Abstract
The mechanisms underlying ethanol self-administration are not fully understood; however, it is clear that ethanol self-administration stimulates nucleus accumbens dopamine release in well trained animals. During operant sweetened ethanol self-administration behavior, an adaptation in the nucleus accumbens dopamine system occurs between the first and second exposure paralleling a dramatic increase in sweetened ethanol intake, which suggests a single exposure to sweetened ethanol may be sufficient to learn the association between sweetened ethanol cues and its reinforcing properties. In the present experiment, we test the effects of blockade of nucleus accumbens dopamine D1 receptors on operant sweetened ethanol self-administration behavior during the first two days of exposure. Adult male Long-Evans rats were first trained to self-administer 10% sucrose (10S) across six days in an appetitive and consummatory operant model (appetitive interval: 10 min pre-drinking wait period and a lever response requirement of 4; consummatory interval: 20 min access to the drinking solution). After training on 10S, the drinking solution was switched to 10% sucrose plus 10% ethanol (10S10E); control rats remained drinking 10S throughout the experiment. Bilateral nucleus accumbens microinjections of the dopamine D1 antagonist, SCH-23390 (0, 1.0, or 3.0 μg/side), immediately preceded the first two sessions of drinking 10S10E. Results show that blocking nucleus accumbens dopamine D1 receptors has little or no influence on consumption during the first two days of exposure to the sweetened ethanol solution or maintenance of sucrose only drinking. Furthermore, the high dose of SCH-23390, 3.0 μg/side, reduced open field locomotor activity. In conclusion, we found no evidence to suggest that nucleus accumbens D1 receptor activation is involved in consumption of a sweetened ethanol solution during the first two days of exposure or maintenance of sucrose drinking, but rather D1 receptors seem needed for general locomotor activity that contributes to initiation of appetitive behavior.
Keywords: ethanol, alcohol, microinjection, initiation, dopamine, nucleus accumbens
Introduction
Mechanisms that underlie ethanol self-administration are not fully understood; however, it is clear ethanol self-administration stimulates dopamine release in the nucleus accumbens (Gonzales and Weiss, 1998; Gonzales et al., 2004). Dopamine within the nucleus accumbens serves to modulate motor, reinforcement, and learning behavior (Willuhn et al., 2010). In well-trained animals tested for weeks, the dopamine signal in the mesolimbic system increases during unexpected reward presentation, then after many pairings the dopamine signal switches from responding to the reward to responding to cues predicting the reward (Schultz, 2007; Day et al., 2007). In naïve rats learning a pavlovian conditioning task with sucrose, phasic dopamine levels in the nucleus accumbens switch from responding to the reward, to responding to the cue predicting the reward (Stuber et al., 2008). Dopamine D1-like (D1 and D5) and D2-like (D2, D3, D4) receptor subtypes are expressed throughout the brain, with a high level of expression of D1- and D2-like receptors in the nucleus accumbens (Fremeau et al., 1991). The role of mesolimbic dopamine as a reward-prediction signal suggests dopamine receptors may also play a critical role in learning cue-reward associations during acquisition of ethanol-reinforced behavior, but it is unclear which subtypes might be involved.
Our previous work supports the reward-prediction role of nucleus accumbens dopamine in ethanol self-administration. In well-trained rats, a transient increase in dopamine levels in nucleus accumbens is correlated with exposure to the stimulus properties of ethanol rather than the brain concentration of ethanol (Doyon et al., 2003, 2005, 2006; Howard et al., 2009), which suggests dopamine is playing a sensory or physiological role that predicts the rewarding effects of ethanol that will be experienced as brain ethanol concentrations rise. We also measured dopamine during a switch from sucrose alone to sweetened ethanol self-administration behavior, and showed a significant increase or adaptation in dopamine activity in nucleus accumbens occurs between the first and second self-administration exposure, paralleling the dramatic increase in sweetened ethanol consumption between day 1 and 2 (Carrillo and Gonzales, 2011). Therefore, a single exposure to sweetened ethanol may be sufficient to induce dopamine plasticity during learning of the association between sweetened ethanol cues and its reinforcing properties.
Most work examining possible mechanisms underlying acquisition of reinforced or conditioned behavior has focused on psychostimulant or food motivated behaviors. Acquisition is postulated to be a measure of the rewarding value of a drug (Campbell and Carroll, 2000; Di Chiara and Bassareo, 2007); faster acquisition of drug- or food-reinforced behavior correlates to higher rewarding value, and dopamine seems important during this process (e.g. Kosten et al., 1997; Walker and Ettenberg, 2007; Weitemier and Murphy, 2009; Veeneman et al., 2012;Smith-Roe and Kelley, 2000; but see Crespo et al., 2006 for non-dopamine). Acquisition of operant self-administration of the natural reinforcer sucrose was dependent upon nucleus accumbensD1 receptor activation, in conjunction with NMDA receptor activation (Smith-Roe and Kelley, 2000). However, the mechanisms important for acquisition of ethanol self-administration behavior are largely unknown. Lesion studies testing two bottle choice ethanol intake have reported mixed results. Ikemoto et al. (1997) show 6-hydroxydopamine (6-OHDA) lesion of nucleus accumbens disrupts acquisition of ethanol self-administration in rats, however, Koistinen et al. (2001) show the same lesion procedure has no effect on ethanol intake. Also, ethanol conditioned place preference studies in mice show D1-like (not D2-like) receptors in nucleus accumbens are important in the learning context-ethanol associations (Young et al., 2013). Thus, very little conclusive evidence exists about acquisition of ethanol reinforcement utilizing what may be the most behaviorally relevant model of drug and alcohol abuse liability, operant self-administration (Schuster and Thompson, 1969; Meisch, 1982; Ator and Griffiths, 2003).
In the present experiment, we hypothesize that nucleus accumbens dopamine activation of D1 receptors contributes to the behavioral adaptation that occurs with a switch of liquid reinforcer from sucrose alone to sweetened ethanol. If a transient nucleus accumbens dopamine release signal is important in promoting the establishment of behavior reinforced by sweetened ethanol, pharmacological blockade of the dopamine signal should prevent or retard the changes in behavior that accompany the switch from sucrose alone to sweetened ethanol. Pharmacological manipulations of the mesolimbic dopamine pathway in well-trained rats have generally supported the idea that some, but not all, aspects of ethanol self-administration behavior are influenced by dopamine. In well-trained rats, dopamine agonists microinjected into nucleus accumbens tend to increase ethanol intake, while dopamine antagonists decrease ethanol intake (Hodge et al., 1992, 1997; Rassnick et al., 1992; Samson et al., 1992; Czachowski et al., 2001; Samson and Chappell, 2003, 2004). We tested our hypothesis by measuring operant self-administration of sucrose and sweetened ethanol in the presence of a selective antagonist of dopamine D1/D5 receptors, SCH-23390, which was microinjected into the nucleus accumbens. We used an operant self-administration model that separated the appetitive (anticipatory/seeking) and consummatory phases within session across time (Czachowski and Samson, 1999; Doyon et al., 2003). We administered the fewest number of operant training sessions on a sucrose only solution to be confident of stable responding before switching rats to drinking a sweetened ethanol solution (6 days of training; Carrillo and Gonzales, 2011); thus, we focus on acquisition of sweetened ethanol reinforced behavior, as opposed to ethanol reinforced behavior after many weeks of operant training (well-trained). We define our use of the term ‘acquisition’ as a behavioral adaptation during a switch to a new liquid reinforcer (sucrose to sweetened ethanol), rather than the learning of the association between access to the reinforcer and the operant response.
Methods and Materials
Animals and surgery
Fifty-six adult male Long-Evans rats were used (290–301 g on arrival; Charles River Laboratories, Raleigh, NC). Rats were housed individually after surgery in a temperature (25°C) and light (12 h light/12 h dark; lights on 0700 h) controlled environment. Food and water were available ad libitum in home cages, except noted below for brief water deprivation. Rats were handled for a minimum of five days before surgery, and body weights were recorded daily. All behavioral testing occurred at approximately 1000–1600 h. Principles of laboratory animal care were followed; all procedures complied with NIH Guide for Care and Use of Laboratory Animals (7th Ed., 1998) and were approved by the Institutional Animal Care and Use Committee of the University of Texas at Austin.
Prior to operant training, stainless steel, bilateral, guide cannula were surgically implanted (26 gauge; Plastics One, Roanoke, VA, USA) above the nucleus accumbens core-shell border. The core-shell border was our region of interest because the dopamine response occurs at the time of the cue and consumption of the reinforcer (Howard et al., 2009). Surgery occurred while rats were under isoflurane gas anesthesia, using standard stereotaxic equipment and procedures (Howard et al., 2008). Coordinates in mm relative to bregma, flat-skull orientation: +2.0 anterior, +1.0–1.2 lateral to midline, −5.5–6.5 ventral to skull surface (Paxinos et al.,1999). The range in coordinates is due to the use of two different sets of Plastics One bilateral cannulae and injectors, and we optimized the coordinates accordingly to maximize the hit rate for the nucleus accumbens core-shell border at a targeted DV depth of 7.5 mm. Cannulae were secured to the skull with dental acrylic and stainless steel screws, and a dummy cannula occluded the guide to maintain patency. Carprofen (5 mg/kg, subcutaneous; Pfizer, New York, NY, USA) was administered to minimize post-surgical pain. Rats began operant training after 5–7 days of recovery.
Drugs and microinjections
The D1-family selective receptor antagonist R(+)-SCH-23390 hydrochloride (SCH; Sigma-Aldrich, Saint Louis, MO, USA) was dissolved in sterile artificial cerebral spinal fluid (2.8 mMKCl, 149 mMNaCl, 1.2 mM CaCl2, and 1.2 mM MgCl2) and kept at 4°C in 400 μl aliquots. Intracerebral microinjections of drug or vehicle (VEH; artificial cerebral spinal fluid) were administered by lowering a 33 gauge bilateral injector cannula (Plastics One) to the site of infusion (7.5 mm ventral from skull using a 1–2 mm projection), and injecting a volume of 1.0 μl/side across 4 min, followed by 1 min of diffusion, using Exmire microsyringes (Ito Corp., Fuji, Japan) attached to PE50 tubing and a CMA pump (CMA Microdialysis, Acton, MA, USA). Rats were free to move about their home cage during the microinjection procedure by utilizing a rotating connector assembly (Plastics One). Drugs were administered in a between groups design (VEH: 10S n=7, 10S10E n=8), SCH 1.0 μg/side (SCH 1.0: 10S n=4, 10S10E n=6), or SCH 3.0 μg/side (SCH 3.0: 10S n=5, 10S10E n=7), and SCH doses were based on prior reports (Hodge et al., 1997; Smith-Roe and Kelley, 2000). Following microinjection, the dummy cannula was replaced, and rats were immediately placed into operant chambers. Rats were gradually habituated to the microinjection procedure in the three weeks prior to injections.
Equipment
Self-administration was conducted in standard rat operant conditioning chambers housed in sound-attenuating cubicles with the exterior doors removed (MedAssociates, St Albans, VT, USA). Each chamber was equipped with one retractable lever on the left side of one wall (6 cm above the grid floor). After the appropriate lever response requirement was obtained, a retractable ball point drinking tube (Ancare Corp., Bellmore, NY, USA) attached to a 50 ml conical vial entered the chamber on the right side of the same wall (6 cm above the grid floor). The metal grid floor bars were connected to the metal spout of the drinking bottle through a lickometer circuit (MedAssociates). An interior chamber light and sound-attenuating fan were activated with the start of each operant session, and lever presses activated a cue light 4 cm above the lever for 0.1 sec. Operant chamber components and data collection were controlled by a computer using MedAssociates software (Med PC IV).
Locomotor activity was tested in clean Plexiglas chambers (41 L x 41 W x 31 H cm; clean bedding on the floor). Activity was videotaped by an overhead-mounted camera and quantified as crossings over four evenly spaced matrix quadrants (20 cm2).
Self-administration
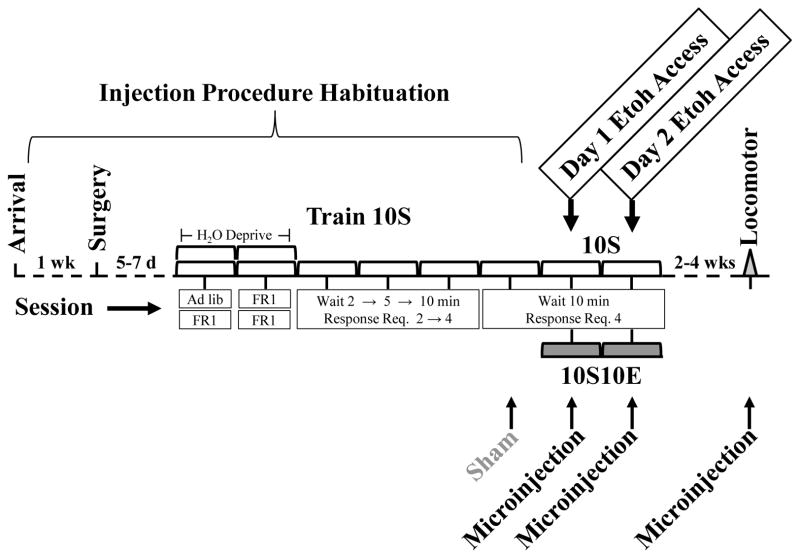
The training procedure was similar to our previous studies (Carrillo et al., 2008; Carrillo and Gonzales, 2011) (Fig. 1). Drinking solutions (10% sucrose (w/v) (10S) or 10% sucrose + 10% ethanol (v/v) (10S10E)) were made from 95% ethanol (AAPER, Shelbyville, KY, USA), ultra-pure sucrose (MP Biomedicals, Solon, OH, USA), and distilled water. Operant sessions were 20 min in length and occurred once a day, five days per week (except noted below). All rats were initially trained to lever press for access to the 10S solution. Rats were water deprived (22 h/d) during the first 2 days of training to facilitate learning of the operant response. Day 1 of initial training began with a habituation session (40 min) where both the lever and the drinking tube were inserted into the chamber for the entire session; lever presses had no consequence, and only the 10S solution was available to drink. Approximately 3–4 h later, all rats were trained to lever press for access to the 10S solution on a fixed ratio one (FR1) schedule of reinforcement, with each lever press yielding 10 sec access to the sipper tube. Day 2 of training consisted of a morning and an afternoon FR1 schedule test. A reliable lever-pressing response for 10S occurred within these initial 1–2 days using two 40 min sessions per day; once established, rats were given water ad libitum for the remainder of the experiment. Subsequent to the initial two days, all rats had four days of 10S self-administration (one session per day). During days 3–6, rats were gradually habituated to a 10-min wait period which preceded access to the lever and drinking solution, and the lever response requirement was progressively increased to four (wait period (W) and response requirement (RR) sequence: Day 3 W 2 min, RR 2; Day 4 W 5 min, RR 4; Days 5 and 6 W 10 min, RR 4). The 10 min wait period, lever responding, and time to first lick served as the appetitive phase of the session. When the response requirement was completed, the lever retracted, the drinking spout extended into the chamber, and rats had a single 20 min access period to the drinking solution, i.e. the consummatory phase. Following the consummatory period, the drinking spout retracted, and rats remained in the chambers for a 5 min post drink period without the lever or drinking solution. On the sixth training day on 10S, a sham microinjection was performed where an injector was lowered into the nucleus accumbens, but no solution was infused. After the sixth training session on 10S, the ethanol groups were switched to the 10S10E drinking solution for 2 days, while the sucrose controls remained drinking 10S throughout the study; microinjections of VEH, SCH 1.0, or SCH 3.0 occurred before access to the operant chamber on these last 2 days of self-administration. Thus, all rats received two microinjections plus one sham microinjection. Consumption of drinking solutions was monitored by the lickometer circuit and by measuring the volume of liquid in the drinking bottle before and after the session (resolution of 0.1 mL, accounting for spillage).
Fig. 1.
Timeline of testing. Self-administration training and testing occurred for eight total days, with only two days of access to the sweetened ethanol solution in 10S10E groups. All rats received microinjections prior to self-administration testing on three consecutive days, consisting of a sham day (no fluid injected), and two days of microinjection treatment. A subset of rats also received one microinjection prior to an open field locomotor test. See text for detailed description of the testing session. 10S = 10% sucrose, 10S10E = 10% sucrose +10% ethanol.
Locomotor activity
Locomotor activity was recorded in a subset of rats (counterbalanced across treatment groups) two-four weeks after the end of operant conditioning. Rats first underwent a baseline locomotor test for 30 min. Rats were then removed from the Plexiglas chamber and microinjected with SCH 3.0 or VEH (same procedure as above), and immediately returned to the chamber for 60 min.
Histology
One to three days after the end of self-administration testing or the locomotor test, rats were overdosed using a sodium pentobarbital euthanasia solution (Euthanasia-III, intraperitoneal; Med-Pharmex, Pomona, CA, USA). Rats were perfused intracardially with saline and then 10% formalin, and brains were extracted and submerged in 10% formalin for 1–3 days. Brains were sectioned with a vibratome (100–120 μm thick;Leica, Nussloch, Germany) and stained with cresyl violet to confirm injector placement. The tips of the injectors were mapped onto an adapted atlas of Paxinos et al. (1999). The diameter of the dots (0.5 mm) represents a minimal drug diffusion area for our injection parameters (Caine et al., 1995).
Statistics
Nineteen of 56 rats were excluded from data analyses (n=8 histological verification outside nucleus accumbens core-shell border; n=6 complications due to surgery or microinjections; n=1 did not consume ≥ 0.3 g/kg ethanol averaged across the 2 days; n=4 did not complete the lever response requirement on day 2 within 80 min [1 from 10S SCH 1.0, 2 from 10S SCH 3.0, 1 from 10S10E SCH 3.0]). Measures of consumption (total volume consumed and number of licks) and measures of appetitive behavior (delay to first lever press, inter-response interval (IRI) for lever presses, and delay to first lick) were analyzed using three-way mixed-measures analyses of variance (ANOVA), with dose (VEH, SCH 1.0, and SCH 3.0) and drinking solution (10S and 10S10E) as between-subjects factors, and day (Sham, and Day 1 and 2 of EtOH Access) as a within-subjects repeated measure (RM). Amount of ethanol consumed (g/kg in 20 min) was analyzed using a two-way ANOVA, with dose as between-subjects factor, and day (Day 1 and 2 of EtOH Access) as a within-subjects RM. Baseline locomotor activity did not differ between treatment groups; therefore, we expressed locomotor activity during the 60 min test as a percentage of activity during the baseline test for each rat, and compared treatment groups using an independent t-test. Locomotor activity was also analyzed using a two-way ANOVA, with dose (VEH and SCH 3.0) as between-subjects factor, and time point (four 15 min bins) as a within-subjects RM. Follow-up post-hoc comparisons and tests of simple effects, with Bonferroni’s correction and pooled error, were used as appropriate. Results were significant if p≤0.05, except in cases where violations of Levene’s test of equality of error variance occurred, for which we adjusted the alpha to p<0.025 to reduce the possibility of making Type I errors (Rogan and Keselman, 1977).
Results
Consumption – dose, amount, and licking behavior
The major hypothesis of this study is that dopamine signaling through D1-like receptors in the core-shell border of the nucleus accumbens during the initial two days of exposure to a sweetened ethanol solution is necessary for the increase in sweetened ethanol consumption that is observed across these sessions. The major finding of this study demonstrates that blocking dopamine D1 receptors in nucleus accumbens had little or no effect on consumption of 10S10E during the first two days of exposure (Fig. 2a,b). As expected, rats switched from drinking 10S to drinking 10S10E decreased the amount consumed the first day, and then increased the amount consumed from day 1 to day 2 indicating acquisition of sweetened ethanol self-administration. This pattern of consumption of sweetened ethanol and lack of effect of D1 antagonism was reflected in all measures of drinking behavior.
Fig. 2.
Total volume consumed and grams per kilogram ethanol. Treatment with SCH did not influence consumption behavior. Only effects of day differed within groups drinking 10S10E. Total ethanol consumed in grams per kilogram (g/kg in 20 min) in rats drinking 10S10E (a). Total volume consumed (mls) in 20 min in rats drinking 10S10E (b) or 10S (c). Day 1 EtOH Access vs. Day 2 EtOH Access (#p<0.001). 10S10E = 10% sucrose +10% ethanol, 10S = 10% sucrose. VEH: 10S n=7, 10S10E n=8, SCH 1.0: 10S n=4, 10S10E n=6, SCH 3.0: 10S n=5, 10S10E n=7. Points represent mean ± SEM.
The dose (g/kg) of ethanol consumed in 20 min significantly increased from day 1 to day 2 (Fig. 2a) (F1,18=18.20, p<0.001, two-way RM ANOVA), but there was no apparent effect of blocking nucleus accumbens dopamine D1 receptors (non-significant main effect of SCH dose and dose X day interaction). Analysis of total volume consumed (mls) in both the control sucrose drinkers and the sweetened ethanol drinkers confirmed the changes in ethanol consumption across the first two days of exposure to the ethanol solution (Fig. 2b), while maintenance of sucrose consumption alone was stable across days of the study that matched the ethanol exposures (Fig 2c) (day X drinking solution interaction, F2,62=28.57, p<0.001; three-way RM ANOVA). Follow-up post hoc analyses with Bonferroni’s correction confirmed significant effects of day only within the 10S10E groups (Day 1 EtOH Access vs. Day 2 EtOH Access F1,62=30.69, p<0.001).
Appetitive behavior - Delay to first lever press, inter response interval (IRI) of lever presses, and delay to first lick
Initiation of responding on the lever during the appetitive interval prior to consumption was dose-dependently delayed by blocking dopamine D1 receptors in nucleus accumbens, independent of drinking solution (Fig. 3a, b); however, inter-response interval (IRI) of lever presses and delay to first lick were not significantly influenced by SCH dose (Table 1). A three-way ANOVA confirmed the delay to first lever press differed according to dose (F2,31=8.63, p<0.01), and an interaction of day X dose (F4,62=3.84, p<0.01). Follow-up post hoc analyses with Bonferroni’s correction confirmed groups treated with SCH 3.0 exhibited a significant delay to first lever press compared to VEH (F1,80=20.65, p<0.001) and SCH 1.0 groups (F1,80=16.10, p<0.001). The effect of dose was only significant when comparing Sham vs. Day 2 EtOH Access (F1,62=20.36, p<0.001). The IRI of lever presses and delay to first lick did not differ between groups (Table 1). Three-way ANOVAs showed no significant main effects or interactions. On Day 1 EtOH Access, 2 of 7 rats in the 10S10E SCH 3.0 group exhibited IRI values more than two times higher than the group standard deviation.
Fig. 3.
Delay to first lever press. Delay to first lever press (min) in rats drinking 10S10E (a) or 10S (b). Regardless of drinking solution, nucleus accumbens microinjection of SCH 3.0 increased the delay to first lever press vs. VEH (# p<0.001), and vs. SCH 1.0 (* p<0.001) on Day 2 EtOH Access vs. Sham. 10S10E = 10% sucrose +10% ethanol, 10S = 10% sucrose. VEH: 10S n=7, 10S10E n=8, SCH 1.0: 10S n=4, 10S10E n=6, SCH 3.0: 10S n=5, 10S10E n=7. Points represent mean ± SEM.
Table 1.
Inter-response interval (IRI) of lever presses and delay to first lick appetitive behavior.
| 10S | 10S10E | ||||||
|---|---|---|---|---|---|---|---|
| VEH | SCH 1.0 | SCH 3.0 | VEH | SCH 1.0 | SCH 3.0 | ||
| IRI (sec) | Sham | 2.9 ± 0.7 | 2.4± 0.8 | 2.9 ± 1.0 | 2.6 ± 0.9 | 2.7± 0.4 | 5.3 ± 1.6 |
| Day 1 | 4.8 ± 2.2 | 5.1 ± 3.0 | 7.3 ± 2.8 | 2.7± 0.7 | 2.4± 0.5 | 98.4 ± 60.4 | |
| Day 2 | 3.0 ± 0.9 | 2.2± 1.1 | 5.3 ± 2.5 | 4.0 ± 1.8 | 2.1 ± 4.0 | 8.9 ± 2.6 | |
| Delay to 1st Lick (sec) | Sham | 2.6 ± 1.1 | 2.4 ± 0.4 | 6.4 ± 2.1 | 4.6 ± 1.2 | 2.2± 0.4 | 4.6 ± 0.8 |
| Day 1 | 3.4 ± 0.5 | 12.6 ± 10.2 | 3.8 ± 1.3 | 2.9 ± 0.3 | 3.5± 0.7 | 17.8 ± 13.4 | |
| Day 2 | 2.2 ± 0.3 | 10.9 ± 9.1 | 6.5 ± 1.7 | 6.8 ± 4.3 | 8.1 ± 4.8 | 6.4 ± 2.6 | |
10S = 10% sucrose, 10S10E = 10% sucrose +10% ethanol. VEH: 10S n=7, 10S10E n=8, SCH 1.0: 10S n=4, 10S10E n=6, SCH 3.0: 10S n=5, 10S10E n=7. Values represent mean ± SEM.
Locomotor activity
Open field locomotor activity was attenuated by blocking nucleus accumbens dopamine D1 receptors in a subset of rats that had self-administered 10S or 10S10E 2-4 weeks earlier (Fig. 4). Matrix crossings did not differ between the two dose groups at baseline testing, (VEH n=10 mean ± SEM: 161±12; SCH 3.0 n=9 mean ± SEM: 162 ± 15); thus, we expressed locomotor activity as a percentage of baseline activity, and microinjection of SCH 3.0 into nucleus accumbens significantly decreased total activity (t17=2.85, p<0.05). A two-way ANOVA also confirmed that locomotor activity in both treatment groups decreased across time (main effect of time, F3,51=29.17, p<0.001). Follow-up analyses, collapsing across dose, confirmed that the greatest activity occurred during the first 15 min of testing (bin 1 vs. bin 2: F1,51=62.00, p<0.001; bin 1 vs. bin 3: F1,51=47.80, p<0.001; bin 1 vs. bin 4: F1,51=67.25, p<0.001; no other differences between bins occurred).
Fig. 4.

Locomotor activity. INSET Nucleus accumbens microinjection of SCH 3.0 decreased locomotor activity compared to VEH (* p<0.05; expressed as a percentage of baseline crossings). Both treatment groups also decreased activity across four 15 min time bins. VEH: n=10, SCH 3.0: n=9. Bars and points represent mean ± SEM.
Histology
Figure 5 and 6 shows representation of injector tip locations within the nucleus accumbens across all rats drinking 10S10E or 10S, respectively, with all tip placements spanning the core-shell border subregion.
Fig. 5.
Schematic representation of injector tips (black dots) spanning the core-shell border subregion of nucleus accumbens from all rats drinking 10S10E. Numbers denote location in millimeter from bregma. Figure adapted from the rat forebrain atlas of Paxinos et al. (1999). 10S10E = 10% sucrose +10% ethanol. VEH 10S10E n=8, SCH 1.0 10S10E n=6, SCH 3.0 10S10E n=7. Scale bar = 1mm.
Fig. 6.
Schematic representation of injector tips (black dots) spanning the core-shell border subregion of nucleus accumbens from all rats drinking 10S. Numbers denote location in millimeter from bregma. Figure adapted from the rat forebrain atlas of Paxinos et al. (1999). 10S = 10% sucrose. VEH 10S n=7, SCH 1.0 10S n=4, SCH 3.0 10S n=5. Scale bar = 1mm.
Discussion
The major goal of this study was to determine if blocking dopamine D1 receptor activation within the nucleus accumbens influences consumption of a sweetened ethanol solution during the first two days of exposure. We found no evidence to suggest that D1 receptor activation is involved in consumption of a sweetened ethanol solution during the first two days after a switch of the drinking solution from sucrose alone to sweetened ethanol. Similarly, blocking dopamine D1 receptors in nucleus accumbens had little or no influence on consumption of a control sucrose only solution. Rather, D1 receptors seem needed for general locomotor activity that contributes to initiation of appetitive behavior. Together with our previous study showing an adaptation in dopamine release in nucleus accumbens during the first two days of operant self-administration of sweetened ethanol, our results suggest plasticity in dopamine circuits associated with consumption of ethanol during the first two days may not involve D1 receptors.
Rats in the present study self-administered amounts of 10S10E comparable to our previous studies (Carrillo et al., 2008; Carrillo and Gonzales, 2011). The amounts self-administered on both days (~ 0.75 g/kg and ~1.5 g/kg ethanol in 20 min on Day 1 and Day 2 respectively) are pharmacologically relevant amounts of ethanol, sufficient enough to reach the brain and reinforce behavior (Czachowski et al., 2001, 2003). The fact that consumption dramatically increased on day 2 might suggest that rewarding sensations were experienced on day 1 of ethanol consumption, and associations between the rewarding effects of ethanol and cues present during consumption of the novel ethanol solution were formed. If rewarding sensations were experienced on day 1, we speculate that increased ethanol intake on day 2 may be due to enhanced motivation to seek ethanol because of the newly formed association. Thus, the dopamine response we previously recorded, present on day 2, but not day 1 of 10S10E (Carrillo and Gonzales, 2011), may reflect the reward-prediction role of mesolimbic dopamine that developed after the single pairing between the stimulus cues of ethanol during consumption and the subsequent reward produced by ethanol after a sufficient concentration has reached the brain (Horvitz et al., 2007; Schultz, 2007; Stuber et al., 2008). An alternative explanation of why rats dramatically increase ethanol intake on day 2 is habituation to the novelty or aversive taste of the added ethanol on day 1. Analysis of the first bout lick rate would support this idea, as lick rate was reduced during the first exposure to the 10S10E solution compared to 10S controls and 10S10E on day 2 (10S10E sham 279 ± 15, day 1 162 ± 13, day 2 245 ± 17; 10S sham 279 ± 16, day 1 253 ± 18, day 2 282 ± 18). Habituation to aversive fluids occurs only to a limited degree in Long-Evans rats (Gartside and Laycock, 1987), thus habituation cannot explain all the behavior. Numerous factors contribute to enhancement of sweetened ethanol consumption during the first two days of access, including increased positive reinforcement and habituation to aversive taste, but the balance would likely change to predominately positive reinforcement with continued drinking.
Microinjection of SCH into nucleus accumbens inhibited locomotor activity, thus providing an important positive control for the self-administration behavior. Based on the locomotor results, we can assume that nucleus accumbens D1 receptors were also blocked during the appetitive and consumption phases of operant testing. Microinjection of SCH into the brain produces rapid, time dependent effects on reinforced behavior (cocaine self-administration; Caine et al., 1995), and we replicated previous results of microinjection of SCH into nucleus accumbens attenuating locomotor activity for at least 30 min in mice (Young et al., 2013) and up to 50 min in rats (Meyer, 1993). Microinjection of SCH into brain exhibits significant dopamine receptor binding and a robust autoradiographic signal at the injection site up to at least 60 min post microinjection, and the maximum effect of SCH attenuating cocaine self-administration occurs at ~20 min which corresponds to the time our rats would be drinking the majority of their drinking solution (first bout) (Caine et al., 1995). Therefore, we suggest that D1 receptor mediated neurotransmission in nucleus accumbens may not be needed for consumption of a sweetened ethanol solution during the first two days of this operant behavior, or maintenance of sucrose drinking behavior. In contrast, D1 receptors seem to be needed for general locomotor activity that contributes to initiation of appetitive behavior for reinforcing substances (i.e. delay to first lever press). Alternatively, the increased delay to first lever press behavior produced by two days of high dose intra-accumbens SCH could be due to aversive conditioning or sensitization. Intra-accumbens SCH administered at doses lower than our high dose produces conditioned place aversion (Shippenberg et al., 1991; Shippenberg et al., 1993). We cannot rule out sensitization, but sensitization seems unlikely because the acute effects of SCH on locomotor behavior are apparent at first exposure (Meyer, 1993; Young et al., 2013). Important for our consumption data, once lever pressing begins the motor-impairing effects of intra-accumbens D1 blockade are overcome and no robust treatment effects occurred on inter response interval of lever presses or delay to first lick.
Our present results using an appetitive/consummatory operant model may appear at first to disagree with reports using more traditional operant models testing for acquisition of self-administration behavior. Nucleus accumbens infusion of the dopamine antagonist α-flupenthixol inhibits the acquisition of cocaine self-administration (Veeneman et al., 2012). Furthermore, nucleus accumbens infusion of SCH attenuates responding during acquisition of sucrose self-administration behavior, although blocking D1 activity was not sufficient as concurrent glutamate receptor blockade was also necessary, and the rats were motivated by hunger (Smith-Roe and Kelley, 2000). In contrast, our results do not provide evidence for a role for nucleus accumbens D1 mediated neurotransmission during the initial two days of operant sweetened ethanol self-administration. The disadvantage of using traditional operant models is the inability to distinguish between responding for vs. consumption of a reinforcing substance, which is why we employed the appetitive/consummatory operant model. A likely reason why acquisition of cocaine and sucrose self-administration was attenuated by blocking dopamine receptors is due to impairment of responding, which would lead to reduced consumption. Models using two bottle choice procedures or conditioned place preference offer mixed results compared to our operant self-administration behavior. One study supports our data; acquisition of two bottle choice ethanol intake was not affected in rats following lesion of nucleus accumbens (Koistinen et al., 2001), however, another study using the same lesion procedure shows attenuated acquisition of ethanol intake (Ikemoto et al., 1997). Furthermore, ethanol conditioned place preference studies in mice showed D1-like (not D2-like) receptors in the nucleus accumbens are important in learning context-ethanol associations (Young et al., 2013).
The novel focus of the current study is we investigated the role of nucleus accumbens D1 receptors during the initial two days of operant sweetened ethanol self-administration behavior. If we compare our results (operant training with 10S for six days, only two days sweetened ethanol access) with previous studies using well-trained (training for many weeks) rats employing dopamine D1-family receptor agonists and antagonists, we must distinguish between systemic vs. intra-accumbens administration, and between responding for vs. consumption of reinforcing solutions. Systemic administration of D1-family receptor agonists and antagonists have produced inconsistent results; two studies show the dopamine D1 partial agonist SKF-38393 decreased ethanol intake, while the dopamine D1/D5 antagonist SCH had no effect on ethanol intake (Linseman, 1990; Silvestre et al., 1996), and another study showed both SKF-38393 and SCH decreased ethanol intake in selectively bred high alcohol drinking female rats (Dyret al., 1993). Intra-accumbens microinjection of dopamine D1-family receptor agonists and antagonists modulates self-administration behavior by affecting responding for reinforcing substances, but not consumption. Our present results during the initial two days of sweetened ethanol self-administration or maintenance of sucrose drinking are consistent with results during long-term established drinking; microinjection of SCH into the nucleus accumbens at doses of 0.25 and 1.0 μg/side decreased total lever press responding and the duration of responding, but had no effect on response rate, using a fixed ratio four response requirement in well-trained rats (Hodge et al., 1997). Microinjection of SCH into nucleus accumbens at doses of 0.1–2.0 μg/side in female alcohol-preferring P rats also did not affect ethanol intake (Levy et al., 1991). Interestingly, microinjection of SKF-38393 into nucleus accumbens did not affect responding for ethanol (Hodge et al., 1997), while the broad dopamine receptor blocker fluphenazine attenuated the development of intracerebral ethanol conditioned place preference (Walker and Ettenberg, 2007). Overall, our results are consistent with numerous studies implicating mesolimbic dopamine, specifically in nucleus accumbens, as more important for general locomotor activity and the appetitive phase compared to the consummatory phase of motivated behaviors (Blackburn et al., 1989; Ikemoto and Panksepp, 1996; Salamone et al., 1991). We should note that one limitation of the present study is that our finding of a nonsignificant effect of microinjection of SCH on consumption of either sweetened ethanol or sucrose alone does not allow us to firmly conclude that dopamine D1 receptors are not required for these behaviors. We cannot rule out the possibility that small effects of D1 receptor blockade might be observed with larger sample sizes. For future studies, we hypothesize that D2 (and maybe D3) receptors could be important for responding for ethanol, but not consumption, during the initial two days of exposure to operant self-administration of sweetened ethanol. D2-family receptors have been reported to influence responding for ethanol, but not consumption, in well-trained rats (Samson et al., 1992; Hodge et al., 1997; Czachowski et al., 2001; Samson and Chappell, 2003, 2004).
In summary, we found no evidence to suggest that nucleus accumbens D1 receptor activation is involved in consumption of a sweetened ethanol solution during the first two days of exposure, but rather D1 receptors may be needed for general locomotor activity that contributes to initiation of appetitive behavior. Together with our previous study showing an adaptation in dopamine release in nucleus accumbens during the first two days of exposure to sweetened ethanol self-administration, our results suggest plasticity in dopamine circuits associated with enhanced consumption of sweetened ethanol during the first two days may not involve D1 receptors.
Acknowledgments
Funding and support provided by a Postdoctoral Fellowship NIH-NIAAA T32 AA00747 to JMD, NIH-NIAAA R37 AA11852 to RAG, the Waggoner Center for Alcohol and Addiction Research, and College of Pharmacy at the University of Texas at Austin. Authors thank John Valenta, Wonbin Song, Roberto Cofresi, Geoff Dilly, and Drs. Regina Mangieri, and Christy Schier, for technical and editorial assistance. The authors also thank Dr. Michael Mahometa for assistance with statistical analysis.
References
- Ator NA, Griffiths RR. Principles of drug abuse liability assessment in laboratory animals. Drug and Alcohol Depend. 2003;70(3 Suppl):S55–72. doi: 10.1016/s0376-8716(03)00099-1. [DOI] [PubMed] [Google Scholar]
- Blackburn JR, Phillips AG, Jakubovic A, Fibiger HC. Dopamine and preparatory behavior: II. A neurochemical analysis. Behav Neurosci. 1989;103(1):15–23. doi: 10.1037//0735-7044.103.1.15. [DOI] [PubMed] [Google Scholar]
- Caine SB, Heinrichs SC, Coffin VL, Koob GF. Effects of the dopamine D-1 antagonist SCH 23390 microinjected into the accumbens, amygdala or striatum on cocaine self-administration in the rat. Brain Res. 1995;692(1–2):47–56. doi: 10.1016/0006-8993(95)00598-k. [DOI] [PubMed] [Google Scholar]
- Campbell UC, Carroll ME. Acquisition of drug self-administration: Environmental and pharmacological interventions. Exp Clin Psychopharmacol. 2000;8(3):312–325. doi: 10.1037//1064-1297.8.3.312. [DOI] [PubMed] [Google Scholar]
- Carrillo J, Gonzales RA. A single exposure to voluntary ethanol self-administration produces adaptations in ethanol consumption and accumbal dopamine signaling. Alcohol. 2011;45(6):559–566. doi: 10.1016/j.alcohol.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrillo J, Howard EC, Moten M, Houck BD, Czachowski CL, Gonzales RA. A 3-day exposure to 10% ethanol with 10% sucrose successfully initiates ethanol self-administration. Alcohol. 2008;42(3):171–178. doi: 10.1016/j.alcohol.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespo JA, Sturm K, Saria A, Zernig G. Activation of muscarinic and nicotinic acetylcholine receptors in the nucleus accumbens core is necessary for the acquisition of Drug Reinforcement. J Neurosci. 2006;26(22):6004–6010. doi: 10.1523/JNEUROSCI.4494-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czachowski CL, Chappell AM, Samson HH. Effects of raclopride in the nucleus accumbens on ethanol seeking and consumption. Alcohol Clin Exp Res. 2001;25(10):1431–1440. doi: 10.1097/00000374-200110000-00005. [DOI] [PubMed] [Google Scholar]
- Czachowski CL, Legg BH, Samson HH. Assessment of sucrose and ethanol reinforcement: the across-session breakpoint procedure. Physiol Behav. 2003;78(1):51–59. doi: 10.1016/s0031-9384(02)00963-0. [DOI] [PubMed] [Google Scholar]
- Czachowski CL, Samson HH. Breakpoint determination and ethanol self-administration using an across-session progressive ratio procedure in the rat. Alcohol Clin Exp Res. 1999;23(10):1580–1586. [PubMed] [Google Scholar]
- Day JJ, Roitman MF, Wightman RM, Carelli RM. Associative learning mediates dynamic shifts in dopamine signaling in the nucleus accumbens. Nat Neurosci. 2007;10(8):1020–1028. doi: 10.1038/nn1923. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Bassareo V. Reward system and addiction: what dopamine does and doesn’t do. Curr Opin Pharmacol. 2007;7(1):69–76. doi: 10.1016/j.coph.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Doyon WM, Anders SK, Ramachandra VS, Czachowski CL, Gonzales RA. Effect of operant self-administration of 10% ethanol plus 10% sucrose on dopamine and ethanol concentrations in the nucleus accumbens. J Neurochem. 2005;93(6):1469–1481. doi: 10.1111/j.1471-4159.2005.03137.x. [DOI] [PubMed] [Google Scholar]
- Doyon WM, Howard EC, Shippenberg TS, Gonzales RA. Kappa-Opioid receptor modulation of accumbal dopamine concentration during operant ethanol self-administration. Neuropharmacology. 2006;51(3):487–496. doi: 10.1016/j.neuropharm.2006.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyon WM, York JL, Diaz LM, Samson HH, Czachowski CL, Gonzales RA. Dopamine activity in the nucleus accumbens during consummatory phases of oral ethanol self-administration. Alcohol Clin Exp Res. 2003;27(10):1573–1582. doi: 10.1097/01.ALC.0000089959.66222.B8. [DOI] [PubMed] [Google Scholar]
- Dyr W, McBride WJ, Lumeng L, Li TK, Murphy JM. Effects of D1 and D2 dopamine receptor agents on ethanol consumption in the high-alcohol-drinking (HAD) line of rats. Alcohol. 1993;10(3):207–212. doi: 10.1016/0741-8329(93)90037-o. [DOI] [PubMed] [Google Scholar]
- Fremeau RT, Duncan GE, Fornaretto MG, Dearry A, Gingrich JA, Breese GR, Caron MG. Localization of D1 dopamine receptor mRNA in brain supports a role in cognitive, affective, and neuroendocrine aspects of dopaminergic neurotransmission. Proc Natl Acad Sci. 1991;88(9):3772–3776. doi: 10.1073/pnas.88.9.3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartside IB, Laycock JF. Increased aversion to bitter tasting fluids in the Brattleboro rat. Physiol Behav. 1987;39:571–577. doi: 10.1016/0031-9384(87)90155-7. [DOI] [PubMed] [Google Scholar]
- Gonzales RA, Job MO, Doyon WM. The role of mesolimbic dopamine in the development and maintenance of ethanol reinforcement. Pharmacol Ther. 2004;103(2):121–146. doi: 10.1016/j.pharmthera.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Gonzales RA, Weiss F. Suppression of ethanol-reinforced behavior by naltrexone is associated with attenuation of the ethanol-induced increase in dialysate dopamine levels in the nucleus accumbens. J Neurosci. 1998;18(24):10663–10671. doi: 10.1523/JNEUROSCI.18-24-10663.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge CW, Samson HH, Haraguchi M. Microinjections of dopamine agonists in the nucleus accumbens increase ethanol-reinforced responding. Pharmacol Biochem Behav. 1992;43(1):249–254. doi: 10.1016/0091-3057(92)90665-3. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Samson HH, Chappelle AM. Alcohol self-administration: further examination of the role of dopamine receptors in the nucleus accumbens. Alcohol Clin Exp Res. 1997;21(6):1083–1091. doi: 10.1111/j.1530-0277.1997.tb04257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvitz JC, Choi WY, Morvan C, Eyny Y, Balsam PD. A “good parent” function of dopamine: transient modulation of learning and performance during early stages of training. Ann N Y Acad Sci. 2007;1104:270–288. doi: 10.1196/annals.1390.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard EC, Schier CJ, Wetzel JS, Duvauchelle CL, Gonzales RA. The shell of the nucleus accumbens has a higher dopamine response compared with the core after non-contingent intravenous ethanol administration. Neuroscience. 2008;154(3):1042–1053. doi: 10.1016/j.neuroscience.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard EC, Schier CJ, Wetzel JS, Gonzales RA. The dopamine response in the nucleus accumbens core–shell border differs from that in the core and shell during operant ethanol self-administration. Alcohol Clin Exp Res. 2009;33(8):1355–1365. doi: 10.1111/j.1530-0277.2009.00965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S, Panksepp J. Dissociations between appetitive and consummatory responses by pharmacological manipulations of reward-relevant brain regions. Behav Neurosci. 1996;110(2):331–345. doi: 10.1037//0735-7044.110.2.331. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, McBride WJ, Murphy JM, Lumeng L, Li TK. 6-OHDA-Lesions of the nucleus accumbens disrupt the acquisition but not the maintenance of ethanol consumption in the alcohol-preferring P line of rats. Alcohol Clin Exp Res. 1997;21(6):1042–1046. [PubMed] [Google Scholar]
- Koistinen M, Tuomainen p, Hyytiä p, Kiianmaa K. Naltrexone suppresses ethanol intake in 6-hydroxydopamine-treated rats. Alcohol Clin Exp Res. 2001;25(11):1605–1612. [PubMed] [Google Scholar]
- Kosten TA, Miserendino MJD, Haile CN, DeCaprio JL, Jatlow PI, Nestler EJ. Acquisition and maintenance of intravenous cocaine self-administration in Lewis and Fischer inbred rat strains. Brain Res. 1997;778(2):418–429. doi: 10.1016/s0006-8993(97)01205-5. [DOI] [PubMed] [Google Scholar]
- Levy AD, Murphy JM, McBride WJ, Lumeng L, Li TK. Microinjection of sulpiride into the nucleus accumbens increases ethanol drinking in alcohol-preferring (P) rats. Alcohol Alcohol Suppl. 1991;1:417–420. [PubMed] [Google Scholar]
- Linseman MA. Effects of dopaminergic agents on alcohol consumption by rats in a limited access paradigm. Psychopharmacology (Berl) 1990;100(2):195–200. doi: 10.1007/BF02244405. [DOI] [PubMed] [Google Scholar]
- Meisch RA. Animal studies of alcohol intake. Br J Psychiatry. 1982;141:113–120. doi: 10.1192/bjp.141.2.113. [DOI] [PubMed] [Google Scholar]
- Meyer ME. Effects of intraaccumbens dopamine agonist SK&F38393 and antagonist SCH23390 on locomotor activities in rats. Pharmacol Biochem Behav. 1993;45(4):843–847. doi: 10.1016/0091-3057(93)90130-l. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Kus L, Ashwell KWS, Watson C. Chemoarchitectonic Atlas of The Rat Forebrain. Academic Press; San Diego: 1999. [Google Scholar]
- Rassnick S, Pulvirenti L, Koob G. Oral ethanol self-administration in rats is reduced by the administration of dopamine and glutamate receptor antagonists into the nucleus accumbens. Psychopharmacology (Berl) 1992;109(1):92–98. doi: 10.1007/BF02245485. [DOI] [PubMed] [Google Scholar]
- Rogan JC, Keselman HJ. Is the ANOVA F-Test Robust to Variance Heterogeneity When Sample Sizes are Equal?: An Investigation via a Coefficient of Variation. Am Educ Res J. 1977;14(4):493–498. [Google Scholar]
- Salamone JD, Steinpreis RE, McCullough LD, Smith P, Grebel D, Mahan K. Haloperidol and nucleus accumbens dopamine depletion suppress lever pressing for food but increase free food consumption in a novel food choice procedure. Psychopharmacology (Berl) 1991;104(4):515–521. doi: 10.1007/BF02245659. [DOI] [PubMed] [Google Scholar]
- Samson HH, Chappell A. Dopaminergic involvement in medial prefrontal cortex and core of the nucleus accumbens in the regulation of ethanol self-administration: a dual-site microinjection study in the rat. Physiol Behav. 2003;79(4–5):581–590. doi: 10.1016/s0031-9384(03)00126-4. [DOI] [PubMed] [Google Scholar]
- Samson HH, Chappell AM. Effects of raclopride in the core of the nucleus accumbens on ethanol seeking and consumption: the use of extinction trials to measure seeking. Alcohol Clin Exp Res. 2004;28(4):544–549. doi: 10.1097/01.alc.0000121649.81642.3f. [DOI] [PubMed] [Google Scholar]
- Samson HH, Tolliver GA, Haraguchi M, Hodge CW. Alcohol self-administration: role of mesolimbic dopamine. Ann N Y Acad Sci. 1992;654(1):242–253. doi: 10.1111/j.1749-6632.1992.tb25971.x. [DOI] [PubMed] [Google Scholar]
- Schultz W. Behavioral dopamine signals. Trends Neurosci. 2007;30(5):203–210. doi: 10.1016/j.tins.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Schuster CR, Thompson T. Self administration of and behavioral dependence on drugs. Annu Rev Pharmacol. 1969;9:483–502. doi: 10.1146/annurev.pa.09.040169.002411. [DOI] [PubMed] [Google Scholar]
- Shippenberg TS, Bals-Kubik R, Huber A, Herz A. Neuroanatomical substrates mediating the aversive effects of D-1 dopamine receptor antagonists. Psychopharmacology (Berl) 1991;103(2):209–214. doi: 10.1007/BF02244205. [DOI] [PubMed] [Google Scholar]
- Shippenberg TS, Bals-Kubik R, Herz A. Examination of the neurochemical substrates mediating the motivational effects of opioids: role of the mesolimbic dopamine system and D-1 vs. D-2 dopamine receptors. J Pharmacol Exp Ther. 1993;265(1):53–59. [PubMed] [Google Scholar]
- Silvestre JS, O’Neill MF, Fernandez AG, Palacios JM. Effects of a range of dopamine receptor agonists and antagonists on ethanol intake in the rat. Eur J Pharmacol. 1996;318(2–3):257–265. doi: 10.1016/s0014-2999(96)00821-7. [DOI] [PubMed] [Google Scholar]
- Smith-Roe SL, Kelley AE. Coincident activation of NMDA and dopamine D1receptors within the nucleus accumbens core is required for appetitive instrumental learning. J Neurosci. 2000;20(20):7737–7742. doi: 10.1523/JNEUROSCI.20-20-07737.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber GD, Klanker M, de Ridder B, Bowers MS, Joosten RN, Feenstra MG, Bonci A. Reward-predictive cues enhance excitatory synaptic strength onto midbrain dopamine neurons. Science. 2008;321(5896):1690–1692. doi: 10.1126/science.1160873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeneman MMJ, Broekhoven MH, Damsteegt R, Vanderschuren LJMJ. Distinct contributions of dopamine in the dorsolateral striatum and nucleus accumbens shell to the reinforcing properties of cocaine. Neuropsychopharmacology. 2012;37(2):487–498. doi: 10.1038/npp.2011.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Ettenberg A. Intracerebroventricular ethanol-induced conditioned place preferences are prevented by fluphenazine infusions into the nucleus accumbens of rats. Behav Neurosci. 2007;121(2):401–410. doi: 10.1037/0735-7044.121.2.401. [DOI] [PubMed] [Google Scholar]
- Weitemier AZ, Murphy NP. Accumbal dopamine and serotonin activity throughout acquisition and expression of place conditioning: correlative relationships with preference and aversion. Eur J Neurosci. 2009;29(5):1015–1026. doi: 10.1111/j.1460-9568.2009.06652.x. [DOI] [PubMed] [Google Scholar]
- Willuhn I, Wanat MJ, Clark JJ, Phillips PE. Dopamine signaling in the nucleus accumbens of animals self-administering drugs of abuse. Curr Top Behav Neurosci. 2010;3:29–71. doi: 10.1007/7854_2009_27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young EA, Dreumont SE, Cunningham CL. Role of nucleus accumbens dopamine receptor subtypes in the learning and expression of alcohol-seeking behavior. Neurobiol Learn Mem. 2014;108:28–37. doi: 10.1016/j.nlm.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]