Abstract
Background
Nonfasting triglycerides are similar to or superior to fasting triglycerides at predicting cardiovascular events. However, diagnostic cutpoints are based on fasting triglycerides. We examined the optimal cutpoint for increased nonfasting triglycerides.
Methods
Baseline nonfasting (<8 hours since last meal) samples were obtained from 6,391 participants in the Women’s Health Study, followed prospectively for up to 17 years. The optimal diagnostic threshold for nonfasting triglycerides, determined by logistic regression models using c-statistics and Youden index (sum of sensitivity and specificity minus one), was used to calculate hazard ratios for incident cardiovascular events. Performance was compared to thresholds recommended by the American Heart Association (AHA) and European guidelines.
Results
The optimal threshold was 175 mg/dL (1.98 mmol/L), corresponding to a c-statistic of 0.656 that was statistically better than the AHA cutpoint of 200 mg/dL (c-statistic of 0.628). For nonfasting triglycerides above and below 175 mg/dL, adjusting for age, hypertension, smoking, hormone use, and menopausal status, the hazard ratio for cardiovascular events was 1.88 (95% CI, 1.52–2.33, P<0.001), and for triglycerides measured at 0–4 and 4–8 hours since last meal, hazard ratios (95%CIs) were 2.05 (1.54– 2.74) and 1.68 (1.21–2.32), respectively. Performance of this optimal cutpoint was validated using ten-fold cross-validation and bootstrapping of multivariable models that included standard risk factors plus total and HDL cholesterol, diabetes, body-mass index, and C-reactive protein.
Conclusions
In this study of middle aged and older apparently healthy women, we identified a diagnostic threshold for nonfasting hypertriglyceridemia of 175 mg/dL (1.98 mmol/L), with the potential to more accurately identify cases than the currently recommended AHA cutpoint.
High triglycerides may promote atherosclerosis via the accumulation of triglyceride-rich remnant particles within the endothelium. 1–9 The nonfasting state provides a better indication of average lipid concentrations in the blood since most people only fast for a few hours in the early morning.1, 10, 11 From a purely practical standpoint, nonfasting samples are much easier to obtain; yet fasting samples remain the standard for measurement of triglycerides and cholesterol because measuring lipids in the fasting state reduces variability and allows for a more accurate derivation of the Friedewald equation.10 However, studies suggest that the variability missed in fasting samples may capture important information about an individual’s metabolic capacity (e.g. through delayed clearance of triglyceride remnants).12, 13 An accumulation of recent evidence from prospective cohort data has shown nonfasting triglycerides to be equivalent, if not superior, to fasting lipids at predicting cardiovascular disease.12–18 Furthermore, triglycerides and triglyceride-rich remnant lipoproteins have not just been associated with, but also may be causally linked to the development of atherosclerosis and even all-cause mortality.19–23
Thus, it is important to determine appropriate clinical cutpoints at which to diagnose and treat nonfasting hypertriglyceridemia. Current national guidelines set the value for defining borderline high fasting triglycerides at 150 mg/dL (1.70 mmol/L) and high at 200 mg/dL (2.28mmol/L) based on population percentiles.8 Together, these two groups roughly correspond to the top one third of the population in developed nations.8, 24–27 Different panels have proposed various cutpoints for nonfasting hypertriglyceridemia at 175 mg/dL (1.98 mmol/L) (European Atherosclerosis Society),28 180mg/dL (2.03 mmol/L) (Athens Expert Panel),11 and 200 mg/dL (2.26 mmol/L) (American Heart Association [AHA]),8 although the underlying rationale for these cutpoints is unclear. It is possible that these values were extrapolated from the fasting cutoffs given that triglycerides increase by approximately 20–30% from their baseline fasting levels and remain stable for two to four hours after a meal.29 To our knowledge, no evidence exists to suggest that any of the above diagnostic cutpoints for increases in nonfasting triglycerides is superior to the others at predicting cardiovascular outcomes. Therefore, in a prospective cohort of 28,345 apparently healthy women followed for up to 17 years for incident cardiovascular disease (CVD) events, we attempted to determine the optimal diagnostic cutpoint for hypertriglyceridemia in the nonfasting state.
Materials and Methods
Study Participants
The study cohort was derived from participants in the Women’s Health Study, a previously completed randomized controlled trial of aspirin and vitamin E in the primary prevention of CVD and cancer among 39,876 apparently healthy women.30 The study protocol was approved by the institutional review board of Brigham and Women’s Hospital (Boston, MA), and all participants provided written informed consent.
Baseline demographic data and health histories were obtained from women at the time of enrollment. They also gave blood samples at that time which were the source of the lipid measurements. Participants were asked to provide a blood sample, if they were willing; 28,345 (71.1%) women provided these. The number of hours since their last meal before the blood draw was self-reported. Fasting participants were defined as those whose last meal was 8 hours or more prior to their blood draw (n=20,118). Nonfasting participants consisted of those who had eaten within 8 hours of their blood draw (n=6,391). Those with unknown time since last meal or missing baseline lipid measurements (n=1,836) were excluded from this analysis.
Laboratory methods
Blood samples were collected at enrollment in tubes containing ethylenediaminetetraacetic acid. The samples were centrifuged upon collection and the plasma was stored in liquid nitrogen (−170° C) until time of analysis. Subsequently, in a core laboratory certified by the National Heart, Lung, and Blood Institute/Centers for Disease Control and Prevention Lipid Standardization program, samples were thawed and analyzed for standard lipids as previously described.12 Direct determination of concentrations of total cholesterol, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), and triglycerides was simultaneously performed on the Hitachi 917 analyzer using reagents and calibrators from Roche Diagnostics. Triglycerides were measured enzymatically with correction for endogenous glycerol, using a Hitachi 917 analyzer and reagents and calibrators from Roche Diagnostics. Triglycerides at concentrations of 84.0 and 201.8 mg/dL (0.95 and 2.28 mmol/L) were determined in the laboratory with a day-to-day reproducibility of 1.8% (SD 1.6 mg/dL or 0.2 mmol/L) and 1.7% (SD, 2.5 mg/dL or 0.3 mmol/L), respectively. Total cholesterol and high-density lipoprotein cholesterol (HDL-C) were measured enzymatically on a Hitachi 911 autoanalyzer (Roche Diagnostics, Basel, Switzerland), and LDL-C was determined by a homogenous direct method from Roche Diagnostics.
Outcomes
The primary outcome of interest was total CVD events (nonfatal myocardial infarction, nonfatal ischemic stroke, coronary revascularization, and death due to cardiovascular causes).12, 14 Myocardial infarction was defined by World Health Organization criteria of characteristic symptoms of chest pain accompanied by increased concentrations of cardiac enzymes or by diagnostic electrocardiographic changes. Stroke was defined as a new neurologic deficit of sudden onset that persisted for at least 24 hours. Computed tomography scans or magnetic resonance images of the head were available for most events and used to distinguish ischemic from hemorrhagic strokes. Coronary revascularization included percutaneous coronary interventions and coronary artery bypass graft surgery. All events were adjudicated by an end points committee. In participants with more than one cardiovascular event, only the first was used in these analyses. Follow-up morbidity and mortality data were available for 97.2% and 99.4% respectively, of the Women’s Health Study participants in this study.
Statistical Analyses
All analyses were performed on nonfasting participants with the exception of baseline characteristics which included fasting and nonfasting participants. Baseline characteristics included age, hypertension status, smoking status, diagnosis of diabetes mellitus, postmenopausal status, postmenopausal hormone use, and high-sensitivity C-reactive protein (hsCRP). Differences between baseline characteristics of participants in the nonfasting and fasting populations were analyzed using the t-test in two group comparisons or Pearson Chi square test for proportions.
Follow-up of this cohort was virtually complete through 8 years and event rate was low. In this scenario both logistic regression and Cox proportional hazards regression produce asymptotically consistent estimates31 so we used more user-friendly logistic regression to estimate the optimal threshold. The optimal diagnostic threshold for nonfasting triglycerides was determined by evaluating the area under the receiver operating characteristic (ROC) curve (c-statistic)32 in univariable logistic regression models with a composite eight-year CVD event as a dependent variable and the dichotomized concentration of non-fasting triglycerides as an independent predictor. Subsequent analyses evaluated this optimal threshold in the full 17 year follow-up using Cox proportional hazard models for incident CVD.
By varying the dichotomization threshold from 100 to 300 mg/dL (1.13 to 3.39 mmol/L) by increments of 25 mg/dL (0.28 mmol/L), we obtained the concentration of nonfasting triglycerides that optimized the c-statistic and equivalently the Youden index (sum of sensitivity plus specificity minus 1). This value has the optimal balance of sensitivity and specificity. Next, in a multivariable analysis, we used Cox proportional hazards regression models to compare dichotomized triglycerides at this optimal cutpoint value with the following alternative cutpoints selected by expert panels: 175mg/dL (1.98 mmol/L) (European Atherosclerosis Society),27 180mg/dL (2.03 mmol/L) (Athens Expert Panel),11 and 200 mg/dL (2.26 mmol/L) (AHA).8 Three multivariable models were used to control for potential confounders and/or mediators. Model 1 adjusted for age, postmenopausal status, hormone replacement therapy use, smoking status, and hypertension (defined as history of hypertension or on antihypertensive medication). To determine the predictive value of triglycerides independent of other lipids, model 2 was additionally adjusted for total cholesterol and HDL-C. Model 3 adjusted for covariates in model 2 plus diabetes mellitus, BMI, and hsCRP as these variables may be in the causal pathway for the association of triglycerides with CVD.
Additionally, in order to validate the results and avoid over-optimism, we compared three different non-fasting triglyceride thresholds in the three models using 10-fold cross-validation and bootstrapping: differences in cross-validated c-index between the three thresholds were calculated and their 95% confidence intervals were estimated by the bootstrap method. Because there was almost no censoring, c-index is an appropriate performance measure of the survival model.32 A better threshold is expected to produce higher c-index, cross-validation mitigates over-optimism, and bootstrap produces a confidence interval with a better coverage probability in this setting.33–36
To further explore the association of time since last meal with future CVD events in nonfasting individuals, Cox proportional hazard regression models were used to study effect modification of nonfasting triglycerides by time since last meal (0 to <4 hours, or 4 to <8 hours). To assess interaction between nonfasting triglycerides and hours since last meal, we included a cross product term. To ascertain the independence of triglycerides from HDL-C and to test for multiplicative interactions in predicting CVD risk, the fully adjusted model for the optimal threshold of nonfasting triglycerides was also run using pre-specified clinical categories of HDL-C (less than or greater than or equal to 50 mg/dL (1.30 mmol/L). All P values were 2-tailed, and a P<0.05 was considered statistically significant. All statistical analyses were performed using SAS version 9.3 (SAS Institute Inc).
Results
Baseline characteristics of participants in the study (Table 1) were similar between fasting and nonfasting individuals for all of the variables examined (at a 0.05 significance level). Compared with the fasting group, the nonfasting participants tended to be slightly younger, were less likely to have hypertension, had lower concentrations of LDL-C, and were more likely to be diabetic.
Table 1.
Baseline characteristics of participants according to fasting status.
| Characteristic | All (N=26509) |
Nonfasting (N=6391) |
Fasting (N=20118) |
|---|---|---|---|
| Age, median (25th–75th percentile), year | 53 (49−59) | 52 (48−58) | 53 (49−59) |
| Hypertension, No. (%) | 6672 (27.2) | 1437 (22.5) | 5235 (26.0) |
| Current smoking, No. (%) | 3075 (11.6) | 713(11.2) | 2362 (11.8) |
| Diabetes mellitus, No. (%) | 635 (2.4) | 174 (2.7) | 461 (2.3) |
| Postmenopausal, No. (%) | 14420 (54.5) | 3241 (50.8) | 11179 (55.6) |
| Postmenopausal hormone use, No (%) | 11564 (43.7) | 2817 (44.2) | 8747 (43.6) |
| Total cholesterol, median (25th–75th percentile), mg/dL | 208 (184−236) | 205 (181−234) | 209 (185−236) |
| LDL cholesterol, median (25th–75th percentile), mg/dL | 121.5 (100.8−144.6) | 117.1 (96.8−139.8) | 122.9 (102.1–145.9) |
| HDL cholesterol, median (25th–75th percentile), mg/dL | 51.9 (43.2−62.4) | 51.8 (42.9−62.2) | 52.0 (43.3−62.4) |
| Body mass index, median (25th–75th percentile) | 24.9 (22.5−28.3) | 24.9 (22.3−28.3) | 24.9 (22.5 −28.3) |
| High-sensitivity CRP, median (25th–75th percentile), mg/L | 2.02 (0.81−4.37) | 1.96 (0.78–4.33) | 2.03 (0.82−4.39) |
Abbreviations: CRP, C-reactive protein. To convert triglycerides to mmol/L, multiply by 0.0113; LDL-C and HDL-C to mmol/L, multiply by 0.0259; and high sensitivity CRP, multiply by 9.524.
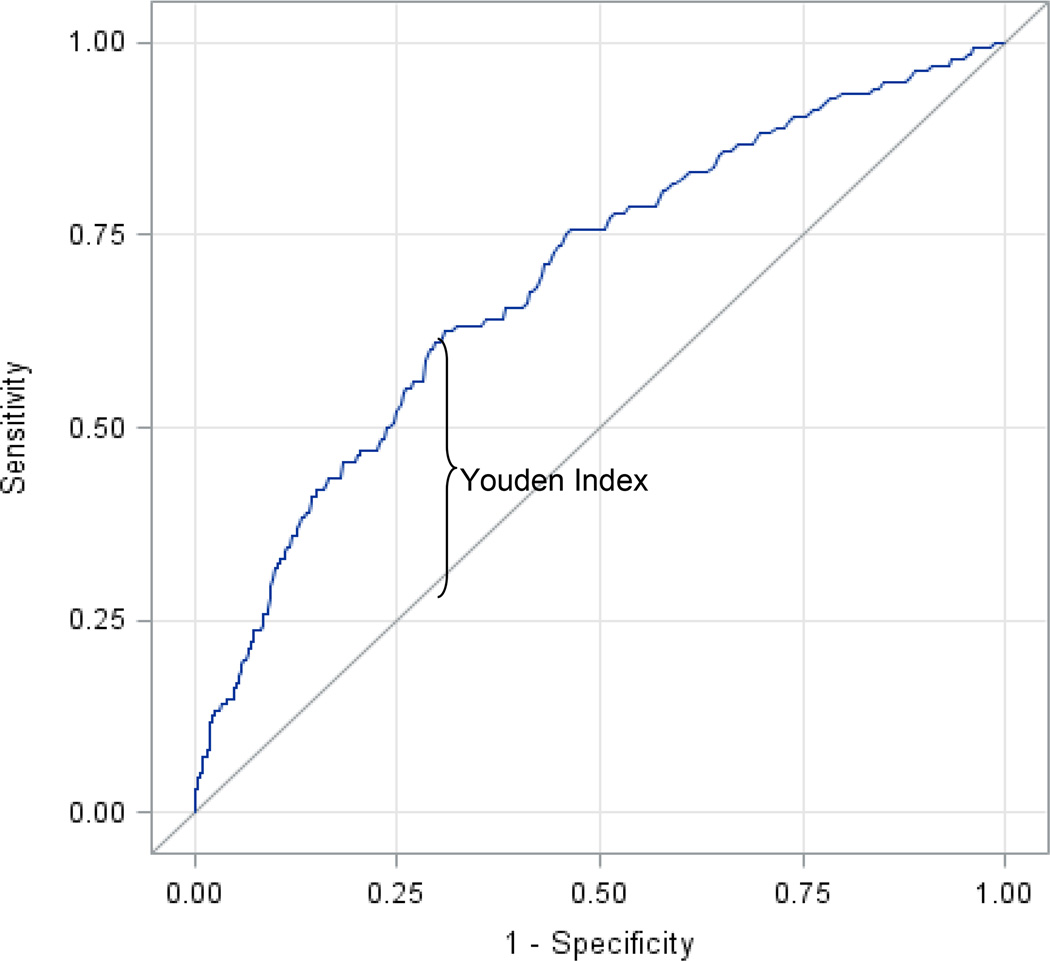
Of the 6,391 nonfasting participants, 136 developed incident CVD in eight years and 353 developed incident CVD in 17 years. The ROC curve over all levels of nonfasting triglycerides is shown in Figure 1 (c-statistic = 0.656). The optimal threshold for triglyceride value for predicting the incidence of CVD during this duration was 175 mg/dL (1.98 mmol/L), corresponding to the maximum Youden index and c-statistic using dichotomized triglycerides (Table 2) over a composite eight-year CVD event period which minimized censoring (censoring rate 94%). It also corresponded to the value proposed by the European Atherosclerosis Society.27 The other proposed values of 180 mg/dL (2.03 mmol/L) and 200 mg/dL (2.26 mmol/L) were also examined, but each produced a lower c-statistic than 175 mg/dL (1.98 mmol/L). Because model fitting and model evaluation were performed in the same dataset, we implemented additionally a bootstrap with ten-fold cross validation in order to avoid over optimism. Results are reported in Table 3.
Figure 1.
Receiver operating characteristic curve for nonfasting triglycerides (c = 0.656) corresponding to the maximal Youden Index (0.313) for dichotomized nonfasting triglycerides.
Table 2.
Identification of an optimal nonfasting triglyceride threshold for predicting CVD
| Nonfasting Triglycerides (mg/dL) |
Population percentile |
Sensitivity (%) | Specificity (%) | c-statistic | Youden Index (J) |
|---|---|---|---|---|---|
| 100 | 30 | 88 | 30 | 0.593 | 0.186 |
| 125 | 45 | 79 | 46 | 0.623 | 0.246 |
| 150 | 58 | 68 | 58 | 0.630 | 0.259 |
| 175 | 68 | 63 | 69 | 0.656 | 0.313 |
| 180 | 70 | 60 | 71 | 0.655 | 0.309 |
| 200 | 76 | 49 | 77 | 0.628 | 0.255 |
| 225 | 82 | 43 | 83 | 0.630 | 0.259 |
| 250 | 86 | 38 | 87 | 0.625 | 0.250 |
| 275 | 89 | 32 | 90 | 0.608 | 0.216 |
| 300 | 92 | 24 | 92 | 0.579 | 0.158 |
The optimal diagnostic threshold for nonfasting triglycerides was determined by evaluating the maximal area under the receiver operating characteristic (ROC) curve (ie, c-statistic)40 for univariable logistic regression models with a composite eight-year CVD event as a dependent variable and the dichotomized level of non-fasting triglycerides as an independent predictor. The Youden index is proportional to the c-statistic and defined as sensitivity + specificity −1.
Table 3.
Cross-validated difference in the c-indices comparing the nonfasting triglyceride cutpoint of 175 mg/dL with the other proposed cutpoints of 180 mg/dL and 200 mg/dL in multivariable models using bootstrapping methodology.
| C-index comparing 175 (optimal) with 180 mg/dL (comparison) cutpoints* |
C-index, comparing 175 (optimal) with 200 (comparison) cutpoints* |
|||||||
|---|---|---|---|---|---|---|---|---|
| Optimal Cutpoint |
Comparison Cutpoint |
Mean Difference (95% CI) |
Optimal Cutpoint |
Comparison Cutpoint |
Mean Difference (95% CI) |
|||
| Model 1a | 0.783 | 0.782 | 0.001 (−0.005 to 0.010) |
0.783 | 0.773 | 0.010 (−0.005 to 0.026) |
||
| Model 2b | 0.788 | 0.787 | 0.001 (−0.005 to 0.010) |
0.788 | 0.779 | 0.008 (−0.004 to 0.026) |
||
| Model 3c | 0.813 | 0.811 | 0.002 (−0.003 to 0.010) |
0.813 | 0.802 | 0.011 (0.000 to 0.028) |
||
Risk estimated at 8 years of follow-up.
Adjusted for age, history of hypertension, smoking, use of hormone therapy, and postmenopausal status.
Adjusted for covariates in model 1 plus total and high-density lipoprotein cholesterol.
Adjusted for covariates in model 2 plus diabetes mellitus, body mass index, and high-sensitivity C-reactive protein.
To convert triglyceride concentrations to millimoles per liter, multiply by 0.0113
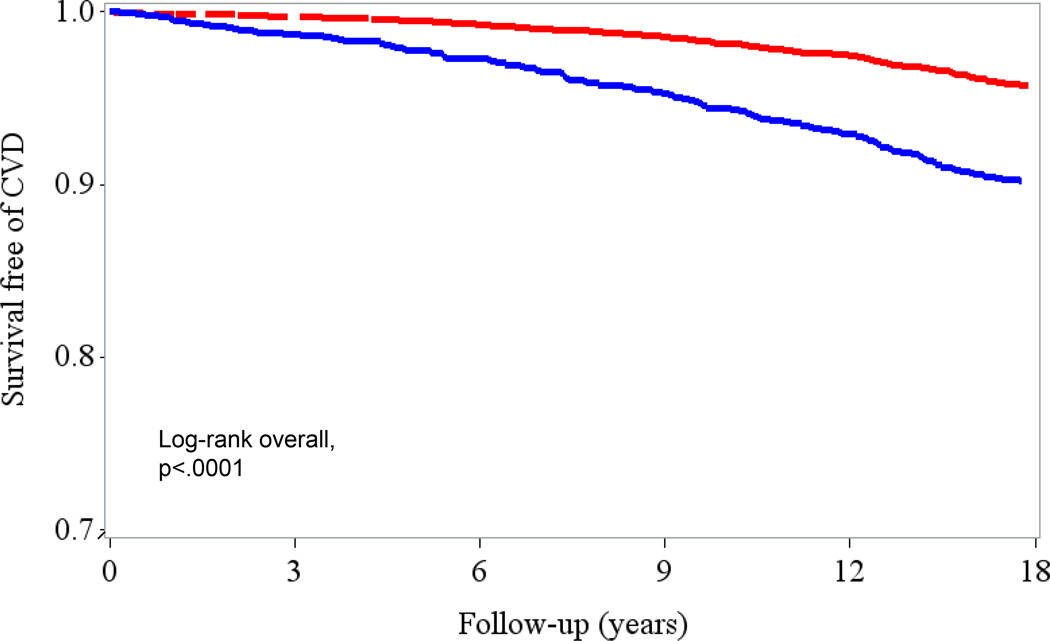
Hazard ratios (HRs) and 95% confidence intervals using the 17 year follow-up were estimated for the optimal cutoff of 175 mg/dL (1.98 mmol/L) in association with incident CVD events both crudely (Figure 2) and adjusted for multiple factors (Table 4). After adjusting for age, history of hypertension, smoking, use of hormone therapy and postmenopausal status (model 1), nonfasting triglyceride concentrations ≥ 175 mg/dL (≥ 1.98 mmol/L) were strongly associated with CVD events 1.88 (95% CI, 1.52–2.33, P<0.001). In model 2, after adjusting for total cholesterol and HDL-C in addition to the variables in model 1, the association was somewhat attenuated, but nonfasting triglycerides ≥ 175 mg/dL (≥ 1.98 mmol/L) remained significantly associated with CVD (HR 1.36 [95% CI 1.06–1.75], P=0.02. After adjusting for variables in the causal pathways (diabetes, BMI, and CRP) in addition to model 2 variables, the HR (95% CI, P value) for incident CVD events over 17 year follow-up for nonfasting triglycerides ≥175 mg/dL (≥ 1.98 mmol/L) was further attenuated 1.25 [(95% CI, 0.96–1.62)], P=0.10. There was no statistical interaction between hours since last meal (0 to <4 and 4 to <8 hours) and the association of triglycerides with CVD events (P for interaction>0.05 for all three models).
Figure 2.
Kaplan Meier curve demonstrating survival free of cardiovascular events (myocardial infarction, ischemic stroke, revascularization, or death due to cardiovascular causes) at the optimal 175 mg/dL(1.98 mmol/L) cutoff. Survival is significantly decreased in individuals with nonfasting triglycerides greater than or equal to the optimal threshold of 175mg/dL (blue line) compared to those with nonfasting triglycerides less than 175 mg/dL (red line).
Table 4.
Association of nonfasting triglycerides with incident CVD above or below an optimal threshold of 175 mg/dL*
| Time since last meal |
Model 1a | Model 2b | Model 3c | |||
|---|---|---|---|---|---|---|
| All nonfasting (0–8 h) N=6391 |
1.88 (1.52–2.33) |
P<.0001 | 1.36 (1.06–1.75) |
P=0.02 | 1.25 (0.96–1.62) |
P=0.10 |
| (0 to <4 h) N =3797 |
2.05 (1.54–2.74) |
P<0.0001 | 1.55 (1.10–2.18) |
P=0.01 | 1.41 (0.99–2.00) |
P=0.06 |
| (4 to <8 h) N=2594 |
1.68 (1.21–2.32) |
P=0.002 | 1.15 (0.78–1.70) |
P=0.47 | 1.05 (0.71–1.57) |
P=0.80 |
| P for Interaction* | P = 0.51 | P = 0.54 | P = 0.71 | |||
Hazard ratios with 95% confidence intervals demonstrating association with incident CVD above or below optimal diagnostic threshold of 175 mg/dL for nonfasting triglycerides over a 17 year follow up period using adjusted models and stratified by time since last meal. There is no interaction between hours since last meal the association of triglycerides with CVD events (this should be a footnote in the table not the title).
P for interaction between hours since last meal (defined as 0–<4 hrs vs 4–<8h ) and triglycerides ≥175 mg/dL. To convert triglyceride concentrations to millimoles per liter, multiply by 0.0113.
Adjusted for age, history of hypertension, smoking, use of hormone therapy, and postmenopausal status.
Adjusted for covariates in model 1 plus total and high-density lipoprotein cholesterol.
Adjusted for covariates in model 2 plus diabetes mellitus, body mass index, and high-sensitivity C-reactive protein.
Discussion
In this prospective cohort of 28,345 apparently healthy women followed for up to 17 years, we observed that the optimal threshold for the diagnosis of hypertriglyceridemia in the nonfasting state was 175 mg/dL (1.98 mmol/L), which more accurately predicted CVD compared with the currently recommended AHA value of 200 mg/dL (2.26 mmol/L). To our knowledge, this is the first study that has prospectively validated a diagnostic cutpoint for nonfasting triglycerides in relation to incident CVD events in a healthy population. Furthermore, the association of the identified threshold with incident CVD was not affected by postprandial duration.
Increased nonfasting triglycerides are associated with higher cardiovascular risk in several studies.12–18 The most plausible explanation for this increased risk is that nonfasting triglycerides signify the presence of atherogenic remnant lipoproteins. These lipoproteins contain a degree of cholesterol that is not accounted for in typical fasting triglyceride samples or LDL-specific measurements. Because all human cells can degrade triglycerides but not cholesterol, it is likely that the cholesterol content of the triglyceride-rich remnant particles enters the arterial intima and contributes to atherosclerosis. Once trapped inside the intima, there is evidence to suggest that remnant particles may be preferentially trapped inside the arterial wall compared to LDL, simply because of their larger size and attachment to extracellular proteoglycans.6 Unlike with LDL particles, triglyceride-rich remnant molecules can be taken up directly by macrophages leading to foam cell formation.33 Another novel mechanism by which triglycerides may predispose an individual to CVD involves the concept that lipoprotein lipase activity at the surface of triglyceride-rich remnant particles acts on the vascular endothelium or within the intima to precipitate the release of free fatty acids, resulting in local injury and inflammation.1, 33, 37
There are several clinical implications to this study. Practitioners who would like to incorporate nonfasting lipid measurements into their practice are hindered by the fact that they have to rely on the same fasting triglyceride cutpoints which have not been studied or validated in nonfasting populations. Patient compliance may become a hindrance to the interpretation of fasting samples. Furthermore, the use of simplified diagnostic criteria with clinical relevance will be more accessible to the increasingly overburdened physician.38 This study builds upon the evidence that nonfasting samples can accurately capture prognostic data for both triglycerides and cholesterol by establishing validated cutpoints that can now be used to help guide clinical decision-making.12, 39 Indeed, a nonfasting lipid profile has been the standard in Denmark since 2009.1
Given that hypertriglyceridemia is a cardiovascular risk factor of evolving significance, it is necessary to create diagnostic tools to assess when these levels place a person at risk for clinically important endpoints (MI, ischemic stroke, or death by cardiovascular causes). Nonfasting triglycerides provide a more accurate assessment of an individual’s average metabolic state.11 One explanation for this phenomenon is that triglycerides do not return to basal levels until at least 8 hours after a meal and clearance of triglycerides from the bloodstream can be delayed as long as 12 hours or more in patients with insulin resistance or a predisposition to producing remnant particles.10 Thus, we spend the vast majority of our time in nonfasting conditions. However, fasting samples have been the standard for measurement of triglycerides and cholesterol because measuring these lipids in the fasting state (1) reduces variability and thus increases precision and (2) allows for a more accurate derivation of the Friedewald equation for calculating LDL-C. Recent data also suggest that nonfasting LDL-C has prognostic value similar to that of fasting LDL-C.40 Triglyceride rich remnant molecules, composed of triglycerides, cholesterol, and proteins are associated with the increased cardiovascular risk in multiple studies.12–18 New advances in genetics have shown that triglycerides are only one component of the causal pathway to CVD and that mutations involving lipoprotein metabolism and function directly impact phenotype.20–23 This has important implications for future therapeutic targets aimed at reducing triglyceride rich remnant molecules.
The strengths of the study include its large sample size, prospective design, and extended follow-up time. Application of rigorous methods such as bootstrap and ten-fold cross validation further supports the optimality of the 175 mg/dL (1.98 mmol/L) threshold. Nonetheless, limitations of this study also merit consideration. First, participants were not randomly assigned to fasting or nonfasting status. The participants chose whether or not to fast and for how long, which may introduce sampling bias. However, the baseline characteristics of all the participants were similar and there was no interaction between hours since the last meal within our nonfasting cohort. Also, there was no standardization of the meal given (e.g. proportion of fat, etc.), but that would result, if anything, in an underestimation of the true effect. Given the variability of triglyceride concentrations, the single measurement of concentrations at study enrollment without repeated sampling could lead to regression dilution bias, but this would again bias the results toward a null finding. Our study population was limited to mostly Caucasians and all women. Further studies should examine outcomes in men and other ethnicities. Lastly, this study focuses on diagnosis, but areas of therapeutic intervention continue to be controversial. The most important lifestyle modification (and least controversial) is to lose weight through eating less and exercising more.1 Other treatments for lowering triglycerides include omega 3 fatty acids (fish oils), statins, fibrates, and niacin.8
In summary, this is the first study to identify a diagnostic threshold for nonfasting hypertriglyceridemia in a large, prospective cohort of apparently healthy individuals. As the evidence for the link between triglycerides and cardiovascular disease increases, identifying early points of intervention in the prevention of CVD are crucial for preventative public health efforts. Given the study entry criteria, additional studies should be done to assess the generalizability of our results in women younger than 45 years, men, and more ethnically diverse populations.
Acknowledgements
We thank the participants, staff, and investigators of the Women’s Health Study for their valuable contributions.
Dr Akinkuolie was supported by the NHLBI (T32 HL007575). Dr Ridker has significant research support from Novartis, AstraZeneca, Aegerion, ISIS, Boeringer, Pfizer, NHLBI, NCI, Amgen. He has modest honoraria from Lilly, Genetech. He is a consultant/advisory board member for ISIS, Boston Heart, Genzyme, Jannson. He is also listed as coinventor on patents held by the Brigham and Women’s Hospital that relate to the use of inflammatory biomarkers to cardiovascular disease. Dr Mora has received significant research support from NHLBI, AstraZeneca, Atherotech Diagnostics, and has served as consultant (modest) to Genzyme, Pfizer, Lilly, Quest Diagnostics, Cerenis Therapeutics.
Funding/Support: The Women’s Health Study is supported by grants HL117861, HL43851, HL 080467, and CA 47988 from the NHLBI and NCI. The funding agencies played no role in the design, conduct, data management, or analysis.
Footnotes
Financial disclosures: Drs White, Demler, Moorthy, and Cook have no relevant financial disclosures.
Contributor Information
Khendi T. White, Email: ktwhite@partners.org.
M.V. Moorthy, Email: MVMoorthy@research.bwh.harvard.edu.
Akintunde O. Akinkuolie, Email: Aoakinkuolie@PARTNERS.ORG.
Olga Demler, Email: Odemler@partners.org.
Paul M Ridker, Email: pridker@partnrers.org.
References
- 1.Nordestgaard BG, Varbo A. Triglycerides and cardiovascular disease. Lancet. 2014;384:626–635. doi: 10.1016/S0140-6736(14)61177-6. [DOI] [PubMed] [Google Scholar]
- 2.Shai I, Rimm EB, Hankinson SE, Cannuscio C, Curhan G, Manson JE, Rifai N, Stampfer MJ, Ma J. Lipoprotein (a) and coronary heart disease among women: Beyond a cholesterol carrier? Eur Heart J. 2005;26:1633–1639. doi: 10.1093/eurheartj/ehi222. [DOI] [PubMed] [Google Scholar]
- 3.Ridker PM, Rifai N, Cook NR, Bradwin G, Buring JE. Non-HDL cholesterol, apolipoproteins A-I and B100, standard lipid measures, lipid ratios, and CRP as risk factors for cardiovascular disease in women. JAMA. 2005;294:326–333. doi: 10.1001/jama.294.3.326. [DOI] [PubMed] [Google Scholar]
- 4.Alipour A, van Oostrom AJHHM, Izraeljan A, Verseyden C, Collins JM, Frayn KN, Plokker TWM, Elte JWF, Castro Cabezas M. Leukocyte activation by triglyceride-rich lipoproteins. Arterioscler Thromb Vasc Biol. 2008;28:792–797. doi: 10.1161/ATVBAHA.107.159749. [DOI] [PubMed] [Google Scholar]
- 5.Wang L, Gill R, Pedersen TL, Higgins LJ, Newman JW, Rutledge JC. Triglyceride-rich lipoprotein lipolysis releases neutral and oxidized ffas that induce endothelial cell inflammation. J Lipid Res. 2009;50:204–213. doi: 10.1194/jlr.M700505-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nordestgaard BG, Wootton R, Lewis B. Selective retention of VLDL, IDL, and LDL in the arterial intima of genetically hyperlipidemic rabbits in vivo. Molecular size as a determinant of fractional loss from the intima-inner media. Arterioscler Thromb Vasc Biol. 1995;15:534–542. doi: 10.1161/01.atv.15.4.534. [DOI] [PubMed] [Google Scholar]
- 7.Steiner G, Schwartz L, Shumak S, Poapst M. The association of increased levels of intermediate-density lipoproteins with smoking and with coronary artery disease. Circulation. 1987;75:124–1230. doi: 10.1161/01.cir.75.1.124. [DOI] [PubMed] [Google Scholar]
- 8.Miller M, Stone NJ, Ballantyne C, Bittner V, Criqui MH, Ginsberg HN, Goldberg AC, et al. American Heart Association Clinical Lipidology T, Prevention Committee of the Council on Nutrition PA, Metabolism, Council on Arteriosclerosis T, Vascular B, Council on Cardiovascular N, Council on the Kidney in Cardiovascular D, Triglycerides and cardiovascular disease: A scientific statement from the american heart association. Circulation. 2011;123:2292–2333. doi: 10.1161/CIR.0b013e3182160726. [DOI] [PubMed] [Google Scholar]
- 9.Sniderman AD. Apolipoprotein B versus non-high-density lipoprotein cholesterol: And the winner is. Circulation. 2005;112:3366–3367. doi: 10.1161/CIRCULATIONAHA.105.583336. [DOI] [PubMed] [Google Scholar]
- 10.McBride PE. Triglycerides and risk for coronary heart disease. JAMA. 2007;298:336–338. doi: 10.1001/jama.298.3.336. [DOI] [PubMed] [Google Scholar]
- 11.Kolovou GD, Mikhailidis DP, Kovar J, Lairon D, Nordestgaard BG, Ooi TC, Perez-Martinez P, et al. Assessment and clinical relevance of non-fasting and postprandial triglycerides: An expert panel statement. Curr Vasc Pharmacol. 2011;9:258–270. doi: 10.2174/157016111795495549. [DOI] [PubMed] [Google Scholar]
- 12.Bansal S, Buring JE, Rifai N, Mora S, Sacks FM, Ridker PM. Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. JAMA. 2007;298:309–316. doi: 10.1001/jama.298.3.309. [DOI] [PubMed] [Google Scholar]
- 13.Nordestrgaad BG, Benn M, Schnohr P, Tybaerg-Hansen A. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease and death in men and women. JAMA. 2007;298:299–308. doi: 10.1001/jama.298.3.299. [DOI] [PubMed] [Google Scholar]
- 14.Mora S, Rifai N, Buring JE, Ridker PM. Fasting compared with nonfasting lipids and apolipoproteins for predicting incident cardiovascular events. Circulation. 2008;118:993–1001. doi: 10.1161/CIRCULATIONAHA.108.777334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eberly LE, Stamler J, Neaton JD. Multiple Risk Factor Intervention Trial Research G. Relation of triglyceride levels, fasting and nonfasting, to fatal and nonfatal coronary heart disease. Arch Intern Med. 2003;163:1077–1083. doi: 10.1001/archinte.163.9.1077. [DOI] [PubMed] [Google Scholar]
- 16.Stensvold I, Tverdal A, Urdal P, Graff-Iversen S. Non-fasting serum triglyceride concentration and mortality from coronary heart disease and any cause in middle aged norwegian women. BMJ (Clinical research ed) 1993;307:1318–1322. doi: 10.1136/bmj.307.6915.1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Langsted A, Freiberg JJ, Tybjaerg-Hansen A, Schnohr P, Jensen GB, Nordestgaard BG. Nonfasting cholesterol and triglycerides and association with risk of myocardial infarction and total mortality: The Copenhagen City Heart Study with 31 years of follow-up. J Intern Med. 2011;270:65–75. doi: 10.1111/j.1365-2796.2010.02333.x. [DOI] [PubMed] [Google Scholar]
- 18.Stalenhoef AFH, de Graaf J. Association of fasting and nonfasting serum triglycerides with cardiovascular disease and the role of remnant-like lipoproteins and small dense LDL. Curr Opin Lipidol. 2008;19:355–361. doi: 10.1097/MOL.0b013e328304b63c. [DOI] [PubMed] [Google Scholar]
- 19.Do R, Willer CJ, Schmidt EM, Sengupta S, Gao C, Peloso GM, Gustafsson S, et al. Common variants associated with plasma triglycerides and risk for coronary artery disease. Nat Genet. 2013;45:1345–1352. doi: 10.1038/ng.2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosenson RS, Davidson MH, Hirsh BJ, Kathiresan S, Gaudet D. Genetics and causality of triglyceride-rich lipoproteins in atherosclerotic cardiovascular disease. J Am Coll Cardiol. 2014;64:2525–2540. doi: 10.1016/j.jacc.2014.09.042. [DOI] [PubMed] [Google Scholar]
- 21.Varbo A, Benn M, Tybjaerg-Hansen A, Jorgensen AB, Frikke-Schmidt R, Nordestgaard BG. Remnant cholesterol as a causal risk factor for ischemic heart disease. J Am Coll Cardiol. 2013;61:427–436. doi: 10.1016/j.jacc.2012.08.1026. [DOI] [PubMed] [Google Scholar]
- 22.Thomsen M, Varbo A, Tybjaerg-Hansen A, Nordestgaard BG. Low nonfasting triglycerides and reduced all-cause mortality: A mendelian randomization study. Clin Chem. 2014;60:737–746. doi: 10.1373/clinchem.2013.219881. [DOI] [PubMed] [Google Scholar]
- 23.Jorgensen AB, Frikke-Schmidt R, West AS, Grande P, Nordestgaard BG, Tybjaerg-Hansen A. Genetically elevated non-fasting triglycerides and calculated remnant cholesterol as causal risk factors for myocardial infarction. Eur Heart J. 2013;34:1826–1833. doi: 10.1093/eurheartj/ehs431. [DOI] [PubMed] [Google Scholar]
- 24.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in adults (Adult Treatment Panel III) Final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 25.Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, et al. American College of Cardiology/American Heart Association Task Force on Practice G. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the american college of cardiology/american heart association task force on practice guidelines. J Am Coll Cardiol. 2014;63:2889–2934. doi: 10.1016/j.jacc.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 26.Anderson TJ, Gregoire J, Hegele RA, Couture P, Mancini GB, McPherson R, Francis GA, et al. 2012 update of the canadian cardiovascular society guidelines for the diagnosis and treatment of dyslipidemia for the prevention of cardiovascular disease in the adult. Can J Cardiol. 2013;29:151–167. doi: 10.1016/j.cjca.2012.11.032. [DOI] [PubMed] [Google Scholar]
- 27.European Association for Cardiovascular P, Rehabilitation. Reiner Z, Catapano AL, De Backer G, Graham I, Taskinen MR, Wiklund O, Agewall S, Alegria E, et al. Guidelines ESCCFP, Committees. ESC/EAS guidelines for the management of dyslipidaemias: The task force for the management of dyslipidaemias of the european society of cardiology (ESC) and the european atherosclerosis society (EAS) Eur Heart J. 2011;32:1769–1818. doi: 10.1093/eurheartj/ehr158. [DOI] [PubMed] [Google Scholar]
- 28.Hegele RA, Ginsberg HN, Chapman JM, et al. The polygenic nature of hypertriglyceridaemia: Implications for definition, diagnosis, and management. Lancet Diabetes Endocrin. 2013;13:70191–70198. doi: 10.1016/S2213-8587(13)70191-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lima-Oliveira G, Salvagno GL, Lippi G, Gelati M, Montagnana M, Danese E, Picheth G, Guidi GC. Influence of a regular, standardized meal on Clin Chem analytes. Ann Lab Med. 2012;32:250–256. doi: 10.3343/alm.2012.32.4.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cook NR, Lee IM, Gaziano JM. Low-dose aspirin in the primary prevention of cancer. The Women's Health Study A randomized controlled trial JAMA. 2005;294:47–55. doi: 10.1001/jama.294.1.47. [DOI] [PubMed] [Google Scholar]
- 31.Green MS, Symons MJ. A comparison of the logistic risk function and the proportional hazards model in prospective epidemiologic studies. J Chronic Dis. 1983;36:715–723. doi: 10.1016/0021-9681(83)90165-0. [DOI] [PubMed] [Google Scholar]
- 32.Pepe MS, York NY. The statistical evaluation of medical tests for classification and prediction. 2004 [Google Scholar]
- 33.Goldberg IJ, Eckel RH, McPherson R. Triglycerides and heart disease: Still a hypothesis? Arterioscler Thromb Vasc Biol. 2011;31:1716–1725. doi: 10.1161/ATVBAHA.111.226100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stone BJ. Cross-validatory choice and assessment of statistical predictions. Stat Soc. 1974;36:111–147. [Google Scholar]
- 35.Efron DT, Barbul A. Modulation of inflammation and immunity by arginine supplements. Curr Opin Clin Nutr Metab Care. 1998;1:531–538. doi: 10.1097/00075197-199811000-00010. [DOI] [PubMed] [Google Scholar]
- 36.Hastie T, Friedman J, Tibshirani R, York NY. The elements of statistical learning. New Springer. 2009 [Google Scholar]
- 37.Saraswathi V, Hasty AH. The role of lipolysis in mediating the proinflammatory effects of very low density lipoproteins in mouse peritoneal macrophages. J Lipid Res. 2006;47:1406–1415. doi: 10.1194/jlr.M600159-JLR200. [DOI] [PubMed] [Google Scholar]
- 38.Gupta M, Singh N, Tsigoulis M, Kajil M, Hirjikaka S, Quan A, Teoh H, Verma S. Perceptions of canadian primary care physicians towards cardiovascular risk assessment and lipid management. Can J Cardiol. 2012;28:14–19. doi: 10.1016/j.cjca.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 39.Doran B, Guo Y, Xu J, Weintraub H, Mora S, Maron DJ, Bangalore S. Prognostic value of fasting versus nonfasting low-density lipoprotein cholesterol levels on long-term mortality: Insight from the national health and nutrition examination survey III (NHANES-III) Circulation. 2014;130:546–553. doi: 10.1161/CIRCULATIONAHA.114.010001. [DOI] [PubMed] [Google Scholar]
- 40.Nordestgaard BG, Langsted A, Freiberg JJ. Nonfasting hyperlipidemia and cardiovascular disease. Curr Drug Targets. 2009;10:328–335. doi: 10.2174/138945009787846434. [DOI] [PubMed] [Google Scholar]