Abstract
The neuronal Ca2+-binding protein Recoverin has been shown to regulate phototransduction termination in mammalian rods. Here we identify four recoverin genes in the zebrafish genome, rcv1a, rcv1b, rcv2a and rcv2b, and investigate their role in modulating the cone phototransduction cascade. While Recoverin-1b is only found in the adult retina, the other Recoverins are expressed throughout development in all four cone types, except Recoverin-1a, which is expressed only in rods and UV cones. Applying a double flash electroretinogram (ERG) paradigm, downregulation of Recoverin-2a or 2b accelerates cone photoresponse recovery, albeit at different light intensities. Exclusive recording from UV cones via spectral ERG reveals that knockdown of Recoverin-1a alone has no effect, but Recoverin-1a/2a double-knockdowns showed an even shorter recovery time than Recoverin-2a-deficient larvae. We also showed that UV cone photoresponse kinetics depend on Recoverin-2a function via cone-specific kinase Grk7a. This is the first in vivo study demonstrating that cone opsin deactivation kinetics determine overall photoresponse shut off kinetics.
Keywords: phototransduction termination, zebrafish, cone photoreceptor, electroretinogram
1. Introduction
The vertebrate retina contains two classes of photoreceptors, rods and cones, which function at low and bright light conditions, respectively. Although both share a similar G-protein-coupled phototransduction pathway, the cone photoresponse is characterized by lower sensitivity and faster kinetics, which allows cones to function over almost 9 orders of illumination magnitude [1]. The rate-limiting step of photoresponse recovery is also different between these two cell types [2–4]. Rods and cones use cell-type-specific molecules in the phototransduction cascade, which may account for these differences.
The cascade is initiated by light-activated rhodopsin (Rh*), which induces the detachment of trimeric G-protein Transducin α-subunit. The Transducin α-subunit in turn binds to phosphodiesterase (PDE), causing a decrease in the second messenger cGMP. This drop in cGMP levels leads to the closure of CNG cation channels, hyperpolarizing the photoreceptor and lowering [Ca2+]i [5]. The deactivation of both Rh* and the PDE–Transducin complex is required to terminate the phototransduction cascade. Rh* in rods is initially phosphorylated by Rhodopsin kinase Grk1 [6] in a Ca2+-dependent manner via the small Ca2+-binding protein Recoverin [7,8]. Rod Recoverin is proposed to inhibit Grk1 in darkness when outer segment [Ca2+]i is high and is released from Grk1 during light response when [Ca2+]i is decreasing [9,10]. Modulation of Rhodopsin lifetime during light adaption is abolished in Recoverin-deficient mice [11]. The final deactivation of Rh* is achieved by the binding of Arrestin [12], while intrinsic GTPase activity ends the ability of PDE–Transducin complex to hydrolyse cGMP [13].
Cone-specific opsin kinase Grk7 has similar functions as rod Grk1 [14,15]. Interestingly, cone Arrestin is not essential to deactivate short-wavelength opsin in mice [16,17], but its functional loss induces delayed cone photoresponse recovery in cone-dominant zebrafish [18]. A Ca2+-sensitive phosphorylation of cone visual pigment in vivo in zebrafish [19] indicates a possible role of Recoverin in regulating cone opsin quenching via direct control of [Ca2+]i during light response.
In this study, we take advantage of the cone-dominant retina of zebrafish to study Recoverin function in cone vision. The zebrafish genome harbours four rcv genes. While Recoverin-1b (Rcv1b) is only expressed in the adult retina the other Recoverins are present throughout development in all cone types, with the exception of Rcv1a, which is expressed in rods and UV cones only.
We determined photoresponse recovery with a double flash electroretinogram (ERG) paradigm [14] analysing the role of Recoverin proteins. Our results establish that the cone photoresponse kinetics are shaped by photopigment quenching and a loss of Recoverin leads to an accelerated photoresponse recovery.
2. Material and methods
2.1. Zebrafish care
Zebrafish (Danio rerio) were kept at a 14 L : 10 D cycle at 28°C [20]. Embryos of WIK wild-type fish were raised in E3 medium containing 0.01% methylene blue or with 0.2 mM PTU (1-phenyl-2-thiourea; Sigma-Aldrich) to avoid pigmentation. Adult zebrafish were sacrificed by fish system water containing 0.4% 3-aminobenzoic acid methyl ester (MESAB, Sigma-Aldrich) and 4.6 mM NaHCO3.
2.2. Cloning of recoverin genes and in situ hybridization
Cloning was performed as described in [21] using oligonucleotide primers listed in electronic supplementary material S1. Digoxigenin-labelled in situ hybridization (ISH) RNA probes were generated according to supplier's instructions (DIG RNA Labelling Mix, Roche) and used on zebrafish larvae as previously described [22].
2.3. Generation of antibodies
Custom polyclonal peptide antibodies were raised by Eurogentec (Seraing, Belgium). Rabbits were immunized with the Rcv1a peptide CIQYDEPKKIQEKLKEKKH or Rcv2b peptide CKLIPKDKQTSLPNDES. Guinea pigs were immunized with the Rcv1b peptide CIQFDKPQKVQEKLKEKTQ or Rcv2a peptide CKLIPKEDQESLPADEN. Antibodies were affinity-purified.
2.4. Immunohistochemistry
Section immunohistochemistry was carried out as described previously [14], except for the following modifications. Adult zebrafish eyes were fixed in 2% trichloroacetic acid (Sigma-Aldrich, Switzerland) for 30 min at room temperature. Primary Recoverin antibodies were diluted 1 : 400. Chicken anti-GFP antibody (Sigma-Aldrich, dilution 1 : 500) and mouse anti-PCKalpha (MC5) antibody (Novus Biologicals, NB200-586; 1 : 500) were used. All the secondary antibodies (Invitrogen) were diluted 1 : 1000 in PBS.
Whole-mount immunohistochemistry was performed on 5 days post-fertilization (dpf) larvae. The larvae were fixed in 2% tricholoroacetic acid at 4°C for 30 min, dehydrated in a methanol series and stored in 100% methanol for at least 24 h, then incubated in ice-cold acetone for 7 min before blocking.
2.5. Microscopy
ISH images were taken by a light microscope (BX61, Olympus) with a CCD camera (ColorView IIIu, Soft Imaging System, Olympus) and processed by Adobe Photoshop CS3. Fluorescence Z-stacks photos were taken by a confocal laser scanning microscope (Leica SP5, Leica Microsystems) and processed by Imaris 7.6.3 (Bitplane, Zurich, Switzerland).
2.6. Targeted gene knockdown
Antisense morpholino oligonucleotides (Gene Tools, Philomath, OR, USA) were designed against translational start sides and injected into one cell-stage embryos (electronic supplementary material S1).
Amounts of morpholino per embryo were rcv1a (20 ng), rcv2a (7.4 ng or 3.6 ng), grk7a (2.4 ng), rcv2b (1.2 ng) and control (7.4 ng). For double knockdowns, rcv2a (3.6 ng)/grk7a (2.4 ng) or rcv1a (11 ng)/rcv2a (7.4 ng) were injected.
2.7. Western blot
Twenty to forty 5 dpf larvae were homogenized in 150 µl RIPA buffer (150 mM NaCl, 1% Triton-X, 0.5% sodium deoxycholate, 50 mM Tris (pH 8), 1 mM EDTA, 0.1% SDS). β-Actin (approx. 42 kDa) was used as a loading control. Primary antibodies were diluted to the following concentrations: Rcv1a: 1 : 1,000; Rcv2a: 1 : 2000; Rcv2b: 1 : 1000 β-Actin: 1 : 3000. Secondary horseradish peroxidase (HPR)-linked antibodies (Invitrogen) were diluted in blocking buffer (goat anti-rabbit: 1 : 15 000; rabbit anti-guinea pig: 1 : 25 000; goat anti-mouse: 1 : 10 000). Signal was detected by the LAS 4000 Chemiluminescence Imager (software: Image Quant LAS 4000, automatic exposure) and knockdown levels were semi-quantified by ImageJ [23].
2.8. Normal and spectrum electroretinography
Normal (white) ERG was recorded as previously described [24]. Full light intensity (light source: Zeiss XBO 75 W) was measured by spectrometer (Ocean Optics, USB2000+) with spectrum shown in S2A (SpectraSuite, Ocean Optics). For the 100% double flash paradigm, pairs of two light flashes with the same intensity and duration (500 ms) were given [14]. The interval between two flashes was increasing (100, 200, 300, 500, 1000, 2000, 3500 and 5000 ms). A neutral density filter was applied to have 0.1% double white flash paradigm and the interval between two flashes was progressively increasing (100, 150, 200, 250, 300, 350, 400, 450 and 500 ms). The interval between two pairs was always 10 s for both bright light and dim light response. Spectrum ERG used the similar set-up but additional background light source (Philips projection lamp type 6958, 20 V, 250 W; housing: Liesegang Diafant 250) with a short wavelength absorbing filter and an UV light filter in front of white light source (electronic supplementary material S2B). After calibration, the spectrum graph showed that most of the light above 385 nm in the visible range was blocked. There was indeed a peak at around 720 nm. To make sure this peak cannot generate any electrical response in the retina, another short wavelength absorbing filter was applied together with this UV light filter to receive only the light at around 720 nm. But even in darkness this light did not give any ERG response. The background light was used to adapt the blue, green and red cones with minimal activation for UV cones. For paired UV flash recordings, flash interval and duration were the same as for dim flash recordings with one extra interval of 750 ms. All the experiments were performed at room temperature (22°C).
3. Results
In order to explore the role of Recoverins (Rcvs) in the termination of the visual transduction cascade, we cloned four zebrafish recoverin (rcv) orthologues, namely rcv1a, rcv1b, rcv2a and rcv2b.
3.1. Recoverins are expressed in photosensitive organs
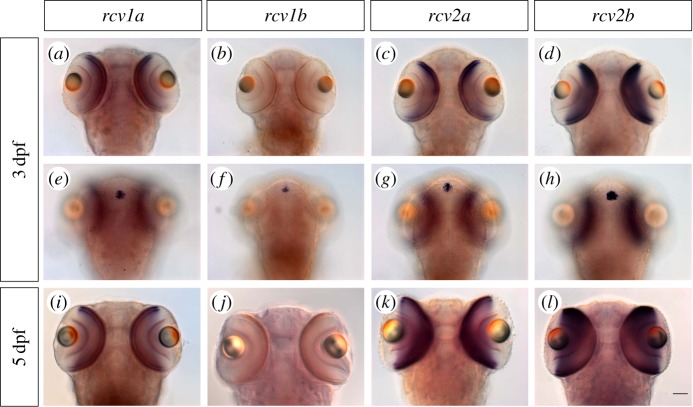
We determined both the RNA and protein expression of the Recoverins in larval and adult zebrafish. All four rcvs transcripts are expressed in the pineal gland, a photosensitive organ, starting at around 2 dpf. At around 3 dpf, expression is initiated in the ventral outer retina, with the exception of rcv1b transcripts, which are absent from the larval retina (figure 1). At 5 dpf, when the retina becomes fully functional with the exception of rods that do not significantly contribute to visual function at this stage [25], rcv1a, rcv2a and rcv2b transcripts are expressed throughout the photoreceptor cell layer.
Figure 1.
Expression of rcv genes in 3 dpf and 5 dpf zebrafish larvae. (a–h) All the rcv genes except rcv1b showed expression in 3 dpf larvae retina. All the rcv genes were expressed in the pineal gland. (i–l) rcv1b still showed no expression in the 5 dpf larval retina. Scale bar (=50 µm) applies to all panels.
The zebrafish retina contains one rod and four cone types, namely UV-sensitive (expressing the opn1sw1 opsin gene) and blue-sensitive (opn1sw2) short single cones and double cones that have a red- (opn1lw) and green- (opn1mw) sensitive member [26].
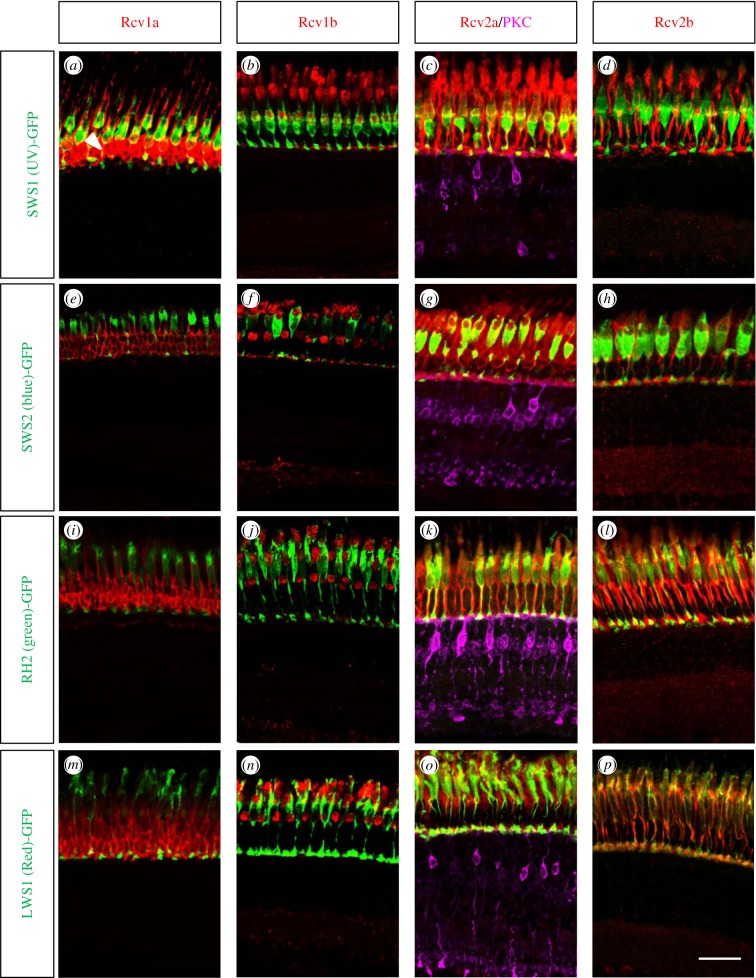
In order to determine the cellular and subcellular localization of the Rcv proteins, we generated paralogue-specific antibodies against all four Rcvs and performed immunohistochemistry on adult retinal section of different transgenic lines with GFP-labelled cone subtypes [27–30]. We detected Rcv1a expression in rods and UV cones (figure 2), while the two Rcv2 paralogues were expressed in all cone subtypes. Rcv2a also showed staining in the inner nuclear layer at the level of bipolar cells (electronic supplementary material S3), where the labelling partially colocalized with the ON-bipolar cell marker protein kinase C alpha (PKC). Interestingly, this inner retinal staining was neither detected by ISH (figure 1) nor immunohistochemical staining in the larval retina (electronic supplementary material S4A). Rcv1b protein was found in all adult cone photoreceptors (figure 2), while being absent in the larval retina (figure 1).
Figure 2.
Zebrafish Recoverins are expressed in the different cone subtypes. Adult retinal sections from transgenic zebrafish highlighting the different cone subtypes were co-stained with Rcv antibodies. White arrowhead in (a) marked the rod photoreceptors. While Rcv1b, Rcv2a and Rcv2b proteins are present in all cone subtypes, Rcv1a is only expressed in rods and UV cones. In order to highlight also ON-bipolar cells, Rcv2a antibodies were supplemented with PKC antibodies (violet staining). Scale bar (=20 µm) applies to all panels.
3.2. UV cone photoresponse recovery is accelerated in Rcv-deficient zebrafish larvae
To study the function of Recoverin proteins, we designed morpholinos against rcvs and cone-specific opsin kinase Grk7 [14]. The knockdown efficiency at 5 dpf was evaluated by Western blot and whole-mount immunohistochemistry (electronic supplementary material S4). The levels of each Rcv protein were largely reduced when it was knocked down while levels of the other Rcv proteins were maintained, indicative of both knockdown efficacy and antibody specificity. The knockdown efficiency was in the range of 90% (Rcv1a, 93%; Rcv2a, 97%; Rcv2b, 88%), as semi-quantified by Western blots using β-actin as loading control (electronic supplementary material S4B).
In order to study the function of the only mammalian orthologue Rcv1a in larvae and to investigate how different Recoverins modulate opsin lifetime in the same cell, we used spectral ERG to isolate photoresponse, which was largely contributed by UV cones. At 5 dpf, photoresponses are considered to be purely cone driven [25]. Additionally, UV cones can be selectively stimulated by UV light with minimal activation of the other cone subtypes.
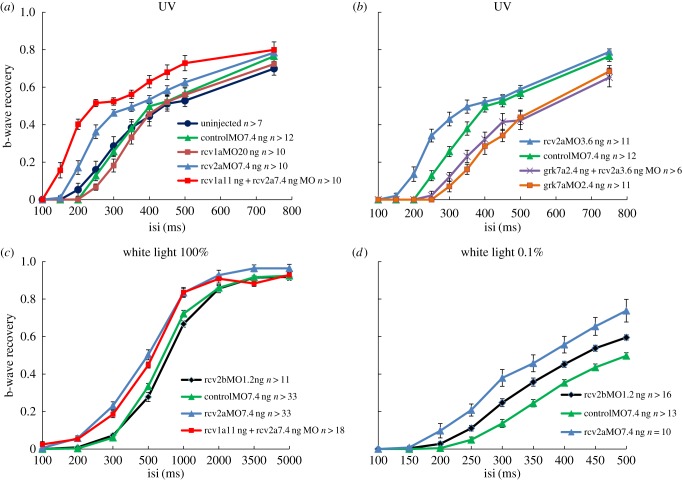
In the double flash paradigm [14], uninjected WT larvae showed similar b-wave recovery compared with control morphants (p > 0.05; figure 3a). Although there was less than 10% protein left in Rcv1a morphants, they did not show any significant acceleration of photoresponse recovery (p > 0.05). When Rcv2a was knocked down (figure 3a), b-wave recovery was accelerated (p < 0.005 at interval 200–300 ms). Double knockdown of Rcv1a and Rcv2a further accelerated the recovery time (p < 0.005 at interval 150 ms and 200 ms compared with Rcv2a single knockdown). In order to recover to 40% of their dark levels, Rcv1a and Rcv2a double knockdown, Rcv2a single knockdown and control morphants required around 200 ms, 275 ms and 350 ms, respectively. Rcv1a knockdown did not induce any phenotype, but Rcv1a and Rcv2a double defect larvae showed significant acceleration compared with Rcv2a single knockdown, indicating that the function of Rcv1a can be replaced by Rcv2a. Concomitant reduction of Rcv2a and Rcv1a cannot be compensated any more. The fact that recovery was faster rather than slower in morphants argues against a toxic side effect of morpholino injection.
Figure 3.
Cone photoresponse recovery is accelerated in Rcv-deficient larvae. Time course of b-wave recovery in (a,b) UV spectral ERG, (c) maximum white light ERG and (d) dim white light ERG are shown. Under UV light conditions recoverin2a and combination of recoverin1a and recoverin2a are effective in accelerating the response recovery (a), whereas depleting the effort kinase Grk7a as expected prolongs the recovery time (b). Note that Rcv2a knockdown accelerates response recovery under normal and dim white light conditions, whereas Rcv2b only accelerates recovery under dim light conditions (c,d). Data are presented as mean ± s.e.m.
Because Rcv proteins are highly expressed in the photoreceptor synaptic terminal (figure 2; electronic supplementary material, figure S4) and Rcv1 in mice has been reported to modulate synaptic transmission in the rod pathway [31], it is possible that the faster b-wave recovery in the morphants not only comes from the faster phototransduction decay, but is additionally mediated by an effect on synaptic transmission. The contribution of Rcv downregulation to synaptic transmission can be easily evaluated by quantifying the b-wave amplitude and time-to-peak (table 1). Indeed in this experiment, we did not find any difference between morphants and controls, arguing that synaptic transmission is not affected, which suggested the effect we observed in b-wave recovery is purely contributed by the accelerated phototransduction termination.
Table 1.
Amplitude and time course of ERG b-wave in Rcv-deficient larvae is not significantly different with control. All the data are shown as mean ± s.e.m. One-way ANOVA shows p > 0.05 in all cases.
| UV |
|||||
|---|---|---|---|---|---|
| control | Rcv2aMO | Rcv1aMO | Rcv1a & 2aMO | ||
| amplitude (µV) | 92.6 ± 20.1 | 130.4 ± 13.4 | 96.5 ± 14.3 | 85.3 ± 14.1 | |
| Tpeak (ms) | 249.3 ± 8.0 | 250.6 ± 7.9 | 253.6 ± 5.4 | 246.6 ± 5.9 | |
| 100% white |
0.1% white |
||||
|---|---|---|---|---|---|
| control | Rcv2aMO | control | Rcv2aMO | Rcv2bMO | |
| amplitude (µV) | 716.0 ± 49.0 | 765.6 ± 72.0 | 182.4 ± 22.4 | 210.2 ± 51.8 | 149.9 ± 20.4 |
| Tpeak (ms) | 206.0 ± 4.4 | 200.8 ± 6.0 | 243.2 ± 8.8 | 249.0 ± 8.6 | 253.0 ± 11.2 |
3.3. Recoverin affects SWS1 recovery via Grk7a
In rod photoreceptors, it is known that Recoverin regulates the lifetime of activated rhodopsin via Rhodopsin kinase [7]. In order to confirm that cone-specific visual pigment kinase Grk7a (electronic supplementary material S5) interacts with Recoverin, we compared the photoresponse recovery in Grk7a single knockdown larvae with the recovery time in double knockdowns of Grk7a and Rcv2a (figure 3b).
We confirmed that the response recovery is significantly delayed in the absence of Grk7a not only under normal ERG [14] but also in UV spectrum ERG (p < 0.05 at interval 250–500 ms), indicating Grk7a is a general cone opsin kinase (figure 3b). Importantly, the photoresponse recovery was not influenced by the additional reduction of Rcv2a (p > 0.5), indicating that Rcv2a acts via Grk7a. Hence, Recoverin regulates SWS1 opsin lifetime via cone opsin kinase.
3.4. Rcv2a and Rcv2b depletion accelerate photoresponse recovery under varying light conditions
It was suggested that white light ERG is dominated by the double cone photoresponse [18]. The fact that (unlike UV response) there was no significant difference in b-wave recovery between Rcv1a and Rcv2a double knockdown and Rcv2a single knockdown larvae in the double white flash paradigm proved this notion for the first time (figure 3c). Indeed, there was a significant acceleration of b-wave response recovery in rcv2a morphants with paired saturating flashes compared with control morphants (figure 3c). The rcv2a morphant recovery at an interval of 300 ms was three times faster than in control morphants. However, normal response kinetics were observed in rcv2b morphants (p > 0.1). Nevertheless, under dim flash conditions (0.1% of maximal light intensity) downregulation of rcv2a and rcv2b both accelerated the response decay (p < 0.05 at intervals from 250 ms to 500 ms; figure 3d). It took about 400 ms for rcv2a morphants, 450 ms for rcv2b morphants and 550 ms for the controls to recover about half of the photoresponse, suggesting a role of rcv2b in modulating the photoresponse kinetics only at a smaller Ca2+ dynamic range.
4. Discussion
Rhodopsin quenching requires the phosphorylation by rhodopsin kinase [6,32] and subsequent binding of Arrestin [33]. Rhodopsin kinase is regulated by Recoverin in a Ca2+-dependent manner [7,8]. Exogenous Recoverin prolongs the rod response [34] and genetic deletion does the opposite [35]. Before this study, little was known about the function of Recoverin in cones. But both the Ca2+-sensitive cone opsin phosphorylation in zebrafish [19] and the Ca2+-sensitive cone opsin quenching step dominating the overall response kinetics in salamander [3,4] imply an important role of Recoverin in shaping the cone photoresponse.
Four recoverins were found in the zebrafish genome. Larval zebrafish double cones exclusively express rcv2a and rcv2b. During bright light conditions, Rcv2a works to delay red and green opsin quenching, while both Rcv2a and Rcv2b work under dim light conditions. Hence Rcv2b contributes only when Ca2+ dynamic range is small [36,37] and less opsin is bleached, suggesting that Rcv2a may be the primary Recoverin and works over broader light conditions. This suggests variations in Ca2+ sensitivity among Recoverin isoforms, which would correlate with different numbers of lysine residues at the C-terminus of various Rcvs. The number of positively charged lysine residues indirectly determines the differences in membrane affinity and efficiency of rhodopsin kinase inhibition between salamander rod and cone Recoverin [38,39]. A reduced number of lysine residues results in decreased Ca2+ sensitivity and requires higher Ca2+ concentrations to inhibit rhodopsin kinase [40]. Rcv2a and Rcv2b possess 2 and 1 lysine residues at the C-terminus, respectively. Another consistent mechanism for Rcv2b working in dim light conditions could be different functional pairs of Grk and Recoverin. Two paralogous genes of grk7 (grk7a and grk7b) and one paralogue for grk1 (grk1b) were found to be expressed in zebrafish cones, while grk1a is restricted to rods [14,41]. The detailed cellular distribution of Grks in cones is not known, but Grk7a is clearly expressed in all cone subtypes (electronic supplementary material S5). According to Rcvs expression pattern and functional analysis, there are at least two functional pairs in zebrafish: Grk1a-Rcv1a exclusively in rods and Grk7a-Rcv2a in UV cones. The specific activity of Grk7a for rhodopsin phosphorylation is around 30 times higher than that of all the other Grks [41]. Rcv2b may regulate Grk1b or Grk7b instead of Grk7a, which would be consistent with Rcv2b working under different conditions than Rcv2a. Different Ca2+ sensitivity and Grk affinity may both contribute to different working ranges between Rcv2a and Rcv2b.
In rods, it is believed that Ca2+ regulates light response and light adaptation via three mechanisms: the dynamic drop in intracellular Ca2+ concentration accelerates rhodopsin phosphorylation [7], speeds up the synthesis of cGMP through guanylyl cyclase [42] and enhances the cGMP affinity of CNG-channels [43]. In cone photoreceptors there is a larger fraction of dark current carried by Ca2+ [44], a faster light-induced Ca2+ dynamic decline and a bigger Ca2+ dynamic range than in rods [37,45]. Furthermore, cones show lower sensitivity, faster response kinetics and adaption to a much wider range of light intensity than rods. It is expected that there is a more powerful Ca2+ negative feedback in cones. The Ca2+-dependent regulation on CNG channel ligand sensitivity is significantly more potent in cones with CNG-modulin as a modulator than in rods using calmodulin [46,47]. However, the Ca2+-sensitive guanylyl cyclase activating proteins (GCAPs) show weaker modulation in mammalian cones than in rods [48]. Ca2+ probably meditates a more powerful feedback via visual pigment phosphorylation in cones than in rods because the rate-limiting step of bright light response recovery in cones has been found to be opsin quenching [3,4], instead of Ca2+ insensitive PDE–Transducin deactivation in rods [2].
Our study is the first in vivo demonstration that cone opsin phosphorylation is indeed regulated by the Ca2+-binding protein Recoverin, which allows Ca2+ to directly control cone response kinetics and modulate the time scale during light response and adaptation.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We are grateful to Kara Dannenhauer for excellent fish care and laboratory management, and the whole Neuhauss group for scientific discussion.
Data accessibility
Genbank accession numbers: KT325590–KT325593.
Authors' contributions
J.Z. carried out the molecular, imaging and electrophysiological work, data analysis and design of the study, and drafted the manuscript; J.K. participated in imaging and electrophysiological work; E.K. contributed to cloning; M.G. contributed to molecular analysis, antibody design and drafted the manuscript; S.C.F.N. conceived, designed and coordinated the study, and drafted the manuscript. All authors gave final approval for publication.
Competing interests
We have no competing interests.
Funding
This work was supported by EU FP7 ZF-HEALTH, the Swiss National Science Foundation 31003A_153289/1 and EMBO ALTF 326-2010 (E.K.).
References
- 1.Fu Y, Yau KW. 2007. Phototransduction in mouse rods and cones. Pflugers Arch. 454, 805–819. (doi:10.1007/s00424-006-0194-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krispel CM, et al. 2006. RGS expression rate-limits recovery of rod photoresponses. Neuron 51, 409–416. (doi:10.1016/j.neuron.2006.07.010) [DOI] [PubMed] [Google Scholar]
- 3.Matthews HR, Sampath AP. 2010. Photopigment quenching is Ca2+ dependent and controls response duration in salamander L-cone photoreceptors. J. Gen. Physiol. 135, 355–366. (doi:10.1085/jgp.200910394) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zang J, Matthews HR. 2012. Origin and control of the dominant time constant of salamander cone photoreceptors. J. Gen. Physiol. 140, 219–233. (doi:10.1085/jgp.201110762) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fain GL, Matthews HR, Cornwall MC, Koutalos Y. 2001. Adaptation in vertebrate photoreceptors. Physiol. Rev. 81, 117–151. [DOI] [PubMed] [Google Scholar]
- 6.Bownds D, Dawes J, Miller J, Stahlman M. 1972. Phosphorylation of frog photoreceptor membranes induced by light. Nat. New Biol. 237, 125–127. (doi:10.1038/newbio237125a0) [DOI] [PubMed] [Google Scholar]
- 7.Chen CK, Inglese J, Lefkowitz RJ, Hurley JB. 1995. Ca2+-dependent interaction of recoverin with rhodopsin kinase. J. Biol. Chem. 270, 18 060–18 066. (doi:10.1074/jbc.270.30.18060) [DOI] [PubMed] [Google Scholar]
- 8.Kawamura S. 1993. Rhodopsin phosphorylation as a mechanism of cyclic GMP phosphodiesterase regulation by S-modulin. Nature 362, 855–857. (doi:10.1038/362855a0) [DOI] [PubMed] [Google Scholar]
- 9.Ames JB, Levay K, Wingard JN, Lusin JD, Slepak VZ. 2006. Structural basis for calcium-induced inhibition of rhodopsin kinase by recoverin. J. Biol. Chem. 281, 37 237–37 245. (doi:10.1074/jbc.M606913200) [DOI] [PubMed] [Google Scholar]
- 10.Strissel KJ, Lishko PV, Trieu LH, Kennedy MJ, Hurley JB, Arshavsky VY. 2005. Recoverin undergoes light-dependent intracellular translocation in rod photoreceptors. J. Biol. Chem. 280, 29 250–29 255. (doi:10.1074/jbc.M501789200) [DOI] [PubMed] [Google Scholar]
- 11.Chen CK, Woodruff ML, Chen FS, Chen D, Fain GL. 2010. Background light produces a recoverin-dependent modulation of activated-rhodopsin lifetime in mouse rods. J. Neurosci. 30, 1213–1220. (doi:10.1523/JNEUROSCI.4353-09.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuhn H. 1978. Light-regulated binding of rhodopsin kinase and other proteins to cattle photoreceptor membranes. Biochemistry 17, 4389–4395. (doi:10.1021/bi00614a006) [DOI] [PubMed] [Google Scholar]
- 13.Arshavsky V, Antoch MP, Philippov PP. 1987. On the role of transducin GTPase in the quenching of a phosphodiesterase cascade of vision. FEBS Lett. 224, 19–22. (doi:10.1016/0014-5793(87)80414-3) [DOI] [PubMed] [Google Scholar]
- 14.Rinner O, Makhankov YV, Biehlmaier O, Neuhauss SC. 2005. Knockdown of cone-specific kinase GRK7 in larval zebrafish leads to impaired cone response recovery and delayed dark adaptation. Neuron 47, 231–242. (doi:10.1016/j.neuron.2005.06.010) [DOI] [PubMed] [Google Scholar]
- 15.Tachibanaki S, Shimauchi-Matsukawa Y, Arinobu D, Kawamura S. 2007. Molecular mechanisms characterizing cone photoresponses. Photochem. Photobiol. 83, 19–26. [DOI] [PubMed] [Google Scholar]
- 16.Nikonov SS, Brown BM, Davis JA, Zuniga FI, Bragin A, Pugh EN, Craft CM. 2008. Mouse cones require an arrestin for normal inactivation of phototransduction. Neuron 59, 462–474. (doi:10.1016/j.neuron.2008.06.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi G, Yau KW, Chen J, Kefalov VJ. 2007. Signaling properties of a short-wave cone visual pigment and its role in phototransduction. J. Neurosci. 27, 10 084–10 093. (doi:10.1523/JNEUROSCI.2211-07.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Renninger SL, Gesemann M, Neuhauss SC. 2011. Cone arrestin confers cone vision of high temporal resolution in zebrafish larvae. Eur. J. Neurosci. 33, 658–667. (doi:10.1111/j.1460-9568.2010.07574.x) [DOI] [PubMed] [Google Scholar]
- 19.Kennedy MJ, Dunn FA, Hurley JB. 2004. Visual pigment phosphorylation but not transducin translocation can contribute to light adaptation in zebrafish cones. Neuron 41, 915–928. (doi:10.1016/S0896-6273(04)00086-8) [DOI] [PubMed] [Google Scholar]
- 20.Westerfield M. 2007. The zebrafish book: a guide for the laboratory use of zebrafish (Danio rerio), 5th edn Eugene, OR: University of Oregon Press. [Google Scholar]
- 21.Kastenhuber E, Gesemann M, Mickoleit M, Neuhauss SC. 2013. Phylogenetic analysis and expression of zebrafish transient receptor potential melastatin family genes. Dev. Dyn. 242, 1236–1249. (doi:10.1002/dvdy.24020) [DOI] [PubMed] [Google Scholar]
- 22.Haug MF, Gesemann M, Mueller T, Neuhauss SC. 2013. Phylogeny and expression divergence of metabotropic glutamate receptor genes in the brain of zebrafish (Danio rerio). J. Comp. Neurol. 521, 1533–1560. (doi:10.1002/cne.23240) [DOI] [PubMed] [Google Scholar]
- 23.Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675. (doi:10.1038/nmeth.2089) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sirisi S, et al. 2014. Megalencephalic leukoencephalopathy with subcortical cysts protein 1 regulates glial surface localization of GLIALCAM from fish to humans. Hum. Mol. Genet. 23, 5069–5086. (doi:10.1093/hmg/ddu231) [DOI] [PubMed] [Google Scholar]
- 25.Bilotta J, Saszik S, Sutherland SE. 2001. Rod contributions to the electroretinogram of the dark-adapted developing zebrafish. Dev. Dyn. 222, 564–570. (doi:10.1002/dvdy.1188) [DOI] [PubMed] [Google Scholar]
- 26.Gestri G, Link BA, Neuhauss SC. 2012. The visual system of zebrafish and its use to model human ocular diseases. Dev. Neurobiol. 72, 302–327. (doi:10.1002/dneu.20919) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takechi M, Hamaoka T, Kawamura S. 2003. Fluorescence visualization of ultraviolet-sensitive cone photoreceptor development in living zebrafish. FEBS Lett. 553, 90–94. (doi:10.1016/S0014-5793(03)00977-3) [DOI] [PubMed] [Google Scholar]
- 28.Takechi M, Seno S, Kawamura S. 2008. Identification of cis-acting elements repressing blue opsin expression in zebrafish UV cones and pineal cells. J. Biol. Chem. 283, 31 625–31 632. (doi:10.1074/jbc.M806226200) [DOI] [PubMed] [Google Scholar]
- 29.Tsujimura T, Chinen A, Kawamura S. 2007. Identification of a locus control region for quadruplicated green-sensitive opsin genes in zebrafish. Proc. Natl Acad. Sci. USA 104, 12 813–12 818. (doi:10.1073/pnas.0704061104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsujimura T, Hosoya T, Kawamura S. 2010. A single enhancer regulating the differential expression of duplicated red-sensitive opsin genes in zebrafish. PLoS Genet. 6, e1001245 (doi:10.1371/journal.pgen.1001245) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sampath AP, et al. 2005. Recoverin improves rod-mediated vision by enhancing signal transmission in the mouse retina. Neuron 46, 413–420. (doi:10.1016/j.neuron.2005.04.006) [DOI] [PubMed] [Google Scholar]
- 32.Kuhn H, Dreyer WJ. 1972. Light dependent phosphorylation of rhodopsin by ATP. FEBS Lett. 20, 1–6. (doi:10.1016/0014-5793(72)80002-4) [DOI] [PubMed] [Google Scholar]
- 33.Kuhn H, Hall SW, Wilden U. 1984. Light-induced binding of 48-kDa protein to photoreceptor membranes is highly enhanced by phosphorylation of rhodopsin. FEBS Lett. 176, 473–478. (doi:10.1016/0014-5793(84)81221-1) [DOI] [PubMed] [Google Scholar]
- 34.Gray-Keller MP, Polans AS, Palczewski K, Detwiler PB. 1993. The effect of recoverin-like calcium-binding proteins on the photoresponse of retinal rods. Neuron 10, 523–531. (doi:10.1016/0896-6273(93)90339-S) [DOI] [PubMed] [Google Scholar]
- 35.Makino CL, et al. 2004. Recoverin regulates light-dependent phosphodiesterase activity in retinal rods. J. Gen. Physiol. 123, 729–741. (doi:10.1085/jgp.200308994) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leung YT, Fain GL, Matthews HR. 2007. Simultaneous measurement of current and calcium in the ultraviolet-sensitive cones of zebrafish. J. Physiol. 579, 15–27. (doi:10.1113/jphysiol.2006.120162) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sampath AP, Matthews HR, Cornwall MC, Fain GL. 1998. Bleached pigment produces a maintained decrease in outer segment Ca2+ in salamander rods. J. Gen. Physiol. 111, 53–64. (doi:10.1085/jgp.111.1.53) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matsuda S, Hisatomi O, Tokunaga F. 1999. Role of carboxyl-terminal charges on S-modulin membrane affinity and inhibition of rhodopsin phosphorylation. Biochemistry 38, 1310–1315. (doi:10.1021/bi982117u) [DOI] [PubMed] [Google Scholar]
- 39.Valentine KG, Mesleh MF, Opella SJ, Ikura M, Ames JB. 2003. Structure, topology, and dynamics of myristoylated recoverin bound to phospholipid bilayers. Biochemistry 42, 6333–6340. (doi:10.1021/bi0206816) [DOI] [PubMed] [Google Scholar]
- 40.Weiergraber OH, et al. 2006. Tuning of a neuronal calcium sensor. J. Biol. Chem. 281, 37 594–37 602. (doi:10.1074/jbc.M603700200) [DOI] [PubMed] [Google Scholar]
- 41.Wada Y, Sugiyama J, Okano T, Fukada Y. 2006. GRK1 and GRK7: unique cellular distribution and widely different activities of opsin phosphorylation in the zebrafish rods and cones. J. Neurochem. 98, 824–837. (doi:10.1111/j.1471-4159.2006.03920.x) [DOI] [PubMed] [Google Scholar]
- 42.Koch KW, Stryer L. 1988. Highly cooperative feedback control of retinal rod guanylate cyclase by calcium ions. Nature 334, 64–66. (doi:10.1038/334064a0) [DOI] [PubMed] [Google Scholar]
- 43.Hsu YT, Molday RS. 1993. Modulation of the cGMP-gated channel of rod photoreceptor cells by calmodulin. Nature 361, 76–79. (doi:10.1038/361076a0) [DOI] [PubMed] [Google Scholar]
- 44.Korenbrot JI, Rebrik TI. 2002. Tuning outer segment Ca2+ homeostasis to phototransduction in rods and cones. Adv. Exp. Med. Biol. 514, 179–203. (doi:10.1007/978-1-4615-0121-3_11) [DOI] [PubMed] [Google Scholar]
- 45.Sampath AP, Matthews HR, Cornwall MC, Bandarchi J, Fain GL. 1999. Light-dependent changes in outer segment free-Ca2+ concentration in salamander cone photoreceptors. J. Gen. Physiol. 113, 267–277. (doi:10.1085/jgp.113.2.267) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gordon SE, Downing-Park J, Zimmerman AL. 1995. Modulation of the cGMP-gated ion channel in frog rods by calmodulin and an endogenous inhibitory factor. J Physiol. 486, 533–546. (doi:10.1113/jphysiol.1995.sp020832) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rebrik TI, Botchkina I, Arshavsky VY, Craft CM, Korenbrot JI. 2012. CNG-modulin: a novel Ca-dependent modulator of ligand sensitivity in cone photoreceptor cGMP-gated ion channels. J. Neurosci. 32, 3142–3153. (doi:10.1523/JNEUROSCI.5518-11.2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sakurai K, Chen J, Kefalov VJ. 2011. Role of guanylyl cyclase modulation in mouse cone phototransduction. J. Neurosci. 31, 7991–8000. (doi:10.1523/JNEUROSCI.6650-10.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Genbank accession numbers: KT325590–KT325593.