Abstract
Oxidative stress figures prominently in retinal diseases including diabetic retinopathy and glaucoma. Ligands for σ1R, a unique transmembrane protein localized to the ER, mitochondria, nuclear and plasma membrane, have profound retinal neuroprotective properties in vitro and in vivo. Studies to determine the mechanism of σ1R-mediated retinal neuroprotection have focused mainly on neurons. Little is known about effects of σ1R on Müller cell function, yet these radial glial cells are essential for homeostatic support of the retina. Here we investigated whether σ1R mediates the oxidative stress response of Müller cells using wildtype (WT) and σ1R knockout (σ1RKO) mice. We observed increased endogenous ROS levels in σ1RKO Müller cells compared to WT, which was accompanied by decreased expression of Sod1, Catalase, Nqo1, Hmox1, Gstm6 and Gpx1. The protein levels of SOD1, CAT, NQO1 and GPX1 were also significantly decreased. The genes encoding these antioxidants contain an antioxidant response element (ARE), which under stress is activated by NRF2, a transcription factor that typically resides in the cytoplasm bound by KEAP1. In the σ1RKO Müller cells Nrf2 expression was decreased significantly at the gene (and protein) level, while Keap1 gene (and protein) levels were markedly increased. NRF2-ARE binding affinity was decreased markedly in σ1RKO Müller cells. We investigated system xc−, the cystine-glutamate exchanger important for synthesis of GSH, and observed decreased function in σ1RKO Müller cells compared to WT as well as decreased GSH and GSH/GSSG ratios. This was accompanied by decreased gene and protein levels of xCT, the unique component of system xc−. We conclude that Müller glial cells lacking σ1R manifest elevated ROS, perturbation of antioxidant balance, suppression of NRF2 signaling and impaired function of system xc−. The data suggest that the oxidative stress-mediating function of retinal Müller glial cells may be compromised in the absence of σ1R. The neuroprotective role of σ1R may be linked directly to the oxidative stress-mediating properties of supportive glial cells.
Keywords: retina, retinal Müller glial cells, sigma 1 receptor (σ1R), system xc−, xCT, Nrf2, mouse
Graphical abstract
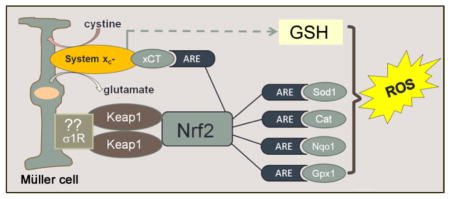
Schematic of paper: Oxidative stress in the form of reactive oxygen species (ROS) figures prominently in retinal diseases. The Müller glial cell is a major mediator of retinal homeostasis. In this paper, Müller cells harvested from mice lacking σ1R, a putative molecular chaperone, showed an increase endogenous production of ROS. This was accompanied by decreased expression of a number of antioxidant proteins, which are known to harbor antioxidant response elements (ARE). Nrf2, which is a major regulator of oxidative stress through its activation of AREs was decreased at the gene, protein and activity level; its major regulatory protein Keap1 was increased. The expression and activity of the cystine-glutamate exchanger was also decreased in Müller cells lacking σ1R. Taken collectively, the data support a key role for σ1R in modulation of oxidative stress in retina.
Introduction
Oxidative stress is implicated in a number of devastating neurodegenerative diseases including those of the retina such as age-related macular degeneration, glaucoma, diabetic retinopathy and retinitis pigmentosa. While the underlying causes of these retinopathies vary, oxidative stress, activation of pro-apoptotic pathways and increased ER stress are features common to all of these diseases [1,2]. Sigma-1 receptor (σ1R) has emerged as a promising target for treatment of neurodegenerative diseases owing to its role in cellular survival [3]. σ1R is a unique 223-amino acid integral membrane protein that spans the membrane twice and also has a third membrane flanking domain [4]. It has been cloned in guinea pig, mouse and human [5–7]. σ1R shares no homology with any other mammalian receptor systems. The hallucinogen N,N-dimethyltryptamine was reported as an endogenous agonist for σ1R [8], but no other natural ligands have been identified.
σ1R appears to play key roles in a number of critical cellular functions. For example, ligands for σ1R can prevent apoptotic cell death in brain-derived neuronal cultures [9–11] as well as in retinal neurons in vitro and in vivo [12–15]. It has been postulated that σ1R functions as a ligand-operated chaperone in complex with the master endoplasmic reticulum (ER) regulatory protein, BiP [16]. ER stress is inexorably linked to oxidative stress. As Malhotra and Kaufman [17] review, the ER is a “protein-folding machine” composed of chaperone proteins, proteins that catalyze protein folding, and sensors that detect the presence of misfolded or unfolded proteins. The ER provides a unique oxidizing folding-environment that favors the formation of the disulfide bonds; protein folding and generation of reactive oxygen species (ROS) as a byproduct of protein oxidation in the ER are closely linked. σ1R can stabilize the inositol 1,4,5-triphosphate type 3 receptor and its ligands modulate ER stress in a variety of cellular systems including retina [16, 18–25]. Of particular interest for the current study are reports that ligands for σ1R can suppress production of reactive oxygen species (ROS) in many tissue types including lung, liver [26], cultured lens cells [18], retinal pigment epithelial cells [27] and retinal neurons [25].
The mechanism by which σ1R modulates oxidative stress was investigated recently in lung and liver using mice that lack σ1R (σ1R knockout (KO) mice) [26]. Metabolomics studies showed an increase in oxidative stress markers (including oxidized glutathione (GSSG) and glutamate) in lung and liver of σ1RKO mice compared to wild type (WT) mice. In that same study, COS-7 cells, which were transfected with σ1R, demonstrated an activation of antioxidant response elements (ARE) in the presence of ligands for σ1R. Sigma1r-transfected cells upregulated genes encoding two important antioxidant proteins, NAD(P)H quinone oxidoreductase (NQO1) and superoxide dismutase (SOD-1) when they were treated with σ1R ligands. These findings are relevant to retinal disease because ligand binding activity to σ1R increases in retina, specifically in retinal Müller glial cells, following exposure to donors of nitric oxide and ROS [28].
The current study examined the role of σ1R in modulating oxidative stress in retinal Müller cells, the major glial cell of the retina. Müller cells are radial macroglial cells offering stability to the complex retinal architecture and providing support for the function and metabolism of retinal neurons and blood vessels [29]. Müller cells play a key role in normal retinal function and become activated in response to pathological stimuli. They hypertrophy and proliferate under pathologic conditions leading to formation of glial scars, which fill the spaces left by dying neurons and dysfunctional synapses. Understanding the role of σ1R in these sustentacular cells may facilitate development of novel neuroprotective approaches for oxidative stress-induced retinal disease. In this study we report elevated endogenous ROS levels and reduced glutathione (GSH) levels in Müller cells harvested from σ1RKO mice compared to WT. We also observed decreased expression of several antioxidant genes leading to investigation of the nuclear factor erythoid-2 related factor (NRF2)-Kelch like-ECH-associated protein 1 (KEAP1) pathway, which was markedly perturbed in glial cells lacking σ1R. We investigated also the activity of the cystine-glutamate exchanger, system xc−, because of its role in synthesis of GSH [30,31] and found significantly reduced activity in σ1RKO cells. Our data provide strong evidence that σ1R regulates oxidative stress through modulation of the NRF2-KEAP1 pathway as well as system xc−.
The findings of the present study are not only important with respect to blinding retinal diseases, which exact a devastating toll on quality of life, but also to cancer, cardiovascular diseases, and neurodegenerative diseases because oxidative stress is overwhelmingly implicated in their pathogenesis [32]. While the current studies focused on a specific cell type within retina, the findings have far-reaching implications with respect to σ1R function and support the notion that σ1R is a modulator of oxidative stress in multiple tissue types including liver, brain, lung [16, 23, 26] and retina [25]. Our findings that absence of σ1R may be linked to alterations in expression of the Nrf2-Keap1 pathway are novel and highly significant. The expression and activity of Nrf2 are decreased significantly in retinal Müller glial cells lacking σ1R. Nrf2 is arguably the most important regulator of the expression of antioxidant molecules [33], thus this first report that lack of σ1R is linked to decreased Nrf2 levels could provide important clues to the mechanism by which σ1R acts as a mediator of oxidative stress as well as to the mechanism by which some of its ligands mediate cellular protection.
Materials and methods
Isolation of primary mouse Müller cells
Wildtype (σ1R+/+, WT) and σ1R knockout (σ1R−/−, σ1RKO) mice were used for isolation of Müller cells. The σR1−/− mice were generated by gene trapping (Oprs1Gt(IRESBetageo)33Lex/Oprs1Gt(IRESBetageo)33Lex) conducted at Lexicon Genetics Corp. (The Woodlands, TX; http://www.informatics.jax.org/searches/accession_report.cgi?id=MGI:3529055). Heterozygote Oprs1 mutant (+/−) Oprs1Gt(IRESBetageo)33Lex embryos on a C57BL/6J × 129S/SvEv mixed background were obtained from the Mutant Mouse Resource Regional Center and implanted into female C57BL/6J mice (Jackson Laboratories, Bar Harbor, ME) at The Scripps Research Institute as reported [34]. Founder heterozygous mice were transferred to the animal facility at Georgia Regents University, and colonies of wild-type (σR1+/+), heterozygous (σR1+/−), and homozygous (σR1−/−) mice were established. Genotyping of mice followed our published protocol [35]. Maintenance of animals adhered to institutional guidelines for the humane treatment of animals and to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research (http://www.arvo.org/About_ARVO/Policies/Statement_for_the_Use_of_Animals_in_Ophthalmic_and_Visual_Research). Müller cells were isolated from 5~7-day-old pups and cultured per our method [36] based upon the original report by Hicks and Cortois [37]. Briefly, eyeballs were removed, placed in serum-free Dulbecco’s modified Eagle medium (DMEM) with penicillin/streptomycin and soaked overnight at 25 °C in the dark. They were rinsed in phosphate-buffered saline (PBS) and incubated in buffer containing trypsin, EDTA and collagenase. Retinas were removed from eyeballs, placed in DMEM supplemented with glucose, fetal bovine serum and penicillin/streptomycin and gently pipetted into small aggregates. Cultures were washed vigorously with medium until only a strongly adherent, flat, cell population remained. Cells were used at passage 4–5. The purity of cultures has been verified in earlier studies [28, 36] in which antibodies that are known markers of Müller cells were used: cellular retinaldehyde binding protein (CRALBP) (Laboratory of Dr. J Saari, U. Washington), vimentin (Millipore, Billerica, MA, Cat. No: AB-1620), glutamine synthetase (Santa Cruz Corp., Santa Cruz, CA, Cat. No. SC- C20), glutamate-aspartate transporter ((GLAST), Alpha Diagnostics, San Antonio, TX, Cat. No: 11-S)). Earlier studies used immunocytochemical analysis of markers for neurons (NF-L (neurofilament-light), SC-H70), a major component of neuronal cytoskeleton), retinal pigment epithelial protein 65 (RPE-65, gift from Dr. M. Redmond, NEI, NIH) and microglial cells (ionized calcium binding adaptor molecule 1 (IBA1, #019-19741, Wako Chemicals, Richmond, VA)) and showed minimal detection in cultures [28, 36].
Immunocytochemical confirmation of σR1 in retinal Müller cells
Müller cells from WT and σ1RKO mice were seeded on coverslips, grown for 24 h, fixed with ice-cold 4% paraformaldehyde (PFA, Electron Microscopy Science, Hatfield, PA), washed with PBS-Triton X-100, incubated with Power Block, (BioGenex, Fremont, CA) and then incubated overnight at 4°C with rabbit polyclonal σR1 (1:1000) [38] or with goat polyclonal anti-vimentin (1:200) antibody. Cells were washed thrice with PBS-Triton X-100 followed by incubation with secondary antibodies (goat anti-rabbit IgG coupled to Alexa Fluor 568 and donkey anti-goat IgG coupled to Alexa Fluor 488 (1:1000) for 1 h at 37 °C. Cells were washed with PBS-Triton X-100 and coverslipped with Fluoroshield with DAPI (Sigma-Aldrich, St. Louis, MO) to label nuclei. Negative controls were treated identically except that PBS replaced the primary antibodies. Immunofluorescent signals were visualized using an Axioplan-2 fluorescent microscope (Carl Zeiss, Göttingen, Germany) equipped with an HRM camera. Images were captured and processed using Zeiss Axiovision digital image processing software (version 4.7).
ROS detection
Müller cells harvested from WT and σ1RKO mice were seeded on coverslips. They were exposed to media containing hydrogen peroxide (H2O2, 200μm) for 6 h. In companion studies, cells were pre-treated 30 min with (+)-pentazocine ((+)-PTZ, 10μM, Sigma-Aldrich, St. Louis, MO, Cat. No: P-127), a high affinity σ1R ligand, alone or were exposed to H2O2 plus (+)-PTZ for 6 h. Control experiments were conducted in parallel in which H2O2 and (+)-PTZ were omitted from the media. At the end of the 6 h, cells were rinsed with PBS so that exogenous H2O2 was removed. Intracellular ROS was detected in cells using 5 μM CellROX Green Reagent (Molecular Probes, Life Technologies, NY, NY; 30 min incubation, followed by fixation). CellROX detects hydroxyl, peroxyl, peroxynitrite and hydroxyl radicals. DAPI was used to stain nuclei. Green fluorescent signals representing ROS were visualized using an Axioplan-2 fluorescent microscope (described above). Fluorescence intensity was quantified using Image J 1.48v software (National Institutes of Health, Bethesda, MD, USA).
To quantify endogenous ROS in WT and σ1RKO Müller cells, cells were seeded in a black-sided, clear-bottomed 24-well plate (5×104 cells per well) overnight. Cell culture media was removed by aspiration. Certain wells of WT and σ1RKO Müller cells were used for protein determination, while the remaining wells were rinsed twice with warm (37°C) HBSS, were then incubated with 20 μM 6-carboxy-2′,7′-dichlorodihydrofluorescein diacetate (carboxy-H2DCFDA) (C-400, Molecular Probes) in HBSS (1h, 37°C) in the dark according to the method of Tetz [39]. This probe measures formation of ROS such as peroxynitrite, hydroxide radicals, and molecules that include peroxyl, alkoxyl, carbonate (CO3·−) and NO2· groups. Fluorescence readings of carboxy-DCFH-DA (495 nm excitation and 527 nm emission) of WT and σ1RKO Müller cells were taken at 1 h intervals for 6 h at 37°C using a Synergy H1 Hybrid multi-mode microplate reader (BioTek, Winooski, VT, USA). The fluorescence of carboxy-H2DCFDA without any samples was taken as the blank. Results were shown as fluorescence (arbitrary units) per μg cell lysate in σ1RKO mice compared to WT Müller cells.
Quantitative real-time RT-PCR
Expression levels of mRNA transcripts specific for several key genes (Sod1, Cat, Nqo1, Hmox1, Gstm3, Gstm6, Gstt3, Gpx1, Gpx2, Gpx3, Nrf2, Keap1 and xCT) involved in the antioxidant response, the NRF2 pathway and system xc- were examined in WT and σ1RKO Müller cells. Total RNA was purified using Trizol (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol and quantified. RNA (2 μg) was reverse-transcribed using the iScript Synthesis kit (BioRad Laboratories, Hercules, Calif., USA). cDNAs were amplified for 40 cycles by using SsoAdvanced™ SYBR Green Supermix (BioRad Laboratories) and gene-specific primers (Table 1) in a CFX96 Touch™ Real-Time PCR Detection System (BioRad Laboratories). Expression levels were calculated by comparison of Ct values (delta-delta Ct) [25].
Table 1.
Sequences of primers used for real-time quantitative reverse transcription plus the polymerase chain reaction
| Gene | NCBI Accession Number | Primer Sequence | Product size (bp) | |
|---|---|---|---|---|
| Sod1 | NM_011434 | Forward Reverse |
5′-AACCAGTTGTGTTGTCAGGAC-3′ 5′-CCACCATGTTTCTTAGAGTGAGG-3′ |
139 |
| Catalase | NM_009804 | Forward Reverse |
5′-AGCGACCAGATGAAGCAGTG-3′ 5′-TCCGCTCTCTGTCAAAGTGTG-3′ |
181 |
| Nqo1 | NM_008706 | Forward Reverse |
5′-AGGATGGGAGGTACTCGAATC-3′ 5′-AGGCGTCCTTCCTTATATGCTA-3′ |
144 |
| Hmox1 | NM_010442 | Forward Reverse |
5′-AAGCCGAGAATGCTGAGTTCA-3′ 5′-GCCGTGTAGATATGGTACAAGGA-3′ |
100 |
| Gstm3 | NM_010359 | Forward Reverse |
5′-CCCCAACTTTTACCTAAGC-3′ 5′-GGTGTCCATAACTTGGTTCTCCA-3′ |
208 |
| Gstm6 | NM_008184 | Forward Reverse |
5′-ACAGTTCATGTACACTCGAAT-3′ 5′-TGGCTTCCGTTTCTCAAAGTC-3′ |
70 |
| Gstt3 | NM_133994 | Forward Reverse |
5′-GGATGGGGACTTCGTCTTGG-3′ 5′-TCAGGAGGTACGGGCTGTC-3′ |
219 |
| Nrf2 | NM_010902 | Forward Reverse |
5′-TAGATGACCATGAGTCGCTTGC-3′ 5′-GCCAAACTTGCTCCATGTCC-3′ |
153 |
| Keap1 | NM_016679 | Forward Reverse |
5′-TGCCCCTGTGGTCAAAGTG-3′ 5′-GGTTCGGTTACCGTCCTGC-3′ |
104 |
| xCT | NM_011990 | Forward Reverse |
5′-GGCACCGTCATCGGATCAG-3′ 5′-CTCCACAGGCAGACCAGAAAA-3′ |
100 |
| Gpx1 | NM_008160 | Forward Reverse |
5′-AGTCCACCGTGTATGCCTTCT-3′ 5′-GAGACGCGACATTCTCAATGA-3′ |
105 |
| Gpx2 | NM_030677 | Forward Reverse |
5′-GCCTCAAGTATGTCCGACCTG-3′ 5′-GGAGAACGGGTCATCATAAGGG-3′ |
143 |
| Gpx3 | NM_008161 | Forward Reverse |
5′-CCTTTTAATCAGTATGCAGGCA-3′ 5′-CAAGCCAAATGGCCCAAGTT-3′ |
120 |
| GAPDH | NM_008084 | Forward Reverse |
5′-AGGTCGGTGTGAACGGATTTG-3′ 5′-TGTAGACCATGTAGTTGAGGTCA-3′ |
123 |
Western blot analysis of antioxidant proteins
Protein was extracted from Müller cells and neural retina isolated from WT and σ1RKO mice per our method [20]. Proteins were subjected to SDS-polyacrylamide gel electrophoresis. Immunoblotting was performed to assess the levels of the following proteins: σ1R, SOD1, Catalase, NQO1, HMOX-1, GPX1, GPX2, NRF2, KEAP1, xCT and GAPDH. Nitrocellulose membranes to which the proteins had been transferred were incubated with primary antibodies at the concentrations listed in Table 2. They were incubated with horseradish-peroxidase-conjugated goat anti-rabbit or goat anti-mouse IgG antibody (Santa Cruz Corp., Santa Cruz, CA, USA). Proteins were visualized by using the SuperSignalWest Pico Chemiluminescent Substrate detection system (Pierce Biotechnology, Rockford, Ill., USA). All western blotting images are representative of three or more independent experiments. The bands from western blotting were quantified using the Image J 1.48v software.
Table 2.
List of antibodies used in this study.
| Primary Antibody | Supplier | Dilution |
|---|---|---|
| Vimentin (AB1620) | Millipore, CA | (1:200) |
| SOD1 (sc-11407) | Santa Cruz Biotechnology, CA | (1:1000) |
| Catalase (sc-50508) | Santa Cruz Biotechnology, CA | (1:2000) |
| NQO1 (ab34173) | Abcam, Cambridge, MA | (1:1000) |
| NRF2 (ab31163) | Abcam, Cambridge, MA | (1:500) |
| KEAP1 (sc-15246) | Santa Cruz Biotechnology, CA | (1:800) |
| HMOX1(AF3776) | R&D Systems, Inc, MN | (1:200) |
| GPX1(AF3798) | R&D Systems, Inc, MN | (1:100) |
| GPX2 (MAB5470) | R&D Systems, Inc, MN | (1:200) |
| GAPDH (MAB374) | EMD Millipore, CA | (1:3000) |
| Secondary Antibody | Supplier | Dilution |
|---|---|---|
| HRP-conjugated anti-rabbit IgG (sc-2004) | Santa Cruz Biotechnology, CA | (1:2000) |
| HRP-conjugated anti-mouse IgG (sc-2005) | Santa Cruz Biotechnology, CA | (1:2000) |
| HRP-conjugated anti-goat IgG (sc-2020) | Santa Cruz Biotechnology, CA | (1:2000) |
| Alexa Fluo 546 anti-rabbit IgG (H+L) | Invitrogen Molecular Probes, NY | (1:1000) |
| Alexa Fluo 488 anti-rabbit IgG (H+L) | Invitrogen Molecular Probes, NY | (1:1000) |
| Alexa Fluo 488 anti-goat IgG (H+L) | Invitrogen Molecular Probes, NY | (1:1000) |
Immunofluorescent detection of NRF2 and KEAP1
Müller cells harvested WT and σ1R KO mice were seeded on coverslips, grown for 24 h, fixed with ice-cold 4% PFA, washed with PBS-Triton X-100, incubated with Power Block and incubated overnight at 4°C with antibodies against NRF2 and KEAP1 (vendors and concentrations provided in Table 2). The cells were washed three times with PBS-Triton X-100 followed by incubation with secondary antibodies (goat anti-rabbit IgG coupled to Alexa Fluor 568 and goat anti-rabbit IgG coupled to Alexa Fluor 488 (1:1000)) for 1 h at 37 °C. Cells were washed with PBS-Triton X-100 three times and coverslipped with Fluoroshield with DAPI (Sigma-Aldrich) to label nuclei. Negative controls were treated identically except that PBS replaced the primary antibodies. Microscopic detection was performed using the Axioplan-2 fluorescent microscope as described above.
Analysis of NRF2 binding activity with ARE by trans-activation assay
NRF2 activation and antioxidant response element (ARE) binding efficacy in WT and σ1RKO Müller cells were evaluated in nuclear extracts using a TransAM® NRF2 Kit (50296, Active Motid, Carlsbad, CA). 5, 10, 20 and 40-μg aliquot of nuclear protein were incubated with immobilized oligonucleotides containing the ARE consensus binding site (5′-GTC ACA GTA CTC AGC AGA ATC TG-3′), separately. The active form of NRF2 that bound to the oligonucleotides was detected using anti-NRF2 primary antibody followed by incubation with HRP-conjugated secondary antibody. The activity of NRF2 in the nuclear extracts, demonstrated by DNA binding activity of NRF2, was measured by colorimetric detection using a VersaMax microplate reader (Molecular Devices, Sunnyvale CA) at 450 nm; absorbance values reflected the activity of NRF2.
Functional assay to determine transport activity and kinetics of system xc−
Uptake of glutamate by the Na+-independent system xc− was performed in cultured Müller cells using an uptake buffer containing: 25 mM 4-(2-hydroxyethyl)-1-piperazineethansulfonic acid (HEPES)/Tris, 140 mM N-methyl-D-glucamine chloride, 5.4 mM KCl, 1.8 mM CaCl2, 0.8 mM MgSO4, and 5 mM glucose, pH 7.5. System xc− is a freely reversible exchanger for cystine and glutamate; its transport function can be studied by monitoring the influx of radiolabeled cystine in exchange for the efflux of cellular glutamate or by monitoring either the influx or efflux of radiolabeled glutamate (since system xc− can also function as a Na+-independent glutamate/glutamate exchanger). (Note that once inside the cell, cystine is converted to cysteine, which is not transported by system xc−.) The intracellular level of cystine is necessarily low and hence would not be an easily measured substrate for the transporter. The most accurate method to study the transporter function is to measure influx of [3H]-labeled substrate, rather than the highly unstable [35S]-labeled cystine. Even though Na+-independent glutamate uptake can occur via AGT1 (alpha-glucoside transporter), there is no evidence for expression of this transporter in the retina [40], thus Na+-independent uptake of glutamate in Müller cells represents the function of system xc− exclusively [41]. L-[G-3H]-glutamic acid (specific radioactivity 27.0 Ci/mmol, concentration 1.0 mCi/ml, Moravek Biochemicals and Radiochemicals, Brea, CA) was used as the substrate for uptake experiments. Uptake was initiated by adding 250 μl of uptake buffer containing 2.5 μM glutamate spiked with 4.0 μCi/ml of radiolabeled [3H]-glutamate. Müller cells were incubated for 15 min at 37°C, after which, buffer was removed and cells were washed twice with ice-cold uptake buffer (to stop uptake). The cells were solubilized with 0.5 ml of 1% sodium dodecyl sulfate-0.2N NaOH (SDS/NaOH) and radioactivity was determined by liquid scintillation spectrometry (Beckman LS 6500 scintillation counter, Beckman Instruments, Brea CA). Protein was measured using the Bio-Rad protein assay reagent. Uptake by system xc− was calculated directly from the radioactivity (cpm) data and expressed as pmol/mg protein/15 min.
Kinetic analysis of system xc− was performed in Müller cells harvested from WT and σ1RKO mice and activity was assessed using increasing amounts of cold glutamate as a competitor ranging from 2.5 μM to 1000 μM. SigmaPlot 2002 for Windows XP Professional (SPSS Inc., Chicago, IL) was used to calculate Km and Vmax from an Eadie-Hofstee plot of V, uptake velocity (pmol/mg protein/15 min) versus V/S where S is cold glutamate concentration (μM). The formula underlying the Eadie-Hofstee plot is v = Km v/[S] + Vmax where v represents reaction velocity, Km is the Michaelis-Menten constant, [S] is the substrate concentration, and Vmax is the maximum reaction velocity. Experiments were repeated three times; results are expressed as the mean ± SE.
Assessment of intracellular GSH levels
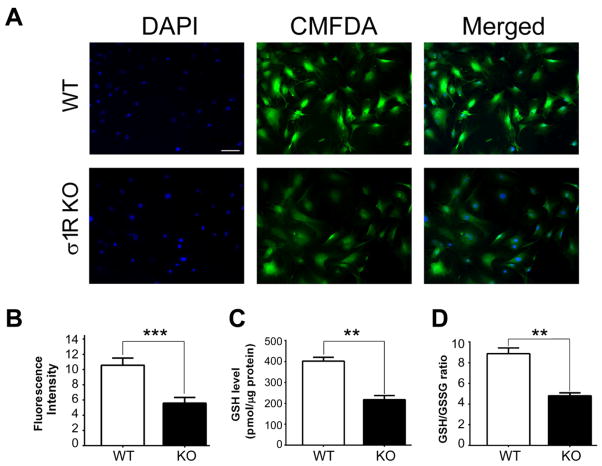
To estimate levels of GSH in Müller cells harvested from WT and σ1RKO mice, CellTracker™ Green CMFDA dye (5-Chloromethylfluorescein Diacetate) (Molecular Probes®, Life technologies, NY, USA) was used. Cells were incubated with 1 μM CMFDA at 37°C for 40 min, incubated with fresh pre-warmed medium for 30 min followed by fixation with ice-cold 4% PFA. Nuclei were stained with DAPI. Fluorescence detection was performed using the Axioplan-2 fluorescent microscope as described above. Fluorescence intensity was quantified using Image J 1.48v software. To verify these findings directly, cellular GSH levels per protein and glutathione redox state (GSH/GSSG) were determined using the Glutathione (GSSG/GSH) Detection Kit (ADI-900–160, Enzo Life Sciences, Farmingdale, NY) according to the manufacturer’s instructions. WT and σ1RKO Müller cells were harvested and a small aliquot of the cell suspension was used for protein determination; the remaining samples were treated with 5% (w/v) MPA (Metaphosphoric acid, Cat. No.239275, Sigma Chem. Corp.) to precipitate proteins, which interfere with the assay. A known volume of the MPA extract was treated without (for total GSH) or with 4-vinylpyridine (only for GSSG analysis), and appropriate GSSG standards were treated similarly to prepare a standard curve. After adding appropriate volumes of freshly-prepared reaction mix (glutathione reductase with reaction mix buffer), a kinetic GSH-reductase recycling assay was performed following the manufacturer’s instruction using a VersaMax microplate reader (Molecular Devices, Sunnyvale CA) set at 405nm and read at 1min intervals over a 15 min time period).
Statistical analysis
Data (with the exception of system xc− kinetic analysis) were analyzed by one- or two-way analysis of variance (ANOVA) as appropriate; Tukey HSD was the post-hoc test. Statistical analyses were conducted using the GraphPad Prism analytical program (LaJolla, Calif., USA). A p value <0.05 was considered significant.
Results
Detection of σ1R in retinal Müller glial cells
Immunofluorescent detection for vimentin was positive in Müller cells harvested from WT and σ1RKO mice confirming the glial origin of the cells (Fig. 1A). Immunocytochemical analysis confirmed that σ1R is expressed in WT Müller cells but is not present in Müller cells isolated from σ1RKO mice (Fig. 1A). The labeling observed (primarily in the nuclear membrane and the perinuclear region is consistent with an earlier report that σ1R is present in the nuclear and ER membranes [28]. Western blot analysis of proteins isolated from WT cells using an antibody specific for σ1R yielded a band of the expected size (Mr 25–27 kD) corresponding to σ1R. σ1R was not detected in σ1RKO Müller cells. Equivalent amounts of protein were loaded on the gels as detected by GAPDH (Fig. 1B). The data verify that σ1R is not present in Müller cells of the σ1RKO mouse and thus provide a powerful tool to evaluate the role of σ1R in modulating oxidative stress.
Fig. 1. Analysis of σ1R expression in mouse Müller cells.

(A) Müller cells harvested from WT (wildtype) and σ1RKO (knockout) mice were cultured as described and subjected to immunocytochemistry to confirm the glial origin of the cells using vimentin (green fluorescence) or to detect σ1R (red fluorescence); DAPI was used to label nuclei blue. Calibration bar = 100 μm. (B) Immunoblotting analysis to detect σ1R in WT and σ1RKO Müller cells, the single band detected in WT samples has the expected size Mr 25–27 kD. Membranes were reprobed with antibody against GAPDH as a loading control.
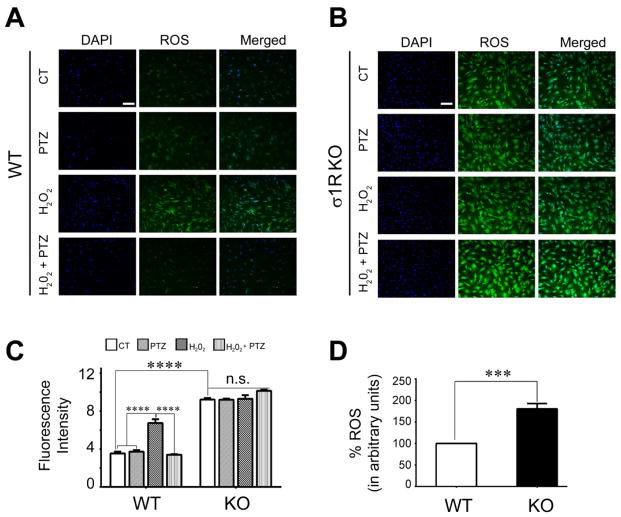
Elevated endogenous ROS in σ1RKO Müller cells compared to WT
In our initial studies of oxidative stress, WT and σ1RKO Müller cells were treated with H2O2, subsequently ROS levels were detected using CellROX reagent followed by immunofluorescence detection. Experiments were conducted using H2O2 in the presence (or absence) of the σ1R ligand (+)-PTZ. In the WT cells, there was very little ROS detected in control (non-treated cells) and (+)-PTZ treated cells, but a significant increase in cells exposed to H2O2 (Fig. 2A). H2O2-exposed cells treated with (+)-PTZ showed a marked decrease in ROS production compared to H2O2–exposed cells that did not receive (+)-PTZ treatment suggesting that activation of σ1R attenuates oxidative stress in Müller cells (Fig. 2A). The same experiments conducted with σ1RKO Müller cells yielded intriguing results. First, as anticipated there was a significant increase in ROS in the H2O2-exposed σ1RKO cells compared to WT control cells. Second, there was no attenuation of ROS in the presence of the σ1R ligand (+)-PTZ, a result predicted because the cells do not express σ1R and previous studies showed that the receptor is required for neuroprotective effects of (+)-PTZ [42,43]. The surprising result was that there was a significantly greater level of ROS detected in σ1RKO cells that had received no treatment (i.e. control, no H202 or (+)-PTZ) compared to control WT cells. That is, using the CellROX® Green Reagent detection assay, we observed significantly higher endogenous levels of ROS in cells lacking σ1R compared to σ1R expressing cells (Fig. 2B). The data from this experiment were quantified as fluorescent intensity. The ROS levels in Müller cells from σR1KO mice were significantly increased (nearly two-fold) compared to WT (Fig. 2C). We confirmed the observation that basal ROS levels in σ1RKO Müller cells are significantly higher than WT by measuring ROS quantitatively (e.g. measuring the oxidative conversion of carboxyl-DCFH-DA to the highly fluorescent carboxyl-DCF). We observed a significant increase in ROS in σ1RKO Müller cells compared to WT (Fig. 2D). These data led us to investigate oxidative stress mediators in σ1RKO Müller cells compared to WT.
Fig. 2. Analysis of ROS levels in WT and σ1RKO mouse Müller cells.
Fluorescent detection of reactive oxygen species (ROS) using CellROX® Green Reagent. Müller cells from WT (wildtype, A) or σR1KO (knockout, B) mice were seeded on coverslips for 18 h. Cells either were or were not (control) exposed for 6 h to 200μM H202 in the presence or absence of (+)-PTZ, including cells treated with (+)-PTZ alone, they were incubated with CellROX® Green Reagent to detect ROS as described. Green fluorescent signals of ROS in WT and σ1RKO cells were visualized by epifluorescence; DAPI was used to label nuclei (blue). Scale bar, 100 μm. (C) Quantification of fluorescent intensity reflecting ROS levels of data shown in panels (A) and (B) in Müller cells. (D) ROS levels were quantified in WT and σ1RKO Müller cells using an assay to detect the oxidative conversion of carboxyl-DCFH-DA to the highly fluorescent carboxyl-DCF (495nm excitation/527nm emission). Data are mean ± S.E.M of triplicate measurements *** p< 0.001; ****p<0.0001. (CT = control, PTZ = (+)-pentazocine treated).
Perturbation of antioxidant balance in σ1RKO Müller glia
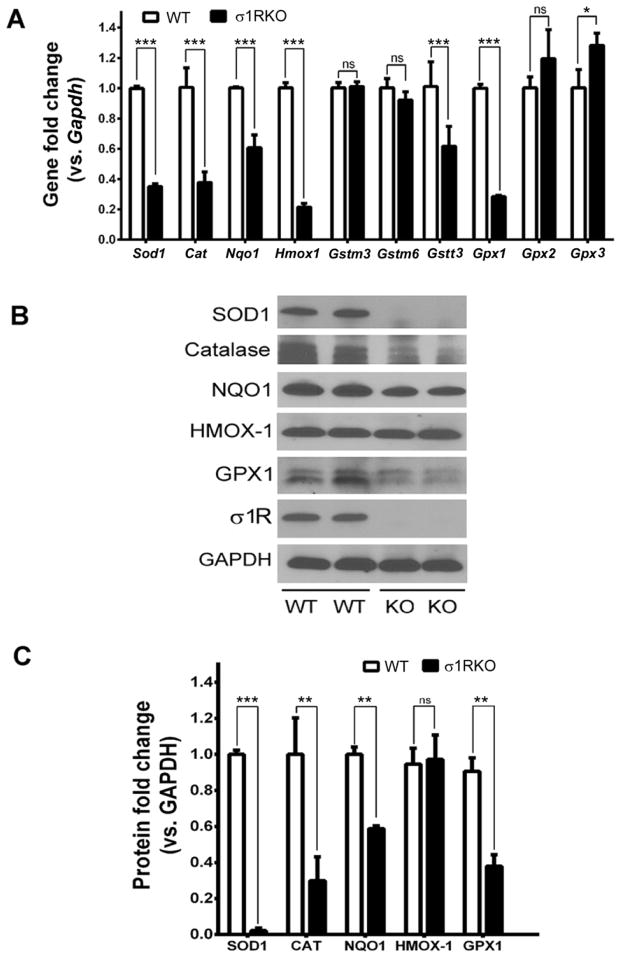
We asked whether σ1R is involved in regulating antioxidant balance by examining the expression of genes encoding antioxidant proteins including SOD1, catalase (CAT), NQO1, hemeoxygenase-1 (HMOX1), glutathione-S-transferases (GST) (including GSTm3, GSTm6, GSTT3), and glutathione peroxidases (GPX1, GPX2, GPX3) using σ1RKO and WT Müller cells. qRT-PCR analysis showed that the expression of several genes was decreased significantly in σ1RKO Müller cells compared to WT (Fig. 3A), i.e. Sod1 (decreased by ~65%), Cat (decreased by ~65%), Nqo1 (decreased by ~40%), Hmox1 (decreased by ~80%), Gstt3 (decreased by ~40%) and Gpx1 (decreased by ~70%) (p<0.001). Gpx3 expression increased slightly. Expression of Gstm3, Gstm6, Gpx2 did not differ between WT and σ1RKO Müller cells. We measured the protein levels of SOD1, CAT and NQO1 and HMOX1, GPX1 and GPX2 in WT and σ1RKO Müller cells and observed a marked decrease in protein levels of SOD1 (decreased by ~ 90%), CAT (decreased by ~70%), NQO1 (decreased by ~40%) and GPX1 (decreased by ~60%) in σ1RKO Müller cells compared to WT (Fig. 3B). Protein levels of HMOX-1 did not differ significantly between WT and σ1RKO Müller cells (Fig. 3B). (It is unclear if this anti-HMOX1 Ab cross reacted to HMOX2, resulting in the discrepancy between HMOX1 mRNA and protein levels.) The quantification of the immunoblotting data (three independent experiments) is shown (Fig. 3C). Detection for GPX2 showed very low protein levels in both WT and σ1RKO Müller cells (e.g. barely detectable by immunoblotting, data not shown).
Fig. 3. Analysis of antioxidant genes and proteins in WT and σ1RKO Müller cells.
(A) Quantitative real-time RT-PCR analysis of Sod1, Cat, Nqo1, Hmox1, Gstm3, Gstm6, Gstt3, Gpx1, Gpx2, Gpx3 mRNAs in WT (wildtype) and σ1RKO (knockout) mouse Müller cells. Error bars represent mean ± S.E.M from four separate experiments (n=4). Representative immunoblots detecting levels of SOD1, CAT, NQO1, HMOX1, GPX1 and σ1R in WT and σ1RKO Müller cells (B) and quantification of band densities (C). Error bars represent mean ± S.E.M from three separate experiments (n=3). *p<0.05, ** p< 0.01, ***p< 0.001.
(.) Otherwise, the discrepancy between mRNA an
Suppression of NRF2 signaling in σ1RKO Müller glia
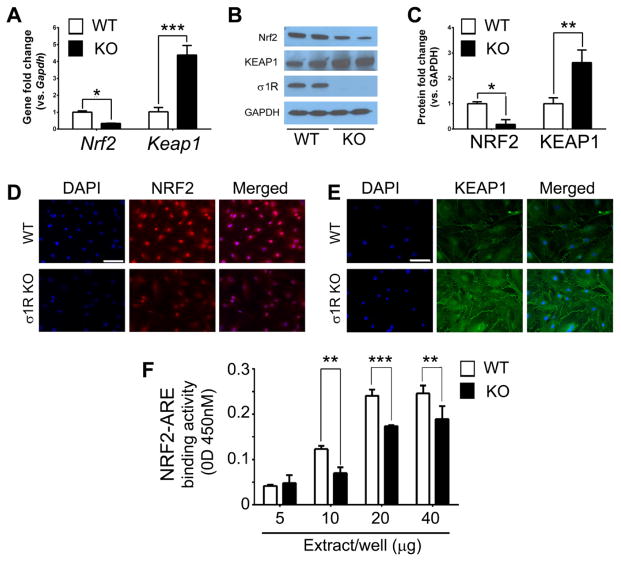
We investigated NRF2 signaling in σ1RKO Müller glia versus WT because NRF2 binds the AREs found in the 5′-flanking region of Sod1, Cat, Nqo1, Hmox1, Gstt3 and Gpx1 [32, 44, 45]. Given the significant decrease in expression of these antioxidant genes in σR1KO Müller cells (Fig. 3), we predicted that NRF2 levels would be altered. We also investigated KEAP1, the well-recognized inhibitor of NRF2, in σ1RKO versus WT Müller cells. There was a significant decrease (~60%) in Nrf2 expression in σ1RKO cells compared to WT (Fig. 4A). Concomitantly there was a marked increase in Keap1 expression (4.38±0.56 fold change in σ1RKO Müller cells versus WT) (Fig. 4A). Western blotting results showed a similar change in NRF2 (decrease) and Keap1 (increase) protein levels (Fig. 4B). Densitometry reflected ~80% decrease in NRF2 levels and ~2.5 fold increase KEAP1 in σ1RKO Müller cells compared to WT (Fig. 4C). We examined NRF2 and KEAP1 using an immunocytochemical approach in Müller cells harvested from σ1RKO and WT mice. We observed a marked decrease in NRF2 in σ1RKO cells compared to WT (Fig. 4D) and conversely a substantial increase in KEAP1 (Fig. 4E) in σ1RKO cells vs. WT. We investigated NRF2 activity using an ARE oligonucleotide-based trans-activation assay in nuclear extracts of σ1RKO and WT Müller cells. There was a significant decrease (~50%) in NRF2 activity in σ1RKO cells compared to WT (p<0.01, p<0.001) (Fig. 4F). The data suggest a role for σ1R in modulating NRF2 transcriptional regulation of AREs of several antioxidant genes.
Fig. 4. Analysis of Nrf2 and Keap1 (gene and protein) in WT and σ1RKO Müller cells.
(A) Quantitative real-time RT-PCR analysis of Nrf2 and Keap1 mRNA extracted from WT (wildtype) and σ1RKO (knockout) mouse Müller cells. The data are mean ± S.E.M from four separate experiments (n=4). (B) Representative immunoblotting to detect NRF2 and KEAP1 following protein extraction from WT and σ1RKO Müller cells. (C) Quantification of immunoblotting data. Error bars represent mean ± S.E.M from three separate experiments (n=3). Immunocytochemistry to detect NRF2 (D) and KEAP1 (E) in WT and σR1KO Müller cells. Scale bar = 100 μm. (F) NRF2/ARE binding activity, assayed as described in the text; the data represent values obtained at 450 nm (mean ± SEM) for three separate experiments. (* p< 0.05, **p< 0.01, ***p<0.001).
Decreased system xc− activity and xCT levels in σ1RKO Müller glia
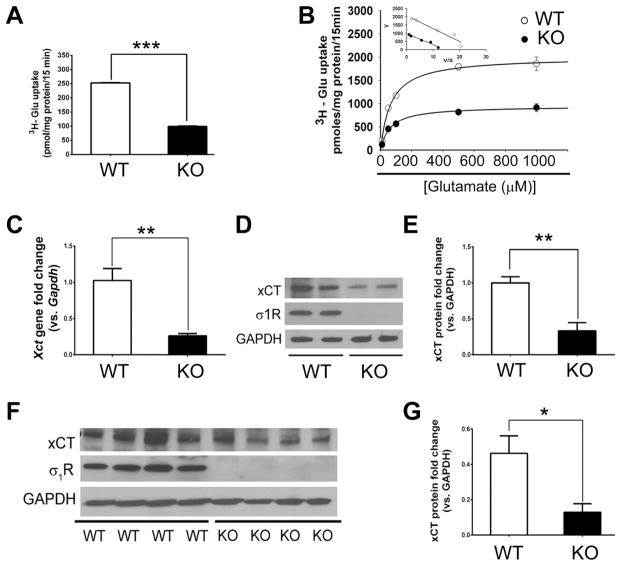
The cystine-glutamate exchanger, system xc− is comprised of two proteins, xCT and 4F2hc (4F2 cell-surface antigen heavy chain, encoded by the Slc3a2 (solute carrier family 3 member 2) gene) [46]. xCT is the unique component of the system; it has an ARE that can be activated by NRF2 [31]. We investigated the activity of the transporter and as well as xCT expression. Since system xc− is a freely reversible exchanger for cystine and glutamate we were able to evaluate its transport function by monitoring the Na+-independent influx of radiolabeled glutamate as described in the methods section. We cultured WT and σ1RKO Müller cells and determined that uptake of radiolabeled glutamate in WT cells was significantly greater than in σ1RKO cells (252.63 ± 2.45 pmol/mg protein/15 min versus 98.86 ± 4.95 pmol/mg protein/15 min) (Fig. 5A). The kinetics of system xc− activity was analyzed in WT and σ1RKO Müller cells. The transport function of system xc− was monitored by measuring the Na+_independent uptake of radiolabeled glutamate in the presence of increasing concentrations of un-labeled glutamate. The decreased transport activity of system xc− observed in σ1RKO Müller cells compared with WT cells was associated with a significant decrease in the maximal velocity (Vmax) of the transporter but no significant difference in the substrate affinity (Km). The maximal velocity of glutamate uptake was ~50% less in σ1RKO Müller cells (955.9 pmol/mg protein/15 min ± 52.1) compared with WT (2062.9 pmol/mg protein/15 min ± 168.8). The Michaelis constant for glutamate in σ1RKO cells (65.0 μM ± 7.2) was similar to WT cells (78.6 μM ± 12.6). Vmax is directly proportional to the concentration of the protein on the membrane. The unique protein component of system xc− is xCT, therefore we examined expression of the gene encoding xCT and the level of the protein in Müller cells from WT and σ1RKO mice. The expression of xCT in σ1RKO Müller cells was markedly reduced (by ~75%) compared to WT (Fig. 5C). There was a significant decrease in the level of xCT protein as well. Fig. 5D shows representative immunoblots detecting xCT in cells isolated from WT versus σ1RKO mice; quantification of band densities is shown (Fig. 5E). We evaluated xCT protein levels in whole retina tissue of WT and σ1R KO mice and observed a decrease in xCT levels in retinas of σ1RKO mice compared to WT (Fig. 5F, 5G). The functional and molecular data suggest that activity and expression of the cystine-glutamate exchanger may be regulated by σ1R.
Fig. 5. Inhibition of xCT uptake and expression level in σ1RKO Müller cells and neural retina.
(A) WT (wildtype) and σ1RKO (knockout) mouse Müller cells were cultured as described and uptake of [3H] glutamate by xCT was assessed as described. (B) Kinetic analysis of system xc− in WT and σ1RKO Müller cells; the uptake of [3H]-glutamate by the Na+-independent system xc− was assessed using increasing amounts of non-radioactive glutamate ranging from 10.0 μM to 1000 μM. The rate of glutamate uptake (pmole/mg/15 min) is shown as a function of glutamate concentration (μM). The inset is an Eadie-Hofstee plot of V, uptake velocity (pmole/mg/15 min) versus V/S where S is non-radioactive glutamate concentration (μM). Values presented are means ± SE from 3 independent experiments performed in duplicate. (C) qRT-PCR analysis of Xct mRNA isolated from WT and σ1RKO Müller cells. Error bars represents mean ± S.E.M from three separate experiments. Immunoblotting (D) and quantification (E) indicated a significant decrease in xCT in σ1RKO Müller cells. Error bars represents mean ± S.E.M from four separate experiments (n=4). Immunoblotting (F) and quantification (G) demonstrated a marked decrease of xCT in whole retinal tissue isolated from σR1KO mice compared with WT. Error bars represents mean ± S.E.M from four separate experiments (n=4). * p< 0.05; ** p< 0.01, *** p<0.001.
Decreased GSH levels in σ1RKO Müller glia
The decreased activity of system xc−, which is important for synthesis of GSH prompted investigation of the levels of this key antioxidant in WT versus σ1RKO Müller cells. We detected GSH indirectly using CMFDA, a cell-permeant chloromethyl derivative of fluorescein diacetate. Immunofluorescent imaging showed a significant decrease in cellular levels of GSH in σ1RKO Müller cells from WT mice (Fig. 6A). Quantification of fluorescent intensity data are provided in Fig. 6B. We then determined intracellular GSH levels and the GSH/GSSG ratio directly using a biochemical assay and observed a dramatic depletion of GSH (WT: 401.7±31.9; σ1RKO: 217.7±33.2, p<0.01) (Fig. 6C), and decreased GSH/GSSG ratio (8.9:1 vs 4.8:1; WT vs σ1RKO, p<0.01) (Fig. 6D) were observed in σ1RKO Müller cells when compared to WT. The results of these indirect and direct assessments suggest that there is a marked decrease in the levels of GSH in Müller cells lacking σ1R.
Fig. 6. Detection of GSH levels in WT and σ1RKO Müller cells.
(A) WT (wildtype) and σ1RKO (knockout) mouse Müller cells were cultured as described, cellular GSH levels were assessed using fluorescence detection of CMFDA (1 μM). Scale bar = 100 μm. (B) Quantification of fluorescent intensity. (p<0.001). Cellular GSH levels and GSH/GSSG were determined as described in the text. (C) GSH levels (pmol/μg protein) detection in WT and σ1RKO Müller cells (p<0.01). (D) Glutathione redox ratio (GSH/GSSG) determined in WT and σ1RKO Müller cells (p<0.01). Each experiment was performed in triplicate.
Discussion
This study provides the first evidence that in the absence of σ1R, NRF2 and KEAP1 gene and protein levels are significantly altered. The data are important because they be relevant to the remarkable neuroprotective properties observed when some ligands for the receptor are used therapeutically in models of retinopathies [10–15, 25, 27]. It is acknowledged that the data presented herein were obtained from isolated cells, which may become activated simply by being maintained under cell culture conditions. Other studies report that the level of GFAP, a marker of Müller cell activation, is very modest in cultures of Müller cells used at passage 4–5, as used in the current study [47]. Thus, useful conclusions can be made from the data that are relevant to retinal disease.
Several important findings emerge from this study. First, there was significant elevation of endogenous ROS in Müller cells isolated from σ1RKO mice. Our study was initially undertaken to compare the effects of (+)-PTZ in attenuating oxidative stress in retinal Müller glial cells. We observed a decrease in ROS when WT cells were pretreated with this high affinity σ1R ligand. We used Müller cells isolated from σ1RKO mice in this initial experiment to test the specificity of (+)-PTZ for σ1R in conferring its protective effects. As predicted, we found that (+)-PTZ treatment of σ1RKO Müller cells had no effect in attenuating ROS production in H2O2-exposed cells. This confirmed earlier reports that σ1R was required for (+)-PTZ to mediate anti-apoptotic effects in retinal ganglion cells [42,43]. What was not expected from these experiments, however, was that endogenous ROS would be elevated in Müller cells that were harvested from mice lacking σ1R. This suggested that absence of σ1R might be linked with an inherent increased level of oxidative stress in these retinal glial cells.
These intriguing findings led to a systematic evaluation of the oxidative stress response in σ1RKO Müller glial cells compared to WT. We investigated the expression of genes encoding a number of well-characterized antioxidant proteins and observed significantly decreased expression of Sod1, Cat, Nqo1, Hmox1, Gstt3, and Gpx1 mRNA. Several of the proteins encoded by these genes (SOD1, CAT, NQO1, GPX1) were also decreased significantly. Regarding expression of Gpx2 and Gpx3, these are both members of the glutathione peroxidase (GPx) family of enzymes, which catalyze the reduction of hydrogen peroxide to water (or alcohol) typically using GSH as the reductant. In our previous microarray analysis of the σ1RKO neural retina [20] we observed a significant increase in Gpx3 in retinas of σ1RKO mice (2.34 fold). GPx3 is an intracellular enzyme actively released into the plasma so the significance of its elevation in the σ1RKO neural retina is not clear, nor its elevation in the present study of Müller cells. Most studies of glutathione peroxidase in retina have focused on GPx1 [48,49], therefore in our studies we investigated its expression in σ1RKO Müller cells and found a significant decrease at the gene and protein level. In our investigation of Gpx2 gene expression; we observed no significant difference between σ1RKO Müller cells versus WT. Indeed, the level of the GPX2 protein was minimally detected in the Müller cells. GPx2 is mainly expressed in the epithelium lining the gastrointestinal tract [50], thus its expression in retinal Müller cells may be quite low. Also observed in the earlier microarray screening of σ1RKO neural retina [20] was a slight increase in expression of two glutathione-s-transferase genes Gstm6 (1.47 fold, p= 0.049) and Gstm3 (1.45 fold, p=0.055). In the current study using Müller cells we investigated the expression of these genes and found no change in gene expression. This suggests that within retinas of σ1RKO mice, there are cells that are changing expression of genes encoding the two GSTs, but they are not likely Müller cells.
Each of these antioxidant genes harbors an antioxidant response element, which is located in the 5′ flanking region of many phase II detoxifying and antioxidant genes [47]. ARE activation triggers the transcription of genes involved in antioxidant defense including those observed in this study (Sod1, Cat, Nqo1, Hmox1, Gstt3, Gpx1) by a mechanism that involves the NRF2-KEAP1 pathway [44, 51,52]. We examined the levels of these two proteins in Müller cells isolated from WT and σ1RKO mice. NRF2 is a ubiquitously expressed member of the Cap’n’Collar family of bZIP proteins [53]. Under basal conditions, NRF2-dependent transcription is repressed by KEAP1. Under oxidative stress conditions, NRF2 protein escapes KEAP1-mediated repression, translocates to the nucleus and induces ARE-dependent gene expression [51]. We observed a significant decrease in NRF2 gene and protein expression in the σ1RKO Müller cells compared with WT, while at the same time observed an increase in KEAP1 gene and protein expression in these cells. We also observed a significant decrease in NRF2-ARE binding activity in σ1RKO Müller cells compared with WT. We speculate that the altered antioxidant gene levels observed in σ1RKO Müller cells reflect perturbation of the NRF2-KEAP1 pathway. In related experiments, it has been demonstrated that when COS-7 cells are transfected with Sigma1r, there is a concomitant upregulation of Nqo1 and Sod1 via the activation of ARE [26]. Taken collectively, it appears that σ1R modulates oxidative stress genes via it action on NRF2-KEAP1.
Another protein that is actively involved in the antioxidant cellular response and whose transcription is regulated by NRF2 binding to ARE is xCT [31]. xCT is a 502 amino acid protein also named SLC7A11. It is the specific light chain subunit of the cystine-glutamate exchanger known as system xc−. System xc− is a sodium-independent, chloride-dependent antiporter of cystine and glutamate. It is comprised of xCT as one unit and the promiscuous 4F2 heavy chain (4F2hc/CD98/SCL3A2) as the other unit [30]. System xc− is particularly important for the synthesis of GSH, which is present in millimolar quantities in cells and is one of the most important small molecule cellular antioxidants. GSH is a tripeptide consisting of the amino acids glutamate, glycine and cysteine. The rate-limiting amino acid for GSH synthesis is cysteine, which can be imported into cells directly or in its oxidized form (cystine) via system xc−. System xc− is known to be present in retinal Müller glial cells [54,55]. We studied the function of system xc− in Müller cells harvested from σ1RKO and WT mice and also examined the expression of xCT at the gene and protein level. We observed a marked reduction in the activity of system xc− in σ1RKO cells compared to WT. Our kinetic analysis suggested a decrease in Vmax, which was accompanied by a decrease, not only in the expression of the gene encoding xCT, but also in the level of the protein as well. The decreased GSH levels that we observed in the σ1RKO Müller glia may reflect the diminished activity of this key cystine uptake system. The current data regarding decreased xCT gene and protein levels using Müller cells are consistent with our earlier microarray analysis showing significantly decreased expression of Slc7a11 (−1.94, p=0.01) in σ1RKO neural retina compared to WT [20]. In the present study, we confirmed the decreased xCT gene and protein levels in neural retina also of σ1RKO mice compared to WT.
The compromised mechanisms for managing oxidative stress detected in retinal Müller glial cells may play a role in the late onset retinal degeneration that is observed in the σ1RKO mouse [35]. Though no overt retinal pathology is observed during the early post-natal period, ultrastructural evidence of axonal disruption is evident in the optic nerve head of σ1RKO mice as early as 6 months of age. By 12 months significantly decreased ERG b-wave amplitudes and diminished negative scotopic threshold responses, consistent with inner retinal dysfunction, are detected in σ1RKO mice. These mutant mice also demonstrate increased susceptibility to stress as seen by more rapid retinal degeneration in mice with diabetes [42] or to optic nerve crush [56].
σ1R is an enigmatic protein. Although its expression levels differ between tissues, it appears to be expressed in all cell types examined to date and in several organelles (plasma membrane, nucleus, mitochondria and the endoplasmic reticulum). Indeed, its localization to the ER and mitochondrial associated membrane has resulted in many investigations of σ1R as a mediator of ER stress [16, 57]. σ1R has no other known family members, since “σ2R”, which shares some ligand binding properties with σ1R, is suggested to be the binding site in the progesterone receptor membrane component 1 (PGRMC1) protein complex [58].
The current findings provide compelling evidence that levels of Nrf2 and Keap1 are altered in the absence of σ1R, which sets the stage for future studies of σ1R as a major regulator of the Nrf2-Keap1 pathway. Although the current data were obtained in retinal glial cells, they are relevant to other organs/tissues as well. Indeed, in many diseases in which oxidative stress is implicated, there may be a major role of σ1R as a mediator of the key antioxidant pathway Nrf2-Keap1. These present findings in conjunction with the reports that σ1R acts a modulator of oxidative stress and activates ARE [26] support this putative role.
Highlights.
Oxidative stress is a major contributor to retinal disease.
The role of σ1R in mediating oxidative stress was explored in σ1RKO mouse retinal Müller cells (MC).
σ1RKO MCs manifest elevated ROS, perturbed antioxidant balance and suppression of Nrf2 signaling.
Function and expression of system xc−, the cystine-glutamate exchanger is impaired in σ1RKO MCs.
Oxidative stress-mediating function of retinal MCs may be compromised in the absence of σ1R.
Schematic of paper.
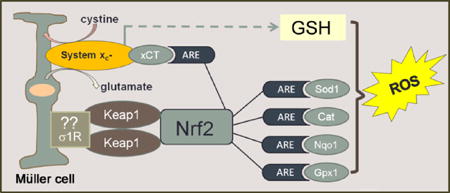
Oxidative stress in the form of reactive oxygen species (ROS) figures prominently in retinal diseases. The Müller glial cell is a major mediator of retinal homeostasis. In this paper, Müller cells harvested from mice lacking σ1R, a putative molecular chaperone, showed an increase endogenous production of ROS. This was accompanied by decreased expression of a number of antioxidant proteins, which are known to harbor antioxidant response elements (ARE). Nrf2, which is a major regulator of oxidative stress through its activation of AREs was decreased at the gene, protein and activity level; its major regulatory protein Keap1 was increased. The expression and activity of the cystine-glutamate exchanger was also decreased in Müller cells lacking σ1R. Taken collectively, the data support a key role for σ1R in modulation of oxidative stress in retina.
Acknowledgments
Acknowledgement of research support: NIH grant R01 EY014560 and the James and Jean Culver Vision Discovery Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature cited
- 1.Frey T, Antonetti DA. Alterations to the blood-retinal barrier in diabetes: cytokines and reactive oxygen species. Antioxid Redox Signal. 2011;15:1271–1284. doi: 10.1089/ars.2011.3906. [DOI] [PubMed] [Google Scholar]
- 2.Cuenca N, Fernández-Sánchez L, Campello L, Maneu V, De la Villa P, Lax P, Pinilla I. Cellular responses following retinal injuries and therapeutic approaches for neurodegenerative diseases. Prog Retin Eye Res. 2014 doi: 10.1016/j.preteyeres.2014.07.001. In Press. [DOI] [PubMed] [Google Scholar]
- 3.Tsai SY, Hayashi T, Harvey BK, Wang Y, Wu WW, Shen RF, Zhang Y, Becker KG, Hoffer BJ, Su TP. Sigma-1 receptors regulate hippocampal dendritic spine formation via a free radical-sensitive mechanism involving Rac1xGTP pathway. Proc Natl Acad Sci. 2009;106:22468–73. doi: 10.1073/pnas.0909089106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brune S, Pricl S, Wünsch B. Structure of the σ1 receptor and its ligand binding site. J Med Chem. 2013;56:9809–9819. doi: 10.1021/jm400660u. [DOI] [PubMed] [Google Scholar]
- 5.Hanner M, Moebius FF, Flandorfer A, Knaus HG, Striessnig J, Kempner E, Glossmann H. Purification, molecular cloning, and expression of the mammalian sigma1-binding site. Proc Natl Acad Sci. 1996;93:8072–8077. doi: 10.1073/pnas.93.15.8072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kekuda R, Prasad PD, Fei YJ, Leibach FH, Ganapathy V. Cloning and functional expression of the human type 1 sigma receptor (hSigmaR1) Biochem Biophys Res Commun. 1996;229:553–558. doi: 10.1006/bbrc.1996.1842. [DOI] [PubMed] [Google Scholar]
- 7.Seth P, Leibach FH, Ganapathy V. Cloning and structural analysis of the cDNA and the gene encoding the murine type 1 sigma receptor. Biochem Biophys Res Commun. 1997;241:535–540. doi: 10.1006/bbrc.1997.7840. [DOI] [PubMed] [Google Scholar]
- 8.Fontanilla D, Johannessen M, Hajipour AR, Cozzi NV, Jackson MB, Ruoho AE. The hallucinogen N,N-dimethyltryptamine (DMT) is an endogenous sigma-1 receptor regulator. Science. 2009;323:934–937. doi: 10.1126/science.1166127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Griesmaier E, Posod A, Gross M, Neubauer V, Wegleiter K, Hermann M, Urbanek M, Keller M, Kiechl-Kohlendorfer U. Neuroprotective effects of the sigma-1 receptor ligand PRE-084 against excitotoxic perinatal brain injury in newborn mice. Exp Neurol. 2012;237:388–395. doi: 10.1016/j.expneurol.2012.06.030. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y, Shi Y, Qiao L, Sun Y, Ding W, Zhang H, Li N, Chen D. Sigma-1 receptor agonists provide neuroprotection against gp120 via a change in bcl-2 expression in mouse neuronal cultures. Brain Res. 2012;1431:13–22. doi: 10.1016/j.brainres.2011.10.053. [DOI] [PubMed] [Google Scholar]
- 11.Yang S, Bhardwaj A, Cheng J, Alkayed NJ, Hurn PD, Kirsch JR. Sigma receptor agonists provide neuroprotection in vitro by preserving bcl-2. Anesth Analg. 2007;104:1179–84. doi: 10.1213/01.ane.0000260267.71185.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tchedre KT, Yorio T. Sigma-1 receptors protect RGC-5 cells from apoptosis by regulating intracellular calcium, Bax levels, and caspase-3 activation. Invest Ophthalmol Vis Sci. 2008;49:2577–2588. doi: 10.1167/iovs.07-1101. [DOI] [PubMed] [Google Scholar]
- 13.Dun Y, Thangaraju M, Prasad P, Ganapathy V, Smith SB. Prevention of excitotoxicity in primary retinal ganglion cells by (+)-pentazocine, a sigma receptor-1 specific ligand. Invest Ophthalmol Vis Sci. 2007;48:4785–4794. doi: 10.1167/iovs.07-0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cantarella G, Bucolo C, Di Benedetto G, Pezzino S, Lempereur L, Calvagna R, Clementi S, Pavone P, Fiore L, Bernardini R. Protective effects of the sigma agonist Pre-084 in the rat retina. Br J Ophthalmol. 2007;91:382–4. doi: 10.1136/bjo.2007.118570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith SB, Duplantier J, Dun Y, Mysona B, Roon P, Martin PM, Ganapathy V. In vivo protection against retinal neurodegeneration by sigma receptor 1 ligand (+)-pentazocine. Invest Ophthalmol Vis Sci. 2008;49:4154–4161. doi: 10.1167/iovs.08-1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hayashi T, Su TP. Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca(2+) signaling and cell survival. Cell. 2007;131:596–610. doi: 10.1016/j.cell.2007.08.036. [DOI] [PubMed] [Google Scholar]
- 17.Malhotra JD, Kaufman RJ. Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxid Redox Signal. 2007;9:2277–2293. doi: 10.1089/ars.2007.1782. [DOI] [PubMed] [Google Scholar]
- 18.Wang L, Eldred JA, Sidaway P, Sanderson J, Smith AJ, Bowater RP, Reddan JR, Wormstone IM. Sigma 1 receptor stimulation protects against oxidative damage through suppression of the ER stress responses in the human lens. Mech Ageing Dev. 2012;133:665–74. doi: 10.1016/j.mad.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 19.Omi T, Tanimukai H, Kanayama D, Sakagami Y, Tagami S, Okochi M, Morihara T, Sato M, Yanagida K, Kitasyoji A, Hara H, Imaizumi K, Maurice T, Chevallier N, Marchal S, Takeda M, Kudo T. Fluvoxamine alleviates ER stress via induction of Sigma-1 receptor. Cell Death Dis. 2014;5:e1332. doi: 10.1038/cddis.2014.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ha Y, Shanmugam AK, Markand S, Zorrilla E, Ganapathy V, Smith SB. Sigma receptor 1 modulates ER stress and Bcl2 in murine retina. Cell Tissue Res. 2014;356:15–27. doi: 10.1007/s00441-013-1774-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mori T, Hayashi T, Hayashi E, Su TP. Sigma-1 receptor chaperone at the ER-mitochondrion interface mediates the mitochondrion-ER-nucleus signaling for cellular survival. PLoS One. 2013;8:e76941. doi: 10.1371/journal.pone.0076941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ortega-Roldan JL, Ossa F, Schnell JR. Characterization of the human sigma-1 receptor chaperone domain structure and binding immunoglobulin protein (BiP) interactions. J Biol Chem. 2013;288:21448–41457. doi: 10.1074/jbc.M113.450379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hayashi T, Hayashi E, Fujimoto M, Sprong H, Su TP. The lifetime of UDP-galactose:ceramide galactosyltransferase is controlled by a distinct endoplasmic reticulum-associated degradation (ERAD) regulated by sigma-1 receptor chaperones. J Biol Chem. 2012;287:43156–43169. doi: 10.1074/jbc.M112.380444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mitsuda T, Omi T, Tanimukai H, Sakagami Y, Tagami S, Okochi M, Kudo T, Takeda M. Sigma-1Rs are upregulated via PERK/eIF2α/ATF4 pathway and execute protective function in ER stress. Biochem Biophys Res Commun. 2011;415:519–525. doi: 10.1016/j.bbrc.2011.10.113. [DOI] [PubMed] [Google Scholar]
- 25.Ha Y, Dun Y, Thangaraju M, Duplantier J, Dong Z, Liu K, Ganapathy V, Smith SB. Sigma receptor 1 modulates endoplasmic reticulum stress in retinal neurons. Invest Ophthalmol Vis Sci. 2011;52:527–540. doi: 10.1167/iovs.10-5731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pal A, Fontanilla D, Gopalakrishnan A, Chae YK, Markley JL, Ruoho AE. The sigma-1 receptor protects against cellular oxidative stress and activates antioxidant response elements. Eur J Pharmacol. 2012;682:12–20. doi: 10.1016/j.ejphar.2012.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bucolo C, Drago F, Lin LR, Reddy VN. Sigma receptor ligands protect human retinal cells against oxidative stress. Neuroreport. 2006;17:287–291. doi: 10.1097/01.wnr.0000199469.21734.e1. [DOI] [PubMed] [Google Scholar]
- 28.Jiang G, Mysona B, Dun Y, Gnana-Prakasam JP, Pabla N, Li W, Dong Z, Ganapathy V, Smith SB. Expression, subcellular localization, and regulation of sigma receptor in retinal Müller cells. Invest Ophthalmol Vis Sci. 2006;47:5576–5582. doi: 10.1167/iovs.06-0608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reichenbach A, Bringmann A. New functions of Müller cells. Glia. 2013;61:651–678. doi: 10.1002/glia.22477. [DOI] [PubMed] [Google Scholar]
- 30.Bannai S, Kitamura E. Transport interaction of L-cystine and L-glutamate in human diploid fibroblasts in culture. J Biol Chem. 1980;255:2372–2376. [PubMed] [Google Scholar]
- 31.Lewerenz J, Hewett SJ, Huang Y, Lambros M, Gout PW, Kalivas PW, Massie A, Smolders I, Methner A, Pergande M, Smith SB, Ganapathy V, Maher P. The cystine/glutamate antiporter system x(c)(−) in health and disease: from molecular mechanisms to novel therapeutic opportunities. Antioxid Redox Signal. 2013;18:522–555. doi: 10.1089/ars.2011.4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gupta RK, Patel AK, Shah N, Chaudhary AK, Jha UK, Yadav UC, Gupta PK, Pakuwal U. Oxidative stress and antioxidants in disease and cancer: a review. Asian Pac J Cancer Prev. 2014;15:4405–4409. doi: 10.7314/apjcp.2014.15.11.4405. [DOI] [PubMed] [Google Scholar]
- 33.Sporn MB, Liby KT. NRF2 and cancer: the good, the bad and the importance of context. Nat Rev Cancer. 2012;12:564–571. doi: 10.1038/nrc3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sabino V, Cottone P, Parylak SL, Steardo L, Zorrilla EP. Sigma-1 receptor knockout mice display a depressive-like phenotype. Behav Brain Res. 2009;198:472–476. doi: 10.1016/j.bbr.2008.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ha Y, Saul A, Tawfik A, Williams C, Bollinger K, Smith R, Tachikawa M, Zorrilla E, Ganapathy V, Smith SB. Late-onset inner retinal dysfunction in mice lacking sigma receptor 1 (σR1) Invest Ophthalmol Vis Sci. 2011;52:7749–7760. doi: 10.1167/iovs.11-8169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shanmugam A, Wang J, Markand S, Perry RL, Tawfik A, Zorrilla E, Ganapathy V, Smith SB. Sigma Receptor 1 activation attenuates release of inflammatory cytokines MIP1, MIP2, MIP3α and IL12 (p40/p70) by retinal Müller glial cells. J Neurochem. 2015 doi: 10.1111/jnc.13002. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hicks D, Courtois Y. The growth and behaviour of rat retinal Müller cells in vitro. 1. An improved method for isolation and culture. Exp Eye Res. 1990;51:119–129. doi: 10.1016/0014-4835(90)90063-z. [DOI] [PubMed] [Google Scholar]
- 38.Ola MS, Moore PM, Maddox D, El-Sherbeny A, Huang W, Roon P, Agarwal N, Ganapathy V, Smith SB. Analysis of Sigma Receptor (σR1) expression in retinal ganglion cells cultured under hyperglycemic conditions and in diabetic mice. Brain Res Mol Brain Res. 2002;107:97–107. doi: 10.1016/s0169-328x(02)00444-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tetz LM, Kamau PW, Cheng AA, Meeker JD, Loch-Caruso R. Troubleshooting the dichlorofluorescein assay to avoid artifacts in measurement of toxicant-stimulated cellular production of reactive oxidant species. J Pharmacol Toxicol Methods. 2013;67:56–60. doi: 10.1016/j.vascn.2013.01.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sarthy VP, Pignataro L, Pannicke T, Weick M, Reichenbach A, Harada T, Tanaka K, Marc R. Glutamate transport by retinal Muller cells in glutamate/aspartate transporter-knockout mice. Glia. 2005;49:184–196. doi: 10.1002/glia.20097. [DOI] [PubMed] [Google Scholar]
- 41.Bridges CC, Kekuda R, Wang H, Prasad PD, Mehta P, Huang W, Smith SB, Ganapathy V. Structure, function, and regulation of human cystine/glutamate transporter in retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2001;42:47–54. [PubMed] [Google Scholar]
- 42.Ha Y, Saul A, Tawfik A, Zorrilla EP, Ganapathy V, Smith SB. Diabetes accelerates retinal ganglion cell dysfunction in mice lacking sigma receptor 1. Mol Vis. 2012;18:2860–2870. [PMC free article] [PubMed] [Google Scholar]
- 43.Mueller BH, Park Y, Daudt DR, Ma HY, Akopova I, Stankowska DL, Clark AF, Yorio T. Sigma-1 receptor stimulation attenuates calcium influx through activated L-type Voltage Gated Calcium Channels in purified retinal ganglion cells. Exp Eye Res. 2013;107:21–31. doi: 10.1016/j.exer.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 44.Muthusamy VR, Kannan S, Sadhaasivam K, Gounder SS, Davidson CJ, Boeheme C, Hoidal JR, Wang L, Rajasekaran NS. Acute exercise stress activates Nrf2/ARE signaling and promotes antioxidant mechanisms in the myocardium. Free Rad Biol Med. 2012;52:366–376. doi: 10.1016/j.freeradbiomed.2011.10.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Banning A, Deubel S, Kluth D, Zhou Z, Brigelius-Flohe R. The GI-GPx gene is a target for Nrf2. Mol Cell Bio. 2005;25:4914–4923. doi: 10.1128/MCB.25.12.4914-4923.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bridges RJ, Natale NR, Patel SA. System Xc- cystine/glutamate antiporter: an update on molecular pharmacology and roles within the CNS. British J Pharmacology. 2012;165:20–34. doi: 10.1111/j.1476-5381.2011.01480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Umapathy NS, Li W, Mysona BA, Smith SB, Ganapathy V. Expression and functionof glutamine transporters SN1 (SNAT3) and SN2 (SNAT5) in retinal Müller cells. Invest Ophthalmol Vis Sci. 2005;46:3980–3987. doi: 10.1167/iovs.05-0488. [DOI] [PubMed] [Google Scholar]
- 48.Tan SM, Stefanovic N, Tan G, Wilkinson-Berka JL, de Haan JB. Lack of the antioxidant glutathione peroxidase-1 (GPx1) exacerbates retinopathy of prematurity in mice. Invest Ophthalmol Vis Sci. 2013;54:555–62. doi: 10.1167/iovs.12-10685. [DOI] [PubMed] [Google Scholar]
- 49.Gosbell AD, Stefanovic N, Scurr LL, Pete J, Kola I, Favilla I, de Haan JB. Retinal light damage: structural and functional effects of the antioxidant glutathione peroxidase-1. Invest Ophthalmol Vis Sci. 2006;47:2613–22. doi: 10.1167/iovs.05-0962. [DOI] [PubMed] [Google Scholar]
- 50.Florian S, Wingler K, Schmehl K, Jacobasch G, Kreuzer OJ, Meyerhof W, Brigelius-Flohé R. Cellular and subcellular localization of gastrointestinal glutathione peroxidase in normal and malignant human intestinal tissue. Free Radic Res. 2001 Dec;35(6):655–63. doi: 10.1080/10715760100301181. [DOI] [PubMed] [Google Scholar]
- 51.Rushmore TH, Morton MR, Pickett CB. The antioxidant responsive element: Activation by oxidative stress and identification of the DNA consensus sequence required for functional activity. J Biol Chem. 1991;266:1632–1163. [PubMed] [Google Scholar]
- 52.Gan L, Johnson JA. Oxidative damage and the Nrf2-ARE pathway in neurodegenerative diseases. Biochim Biophys Acta. 2014;1842:1208–1218. doi: 10.1016/j.bbadis.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 53.Zhang DD. Mechanistic studies of the Nrf2-Keap1 signaling pathway. Drug Metab Rev. 2006;38:769–789. doi: 10.1080/03602530600971974. [DOI] [PubMed] [Google Scholar]
- 54.Tomi M, Funaki T, Abukawa H, Katayama K, Kondo T, Ohtsuki S, Ueda M, Obinata M, Terasaki T, Hosoya K. Expression and regulation of L-cystine transporter,system xc-, in the newly developed rat retinal Müller cell line (TR-MUL) Glia. 2003;43:208–17. doi: 10.1002/glia.10253. [DOI] [PubMed] [Google Scholar]
- 55.Mysona B, Dun Y, Duplantier J, Ganapathy V, Smith SB. Effects of hyperglycemia and oxidative stress on the glutamate transporters GLAST and system xc- in mouse retinal Müller glial cells. Cell Tissue Res. 2009;335:477–488. doi: 10.1007/s00441-008-0742-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mavlyutov TA, Nickells RW, Guo LW. Accelerated retinal ganglion cell death in mice deficient in the Sigma-1 receptor. Mol Vis. 2011;26:1034–1043. [PMC free article] [PubMed] [Google Scholar]
- 57.Mori T, Hayashi T, Hayashi E, Su TP. Sigma-1 receptor chaperone at the ER-mitochondrion interface mediates the mitochondrion-ER-nucleus signaling for cellular survival. PLoS One. 2013;8(10) doi: 10.1371/journal.pone.0076941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xu J, Zeng C, Chu W, Pan F, Rothfuss JM, Zhang F, Tu Z, Zhou D, Zeng D, Vangveravong S, Johnston F, Spitzer D, Chang KC, Hotchkiss RS, Hawkins WG, Wheeler KT, Mach RH. Identification of the PGRMC1 protein complex as the putative sigma-2 receptor binding site. Nat Commun. 2011;2:380. doi: 10.1038/ncomms1386. [DOI] [PMC free article] [PubMed] [Google Scholar]