Highlights
-
•
Beclin orthologs are crucial regulators of autophagy and related membrane-trafficking pathways.
-
•
Multiple signaling pathways converge on Beclin 1 to regulate cellular stress responses, membrane trafficking, and physiology.
Keywords: autophagy, autophagic maturation, endocytic maturation, LC3-associated phagocytosis, endolysosomal trafficking
Abstract
The Beclin family, including yeast Atg6 (autophagy related gene 6), its orthologs in higher eukaryotic species, and the more recently characterized mammalian-specific Beclin 2, are essential molecules in autophagy and other membrane-trafficking events. Extensive studies of Beclin orthologs have provided considerable insights into the regulation of autophagy, the diverse roles of autophagy in physiology and disease, and potential new strategies to modulate autophagy in a variety of clinical diseases. In this review we discuss the functions of Beclin orthologs, the regulation of such functions by diverse cellular signaling pathways, and the effects of such regulation on downstream cellular processes including tumor suppression and metabolism. These findings suggest that Beclin orthologs serve as crucial molecules that integrate diverse environmental signals with membrane trafficking events to ensure optimal responses of the cell to stressful stimuli.
Functional diversity of Beclin orthologs
The lysosomal degradation pathway of autophagy is an evolutionarily conserved pathway that functions in protein and organelle quality control, cellular and tissue homeostasis, differentiation and development, and in diverse aspects of physiology and pathophysiology 1, 2. One of the most-extensively studied autophagy gene products, mammalian Beclin 1 [the ortholog of yeast Atg6/Vps30 (vacuolar protein sorting 30)], is part of a lipid kinase complex that mediates the initial stages of autophagosome formation [3]. Historically, loss-of-function genetic studies with Atg6/Beclin 1 orthologs were crucial in suggesting a role for the autophagy pathway in tumor suppression, dauer development, fruiting body formation, longevity, innate immunity, and the pathophysiology of neurodegenerative and cardiac diseases 1, 4, 5 (Table 1 ). Beyond autophagy, there is expanding evidence that Beclin 1 functions in other membrane-trafficking pathways such as endocytic and phagosomal maturation 6, 7, 8. Moreover, a mammalian-specific close cousin of Beclin 1 has been discovered, Beclin 2, that functions in endolysosomal trafficking of G protein-coupled receptors [9]. There is also increasing evidence that diverse signaling pathways, including oncogenic/tumor-suppressive signals, immune signals, and stress-responsive signals, converge on Beclin 1 to regulate autophagy, allowing Beclin 1 to serve as a central hub that coordinates environmental stimuli with downstream physiological outputs. We discuss recent studies related to the functional diversity of Beclin orthologs, their mechanisms of regulation, and the implications of these studies for understanding the role of Beclin orthologs in physiological processes.
Table 1.
Developmental and disease phenotypes of Atg6/Vps30/Becn1 mutant organisms
| Mutations or RNAi | Phenotypes | Refs |
|---|---|---|
| S. cerevisiae | ||
| atg6/vps30Δ | Defective autophagy Defective sporulation in homozygous mutant diploids |
10, 12, 13 [10] |
| Defective vacuolar protein sorting | 11, 12, 91 | |
| Defective G1/G0 quiescence entry in response to nitrogen starvation | [78] | |
| Plants(N. benthamiana,A. thaliana) | ||
| VIGS-NbATG6, AtATG6-AS | Accelerated leaf senescence Uncontrolled cell death during the hypersensitive immune response to pathogen infection |
80, 92 |
|
AtATG6/atatg6, atatg6/atatg6 |
Defective pollen germination and development | 18, 93 |
| D. discoideum | ||
| atg6Δ | Defective fruiting body formation | [79] |
| Increased Salmonella typhimurium intracellular replication | [94] | |
| D. melanogaster | ||
| +/Atg61, Atg61/Atg61 | Defective protein secretion, enlarged lymph glands and melanotic blood cell mass formation | [19] |
| Atg6 (RNAi), Atg61 | Impaired cell polarity during wing development | [83] |
| C. elegans | ||
| bec-1 RNAi | Defective dauer development | [81] |
| Increased S. typhimurium intestinal epithelial cell replication/invasion | [94] | |
| Abrogates insulin signaling-regulated pathogen resistance | [94] | |
| Decreased animal survival after a severe hypoxic insult | [95] | |
| Increased apoptotic cell death | [96] | |
| Decreased lifespan extension by aberrant insulin/IGF-1 signaling, caloric restriction, resveratrol treatment, sirtuin-1 overexpression, pyrazinamidase/nicotinamidase overexpression, calcineurin null mutation, p53 deficiency, and frataxin silencing | 81, 82, 97, 98, 99, 100, 101 | |
| ok691 (bec-1 null) | Impaired clearance of apoptotic corpses during embryonic development | 20, 102 |
| Impaired retrograde transportation from endosomes to the Golgi | [20] | |
| Reduced cell size | [103] | |
| Increased apoptotic cell death | [96] | |
| Decreased lifespan of daf-2 mutants, germline-less glp-1/Notch mutants, caloric restriction mutants, and lowered mitochondrial respiration mutants | 104, 105 | |
| D. rerio | ||
| becn1 morpholino | Defective cardiac morphogenesis | [106] |
| Mus musculus | ||
| Becn1−/− | Early embryonic lethality Embryoid bodies fails to cavitate |
84, 85 [88] |
| Becn1+/− | Increased frequency of spontaneous malignancies (lung carcinomas, liver carcinomas, lymphomas, and breast carcinomas) | 84, 85, 86 |
| Increased hypoxia-induced angiogenesis | [107] | |
| Increased renal fibrosis following ureteral obstruction | [108] | |
| Increased susceptibility to light-induced retinal damage | [109] | |
| Increased lung pathology during RSV infection | [110] | |
| Resistant to T regulatory cell suppression | [111] | |
| Impaired maintenance of early lymphocyte progenitor populations | [112] | |
| Reduced exercise endurance and impaired exercise-induced insulin sensitivity | [60] | |
| Reduced cardiac injury during ischemia and reperfusion | 46, 113 | |
| Reduced diabetic cardiac damage | [114] | |
| Reduced pressure-stressed pathological remodeling | [115] | |
| Impaired extracellular amyloid β (Aβ) clearance and phagocytosis in brain | [116] | |
| APP+Becn1+/− | Increased (Aβ) accumulation, extracellular Aβ deposition, and neurodegeneration | [117] |
| CD4–Cre.Becn1fl/fl | Impaired peripheral T cell homeostasis | [118] |
| Cyp19–iCre.Becn1fl/fl, | Defective progesterone production and subsequent preterm labor | [119] |
| αMHC–CryABR120GBecn1+/− | Accelerated desmin-related cardiomyopathy | [120] |
| SOD1G86RBecn1+/− | Increased lifespan of mutant SOD1 transgenic mice | [121] |
| Tsc2+/−Becn1+/− | Reduced spontaneous renal tumorigenesis in Tsc2+/− mice | [122] |
Discovery of yeast Atg6/Vps30 and human Beclin 1
Yeast ATG6/VPS30 was discovered in two independent genetic screens, including one to identify genes required for survival during starvation, which led to the discovery of ATG6 [10], and one to identify genes required for endosome to Golgi retrieval of the vacuolar protein sorting receptor, Vps10p, which led to the discovery of VPS30 [11]. In yeast, Atg6/Vps30 is part of two distinct phosphatidylinositol 3-kinase (PI3K) complexes [12]. During autophagy, in complex I, Atg14 interacts with Atg6/Vps30 and targets the PI3K Vps34 and its membrane anchor, Vps15, to the pre-autophagosomal structure (PAS) 13, 14. The newly identified subunit Atg38 bridges the interaction between the Atg14–Atg6 subcomplex and the Vps34–Vps15 subcomplex through dual binding of Atg14 and Vps34 [15]. During vacuolar protein sorting, in complex II, Vps38 is present instead of Atg14 and targets Vps34–Vps15–Atg6 to the endosomal membrane [12].
Beclin 1, the mammalian ortholog of yeast Atg6/Vps30, was discovered in a yeast two-hybrid screen using the anti-apoptotic protein Bcl-2 (B cell lymphoma 2) as bait [16]. Shortly thereafter, Beclin 1 was shown to rescue autophagy, but not vacuolar protein sorting, in yeasts lacking ATG6/VPS30, and to rescue starvation-induced autophagy in human breast carcinoma cells with low endogenous Beclin 1 expression [17]. Subsequently, genetic loss-of-function studies revealed an evolutionarily conserved role for Atg6/Beclin 1 orthologs in autophagy in multiple eukaryotic species including plants, slime molds, nematodes, fruit flies, and mice [4]. The lack of vacuolar protein sorting function of human Beclin 1 in ATG6 null yeast may simply reflect the sequence divergence between yeast Atg6 and human Beclin 1 (24.4% identity and 39.1% similarity) as Atg6/Beclin 1 orthologs have been shown to function in endocytosis in multicellular organisms including A. thaliana [18], Drosophila [19], C. elegans [20], and mice [21].
Similar to its role in yeast, mammalian Beclin 1 is part of distinct Vps34 (also known as PI3KC3)-containing complexes (PI3KC3-C1 and PI3KC3-C2) that function differentially in autophagy and endocytic trafficking, respectively (Figure 1 ) 22, 23, 24, 25. The precise subunits of each complex vary somewhat in different reports but, in general, there is consensus that mammalian Atg14 [also known as Atg14L or Barkor (Beclin 1 interacting autophagy related key regulator)] functions similarly to yeast Atg14 as an autophagy-specific component that targets Beclin 1–Vps34 to the sites of initiation of phagophore biogenesis, whereas mammalian UVRAG functions similarly to yeast Vps38 as a subunit involved in endocytic maturation 22, 23, 24, 25. In addition to its function in early autophagosomal biogenesis, Atg14 appears on mature autophagosomes where it interacts with autophagic SNAREs [soluble NSF attachment protein (SNAP) receptors], syntaxin-17 and SNAP29 to promote autophagosome-lysosome tethering and fusion [26]. NRBF2 (nuclear receptor binding factor 2), the mammalian counterpart of Atg38, also bridges the interaction between Vps34–Vps15 and Beclin 1–Atg14 27, 28. UVRAG (UV radiation resistance associated gene) positively regulates Vps34 activity on endosomes and lysosomes [22], and another protein, Rubicon, that lacks an obvious yeast counterpart, counteracts this function of UVRAG 24, 25, 29, 30. The UVRAG-containing Beclin 1–Vps34 complex is currently believed to function in both endocytic trafficking and autophagosomal maturation, although it is still a matter of debate whether UVRAG functions in autophagosome maturation directly or indirectly through its endocytic functions. In addition to autophagy and endocytic trafficking, mammalian Beclin 1 and its binding partners in the class III PI3K complex also participate in a process termed ‘LC3 (named from microtubule-associated protein 1 light chain 3)-associated phagocytosis’ [7], which involves the maturation of phagosomes containing intracellular pathogens, apoptotic cells, or entotic cells (Figure 2 ).
Figure 1.
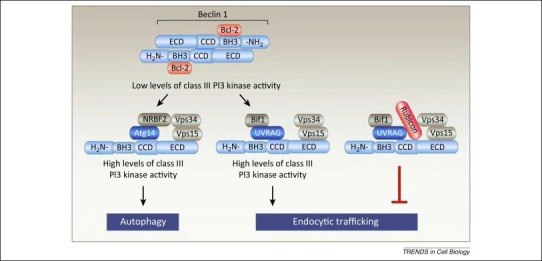
Model of mammalian Beclin 1 class III phosphatidylinositol 3-kinase (PI3K) complexes. Beclin 1 homodimerization (top) favors binding to Bcl-2 and disfavors binding to the Vps34-containing class III PI3K complex. Beclin 1 monomers form distinct class III PI3K complexes (often referred to as PI3KC3-C1 and PI3KC3-C2), including an autophagy-active complex containing Atg14, Vps15, Vps34, and NRBF2 (left) as well as a complex that functions in endocytic trafficking containing UVRAG, Bif1, Vps15, and Vps34 (middle). The Beclin 1-containing class III PI3K complex (PI3KC3-C2) that functions in endocytic trafficking is inhibited by Rubicon, which also preferentially binds to Beclin 1 dimers, not shown here (right). The complete structure of these complexes has not been solved, and therefore the precise spatial arrangement of proteins in these complexes is not yet known.
Figure 2.

Functions of Atg6/Beclin 1 and Beclin 2 in diverse membrane trafficking processes. Yeast Atg6 and mammalian Beclin 1 function in autophagy and vacuolar protein sorting, and mammalian Beclin 1 functions in LC3-associated phagocytosis. The mammalian-specific protein Beclin 2 functions in autophagy, and in a class III PI3K-independent manner, in GPCR endolysosomal trafficking. The autophagy protein names are shown as yeast or mouse; similar functions exist for the human orthologs. Abbreviations: ER, endoplasmic reticulum; GPCR, G protein-coupled receptor; LC3, microtubule-associated protein 1 light chain 3; PE, phosphatidylethanolamine; PI3K, phosphatidylinositol 3-kinase.
The function of Atg6/Beclin 1 in autophagy
The central mechanism by which Atg6/Beclin 1 functions in autophagy and probably in endocytic maturation seems to be via the allosteric regulation of organelle-specific lipid kinase activity of the catalytic subunit of Vps34. Atg6/Beclin 1 is a peripheral membrane protein containing an N-terminal intrinsically disordered region [31], a BH3 (Bcl-2 homology 3) domain [32], a coiled-coil domain [33], and a C-terminal BARA (β-α repeated, autophagy-specific) domain 34, 35. It is generally believed that the Vps34–Vps15–Atg6/Beclin 1 complex is recruited to autophagic or endocytic membranes through interaction between the coiled-coil domain of Beclin 1 with either Atg14 or Vps38/UVRAG, thereby specifying the organellar site of Vps34 lipid kinase activity. Atg14 directly interacts with membrane via the hydrophobic surface of an ALPS (amphipathic lipid-packing sensor) motif within its BATS (Barkor/Atg14 autophagosome targeting sequence) domain [36], and UVRAG likely interacts with membrane through its phospholipid-binding C2 domain, or indirectly through its interaction with Bif-1/endophilin B1 that contains an N-terminal N-BAR (Bin–amphiphysin–Rvs) domain and a C-terminal SH3 (Src-homology 3) domain, which display membrane binding and bending activities 37, 38. Beclin 1 may also bridge the membrane interaction of the PI3KC3 complex through aromatic amino acids at the tip of a surface loop in its BARA domain [34].
Recently, the structure of the PI3KC3-C1 complex was determined by single-particle electron microscopy of ordered regions of the Beclin 1, Atg14, Vps15, and Vps34 subunits, and its dynamics were analyzed by hydrogen exchange [39]. While higher-resolution structures that include the full-length proteins of each of these subunits as well as of NRBF2 will be helpful for a more complete understanding, these studies reveal an architectural and dynamic model that is consistent with a growing body of evidence that modifications in the N terminus of Beclin 1 are crucial for regulating the lipid kinase activity of PI3KC3-C1. According to the model proposed by Baskaran et al., the complex has a V-shaped architecture; Vps15 forms a bridge between Vps34 and an Atg14–Beclin 1 subcomplex; dynamic transitions occur during which the lipid kinase domain of Vps34 is ejected from the complex and Vps15 pivots at the base of the V; and the N-terminal disordered region of Beclin 1 is predicted to reside near the pivot point, thereby allosterically regulating the lipid kinase activity of PI3KC3-C1.
As discussed below and shown in Figure 3 , the N-terminal region of Beclin 1 contains many residues that are targets of autophagy regulatory kinases [e.g., ULK1, MAP kinase activated protein kinases 2/3 (MAPKAPK2/MAPKAPK3 – MK2/MK3), AMPK, DAPK, ROCK1, and MST1], as well as the BH3 domain which mediates its interactions with a crucial family of negative regulators of autophagy, Bcl-2, and its related cellular and viral anti-apoptotic proteins. Most autophagy stimulatory phosphorylation events enhance Beclin 1-associated Vps34 lipid kinase activity, whereas most autophagy inhibitory phosphorylation events as well as Bcl-2 binding inhibit Beclin 1-associated Vps34 lipid kinase activity. Furthermore, Bcl-2 binding, which stabilizes Beclin 1 homodimers, blocks N-terminal autophagy stimulatory phosphorylation (e.g., MK2/MK3-dependent Beclin 1 S90 phosphorylation [40]) whereas Atg14–Beclin 1 heterodimerization enhances N-terminal autophagy stimulatory phosphorylation (e.g., ULK1-dependent Beclin 1 S14 phosphorylation [41], AMPK-dependent Beclin 1 S93 and S96 phosphorylation [42]). Thus, both structural and biochemical data point to a crucial role of regulatory signaling input into Beclin 1 as a key mechanism for regulating Vps34 lipid kinase activity at membranes involved in autophagosomal biogenesis and endocytic maturation. The stimulation of Vps34 lipid kinase activity and resulting phosphatidylinositol 3-phosphate (PI3P) production then allows downstream effectors to complete the processes of membrane extension, cargo recruitment, and autophagosome maturation [43]. Of note (and an important experimental limitation for autophagy detection by Atg8–phosphatidylethanolamine (PE)/LC3-II western blots), Atg6 and Beclin 1 are essential for the recruitment of lipidated Atg8 and LC3, respectively, to the autophagosome, but Atg6 is not essential in yeast 44, 45 and Beclin 1 is not essential in mouse embryonic stem cells [46] for the Atg8/LC3 lipidation process itself.
Figure 3.
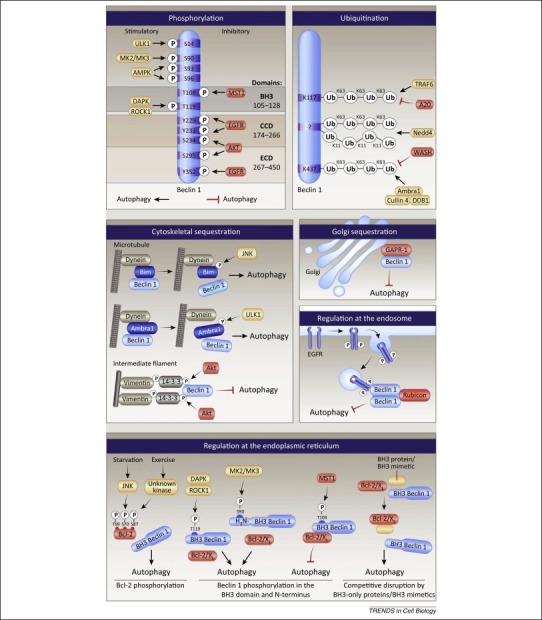
Mechanisms of regulation of Beclin 1 function. The autophagy activity of Beclin 1 is regulated by several kinases that phosphorylate (P) Beclin 1 (top left box), by ubiquitination modifications (top right box), by post-translational modifications or protein–protein interactions that sequester Beclin 1 in the cytoskeleton (left middle box) or Golgi (left middle right box), by post-translation modifications that promote Beclin 1–Rubicon interaction at the endosome (left middle right box), and by several mechanisms that regulate the interaction between Bcl-2/Bcl-XL and Beclin 1 at the endoplasmic reticulum (lower box).
Divergent modes of regulation of Beclin 1 function
Given the complexity of mammals, it is not surprising that mammalian Beclin 1 undergoes multiple layers of regulation in addition to its interactions with other core subunits of the PI3KC3 complexes. Diverse cellular stimuli communicate with the PI3KC3 complexes to modulate autophagy and endocytic trafficking via post-translational modifications of Beclin 1 (e.g., phosphorylation, ubiquitination), sequestration of Beclin 1 in other subcellular locations (e.g., cytoskeleton, Golgi apparatus), or via protein–protein interactions that alter its interactions with other core components of PI3KC3 complexes or directly affect Beclin 1-associated Vps34 lipid kinase activity (Figure 3). The wide and continuously growing spectrum of bona fide Beclin 1-interacting partners (Table 2 ) that contribute to such regulation underscores the importance of crosstalk with Beclin 1 and autophagy in growth signaling, metabolism, mitophagy, innate immune signaling, and microbial pathogenesis. At present, it is unclear, given the multitude of post-translational modifications and protein–protein interactions that regulate the function of Beclin 1, what the precise temporal, spatial, and hierarchical relationships are between different modes of Beclin 1 regulation, and how such relationships may be altered in different cell types and physiological/pathophysiological contexts. In the sections below we highlight a few key mechanisms underlying the regulation of Beclin 1 activity that relate to metabolism and cancer, including binding of Bcl-2 family members and phosphorylation by oncogenic and candidate tumor-suppressor kinases. For a more complete review of the Beclin 1 interactome (Table 2) and regulation of Beclin 1 by ubiquitination (summarized in Figure 3) we refer readers to other excellent recent reviews on this topic 47, 48.
Table 2.
Beclin 1-binding Partnersa
| Protein | Impact on Autophagy | Refs |
|---|---|---|
| PI3KC3 components | ||
| Vps34 | Required for autophagy | [3] |
| Vps15 | Required for autophagy | [3] |
| Atg14/Barkor | Required for autophagy | 23, 24, 25, 53 |
| UVRAG | Involved in autophagy induction and/or autophagosomal maturation | 22, 24, 54 |
| AMBRA1 | Stimulates autophagy | [123] |
| Bif-1/endophilin B1 | Stimulates autophagy | [37] |
| Rubicon | Inhibits autophagy, endosome maturation and autophagosomal maturation | 24, 25, 29, 30 |
| NRBF2 | Stimulates and/or inhibits autophagy | 27, 28, 124 |
| Bcl-2 family members | ||
| Bcl-2 | Inhibits autophagy | 56, 64 |
| Bcl-XL | Inhibits autophagy | 56, 64 |
| Bim | Inhibits autophagy | [52] |
| Viral Bcl-2 homologs | ||
| Adenovirus E1B19K | Stimulates autophagy | [125] |
| ASFV A179L | Inhibits autophagy | [126] |
| γHV68 M11 | Inhibits autophagy | 127, 128 |
| KSHV vBcl-2 | Inhibits autophagy | [56] |
| Kinases | ||
| AKT | Inhibits autophagy | [68] |
| AMPKb | Stimulates autophagy | [42] |
| DAPK | Stimulates autophagy | [61] |
| EGFR | Inhibits autophagy | [55] |
| MK2/MK3b | Stimulates autophagy | [40] |
| HER2 | Inhibits autophagy | [129] |
| MST1 | Inhibits autophagy | [65] |
| PINK1 | Stimulates mitophagy | [130] |
| ROCK1 | Stimulates autophagy | [66] |
| ULK1 | Stimulates autophagy | [41] |
| Ubiquitinating/deubiquitinating enzymes | ||
| A20 | Inhibits autophagy | [131] |
| PARK2 | Stimulates mitophagy | [132] |
| TRAF6 | Stimulates autophagy | [131] |
| USP9X | Unknown | [133] |
| WASH | Inhibits autophagy | [134] |
| Immunity-related molecules | ||
| cGAS | Stimulates autophagy | [135] |
| MyD88 | Stimulates autophagy | [62] |
| NLRP4 | Inhibits autophagy | [136] |
| PYCARD/ASC | Unknown | [137] |
| SLAMF1 | Stimulates LC3-associated phagocytosis | [138] |
| Trif | Stimulates autophagy | [62] |
| TRIM5α | Stimulates autophagy | [63] |
| Other cellular proteins | ||
| 14-3-3 | Inhibits autophagy | [68] |
| β-Arrestin-1 | Stimulates autophagy | [139] |
| Dapper1 (Dpr1) | Stimulates autophagy | [140] |
| GLIPR2 (GAPR-1) | Inhibits autophagy | [90] |
| HMGB1 | Stimulates autophagy | [141] |
| Ins(1,4,5)P3R | Inhibits autophagy | [142] |
| LRPPRC | Inhibits autophagy | [143] |
| nPIST | Stimulates autophagy | [144] |
| p53 | Inhibits autophagy | [145] |
| Rhes | Stimulates autophagy | [146] |
| TAB2/TAB3 | Inhibits autophagy | [147] |
| VMP1 | Stimulates autophagy | [148] |
| Other microbial proteins | ||
| Coronavirus PLP2-TM | Stimulates autophagy | [149] |
| FMDV 2C | Unknown | [150] |
| HCMV TRS1 | Inhibits autophagy | [151] |
| HIV-1 Nef | Inhibits autophagosomal maturation | [152] |
| HSV-1 ICP34.5 | Inhibits autophagy and autophagosomal maturation | 153, 154 |
| Influenza A M2 | Inhibits autophagosomal maturation | [155] |
Includes only cellular and viral proteins shown to interact with endogenous Beclin 1, unless otherwise noted.
These kinases have been shown to directly phosphorylate Beclin 1 in vitro, but their interaction with Beclin 1 has not been demonstrated.
Regulation of Beclin 1 by Bcl-2
Beclin 1 was originally identified by virtue of its interaction with Bcl-2 [16], and several Bcl-2 family members, including Bcl-2, Bcl-XL, and virus-encoded Bcl-2 proteins, inhibit autophagy through a direct interaction with the BH3 domain of Beclin 1 [49]. Unlike other BH3-only proteins, Beclin 1 has no direct effects on the anti-apoptotic function of Bcl-2 or on apoptosis [50], whereas, in contrast, the apoptotic machinery may negatively regulate autophagy through caspase cleavage of Beclin 1 [51] or Bim (Bcl-2 interacting mediator of cell death)-mediated sequestration of Beclin 1 to microtubules [52]. The favored hypothesis is that Bcl-2 family members interact with the dimerized form of Beclin 1 in unstressed cells, and upon exposure to stress stimuli the Bcl-2/Beclin 1 complex, as well as Beclin 1 homodimerization, is disrupted, leading to heterodimerization with Atg14 or UVRAG and enhanced Vps34 lipid kinase activity on distinct membrane structures to activate autophagy or endocytosis 23, 24, 30, 53, 54, 55. Recent evidence also indicates that the binding of Bcl-2 to Beclin 1 blocks a newly defined signaling event both in vitro and in mouse tissues that is essential for initiation of amino acid starvation-induced autophagy, namely, MK2/MK3-mediated Beclin 1 S90 serine phosphorylation [40]. Endoplasmic reticulum (ER)-localized Bcl-2, but not mitochondrion-localized Bcl-2, inhibits autophagy [56], and this is consistent with a crucial role of ER-associated PI3KC3 activity in autophagosome initiation 57, 58.
The dissociation of Bcl-2/Beclin 1 represents a central switch that turns autophagy on in response to diverse stress signals, including amino acid starvation 59, 60, exercise [60], death signaling molecules (e.g., DAPK) [61], toll-like receptor adaptor signaling molecules (e.g., MyD88 and Trif) [62] and the host restriction molecule, TRIM5α (tripartite motif-containing protein 5α) [63]. Several different mechanisms regulate the interaction between Bcl-2 and the BH3 domain of Beclin 1, including competitive disruption by BH3-only proteins [64], phosphorylation events within the BH3 domain of Beclin 1 that either promote (e.g., MST1) or inhibit (e.g., DAPK, ROCK1) its binding to Bcl-2 61, 65, 66, or by multisite phosphorylation of the non-structured loop of Bcl-2, which prevents its binding to Beclin 1 [59]. During starvation conditions, this is mediated by the stress-signaling molecule, JNK1 (c-Jun N-terminal kinase 1) [59], whereas the kinase that leads to Bcl-2 phosphorylation and disruption of the Bcl-2/Beclin 1 complex during exercise is not yet known [60].
The physiological relevance of Bcl-2 multisite phosphorylation in regulating stimulus-induced autophagy has been demonstrated in vivo using knock-in mice containing non-phosphorylatable mutations in Bcl-2 (T69A, S70A and S84A; Bcl-2 AAA) [60]. These mice have normal basal levels of autophagy but are severely deficient in starvation- and exercise-induced autophagy. This deficiency in exercise-induced autophagy in mice with normal basal levels of autophagy and normal tissue development led to the discovery of a novel role of Beclin 1 and autophagy in metabolism. Unlike wild type mice, acute exercise in Bcl-2 AAA mice fails to result in increased insulin sensitivity in skeletal muscle, as measured by glucose uptake, plasma membrane localization of the glucose transporter Glut4, and activation of AMP-activated protein kinase (AMPK). Similar results were also observed in mice with allelic loss of the Beclin 1 gene Becn1 or a hypomorphic allele of another autophagy gene, Atg16L1, suggesting that these phenotypes are indeed likely due to deficient autophagy rather than an unknown effect of the Bcl-2 AAA mutation. Moreover, chronic exercise has beneficial effects in wild type mice but not in Bcl-2 AAA mice against high-fat diet-induced diabetes and other metabolic abnormalities. These findings raise the possibility that autophagy may act not only downstream of AMPK signaling but also upstream of AMPK signaling. Such a feed-forward mechanism of autophagy on AMPK activation may help to explain the biochemical mechanisms underlying the more broad observations of beneficial effects of autophagy not only on metabolism but also on tumor-suppression and lifespan extension.
Regulation of Beclin 1 by oncogenic kinases
The discovery of Akt (protein kinase B)-mediated and epidermal growth factor receptor (EGFR)-mediated phosphorylation and inhibition of Beclin 1 provides insights into the biochemical mechanisms connecting cell growth control with autophagy regulation, and also allows dissection of the role of oncogenic signaling mediated inhibition of autophagy in different stages of tumorigenesis. Many oncogenic receptor tyrosine kinases and growth signaling molecules in the class I PI3K signaling pathway have been long known to suppress autophagy [67]; however, because these signals activate mTOR (mechanistic target of rapamycin), which not only inhibits autophagy but also stimulates translation and other processes involved in anabolic growth, the relevance of oncogenic signaling suppression of autophagy per se in tumorigenesis has been unclear.
The oncoprotein Akt is activated in the majority of human epithelial tumors, and such activation results in Beclin 1 phosphorylation on serines S234 and S295, the binding of Beclin 1 to 14-3-3 and intermediate filament proteins, its sequestration in the cytoskeleton, and mTOR-independent autophagy inhibition [68]. Importantly, the ability of Akt to transform fibroblasts and the ability of Akt-transformed fibroblasts to form fibrosarcomas when implanted into immunodeficient mice is severely impaired in cells expressing mutant forms of Beclin 1 that are resistant to Akt-mediated phosphorylation and autophagy suppression [68]. This suggests that the suppression of autophagy by oncogenic signaling may directly contribute to oncogenic transformation.
The oncogenic receptor tyrosine kinase, EGFR, is amplified in many solid tumors, and constitutive active mutations in its tyrosine kinase domain are driver mutations in non-small cell lung carcinomas (NSCLCs) that occur in non-smokers [69]. Activated EGFR phosphorylates three Beclin 1 tyrosine residues, Y229, Y233, and Y352, which promotes Beclin 1 homodimerization and Rubicon binding, leading to decreased formation of the autophagy-active Beclin 1-containing PI3KC3-C1 complex [55]. These biochemical data are consistent with predictions from the crystal structure of the Beclin 1 coiled-coil domain, which suggest that it forms a metastable antiparallel dimer that can be disrupted by Atg14 or UVRAG to form heterodimers [33]. The two Beclin 1 tyrosine phosphorylation sites, Y229 and Y233, are at the interface of the Beclin 1 coiled-coil domain and stabilize the homodimers [70]. Constitutive activation of Beclin 1 tyrosine phosphorylation functions as a dominant negative mutant by promoting Beclin 1 homodimerization, and the expression of such a mutant in NSCLC xenografts leads to autophagy suppression, enhanced tumor growth, increased cell proliferation, and tumor dedifferentiation from an adenocarcinoma to a more aggressive adenosquamous phenotype [55]. In addition, the inducible expression of a Beclin 1 tyrosine phosphomimetic mutant in established NSCLC xenografts results in partial chemoresistance to EGFR tyrosine kinase inhibitor therapy [55]. Thus, Beclin 1 not only acts as a suppressor of tumor initiation, but, at least in the setting of NSCLC driven by an active EGFR mutation, also can prevent the progression of established tumors and help to mediate chemotherapeutic responses.
In contrast to active EGFR, which inhibits autophagy through Beclin 1 tyrosine phosphorylation, inactive EGFR may function in basal and starvation-induced autophagy [71]. Inactive EGFR forms an endosomal-localized complex with LAPTM4B (lysosomal protein transmembrane 4b) and Sec5 (also known as EXOC2, exocyst complex component 2), which promotes EGFR association with Rubicon, leading to Beclin 1 dissociation from Rubicon and autophagy initiation. This function of inactive EGFR is postulated to promote tumor cell survival upon serum starvation or metabolic stress; however, this concept has not yet been tested in tumorigenesis in vivo. The opposing regulation of Beclin 1 by inactive EGFR versus active EGFR raises the broader question of whether oncogenic signals may function more generally as ‘on/off’ switches that tightly coordinate autophagic responses with cell growth control.
Regulation of Beclin 1 by tumor-suppressor signaling molecules
Whereas there is emerging evidence that oncogenic signaling molecules may converge on Beclin 1 to suppress autophagy, there is also emerging evidence that tumor-suppressor signaling molecules converge on Beclin 1 to stimulate autophagy. The death-associated protein kinase (DAPK) is a serine/threonine kinase that induces different types of cell death (including autophagic cell death), functions as a tumor-suppressor, and is deleted in many cancer types [72]; this kinase induces autophagy by Beclin 1 threonine T119 phosphorylation, which disrupts its binding to Bcl-2 and Bcl-XL [61]. The liver kinase B1 (LKB1)/AMPK signaling pathway is an important tumor-suppressor signaling pathway that has multiple downstream targets including p53 and inhibition of mTOR [73]. AMPK has also been shown to promote autophagy by phosphorylating components of the autophagy machinery, including the upstream kinase, ULK1 (unc-51 like autophagy activating kinase 1) 74, 75, and, during glucose starvation, Beclin 1 serines S93 and S96 [42]. The stress-related kinase, MK3, was originally discovered by virtue of its frequent deletions in small cell lung carcinomas, is lost in a variety of other tumors, and is postulated to have tumor suppressor function [76]. This kinase, as well as its close relative, MK2, is required for amino acid starvation-induced autophagy via direct phosphorylation of Beclin 1 S90 [40]. Furthermore, mutation of Beclin 1 S90 blocks the ability of Beclin 1 to rescue starvation-induced autophagy in Beclin 1-deficient cells and blocks the tumor-suppressor activity of Beclin 1 in MCF7 human breast carcinoma xenografts [40]. While further studies are warranted to determine the precise role of Beclin 1 activation/phosphorylation by tumor-suppressor signaling molecules in mediating their tumor-suppressor function, it is notable that environmental stress signals – such as glucose and/or amino acid starvation – may dually activate autophagy and inhibit tumorigenesis. The overlapping requirement of specific post-translational modifications of Beclin 1 for its autophagy and tumor-suppressor activity may suggest a mechanistic link between these two functions, although more general effects on regulation of Vps34 activity in other trafficking events cannot be ruled out.
The discovery of Beclin 2
Another layer of complexity in higher eukaryotic organisms, beyond the diversity of signaling inputs to Beclin 1, has emerged from the identification of mammalian specific autophagy proteins such as AMBRA1 (activating molecule in Beclin 1-regulated autophagy) that activate the Beclin 1-containing PI3KC3-C1 complex (reviewed in [77]) and the recent identification of a mammalian-specific Beclin 1 homolog, Beclin 2 [9]. Beclin 2 shares ∼57% sequence identity with Beclin 1 and, similarly to Beclin 2, contains a predicted BH3 domain, a central coiled-coil domain, and a C-terminal BARA domain [9]. Beclin 2 interacts with Atg14, Vps34, UVRAG, and AMBRA1, but not Rubicon, and is required for autophagy [9]. The distinct tissue expression pattern of Beclin 2 versus Beclin 1 [9] may reflect distinct roles of different Beclin family members in different tissues; however, it is unclear why these proteins are not functionally redundant in autophagy in cells that express both proteins given their similarity in the coiled-coil and BARA domains.
The most notable sequence divergence between Beclin 1 and Beclin 2 is in their N-terminal regions. The N terminus of Beclin 2, but not Beclin 1, interacts with GASP1 (G-protein-coupled receptor-associated sorting protein 1), and Beclin 2, but not Beclin 1, is required for the endolysosomal trafficking and degradation of GASP1-dependent G protein-coupled receptors (GPCRs), including the δ-opioid receptor, the cannabinoid type 1 receptor (CB1R), and the non-recycling mutant β-adrenergic receptor [9]. This function of Beclin 2 in lysosomal degradation of particular GPCRs is not shared by other members of the autophagic PI3KC3 complex such as Atg14, Beclin 1, and Vps34. Thus, GPCR endolysosomal trafficking represents the first Vps34 kinase-independent trafficking function described for members of the Atg6/Beclin family. This dual use of a mammalian-specific protein in an autophagy PI3KC3 complex and in autophagy-independent, PI3KC3-independent, endolysosomal degradation of an important class of cell surface receptors may reflect a need for mammalian cells to integrate diverse lysosomal degradation pathways to optimally fine-tune cellular responses to complex environmental signals.
Physiological functions of mammalian Beclin 1 and Beclin 2
Atg6/Beclin 1 null mutations in lower eukaryotes result in several important developmental and disease phenotypes (Table 1), likely reflecting its diverse functions in autophagy, vacuolar protein sorting, and endocytic maturation. Many of these phenotypes, such as defective sporulation in yeasts [10], proper entry into G0/G1 quiescence during nitrogen starvation in yeasts [78], defective fruiting body formation in Dictyostelium [79], uncontrolled cell death during the plant hypersensitive response [80], and defective dauer development and longevity phenotypes in C. elegans 81, 82, are identical to those observed with mutation of other core autophagy genes. However, some phenotypes such as defective pollen germination in plants [18], impaired cell polarity in Drosophila [83], and defects in retrograde transportation from endosomes to Golgi in C. elegans [20] are not shared by other autophagy gene mutant organisms.
In mice, biallelic loss of Becn1 results in early embryonic lethality 84, 85. Monoallelic deletion of Becn1 results in increased incidence of spontaneous malignancies including lung carcinomas, lymphomas, hepatocellular carcinomas, and breast carcinomas that have basal like features 84, 85, 86. This latter phenotype is consistent with recent data showing that decreased BECN1 mRNA expression in patients is strongly associated with increased risk of the more aggressive basal-like subtypes of sporadic breast cancer and with worse patient survival [87]. In addition, allelic loss of Becn1 in mice results in increased susceptibility to Alzheimer's-like disease, increased severity of Desmin-related cardiomyopathy, increased renal fibrosis following ureteral obstruction, increased lung pathology during respiratory syncytial virus (RSV) infection, reduced exercise endurance, and impaired exercise-induced insulin sensitivity, among other phenotypes (Table 1) [5].
It is difficult to ascertain whether most of these phenotypes are due to defects in autophagy or other trafficking functions of Beclin 1 because comparable disease models have rarely been studied in mutant animals with partial loss of function of other autophagy genes. However, tissue-specific deletion of other autophagy genes in mice generally confirms an important role for autophagy in tumor suppression and in protection against neurodegenerative, infectious, cardiac, muscular, and metabolic diseases 1, 2. Nonetheless, a recent report describes a role for Beclin 1 in Rab5 (Ras-related in brain 5) GTPase-associated endosome formation and endosome maturation in vivo in mouse neurons [21]. Thus, the spectrum of phenotypes of Beclin 1-deficient mice most likely reflects its diverse roles in autophagy, endocytic trafficking, and LC3-associated phagocytosis. Although Beclin 1-dependent LC3-associated phagocytosis is thought to be essential for apoptotic corpse clearance [7], the requirement for cell autonomous ATP-production that requires both Beclin 1 and the autophagy protein, ATG5, in apoptotic clearance in mammalian embryonic body cavitation suggests that the autophagy process itself is important in this aspect of early development [88].
Interestingly, mice with allelic loss of Becn2 have a metabolic phenotype not shared by mice with allelic loss of Becn1, even though Becn1 +/− mice are more deficient in autophagy [9]. Becn2 heterozygous-deficient mice have increased brain levels of CB1R [9] and, consistent with the known effects of excessive CB1R signaling [89], have increased food intake, obesity, impaired glucose tolerance, and decreased insulin sensitivity [9]. These findings suggest that Beclin 2-mediated lysosomal degradation of CB1R, and potentially other GPCRs, may be important in vivo in preventing diseases caused by excessive GPCR signaling. Indeed, the genetic locus that contains human Beclin 2, chromosome 1q43, has been linked to obesity and diabetes in multiple ethnic populations [9], warranting further investigation of a possible role for Beclin 2 as a regulator of body weight and glucose homeostasis in humans.
Therapeutic potential of Beclin 1-derived autophagy activation
Given the broad involvement of autophagy in defense against infection, neurodegenerative disorders, diabetes, cancer, and aging, agents that specifically induce autophagy may have wide therapeutic applications [5]. Proof-of-principle for the concept that enhanced Beclin 1 activity may be therapeutically beneficial is provided by rodent Becn1 gene therapy studies that have shown a protective effect in different neurodegenerative diseases (α-synuclein models of Parkinson's disease, spinocerebellar ataxia type 3 disease), cystic fibrosis, K-ras-driven lung tumors, and collagen VI muscular dystrophies [5]. Moreover, the central role of Beclin 1 as a rate-limiting protein in autophagy initiation that is tightly regulated by protein–protein interactions and post-translational modifications provides a unique opportunity to translate knowledge gained from mechanistic studies of its regulation into new therapeutic approaches. Several drugs already in clinical use may activate autophagy at the level of Beclin 1 through disrupting Bcl-2/Beclin 1 interactions, inhibiting Akt, inhibiting EGFR and HER2 (human epidermal growth factor 2), or activating AMPK, and with recent advances in understanding the precise molecular of Beclin 1 regulation it should be possible to design even more specific activators in the future.
Another therapeutic strategy for increasing autophagy by increasing Beclin 1 function has emerged from the identification of a cell permeable peptide, Tat-Beclin 1, composed of 18 amino acids from the BARA domain of Beclin 1, that likely induces autophagy by competitively disrupting the binding of endogenous Beclin 1, and a newly identified negative regulator, the Golgi-associated plant pathogenesis-related protein 1 (GAPR-1, also known as GLIPR2) [90]. Tat-Beclin 1 decreases the accumulation of polyglutamine expansion protein aggregates and the replication of several pathogens (including HIV-1) in vitro, and reduces the mortality of mice infected with chikungunya virus or West Nile virus. Thus, this autophagy-inducing peptide (or derivatives thereof) may have potential efficacy in the treatment of human diseases.
Concluding remarks
Significant recent progress has been made in understanding the architecture of Beclin 1/PI3KC3 complexes, the functional diversity of Beclin 1 and Beclin 2 in different membrane trafficking events, and the stress-induced signaling events that alter the conformation and function of Beclin 1/PI3KC3 complexes. An emerging principle is that different upstream trafficking events, including endocytic maturation, phagosomal maturation, autophagosomal biogenesis, and endolysosomal GPCR protein sorting, which converge at the stage of lysosomal degradation, may all involve protein complexes that contain either Beclin 1 (and associated subunits in the PI3KC3 complex) or Beclin 2. The complex integration of cell signaling molecules that regulate these complexes and their functional outputs is likely to be essential for a wide range of physiological processes.
Acknowledgments
We thank Haley Harrington for assistance with manuscript preparation and Angela Diehl for expert scientific illustration. This work was supported by National Institutes of Health grants RO1 CA109618 (B.L.), RO1 CA133228 (Q.Z.), and U19AI109725 (B.L.); The Welch Foundation I-1864 (Q.Z.); CPRIT RP120718 (B.L.); and RP140320 (Q.Z.); and the American Cancer Society Research Scholar Grant RSG-11-274-01-CCG (Q.Z.). We apologize to those authors whose work could not be cited owing to space limitations.
References
- 1.Levine B., Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mizushima N., Komatsu M. Autophagy: renovation of cells and tissues. Cell. 2011;147:728–741. doi: 10.1016/j.cell.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 3.Kihara A. Beclin-phosphatidylinositol 3-kinase complex functions at the trans-Golgi network. EMBO Rep. 2001;2:330–335. doi: 10.1093/embo-reports/kve061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levine B., Klionsky D.J. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev. Cell. 2004;6:463–477. doi: 10.1016/s1534-5807(04)00099-1. [DOI] [PubMed] [Google Scholar]
- 5.Levine B. Development of autophagy inducers in clinical medicine. J. Clin. Invest. 2015;125:14–24. doi: 10.1172/JCI73938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Funderburk S.F. The Beclin 1-VPS34 complex – at the crossroads of autophagy and beyond. Trends Cell Biol. 2010;20:355–362. doi: 10.1016/j.tcb.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Green D.R., Levine B. To be or not to be? How selective autophagy and cell death govern cell fate. Cell. 2014;157:65–75. doi: 10.1016/j.cell.2014.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thoresen S.B. A phosphatidylinositol 3-kinase class III sub-complex containing VPS15, VPS34, Beclin 1, UVRAG and BIF-1 regulates cytokinesis and degradative endocytic traffic. Exp. Cell Res. 2010;316:3368–3378. doi: 10.1016/j.yexcr.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 9.He C. Beclin 2 functions in autophagy, degradation of G protein-coupled receptors, and metabolism. Cell. 2013;154:1085–1099. doi: 10.1016/j.cell.2013.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsukada M., Ohsumi Y. Isolation and characterization of autophagy-defective mutants of Saccharomyces cerevisiae. FEBS Lett. 1993;333:169–174. doi: 10.1016/0014-5793(93)80398-e. [DOI] [PubMed] [Google Scholar]
- 11.Robinson J.S. Protein sorting in Saccharomyces cerevisiae: isolation of mutants defective in the delivery and processing of multiple vacuolar hydrolases. Mol. Cell. Biol. 1988;8:4936–4948. doi: 10.1128/mcb.8.11.4936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kihara A. Two distinct Vps34 phosphatidylinositol 3-kinase complexes function in autophagy and carboxypeptidase Y sorting in Saccharomyces cerevisiae. J. Cell Biol. 2001;152:519–530. doi: 10.1083/jcb.152.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kametaka S. Apg14p and Apg6/Vps30p form a protein complex essential for autophagy in the yeast, Saccharomyces cerevisiae. J. Biol. Chem. 1998;273:22284–22291. doi: 10.1074/jbc.273.35.22284. [DOI] [PubMed] [Google Scholar]
- 14.Obara K. Assortment of phosphatidylinositol 3-kinase complexes – Atg14p directs association of complex I to the pre-autophagosomal structure in Saccharomyces cerevisiae. Mol. Biol. Cell. 2006;17:1527–1539. doi: 10.1091/mbc.E05-09-0841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Araki Y. Atg38 is required for autophagy-specific phosphatidylinositol 3-kinase complex integrity. J. Cell Biol. 2013;203:299–313. doi: 10.1083/jcb.201304123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liang X.H. Protection against fatal Sindbis virus encephalitis by Beclin, a novel Bcl-2-interacting protein. J. Virol. 1998;72:8586–8596. doi: 10.1128/jvi.72.11.8586-8596.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liang X.H. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature. 1999;402:672–676. doi: 10.1038/45257. [DOI] [PubMed] [Google Scholar]
- 18.Fujiki Y. An Arabidopsis homolog of yeast ATG6/VPS30 is essential for pollen germination. Plant Physiol. 2007;143:1132–1139. doi: 10.1104/pp.106.093864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shravage B.V. Atg6 is required for multiple vesicle trafficking pathways and hematopoiesis in Drosophila. Development. 2013;140:1321–1329. doi: 10.1242/dev.089490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruck A. The Atg6/Vps30/Beclin 1 ortholog BEC-1 mediates endocytic retrograde transport in addition to autophagy in C. elegans. Autophagy. 2011;7:386–400. doi: 10.4161/auto.7.4.14391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McKnight N.C. Beclin 1 is required for neuron viability and regulates endosome pathways via the UVRAG–VPS34 complex. PLoS Genet. 2014;10:e1004626. doi: 10.1371/journal.pgen.1004626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Itakura E., Mizushima N. Atg14 and UVRAG: mutually exclusive subunits of mammalian Beclin 1-PI3K complexes. Autophagy. 2009;5:534–536. doi: 10.4161/auto.5.4.8062. [DOI] [PubMed] [Google Scholar]
- 23.Sun Q. Identification of Barkor as a mammalian autophagy-specific factor for Beclin 1 and class III phosphatidylinositol 3-kinase. Proc. Natl. Acad. Sci. U.S.A. 2008;105:19211–19216. doi: 10.1073/pnas.0810452105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsunaga K. Two Beclin 1-binding proteins, Atg14L and Rubicon, reciprocally regulate autophagy at different stages. Nat. Cell Biol. 2009;11:385–396. doi: 10.1038/ncb1846. [DOI] [PubMed] [Google Scholar]
- 25.Zhong Y. Distinct regulation of autophagic activity by Atg14L and Rubicon associated with Beclin 1-phosphatidylinositol-3-kinase complex. Nat. Cell Biol. 2009;11:468–476. doi: 10.1038/ncb1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diao J. ATG14 promotes membrane tethering and fusion of autophagosomes to endolysosomes. Nature. 2015;520:563–566. doi: 10.1038/nature14147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cao Y. NRBF2 regulates macroautophagy as a component of Vps34 complex I. Biochem. J. 2014;461:315–322. doi: 10.1042/BJ20140515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu J. NRBF2 regulates autophagy and prevents liver injury by modulating Atg14L-linked phosphatidylinositol-3 kinase III activity. Nat. Commun. 2014;5:3920. doi: 10.1038/ncomms4920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun Q. Rubicon controls endosome maturation as a Rab7 effector. Proc. Natl. Acad. Sci. U.S.A. 2010;107:19338–19343. doi: 10.1073/pnas.1010554107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun Q. The RUN domain of rubicon is important for hVps34 binding, lipid kinase inhibition, and autophagy suppression. J. Biol. Chem. 2011;286:185–191. doi: 10.1074/jbc.M110.126425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mei Y. Intrinsically disordered regions in autophagy proteins. Proteins. 2014;82:565–578. doi: 10.1002/prot.24424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oberstein A. Crystal structure of the Bcl-XL–Beclin 1 peptide complex: Beclin 1 is a novel BH3-only protein. J. Biol. Chem. 2007;282:13123–13132. doi: 10.1074/jbc.M700492200. [DOI] [PubMed] [Google Scholar]
- 33.Li X. Imperfect interface of Beclin1 coiled-coil domain regulates homodimer and heterodimer formation with Atg14L and UVRAG. Nat. Commun. 2012;3:662. doi: 10.1038/ncomms1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang W. Crystal structure and biochemical analyses reveal Beclin 1 as a novel membrane binding protein. Cell Res. 2012;22:473–489. doi: 10.1038/cr.2012.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Noda N.N. Structure of the novel C-terminal domain of vacuolar protein sorting 30/autophagy-related protein 6 and its specific role in autophagy. J. Biol. Chem. 2012;287:16256–16266. doi: 10.1074/jbc.M112.348250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fan W. Autophagosome targeting and membrane curvature sensing by Barkor/Atg14(L) Proc. Natl. Acad. Sci. U.S.A. 2011;108:7769–7774. doi: 10.1073/pnas.1016472108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takahashi Y. Bif-1 interacts with Beclin 1 through UVRAG and regulates autophagy and tumorigenesis. Nat. Cell Biol. 2007;9:1142–1151. doi: 10.1038/ncb1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Farsad K. Generation of high curvature membranes mediated by direct endophilin bilayer interactions. J. Cell Biol. 2001;155:193–200. doi: 10.1083/jcb.200107075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baskaran S. Architecture and dynamics of the autophagic phosphatidylinositol 3-kinase complex. Elife. 2014;3:e05115. doi: 10.7554/eLife.05115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wei Y. The stress-responsive kinases MAPKAPK2/MAPKAPK3 activate starvation-induced autophagy through Beclin 1 phosphorylation. Elife. 2015;4:e05289. doi: 10.7554/eLife.05289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Russell R.C. ULK1 induces autophagy by phosphorylating Beclin-1 and activating VPS34 lipid kinase. Nat. Cell Biol. 2013;15:741–750. doi: 10.1038/ncb2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim J. Differential regulation of distinct Vps34 complexes by AMPK in nutrient stress and autophagy. Cell. 2013;152:290–303. doi: 10.1016/j.cell.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lamb C.A. The autophagosome: origins unknown, biogenesis complex. Nat. Rev. Mol. Cell Biol. 2013;14:759–774. doi: 10.1038/nrm3696. [DOI] [PubMed] [Google Scholar]
- 44.Suzuki K. The pre-autophagosomal structure organized by concerted functions of APG genes is essential for autophagosome formation. EMBO J. 2001;20:5971–5981. doi: 10.1093/emboj/20.21.5971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suzuki K. Interrelationships among Atg proteins during autophagy in Saccharomyces cerevisiae. Yeast. 2004;21:1057–1065. doi: 10.1002/yea.1152. [DOI] [PubMed] [Google Scholar]
- 46.Matsui Y. Distinct roles of autophagy in the heart during ischemia and reperfusion: roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ. Res. 2007;100:914–922. doi: 10.1161/01.RES.0000261924.76669.36. [DOI] [PubMed] [Google Scholar]
- 47.Kang R. The Beclin 1 network regulates autophagy and apoptosis. Cell Death Differ. 2011;18:571–580. doi: 10.1038/cdd.2010.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reidick C. Regulation of the tumor-suppressor function of the class III phosphatidylinositol 3-kinase complex by ubiquitin and SUMO. Cancers. 2014;7:1–29. doi: 10.3390/cancers7010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Levine B. Bcl-2 family members: dual regulators of apoptosis and autophagy. Autophagy. 2008;4:600–606. doi: 10.4161/auto.6260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ciechomska I.A. Bcl-2 complexed with Beclin-1 maintains full anti-apoptotic function. Oncogene. 2009;28:2128–2141. doi: 10.1038/onc.2009.60. [DOI] [PubMed] [Google Scholar]
- 51.Luo S., Rubinsztein D.C. Apoptosis blocks Beclin 1-dependent autophagosome synthesis: an effect rescued by Bcl-xL. Cell Death Differ. 2010;17:268–277. doi: 10.1038/cdd.2009.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Luo S. Bim inhibits autophagy by recruiting Beclin 1 to microtubules. Mol. Cell. 2012;47:359–370. doi: 10.1016/j.molcel.2012.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Itakura E. Beclin 1 forms two distinct phosphatidylinositol 3-kinase complexes with mammalian Atg14 and UVRAG. Mol. Biol. Cell. 2008;19:5360–5372. doi: 10.1091/mbc.E08-01-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liang C. Autophagic and tumour suppressor activity of a novel Beclin1-binding protein UVRAG. Nat. Cell Biol. 2006;8:688–699. doi: 10.1038/ncb1426. [DOI] [PubMed] [Google Scholar]
- 55.Wei Y. EGFR-mediated Beclin 1 phosphorylation in autophagy suppression, tumor progression, and tumor chemoresistance. Cell. 2013;154:1269–1284. doi: 10.1016/j.cell.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pattingre S. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122:927–939. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 57.Matsunaga K. Autophagy requires endoplasmic reticulum targeting of the PI3-kinase complex via Atg14L. J. Cell Biol. 2010;190:511–521. doi: 10.1083/jcb.200911141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hayashi-Nishino M. A subdomain of the endoplasmic reticulum forms a cradle for autophagosome formation. Nat. Cell Biol. 2009;11:1433–1437. doi: 10.1038/ncb1991. [DOI] [PubMed] [Google Scholar]
- 59.Wei Y. JNK1-mediated phosphorylation of Bcl-2 regulates starvation-induced autophagy. Mol. Cell. 2008;30:678–688. doi: 10.1016/j.molcel.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.He C. Exercise-induced BCL2-regulated autophagy is required for muscle glucose homeostasis. Nature. 2012;481:511–515. doi: 10.1038/nature10758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zalckvar E. DAP-kinase-mediated phosphorylation on the BH3 domain of beclin 1 promotes dissociation of beclin 1 from Bcl-XL and induction of autophagy. EMBO Rep. 2009;10:285–292. doi: 10.1038/embor.2008.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shi C.S., Kehrl J.H. MyD88 and Trif target Beclin 1 to trigger autophagy in macrophages. J. Biol. Chem. 2008;283:33175–33182. doi: 10.1074/jbc.M804478200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mandell M.A. TRIM proteins regulate autophagy and can target autophagic substrates by direct recognition. Dev. Cell. 2014;30:394–409. doi: 10.1016/j.devcel.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Maiuri M.C. Functional and physical interaction between Bcl-X(L) and a BH3-like domain in Beclin-1. EMBO J. 2007;26:2527–2539. doi: 10.1038/sj.emboj.7601689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Maejima Y. Mst1 inhibits autophagy by promoting the interaction between Beclin1 and Bcl-2. Nat. Med. 2013;19:1478–1488. doi: 10.1038/nm.3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gurkar A.U. Identification of ROCK1 kinase as a critical regulator of Beclin1-mediated autophagy during metabolic stress. Nat. Commun. 2013;4:2189. doi: 10.1038/ncomms3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Botti J. Autophagy signaling and the cogwheels of cancer. Autophagy. 2006;2:67–73. doi: 10.4161/auto.2.2.2458. [DOI] [PubMed] [Google Scholar]
- 68.Wang R.C. Akt-mediated regulation of autophagy and tumorigenesis through Beclin 1 phosphorylation. Science. 2012;338:956–959. doi: 10.1126/science.1225967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lynch T.J. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N. Engl. J. Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 70.Hurley J.H., Schulman B.A. Atomistic autophagy: the structures of cellular self-digestion. Cell. 2014;157:300–311. doi: 10.1016/j.cell.2014.01.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tan X. A kinase-independent role for EGF receptor in autophagy initiation. Cell. 2015;160:145–160. doi: 10.1016/j.cell.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bialik S., Kimchi A. The death-associated protein kinases: structure, function, and beyond. Annu. Rev. Biochem. 2006;75:189–210. doi: 10.1146/annurev.biochem.75.103004.142615. [DOI] [PubMed] [Google Scholar]
- 73.Mihaylova M.M., Shaw R.J. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat. Cell Biol. 2011;13:1016–1023. doi: 10.1038/ncb2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Egan D.F. Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science. 2011;331:456–461. doi: 10.1126/science.1196371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kim J. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 2011;13:132–141. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cargnello M., Roux P.P. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol. Mol. Biol. Rev. 2011;75:50–83. doi: 10.1128/MMBR.00031-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fimia G.M. Ambra1 at the crossroad between autophagy and cell death. Oncogene. 2013;32:3311–3318. doi: 10.1038/onc.2012.455. [DOI] [PubMed] [Google Scholar]
- 78.An Z. Autophagy is required for G1/G0 quiescence in response to nitrogen starvation in Saccharomyces cerevisiae. Autophagy. 2014;10:1702–1711. doi: 10.4161/auto.32122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Otto G.P. Macroautophagy is required for multicellular development of the social amoeba Dictyostelium discoideum. J. Biol. Chem. 2003;278:17636–17645. doi: 10.1074/jbc.M212467200. [DOI] [PubMed] [Google Scholar]
- 80.Liu Y. Autophagy regulates programmed cell death during the plant innate immune response. Cell. 2005;121:567–577. doi: 10.1016/j.cell.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 81.Melendez A. Autophagy genes are essential for dauer development and life-span extension in C. elegans. Science. 2003;301:1387–1391. doi: 10.1126/science.1087782. [DOI] [PubMed] [Google Scholar]
- 82.Jia K., Levine B. Autophagy is required for dietary restriction-mediated life span extension in C. elegans. Autophagy. 2007;3:597–599. doi: 10.4161/auto.4989. [DOI] [PubMed] [Google Scholar]
- 83.Lorincz P. Atg6/UVRAG/Vps34-containing lipid kinase complex is required for receptor downregulation through endolysosomal degradation and epithelial polarity during Drosophila wing development. BioMed Res. Int. 2014;2014:851349. doi: 10.1155/2014/851349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Qu X. Promotion of tumorigenesis by heterozygous disruption of the beclin 1 autophagy gene. J. Clin. Invest. 2003;112:1809–1820. doi: 10.1172/JCI20039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yue Z. Beclin 1, an autophagy gene essential for early embryonic development, is a haploinsufficient tumor suppressor. Proc. Natl. Acad. Sci. U.S.A. 2003;100:15077–15082. doi: 10.1073/pnas.2436255100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cicchini M. Autophagy regulator BECN1 suppresses mammary tumorigenesis driven by WNT1 activation and following parity. Autophagy. 2014;10:2036–2052. doi: 10.4161/auto.34398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tang H. Decreased BECN1 mRNA expression in human breast cancer is associated with estrogen receptor-negative subtypes and poor prognosis. EBioMedicine. 2015;2:255–263. doi: 10.1016/j.ebiom.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Qu X. Autophagy gene-dependent clearance of apoptotic cells during embryonic development. Cell. 2007;128:931–946. doi: 10.1016/j.cell.2006.12.044. [DOI] [PubMed] [Google Scholar]
- 89.Maccarrone M. The endocannabinoid system and its relevance for nutrition. Annu. Rev. Nutr. 2010;30:423–440. doi: 10.1146/annurev.nutr.012809.104701. [DOI] [PubMed] [Google Scholar]
- 90.Shoji-Kawata S. Identification of a candidate therapeutic autophagy-inducing peptide. Nature. 2013;494:201–206. doi: 10.1038/nature11866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Seaman M.N. Endosome to Golgi retrieval of the vacuolar protein sorting receptor, Vps10p, requires the function of the VPS29, VPS30, and VPS35 gene products. J. Cell Biol. 1997;137:79–92. doi: 10.1083/jcb.137.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Patel S., Dinesh-Kumar S.P. Arabidopsis ATG6 is required to limit the pathogen-associated cell death response. Autophagy. 2008;4:20–27. doi: 10.4161/auto.5056. [DOI] [PubMed] [Google Scholar]
- 93.Qin G. Arabidopsis AtBECLIN 1/AtAtg6/AtVps30 is essential for pollen germination and plant development. Cell Res. 2007;17:249–263. doi: 10.1038/cr.2007.7. [DOI] [PubMed] [Google Scholar]
- 94.Jia K. Autophagy genes protect against Salmonella typhimurium infection and mediate insulin signaling-regulated pathogen resistance. Proc. Natl. Acad. Sci. U.S.A. 2009;106:14564–14569. doi: 10.1073/pnas.0813319106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Samokhvalov V. Autophagy protects against hypoxic injury in C. elegans. Autophagy. 2008;4:1034–1041. doi: 10.4161/auto.6994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Takacs-Vellai K. Inactivation of the autophagy gene bec-1 triggers apoptotic cell death in C. elegans. Curr. Biol. 2005;15:1513–1517. doi: 10.1016/j.cub.2005.07.035. [DOI] [PubMed] [Google Scholar]
- 97.Hansen M. A role for autophagy in the extension of lifespan by dietary restriction in C. elegans. PLoS Genet. 2008;4:e24. doi: 10.1371/journal.pgen.0040024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Morselli E. Caloric restriction and resveratrol promote longevity through the Sirtuin-1-dependent induction of autophagy. Cell Death Dis. 2010;1:e10. doi: 10.1038/cddis.2009.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dwivedi M. Autophagy genes mediate the effect of calcineurin on life span in C. elegans. Autophagy. 2009;5:604–607. doi: 10.4161/auto.5.5.8157. [DOI] [PubMed] [Google Scholar]
- 100.Tavernarakis N. The effects of p53 on whole organism longevity are mediated by autophagy. Autophagy. 2008;4:870–873. doi: 10.4161/auto.6730. [DOI] [PubMed] [Google Scholar]
- 101.Schiavi A. Autophagy induction extends lifespan and reduces lipid content in response to frataxin silencing in C. elegans. Exp. Gerontol. 2013;48:191–201. doi: 10.1016/j.exger.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Huang S. Autophagy genes function in apoptotic cell corpse clearance during C. elegans embryonic development. Autophagy. 2013;9:138–149. doi: 10.4161/auto.22352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Aladzsity I. Autophagy genes unc-51 and bec-1 are required for normal cell size in Caenorhabditis elegans. Genetics. 2007;177:655–660. doi: 10.1534/genetics.107.075762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Toth M.L. Longevity pathways converge on autophagy genes to regulate life span in Caenorhabditis elegans. Autophagy. 2008;4:330–338. doi: 10.4161/auto.5618. [DOI] [PubMed] [Google Scholar]
- 105.Lapierre L.R. Autophagy genes are required for normal lipid levels in C. elegans. Autophagy. 2013;9:278–286. doi: 10.4161/auto.22930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lee E. Autophagy is essential for cardiac morphogenesis during vertebrate development. Autophagy. 2014;10:572–587. doi: 10.4161/auto.27649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lee S.J. Beclin 1 deficiency is associated with increased hypoxia-induced angiogenesis. Autophagy. 2011;7:829–839. doi: 10.4161/auto.7.8.15598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ding Y. Autophagy regulates TGF-β expression and suppresses kidney fibrosis induced by unilateral ureteral obstruction. J. Am. Soc. Nephrol. 2014;12:2835–2846. doi: 10.1681/ASN.2013101068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chen Y. Autophagy protects the retina from light-induced degeneration. J. Biol. Chem. 2013;288:7506–7518. doi: 10.1074/jbc.M112.439935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Reed M. Autophagy-inducing protein beclin-1 in dendritic cells regulates CD4 T cell responses and disease severity during respiratory syncytial virus infection. J. Immunol. 2013;191:2526–2537. doi: 10.4049/jimmunol.1300477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Verghese D.A. Costimulatory blockade-induced allograft survival requires Beclin1. Am. J. Transplant. 2014;14:545–553. doi: 10.1111/ajt.12610. [DOI] [PubMed] [Google Scholar]
- 112.Arsov I. A role for autophagic protein beclin 1 early in lymphocyte development. J. Immunol. 2011;186:2201–2209. doi: 10.4049/jimmunol.1002223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hariharan N. Oxidative stress stimulates autophagic flux during ischemia/reperfusion. Antioxid. Redox. Signal. 2011;14:2179–2190. doi: 10.1089/ars.2010.3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Xu X. Diminished autophagy limits cardiac injury in mouse models of type 1 diabetes. J. Biol. Chem. 2013;288:18077–18092. doi: 10.1074/jbc.M113.474650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhu H. Cardiac autophagy is a maladaptive response to hemodynamic stress. J. Clin. Invest. 2007;117:1782–1793. doi: 10.1172/JCI27523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lucin K.M. Microglial beclin 1 regulates retromer trafficking and phagocytosis and is impaired in Alzheimer's disease. Neuron. 2013;79:873–886. doi: 10.1016/j.neuron.2013.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pickford F. The autophagy-related protein beclin 1 shows reduced expression in early Alzheimer disease and regulates amyloid beta accumulation in mice. J. Clin. Invest. 2008;118:2190–2199. doi: 10.1172/JCI33585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kovacs J.R. Autophagy promotes T-cell survival through degradation of proteins of the cell death machinery. Cell Death Differ. 2012;19:144–152. doi: 10.1038/cdd.2011.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gawriluk T.R. Beclin-1 deficiency in the murine ovary results in the reduction of progesterone production to promote preterm labor. Proc. Natl. Acad. Sci. U.S.A. 2014;111:E4194–E4203. doi: 10.1073/pnas.1409323111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tannous P. Autophagy is an adaptive response in desmin-related cardiomyopathy. Proc. Natl. Acad. Sci. U.S.A. 2008;105:9745–9750. doi: 10.1073/pnas.0706802105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nassif M. Pathogenic role of BECN1/Beclin 1 in the development of amyotrophic lateral sclerosis. Autophagy. 2014;10:1256–1271. doi: 10.4161/auto.28784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Parkhitko A. Tumorigenesis in tuberous sclerosis complex is autophagy and p62/sequestosome 1 (SQSTM1)-dependent. Proc. Natl. Acad. Sci. U.S.A. 2011;108:12455–12460. doi: 10.1073/pnas.1104361108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Fimia G.M. Ambra1 regulates autophagy and development of the nervous system. Nature. 2007;447:1121–1125. doi: 10.1038/nature05925. [DOI] [PubMed] [Google Scholar]
- 124.Zhong Y. Nrbf2 protein suppresses autophagy by modulating Atg14L protein-containing Beclin 1–Vps34 complex architecture and reducing intracellular phosphatidylinositol-3 phosphate Levels. J. Biol. Chem. 2014;289:26021–26037. doi: 10.1074/jbc.M114.561134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Piya S. The E1B19K oncoprotein complexes with Beclin 1 to regulate autophagy in adenovirus-infected cells. PLoS ONE. 2011;6:e29467. doi: 10.1371/journal.pone.0029467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hernaez B. A179L, a new viral Bcl2 homolog targeting Beclin 1 autophagy related protein. Curr. Mol. Med. 2013;13:305–316. [PubMed] [Google Scholar]
- 127.Ku B. Structural and biochemical bases for the inhibition of autophagy and apoptosis by viral BCL-2 of murine gamma-herpesvirus 68. PLoS Pathog. 2008;4:e25. doi: 10.1371/journal.ppat.0040025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sinha S. Molecular basis of the regulation of Beclin 1-dependent autophagy by the gamma-herpesvirus 68 Bcl-2 homolog M11. Autophagy. 2008;4:989–997. doi: 10.4161/auto.6803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Han J. Interaction between Her2 and Beclin-1 proteins underlies a new mechanism of reciprocal regulation. J. Biol. Chem. 2013;288:20315–20325. doi: 10.1074/jbc.M113.461350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Michiorri S. The Parkinson-associated protein PINK1 interacts with Beclin1 and promotes autophagy. Cell Death Differ. 2010;17:962–974. doi: 10.1038/cdd.2009.200. [DOI] [PubMed] [Google Scholar]
- 131.Shi C.S., Kehrl J.H. TRAF6 and A20 regulate lysine 63-linked ubiquitination of Beclin-1 to control TLR4-induced autophagy. Sci. Signal. 2010;3:ra42. doi: 10.1126/scisignal.2000751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Choubey V. BECN1 is involved in the initiation of mitophagy: it facilitates PARK2 translocation to mitochondria. Autophagy. 2014;10:1105–1119. doi: 10.4161/auto.28615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Elgendy M. Beclin 1 restrains tumorigenesis through Mcl-1 destabilization in an autophagy-independent reciprocal manner. Nat. Commun. 2014;5:5637. doi: 10.1038/ncomms6637. [DOI] [PubMed] [Google Scholar]
- 134.Xia P. WASH inhibits autophagy through suppression of Beclin 1 ubiquitination. EMBO J. 2013;32:2685–2696. doi: 10.1038/emboj.2013.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Liang Q. Crosstalk between the cGAS DNA sensor and Beclin-1 autophagy protein shapes innate antimicrobial immune responses. Cell Host Microbe. 2014;15:228–238. doi: 10.1016/j.chom.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Jounai N. NLRP4 negatively regulates autophagic processes through an association with beclin1. J. Immunol. 2011;186:1646–1655. doi: 10.4049/jimmunol.1001654. [DOI] [PubMed] [Google Scholar]
- 137.Shi C.S. Activation of autophagy by inflammatory signals limits IL-1β production by targeting ubiquitinated inflammasomes for destruction. Nat. Immunol. 2012;13:255–263. doi: 10.1038/ni.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Berger S.B. SLAM is a microbial sensor that regulates bacterial phagosome functions in macrophages. Nat. Immunol. 2010;11:920–927. doi: 10.1038/ni.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wang P. ARRB1/beta-arrestin-1 mediates neuroprotection through coordination of BECN1-dependent autophagy in cerebral ischemia. Autophagy. 2014;10:1535–1548. doi: 10.4161/auto.29203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ma B. Dapper1 promotes autophagy by enhancing the Beclin1–Vps34–Atg14L complex formation. Cell Res. 2014;24:912–924. doi: 10.1038/cr.2014.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tang D. Endogenous HMGB1 regulates autophagy. J. Cell Biol. 2010;190:881–892. doi: 10.1083/jcb.200911078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Vicencio J.M. The inositol 1,4,5-trisphosphate receptor regulates autophagy through its interaction with Beclin 1. Cell Death Differ. 2009;16:1006–1017. doi: 10.1038/cdd.2009.34. [DOI] [PubMed] [Google Scholar]
- 143.Zou J. Mitochondrion-associated protein LRPPRC suppresses the initiation of basal levels of autophagy via enhancing Bcl-2 stability. Biochem. J. 2013;454:447–457. doi: 10.1042/BJ20130306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yue Z. A novel protein complex linking the delta 2 glutamate receptor and autophagy: implications for neurodegeneration in lurcher mice. Neuron. 2002;35:921–933. doi: 10.1016/s0896-6273(02)00861-9. [DOI] [PubMed] [Google Scholar]
- 145.Tripathi R. Beclin-1-p53 interaction is crucial for cell fate determination in embryonal carcinoma cells. J. Cell Mol. Med. 2014;18:2275–2286. doi: 10.1111/jcmm.12386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mealer R.G. Rhes, a striatal-selective protein implicated in Huntington disease, binds beclin-1 and activates autophagy. J. Biol. Chem. 2014;289:3547–3554. doi: 10.1074/jbc.M113.536912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Criollo A. Inhibition of autophagy by TAB2 and TAB3. EMBO J. 2011;30:4908–4920. doi: 10.1038/emboj.2011.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ropolo A. The pancreatitis-induced vacuole membrane protein 1 triggers autophagy in mammalian cells. J. Biol. Chem. 2007;282:37124–37133. doi: 10.1074/jbc.M706956200. [DOI] [PubMed] [Google Scholar]
- 149.Chen X. Coronavirus membrane-associated papain-like proteases induce autophagy through interacting with Beclin1 to negatively regulate antiviral innate immunity. Protein Cell. 2014;5:912–927. doi: 10.1007/s13238-014-0104-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Gladue D.P. Foot-and-mouth disease virus nonstructural protein 2C interacts with Beclin1, modulating virus replication. J. Virol. 2012;86:12080–12090. doi: 10.1128/JVI.01610-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chaumorcel M. The human cytomegalovirus protein TRS1 inhibits autophagy via its interaction with Beclin 1. J. Virol. 2012;86:2571–2584. doi: 10.1128/JVI.05746-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kyei G.B. Autophagy pathway intersects with HIV-1 biosynthesis and regulates viral yields in macrophages. J. Cell Biol. 2009;186:255–268. doi: 10.1083/jcb.200903070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Orvedahl A. HSV-1 ICP34.5 confers neurovirulence by targeting the Beclin 1 autophagy protein. Cell Host Microbe. 2007;1:23–35. doi: 10.1016/j.chom.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 154.Gobeil P.A., Leib D.A. Herpes simplex virus γ34.5 interferes with autophagosome maturation and antigen presentation in dendritic cells. Mbio. 2012;3:e00267–e312. doi: 10.1128/mBio.00267-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Gannage M. Matrix protein 2 of influenza A virus blocks autophagosome fusion with lysosomes. Cell Host Microbe. 2009;6:367–380. doi: 10.1016/j.chom.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]


