomic and clinical characteristics of the 3 HC hemodynamic subtypes.
SAM: systolic anterior motion of mitral valve, VF: ventricular fibrillation, VT: ventricular tachycardia
Our results urge a re-consideration of several key management decisions in HC: 1) Non-obstructive HC patients may need to be monitored more closely and sudden cardiac death risk adjudication be performed more thoughtfully. 2) Estimation of scar burden via LGE and microvascular ischemia by PET may need to be considered as part of this risk adjudication. 3) Given the relatively benign clinical outcome profile, labile-obstructive HC may warrant a more conservative treatment strategy.
There may be other factors not examined or clearly evident in our analysis that may contribute to these inter-group differences. We did not see differences in deaths or heart failure. This could be a reflection of our follow-up period, sample size and/or cohort case-mix. It is likely that longer follow-up periods may reveal that obstructive patients have higher rates of heart failure (as reported previously), which makes sense given the afterload burden in this group. Nonetheless, our arrhythmia data are convincing and relevant. We did not include genotyping data. At the current time, diagnosis, treatment and prognostication of HC is clinically adjudicated without use of genetic information. Moreover, eliminating gene-negative individuals would exclude about 50–60% of the population at-risk.
Acknowledgements
We thank the sonographers/technologists and nurses of the Johns Hopkins Echocardiography and CMR laboratories for their contributions. We appreciate support from the Dr. Lawrence and Sheila Pakula Foundation and the Hypertrophic Cardiomyopathy Association (HCMA).
Funding Sources: This work was supported in part by a grant from the National Institutes of Health, Bethesda, MD (HL 9804006). Iraklis Pozios was supported in part by a grant from the Hellenic Cardiological Society, Athens, Greece.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.O'Hanlon R, Grasso A, Roughton M, Moon JC, Clark S, Wage R, Webb J, Kulkarni M, Dawson D, Sulaibeekh L, Chandrasekaran B, Bucciarelli-Ducci C, Pasquale F, Cowie MR, McKenna WJ, Sheppard MN, Elliott PM, Pennell DJ, Prasad SK. Prognostic significance of myocardial fibrosis in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2010;56:867–874. doi: 10.1016/j.jacc.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 2.Cecchi F, Olivotto I, Gistri R, Lorenzoni R, Chiriatti G, Camici PG. Coronary microvascular dysfunction and prognosis in hypertrophic cardiomyopathy. N Engl J Med. 2003;349:1027–1035. doi: 10.1056/NEJMoa025050. [DOI] [PubMed] [Google Scholar]
- 3.Bravo PE, Pinheiro A, Higuchi T, Rischpler C, Merrill J, Santaularia-Tomas M, Abraham MR, Wahl RL, Abraham TP, Bengel FM. PET/CT assessment of symptomatic individuals with obstructive and nonobstructive hypertrophic cardiomyopathy. J Nucl Med. 2012;53:407–414. doi: 10.2967/jnumed.111.096156. [DOI] [PubMed] [Google Scholar]
- 4.Serri K, Reant P, Lafitte M, Berhouet M, Le Bouffos V, Roudaut R, Lafitte S. Global and regional myocardial function quantification by two-dimensional strain: application in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2006;47:1175–1181. doi: 10.1016/j.jacc.2005.10.061. [DOI] [PubMed] [Google Scholar]
- 5.Abraham TP, Dimaano VL, Liang HY. Role of tissue Doppler and strain echocardiography in current clinical practice. Circulation. 2007;116:2597–2609. doi: 10.1161/CIRCULATIONAHA.106.647172. [DOI] [PubMed] [Google Scholar]
- 6.Adabag AS, Maron BJ, Appelbaum E, Harrigan CJ, Buros JL, Gibson CM, Lesser JR, Hanna CA, Udelson JE, Manning WJ, Maron MS. Occurrence and frequency of arrhythmias in hypertrophic cardiomyopathy in relation to delayed enhancement on cardiovascular magnetic resonance. J Am Coll Cardiol. 2008;51:1369–1374. doi: 10.1016/j.jacc.2007.11.071. [DOI] [PubMed] [Google Scholar]
- 7.Rubinshtein R, Glockner JF, Ommen SR, Araoz PA, Ackerman MJ, Sorajja P, Bos JM, Tajik AJ, Valeti US, Nishimura RA, Gersh BJ. Characteristics and clinical significance of late gadolinium enhancement by contrast-enhanced magnetic resonance imaging in patients with hypertrophic cardiomyopathy. Circ Heart Fail. 2010;3:51–58. doi: 10.1161/CIRCHEARTFAILURE.109.854026. [DOI] [PubMed] [Google Scholar]
- 8.Maron MS, Olivotto I, Betocchi S, Casey SA, Lesser JR, Losi MA, Cecchi F, Maron BJ. Effect of left ventricular outflow tract obstruction on clinical outcome in hypertrophic cardiomyopathy. N Engl J Med. 2003;348:295–303. doi: 10.1056/NEJMoa021332. [DOI] [PubMed] [Google Scholar]
- 9.Maron MS, Olivotto I, Zenovich AG, Link MS, Pandian NG, Kuvin JT, Nistri S, Cecchi F, Udelson JE, Maron BJ. Hypertrophic cardiomyopathy is predominantly a disease of left ventricular outflow tract obstruction. Circulation. 2006;114:2232–2239. doi: 10.1161/CIRCULATIONAHA.106.644682. [DOI] [PubMed] [Google Scholar]
- 10.Autore C, Bernabo P, Barilla CS, Bruzzi P, Spirito P. The prognostic importance of left ventricular outflow obstruction in hypertrophic cardiomyopathy varies in relation to the severity of symptoms. J Am Coll Cardiol. 2005;45:1076–1080. doi: 10.1016/j.jacc.2004.12.067. [DOI] [PubMed] [Google Scholar]
- 11.American College of Cardiology Foundation/American Heart Association Task Force on Practice, American Association for Thoracic Surgery, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Thoracic Surgeons. Gersh BJ, Maron BJ, Bonow RO, Dearani JA, Fifer MA, Link MS, Naidu SS, Nishimura RA, Ommen SR, Rakowski H, Seidman CE, Towbin JA, Udelson JE, Yancy CW, editors. 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Thorac Cardiovasc Surg. 2011;142:e153–e203. doi: 10.1016/j.jtcvs.2011.10.020. [DOI] [PubMed] [Google Scholar]
- 12.Spirito P, Bellone P, Harris KM, Bernabo P, Bruzzi P, Maron BJ. Magnitude of left ventricular hypertrophy and risk of sudden death in hypertrophic cardiomyopathy. N Engl J Med. 2000;342:1778–1785. doi: 10.1056/NEJM200006153422403. [DOI] [PubMed] [Google Scholar]
- 13.Canepa M, Sorensen LL, Pozios I, Dimaano VL, Luo HC, Pinheiro AC, Strait JB, Brunelli C, Abraham MR, Ferrucci L, Abraham TP. Comparison of clinical presentation, left ventricular morphology, hemodynamics, and exercise tolerance in obese versus nonobese patients with hypertrophic cardiomyopathy. Am J Cardiol. 2013;112:1182–1189. doi: 10.1016/j.amjcard.2013.05.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maron BJ, Shen W, Link MS, Epstein AE, Almquist AK, Daubert JP, Bardy GH, Favale S, Rea RF, Boriani G, Estes NAM, Spirito P, Casey SA, Stanton MS, Betocchi S. Efficacy of Implantable Cardioverter–Defibrillators for the Prevention of Sudden Death in Patients with Hypertrophic Cardiomyopathy. N Engl J Med. 2000;342:365–373. doi: 10.1056/NEJM200002103420601. [DOI] [PubMed] [Google Scholar]
- 15.Maron BJ, Spirito P, Shen WK, Haas TS, Formisano F, Link MS, Epstein AE, Almquist AK, Daubert JP, Lawrenz T, Boriani G, Estes NA, 3rd, Favale S, Piccininno M, Winters SL, Santini M, Betocchi S, Arribas F, Sherrid MV, Buja G, Semsarian C, Bruzzi P. Implantable cardioverter-defibrillators and prevention of sudden cardiac death in hypertrophic cardiomyopathy. JAMA. 2007;298:405–412. doi: 10.1001/jama.298.4.405. [DOI] [PubMed] [Google Scholar]
- 16.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ Chamber Quantification Writing Group, American Society of Echocardiography's Guidelines and Standards Committee, European Association of Echocardiography. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 17.Nagueh SF, Bierig SM, Budoff MJ, Desai M, Dilsizian V, Eidem B, Goldstein SA, Hung J, Maron MS, Ommen SR, Woo A American Society of Echocardiography, American Society of Nuclear Cardiology, Society for Cardiovascular Magnetic Resonance, Society of Cardiovascular Computed Tomography. American Society of Echocardiography clinical recommendations for multimodality cardiovascular imaging of patients with hypertrophic cardiomyopathy: Endorsed by the American Society of Nuclear Cardiology, Society for Cardiovascular Magnetic Resonance, and Society of Cardiovascular Computed Tomography. J Am Soc Echocardiogr. 2011;24:473–498. doi: 10.1016/j.echo.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 18.Shah JS, Esteban MT, Thaman R, Sharma R, Mist B, Pantazis A, Ward D, Kohli SK, Page SP, Demetrescu C, Sevdalis E, Keren A, Pellerin D, McKenna WJ, Elliott PM. Prevalence of exercise-induced left ventricular outflow tract obstruction in symptomatic patients with non-obstructive hypertrophic cardiomyopathy. Heart. 2008;94:1288–1294. doi: 10.1136/hrt.2007.126003. [DOI] [PubMed] [Google Scholar]
- 19.Pellikka PA, Nagueh SF, Elhendy AA, Kuehl CA, Sawada SG American Society of Echocardiography. American Society of Echocardiography recommendations for performance, interpretation, and application of stress echocardiography. J Am Soc Echocardiogr. 2007;20:1021–1041. doi: 10.1016/j.echo.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 20.Marwick TH, Nakatani S, Haluska B, Thomas JD, Lever HM. Provocation of latent left ventricular outflow tract gradients with amyl nitrite and exercise in hypertrophic cardiomyopathy. Am J Cardiol. 1995;75:805–809. doi: 10.1016/s0002-9149(99)80416-0. [DOI] [PubMed] [Google Scholar]
- 21.Bravo PE, Zimmerman SL, Luo H, Pozios I, Rajaram M, Pinheiro A, Steenbergen C, Kamel IR, Wahl RL, Bluemke DA, Bengel FM, Abraham MR, Abraham TP. Relationship of Delayed Enhancement by Magnetic Resonance to Myocardial Perfusion by Positron Emission Tomography in Hypertrophic Cardiomyopathy. Circulation: Cardiovascular Imaging. 2013;6:210–217. doi: 10.1161/CIRCIMAGING.112.000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spiewak M, Malek LA, Misko J, Chojnowska L, Milosz B, Klopotowski M, Petryka J, Dabrowski M, Kepka C, Ruzyllo W. Comparison of different quantification methods of late gadolinium enhancement in patients with hypertrophic cardiomyopathy. Eur J Radiol. 2010;74:e149–e153. doi: 10.1016/j.ejrad.2009.05.035. [DOI] [PubMed] [Google Scholar]
- 23.Hutchins GD, Schwaiger M, Rosenspire KC, Krivokapich J, Schelbert H, Kuhl DE. Noninvasive quantification of regional blood flow in the human heart using N-13 ammonia and dynamic positron emission tomographic imaging. J Am Coll Cardiol. 1990;15:1032–1042. doi: 10.1016/0735-1097(90)90237-j. [DOI] [PubMed] [Google Scholar]
- 24.Bravo PE, Pozios I, Pinheiro A, Merrill J, Tsui BM, Wahl RL, Bengel FM, Abraham MR, Abraham TP. Comparison and effectiveness of regadenoson versus dipyridamole on stress electrocardiographic changes during positron emission tomography evaluation of patients with hypertrophic cardiomyopathy. Am J Cardiol. 2012;110:1033–1039. doi: 10.1016/j.amjcard.2012.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Braunwald E, Lambrew CT, Rockoff SD, Ross J, Jr, Morrow AG. Idiopathic Hypertrophic Subaortic Stenosis. I. a Description of the Disease Based upon an Analysis of 64 Patients. Circulation. 1964;30(SUPPL 4):3–119. doi: 10.1161/01.cir.29.5s4.iv-3. [DOI] [PubMed] [Google Scholar]
- 26.Wigle ED, Sasson Z, Henderson MA, Ruddy TD, Fulop J, Rakowski H, Williams WG. Hypertrophic cardiomyopathy. The importance of the site and the extent of hypertrophy. A review. Prog Cardiovasc Dis. 1985;28:1–83. doi: 10.1016/0033-0620(85)90024-6. [DOI] [PubMed] [Google Scholar]
- 27.Maron BJ, Olivotto I, Spirito P, Casey SA, Bellone P, Gohman TE, Graham KJ, Burton DA, Cecchi F. Epidemiology of Hypertrophic Cardiomyopathy–Related Death: Revisited in a Large Non–Referral-Based Patient Population. Circulation. 2000;102:858–864. doi: 10.1161/01.cir.102.8.858. [DOI] [PubMed] [Google Scholar]
- 28.Spirito P, Autore C, Formisano F, Assenza GE, Biagini E, Haas TS, Bongioanni S, Semsarian C, Devoto E, Musumeci B, Lai F, Yeates L, Conte MR, Rapezzi C, Boni L, Maron BJ. Risk of Sudden Death and Outcome in Patients With Hypertrophic Cardiomyopathy With Benign Presentation and Without Risk Factors. Am J Cardiol. 2014;113:1550–1555. doi: 10.1016/j.amjcard.2014.01.435. [DOI] [PubMed] [Google Scholar]
- 29.Chan RH, Maron BJ, Olivotto I, Pencina MJ, Assenza GE, Haas T, Lesser JR, Gruner C, Crean AM, Rakowski H, Udelson JE, Rowin E, Lombardi M, Cecchi F, Tomberli B, Spirito P, Formisano F, Biagini E, Rapezzi C, De Cecco CN, Autore C, Cook EF, Hong SN, Gibson CM, Manning WJ, Appelbaum E, Maron MS. Prognostic Value of Quantitative Contrast-Enhanced Cardiovascular Magnetic Resonance for the Evaluation of Sudden Death Risk in Patients With Hypertrophic Cardiomyopathy. Circulation. 2014;130:484–495. doi: 10.1161/CIRCULATIONAHA.113.007094. [DOI] [PubMed] [Google Scholar]
- 30.Maron BJ, Olivotto I, Maron MS. The dilemma of left ventricular outflow tract obstruction and sudden death in hypertrophic cardiomyopathy: do patients with gradients really deserve prophylactic defibrillators? Eur Heart J. 2006;27:1895–1897. doi: 10.1093/eurheartj/ehl130. [DOI] [PubMed] [Google Scholar]