Abstract
Although males with fragile X syndrome (FXS) are frequently described as demonstrating autism symptomatology, there is much debate regarding whether the behavioral symptoms representing the core domains of autism are the result of the same or different underlying neurological/psychological mechanisms. The present study used a cross-sectional developmental trajectories approach to compare the profiles of autism symptomatology relative to chronological age (CA), nonverbal IQ, and expressive vocabulary ability between individuals with FXS and individuals with nonsyndromic ASD. Results suggest that the onset of autism symptoms and their developmental trajectories in males with FXS differ in important ways as a function of chronological age, nonverbal cognitive ability, and expressive vocabulary relative to males with nonsyndromic ASD. Theoretical and clinical implications are discussed.
Introduction
Much has been learned since Kanner's initial description (1943) of what is now termed autism spectrum disorder (ASD). Nevertheless, Kanner's characterization of the central features of ASD is still fundamental to a diagnosis. Currently, ASD is considered a lifelong developmental disorder and affects an estimated 1 in 68 children in the United States (CDC, 2014). Although there is considerable variation in its presentation, ASD is defined by a core impairment in social communication accompanied by the presence of repetitive and stereotyped behaviors (American Psychiatric Association, 2013). Despite being behaviorally defined, ASD is now known to be a highly heritable, multifactorial condition (Ronald & Hoestra, 2011), resulting from an interaction among various genetic susceptibilities and environmental risks (Persico & Bourgeron, 2006; Hertz-Picciotto et al., 2006; Schmidt et al., 2011).
Fragile X syndrome (FXS), the leading inherited cause of intellectual disability, results from an expansion of the CGG sequence of nucleotides within the FMR1 gene on the X chromosome to more than 200 repeats (Oostra & Willemson, 2003). Individuals with FXS (particularly males) are at high risk for presenting with symptoms of ASD. The risk of comorbid FXS and ASD is higher than that associated with any other known ASD risk factor, with as many as 60% of males with FXS displaying symptoms that, when utilizing gold standard diagnostic instruments, warrant a comorbid diagnosis of ASD (Clifford et al., 2007; Harris et al., 2008). Furthermore, more than 90% of males with FXS are reported to display at least some behavioral symptoms typically associated with an ASD (Bailey et al., 2000; Feinstein & Reiss, 1998). Nevertheless, this mutation accounts for only a small percentage (2-6%) of all cases of ASD (e.g., Cohen et al., 2005).
Knowing that an individual with FXS has an ASD diagnosis or displays behaviors characteristic of ASD, however, tells us little about the developmental implications of these symptoms. Observable behaviors reflect a complex and continuous interplay among and across different domains and levels of analysis. Thus, one cannot assume that commonalities observed in two conditions reflect the same neuropsychological underpinnings, origins, effects, or developmental course. Conversely, similar neuropsychological underpinnings may have different behavioral endpoints as a function of how other syndrome-specific characteristics predispose a given individual to process incoming sensory information and/or interact with his/her environment. Thus, we must begin to decipher the paths by which behavioral symptoms of ASD emerge in FXS and determine how these developmental trajectories compare to those observed for individuals with nonsyndromic ASD (i.e., cases of ASD for which a single genetic etiology has been ruled out). In addition to having important implications for theory, understanding the developmental paths to and from symptoms of ASD across the two disorders is vital to determining the extent to which similar treatment approaches, both pharmacological and behavioral, may be useful for both conditions. In the present study, we used a cross-sectional developmental trajectories approach (Thomas et al., 2009) to test for differences in the relations between severity of ASD symptomatology and important developmental benchmarks (i.e., chronological age, nonverbal cognition, and expressive vocabulary ability) between boys with FXS and boys with nonsyndromic ASD. These data can contribute insights into similarities and differences in the developmental paths to and/or from ASD symptomatology in the two conditions and provide an embarkation point for longitudinal examinations of the same trajectories.
ASD Symptomatology in Fragile X Syndrome
In 2001, Rogers et al. identified two subgroups of children with FXS, those with ASD and those without ASD, for whom scores on the ASD instruments were comparable to children with general developmental delays. Although researchers have been describing the high rates of comorbid FXS and ASD for several decades (e.g., Brown et al., 1982), there is still much that we do not understand about ASD in FXS. ASD symptoms (e.g., gaze avoidance, perseverative speech, hyperarousal, and stereotyped behaviors) are frequently observed in individuals with FXS (Bailey et al., 2000; Feinstein & Reiss, 1998), even in those who do not meet criteria for an ASD diagnosis. For example, Thurman et al. (in press) reported that, of 9 children with FXS who did not meet criteria for a comorbid diagnosis of ASD, 7 met ASD criteria on one of the two diagnostic measures (ADOS or ADI-R). Thus, it is important to characterize ASD symptomatology in all individuals with FXS in addition to understanding if subgroups exist within FXS.
Data emerging from direct comparisons between individuals with FXS and those with nonsyndromic ASD have also added to the debate surrounding whether the ASD symptoms reflect the same neurodevelopmental mechanisms in FXS as they do in nonsyndromic ASD. To begin, there is a growing body of data indicating that individuals with FXS+ASD (i.e., comorbid FXS and ASD) demonstrate more significant cognitive impairments than do individuals with nonsyndromic ASD (Dissanayake et al., 2009; McDuffie et al., in press; Rogers et al., 2001; Wolff et al., 2012). In addition, there is evidence to suggest that, on average, ASD symptoms are less severe in individuals with FXS+ASD than in those with nonsyndromic ASD. Interestingly, more between-group differences seem to be observed in the social affective domain than in the restricted and repetitive behavior domain (e.g., Dissanayake et al., 2009; Hall et al., 2010; McDuffie et al., in press; Wolff et al., 2012), indicating that individuals with comorbid FXS and ASD may suffer from less severe impairments in social reciprocity relative to individuals with nonsyndromic ASD. In fact, between-group differences in the social affective domain, have been observed both when controlling for between-group differences in IQ and when controlling for between-group differences in overall severity of ASD symptomatology, but with individuals with FXS demonstrating more limited cognitive ability (Dissanayake et al., 2009; McDuffie et al., in press; Wolff et al., 2012).
We have yet to determine what these between-group differences ‘mean’ in terms of the emergence of ASD symptomatology in FXS and in nonsyndromic ASD. Heterogeneity in developmental outcomes are driven by ongoing interactions between different domains of functioning, the social environment, and an individual's adaptation to his/her environment (e.g., Fidler, Lunkenheimer, & Hahn, 2011; Karmiloff-Smith, 1998). Thus, ASD symptoms in FXS and nonsyndromic ASD may not reflect the same neurodevelopmental mechanisms, particularly given the fact that there are clear phenotypic differences between the two conditions.
Developmental Characteristics Associated with ASD Symptomatology in FXS
Chronological age, cognitive ability, and expressive vocabulary ability are three factors that have been considered in the literature as relating to the presence and development of ASD symptomatology. Findings from several research studies have revealed a robust negative relation between ASD symptomatology and IQ in males with FXS, such that lower IQ scores are associated with increased severity of ASD on a number of different measures (McDuffie et al., 2010; Hall et al., 2010; Loesch et al., 2007; Bailey et al.; 1998; Bailey et al., 2001).
Additionally, there has been some research examining the relation between ASD symptomatology and chronological age. In a longitudinal study, Hatton et al. (2006) found a small but significant increase in symptom severity with age when examining data from the Childhood Autism Rating Scale (CARS; Schopler, Reichler, & Reynolds, 1988) in a sample of 116 children with FXS ranging from 1 to 14 years in age; however, the mean age of the participants at the initial visit was less than 5 years, which limits our understanding of what is happening during the school-age period. When comparing lifetime and current item scores from the ADI-R for a group of individuals with FXS only, McDuffie et al. (2010) found significant improvement in ASD symptomatology with age, after controlling for nonverbal IQ, for some items in the Reciprocal Social Interaction and Communication domains. Additionally, for participants with comorbid FXS +ASD, these authors found significant improvements with age for numerous items in all three ADI-R domains. Dissanayake et al. (2009), utilizing cross-sectional data, also found that chronological age was significantly and negatively related to ADOS Communication domain scores for adolescent participants with comorbid FXS and ASD. Taken together, these finding suggest that many symptoms of ASD decline during the childhood and adolescent period for children with FXS regardless of ASD diagnostic status.
Finally, there has been some research examining the relation between ASD symptomatology and expressive vocabulary ability in FXS. Kover and Abbeduto (2010) compared the language abilities of adolescent males with FXS without ASD to adolescent males with FXS and comorbid ASD matched on chronological age, nonverbal IQ, and nonverbal mental age. The authors observed differences between the two groups in intelligibility, but failed to document between-group differences in lexical diversity (number of words used). Similarly, McDuffie et al. (2010) found that, after controlling for chronological age and nonverbal IQ, there were no significant differences between males with FXS only and males with FXS and comorbid ASD on expressive vocabulary. Furthermore, when examining ASD symptomatology as a continuous metric, ASD severity score did not contribute to prediction of expressive vocabulary over and above the effects of chronological age and nonverbal IQ. Although these studies failed to observe a relation between ASD symptomatology and expressive vocabulary, this may reflect their reliance on overall ASD symptomatology rather than on the separate domains of autism symptoms. To date, no studies have examined the relation between expressive vocabulary and social affective and restricted repetitive ASD symptomatology separately.
Although progress has been made in understanding the similarities and differences between the behavioral phenotypes of FXS and nonsyndromic ASD, much remains to be understood. Most studies conducted have utilized within-group comparisons of individuals with FXS. The few studies that have utilized between-group comparisons have focused on item-level comparisons between the two conditions. Information regarding whether the factors attributable to and/or emerging from these symptoms are similar across these two conditions would provide insight into whether the same treatment approaches targeting ASD symptomatology are appropriate in FXS and in nonsyndromic ASD. To begin to address this goal, it is important to directly compare putative predictors (e.g., age, cognition, and language) of ASD symptomatology for individuals with FXS and individuals with nonsyndromic ASD in order to characterize the emergence of ASD symptoms.
The Current Study
The purpose of the present study was to characterize profiles of the severity of ASD symptomatology relative to chronological age, nonverbal cognition, and expressive vocabulary in boys with FXS with a direct comparison to boys with nonsyndromic ASD as a point of reference. In contrast to the traditional group comparisons reviewed above, the present study used the cross-sectional developmental trajectories approach endorsed by Thomas and colleagues (2009). Rather than focusing on the characterization of static phenotypes, this approach utilizes cross-sectional data to focus on understanding developmental relationships by highlighting change in a domain of interest relative to other important developmental benchmarks. In contrast to testing differences in cross-sectional group means, group differences are evaluated in terms of intercepts and rates of development and predictors of intercept and rate. This cross-sectional analysis approach can provide important information about the nature of development. In addition, it can be used to provide the impetus for the future design of prospective longitudinal studies addressing the emergence of phenotypes and exploring how categories of behaviors compare across neurodevelopmental disorders. This technique has been beneficial in defining multiple aspects of behavioral phenotypes (e.g., Kover, McDuffie, Hagerman, & Abbeduto, 2013), but has not yet been used as a method of understanding whether the presence of ASD symptomatology relative to other important aspects of functioning in FXS (i.e., chronological age, nonverbal cognition, and expressive vocabulary) is similar to that observed in nonsyndromic ASD. The current study utilized quantitative measures of severity of ASD symptomatology—social-affective symptoms and restricted, repetitive behavior symptoms— as the outcome variables and addressed the following research questions:
Relative to chronological age, nonverbal cognition, and expressive vocabulary, do the developmental trajectories of the severity of ASD symptoms in the social affective domain differ (in terms of intercept or slope) between boys with FXS and boys with nonsyndromic ASD?
Relative to chronological age, nonverbal cognition, and expressive vocabulary, do the developmental trajectories of the severity of ASD symptoms in the restricted and repetitive behavior domain differ (in terms of intercept or slope) between boys with FXS and boys with nonsyndromic ASD?
Method
Participants
Participants were boys with FXS and boys with nonsyndromic ASD who were drawn from a larger longitudinal study on the social-affective bases of word learning in FXS and nonsyndromic ASD. Boys were recruited nationally at one of two university sites (deleted for review). Only data from the initial visit (Time 1) were included in the present project. Inclusion in this larger project required all participants to meet the following criteria according to parent report: (a) native English speakers; (b) able to comply with simple instructions (e.g., “Give me the ball”); (c) speech was the primary means of communication; (d) produced approximately 10 different words spontaneously within the prior month; (e) no sensory or physical impairments that would limit participation in project activities; and (f) lived at home with biological mother who is a fluent English speaker. In addition, participants were tested by project staff and found to have a pure tone, air conduction threshold of 30 dB HL or better in each ear (averaged across 500, 1000, and 2000 Hz). Approval for the study was obtained from the respective university Institutional Review Boards, and informed consent, was obtained.
Participants with FXS were required to provide documentation of a diagnosis of the FMR1 full mutation (i.e., >200 CGG repeats, with or without mosaicism). Participants with nonsyndromic ASD (1) entered the study with an existing community diagnosis of ASD; (2) had parent-provided documentation of prior genetic testing ruling out FXS; and (3) had other possible syndromic causes of ASD (e.g., Rett's syndrome, tuberous sclerosis) ruled out via dysmorphology and neurological examinations conducted by a project physician at the initial visit.
At the initial project visit, both the Autism Diagnostic Observation Schedule (ADOS; Lord, Rutter, DiLavore, & Risi, 2007) and the Autism Diagnostic Interview-Revised (ADI-R; Rutter et al., 2003) were administered to both groups of participants. ASD status was determined using the caseness criteria proposed by Risi and colleagues (Risi et al., 2006). That is, a research diagnosis of ASD was assigned to a participant if he received an ADOS calibrated severity score of at least 4 and met one of the following benchmarks on the ADI-R: (1) met the autism cutoff for the ADI-R Social Reciprocity Domain and either the Communication or Repetitive Behavior domains; (2) came within one point of the cutoffs for both Social Reciprocity and Communication Domains; or (3) met the autism cutoff on either the Social Reciprocity or Communication domains and came within two points of the cut-off on the other domain. Thus, the Risi caseness criteria were used to verify group membership for participants with ASD and to assign a research classification of ASD to participants with FXS. Project staff who administered both the ADOS and the ADI-R were graduate-level professionals who had completed research reliability training.
Participant selection for the current study
The participant samples for the present study overlap with those of [removed for review], although the focus of the analyses and the primary measures used was different across these reports. Consideration for inclusion in the present study required participants with FXS or nonsyndromic ASD to have complete data from the ADOS, which resulted in the exclusion of 3 boys with FXS. In addition, inclusion for the present study required participants to have a nonverbal IQ score less than or equal to 85, as this cutoff is inclusive of essentially all males with FXS (Hessl et al., 2009), resulting in the exclusion of 3 children with FXS (2 IQ data not available, 1 IQ score > 85) and 16 children with nonsyndromic ASD (all due to IQ scores > 85). These inclusionary and exclusionary criteria resulted in the final participant samples of 53 boys with FXS and 39 boys with nonsyndromic ASD ranging in age from 4 – 11 years. For participants with FXS, the racial/ethnic composition of the sample was 83% Caucasian, 8% Hispanic, 4% Asian, 2% African-American, and 3% Native American. For the participants with nonsyndromic ASD, the racial/ethnic composition of the sample was 70% Caucasian, 10% Hispanic, 10% African-American, 8% Asian, and 2% Native American.
Descriptive between-group analyses were conducted on the developmental benchmarks of chronological age, nonverbal IQ, and expressive vocabulary ability to provide information regarding the comparability of participants in the two groups. Independent samples t-tests indicated that the FXS and nonsyndromic ASD samples were comparable on chronological age (p = .56, d = .12, s2ratio = 1.09). As expected, based on previous findings within the literature, participants with FXS had lower nonverbal IQ scores than did participants with nonsyndromic ASD (p = .050, d = .42, s2ratio = 1.00). Significant differences were not observed between the two groups on expressive vocabulary standard scores (p = .26, d = .25, s2ratio = .46).
Subsamples
For the primary analyses, we were interested in determining if findings would remain unchanged after participants with FXS who did not meet criteria for an ASD were excluded. For this reason, boys with FXS were identified as having met research criteria for ASD utilizing the same procedures used to verify ASD diagnosis in participants with nonsyndromic ASD. It was determined that 41 of the 53 boys in the FXS sample could be classified as also meeting criteria for an ASD (hereafter, the FXS+ASD group). It is important to note that of the 12 children who were not classified as meeting research criteria for an ASD, only 2 meet criteria on neither the ADOS nor the ADI-R. The remaining 10 children were not classified as FXS+ASD for the following reasons: 4 met criteria on the ADOS but were missing ADI-R data, 2 children met criteria on the ADOS but did not meet criteria on the ADI-R, and 4 children met criteria on the ADI-R but did not meet criteria on the ADOS. The descriptive analyses comparing developmental benchmarks for participants with nonsyndromic ASD to those classified with FXS+ASD revealed the same pattern of findings as was observed for the full FXS sample. The chronological ages of the boys with FXS+ASD and the boys with nonsyndromic ASD were comparable (p = .54, d = .13, s2ratio = 1.14), but boys with FXS+ASD were observed, as expected, to have significantly lower nonverbal IQ scores than the boys with nonsyndromic ASD (p = .009, d = .59, s2ratio = 0.96). Once again, significant differences were not observed between the two groups on expressive vocabulary standard scores (p = .61, d = .12, s2ratio = .45).
Finally, we sought to understand if similar patterns of findings would also be observed when participants classified with FXS+ASD and participants with nonsyndromic ASD were matched on overall severity of ASD symptomatology. To achieve this goal, a subsample (n = 21 per group) of participants was selected from the FXS+ASD and the nonsyndromic ASD groups, utilizing the sampling procedures outlined by Mervis and John (2008), to create groups of males with FXS+ASD and males with nonsyndromic ASD matched on overall severity of ASD symptomatology (hereafter referred to as the ASD severity-matched subsamples). The descriptive analyses comparing developmental benchmarks demonstrated similar pattern of findings as reported previously. The chronological ages were not observed to differ significantly in the ASD severity-matched samples (p = .38, d = .28, s2ratio = 1.15). The boys with FXS+ASD in the ASD severity-matched sample were still observed to have significantly lower nonverbal IQ scores than the boys with nonsyndromic ASD (p < .001, d = 1.28, s2ratio = 1.18). Mean scores for expressive vocabulary ability were not observed to differ for the ASD severity-matched subsamples (p = .62, d = .16, s2ratio = .27); however, differences were observed in the variance of these scores. Descriptive statistics for the participant samples are presented in Table 1.
Table 1. Descriptive Statistics (Mean, SD, and Range) for Full Sample of Participants with FXS, FXS+ASD, nonsyndromic ASD.
| FXS1 (n = 53) |
FXS+ASD2 (n = 41) |
FXS+ASD – Severity Match (n = 21) |
Nonsyndromic ASD (n = 39) |
Nonsyndromic ASD – Severity Match (n = 21) |
|
|---|---|---|---|---|---|
| Chronological Age | 7.51 (1.98, 4.06-10.62) |
7.54 (2.03, 4.06-10.63) |
7.39 (1.92, 4.06-10.63) |
7.27 (1.90, 4.02-10.99) |
6.88 (1.79, 4.02-10.99) |
| Nonverbal IQ SS | 57.77 (13.94, 36-83) |
55.39 (13.61, 36-83) |
53.33 (12.87, 36-79) |
63.62 (13.93, 36-85) |
69.24 (11.84, 36-85) |
| Expressive Vocabulary SS | 65.63 (16.24, 20-103) |
62.88 (16.11, 20-103) |
60.85 (12.83, 41-76) |
60.47 (23.88, 20-96) |
63.95 (24.87, 20-96) |
| ADOS Social Affective CSS | 5.96 (2.30, 2-10) |
6.27 (2.10, 2-10) |
7.38 (1.77, 4-10) |
7.82 (1.67, 3-10) |
7.33 (1.80, 3-10) |
| ADOS Restricted and Repetitive Behavior CSS | 7.26 (1.74, 1-10) |
7.54 (1.52, 5-10) |
8.05 (1.77, 5-10) |
8.36 (1.42, 5-10) |
7.85 (1.42, 5-10) |
This sample includes children with FXS who do and do not meet research criteria for a comorbid diagnosis of ASD.
This sample is comprised of children with FXS who meet research criteria for a comorbid diagnosis of ASD and is a subsample of the full sample of participants with FXS.
Assessment Measures
The Leiter International Performance Scale – Revised
The Leiter International Performance Scale – Revised (Leiter-R; Roid & Miller, 1997) is a nonverbally administered standardized assessment of nonverbal intelligence. The subtests comprising the Brief IQ were administered; namely, Figure Ground, Form Completion, Sequential Order, and Repeated Patterns. The Brief IQ standard score from the Leiter-R was utilized in the present study. The mean IQ for the Leiter-R standardization sample is 100, with a standard deviation of 15.
Autism Diagnostic Observation Schedule
The ADOS (Lord, Rutter, DiLavore, & Risi, 2007) is a semi-structured play-based interaction in which a trained examiner creates specific interactive contexts to observe the participant's reciprocal social interaction skills and repetitive behaviors. One of four ADOS modules is administered based upon the participant's expressive language level. In the current project, participants received ADOS modules 1, 2, or 3. The Social Affect Calibrated Severity Scores (SA-CSS) and the Restricted and Repetitive Behavior Calibrated Severity Scores (RRB-CSS), which allow comparisons across different modules, provided by Hus, Gotham, & Lord (2014), were utilized.
The original severity scores generated by Hus et al. (2014) for the Restricted and Repetitive Behaviors domain had a range of 1 to 7 (hereafter referred to as RRB-CSSReduced), unlike the severity scores for the Social Affective domain, which ranged from 1 to 10. Due to concerns that differing ranges across the two domains would cause confusion when interpreting the meaning of severity scores, Hus and colleagues transformed the RRB-CSSReduced scale to the 10-point range observed on the SA-CSS, but with severity scores of 2, 3, and 4 not possible to obtain (i.e., RRB-CSS scores included 1, 5-10). Within a parametric analysis, however, methodological concerns arise when utilizing a dependent variable in which multiple scores are not obtainable. The focus of the present study was on examining the trajectories of calibrated severity scores within each domain as a function of child characteristics, and not on the direct comparison of severity scores between domains; thus, the current study utilized the RRB-CSSReduced scale (i.e. the original, untransformed calibrated severity score for the Restricted and Repetitive Behaviors domain) that ranged from 1 to 7. As such, the severity scores of 2 – 7 from the RRB-CSSReduced scale correspond to the severity scores of 5 – 10 from the RRB-CSS scale described by Hus and colleagues.
Expressive Vocabulary Test, Second Edition
The Expressive Vocabulary Test, Second Edition (EVT-2; Williams, 2007) is an individually administered measure of expressive vocabulary. During this assessment, the participant is instructed to provide a label or synonym for each color picture on the page of an easel book. The mean for standard scores on the EVT-2 is 100, with a standard deviation of 15.
Analysis Plan
Preliminary analyses were conducted to assess average group differences in the SA-CSS and RRB-CSSReduced and to provide information regarding the correlations between the ADOS severity scores and chronological age, nonverbal IQ, and expressive vocabulary ability. Cross-sectional developmental trajectories were then calculated using the procedures outlined by Thomas and colleagues (2009), for both SA-CSS and RRB-CSSReduced relative to chronological age, nonverbal IQ standard score, and expressive vocabulary standard score. This approach is similar to an ANOVA; however, instead of testing the differences between cross-sectional group means, differences are evaluated between the intercept and slope of the lines used to depict the developmental trajectory of each group. Main effects of group (FXS vs. nonsyndromic ASD), main effects of the three continuous predictors (i.e., chronological age, nonverbal IQ, or expressive vocabulary), and the interactions between group and slope were of interest in the current study. In the case of significant findings, follow-up analyses, using one-tailed tests, were conducted to determine if the same pattern of findings was obtained when only the participants with FXS who met research criteria for an ASD diagnosis (FXS+ASD) were included in the analyses. Finally, follow-up analyses, using one-tailed tests, were also conducted to determine if the same pattern of findings were obtained when participants with FXS+ASD and participants with nonsyndromic ASD were matched on overall severity of ASD symptomatology The data from these analyses are considered exploratory because of the small sample size as nearly half of the FXS+ASD and nonsyndromic ASD samples had to be excluded to achieve this match.
Results
Preliminary Analyses
Preliminary comparisons of ASD severity domain scores between the two groups demonstrated that the participants with FXS earned significantly lower ADOS severity scores for both the SA-CSS (t(90) = 4.29, p < .001, d = .92) and RRB-CSSReduced (t(90) = 4.29, p = .002, d = .69) than did the participants with nonsyndromic ASD. In addition, for both the SA-CSS (t(78) = 3.65, p < .001, d = .82) and RRB-CSSReduced (t(78) = 2.51, p = .01, d = .56), these between-group differences remained even after including only participants classified as FXS+ASD. No differences were observed in the social affective (t(40) = -.09, p = .93, d = .03) or the restricted and repetitive behavior (t(40) = -.38, p = .70, d = .13) severity scores in the ASD severity-matched subsamples. Finally, results of correlational analyses between the SA-CSS and RRB-CSSReduced and putative developmental predictors are presented in Tables 2 and 3.
Table 2. Correlation Coefficients between Chronological Age (CA), Nonverbal IQ (NVIQ), and Expressive Vocabulary Standard Scores and ADOS Domain Severity Scores for Males with Nonsyndromic ASD as a function of Matching Sample.
| ADAMS Subscale | CA | Leiter-R NVIQ SS | EVT-2 SS |
|---|---|---|---|
| Social Affective Severity Score | |||
| Full Sample FXS (n = 53) | r = .32* | r = -.44** | r = -.04 |
| FXS+ASD (n = 41) | r = .27 | r = -.31* | r = .07 |
| ASD-Severity Matched Sample | r = .37 | r = -.38 | r = -.05 |
| FXS+ASD (n = 21) | |||
|
| |||
| Restricted and Repetitive Behavior Severity | |||
| Full Sample FXS (n = 53) | r = .02 | r = -.38* | r = -.40* |
| FXS+ASD (n = 41) | r = -.05 | r = -.32* | r = -.43* |
| ASD-Severity Matched Sample | r = .04 | r = -.22 | r = -.45* |
| FXS+ASD (n = 21) | |||
p < .05
Table 3. Correlation Coefficients between Chronological age (CA), Nonverbal IQ (NVIQ), and Expressive Vocabulary Standard Scores and ADOS Domain Severity Scores for Males with Nonsyndromic ASD as a function of Matching Sample.
| ADAMS Subscale | CA | Leiter-R NVIQ SS | EVT-2 SS |
|---|---|---|---|
| Social Affective Severity Score | |||
| Nonsyndromic ASD (n = 39) | r = .02 | r = .03 | r = .06 |
| ASD-Severity Matched Sample | r = .02 | r = .05 | r = .13 |
| Nonsyndromic ASD (n = 21) | |||
|
| |||
| Restricted and Repetitive Behavior Severity | |||
| Nonsyndromic ASD (n = 39) | r = .26 | r = -.30 | r = .00 |
| ASD-Severity Matched Sample | r = .29 | r = .08 | r = .33 |
| Nonsyndromic ASD (n = 21) | |||
Cross-Sectional Developmental Trajectories: Social Affect
Using ADOS calibrated severity scores, severity of social affective symptoms was evaluated relative to chronological age by examining the trajectory intercept at the lowest age of overlap between the groups (i.e., 4 years of age) and within-group trajectory slopes. The intercept of the SA-CSS trajectory was significantly lower for boys with FXS, indicating less impairment on average, than for boys with nonsyndromic ASD, F(1,88) = 7.40, p = .008, partial η2 = .08. For rate of change over time, neither the average relationship between SA-CSS and chronological age, F(1,88) = 3.14, p = .08, partial η2 = .03, nor the interaction between group and chronological age, F(1,88) = 2.66, p = .11, partial η2 = .029, was significant. As seen in Table 4 and Figure 1, level of impairment in the Social Affective domain did not change with chronological age for boys with nonsyndromic ASD. For boys with FXS, however, there was a nonsignificant trend for social affective symptoms to worsen with chronological age. Follow-up analyses, including only boys with comorbid FXS+ASD yielded the same pattern of findings; that is, the intercept of the SA-CSS trajectory was lower for boys with FXS+ASD than for boys with nonsyndromic ASD, F(1,80) = 4.44, p = .02, partial η2 = .06, one tailed. Analyses including the ASD severity-matched subsamples did not indicate a significant effect of intercept of the SA-CSS trajectory, F(1,42) = 1.11, p = .30, partial η2 = .03, one tailed. Intercept and slope data for follow-up analyses are presented in Table 4.
Table 4. Intercept and Slope of Linear Developmental Trajectories Predicting Social Affective Severity Score based on Putative Developmental Predictors as Function of Sample.
| ADAMS Subscale | CA | Leiter NVIQ SS Fragile X Samples |
EVT SS |
|---|---|---|---|
| Full Sample FXS (n = 53) | m = .38 (SE = .14) | m = -.07 (SE = .02) | m = -.006 (SE = .02) |
| b = 4.67, r2 = .11 | b = 10.15, r2 = .19 | b = 6.33, r2 =.002 | |
| FXS+ASD (n = 41) | m = .27 (SE = .15) | m = -.05 (SE = .02) | m = .009 (SE = .02) |
| b = 5.32, r2 = .07 | b = 8.95, r2 = .10 | b = 5.69, r2 = .005 | |
| ASD-Severity Matched Sample | m = .34 (SE = .21) | m = -.05(SE = .03) | m = -.006 (SE = .03) |
| FXS+ASD (n = 21) | b = 4.89, r2 = .13 | b = 10.16, r2 = .14 | b = 7.83, r2 = .002 |
|
| |||
| Nonsyndromic ASD Samples | |||
| Nonsyndromic ASD (n = 39) | m = .02 (SE = .22) | m = .003 (SE = .03) | m = .004 (SE = .02) |
| b = 7.77, r2 <.001 | b = 7.62, r2 <.001 | b = 7.61, r2 <.003 | |
| ASD-Severity Matched Sample | m = .01(SE = .30) | m = .008 (SE = .05) | m = .01(SE = .04) |
| Nonsyndromic ASD (n = 21) | b = 7.23, r2 < .001 | b = 6.78, r2 = .003 | b = 6.75, r2 = .02 |
Figure 1.
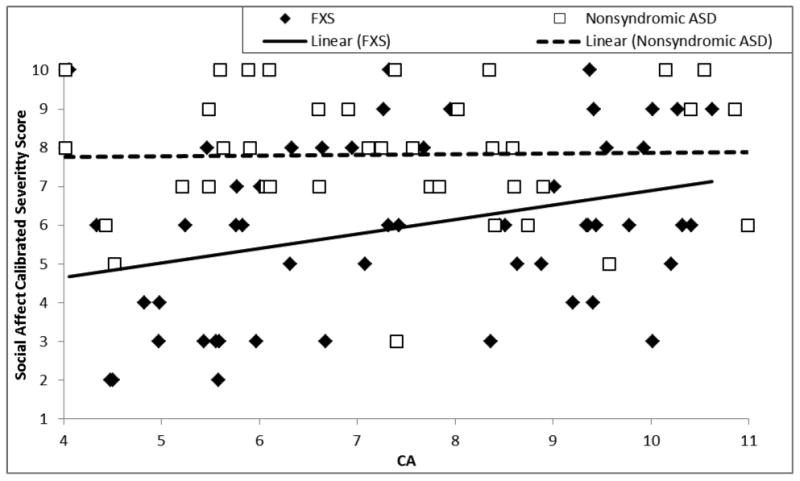
Trajectory of social affect calibrated severity score relative to chronological age for boys with FXS and boys with nonsyndromic ASD.
Severity of social affective symptoms was evaluated against nonverbal cognitive ability in terms of intercept at the lowest point of overlap between the groups (i.e., Leiter-R SS of 36, the lowest possible standard score) and in terms of within-group slopes. The intercept of the SA-CSS trajectory did not differ significantly between the two groups, F(1,88) = 1.87, p = .18, partial η2 = .021. The relationship between SA-CSS and nonverbal cognition was observed to be moderately negative on average, F(1,88) = 5.52, p = .02, partial η2 = .06; however, this relationship differed between participants with FXS and those with nonsyndromic ASD, yielding a significant difference across the two diagnostic groups in the relation between social affective symptoms and nonverbal cognition, F(1,88) = 6.57, p = .01, partial η2 = .07. For boys with FXS, SA-CSSs decreased as nonverbal cognitive ability increased. For boys with nonsyndromic ASD, SA-CSSs remained relatively stable across the full range of nonverbal IQ standard scores. This difference in slopes can be seen in Figure 2 and Table 4. Follow-up analyses including only boys with FXS+ASD again yielded a significant difference in the relation between SA-CSS and nonverbal cognition for FXS and nonsyndromic ASD, F(1,80) = 2.82, p = .048, partial η2 = .036. Analyses including the ASD severity-matched subsamples did not yield a significant difference in the relations between SA-CSS and nonverbal cognition, F(1, 42) = 1.76, p = .096, partial η2 = .044); however, an increase in effect size was observed relative to that observed for the boys with FXS+ASD. Intercept and slope results for follow-up analyses are presented in Table 4.
Figure 2.
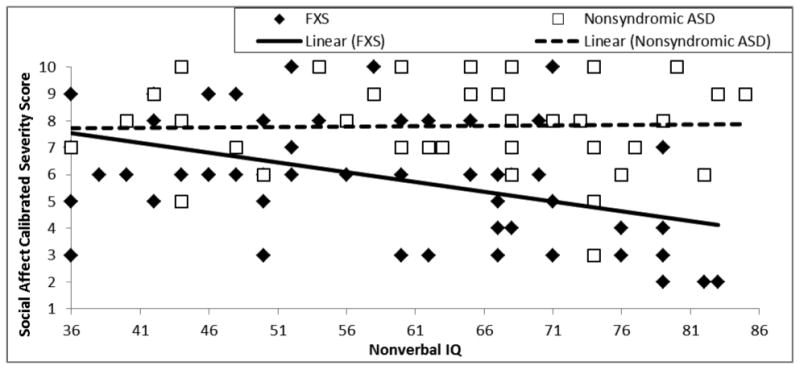
Trajectory of social affect calibrated severity score relative to nonverbal cognitive ability for boys with FXS and boys with nonsyndromic ASD.
Finally, severity of social affective symptoms was evaluated against expressive vocabulary in terms of the intercept at the lowest point of overlap between the groups (i.e. EVT-2 SS of 20, the lowest possible standard score) and in terms of within-group slopes. The intercept of the SA-CSS trajectories did not differ between the two groups, F(1,88) = .66, p = .418, partial η2 = .008, the relationship between SA-CSS and expressive vocabulary was not significant, F(1,88) = .003, p = .96, partial η2 < .001, and no difference was observed between the two groups in the relationship between SA-CSS and expressive vocabulary, F(1,88) = .171, p = .68, partial η2 = .002. These data are shown in Figure 3 and Table 4.
Figure 3.
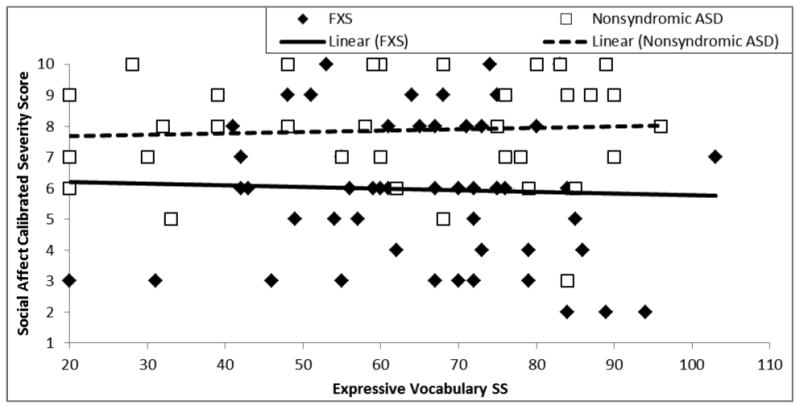
Trajectory of social affect calibrated severity score relative to expressive vocabulary ability for boys with FXS and boys with nonsyndromic ASD.
Cross-Sectional Developmental Trajectories: Restricted and Repetitive Behaviors
Analyses were then conducted to evaluate the presence of between-group differences in the relation between severity of restricted and repetitive behavior symptoms and the developmental benchmarks of interest (chronological age, nonverbal cognition, expressive vocabulary). To begin, severity of restricted and repetitive behavior symptoms was evaluated relative to age by examining the trajectory intercept and within-group trajectory slopes between RRB-CSSReduced and chronological age. The intercept of the RRB-CSSReduced trajectories did not differ between groups, F(1,88) = .03, p = .86, partial η2 < .001. In addition, the relation between RRB-CSSReduced and chronological age was not significant, F(1,88) = 1.60, p = .21, partial η2 = .02, and no between-group difference was observed in this relationship, F(1,88) = 1.10, p = .30, partial η2 = .012 (see Figure 4 and Table 5).
Figure 4.
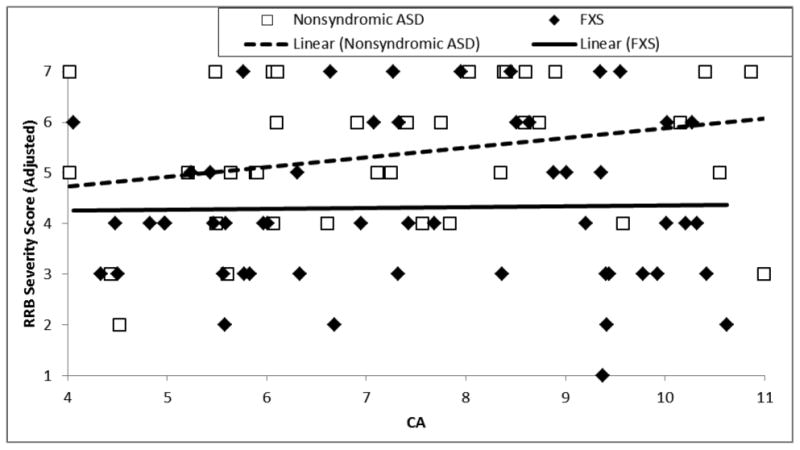
Trajectory of restrictive and repetitive behavior calibrated severity score relative to chronological age for boys with FXS and boys with nonsyndromic ASD.
Table 5. Intercept and Slope of Linear Developmental Trajectories Predicting Restricted and Repetitive Behavior Severity Score based on Putative Developmental Predictors as Function of Sample.
| CA | Leiter-R NVIQ SS Fragile X Samples |
EVT-2 SS | |
|---|---|---|---|
| Full Sample FXS (n = 53) | m = .02 (SE = .11) | m = -.04 (SE = .01) | m = -.04 (SE = .01) |
| b = 4.26, r2 <.001 | b = 6.83, r2 = .15 | b = 6.77, r2 = .16 | |
| FXS+ASD (n = 41) | m = -.04 (SE = .11) | m = -.04 (SE = .02) | m = -.04 (SE = .01) |
| b = 4.68, r2 = .003 | b = 6.54, r2 = .11 | b = 6.96, r2 = .18 | |
| ASD-Severity Matched Sample | m = .04 (SE = .19) | m = -.03 (SE = .03) | m = -.06 (SE = .03) |
| FXS+ASD (n = 21) | b = 4.76, r2 = .002 | b = 6.66, r2 = .05 | b = 8.69, r2 = .20 |
|
| |||
| Nonsyndromic ASD Samples | |||
| Nonsyndromic ASD (n = 39) | m = .19 (SE = .17) | m = -.03 (SE = .02) | m = 0 (SE = .02) |
| b = 4.74, r2 = .07 | b = 7.27, r2 = .09 | b = 5.36, r2 < .001 | |
| ASD-Severity Matched Sample | m = .04 (SE = .28) | m = .01 (SE = .04) | m = .01 (SE = .03) |
| Nonsyndromic ASD (n = 21) | b = 4.76, r2 = .09 | b = 4.17, r2 = .007 | b = 3.68, r2 = .11 |
Next, the severity of restricted and repetitive behavior symptoms was evaluated relative to nonverbal cognition. The intercept of the RRB-CSSReduced trajectories did not differ between the two groups, F(1,88) = .10, p = .75, partial η2 = .001. The relation between RRB-CSSReduced and nonverbal cognition was significant and negative, F(1,88) = 11.33, p = .001, partial η2 = .114, but did not differ significantly between the two groups, F(1,88) = .38, p = .54, partial η2 = .004. As seen in Figure 5 (see also Table 5), RRB-CSSReduced decreases as nonverbal IQ increases for both participants with FXS and participants with nonsyndromic ASD. Follow-up analyses replicated the finding of a negative relationship between nonverbal cognition and RRB-CSSReduced when the FXS sample was restricted to only FXS+ASD, F(1,80) = 8.15, p = .006, partial η2 = .097, one-tailed. For the ASD severity-matched subsamples, the relation between did not indicate a significant relation between nonverbal cognition and RRB-CSSReduced, F(1, 42) = .24, p = .31, partial η2 = .006) and a decrease in effect size relative to that observed for the boys with FXS+ASD. Intercept and slope results for follow-up analyses are presented in Table 5.
Figure 5.
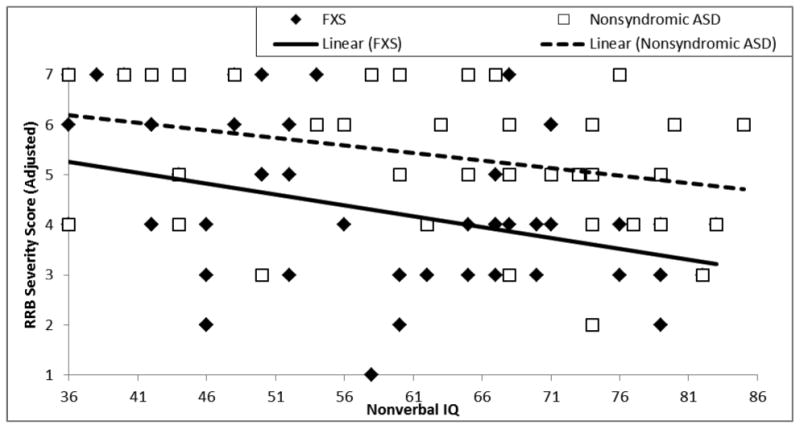
Trajectory of restrictive and repetitive behavior calibrated severity score relative to nonverbal cognitive ability for boys with FXS and boys with nonsyndromic ASD.
Finally, severity of restricted and repetitive behavior symptoms was evaluated against expressive vocabulary ability in terms of intercept at the lowest point of overlap between the groups (i.e., EVT-2 standard score of 20) and in terms of within-group slopes. The intercept of the RRB-CSSReduced trajectories did not significantly differ between the two groups, F(1,88) = 1.76, p = .19, partial η2 = .02. The relation between RRB-CSSReduced and expressive vocabulary ability was observed to be moderately negative on average, F(1,88) = 5.68, p = .02, partial η2 = .063; however, this relationship differed for participants with FXS and participants with nonsyndromic ASD, yielding a significant interaction, F(1,88) = 11.69, p = .02, partial η2 = .063. As seen in Figure 6 (see also Table 5), RRB-CSSReduced decreased as expressive vocabulary ability increased for boys with FXS; however, RRB-CSSReduced remained stable for boys with nonsyndromic ASD. Follow-up analyses, including only boys with FXS+ASD, yielded the same pattern of findings: the relation between RRB-CSSReduced and expressive vocabulary was moderately negative, F(1,76) = 5.37, p = .01, partial η2 = .069, qualified by a significant interaction between diagnosis and expressive vocabulary, F(1,88) = 5.37, p = .01, partial η2 = .063. For the ASD severity-matched subsamples, the relation between RRB-CSSReduced and expressive vocabulary ability was not significant, F(1, 42) = 1.93, p = .086, partial η2 = .051); however, there was a significant interaction between diagnosis and expressive vocabulary, F(1, 42) = 6.97, p = .005, partial η2 = .16), with an increase in effect size relative to that observed for the boys with FXS+ASD. Intercept and slope results for follow-up analyses are presented in Table 5.
Figure 6.
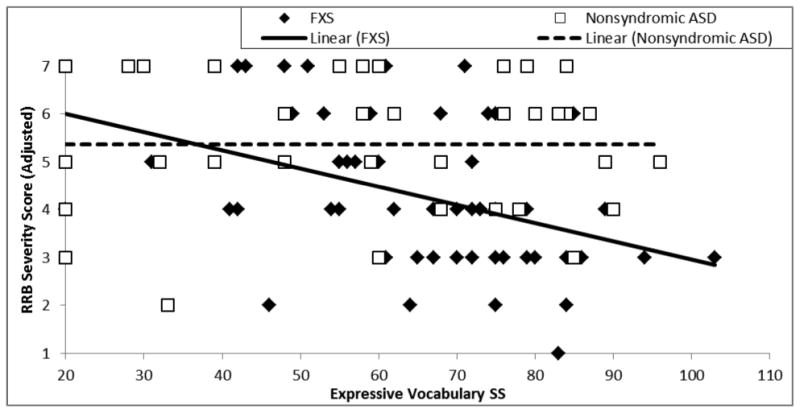
Trajectory of restrictive and repetitive behavior calibrated severity score relative to expressive vocabulary ability for boys with FXS and boys with nonsyndromic ASD.
Discussion
Despite the research that has focused on ASD symptomatology in FXS, we do not fully understand the implications of the behavioral features of ASD in FXS. To begin to address this issue, the present study used a direct between-group comparison to evaluate the relative contributions of three important developmental factors (e.g., chronological age, nonverbal cognition, and expressive vocabulary) that could potentially impact the emergence and course of ASD symptoms in school-age males with FXS and compared these patterns to those observed in males with nonsyndromic ASD.
Social Affective Symptoms
Results from the present study suggest that the onset of ASD symptoms and their developmental trajectories in males with FXS differ as a function of chronological age, nonverbal cognitive ability, and expressive vocabulary relative to males with nonsyndromic ASD. With regard to the social affective symptoms associated with ASD, we found that at 4 years of age, social affective symptoms of ASD were significantly less severe in males with FXS than in males with nonsyndromic ASD. This finding could be attributed to the fact that some boys with FXS did not meet criteria for a comorbid diagnosis of ASD, despite displaying some symptoms of ASD. However, even when restricting the analysis to only participants with FXS who met criteria for a classification of ASD, the between-group difference in severity of social affective symptoms remained significant.
In addition, our results revealed that the severity of social affective impairment did not change with chronological age for males with nonsyndromic ASD. Although not reaching significance within the trajectory analyses, there was a trend for symptom severity in the Social Affective domain to worsen with age for males with FXS. This trend was further supported with the significant correlation between age and severity of social affective symptoms observed in the correlational analyses. This finding, which might emerge as significant in a trajectory study with a larger sample size, is consistent with previous findings by Hall et al. (2009), but is discrepant from the findings reported by McDuffie et al. (2010), who found that symptoms of ASD lessened with age in a within group comparison. The findings of McDuffie et al. (2010), however, were based on data obtained from retrospective parent report in response to queries about current and past behaviors, which has methodological limitations, including the risk of telescoping (Hus, Taylor, & Lord, 2011). In contrast, the current data were derived from direct, examiner-implemented observational samples of behavior. The discrepancy between the current study and McDuffie et al. could also be related to the age range of the participants (i.e., children in the present study were younger than those in McDuffie et al.). Unlike the other comparisons made between FXS and nonsyndromic ASD within the present study, there were no between-group differences at 4 years of age for the severity-matched subsamples (i.e., individuals with FXS+ASD and individuals with nonsyndromic ASD who were matched on overall severity of ASD). It is important to note, however, that achieving a severity match in the current study required the selection of more severely affected individuals with FXS+ASD and less affected individuals with ASD, thereby reducing the variability in within-group severity scores as well as the resultant sample sizes.
In terms of nonverbal cognitive ability, no differences were observed between males with FXS and males with nonsyndromic ASD in severity of social affective symptoms at the lowest bound of the nonverbal IQ distribution (i.e., a standard score of 36). However, a significant between-group difference was observed in the trajectory of change between severity of social affective symptoms and nonverbal IQ. In males with nonsyndromic ASD, severity of social affective symptoms remained stable across the range of nonverbal IQ scores. In contrast, severity of social affective symptoms significantly decreased as nonverbal IQ increased in males with FXS. These findings continued to be significant when only the participants with FXS who met research criteria for a comorbid diagnosis of ASD were included in the analyses. When comparing the ASD severity-matched subsamples, however, the significant between-group differences in the relation between severity of social affective symptoms and nonverbal IQ failed to reach significance. That being said, the effect size obtained in this latter comparison was larger than that obtained in the comparison including all children classified with FXS+ASD. Further support for this finding is observed when examining the correlational analyses for the severity matched groups. In particular, the correlation between nonverbal IQ and the severity of social affective symptoms was higher for the FXS+ASD participants (r = -.38) than for the severity matched participants with nonsyndromic ASD (r = .05). Therefore, it appears that the same patterns of findings are observed in both the diagnostic-matched and severity-matched samples, and that the latter analysis did not have enough power to reach significance because nearly half of the participants were excluded from these samples to achieve a satisfactory severity match. Finally, when examining severity of social affective symptoms as a function of expressive vocabulary, no between-group differences were detected at the lowest bound of the expressive vocabulary distribution or in the trajectory of change in severity of social affective symptoms. For both groups of participants, therefore, severity of social affective symptoms remained stable across the full range of expressive vocabulary ability scores.
Taken together, these findings suggest that differences may exist in the factors associated with the severity of social affective symptoms of ASD in FXS and in nonsyndromic ASD. The severity score from the ADOS was specifically calibrated to reduce the influences of age and developmental level, thereby providing a “purer” measure of core symptoms of ASD. Our data indicate that this score seems to function as intended for the children with nonsyndromic ASD, with severity of social affective symptomatology relatively uninfluenced by age, nonverbal cognitive ability, and expressive vocabulary ability. However, this does not appear to be true for children with FXS. Specifically, cognitive impairments continue to be a significant predictor of the severity of social affective symptoms of ASD in FXS, not only when considering all children with FXS but also when considering children classified as having comorbid FXS+ASD. Furthermore, the relation between nonverbal cognitive ability and severity of social affective symptoms appears to be stronger in children with comorbid FXS+ASD than in children with nonsyndromic ASD even when the two groups are matched on overall severity of ASD symptomatology.
Our findings additionally demonstrate a worsening in the severity of social affective symptoms of ASD with age in males with FXS, but not in nonsyndromic ASD. If replicated, this would not be surprising given that nonverbal IQ declines in males with FXS causing them to fall further behind their chronological age peers over time (Kover, Pierpont, Kim, Brown, & Abbeduto, 2013). This relationship was also observed in the full sample of males with FXS included in the present study (data available upon request). We propose that declining IQ would likely lead to increasing difficulties in adequately responding to the demands of social interactions as a child ages, resulting in more impairments in behaviors that constitute the Social Affective domain of the ADOS.
Restricted and Repetitive Behaviors
When considering severity of restricted and repetitive behaviors, our results indicate that the intercepts in this domain did not differ as a function of chronological age between the groups of participants, with minimal change with chronological age for either group. Similarly, no between-group differences were observed in the severity of symptoms of restricted and repetitive behaviors at the lowest bound of the nonverbal IQ distribution (IQ = 36). In addition, the severity of restricted and repetitive behaviors decreased as nonverbal IQ increased for both groups. Other researchers have observed an inverse relation between nonverbal IQ and restricted and repetitive behaviors in children with ASD (e.g., Bishop, Richler, & Lord, 2006). School-age children with higher nonverbal IQs may have a greater range of interests and activities and, therefore, may be more likely to engage in productive rather than repetitive behaviors relative to children with lower levels of cognitive ability (Bishop et al., 2006). Our data provide additional support for this premise and reveal that a similar pattern is observed for males with FXS. It is important to note, however, that previous research suggests that the relationship between nonverbal cognition and restricted and repetitive behaviors may vary as function of the type of repetitive behavior being considered (e.g., insistence on sameness vs restricted interests; Bishop et al., 2006; Oakes et al., 2014). In the current study, we considered domain level scores and did not consider the impact of individual categories of repetitive behaviors.
When we compared the relation between severity of symptoms of restricted and repetitive behaviors and expressive vocabulary, differences were observed between the participants with FXS and the participants with nonsyndromic ASD. Although no differences were observed at the lowest bound of the distribution for expressive vocabulary (a standard score of 20), between-group differences in the slopes of the trajectories of symptom severity did emerge. For participants with FXS, severity of restricted and repetitive behaviors decreased as expressive vocabulary standard scores increased. In contrast, the severity of restricted and repetitive behaviors symptoms was stable across the full range of expressive vocabulary standard scores for participants with ASD. This finding was replicated not only when analyses were restricted to individuals classified as having comorbid FXS+ASD but also in comparisons of severity-matched children. Once again, it would appear that calibration of a metric that reduces the influence of developmental factors was successful for individuals with nonsyndromic ASD but not those with FXS+ASD.
Across all of the analyses of the present study, symptoms of ASD appeared to be relatively stable and did not tend to covary with other domains of development in individuals with nonsyndromic ASD. In the standardization sample for ADOS severity scores (Hus et al., 2014), verbal ability accounted for less than 10% of the variance in calibrated severity scores for the Social Affective domain and verbal and nonverbal ability accounted for less than 5% of the variance in calibrated severity scores for the Restricted and Repetitive Behaviors domain (Hus et al., 2014). Therefore, our findings of limited associations between domain severity scores and developmental correlates provides additional evidence that Hus et al. (2014) were successful in limiting the influence of developmental characteristics for participants with ASD. Recent cross-sectional research examining the developmental trajectories of ASD symptomatology in two large cohorts of preschool and school-age children with nonsyndromic ASD has additionally revealed that approximately 80% of children demonstrated stable severity scores across time, being classified as having either persistently high or persistently moderate levels of impairment (Gotham, Pickles, & Lord, 2012; Venker, Ray-Subramanian, Bolt, & Ellis Weismer, 2014). Results of the current study, while examining a much smaller sample, also suggest that ASD symptoms may not change significantly as age increases for children with nonsyndromic ASD.
In contrast, our findings revealed that severity of social affective symptoms and restricted and repetitive behaviors covaried with other domains of functioning in males with FXS, indicating that the calibration of severity scores for children with nonsyndromic ASD was not generalizable to this more homogeneous group of children with a single identified genetic etiology. Interestingly, as discussed by Venker et al. (2014), existing research examining the developmental trajectories of overall ASD severity scores for children with nonsyndromic ASD suggests that these trajectories are less stable across time in children presenting with mild to moderate levels of ASD severity and more stable in children presenting with moderate to high levels of ASD severity. Results from the present study are relatively consistent with this interpretation. Males with FXS in the current study demonstrated significantly lower (i.e., less impaired) social affective severity scores than did males with nonsyndromic ASD, even when only participants with FXS who met research standards for a comorbid diagnosis of ASD were included in the sample. Our findings also indicated that males with FXS demonstrated significantly lower restricted and repetitive behavior severity scores than did males with nonsyndromic ASD, even when only the participants with comorbid FXS+ASD were included in the analysis. In contrast to social affective symptom severity, the severity of restricted and repetitive behaviors did not appear to change with chronological age in either participant group; however the between-group difference in symptom severity was smaller for the Restricted and Repetitive Behavior domain than for the Social Affective domain. Although preliminary, our data suggest the presence of between-group differences in the influence of nonverbal cognitive ability on severity of social affective symptoms and in the influence of expressive vocabulary ability on severity of restricted and repetitive behaviors even when children are matched on overall severity of ASD symptomatology.
Within the field, there is ongoing debate regarding how similar males with FXS and males with nonsyndromic ASD actually are at the level of individual behaviors and what these symptoms of ASD mean in FXS (e.g., Hall et al., 2010; Budimirovic & Kaufman, 2011). Collectively, there is growing evidence that although similarities do exist, there are important differences between these two conditions in the behavioral manifestations of ASD symptomatology and their behavioral correlates (e.g., McDuffie et al., in press; Wolff et al., 2013).
Although we have focused on differences between FXS and nonsyndromic ASD, it is important to acknowledge that there are also many commonalities even at the level of individual symptoms (Abbeduto, McDuffie, & Thurman, 2014). This is to be expected given that symptoms associated with ASD are distributed as a continuum across the population and simply rise to a threshold of atypicality in individuals who then meet the cut-offs established for ASD diagnosis. Additionally, common biological substrates are known to exist across the two disorders. For example, levels of FMRP, a protein product which controls the translation of approximately 30% of the genes implicated ASD (Iossifov et al., 2012), have been observed to be lower in the brains of individuals with nonsyndromic ASD as compared to controls (Fatemi & Folsom, 2011). Thus, there are potential commonalities between FXS and nonsyndromic ASD even at the molecular level.
Limitations and Future Directions
The findings of the current study suggest that understanding predictors and trajectories of development has the potential to make especially relevant contributions to our theoretical and clinical understanding of the phenotypes of both FXS and nonsyndromic ASD. However, this line of research is still in its infancy. To begin, it is vital that prospective longitudinal data be collected to confirm between-group differences in the developmental trajectories of ASD symptomatology observed using cross sectional approaches. The present project examined a small number of predictors likely to influence or moderate the presence of ASD symptomatology. There certainly are other important characteristics (e.g., child and environmental) that may also differentially contribute to the emergence of ASD symptomatology across time. An additional limitation of the present study is that research staff was not blind to participant diagnostic group membership. Although blinding of these experienced testers would be difficult to achieve given these tester's familiarity with the physical and behavioral features of FXS and/or the behavioral features of ASD, a study in which clinicians who administered the ADOS and ADI-R were blind to diagnosis or in which clinical expertise was used to confirm ASD status would provide valuable information to support our understanding of ASD in FXS. In such a study, information could be gathered regarding clinical disagreements with the goal of revealing important insights into clinically meaningful differences between the two disorders.
The current study, as well as much of the evidence directly comparing children with FXS to those with nonsyndromic ASD, has utilized the ADOS and ADI-R, two measures designed to identify individuals with nonsyndromic ASD with high specificity. Although we acknowledge that these measures are considered to be the ‘gold standard’ tools for use in ASD diagnostic evaluations, they are but one piece of a larger picture. From a clinical standpoint there are a number of additional differences that become apparent when comparing children with FXS and children with nonsyndromic ASD. What is less apparent is what these differences mean and if they change how we interpret the behaviors that appear to be similar across the two conditions.
In the field of neurodevelopmental disorders, it is not bothersome to acknowledge that important differences can exist across syndromes despite the fact that they are all characterized by the presence of intellectual disability. It may be that ASD symptomatology should be considered similarly. From this perspective, an ASD diagnosis in FXS would merely provide an indication that problems exist in the core features of ASD that negatively impacts children's day to day functioning. Furthermore, this acknowledgement that both individuals with FXS and individuals with nonsyndromic ASD can share a diagnosis of ASD would not negate the possibility of condition-specific phenotypic differences in the individual behaviors that are summed to create this overall diagnosis, much as differences in cognitive profiles are observed in intellectual disability conditions (e.g., FXS and Down syndrome). Thus, in order to answer the questions of what needs to be done to improve children's functioning and whether there are phenotypic differences in the profiles of behaviors relating to an ASD diagnosis, broader measures that allow for a more thorough characterization of social affective skills and repetitive behavior symptoms will need to be utilized.
Alternatively, it may be the case that the behaviors displayed by children with FXS that are captured by “gold standard” measures are actually better explained by another comorbid condition. This determination becomes particularly problematic in the case of FXS due to the increased risk for numerous co-occurring conditions (e.g., intellectual disability, attentional difficulties, anxiety) that one would expect to impact the behaviors we are observing when we consider an ASD diagnosis. Thus, disentangling this complicated clinical presentation would require studies elucidating the roles of each of these factors in the development of the FXS behavioral phenotype. Although this could also be said for nonsyndromic ASD, there is evidence to suggest the severity of these co-occurring non-ASD features is more severe in FXS than they are in nonsyndromic ASD (Thurman et al., 2014).
Conclusion
The current study suggests differences in at least three child characteristics that may moderate the expression of the severity of ASD symptoms in school-aged boys with FXS relative to those with nonsyndromic ASD. These between-group differences suggest that there are between-syndrome differences in developmental pathways to and/or from these symptoms. The picture that emerges thus far not only informs our understanding of the FXS phenotype, but also provides important insights into ASD more generally. More specifically, this line of investigation suggests that the use of diagnostic categories potentially masks meaningful differences that could be revealed if a continuous metric of ASD symptomology were utilized or when individual behavioral symptoms were considered. Indeed, this is particularly apparent when a wide variety of behavioral features must be summarized using a metric that results in a categorical-ordinal classification, as is the case for the ADOS and the ADI-R. In addition, it is important to remember that commonalities in observable behaviors reflect, in essence, the developmental outcomes of a complex interplay of genetic, developmental, and environmental factors. We, therefore, cannot assume that the paths by which these behavioral symptoms have emerged are the same in any two children. This is particularly apparent in the present study as FXS likely represents a more straightforward correspondence between genes and behavior than does a multifactorial disorder like nonsyndromic ASD. Finally, it is our contention that a focus on elucidating differences in symptomatology and in identifying the developmental mechanisms underlying these behavioral symptoms has the potential to make especially important contributions to both theory and clinical practice. Psychopharmacological and behavioral interventions that target the underlying or core symptoms of a given disorder are currently under development. The efficacy of newly developed intervention approaches will depend on the goodness of fit between the components of the intervention and the neurocognitive mechanisms that a given intervention is designed to ameliorate. Moving our knowledge base forward, both in FXS and in nonsyndromic ASD, will likely require a focus on understanding how individual behaviors and/or constellations of behaviors change across time and on identifying the underlying factors that influence developmental changes in these behaviors.
Acknowledgments
This research was supported by grants R01 HD054764 and U54 HD079125 from the National Institute of Child Health and Human Development. We wish to thank the children and their families for their participation in this study. We also thank David Benjamin, Susan Harris, Beth Goodlin-Jones, Claire Hauser, Sara Armson, Eileen Haebig, Ashley Oakes, and Cecilia Compton for assisting with data collection and Susen Schroeder for coordinating all study visits. Leonard Abbeduto has received financial support to develop and implement outcome measures for fragile X syndrome clinical trials from F. Hoffman-LaRoche, Ltd., Roche TCRC, Inc. and Neuren Pharmaceuticals Limited. Randi J. Hagerman has received funding from Novartis, Roche Pharmaceuticals, Alcobra and Seaside Therapeutics to carry out treatment studies in fragile X syndrome and ASD. She has also consulted with Roche/Genetech and Novartis regarding treatment studies in fragile X syndrome.
Footnotes
No other authors have financial disclosures to make.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th. Washington, DC: American Psychiatric Publishing; 2000. text revision. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- Bailey DB, Hatton DD, Mesibov G, Ament N, Skinner M. Early development, temperament, and functional impairment in autism and fragile X syndrome. Journal of Autism and Developmental Disorders. 2000;30:49–59. doi: 10.1023/a:1005412111706. [DOI] [PubMed] [Google Scholar]
- Bailey DB, Hatton DD, Skinner M, Mesibov G. Autistic behavior, FMR1 protein, and developmental trajectories in young males with fragile X syndrome. Journal of Autism and Developmental Disorders. 2001;31:165–174. doi: 10.1023/a:1010747131386. [DOI] [PubMed] [Google Scholar]
- Bailey DB, Mesibov GB, Hatton DD, Clark RD, Roberts JE, Mayhew L. Autistic behavior in young boys with fragile X syndrome. Journal of Autism and developmental Disorders. 1998;28:499–508. doi: 10.1023/a:1026048027397. [DOI] [PubMed] [Google Scholar]
- Berry-Kravis EM, Hessl D, Rathmell B, Zarevics P, Cherubini M, Walton-Bowen K, Hagerman RJ. Effects of STX209 (Arbaclofen) on neurobehavioral function in children and adults with fragile X syndrome: A randomized, controlled, phase 2 trial. Science in Translational Medicine. 2012;4:152ra127. doi: 10.1126/scitranslmed.3004214. [DOI] [PubMed] [Google Scholar]
- Bishop SL, Richler J, Lord C. Association between restricted and repetitive behaviors and nonverbal IQ in children with autism spectrum disorders. Child Neuropsychology. 2006;12(4-5):247–267. doi: 10.1080/09297040600630288. [DOI] [PubMed] [Google Scholar]
- Bodfish JW, Symons FJ, Parker DE, Lewis MH. Varieties of repetitive behavior in autism: Comparisons to mental retardation. Journal of Autism and Developmental Disorders. 2000;30:237–243. doi: 10.1023/a:1005596502855. [DOI] [PubMed] [Google Scholar]
- Brown WT, Friedman E, Jenkins EC, Brooks J, Wisniewski K, Raguthu S, et al. Association of fragile X syndrome with autism. Lancet. 1982;9:100. doi: 10.1016/s0140-6736(82)90231-8. [DOI] [PubMed] [Google Scholar]
- Budimirovic DB, Kaufmann WE. What can we learn about autism from studying fragile X syndrome? Developmental Neuroscience. 2011;33:379–394. doi: 10.1159/000330213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Prevalence of autism spectrum disorder among children aged 8 years – Autism and Developmental Disabilities Monitoring Network, 11 sites, United States, 2010. Morbidity and Mortality Weekly Report Surveillance Summary. 2014;63:1–21. [PubMed] [Google Scholar]
- Clifford S, Dissanyake C, Bui QM, Huggins R, Taylor AK, Loesch DZ. Autism spectrum phenotype in males and females with fragile X full mutation and premutation. Journal of Autism and Developmental Disorders. 2007;37:738–747. doi: 10.1007/s10803-006-0205-z. [DOI] [PubMed] [Google Scholar]
- Cohen D, Pichard N, Tordjman S, Baumann C, Burglen L, Excoffier E, Héron D. Specific genetic disorders and autism: Clinical contribution toward their identifications. Journal of Autism and Developmental Disorders. 2005;35:103–116. doi: 10.1007/s10803-004-1038-2. [DOI] [PubMed] [Google Scholar]
- Constantino JN. The quantitative nature of autistic social impairment. Pediatric Research. 2011;69:55R–62R. doi: 10.1203/PDR.0b013e318212ec6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino JN, Gruber CP, Davis S, Hayes S, Passanante N, Przybeck T. The factor structure of autistic traits. Journal of Child Psychology and Psychiatry. 2004;45(4):719–726. doi: 10.1111/j.1469-7610.2004.00266.x. [DOI] [PubMed] [Google Scholar]
- Dissanayake C, Bui Q, Bulhak-Patterson D, Huggins R, Loesch DZ. Behavioural and cognitive phenotypes in idiopathic autism versus autism associated with fragile X syndrome. Journal of Autism and Developmental Disorders. 2009;28:393–405. doi: 10.1111/j.1469-7610.2008.01988.x. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Folsom TD. Dysregulation of fragile X mental retardation protein and metabotropic glutamate receptor 5 in superior frontal cortex of individuals with autism: A postmortem brain study. Molecular Autism. 2011;2:6. doi: 10.1186/2040-2392-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein C, Reiss AL. Autism: The point of view from fragile X studies. Journal of Autism and Developmental Disorders. 1998;28:393–405. doi: 10.1023/a:1026000404855. [DOI] [PubMed] [Google Scholar]
- Fidler DJ, Lunkenheimer E, Hahn L. Emerging behavioral phenotypes and dynamic systems theory. International Review or Research on Developmental Disabilities. 2011;40:17–42. [Google Scholar]
- Gotham K, Pickles A, Lord C. Standardizing ADOS scores for a measure of severity in autism spectrum disorders. Journal of autism and developmental disorders. 2009;39(5):693–705. doi: 10.1007/s10803-008-0674-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurken CK, Hagerman RJ. Targeted treatments in autism and fragile X syndrome. Research in Autism Spectrum Disorders. 2012;6:1311–1320. doi: 10.1016/j.rasd.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerman R, Lauterborn J, Au J, Berry-Kravis E. Fragile X syndrome and targeted treatments. In: Denman RB, editor. Modeling Fragile X Syndrome. New York: Springer; 2012. pp. 297–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall SS, Lightbody AA, Hirt M, Rezvani A, Reiss AL. Autism in fragile X syndrome: A category mistake? Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49:921–933. doi: 10.1016/j.jaac.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris S, Hessl D, Goodlin-Jones B, Ferranti J, Bacalman S, Barbato I, et al. Autism profiles in males with fragile X syndrome. American Journal on Mental Retardation. 2008;113:427–438. doi: 10.1352/2008.113:427-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatton DD, Sideris J, Skinner M, Mankowski J, Bailey DB, Roberts J, Mirrett P. Autistic behavior in children with fragile X syndrome: Prevalence, stability, and the impact of FMRP. American Journal of Medical Genetics – Part A. 2006;140:1804–1813. doi: 10.1002/ajmg.a.31286. [DOI] [PubMed] [Google Scholar]
- Hertz-Picciotto I, Croen LA, Hansen R, Jones CR, van de Water J, Pessah IN. The CHARGE study: An epidemiological investigation of genetic and environmental factors contributing to autism. Environmental Health Perspectives. 2006;114:1119–1125. doi: 10.1289/ehp.8483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessl D, Nguyen D, Green C, Chavez A, Tassone F, Hagerman R, et al. A solution to limitations of cognitive testing in children with intellectual disabilities: The case of fragile X syndrome. Journal of Neurodevelopmental Disorders. 2009;1:33–45. doi: 10.1007/s11689-008-9001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hus V, Gotham K, Lord C. Standardizing ADOS domain scores: Separating severity of social affect and restricted and repetitive behaviors. Journal of Autism and Developmental Disabilities. 2014;44:2400–2412. doi: 10.1007/s10803-012-1719-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iossifov I, Ronemus M, Levy D, Wang Z, Hakker I, Rosenbaum J, Wilson RK. De novo gene disruptions in children on the autism spectrum. Neuron. 2012;74:285–299. doi: 10.1016/j.neuron.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanner L. Autistic disturbances of affective context. Nervous Child. 1943;2:217–250. [Google Scholar]
- Karmiloff-Smith A. Development itself is the key to understanding developmental disorders. Trends in Cognitive Science. 1998;2:389–398. doi: 10.1016/s1364-6613(98)01230-3. [DOI] [PubMed] [Google Scholar]
- Kover ST, Abbeduto L. Expressive language in male adolescents with fragile X syndrome with and without co-morbid autism. Journal of Intellectual Disability Research. 2010;54(3):246–265. doi: 10.1111/j.1365-2788.2010.01255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kover ST, McDuffie AS, Hagerman RJ, Abbeduto L. Receptive vocabulary in boys with Autism Spectrum Disorder: Cross-sectional developmental trajectories. Journal of Autism and Developmental Disorders. 2013;43:2696–2709. doi: 10.1007/s10803-013-1823-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kover ST, Pierpont EI, Kim JS, Brown WT, Abbeduto L. A neurodevelopmental perspective on acquisition of nonverbal cognitive skills in adolescents with fragile X syndrome. Developmental Neuropsychology. 2013;38:445–460. doi: 10.1080/87565641.2013.820305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loesch DZ, Bui QM, Dissanyake C, Clifford S, Gould E, Bulhak-Paterson D, et al. Molecular and cognitive predictors of the continuum of autistic behaviours in fragile X. Neuroscience and Biobehavioral Reviews. 2007;31:315–326. doi: 10.1016/j.neubiorev.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S. Autism Diagnostic Observation Schedule (ADOS) Los Angeles: Western Psychological Services; 1999. [Google Scholar]
- McDuffie A, Abbeduto L, Lewis P, Kover S, Kim Jee-Seon, Weber A. Autism spectrum disorder in children and adolescents with fragile X syndrome: Within-syndrome differences and age-related changes. American Journal on Intellectual and Developmental Disabilities. 2010;115:307–326. doi: 10.1352/1944-7558-115.4.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDuffie A, Kover ST, Hagerman RJ, Abbeduto L. Investigating word learning in fragile X syndrome: A fast-mapping study. Journal of Autism and Developmental Disorders. 2013;43:1676–1691. doi: 10.1007/s10803-012-1717-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDuffie A, Thurman AJ, Hagerman RJ, Abbeduto L. Symptoms of autism in males with fragile X syndrome: A comparison to nonsyndromic ASD using current ADI-R scores. Journal of Autism and Developmental Disorders. doi: 10.1007/s10803-013-2013-6. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakes AC, Thurman AJ, McDuffie A, Bullard L, Hagerman R, Abbeduto L. Abbeduto L. Characterizing repetitive behaviors in young males with fragile X syndrome. Understanding autism symptomatology in fragile X syndrome; Symposium conducted at the Gatlinburg Conference on Research and Theory in Intellectual Disabilities; Chicago, IL. 2014. Chair. [Google Scholar]
- Oostra BA, Willemson R. A fragile X balance: FMR1 expression levels. Human Molecular Genetics. 2003;12:249–457. doi: 10.1093/hmg/ddg298. [DOI] [PubMed] [Google Scholar]
- Persico AM, Bourgeron T. Searching for ways out of the autism maze: Genetic, epigenetic, and environmental clues. Trends in Neuroscience. 2006;29:349–358. doi: 10.1016/j.tins.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Ray-Subramanian CE, Ellis Weismer S. Receptive and expressive language as predictors of restricted and repetitive behaviors in young children with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2012;42(10):2113–2120. doi: 10.1007/s10803-012-1463-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risi S, Lord C, Gotham K, Corsello C, Chrysler C, Szatmari P, et al. Combining information from multiple sources in the diagnosis of autism spectrum disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 2006;45:1094–1103. doi: 10.1097/01.chi.0000227880.42780.0e. [DOI] [PubMed] [Google Scholar]
- Rogers SJ, Wehner E, Hagerman R. The behavioral phenotype in fragile X: Symptoms of autism in very young children with fragile X syndrome, idiopathic autism, and other developmental delays. Journal of Developmental & Behavioral Pediatrics. 2001;22:409–417. doi: 10.1097/00004703-200112000-00008. [DOI] [PubMed] [Google Scholar]
- Roid G, Miller L. Leiter International Performance Scale – Revised. Wood Dale, IL: Stoelting; 1997. [Google Scholar]
- Ronald A, Hoekstra RA. Autism spectrum disorders and autistic traits: A decade of new twin studies. American Journal of Medical Genetics - Part B. 2011;156:255–274. doi: 10.1002/ajmg.b.31159. [DOI] [PubMed] [Google Scholar]
- Russell G. Contextualising Autism Diagnosis. Autism. 2014;4(128):2. [Google Scholar]
- Rutter M, LeCouteur A, Lord C. Autism Diagnostic Interview-Revised. Los Angeles: Western Psychological Services; 2003. [Google Scholar]
- Schopler E, Reichler R, Renner B. The childhood autism rating scale (CARS) Los Angeles: Western Psychological Services; 1988. [Google Scholar]
- Thomas MS, Annaz D, Ansari D, Scerif G, Jarrold C, Karmiloff-Smith A. Using developmental trajectories to understand developmental disorders. Journal of Speech, Language and Hearing Research. 2009;52:336–358. doi: 10.1044/1092-4388(2009/07-0144). [DOI] [PubMed] [Google Scholar]
- Thurman AJ, McDuffie A, Hagerman RJ, Abbeduto L. Psychiatric symptoms in boys with fragile X syndrome: A comparison with nonsyndromic autism spectrum disorder. Research in Developmental Disabilities. 2014;35:1072–1086. doi: 10.1016/j.ridd.2014.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venker CE, Ray-Subramanian CE, Bolt DM, Ellis Weismer S. Trajectories of autism severity in early childhood. Journal of Autism and Developmental Disorders. 2014;44(3):546–563. doi: 10.1007/s10803-013-1903-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KT. Expressive Vocabulary Test-2nd ed. Minneapolis, MN: Pearson Assessments; 2007. [Google Scholar]
- Wolff JJ, Bodfish JW, Hazlett HC, Lightbody AA, Reiss AL, Piven J. Evidence of a distinct behavioral phenotype in young boys with fragile X syndrome and autism. Journal of the American Academy of Child and Adolescent Psychiatry. 2012;51:1324–1332. doi: 10.1016/j.jaac.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]


