Abstract
Fibrotic remodeling in lung injury is a major cause of morbidity. The mechanism that mediates the ongoing fibrosis is unclear, and there is no available treatment to abate the aberrant repair. Reactive oxygen species (ROS) have a critical role in inducing fibrosis by modulating extracellular matrix deposition. Specifically, mitochondrial hydrogen peroxide (H2O2) production by alveolar macrophages is directly linked to pulmonary fibrosis as inhibition of mitochondrial H2O2 attenuates the fibrotic response in mice. Prior studies indicate that the small GTP-binding protein, Rac1, directly mediates H2O2 generation in the mitochondrial intermembrane space. Geranylgeranylation of the C-terminal cysteine residue (Cys189) is required for the for Rac1 activation and mitochondrial import. We hypothesized that impairment of geranylgeranylation would limit mitochondrial oxidative stress, and, thus, abrogate progression of pulmonary fibrosis. By targeting the isoprenoid pathway with a novel agent, digeranyl bisphosphonate (DGBP), which impairs geranylgeranylation, we demonstrate that Rac1 mitochondrial import, mitochondrial oxidative stress, and progression of the fibrotic response to lung injury are significantly attenuated. These observations reveal that targeting the isoprenoid pathway to alter Rac1 geranylgeranylation halts the progression of pulmonary fibrosis after lung injury.
INTRODUCTION
Pulmonary fibrosis is a devastating lung disease that is increasing in incidence, and no current therapeutic modalities are available to halt its progression. In particular, idiopathic pulmonary fibrosis (IPF), which is the most common form, has a median survival of 3-5 years after the diagnosis (1-3). The factors that regulate the process of tissue remodeling in pulmonary fibrosis are poorly understood. Defining the molecular mechanisms that mediate pulmonary fibrosis is urgently needed to prevent the development and/or halt the progression of the disease.
Reactive oxygen species (ROS) have a crucial role in inducing a fibrotic response to lung injury by modulating extracellular matrix deposition. Alveolar macrophages are critical in regulating host responses to lung injury, and H2O2 production by macrophages is directly linked to pulmonary fibrosis (4,5). The primary source of H2O2 in alveolar macrophages in the setting of fibrosis is the mitochondria (4-6). Moreover, inhibition of mitochondrial H2O2 or administration of catalase attenuates the fibrotic phenotype in mice (4,5,7).
The Rho GTP-binding proteins, including Rac1, play an important role in host defense. Rac1 regulates several cellular functions in macrophages, such as cell adhesion, actin polymerization and migration, and phagocytosis (8-10). Rac1 activation also increases the generation of H2O2 in nearly every cell type (7,11-14). In macrophages, Rac1 directly mediates H2O2 generation in the mitochondrial intermembrane space (6). Rac1 is biologically relevant in that mice harboring a conditional deletion of Rac1 in macrophages are protected from developing asbestos-induced pulmonary fibrosis (6,7).
The C-terminal cysteine residue in Rho GTPases, such as Cys189 in Rac1, can be modified by geranylgeranylation with the requisite geranylgeranyl moiety derived from the isoprenoid pathway. This post-translational modification is necessary for activation, interaction with other proteins, and mitochondrial import (6,15). Because mitochondrial Rac1 activity is linked to the development of the fibrotic phenotype in mice, we sought to target the isoprenoid pathway to inhibit Rac1 mitochondrial import as a therapeutic maneuver to prevent the fibrotic response to lung injury. Statins, which block the rate-limiting enzyme, HMG-CoA reductase, of the isoprenoid pathway, have been associated with interstitial lung abnormalities in smoking individuals likely due to inhibition of several intermediates in the isoprenoid pathway (16). Thus, we chose to use a more specific inhibitor of geranylgeranylation by inhibiting geranylgeranylpyrophosphate (GGPP) synthase, the enzyme that catalyzes the next to the last step in the post-translational modification of Rac1. Our novel observations reveal that targeting the isoprenoid pathway to alter Rac1 geranylgeranylation halts progression of pulmonary fibrosis after lung injury.
MATERIALS AND METHODS
Materials
Bleomycin was obtained from the University of Iowa Hospital and Clinics hospital stores. Chrysotile was provided Dr. Peter S. Thorne, College of Public Health. University of Iowa, Iowa City, IA. p-hydroxylphenyl acetic acid (pHPA), horseradish peroxidase (HRP), α-ketoglutarate and NADPH were purchased from Sigma Chemical Company (St. Louis, MO).
Human subjects
The Human Subjects Review Board of the University of Iowa Carver College of Medicine approved the protocol of obtaining alveolar macrophages from normal volunteers and patients with IPF and asbestosis. Normal volunteers had to meet the following criteria: (1) age between 18 and 55 years; (2) no history of cardiopulmonary disease or other chronic disease; (3) no prescription or nonprescription medication except oral contraceptives; (4) no recent or current evidence of infection; and (5) lifetime nonsmoker. Alveolar macrophages were also obtained from patients with IPF. Patients with IPF had to meet the following criteria: (1) FVC and DLCO at least 50% predicted; (2) current nonsmoker; (3) no recent or current evidence of infection; and (4) evidence of restrictive physiology on pulmonary function tests and interstitial fibrosis on chest computed tomography. Fiberoptic bronchoscopy with bronchoalveolar lavage was performed after subjects received intramuscular atropine (0.6 mg) and local anesthesia. Three sub-segments of the lung were lavaged with five 20-ml aliquots of normal saline, and the first aliquot in each was discarded. The percentage of alveolar macrophages was determined by Wright-Giemsa stain and varied from 90 to 98%.
Mice
Wild-type C57Bl/6 mice were from Jackson Laboratories (Bar Habor, Maine). The University of Iowa Institutional Animal Care and Use Committee approved all protocols. After equilibration, osmotic pumps (Alzet, Cupertino, CA) containing either vehicle (water) or DGBP (0.2 mg/kg/day) were implanted subcutaneously, as describe previously (17). Rac1 null and Rac2 knockout mice (a generous gift from Dr. Michael Glogauer, University of Toronto, Toronto, CA) have been previously described (5,18). Briefly, Rac1 null mice are conditional and were generated using LysMcre to selectively delete Rac1 from cells of the granulocyte/monocyte lineage. The Rac2 knockout mice were generated using conventional gene targeting to delete the Rac2 gene as Rac2 is only expressed in cells of the granulocyte/monocyte lineage. Bleomycin (1.3—2.0 U/kg) or chrysotile (100 μg) was administered intratracheally. Mice were euthanized and fibrosis determined as previously described (6,19).
Cell culture
THP-1 macrophages were obtained from American Type Culture Collection (Manassas, VA). Cells were maintained in RPMI-1640 media supplemented with fetal bovine serum and penicillin/streptomycin. All experiments were performed with media supplemented with 0.5% serum.
Synthesis of digeranyl bisphosphonate (DGBP)
DGBP (U.S. Patent: 7,268,164) was synthesized as previously described (20).
Determination of H2O2 generation
Extracellular H2O2 production was determined fluorometrically, as previously described (4). Mitochondrial H2O2 was measured by suspending mitochondria in phenol-red free Hanks’ balanced salt solution supplemented with 6.5 mM glucose, 1 mM HEPES, 6 mM sodium bicarbonate, 1.6 mM pHPA, 0.95 μg/ml HRP and 5mM α-ketoglutarate.
Isolation of mitochondria and membrane fractions
Mitochondria and cytoplasm were isolated as previously described (4,7).
Rac1 and Rac2 GTPase activation assays
Rac1 and Rac2 activity were determined using a bead pull-down kit (Cytoskeleton Inc.) or Rac1 activity was determined using the G-LISA kit (Cytoskeleton Inc.), according to manufacturer's protocols. Negative and positive lysate controls were incubated with GTPγS or GDP, respectively, during PAK-binding domain-GST pull-down for Rac1 and Rac2. Bound protein was eluted and separated by SDS-PAGE. Immunoblots were probed with an antibody specific to Rac1 or Rac2, and GST expression was determined by Coomassie staining, as a loading control. Active Rac1 was also determined by the binding of Rac1 to PAK-PBD beads immobilized in a 96-well plate using G-LISA. The bound active Rac1 was detected with a Rac1 specific antibody. Absorbance was read at 490 nm and normalized to protein concentration in the lysate sample.
Hydroxyproline assay
Lung tissue was dried to stable weight and acid hydrolyzed with 6N HCl for 24 h at 120 °C. Hydroxyproline concentration normalized to dry weight of the lung was determined as described previously (5).
Immunoblot analysis
Whole cells lysates and sub-cellular fractions were separated by SDS-PAGE and transferred to PVDF membranes. Immunoblot analyses on the membranes were performed with the designated antibodies followed by the appropriate secondary antibody cross-linked to HRP.
ELISA
Active TGF-β in BAL fluid was measured by ELISA (R&D, Minneapolis, MN), according to manufacturer's instructions.
Triton X-114 Separation
The separation of geranylgeranylated and non-geranylgeranylated Rac1 was prepared according to a previously published protocol (21). Briefly, cells were lysed in ice-cold Triton X-114 lysis buffer (20 mM Tris, pH 7.5, 150 mM NaCl, and 1% Triton X-114). Cell lysates were sonicated and cleared by centrifugation. The supernatant was incubated at 37 °C for 10 min and centrifuged at room temperature for 2 min at 12,000 × g. The detergent, or lower phase, was diluted with buffer that did not contain Triton X-114, and the aqueous, or upper phase, was transferred to a new tube.
Statistical analysis
Statistical comparisons were performed using an unpaired, two-tailed t test or one-way ANOVA followed by Tukey's post-test to compare columns. Values in figures are expressed as means with standard errors and p < 0.05 was considered to be significant.
RESULTS
Impaired geranylgeranylation of Rac1 by DGBP attenuates Rac1 activation and H2O2 generation
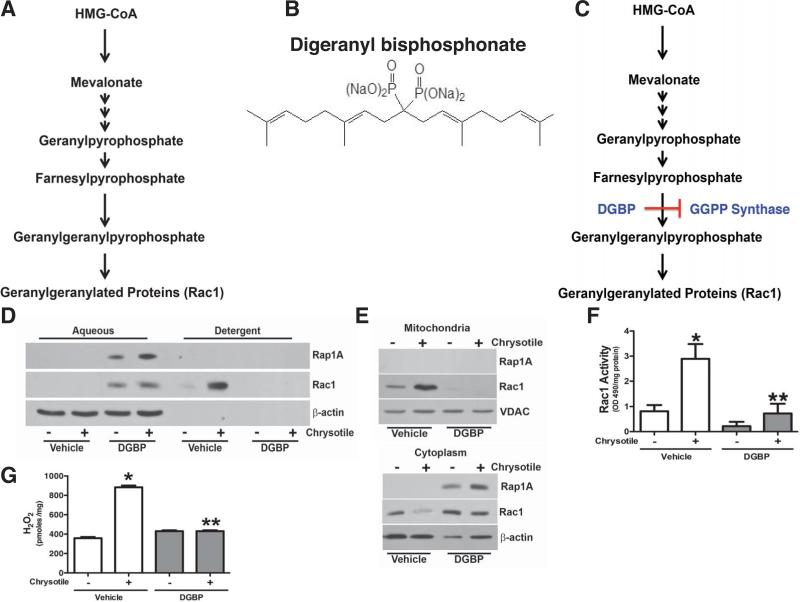
The C-terminal cysteine residues of Rho GTPases, including Rac1, are known to undergo geranylgeranylation, a post-transcriptional modification that is required for activation, interaction with other proteins, and mitochondrial import (6,15). Geranylgeranylation is catalyzed by geranylgeranyltransferase (GGTase), which transfers the geranylgeranyl moiety to the GTPase (Figure 1A). Because previous data demonstrate that the absence of Rac1 in macrophage mitochondria attenuates fibrosis development (6), we utilized a potent inhibitor of geranylgeranylpyrophosphate (GGPP) synthase, digeranyl bisphosphonate (DGBP), to inhibit geranylgeranylation of Rac1 (Figure 1B). DGBP was synthesized as previously described (20) and contains two polar groups that mimic the pyrophosphate and bind to the active site of GGPP synthase, the enzyme that catalyzes the conversion of farnesylpyrophosphate to GGPP (Figure 1C).
Figure 1. Digeranyl bisphosphonate attenuates Rac1 activity and H2O2 production.
(A) Schematic flow diagram of isoprenoid pathway. (B) Chemical diagram of digeranyl bisphophonate (DGBP). U.S. Patent: 7,268,164. (C) Schematic flow diagragm of the isopresnoid pathway showing the location of DGBP inhibition of GGPP synthase. (D) Macrophages were cultured overnight with vehicle (water) or DGBP (10 μM). Cells were exposed to chrysotile (10 μg/cm2) for 30 min. Cells were fractionated into aqueous or detergent phases. Lysates were subjected to immunoblot analysis for Rap 1A and Rac1. (E) Macrophages were cultured overnight with vehicle or DGBP (10 μM) and exposed to chrysotile (10 μg/cm2) for 30 min. Immunoblot analysis was performed for Rap 1A and Rac1 in isolated mitochondria (upper panel) and cytoplasm (lower panel). (F) Macrophages were cultured overnight with vehicle or DGBP (10 μM) and exposed to chrysotile (10 μg/cm2 for 30 min. Rac1 activity was measured by G-LISA and normalized to protein concentration. n = 4, * p < 0.018 vs. - chrysotile (vehicle) and ** p < 0.022 vs. chrysotile (vehicle). (G) Macrophages were cultured in the presence vehicle or DGBP (10 μM) overnight and exposed to chrysotile (10 μg/cm2) for 30 min. H2O2 was measured by pHPA assay and is expressed in pmoles/mg. n = 5, * p < 0.0001 vs. all other conditions.
To determine if DGBP modulates Rac1 geranylgeranylation, we exposed macrophages to vehicle or DGBP overnight followed by chrysotile exposure. Lysates were separated into an aqueous phase (hydrophilic), which contain non-prenylated proteins, and a detergent phase (hydrophobic), which retains the prenylated proteins. An immunoblot analysis for Rac1 and Rap 1A, using a Rap 1A antibody that only recognizes the non-geranylgeranylated protein and is indicative of reduced GGPP levels (21,22), was performed. Both Rac1 and non-geranylated Rap 1A were absent in the vehicle-exposed aqueous phase; whereas DGBP increased Rac1 and Rap 1A in the aqueous phase, which indicates they are non-geranylgeranylated (Figure 1D). In contrast, Rac1 increased in the detergent phase with chrysotile exposure, but it was not present with DGBP treatment.
Because mitochondrial Rac1 has been linked to H2O2 generation and the fibrotic phenotype (6), we determined if DGBP modulated mitochondrial Rac1 and localization. Macrophages were cultured in vehicle or DGBP overnight followed by chrysotile exposure. Chrysotile increased mitochondrial Rac1 content, whereas immunoreactive Rac1 was below control levels in DGBP-treated cells (Figure 1E, upper panel). Rap 1A was not seen in isolated mitochondria. In contrast, chrysotile exposure decreased Rac1 in the cytoplasm, while DGBP treatment increased cytoplasmic Rac1 expression (Figure 1E, lower panel). Non-geranylgeranylated Rap 1A was present in the cytoplasm of DGBP-treated cells, suggesting the geranylgeranylation of Rac1 is necessary for mitochondrial import.
To examine if DGBP modulates Rac1 activity, cells were exposed to vehicle or DGBP. Rac1 activation increased significantly after chrysotile exposure, whereas the activity in DGBP-treated cells was reduced to control levels (Figure 1F). DGBP also decreased H2O2 generation in chrysotile-exposed macrophages (Figure 1G). In aggregate, these results demonstrate that inhibition of geranylgeranylation by altering GGPP synthase activity is an effective way to abrogate Rac1 activation and oxidative stress in macrophages.
Geranylgeranylation of Rac1 is required for chrysotile-induced pulmonary fibrosis
Because Rac1-mediated mitochondrial H2O2 generation requires Rac1 geranylgeranylation and Rac1 null mice are protected from pulmonary fibrosis (6), we investigated the role of DGBP in modulating chrysotile-induced pulmonary fibrosis. WT mice with subcutaneous osmotic pumps delivering vehicle or DGBP were exposed to chrysotile. We first determined if DGBP altered mitochondrial Rac1 localization in alveolar macrophages 21 days after chrysotile exposure. Mitochondria isolated from mice exposed to chrysotile had greater Rac1 content in mitochondria than saline-exposed mice, whereas mitochondrial Rac1 content was markedly reduced in the mice treated with DGBP (Figure 2A). The opposite changes were seen in the cytoplasmic fraction indicating that Rac1 geranylgeranylation is necessary for mitochondrial Rac1 import (Figure 2B). Similar findings were found in vitro using the geranylgeranyl transferase type I inhibitor (GGTI). Chrysotile increased mitochondrial localization of Rac1 in vehicle-treated cells, while macrophages treated with GGTI had decreased Rac1 in the mitochondria (Supplemental Figure S1A) and an increase in the cytoplasmic fraction (Supplemental Figure S1B). Rac1 activity was also increased in the macrophages exposed to chrysotile, whereas activity was significantly reduced in cells treated with GGTI (Supplemental Figure S1C).
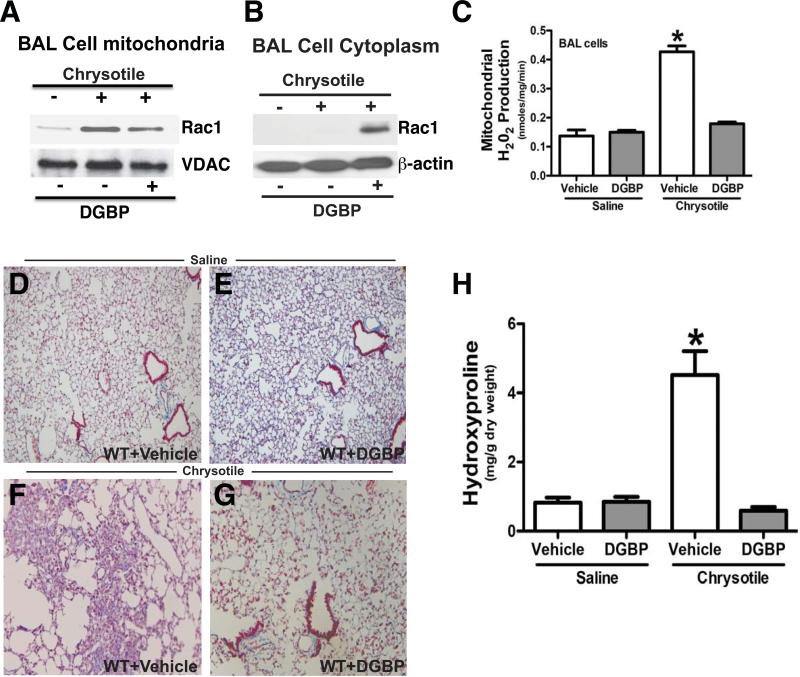
Figure 2. DGBP abrogates macrophage H2O2 generation and development of chrysotile-induced pulmonary fibrosis.
Osmotic pumps containing vehicle or DGBP were implanted subcutaneously in C57Bl/6 WT mice. DGBP was administered at 0.2 mg/kg/day. Mice were exposed to saline or chrysotile (100 μg/50 ml NS) intratracheally. After 21 days, alveolar macrophages were isolated by BAL. An immunoblot analysis for Rac1 was performed in isolated (A) mitochondria or (B) cytoplasm. (C) Mitochondria were isolated from alveolar macrophages obtain from saline+vehicle (n = 5), saline+DGBP (n = 6) and chrysotile+vehicle (n = 6), and chrysotile+DGBP (n = 7) mice. The rate of H2O2 generation was performed by pHPA assay. * p < 0.0001 vs. all other conditions. Lungs were removed and processed for Masson's trichrome staining. Micrographs are representative of (D) saline+vehicle (n = 8), (E) saline+DGBP (n = 8), (F) chrysotile+vehicle (n = 6), and (G) chrysotile+DGBP (n = 6) mice. (H) Lungs were extracted and homogenized for hydroxyproline assay. * p < 0.0004 vs. all other conditions.
To determine if BAL cell mitochondrial H2O2 production was modulated by DGBP, we measured the rate of H2O2 generation and found that vehicle-treated mice exposed to chrysotile had more than 3-fold greater H2O2 production than mice exposed to saline, and DGBP treatment reduced the rate to control levels (Figure 2C).
DGBP protects mice from developing chrysotile-induced pulmonary fibrosis
To further evaluate the effect of DGBP in protecting mice from chrysotile-induced pulmonary fibrosis, the mice were administered vehicle or DGBP subcutaneously in osmotic pumps, and exposed to saline or chrysotile the following day. Mice exposed to saline had normal lung architecture with vehicle (Figure 2D) and DGBP treatment (Figure 2E). Chrysotile-exposed mice that received vehicle had significant architectural changes in their lung parenchyma and large amounts of collagen deposition (Figure 2F), whereas the lungs of the DGBP-treated mice were essentially normal (Figure 2G). The histological findings were confirmed biochemically measuring hydroxyproline content in lung tissue (Figure 2H). In aggregate, these observations suggest that geranylgeranylation of Rac1 has a critical role in development of a fibrotic phenotype after chrysotile-induced lung injury. Moreover, these data suggest that macrophage-derived mitochondrial H2O2 plays an important role in mediating the development of pulmonary fibrosis.
Bleomycin-induced oxidative stress is attenuated by DGBP
To determine if DGBP had similar effects on other forms of lung injury, we measured mitochondrial H2O2 levels in alveolar macrophages obtained after bleomycin exposure. WT mice were treated with saline or bleomycin at a dose of 1.3 or 2.0 U/kg. H2O2 levels were significantly elevated in alveolar macrophages after bleomycin exposure. Further, bleomycin at 2.0 U/kg induced more mitochondrial H2O2 compared to the lower dose (Figure 3A).
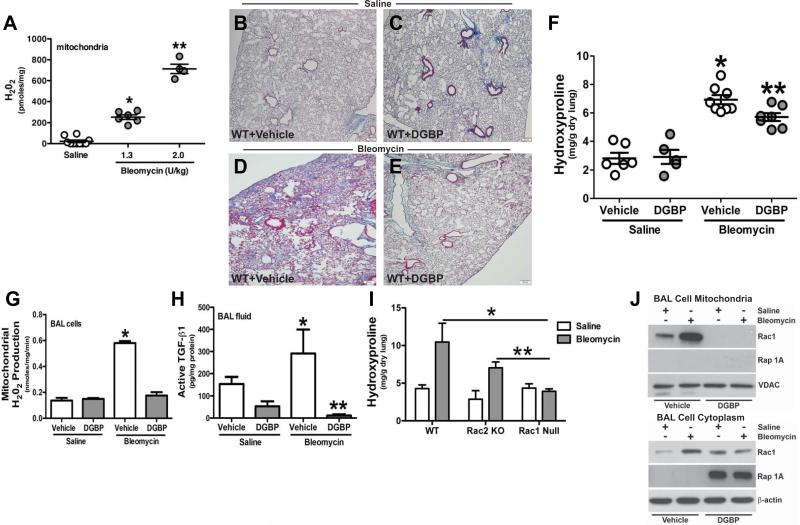
Figure 3. DGBP abrogates macrophage H2O2 generation and development of bleomycin-induced pulmonary fibrosis.
(A) C57Bl/6 WT mice were administered saline (n = 6) or bleomycin (1.3 (n = 6) or 2.0 (n = 4) U/kg) intratracheally. Alveolar macrophages were isolated 21 days later by BAL. Mitochondria were isolated, and H2O2 generation was measured by pHPA assay and is expressed in pmol/mg. * p < 0.0001 vs. saline; ** p < 0.0001 vs.1.3 U/kg. Osmotic pumps containing vehicle (B) and (D) or (C) and (E) DGBP were implanted subcutaneously. DGBP was administered at 0.2 mg/kg/day. Saline or bleomycin (2.0 U/kg) was administered intratracheally. Lungs were extracted and processed for Masson's trichrome staining. Micrographs are representative of saline+vehicle (n = 4), bleomycin+vehicle (n = 6), saline+DGBP (n = 5), and bleomycin+DGBP (n = 6). (F) Lungs were extracted and homogenized for hydroxyproline assay and is expressed in mg/g dry lung weight. Saline+vehicle (n = 6), bleomycin+vehicle (n = 8) and saline+DGBP (n = 5), bleomycin+DGBP (n = 7). * p < 0.008 vs. saline groups and ** p < 0.014 vs. bleomycin (vehicle). (G) Mitochondria were isolated from alveolar macrophages obtain from saline+vehicle (n = 5) saline+DGBP (n = 6) and bleomycin+vehicle (n = 5), bleomycin+DGBP n = 6) mice. The rate of H2O2 generation was performed by pHPA assay. * p < 0.0001 vs. all other conditions. (H) Active TGF-β in BAL fluid was measured by ELISA. n = 4 in all. * p < 0.0001 vs. all other groups and ** p < 0.0001 vs. bleomycin (vehicle). (I) WT, Rac2 KO, and Rac1 null mice were exposed to saline (n = 6, n = 4, n = 6) or bleomycin (n = 4, n = 6, n = 6). After 21d lungs were extracted and homogenized for hydroxyproline assay and is expressed in mg/g. * p < 0.0125 vs. Rac1 null (bleomycin); ** p < 0.0045 vs. Rac1 null (bleomycin). (J) WT mice with osmotic pumps containing vehicle or DGBP were exposed to saline or bleomycin. Alveolar macrophages were obtained after 21 days by BAL. An immunoblot analysis was perfomed for Rac1 and Rap 1A in isolated mitochondria and in isolated cytoplasm.
To investigate the effect of DGBP in bleomycin-induced fibrosis, osmotic pumps containing either vehicle or DGBP were implanted subcutaneously in WT mice. Mice were exposed to saline or bleomycin the following day. Mice exposed to saline had normal lung architecture and no collagen deposition with vehicle (Figure 3B) and DGBP treatment (Figure 3C). Bleomycin treatment resulted in widespread lung architectural destruction and collagen deposition in animals that received vehicle (Figure 3D), whereas the lungs of the DGBP-treated mice showed normal lung architecture and no significant collagen deposition (Figure 3E). The histological observations were verified biochemically by a hydroxyproline assay. DGBP-treated mice showed significantly less hydroxyproline compared to vehicle-treated mice exposed to bleomycin (Figure 3F). Taken together, these data suggest that GGPP synthase is a novel therapeutic target to limit the fibrotic response to bleomycin- induced lung injury.
Because DGBP-treated mice showed reduced pulmonary fibrosis following bleomycin, we determined if mitochondrial H2O2 production was modulated by DGBP. After 21 days, BAL cell mitochondrial H2O2 production rate showed that vehicle-treated mice exposed to bleomycin had more than 4-fold greater H2O2 production than mice exposed to saline, and DGBP treatment reduced the rate to control levels (Figure 3G). These data strongly suggest that the increase flux through the isoprenoid pathway in alveolar macrophages is, in part, accountable for the mitochondrial oxidative stress.
Based on our prior data linking mitochondrial oxidative stress to the development of pulmonary fibrosis (4,6,7,19), we determined if DGBP treatment would limit the fibrotic response to bleomycin-induced lung injury. We measured the pro-fibrotic cytokine, active TGF-β, in BAL fluid. Mice treated with vehicle following bleomycin exposure showed significantly more active TGF-β in BAL fluid than vehicle-treated mice exposed to saline, and DGBP treatment reduced active TGF-β below control levels after bleomycin exposure (Figure 3H). These data suggest that the reduction in macrophage mitochondrial H2O2 production limits the development of a pro-fibrotic environment.
The post-translational modification of geranylgeranylation is common to all Rho GTPases. The two most common GTPases in macrophages are Rac1 and Rac2 (23,24). To determine if modulation of Rac1 and/or Rac2 was linked to the development of pulmonary fibrosis, we exposed WT, Rac2 KO, and Rac1 null mice to saline or bleomycin. WT and Rac2 KO mice showed significant increases in hydroxyproline in the lung tissue after bleomycin exposure, whereas the hydroxyproline content in lungs of conditional Rac1 null mice was not altered by bleomycin (Figure 3I). Moreover, there was no significant difference between the hydroxyproline levels in WT and Rac2 KO mice, while the lungs of Rac1 null mice had a substantial reduction in hydroxyproline content compared to the other two strains of mice. The data demonstrate that Rac1 expression in alveolar macrophages has a critical role in the development of a fibrotic phenotype after bleomycin.
Because Rac1 is linked to pulmonary fibrosis and mitochondrial oxidative stress in vivo after bleomycin, we evaluated if bleomycin modulates Rac1 mitochondrial import in alveolar macrophages from mice. Bleomycin increased Rac1 mitochondrial localization compared to vehicle-treated saline-exposed mice, whereas mice treated with DGBP showed complete absence of immunoreactive Rac1 in mitochondria (Figure 3J). In contrast, Rap 1A is not present in the BAL cell mitochondria in any condition. Compared to the mitochondrial fraction, there was less Rac1 in the cytoplasm in vehicle-treated mice suggesting that bleomycin induces the post-translational modification of Rac1 necessary for mitochondrial import. Furthermore, an immunoblot analysis showed that non-geranylgeranylated Rap 1A was increased in the cytoplasm of BAL cells obtained from mice treated with DGBP (Figure 3J). In aggregate, these data strongly suggest that Rac1-mediated mitochondrial H2O2 is linked to pulmonary fibrosis, and disruption of geranylgeranylation and Rac1 activation with DGBP in alveolar macrophages provides a novel therapeutic target for preventing fibrotic development.
Inhibition of GGPP synthase with DGBP halts progression of fibrosis
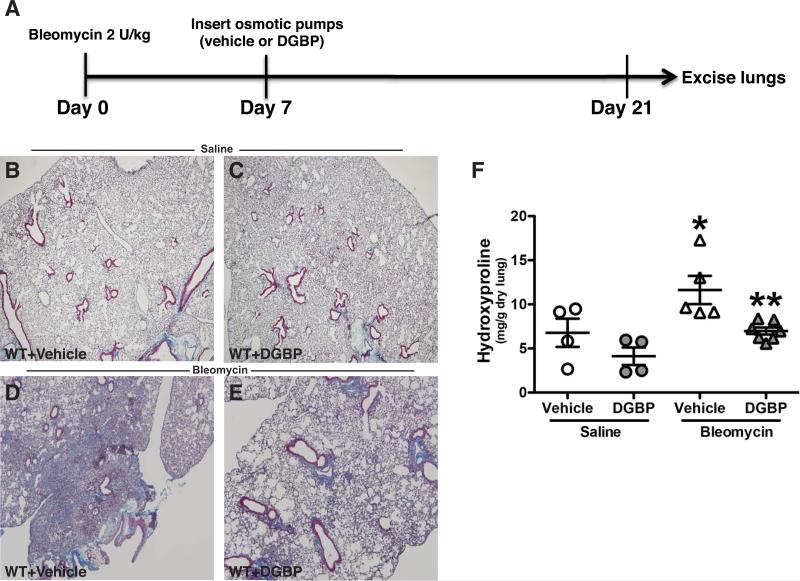
To investigate the therapeutic potential of arresting progression of pulmonary fibrosis by impairing geranylgeranylation, we first exposed mice to bleomycin and then installed osmotic pumps seven days after bleomycin exposure (Figure 4A). Lung injury was present seven days after bleomycin (data not shown). Vehicle- (Figure 4B) and DGBP-treated mice (Figure 4C) exposed to saline had normal lungs without collagen deposition. As expected, bleomycin exposure in vehicle-treated mice showed wide spread lung destruction and collagen deposition compared to the saline-exposed mice (Figure 4D). In contrast, lungs of DGBP-treated mice showed small patches of collagen, but there were significantly less collagen compared to the vehicle-treated mice (Figure 4E). The quantitative measure of lung collagen content by hydroxyproline assay confirmed these histological findings (Figure 4D). Taken together, these observations suggest that the isoprenoid pathway may be a novel target for halting pulmonary fibrosis following lung injury.
Figure 4. DGBP attenuates progression of bleomycin-induced pulmonary fibrosis.
(A) Schematic diagram of experimental design. C57Bl/6 WT mice were administered saline or bleomycin (2.0 U/kg) intra-tracheally. Osmotic pumps containing vehicle or DGBP were implanted subcutaneously seven days later. DGBP was delivered at 0.2 mg/kg/day. Mice were euthanized 21 days after bleomycin. Lungs were removed and processed for Masson's trichrome staining. Micrographs are representative of (B) saline+vehicle (n = 4), (C) saline+DGBP (n = 4), (D) bleomycin+vehicle (n = 5), and (E) bleomycin+DGBP (n = 7). (E) Lungs were extracted and homogenized for hydroxyproline assay. * p < 0.036 vs. all other groups and ** p < 0.004 vs. bleomycin+vehicle.
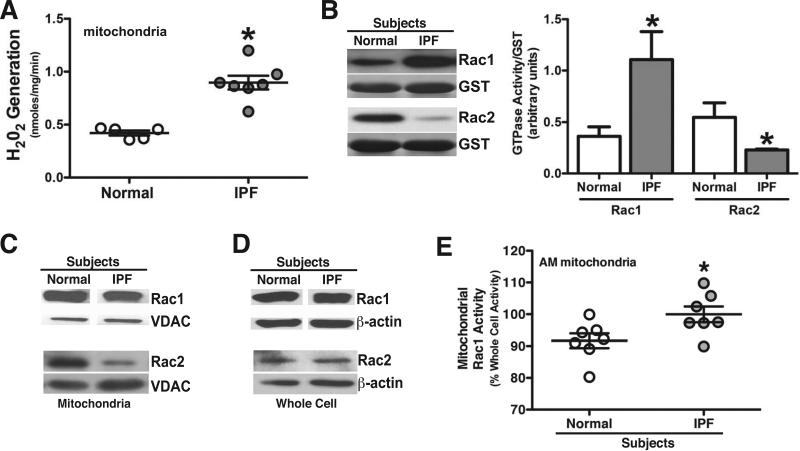
Alveolar macrophages from IPF patients show increased mitochondrial oxidative stress and Rac1 activation
Lungs of patients with IPF reveal an oxidant/antioxidant imbalance resulting from increased oxidant production (25-27); however, the source(s) and type(s) of ROS has not been determined. Because mitochondria-derived H2O2 production in alveolar macrophages contributes to pulmonary fibrosis (4-7,19,28), we measured mitochondrial H2O2 production in alveolar macrophages from IPF patients. Isolated mitochondria from IPF patients showed significantly greater H2O2 levels than normal subjects (Figure 5A). In contrast, there was no difference in H2O2 generation from isolated membrane fractions, which was significantly less than the mitochondrial fraction (data not shown).
Figure 5. Alveolar macrophages from IPF patients have increased mitochondrial H2O2 production and mitochondrial Rac1 activity.
Alveolar macrophages (AM) were obtained by BAL and mitochondria were isolated. (A) Normal subjects (n = 5) and IPF patients (n = 7). H2O2 was measured by pHPA assay and is expressed as nmoles/mg. * p < 0.019 normal vs. IPF mitochondria. (B) Rac1 and Rac2 activity was determined by PAK-binding domain pull-down followed with immunoblot analysis in normal subjects (n = 6) and IPF patients (n = 6). A representative immunoblot analysis of Rac1 and Rac2 activity is shown. Densitometry was performed of Rac1 and Rac2 normalized to GST and is expressed graphically in arbitrary units. * p < 0.0340 compared to normal. Normal subjects (n = 3) and IPF patients (n = 4). (C) Mitochondria and (D) cytoplasm were isolated and immunoblot analyses for Rac1 and Rac2 were performed. Normal subjects (n = 5) and IPF patients (n = 7). Representative immunoblot is shown. (E) Rac1 activity was measured by GLISA in whole cell lysates and isolated mitochondria and is expressed as mitochondrial Rac1 activity as a % of whole cell activity. * p < 0.016. Normal subjects (n = 8) and IPF patients (n = 6).
Rac1 and Rac2 are associated with O2.- and H2O2 generation (6,7,23,24). We found that alveolar macrophages from IPF patients showed significantly greater Rac1 and lower Rac2 activation compared to values obtained for normal subjects (Figure 5B). Because a greater fraction of Rac1 is activated in alveolar macrophages from IPF patients, we determined if localization of Rac1 in the mitochondria is increased in IPF macrophages. Immunoblot analysis showed similar amounts of Rac1 in mitochondria of normal subjects and IPF patients (Figure 5C). This was confirmed by densitometry of immunoblot analyses among multiple normal subjects and patients (data not shown). In contrast, Rac2 content was decreased in alveolar macrophage mitochondria from IPF patients compared to normal subjects. Whole cell Rac1 and Rac2 expression was similar in normal subjects and IPF patients (Figure 5D).
Because mitochondrial Rac1 activity has a direct effect on mitochondrial H2O2 levels (6), we measured Rac1 activity in whole cell lysates and isolated mitochondria. Rac1 activity in IPF mitochondria represented approximately 100% of the whole cell activity and thus, was significantly higher compared to the normal subjects (Figure 5E). In aggregate, these in vivo and ex vivo observations indicate that geranylgeranylation is required for Rac1-mediated oxidative stress in alveolar macrophages, and GGPP synthase is a novel target to attenuate fibrotic remodeling after lung injury.
DISCUSSION
Pulmonary fibrosis is a devastating lung disease that is increasing in incidence. In particular, IPF has a grim prognosis, and supportive care is the primary means of treatment as no current therapeutic modalities are available to halt its progression. The goal in this study was to abrogate the development and progression of pulmonary fibrosis by focusing on the modulation of mitochondrial H2O2 generation in alveolar macrophages, which is a critical determinant of the fibrotic response to lung injury (4,6,7,19). By disrupting the isoprenoid pathway as a therapeutic target, we found that inhibiting geranylgeranylation attenuated Rac1-mediated activation and the progression of pulmonary fibrosis.
The isoprenoid pathway is a target for drug therapy in multiple conditions. Statins are the most widely prescribed drug in the United States and are used to inhibit HMG-CoA reductase, which is the rate-limiting enzyme that converts HMG-CoA to mevalonate. Statins are clearly important in the management of hypercholesterolemia as well as the prevention of stroke (29,30). Although statins reduce GGPP levels and activation of RhoGTPases, the use of statins has been associated with interstitial lung abnormalities in smoking individuals likely due to inhibition of several intermediates in the isoprenoid pathway (16). Because HMG-CoA is a proximal enzyme in the isoprenoid pathway it may alter cell membrane integrity and reduce the N-glycosylation of growth-factor receptors. Statins also activate Akt (31), and multiple studies show that Akt is linked to fibrosis development (32-37). Moreover, statins are potent anti-inflammatory agents (38-40), so it is plausible that statins have a role in the polarization of macrophages to an M2 phenotype, which are anti-inflammatory and repair injured tissue (41-43); however, an imbalance of macrophages with a predominance of an M2 phenotype can promote fibrosis (19,44).
In osteoporosis, the bisphosphonates adsorb to bone mineral and reduce bone resorption by inhibition of farnesyl diphosphate synthase, which synthesizes farnesyl diphosphate through successive condensations of isopentenyl pyrophosphate with dimethylallyl pyrophosphate and geranyl pyrophosphate (45-47). Agents that disrupt the isoprenoid pathway have been used for cancer therapeutically. Farnesyl transferase (FTase), which catalyzes the farnesylation of the Ras proteins, and geranylgeranyltranferase I (GGTase I), which catalyzes the final step in the lipid post-translational modification of Rho GTPases, have been studied because Ras and Rho GTPases have been shown to be essential for cell growth and proliferation (48-51). To date, the isoprenoid pathway has not been targeted as a treatment strategy for pulmonary fibrosis.
DGBP inhibits GGPP synthase by mimicking the substrate farnesyl pyrophosphate with its two polar groups that bind to the active site of GGPP synthase. The hydrophobic chains bind to the interior of the enzyme at the site where GGPP would be released. One study showed that GGTase I deficiency induces pro-inflammatory gene expression in macrophages, and Rac1 and other Rho GTPases were localized to the plasma membrane (52), which contrasts from our observations. In fact, we found that DGBP has no effect on pro-inflammatory gene expression in macrophages stimulated with LPS (Supplemental Figure 2), which suggests that inhibition of isoprenylation of Rac1 does not alter the inflammatory response of macrophages. This difference in their study may be based on the stimulus, their use of bone marrow derived macrophages, or the difference in localization of the macrophages in mice. The localization of Rac1 to the mitochondria, however, was not investigated in that study (52). Our results demonstrate that the inhibition of GGPP synthase reduces the activation of Rac1. Although DGBP has the potential to limit the isoprenylation of other Ras and Rho GTPases, the DGBP concentrations used in our studies had no apparent toxicity in vitro or in vivo. Furthermore, the predominant Rho GTPase in macrophages, Rac2, is not activated in IPF patients. These results suggest that Rac1 is preferentially activated in the mitochondria, there is a larger pool of inactive Rac1 in normal subjects, or other factors are involved increasing Rac1 activity in the mitochondria of alveolar macrophages.
Mitochondrial-derived oxidants are linked to TGF-β activation and Smad signaling in multiple tissues (28,53). This association is critical because a reduction in oxidative stress decreases TGF-β activation. In addition, mitochondrial complex III-mediated O2.- generation is directly associated with TGF-β-mediated Smad signaling in human fibroblasts (28), which results in fibrotic remodeling. To our knowledge, the isoprenoid pathway has not been directly associated with TGF-β activation; however, our results indicate that DGBP treatment in vivo significantly reduces the level of active TGF-β in BAL fluid. In aggregate, these observations indicate that increased flux through the isoprenoid pathway promotes the development of a fibrotic phenotype.
The lungs of IPF patients are considered to have an oxidant/antioxidant imbalance (25-27), but the source of oxidative stress in IPF is not known. We discovered that IPF alveolar macrophages have increased mitochondrial H2O2 levels. The primary source of H2O2 in macrophages is the mitochondria in inflammatory and fibrotic states (19,54), and Rac1, at least in part, regulates mitochondrial H2O2 levels in macrophages (6). A conditional deletion of Rac1 in macrophages significantly attenuates development of pulmonary fibrosis (4,6,54) and highlights the importance of macrophages in aberrant lung repair following injury. Studies show that the alveolar epithelium and fibroblasts have a critical role in pulmonary fibrosis (55-58); however, our findings demonstrate that alveolar macrophage-derived oxidative stress is linked to fibrotic repair. Moreover, these observations uncover a mechanism that mediates pulmonary fibrosis and provide a novel therapy that abrogates progression of the fibrotic phenotype by targeting the isoprenoid pathway.
Supplementary Material
Highlights.
Pulmonary fibrosis is a devastating disease with high morbidity and mortality
Alveolar macrophages have a key role in modulating development of pulmonary fibrosis
The post-translational modification of Rac1 mediates mitochondrial oxidative stress in macrophages
Impairment of the isoprenoid pathway prevent Rac1 activation, mitochondrial oxidative stress, and abrogates development and progression of pulmonary fibrosis
ACKNOWLEDGEMENTS
This work was supported, in whole or in part, by National Institutes of Health Grants 2R01ES015981-07 and R01ES014871. This work was also supported by a Merit Review from the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development Grant 1BX001135-01.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.King TE, Jr., Schwarz MI, Brown K, Tooze JA, Colby TV, Waldron JA, Jr., Flint A, Thurlbeck W, Cherniack RM. Idiopathic pulmonary fibrosis: relationship between histopathologic features and mortality. Am J Respir Crit Care Med. 2001;164:1025–1032. doi: 10.1164/ajrccm.164.6.2001056. [DOI] [PubMed] [Google Scholar]
- 2.King TE, Jr., Tooze JA, Schwarz MI, Brown KR, Cherniack RM. Predicting survival in idiopathic pulmonary fibrosis: scoring system and survival model. Am J Respir Crit Care Med. 2001;164:1171–1181. doi: 10.1164/ajrccm.164.7.2003140. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz DA, Helmers RA, Galvin JR, Van Fossen DS, Frees KL, Dayton CS, Burmeister LF, Hunninghake GW. Determinants of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 1994;149:450–454. doi: 10.1164/ajrccm.149.2.8306044. [DOI] [PubMed] [Google Scholar]
- 4.He C, Murthy S, McCormick ML, Spitz DR, Ryan AJ, Carter AB. Mitochondrial Cu,Zn-Superoxide Dismutase Mediates Pulmonary Fibrosis by Augmenting H2O2 Generation. J Biol Chem. 2011;286:15597–15607. doi: 10.1074/jbc.M110.187377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murthy S, Adamcakova-Dodd A, Perry SS, Tephly LA, Keller RM, Metwali N, Meyerholz DK, Wang Y, Glogauer M, Thorne PS, Carter AB. Modulation of reactive oxygen species by Rac1 or catalase prevents asbestos-induced pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2009;297:L846–855. doi: 10.1152/ajplung.90590.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Osborn-Heaford HL, Ryan AJ, Murthy S, Racila AM, He C, Sieren JC, Spitz DR, Carter AB. Mitochondrial Rac1 GTPase Import and Electron Transfer from Cytochrome c Are Required for Pulmonary Fibrosis. The Journal of biological chemistry. 2012;287:3301–3312. doi: 10.1074/jbc.M111.308387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murthy S, Ryan A, He C, Mallampalli RK, Carter AB. Rac1-mediated Mitochondrial H2O2 Generation Regulates MMP-9 Gene Expression in Macrophages via Inhibition of SP-1 and AP-1. J Biol Chem. 2010;285:25062–25073. doi: 10.1074/jbc.M109.099655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hall A. Small GTP-binding proteins and the regulation of the actin cytoskeleton. Annu Rev Cell Biol. 1994;10:31–54. doi: 10.1146/annurev.cb.10.110194.000335. [DOI] [PubMed] [Google Scholar]
- 9.Roberts AW, Kim C, Zhen L, Lowe JB, Kapur R, Petryniak B, Spaetti A, Pollock JD, Borneo JB, Bradford GB, Atkinson SJ, Dinauer MC, Williams DA. Deficiency of the hematopoietic cell-specific Rho family GTPase Rac2 is characterized by abnormalities in neutrophil function and host defense. Immunity. 1999;10:183–196. doi: 10.1016/s1074-7613(00)80019-9. [DOI] [PubMed] [Google Scholar]
- 10.Wells CM, Walmsley M, Ooi S, Tybulewicz V, Ridley AJ. Rac1-deficient macrophages exhibit defects in cell spreading and membrane ruffling but not migration. Journal of cell science. 2004;117:1259–1268. doi: 10.1242/jcs.00997. [DOI] [PubMed] [Google Scholar]
- 11.Kheradmand F, Werner E, Tremble P, Symons M, Werb Z. Role of Rac1 and oxygen radicals in collagenase-1 expression induced by cell shape change. Science. 1998;280:898–902. doi: 10.1126/science.280.5365.898. [DOI] [PubMed] [Google Scholar]
- 12.Ozaki M, Deshpande SS, Angkeow P, Bellan J, Lowenstein CJ, Dinauer MC, Goldschmidt-Clermont PJ, Irani K. Inhibition of the Rac1 GTPase protects against nonlethal ischemia/reperfusion-induced necrosis and apoptosis in vivo. Faseb J. 2000;14:418–429. doi: 10.1096/fasebj.14.2.418. [DOI] [PubMed] [Google Scholar]
- 13.Ozaki M, Deshpande SS, Angkeow P, Suzuki S, Irani K. Rac1 regulates stress-induced, redox-dependent heat shock factor activation. J Biol Chem. 2000;275:35377–35383. doi: 10.1074/jbc.M005287200. [DOI] [PubMed] [Google Scholar]
- 14.Woo CH, You HJ, Cho SH, Eom YW, Chun JS, Yoo YJ, Kim JH. Leukotriene B(4) stimulates Rac-ERK cascade to generate reactive oxygen species that mediates chemotaxis. J Biol Chem. 2002;277:8572–8578. doi: 10.1074/jbc.M104766200. [DOI] [PubMed] [Google Scholar]
- 15.Zeng PY, Rane N, Du W, Chintapalli J, Prendergast GC. Role for RhoB and PRK in the suppression of epithelial cell transformation by farnesyltransferase inhibitors. Oncogene. 2003;22:1124–1134. doi: 10.1038/sj.onc.1206181. [DOI] [PubMed] [Google Scholar]
- 16.Xu JF, Washko GR, Nakahira K, Hatabu H, Patel AS, Fernandez IE, Nishino M, Okajima Y, Yamashiro T, Ross JC, Estepar RS, Diaz AA, Li HP, Qu JM, Himes BE, Come CE, D'Aco K, Martinez FJ, Han MK, Lynch DA, Crapo JD, Morse D, Ryter SW, Silverman EK, Rosas IO, Choi AM, Hunninghake GM, Investigators CO. Statins and pulmonary fibrosis: the potential role of NLRP3 inflammasome activation. Am J Respir Crit Care Med. 2012;185:547–556. doi: 10.1164/rccm.201108-1574OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Erickson JR, Joiner ML, Guan X, Kutschke W, Yang J, Oddis CV, Bartlett RK, Lowe JS, O'Donnell SE, Aykin-Burns N, Zimmerman MC, Zimmerman K, Ham AJ, Weiss RM, Spitz DR, Shea MA, Colbran RJ, Mohler PJ, Anderson ME. A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell. 2008;133:462–474. doi: 10.1016/j.cell.2008.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glogauer M, Marchal CC, Zhu F, Worku A, Clausen BE, Foerster I, Marks P, Downey GP, Dinauer M, Kwiatkowski DJ. Rac1 deletion in mouse neutrophils has selective effects on neutrophil functions. J Immunol. 2003;170:5652–5657. doi: 10.4049/jimmunol.170.11.5652. [DOI] [PubMed] [Google Scholar]
- 19.He C, Ryan AJ, Murthy S, Carter AB. Accelerated Development of Pulmonary Fibrosis via Cu,Zn-superoxide Dismutase-induced Alternative Activation of Macrophages. J Biol Chem. 2013;288:20745–20757. doi: 10.1074/jbc.M112.410720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shull LW, Wiemer AJ, Hohl RJ, Wiemer DF. Synthesis and biological activity of isoprenoid bisphosphonates. Bioorganic & medicinal chemistry. 2006;14:4130–4136. doi: 10.1016/j.bmc.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 21.Wasko BM, Dudakovic A, Hohl RJ. Bisphosphonates induce autophagy by depleting geranylgeranyl diphosphate. J Pharmacol Exp Ther. 2011;337:540–546. doi: 10.1124/jpet.110.175521. [DOI] [PubMed] [Google Scholar]
- 22.Weivoda MM, Hohl RJ. The effects of direct inhibition of geranylgeranyl pyrophosphate synthase on osteoblast differentiation. Journal of cellular biochemistry. 2011;112:1506–1513. doi: 10.1002/jcb.23087. [DOI] [PubMed] [Google Scholar]
- 23.Yamauchi A, Kim C, Li S, Marchal CC, Towe J, Atkinson SJ, Dinauer MC. Rac2-deficient murine macrophages have selective defects in superoxide production and phagocytosis of opsonized particles. J Immunol. 2004;173:5971–5979. doi: 10.4049/jimmunol.173.10.5971. [DOI] [PubMed] [Google Scholar]
- 24.Zhao X, Carnevale KA, Cathcart MK. Human monocytes use Rac1, not Rac2, in the NADPH oxidase complex. J Biol Chem. 2003;278:40788–40792. doi: 10.1074/jbc.M302208200. [DOI] [PubMed] [Google Scholar]
- 25.Kliment CR, Oury TD. Oxidative stress, extracellular matrix targets, and idiopathic pulmonary fibrosis. Free Radic Biol Med. 2010;49:707–717. doi: 10.1016/j.freeradbiomed.2010.04.036. [DOI] [PubMed] [Google Scholar]
- 26.Psathakis K, Mermigkis D, Papatheodorou G, Loukides S, Panagou P, Polychronopoulos V, Siafakas NM, Bouros D. Exhaled markers of oxidative stress in idiopathic pulmonary fibrosis. European journal of clinical investigation. 2006;36:362–367. doi: 10.1111/j.1365-2362.2006.01636.x. [DOI] [PubMed] [Google Scholar]
- 27.Rahman I, Skwarska E, Henry M, Davis M, O'Connor CM, FitzGerald MX, Greening A, MacNee W. Systemic and pulmonary oxidative stress in idiopathic pulmonary fibrosis. Free Radic Biol Med. 1999;27:60–68. doi: 10.1016/s0891-5849(99)00035-0. [DOI] [PubMed] [Google Scholar]
- 28.Jain M, Rivera S, Monclus EA, Synenki L, Zirk A, Eisenbart J, Feghali-Bostwick C, Mutlu GM, Budinger GR, Chandel NS. Mitochondrial reactive oxygen species regulate transforming growth factor-beta signaling. J Biol Chem. 2013;288:770–777. doi: 10.1074/jbc.M112.431973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blauw GJ, Lagaay AM, Smelt AH, Westendorp RG. Stroke, statins, and cholesterol. A meta-analysis of randomized, placebo-controlled, double-blind trials with HMG-CoA reductase inhibitors. Stroke. 1997;28:946–950. doi: 10.1161/01.str.28.5.946. [DOI] [PubMed] [Google Scholar]
- 30.Nawrocki JW, Weiss SR, Davidson MH, Sprecher DL, Schwartz SL, Lupien PJ, Jones PH, Haber HE, Black DM. Reduction of LDL cholesterol by 25% to 60% in patients with primary hypercholesterolemia by atorvastatin, a new HMG-CoA reductase inhibitor. Arteriosclerosis, thrombosis, and vascular biology. 1995;15:678–682. doi: 10.1161/01.atv.15.5.678. [DOI] [PubMed] [Google Scholar]
- 31.Kureishi Y, Luo Z, Shiojima I, Bialik A, Fulton D, Lefer DJ, Sessa WC, Walsh K. The HMG-CoA reductase inhibitor simvastatin activates the protein kinase Akt and promotes angiogenesis in normocholesterolemic animals. Nature medicine. 2000;6:1004–1010. doi: 10.1038/79510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Larson-Casey JL, Murthy S, Ryan AJ, Carter AB. Modulation of the mevalonate pathway by akt regulates macrophage survival and development of pulmonary fibrosis. J Biol Chem. 2014;289:36204–36219. doi: 10.1074/jbc.M114.593285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Le Cras TD, Korfhagen TR, Davidson C, Schmidt S, Fenchel M, Ikegami M, Whitsett JA, Hardie WD. Inhibition of PI3K by PX-866 prevents transforming growth factor-alpha-induced pulmonary fibrosis. Am J Pathol. 2010;176:679–686. doi: 10.2353/ajpath.2010.090123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li LF, Liao SK, Huang CC, Hung MJ, Quinn DA. Serine/threonine kinase-protein kinase B and extracellular signal-regulated kinase regulate ventilator-induced pulmonary fibrosis after bleomycin-induced acute lung injury: a prospective, controlled animal experiment. Crit Care. 2008;12:R103. doi: 10.1186/cc6983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu Y, Azad N, Wang L, Iyer AK, Castranova V, Jiang BH, Rojanasakul Y. Phosphatidylinositol-3-kinase/akt regulates bleomycin-induced fibroblast proliferation and collagen production. American journal of respiratory cell and molecular biology. 2010;42:432–441. doi: 10.1165/rcmb.2009-0002OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vittal R, Horowitz JC, Moore BB, Zhang H, Martinez FJ, Toews GB, Standiford TJ, Thannickal VJ. Modulation of prosurvival signaling in fibroblasts by a protein kinase inhibitor protects against fibrotic tissue injury. Am J Pathol. 2005;166:367–375. doi: 10.1016/S0002-9440(10)62260-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xia H, Diebold D, Nho R, Perlman D, Kleidon J, Kahm J, Avdulov S, Peterson M, Nerva J, Bitterman P, Henke C. Pathological integrin signaling enhances proliferation of primary lung fibroblasts from patients with idiopathic pulmonary fibrosis. J Exp Med. 2008;205:1659–1672. doi: 10.1084/jem.20080001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pruefer D, Makowski J, Schnell M, Buerke U, Dahm M, Oelert H, Sibelius U, Grandel U, Grimminger F, Seeger W, Meyer J, Darius H, Buerke M. Simvastatin inhibits inflammatory properties of Staphylococcus aureus alpha-toxin. Circulation. 2002;106:2104–2110. doi: 10.1161/01.cir.0000034048.38910.91. [DOI] [PubMed] [Google Scholar]
- 39.Lefer AM, Campbell B, Shin YK, Scalia R, Hayward R, Lefer DJ. Simvastatin preserves the ischemic-reperfused myocardium in normocholesterolemic rat hearts. Circulation. 1999;100:178–184. doi: 10.1161/01.cir.100.2.178. [DOI] [PubMed] [Google Scholar]
- 40.Pruefer D, Scalia R, Lefer AM. Simvastatin inhibits leukocyte endothelial cell interactions and protects against inflammatory processes in normocholesterolemic rats. Arteriosclerosis, thrombosis, and vascular biology. 1999;19:2894–2900. doi: 10.1161/01.atv.19.12.2894. [DOI] [PubMed] [Google Scholar]
- 41.Chensue SW, Warmington K, Ruth JH, Lukacs N, Kunkel SL. Mycobacterial and schistosomal antigen-elicited granuloma formation in IFN-gamma and IL-4 knockout mice: analysis of local and regional cytokine and chemokine networks. J Immunol. 1997;159:3565–3573. [PubMed] [Google Scholar]
- 42.Duffield JS, Forbes SJ, Constandinou CM, Clay S, Partolina M, Vuthoori S, Wu S, Lang R, Iredale JP. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest. 2005;115:56–65. doi: 10.1172/JCI22675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shechter R, Miller O, Yovel G, Rosenzweig N, London A, Ruckh J, Kim KW, Klein E, Kalchenko V, Bendel P, Lira SA, Jung S, Schwartz M. Recruitment of beneficial M2 macrophages to injured spinal cord is orchestrated by remote brain choroid plexus. Immunity. 2013;38:555–569. doi: 10.1016/j.immuni.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prasse A, Pechkovsky DV, Toews GB, Jungraithmayr W, Kollert F, Goldmann T, Vollmer E, Muller-Quernheim J, Zissel G. A vicious circle of alveolar macrophages and fibroblasts perpetuates pulmonary fibrosis via CCL18. American journal of respiratory and critical care medicine. 2006;173:781–792. doi: 10.1164/rccm.200509-1518OC. [DOI] [PubMed] [Google Scholar]
- 45.Hosfield DJ, Zhang Y, Dougan DR, Broun A, Tari LW, Swanson RV, Finn J. Structural basis for bisphosphonate-mediated inhibition of isoprenoid biosynthesis. J Biol Chem. 2004;279:8526–8529. doi: 10.1074/jbc.C300511200. [DOI] [PubMed] [Google Scholar]
- 46.Russell RG, Rogers MJ. Bisphosphonates: from the laboratory to the clinic and back again. Bone. 1999;25:97–106. doi: 10.1016/s8756-3282(99)00116-7. [DOI] [PubMed] [Google Scholar]
- 47.Rogers MJ, Frith JC, Luckman SP, Coxon FP, Benford HL, Monkkonen J, Auriola S, Chilton KM, Russell RG. Molecular mechanisms of action of bisphosphonates. Bone. 1999;24:73S–79S. doi: 10.1016/s8756-3282(99)00070-8. [DOI] [PubMed] [Google Scholar]
- 48.Dan HC, Jiang K, Coppola D, Hamilton A, Nicosia SV, Sebti SM, Cheng JQ. Phosphatidylinositol-3-OH kinase/AKT and survivin pathways as critical targets for geranylgeranyltransferase I inhibitor-induced apoptosis. Oncogene. 2004;23:706–715. doi: 10.1038/sj.onc.1207171. [DOI] [PubMed] [Google Scholar]
- 49.Morgan MA, Wegner J, Aydilek E, Ganser A, Reuter CW. Synergistic cytotoxic effects in myeloid leukemia cells upon cotreatment with farnesyltransferase and geranylgeranyl transferase-I inhibitors. Leukemia. 2003;17:1508–1520. doi: 10.1038/sj.leu.2403022. [DOI] [PubMed] [Google Scholar]
- 50.Sjogren AK, Andersson KM, Liu M, Cutts BA, Karlsson C, Wahlstrom AM, Dalin M, Weinbaum C, Casey PJ, Tarkowski A, Swolin B, Young SG, Bergo MO. GGTase-I deficiency reduces tumor formation and improves survival in mice with K-RAS-induced lung cancer. J Clin Invest. 2007;117:1294–1304. doi: 10.1172/JCI30868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sun J, Ohkanda J, Coppola D, Yin H, Kothare M, Busciglio B, Hamilton AD, Sebti SM. Geranylgeranyltransferase I inhibitor GGTI-2154 induces breast carcinoma apoptosis and tumor regression in H-Ras transgenic mice. Cancer research. 2003;63:8922–8929. [PubMed] [Google Scholar]
- 52.Khan OM, Ibrahim MX, Jonsson IM, Karlsson C, Liu M, Sjogren AK, Olofsson FJ, Brisslert M, Andersson S, Ohlsson C, Hulten LM, Bokarewa M, Bergo MO. Geranylgeranyltransferase type I (GGTase-I) deficiency hyperactivates macrophages and induces erosive arthritis in mice. The Journal of clinical investigation. 2011;121:628–639. doi: 10.1172/JCI43758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hagler MA, Hadley TM, Zhang H, Mehra K, Roos CM, Schaff HV, Suri RM, Miller JD. TGF-beta signalling and reactive oxygen species drive fibrosis and matrix remodelling in myxomatous mitral valves. Cardiovascular research. 2013;99:175–184. doi: 10.1093/cvr/cvt083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Soberanes S, Urich D, Baker CM, Burgess Z, Chiarella SE, Bell EL, Ghio AJ, De Vizcaya-Ruiz A, Liu J, Ridge KM, Kamp DW, Chandel NS, Schumacker PT, Mutlu GM, Budinger GR. Mitochondrial complex III-generated oxidants activate ASK1 and JNK to induce alveolar epithelial cell death following exposure to particulate matter air pollution. J Biol Chem. 2009;284:2176–2186. doi: 10.1074/jbc.M808844200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim KK, Kugler MC, Wolters PJ, Robillard L, Galvez MG, Brumwell AN, Sheppard D, Chapman HA. Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc Natl Acad Sci U S A. 2006;103:13180–13185. doi: 10.1073/pnas.0605669103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li Y, Jiang D, Liang J, Meltzer EB, Gray A, Miura R, Wogensen L, Yamaguchi Y, Noble PW. Severe lung fibrosis requires an invasive fibroblast phenotype regulated by hyaluronan and CD44. J Exp Med. 2011;208:1459–1471. doi: 10.1084/jem.20102510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ramos C, Montano M, Garcia-Alvarez J, Ruiz V, Uhal BD, Selman M, Pardo A. Fibroblasts from idiopathic pulmonary fibrosis and normal lungs differ in growth rate, apoptosis, and tissue inhibitor of metalloproteinases expression. Am J Respir Cell Mol Biol. 2001;24:591–598. doi: 10.1165/ajrcmb.24.5.4333. [DOI] [PubMed] [Google Scholar]
- 58.Zhou Y, Huang X, Hecker L, Kurundkar D, Kurundkar A, Liu H, Jin TH, Desai L, Bernard K, Thannickal VJ. Inhibition of mechanosensitive signaling in myofibroblasts ameliorates experimental pulmonary fibrosis. J Clin Invest. 2013;123:1096–1108. doi: 10.1172/JCI66700. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.