Abstract
Samples of the extremotolerant Antarctic endemite lichen Buellia frigida are currently exposed to low-Earth orbit–space and simulated Mars conditions at the Biology and Mars Experiment (BIOMEX), which is part of the ESA mission EXPOSE-R2 on the International Space Station and was launched on 23 July 2014. In preparation for the mission, several preflight tests (Experimental and Scientific Verification Tests, EVT and SVT) assessed the sample preparation and hardware integration procedures as well as the resistance of the candidate organism toward the abiotic stressors experienced under space and Mars conditions. Therefore, we quantified the post-exposure viability with a live/dead staining technique utilizing FUN-1 and confocal laser scanning microscopy (CLSM). In addition, we used scanning electron microscopy (SEM) to investigate putative patterns of morphological-anatomical damage that lichens may suffer under the extreme exposure conditions. The present results demonstrate that Buellia frigida is capable of surviving the conditions tested in EVT and SVT. The mycobiont showed lower average impairment of its viability than the photobiont (viability rates of >83% and >69%, respectively), and the lichen thallus suffered no significant damage in terms of thalline integrity and symbiotic contact. These results will become essential to substantiate and validate the results prospectively obtained from the returning space mission. Moreover, they will help assess the limits and limitations of terrestrial organisms under space and Mars conditions as well as characterize the adaptive traits that confer lichen extremotolerance. Key Words: Astrobiology—BIOMEX—EXPOSE-R2—Extremotolerance—Lichens. Astrobiology 15, 601–615.
1. Introduction
The Biology and Mars Experiment (BIOMEX) is a current long-term experiment designed to expose a broad variety of organisms and biogenic compounds to low-Earth orbit (LEO) space and simulated Mars conditions. The aim of BIOMEX is to assess the viability of the exposed organisms but also to characterize the damage putatively caused by exposure. BIOMEX is part of ESA's EXPOSE-R2 mission that was launched on 23 July 2014. For exposure to LEO conditions, EXPOSE-R2 is located on the outside of the Russian service module Zvezda at the International Space Station (ISS). The experiment will be completed in about 12–15 months.
In response to the ESA call ILSRA-AO 2009, BIOMEX is a succeeding experiment to the Lichen and Fungi Experiment (LIFE) on EXPOSE-E located on the European Technology Exposure Facility (EuTEF) at the outside of the European Columbus module of the ISS (Onofri et al., 2012). LIFE was the first long-term exposure experiment that exposed lichens, among other extremotolerant organisms such as bacteria, biofilms, and cryptoendolithic fungi, to space and simulated Mars analog conditions for a period of 559 days (Rabbow et al., 2012). During the 1.5-year LIFE mission, fully space-exposed samples of the lichen Xanthoria elegans experienced accumulated PAR400–1000nm doses of c. 2185 MJ m−2, UVR110–400nm doses of c. 634 MJ m−2, vacuum conditions of 10−4 to 10−7 Pa, ionizing radiation of 208±8 mGy, and c. 100 freeze-thaw cycles with temperatures of −22°C to +43°C. Under fully exposed Mars analog conditions, X. elegans experienced accumulated PAR400–1000nm doses of c. 2227 MJ m−2, UVR200–400nm doses of c. 475 MJ m−2, 10 hPa Mars atmosphere, and comparable conditions of ionizing radiation and temperature (data according to Beuselink and van Barvinchove, 2011; Berger et al., 2012; Rabbow et al., 2012; Brandt et al., 2015). Samples in the interior of EXPOSE-E served as a dark control; they were exposed to the very same conditions except insolation. Moreover, a similar set of samples was exposed to simulated space and Mars conditions in ground-based simulation facilities at DLR Cologne (MGR, mission ground reference). The general experimental setup and exposure conditions largely resemble those currently applied in BIOMEX, but due to several modifications at the EXPOSE-R2 facility and to the different orientation of EuTEF and Zvezda on the ISS, the exposure parameters will not be identical.
Previously, short-term space exposure experiments (LICHENS II on BIOPAN-5, STONE and LITHOPANSPERMIA on BIOPAN-6) as well as a significant number of simulation experiments tested the effects of combined and individually applied nonterrestrial stressors on lichens, such as space vacuum, space ultraviolet radiation (UVR), Mars atmosphere, Mars UVR, subzero temperatures, and meteorite impacts (de Vera et al., 2003, 2004a, 2004b, 2008, 2010, 2014; de la Torre Noetzel et al., 2007; Sancho et al., 2007; Stöffler et al., 2007; Horneck et al., 2008; de la Torre et al., 2010; de Vera and Ott, 2010; Raggio et al., 2011; Sánchez et al., 2012, 2014; Meeßen et al., 2014b; Backhaus et al., 2015).
Several of these experiments were designed to test the likelihood of the lithopanspermia hypothesis for its three different phases (Thomson in 1871, revisited by Sancho et al., 2007; Stöffler et al., 2007; Horneck et al., 2008, 2010) and to appraise the possible habitability of the martian surface and its micro-niche environments (sensu Westall, 2013; de Vera et al., 2014). Beyond these subjects, the experiments helped to assess the limits and limitations of terrestrial organisms and characterize adaptive traits that confer extremotolerance. Focusing on lichens, symbiotic organisms that consist of a fungus (mycobiont) and a photoautotrophic partner (photobiont), revealed a remarkable and consistent resistance toward nonterrestrial conditions (see references above). Such resistance toward (simulated) space conditions is attributed to a range of morphological adaptations (Meeßen et al., 2013), a set of protective secondary compounds (Meeßen et al., 2014a) and their ability to pass into anhydrobiosis, an ametabolic state when desiccated (Ertl, 1951; Crowe et al., 1992, 1998; Kranner et al., 2005). To substantiate these findings and assess the patterns of degradation and damage organisms may suffer during space exposure, the current BIOMEX mission again exposes two extremotolerant lichen species to space and simulated Mars conditions. Both lichen species, the Antarctic endemite Buellia frigida and the vagrant lichen Circinaria gyrosa, were previously subjected to several astrobiological studies (de la Torre et al., 2010; de Vera and Ott, 2010; Sánchez et al., 2012, 2014; Meeßen et al., 2013, 2014a, 2014b; Backhaus et al., 2015).
The current study presents the results of Experimental and Scientific Verification Tests (EVT and SVT), which were conducted in preparation for the flight mission EXPOSE-R2. These tests were designed to assess the tolerance of the candidate organisms toward the abiotic stressors experienced in space. They also ensure that all samples are suitably prepared for hardware integration, expected exposure conditions, and the postflight de-integration procedure. The post-exposure viability was tested by live/dead analysis using FUN-1 staining and confocal laser scanning microscopy (CLSM) as previously and successfully applied on lichen samples (de Vera et al., 2004b, 2008, 2010; Onofri et al., 2012; Brandt et al., 2015). Additionally, the potential damage to the lichen thallus was investigated by scanning electron microscopy (SEM). The present results demonstrate that both symbionts of Buellia frigida are capable of surviving the conditions of EVT and SVT without significant damage to the thallus structure.
2. Material and Methods
2.1. Material
2.1.1. Buellia frigida Darb. (1910)
This lichen is an endemic, crustose lichen species that frequently colonizes rocky habitats of maritime and continental Antarctica, where it occurs in latitudes down to 84°S and in altitudes up to 2015 m a.s.l. (Øvstedal and Lewis Smith, 2001). Buellia frigida preferentially grows on rocks that are often fully exposed to wind, low temperatures, and high irradiation levels during the Antarctic summer. By colonizing such niches, B. frigida displays its adaptation to extremely harsh environmental conditions as an extremotolerant organism. The peculiar morphological and anatomical traits of B. frigida as well as its secondary lichen compounds are described elsewhere (Meeßen et al., 2013, 2014a). For the present study, B. frigida was collected by S. Ott in 2009/2010 in the vicinity of the German Gondwana Station at Gerlache Inlet (74°38′S, 164°13′E), in North Victoria Land. The air-dried samples were stored at −25°C until further investigation.
2.2. Methods
2.2.1. Sample preparation
All exposure tests were performed with air-dry lichen material on its natural rock substrate but also on 14 mm pressed pellets [6 t pressure for 15 min in a PP-10 pellet press (Retsch, Germany)] of Sulfatic Mars Regolith Simulant and Phyllosilicatic Mars Regolith Simulant (S-MRS and P-MRS, 0.85 g per pellet) as well as on Lunar Analog Anorthosite (LAA). To ensure contact and thus interaction between the mineral pellet substrates and the lichen sample during exposure, the prepared thallus lobes were carefully and punctually glued on the pellets with space-proofed two-component glue (Wacker RTV-S 691 A/B, Wacker Chemie AG, Germany) to facilitate a close contact between the thalline underside and the pellet's surface. A total of 15–18 carefully prepared and intact areolae of B. frigida as well as 5–6 apothecia were placed on each pellet. It has been proven that LAA pellets lack sufficient stability for safe handling and LEO exposure. Consequently, they were excluded from the flight mission and were not considered in the present study. The composition of S-MRS, P-MRS, and LAA is given in Table 1.
Table 1.
Composition of Sulfatic and Phyllosilicatic Mars Regolith Simulants (S- and P-MRS) as Well as of Lunar Analog Anorthosite (LAA) as Percentage of Mineral Net Weight
| S-MRS | P-MRS | LAA | |||
|---|---|---|---|---|---|
| mineral | weight (%) | mineral | weight (%) | mineral | weight (%) |
| gabbro | 32 | montmorillonite | 45 | plagioclase | 67 |
| gypsum | 30 | chamosite | 20 | volcanic slag | 10 |
| dunite | 15 | quartz | 10 | diopside | 9 |
| hematite | 13 | iron(III)-oxide | 5 | hypersthenes | 6 |
| goethite | 7 | kaolinite | 5 | olivine | 6 |
| quartz | 3 | siderite | 5 | apatite | 1 |
| hydromagnesite | 5 | illmenite | 1 | ||
| gabbro | 3 | iron | 1 | ||
| dunite | 2 | ||||
2.2.2. Experimental Verification Test 1 and 2 (EVT-1 and EVT-2)
EVT-1 and EVT-2 were designed and performed at the Microgravity User Support Center (MUSC) at DLR Cologne. EVT-1 tested several space-simulated parameters: 7 days under vacuum at 10−5 Pa, 7 days under Mars atmosphere (CO2 gas composition) at 103 Pa, temperature cycling between −10°C and +45°C (50 cycles of 8 h each), temperature extremes of −25°C and +60°C (for 1 h), and monochromatic UVC254nm fluences of 10, 100, 1000, and 10,000 J m−2. Since EVT-2 and SVT provided more realistic irradiation in terms of wavelength ranges and overall UVR fluence (see below), and SVT included much longer exposure to vacuum, Mars atmosphere, and temperature cycling, EVT-1 focused on the analyses of the expected temperature extremes during the BIOMEX experiment only. In contrast, EVT-2 focused on irradiation with polychromatic UVR200–400nm (including UVC, UVB, and UVA) by use of the solar simulator SOL2000 at an irradiance of 1271 W m−2 for fluences of 1.4×103, 1.4×104, 1.4×105, 4.5×105, 6.8×105 kJ m−2 for a period of up to 50 d. The maximum fluence was adjusted to the expected mission maximum at the sample site, as calculated according to the data from the two previous EXPOSE missions on the ISS (refer also to Rabbow et al., 2012, 2015). Since UVC irradiation in EVT-1 led to no detectable decrease of viability, the data were not included in the respective results section.
2.2.3. Scientific Verification Tests (SVT)
Basically, SVT are designed to ensure that all samples are prepared and suitable for hardware integration for the conditions experienced during the mission and for postflight de-integration. Besides, the application of mission-equivalent space parameters allows for testing of the samples' resilience toward extreme environmental conditions of space and simulated Mars exposure. Therefore, the samples were transferred to MUSC and integrated in the EXPOSE-E/-R hardware, which is nearly identical to the EXPOSE-R2 hardware (Fig. 1). To simulate a space-like test profile, the samples were exposed to vacuum (4.1×10−5 Pa) and cycling temperatures between −25°C (16 h in the dark) and +10°C (8 h during irradiation) in tray 1, compartment 1. In parallel, Mars test parameters were simulated by subzero temperature (−23°C), Mars atmosphere (95.55% CO2, 2.70% N2, 1.60% Ar, 0.15% O2, ∼370 ppm H2O, Praxair Deutschland GmbH), and Mars-like pressure of 780–930 Pa (performed in tray 2, compartment 1). All conditions were simulated for a period of 38 days. Additionally, the upper layers' sample sets were irradiated with UVR, which simulated a mission period of 12 months by using the solar simulator SOL2000 at an irradiance of 1271 W m−2 for an accumulated period of 5924 min, applying a total fluence of 5.7×105 kJ m−2 (λ200–400nm) to the samples of the upper layer (referred to as “fully exposed,” FE). Below, an identical set of samples was kept in the dark and experienced all simulation parameters except UVR exposure (referred to as “dark exposed,” DE). The samples were as follows: In tray 1, one irradiated rock piece (grown with Buellia frigida) and one LAA pellet were exposed in each layer to space simulation conditions, and in tray 2 one pellet of S-MRS and one of P-MRS were exposed in each layer to Mars simulation conditions. All pellets were provided with lichen samples as described above.
FIG. 1.
Experimental setup of the simulation procedure at the Microgravity User Support Center (MUSC) at DLR Cologne utilizing the EXPOSE-E/-R hardware for ground-based Scientific Verification Tests (SVT). The samples in tray 1 (front row) were exposed to vacuum of 4.1×10−5 Pa, those in tray 2 (middle row) to Mars atmosphere and pressure (780–930 Pa). Additionally, the upper layer was irradiated with a total UVR200–400nm fluence of 5.7×105 kJ m−2, and the samples were subdued to irradiation-dependent temperature cycling temperatures between −25°C in the dark (16 h) and +10°C during irradiation (8 h). (Color images available at www.liebertonline.com/ast)
2.2.4. Viability analysis by FUN-1 staining and confocal laser scanning microscopy
The viability analysis after the respective exposure tests was performed by live/dead staining with the fluorescent dye FUN-1 (Molecular Probes, Oregon, USA), which allows for the differentiation of metabolically inactive and active cells. The dye contains a fluorophore with an excitation wavelength of 488 nm (provided by an argon laser) that changes its emission properties when metabolized. While all cells achieve basic fluorescent labeling in one channel (consistently represented as “green channel,” bandpass of 505–550 nm) by taking up the dye, only metabolically active cells deposit the dye in their vacuoles, whose pH shifts its fluorescence properties to a bandpass of 575–615 nm (“red channel,” details in Millard et al., 1997). Such a shift is represented in the overlay images by golden yellow, orange, or red indicating metabolically active cells. To improve symbiont discrimination, photobiont cells were additionally detected by chlorophyll-based autofluorescence in a separate bandpass of 660–750 nm (“blue channel”). For live/dead analysis, the areolae of Buellia frigida were wetted (provoking metabolic reactivation of surviving cells), sectioned into 120 μm slices with a cryotome (Reichert-Jung, Germany), and immediately dyed with the FUN-1 staining solution (20 μM FUN-1 in HEPES buffer at pH 7.0). The manufacturer's protocol was followed. However, the incubation period was prolonged to 60–120 min due to the poorly penetrable algal cell wall as has been successfully implemented in previous studies (Brandt et al., 2015). The dyed samples were examined by CLSM (LSM 510 Meta, Zeiss, Germany) with the use of virtual slicing, z-stacking, and time series to verify the metabolic activity of both symbionts. For live/dead quantification, 5–23 morphologically representative pictures of each sample (always containing both photobiont cells and mycobiont hyphae) were taken with a 1.0–2.0 pinhole opening and amplifier values according to the fluorescence signal intensity (400–800, corresponding to red and green channels). Metabolically active and inactive cells were interpreted as alive and dead, respectively. Degenerated photobiont artifacts as well as epicortical and medullary mucilage deposits (refer to Fig. 2: dp, e, g) may have led to misinterpretation of the live/dead-ratio. Therefore, quantification was performed manually with the ImageJ software (W. Rasband, NIH, USA) and in careful consideration of the lichen's morphological and anatomical characteristics. Areas of high mucilage deposition were excluded from analysis. The same applies for degenerated photobiont cells, since their advanced stage of degeneration implies impairment before the exposure experiment itself. To assure the method's reliability, the procedure was successfully tested on vital and dead lichen tissue (by thermal sterilization).
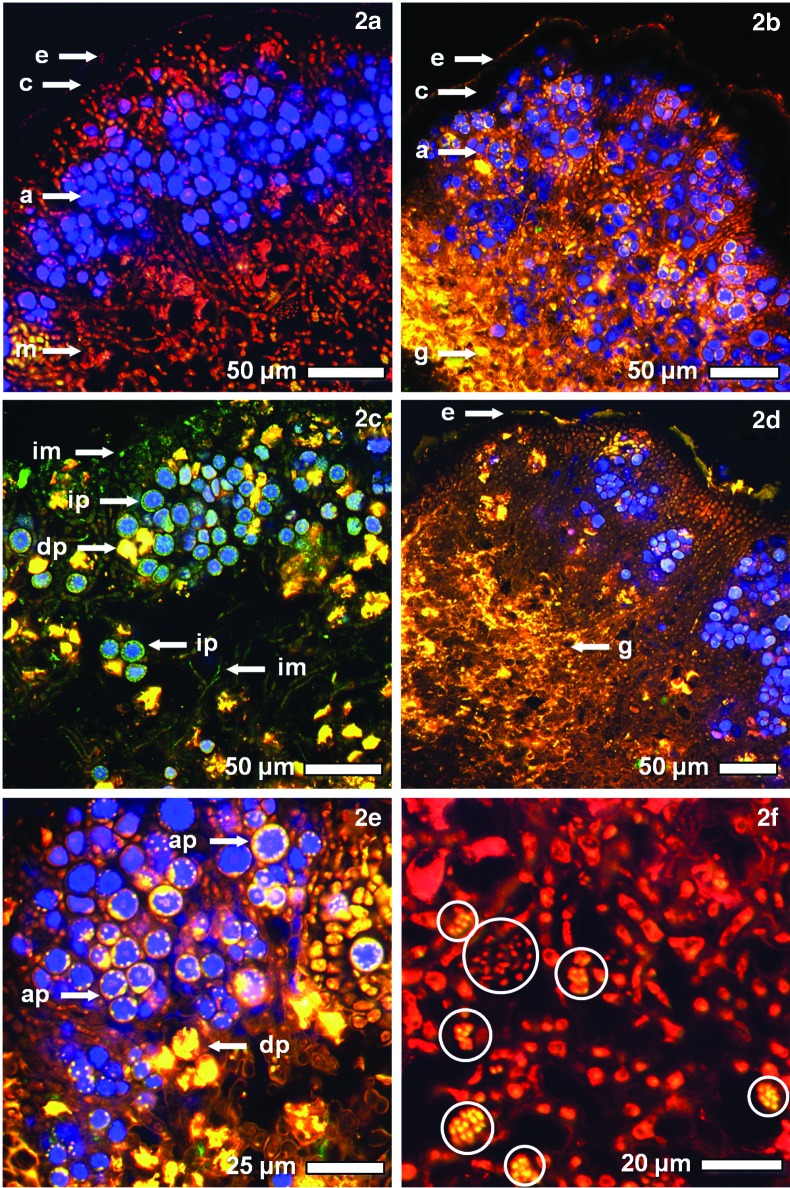
FIG. 2.
Cross sections of B. frigida stained with FUN-1 and documented by CLSM. The micrographs depict mostly vital thallus areas in 2a–2b and 2d–2f and mostly metabolically inactive thallus areas in 2c. The stratification of the thallus is presented by the epicortical gelatinous sheath (e); the cortex (c), which is encrusted with melanin and thus dark in fluorescence microscopy; the layer of clustered algal cells (a); and the medulla (m, 2a–2b). Areas of intense production of extracellular gelatinous substances (g) are found occasionally (2b, 2d). Some areas of the investigated thallus sections show exposure-induced impairment of photobiont vitality and are predominantly metabolically inactive (2c), as demonstrated by the green-colored hyphal cells of the mycobiont (im) and of the photobiont (ip), while degenerating photobionts (dp) are intensely stained by passively accumulating dye (2c, 2e). Metabolically active photobiont cells are characterized by the accumulation and pH-induced change of color in small spherical vacuoles at the margin of the algal cells (ap, 2e). Within the medulla of several samples (2f), yet-unidentified metabolically active cell clusters survive the exposure conditions (encircled areas), neither resembling fungal hyphae nor algal cells. The samples are (2a) SVT, rock, non-irradiated; (2b) EVT-2, P-MRS, transportation control; (2c) EVT-2, P-MRS, 1.4×103 kJ m−2; (2d) EVT-2, S-MRS, 1.4×105 kJ m−2; (2e) EVT-2, P-MRS, 1.4×104 kJ m−2; (2f) SVT, rock, non-irradiated. (Color images available at www.liebertonline.com/ast)
2.2.5. Fixation procedure and scanning electron microscopy
For SEM fixation, the areolae of Buellia frigida were wetted and (a) prepared as a whole from the crustose thallus to investigate the thallus surface as well as (b) prepared and radially sectioned into 40–120 μm slices with a cryotome (Reichert-Jung GmbH) to investigate anatomical changes. Afterward, the samples were transferred into 2.5% glutaraldehyde in 200 mM cacodylate buffer (pH 7.0), fixed under vacuum and under ambient pressure (30 min each), and washed twice for 10 min in cacodylate buffer. The samples were mounted in microporous capsules (plano GmbH) and dehydrated by subsequent dilution series of graduated ethanol (against water) and acetone (against ethanol). Submerged in acetone, the thallus sections were brought into screw cap containers (Dr. W. Hert, Mikrotechnik), closed with 3 mm copper grids, transferred to a critical point drying device (CPD 020, Balzers Union), washed thrice with liquid CO2, and dried at the critical point temperature of 34.5°C. In an alternative to critical point drying, a similar subset of samples was chemically dried with tetramethylsilane (TMS) by substituting the solvent with water-free acetone for 30 min, followed by substitution with 2:1 acetone:TMS and 1:1 acetone:TMS for 30 min each. Finally, the solvent was substituted with 100% TMS, which was allowed to evaporate completely in dry air. The dehydrated samples from both procedures were transferred to specimen holders, air-dried for 8 h, and gold sputter-coated for 180 s at 35 mA (Sputter Coater, Agar Scientific Ltd.), followed by examination of thalline morphology and anatomy by SEM [LEO 1430(VP), LEO Electron Microscopy Ltd.].
3. Results
3.1. General aspects of FUN-1 staining and CLSM analysis
This following paragraph presents qualitative insight into the live/dead staining procedure only. The quantitative approaches, that is, the viability rates of EVT-1, EVT-2, and SVT, are addressed in the three subsequent paragraphs. In general, the live/dead staining procedure with FUN-1 led to good results in the discrimination of metabolically active and inactive myco- and photobiont cells. The former condition is represented by golden yellow to orange and red staining and is interpreted as alive; the latter condition is represented by green staining and is interpreted as dead. The chlorophyll fluorescence of the algal chloroplast is represented in blue. The thallus sections (Fig. 2a, 2b) show the different strata of the lichen thallus. From bottom to top, it is the medulla (m), which is exclusively formed by the mycobiont; the algal layer (a), which is composed of algal cell clusters interwoven with fungal hyphae; and the cortex (c). The gelatinous epicortical sheath (e) shows minor staining by uptake of dye and/or autofluorescence, while the hyphal cell walls of the upper part of the cortex are encrusted with melanin, resulting in the complete extinction of any fluorescence (dark layer). This was also observed in fungal ascospores that are also encrusted with melanin (data not shown). In the mycobiont, FUN-1 is taken up by the complete cell, staining metabolically inactive mycobiont cells (im, Fig. 2c) green. In metabolically active mycobiont cells (yellow to red, Fig. 2a, 2b), the dye is deposited in intra-vacuolar structures of the hyphal cells, which leads to the staining of the hyphal cells by its changing fluorescence properties under the pH conditions of intact vacuoles. In the inactive photobionts (ip, Fig. 2c), the absorbed dye is seen as a green frame in the marginal cytoplasma. In active photobiont cells (ap, Fig. 2e), it is deposited in the small marginal vacuoles that surround the single, centrally located and irregularly shaped chloroplast (Fig. 2c, 2e). Occasionally, degenerated photobionts (dp, Fig. 2c, 2e) take up large amounts of dye, resulting in a bright yellow staining of the cell sheaths where the change of staining is facilitated by the high concentration of dye. Medullary areas with large amounts of extracellular gelatinous substances (g, Fig. 2b, 2d) show a similar effect. Since these effects were also found in control samples and their way of formation is unclear, they are excluded from the live/dead analysis of the photobiont (in accordance with the procedures of Brandt et al., 2015). Particular reproductive structures such as pycnidia and apothecia were frequently observed. However, they were excluded from analysis, as they are composed of fungal tissue only and do not give information on the viability of the photobiont. Moreover, several thallus sections revealed cell clusters of small metabolically active organisms that do not resemble hyphal or algal cells in size, shape, and arrangement (Fig. 2f). Thus, it might be hypothesized that lichen-associated microorganisms also survive the respective exposure conditions, and consequently additional investigations are in progress.
3.2. Viability rates of EVT-1 samples by live/dead staining
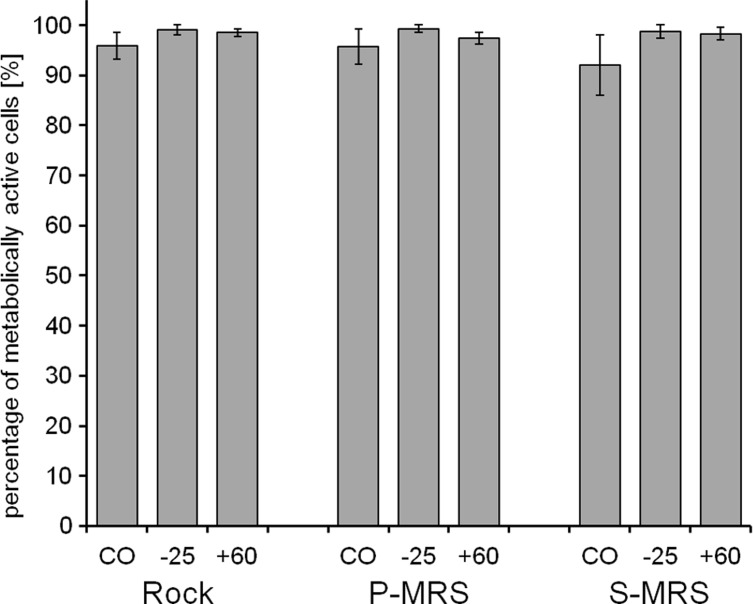
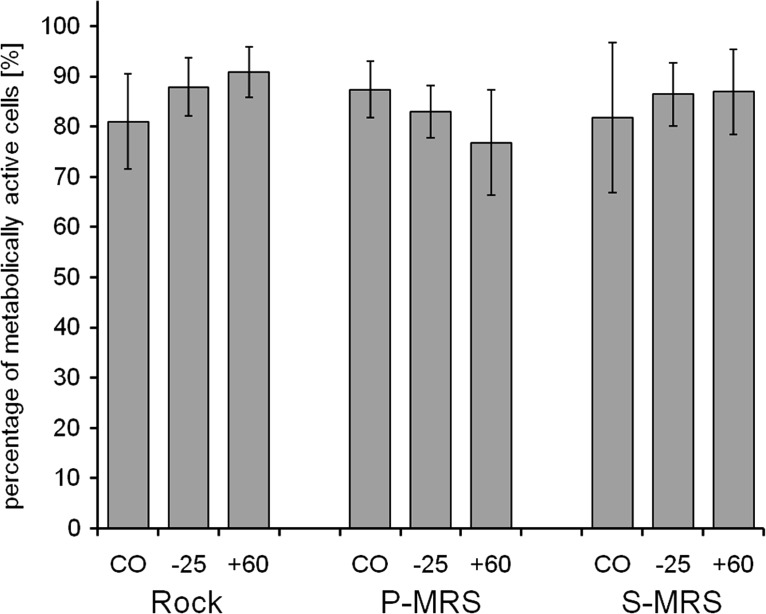
The exposure of the lichen samples toward high to low temperatures showed insignificant effects on the viability of both symbionts (Figs. 3 and 4). After treatment with the subzero temperature of −25°C, the mycobiont revealed slightly higher viability rates from 98.7%±1.3% (S-MRS) via 99.1%±1.0% (rock) to 99.3%±0.7% (P-MRS), while the viability rate of the photobiont ranged from 82.9%±5.2% (P-MRS) via 86.4%±6.3% (S-MRS) to 87.9%±5.8% (rock). The treatment with a high temperature of +60°C showed a comparably low effect on the viability of both symbionts, ranging from 97.4%±1.2% (P-MRS) via 98.3%±1.2% (S-MRS) to 98.5%±0.7% (rock) in the mycobiont and from 76.8%±10.5% (P-MRS) via 86.9%±8.4% (S-MRS) to 90.8%±5.0% (rock) in the photobiont. A significant reduction of viability between the control samples (CO) and the tested conditions was neither observed in the mycobiont (Fig. 3) nor in the photobiont (Fig. 4) of B. frigida. Moreover, the exposure on the different substrates of rock, P-MRS, and S-MRS did not elicit an effect on the symbionts' viability. Nonetheless, the results of the photobiont reveal lower average viability and higher inter-sample variability compared to the mycobiont. This finding was consistently found in all investigations and seems to be a distinctive feature between both symbionts (refer to subsequent Figs. 5–8).
FIG. 3.
Viability of the mycobiont of B. frigida after exposure to EVT-1 conditions of low and high temperatures as percentage of metabolically active cells by live/dead staining with FUN-1. The exposure was performed under simulated space conditions on rock and simulated Mars conditions on P- and S-MRS. The experimental conditions are “control” (CO), exposure for 1 h at −25°C (−25), and exposure for 1 h at +60°C (+60). Percentage given as mean value±standard deviation of n=5–15 thallus sections and 4,660–10,144 cells counted.
FIG. 4.
Viability of the photobiont of B. frigida after exposure to EVT-1 conditions of low and high temperatures as percentage of metabolically active cells by live/dead staining with FUN-1. The exposure was performed under simulated space conditions on rock and simulated Mars conditions on P- and S-MRS. The experimental conditions are “control” (CO), exposure for 1 h at −25°C (−25), and exposure for 1 h at +60°C (+60). Percentage given as mean value±standard deviation of n=5–15 thallus sections and 336–898 cells counted.
FIG. 5.
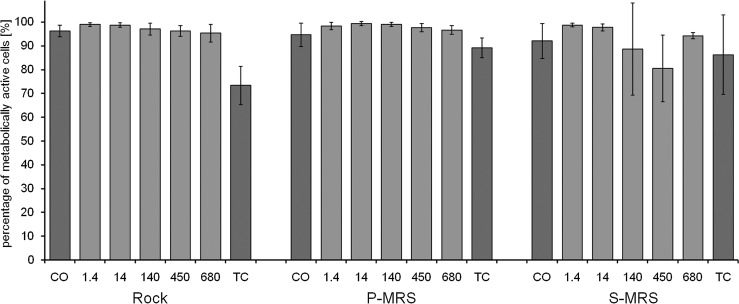
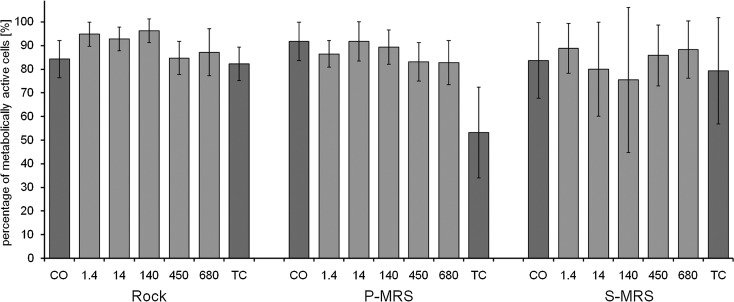
Viability of the mycobiont of B. frigida as percentage of metabolically active cells by live/dead staining with FUN-1 after exposure to EVT-2 conditions (simulated space conditions on rock and simulated Mars conditions on P- and S-MRS). The experimental conditions are “control” (CO), exposure to polychromatic UVR200–400nm for doses of 1.4, 14, 140, 450, and 680 MJ m−2, and a transportation control (TC). Percentage given as mean value±standard deviation of n=5–15 thallus sections and 1,513–10,787 cells counted.
FIG. 6.
Viability of the photobiont of B. frigida as percentage of metabolically active cells by live/dead staining with FUN-1 after exposure to EVT-2 conditions (simulated space conditions on rock and simulated Mars conditions on P- and S-MRS). The experimental conditions are “control” (CO), exposure to polychromatic UVR200–400nm for doses of 1.4, 14, 140, 450, and 680 MJ m−2, and a transportation control (TC). Percentage given as mean value±standard deviation of n=5–15 thallus sections and 236–1178 cells counted.
FIG. 7.
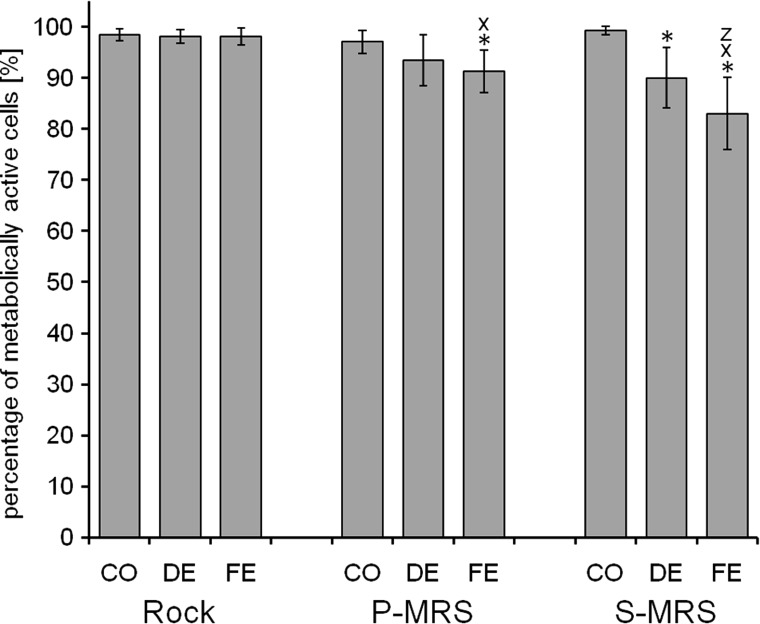
Viability of the mycobiont of B. frigida after exposure to SVT conditions as percentage of metabolically active cells by live/dead staining with FUN-1. The exposure was performed under simulated space conditions on rock and simulated Mars conditions on P- and S-MRS. The experimental conditions are “control” (CO), “dark exposure” (DE), and “full exposure” with a total UVR (λ200–400nm) fluence of 5.7×105 kJ m−2 (FE). Percentage given as mean value±standard deviation of n=5–23 thallus sections and 3,049–16,530 cells counted. Significant differences are calculated by Student t test with H0>0.05: *=significantly lower compared to the respective controls, x=significantly lower compared to FE samples on rock, z=significantly lower compared to the FE samples on P-MRS.
FIG. 8.
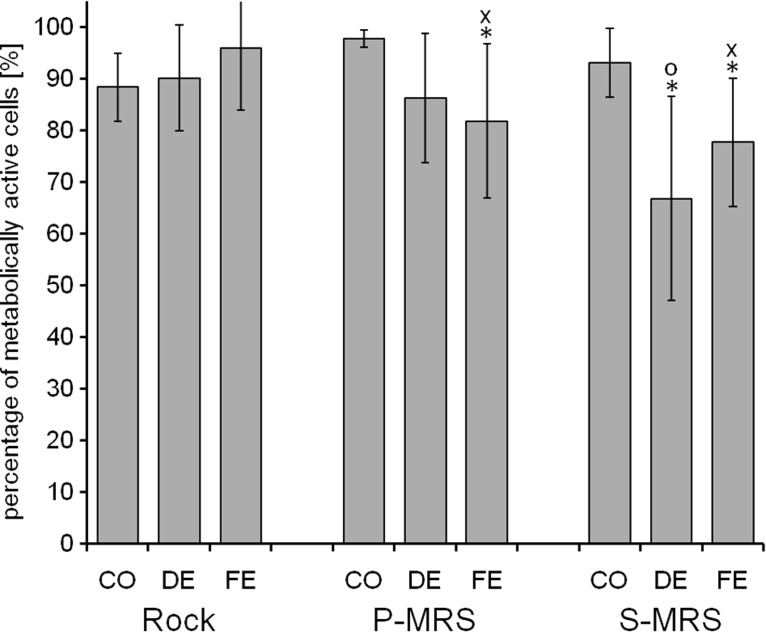
Viability of the photobiont of B. frigida after exposure to SVT conditions as percentage of metabolically active cells by live/dead staining with FUN-1. The exposure was performed under simulated space conditions on rock and simulated Mars conditions on P- and S-MRS. The experimental conditions are “control” (CO), “dark exposure” (DE), and “full exposure” with a total UVR (λ200–400nm) fluence of 5.7×105 kJ m−2 (FE). Percentage given as mean value±standard deviation of n=5–23 thallus sections and 255–1875 cells counted. Significant differences are calculated by Student t test with H0>0.05: *=significantly lower compared to the respective controls, x=significantly lower compared to FE samples on rock, o=significantly lower compared to the dark-exposed samples on rock and on P-MRS.
3.3. Viability rates of EVT-2 samples by live/dead staining
The exposure experiment EVT-2 focused on irradiation with polychromatic UVR200–400nm at fluences up to 6.8×105 kJ m−2 and therefore in the expected range of the maximum exposure during the BIOMEX mission at EXPOSE-R2. The controls (CO) revealed average viability rates from 92.0%±7.4% (S-MRS) to 96.2%±2.4% (rock) in the mycobiont and from 83.6%±16.0% (S-MRS) to 91.7%±8.1% (P-MRS) in the photobiont (Figs. 5 and 6). Despite the exposure of B. frigida to a wide range of UVR from 1.5 to 680 MJ m−2, there is virtually no difference found, and thus no dose-dependent effect on the viability of both symbionts was observed.
While the control (CO) elicited viability rates of 96.2%±2.4%, 94.6%±4.9%, and 92.0%±7.4% for the mycobiont on rock, P-MRS, and S-MRS, the highest applied UVR dose of 680 MJ m−2 showed quite similar results with 95.3%±3.7%, 96.6%±1.8%, and 94.2%±1.3% on the respective substrates (Fig. 5). The results for the photobiont are lower and showed higher standard deviation, but the pattern is comparable: With viability rates of 87.1%±9.9%, 82.7%±17.1%, and 88.3%±12.1% after UVR irradiation with 680 MJ m−2 on rock, P-MRS, and S-MRS, it showed no significant reduction of viability when compared to the respective control values (CO) of 84.2%±7.8%, 91.7%±8.1%, and 83.6%±16.0% (Fig. 6). In respect to the percentage of metabolically active cells, which served as a measure of viability, both lichen symbionts showed no significant impairment by irradiation with UVR. That was even found with a dose roughly resembling an exposure equivalent of 1.5 years in LEO (refer to Brandt et al., 2015, where samples of Xanthoria elegans experienced ∼634 MJ m−2 during 18 months in LEO) and included UVA, UVB, and UVC, which is most harmful with respect to biological damage. Interestingly, the transportation control (TC) revealed lower viability rates for the mycobiont on rock substrate (73.3%±8.0%, Fig. 5) and for the photobiont on P-MRS (53.2%±19.2%, Fig 6). Regarding the comparably high viability rates in all lab controls (CO) and the fact that the exposed samples also demonstrated higher viability rates on average, the transportation control samples may have suffered unfavorable conditions (e.g., moistness by condensation) during the storage period.
3.4. Viability rates of SVT samples by live/dead staining
The control samples of the SVT revealed viability rates in both symbionts that are comparable to those of the previously described experiments. They ranged from 97.1%±2.3% to 99.3%±0.8% in the mycobiont and from 88.4%±6.6% to 97.9%±1.7% in the photobiont (Figs. 7 and 8). On rock substrate, the “dark exposed” samples (DE) as well as the “fully exposed” samples (FE) of the mycobiont demonstrated no effect of the exposure to its viability (98.2% +1.3% and 98.2% +1.7%, respectively, Fig. 7). Regarding the mycobiont, we observed a trend of decreasing viability from CO via DE to FE conditions on both P-MRS and S-MRS. These differences were checked on significance by two-sided t tests with a level of significance of α=5%. Compared to the control, the FE mycobiont on P-MRS revealed a significantly reduced viability rate (CO: 97.1%±2.3%, FE: 91.3%±4.2%). On S-MRS, the dark as well as the FE samples showed a significant decrease of mycobiont viability when compared to its respective control (CO: 99.3%±0.8%, DE: 90.1%±5.9%, FE: 83.1%±7.1%). Moreover, the FE samples on S-MRS revealed significantly reduced mycobiont viability when compared to the FE samples on rock and P-MRS, giving a first hint on a detrimental effect in the samples on S-MRS substrate. In the photobiont, the FE samples on P-MRS (81.9%±14.9%) as well as the DE samples (66.9%±19.8%) and FE samples (77.8%±12.4%) on S-MRS revealed a significant loss of viability when compared to the respective rates of their controls. Both FE samples, on P-MRS as well as on S-MRS, showed significantly lower viability rates compared to the FE samples on rock and the DE samples on S-MRS compared to the DE samples on rock and P-MRS. These findings again suggest a detrimental effect of the Mars analog substrates on the viability of the lichen symbionts. This effect might be due to the preparation procedure where thallus areolae are cut off their natural substrate or to an adverse effect of the substrate itself. As such an effect is not observed in the controls of P- and S-MRS but found to be more pronounced in FE than in DE samples, we suggest that it is the combination of Mars-like simulation parameters and Mars analog substrates that elicits a negative effect on the viability of both lichen symbionts. Nonetheless, it should be outlined that the viability of both symbionts is quite high (minimal viability rate of 83.1%±7.1% for the mycobiont and 66.9%±19.8% for the photobiont) regarding the extreme conditions applied to the samples for 38 days and simulating UVR fluences that were designed to represent a 12-month exposure to LEO-space and Mars conditions.
3.5. Morphological stability after SVT
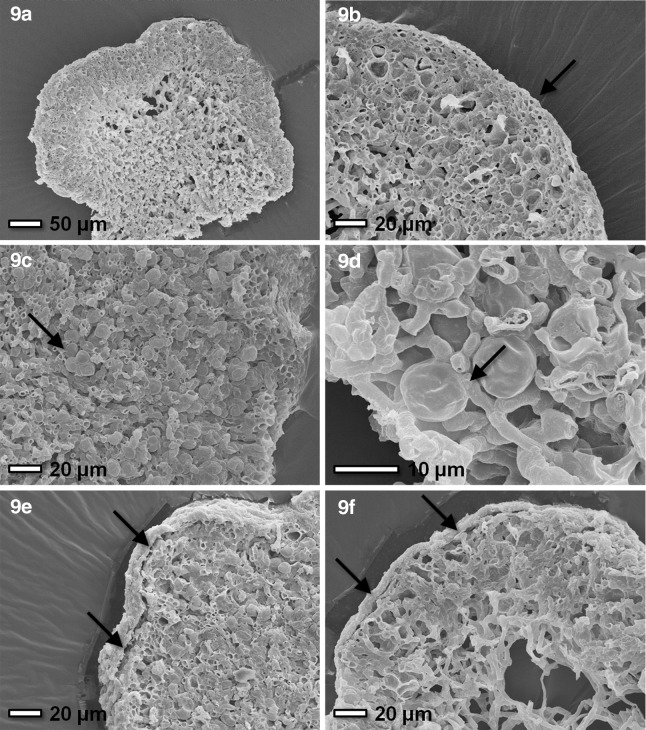
The results of the previously described analyses reveal a certain degree of loss of viability after exposure to the SVT conditions. Therefore, we investigated the morphology and anatomy of these samples to gain insight into the potential damage that might have happened during the experimental period of 38 days. Additionally, we investigated the samples of EVT-2 in the same way. The control samples showed the species' general morphology as described in previous studies (Meeßen et al., 2013). Buellia frigida is a crustose to placoid, epilithic lichen. While the growth zone at the thallus margin is composed of blackish, stretched areolae, the central region is older and formed by granular, mostly grayish areolae and numerous, black apothecia. The thallus surface is mostly covered by a mucilaginous epicortex (Fig. 9a, 9e, 9f), while a paraplectenchymatous cortex of swollen and melanin-encrusted apical cells is located below (Fig. 9b). The following algal layer is composed of clusters of trebouxoid green algae interwoven with highly gelatinized hyphae (Fig. 9c). The clusters are more numerous and denser at the margin but reduced to singled, patchy clusters in a less dense matrix in central thallus parts. Below, the thallus is fixed to the substratum by a thick layer of medullary hyphae that is again denser and more conglutinated in the younger marginal thallus parts. In summary, none of the exposure parameters in the preflight experiments—UVR (5.7×105 kJ m−2, λ200–400nm), temperature (−23°C to +10°C), Mars atmosphere (95.55% CO2 at 780–930 Pa), vacuum (4.1×10−5 Pa)—revealed a remarkable effect on the morphology, anatomy, thalline stability, and the symbiotic contact between the symbionts of B. frigida (Fig. 9d). Severe damage was not elicited by any of the tested conditions. However, the analysis demonstrated several minor defects of the epicortical mucilaginous layer, seen as fissures, ruptures, clefts, and its partial delamination (Fig. 9e, 9f). These impairments were predominantly found in the vacuum-exposed samples on rock but also in the control rock samples. All damage was more pronounced on rock samples when compared to P- and S-MRS.
FIG. 9.
Morphological characteristics of B. frigida in relation to EVT-2 exposure and SVT exposure. 9a: Section of areolae with cortex, highly gelatinated algal layer, and less dense, spongy medulla from the surface inward. 9b: Details of the densely packed cells of the cortical layer (arrow) followed inward by the algal layer with spherical algal cells and the less dense medulla (lower left). 9c: Algal clusters (arrow) in a thick algal layer with medulla (right) and cortex (left). 9d: Mycobiont-photobiont interaction with a fungal appresorium (arrow) attached to an algal cell. 9e: Delamination of the epicortical mucilaginous sheath (arrows) in control samples (rock) of EVT-2. 9f: Delamination of the epicortical mucilaginous sheath (arrows) in rock samples exposed to UVR during the SVT. As a comparison of both pictures shows, impairments of the epicortex cannot clearly be attributed to the preflight test conditions (refer to the text). The samples are (9a) SVT, rock, control; (9b) EVT-2, P-MRS, control; (9c) SVT, S-MRS, control; (9d) SVT, P-MRS, full exposure; (9e) EVT-2, rock, control; (9f) SVT, rock, full exposure.
4. Discussion
The present study demonstrates the high resistance of Buellia frigida—assessed and quantified by the viability rates of both lichen symbionts—toward the hostile conditions found in simulated LEO-space and Mars conditions of EVT and SVT. Therefore, the results form an encouraging basis for the future investigation and interpretation of the B. frigida samples that are currently exposed in the BIOMEX experiment on the ISS. Based on this data, it seems very probable that the returning B. frigida samples from the ISS as well as from the MGR will retain their vitality to some degree.
4.1. Comparison to previous space exposure experiments
Many of the present results mirror recent findings on the viability of the lichen Xanthoria elegans after exposure to LEO-space and Mars analog conditions for 1.5 years outside the European Columbus Module of the ISS (LIFE experiment, Onofri et al., 2012; Brandt et al., 2015). This principal accordance of results again stresses the importance and also the validity of Experimental and Scientific Verification Tests ahead of astrobiological exposure missions. LIFE was the first mission to expose lichens—among several other organisms—for a long-term period to LEO-space and Mars analog conditions (as already specified in the introduction). Investigation of X. elegans in the framework of LIFE focused on postflight viability as measured by live/dead analysis with FUN-1/CLSM and substantiated by additional analyses of chlorophyll a fluorescence and the symbionts' growth capacity (Brandt et al., 2015; Brandt, personal communication). The live/dead analysis of X. elegans produced several results that are comparable to those of the present study on Buellia frigida:
(i) Xanthoria elegans revealed a high overall post-exposure viability. It was 71% in the photobiont and 84% in the mycobiont but ranging from 43% to 83% (photobiont) and 60% to 89% (mycobiont) depending on the experimental condition applied. In the simulation tests on B. frigida, the average viability rates were higher, which is reasonable when comparing a maximum 38 days of space/Mars simulation in the SVT to 559 days of actual space exposure in LIFE (including also ionizing radiation).
(ii) The viability was consistently higher in the mycobiont than in the photobiont, and loss of vitality—if observed—was correlated in both symbionts. This behavior was also shown for B. frigida in the present study. Both findings are also in line with previous results from investigation of the viability of the symbionts of B. frigida, X. elegans, and Peltigera aphthosa—a lichen from habitats only moderately exposed to UVR and photosynthetically active radiation (PAR)—after exposure to UVC254nm at 2–202 J m−2, and both findings demonstrated that the photobionts are more susceptible to UVC254nm than the mycobionts (de Vera and Ott, 2010). Additionally, UVC254nm irradiation of X. elegans in doses of 5, 10, and 20 kJ m−2 induced dose-correlated photoproduct formation in isolated photobionts (≤90 per 104 bp) but not in the isolated mycobiont (de Vera, 2005). Recent observations have shown that the resistance of lichens to high UVR and vacuum can be attributed to protective thallus structures and secondary lichen compounds predominantly formed by the mycobiont (de Vera et al., 2008; Meeßen et al., 2013, 2014a), while the isolated and metabolically active photobiont is clearly impaired by UVC254nm irradiation (Meeßen et al., 2014b).
(iii) The insolation conditions during LIFE (full and 0.1% exposure) had no significant dose-dependent influence on the postflight viability of X. elegans. Such a lack of dose-dependent UVR damage was also demonstrated for B. frigida in EVT-2 when applying up to 680 MJ m−2 of polychromatic UVR200–400nm to the samples (Figs. 5 and 6). A similar effect is also reported from space simulation studies, where no decrease of viability was reported after irradiation with UVR200–400nm up to 10 kJ m−2 and a minor decrease only after UVR160–400nm up to 150 kJ m−2 and under vacuum (de Vera et al., 2003).
(iv) In the ground-based MGR experiment, the average viability of both X. elegans symbionts was higher under space simulation conditions than under Mars analog conditions. This pattern was also found in space vacuum samples of B. frigida of SVT on rock when compared to the Mars analog conditions (Figs. 7 and 8). The conditions of Mars exposure during SVT induced a significant loss of both mycobiont vitality and photobiont vitality when subdued to simulated Mars substrates (P- and S-MRS), atmosphere (95.55% CO2 at 780–930 Pa), temperature conditions [−23°C, to +10°C, as found during summer daytime in (sub-)tropical latitudes], and UVR flux (5.7×105 kJ m−2, mimicking the wavelength spectrum of λ=200–400 nm on the surface, Nier et al., 1976; Owen et al., 1977; Cockell et al., 2000; Cockell and Raven, 2004; Rabbow et al., 2012; Mahaffy et al., 2013). While temperature and irradiation profiles are the same for the simulated space exposure on rock, lacking atmospheric pressure and subsequent extreme desiccation are much more severe. Nonetheless, the impairment of viability rates is lower under space conditions. Therefore, it might be suggested that the more harmful effect of Mars analog conditions is due to the combination of several Mars analog conditions. Since the decrease in symbiont viability is more pronounced under FE conditions, this hypothesis might be supported by proposed UVR-induced decomposition of organic molecules under Mars conditions (Noblet et al., 2012; Poch et al., 2013, 2014). In this respect, the postulated UVR-induced radical formation in Mars substrates (Shkrob et al., 2010) might be mimicked by P- and S-MRS and may affect the vitality of exposed organisms as well.
(v) The lower temperature extreme during LIFE (−22°C) was roughly comparable to those tested during SVT (−23°C) and those applied during EVT-1 (−25°C). The results of EVT-1 show that there is no short-term impairment of the symbionts' vitality by such a subzero temperature. In accordance, ecophysiological investigations have routinely shown that lichens as well as their isolated photobionts endure the extremely low temperatures experienced in their harsh natural habitats, like B. frigida in Antarctica (Kappen and Lange, 1970, 1972; Kappen, 1973, 1993, 2000; Schroeter et al., 1994; Pannewitz et al., 2003; Sadowsky and Ott, 2012). Isolated photobionts were shown to recover better from UVC irradiation when the irradiation is performed under subzero temperatures, indicating an attenuating effect of one stressor (as freezing) toward another (as UVC, Backhaus et al., 2015). Additionally, temperature peaks do not impair the vitality of both symbionts as tested in EVT-1. That was also demonstrated by the high viability of X. elegans after LIFE, indicating that temperature peaking is less harmful as a rare and short-termed event (Rabbow et al., 2012; Brandt et al., 2015). Furthermore, the LIFE experiment showed that lichens survive multiple freezing point transits, a condition normally tolerated by polar, alpine, and desert lichens, including B. frigida, in diurnal and annual courses (Kappen, 1985; Sadowsky and Ott, 2012).
4.2. Effects of preflight tests on lichen morphology
The exposure parameters tested in EVT and SVT revealed no evident effect on Buellia frigida with respect to its morphology, anatomy, and thalline stability as well as the symbiotic contact between the photo- and the mycobiont. The minor damage to the epicortical mucilaginous layer might be due to the extreme desiccation during vacuum exposure but are also found in control samples. The results of previous studies might give some explanation: The overall cortex structure remains stable from younger toward older thallus parts, but its pigmentation ceases while conglutination of cortical cells as well as epicortical mucilage deposition increases. Besides other characteristics of thallus aging (depigmentation, increase of epicortical mucilage formation, and ceasing of the algal layer), the gelatinous epicortex is occasionally interrupted in more central, that is, older, areolae (Meeßen et al., 2013). Such damage to the epicortical and extracellular matrix did not affect the viability of the symbionts' cells but supports the conclusion that epicortical defects are attributed to a natural aging process rather than exposure conditions. This conclusion is also supported by the occurrence of similar defects in control samples and recent SEM investigation of the surface of Circinaria gyrosa, which is also part of BIOMEX and took part in all EVT and SVT. SEM investigation of C. gyrosa showed only minor impairments of the thallus surface (as cracks and fissures) that could not be clearly attributed to single exposure parameters and were also found in the controls (de la Torre et al., personal communication).
4.3. Mechanisms of resistance and survival
In accordance with the present results, lichens have performed consistently well in past and recent astrobiological studies. Initial short-term space exposure experiments like LICHENS II, LITHOPANSPERMIA, and STONE demonstrated high post-exposure viability of both symbionts that was subsequently confirmed in the long-term exposure experiment LIFE (Onofri et al., 2012; Brandt et al., 2015) and is now being tested in the current BIOMEX mission. Complementary simulation studies and results emphasize the high viability of lichens after exposure to single or combined nonterrestrial parameters, such as space vacuum, space UVR, Mars atmosphere, Mars UVR, subzero temperatures, and meteorite impacts (de Vera et al., 2003, 2004a, 2004b, 2008, 2010, 2014; de la Torre Noetzel et al., 2007; Sancho et al., 2007; Stöffler et al., 2007; Horneck et al., 2008; de la Torre et al., 2010; de Vera and Ott, 2010; Raggio et al., 2011; Sánchez et al., 2012, 2014; Meeßen et al., 2014b; Backhaus et al., 2015). Among other areas of investigation, such as testing the lithopanspermia hypothesis and the habitability of martian environments, all these experiments were designed to assess the limits and limitations of terrestrial organisms and to characterize the adaptive traits that confer extremotolerance, and such limits were constantly fathomed and pushed forward by the cited astrobiological studies (see references above). Moreover, the studies give some insight into the mechanisms of resistance toward the extreme stressors found beyond Earth. Besides anhydrobiosis (see below), these mechanisms are based on a range of morphological adaptations and a set of protective secondary compounds. In the case of B. frigida, the protective morphological traits can be specified as mucilaginous epicortex, cortex, algal clustering, and cortical densification when desiccated (Meeßen et al., 2013), while the protective secondary lichen compounds were identified as norstictic acid and melanin (Meeßen et al., 2014a). Melanin is encrusted in the cortex and is known to act as an efficient screening compound against UVR (Henson et al., 1999; Meredith and Riesz, 2004; Wang et al., 2006).
4.4. The role of anhydrobiosis
Vacuum desiccation is the main process that affects biological samples exposed to space vacuum, while pressure conditions similar to Mars also create desiccation-induced problems (Horneck et al., 2010). Therefore, anhydrobiosis may be regarded as another key factor of resistance toward (simulated) space and Mars conditions. Anhydrobiosis describes an organism's ability to pass into an ametabolic state when desiccated (Ertl, 1951; Crowe et al., 1992, 1998; Kranner et al., 2005). It is widely recognized as an adaptation toward harsh terrestrial habitats where water availability is scarce and infrequent. But also, in the context of astrobiology, anhydrobiosis plays an important role in explaining the high survival rates of previous studies, since most experiments were conducted with dry, that is, anhydrobiotic, lichen samples (de Vera et al., 2003, 2004a, 2004b, 2008, 2010; de la Torre Noetzel et al., 2007; Sancho et al., 2007; de la Torre et al., 2010; Raggio et al., 2011; Sánchez et al., 2012; Brandt et al., 2015). Further studies have demonstrated that wet, that is, metabolically active, thalli (de Vera and Ott, 2010; Sánchez et al., 2014) and isolated photobionts (Meeßen et al., 2014b) are more susceptible to UVC. Moreover, it was found that desiccation makes both symbionts less susceptible to stressors that usually accompany drought (as cold, excess light, and UVR, Kranner et al., 2008), preconditions the photosynthetic apparatus to UVR stress (Rao et al., 1996; Vass et al., 2005; Pandey et al., 2010), and attenuates the impairing effect of UVR on the photosynthetic activity of isolated lichen photobionts (Backhaus et al., 2015). In this context, the high viability rates of both symbionts in the present study are explained—at least partially—by the anhydrobiotic and ametabolic state of the lichen samples when exposed to the EVT and SVT. Since the samples of the current BIOMEX missions are exposed in the dry, ametabolic state, some degree of postflight viability might be expected. Nonetheless, the precise mechanisms of metabolic shutdown and the extent of desiccation-induced resistance need to be investigated in detail.
Abbreviations Used
- BIOMEX
Biology and Mars Experiment
- CLSM
confocal laser scanning microscopy
- DE
dark exposed
- EuTEF
European Technology Exposure Facility
- EVT
Experimental Verification Tests
- FE
fully exposed
- ISS
International Space Station
- LAA
Lunar Analog Anorthosite
- LEO
low-Earth orbit
- LIFE
Lichen and Fungi Experiment
- MGR
mission ground reference
- MUSC
Microgravity User Support Center
- PAR
photosynthetically active radiation
- P-MRS
Phyllosilicatic Mars Regolith Simulant
- SEM
scanning electron microscopy
- S-MRS
Sulfatic Mars Regolith Simulant
- SVT
Scientific Verification Tests
- TMS
tetramethylsilane
- UVR
ultraviolet radiation (λ=100–400 nm)
Acknowledgments
The authors would like to thank the German Federal Ministry of Economics and Technology (BMWi) and the German Aerospace Center (DLR) for funding the work of Joachim Meeßen (50BW1153), and thanks to ESA as well as to the DLR for supporting the space experiment BIOMEX (ESA-ILSRA 2009-0834, PI: Dr. J.-P.P. de Vera). S. Ott collected the samples of Buellia frigida during the GANOVEX 10 expedition, which was funded by the German Research Foundation (DFG, OT 96/151) in the framework of the Antarctic Priority Program 1158. Finally, we would also like to thank Annette Brandt for productive discussions and the anonymous reviewers for their comments and suggestions. Some of the results were presented at the 13th European Workshop on Astrobiology (EANA, 2013) and the 10th International Mycological Congress (IMC 10, 2014).
Author Disclosure Statement
No competing financial interests exist.
References
- Backhaus T., de la Torre R., Lyhme K., de Vera J.-P., and Meeßen J. (2015) Desiccation and low temperature attenuate the effect of UVC254nm in the photobiont of the astrobiologically relevant lichens Circinaria gyrosa and Buellia frigida. International Journal of Astrobiology 14:479–488 [Google Scholar]
- Berger T., Hajek M., Bilski P., Körner C., Vanhavere P., and Reitz G. (2012) Cosmic radiation exposure of biological test systems during the EXPOSE-E mission. Astrobiology 12:387–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuselink T. and van Barvinchove C. (2011) EXPOSE: Environmental History by Calculation: EXPOSE-E Simulation Results, EXP-RP-017-RS, Issue A, Revision 2, RedShift Design and Engineering BVBA, Belgium [Google Scholar]
- Brandt A., de Vera J.-P., Onofri S., and Ott S. (2015) Viability of the lichen Xanthoria elegans and its symbionts after 18 months of space exposure and simulated Mars conditions on the ISS. International Journal of Astrobiology 14:411–425 [Google Scholar]
- Cockell C.S. and Raven J.A. (2004) Zones of photosynthetic potential on Mars and the early Earth. Icarus 169:300–310 [Google Scholar]
- Cockell C.S., Catling D., Davis W.L., Kepner R.N., Lee P.C., Snook K., and McKay C.P. (2000) The ultraviolet environment of Mars: biological implications past, present, and future. Icarus 146:343–359 [DOI] [PubMed] [Google Scholar]
- Crowe J.H., Hoekstra F.A., and Crowe L.M. (1992) Anhydrobiosis. Annu Rev Physiol 54:579–599 [DOI] [PubMed] [Google Scholar]
- Crowe J.H., Carpenter J.F., and Crowe L.M. (1998) The role of vitrification in anhydrobiosis. Annu Rev Physiol 60:73–103 [DOI] [PubMed] [Google Scholar]
- de la Torre R., Sancho L.G., Horneck G., de los Ríos A., Wierzchos J., Olsson-Francis K., Cockell C., Rettberg P., Berger T., de Vera J.-P., Ott S., Frías J.M., Gonzalez P.M., Lucas M.M., Reina M., Pintado A., and Demets R. (2010) Survival of lichens and bacteria exposed to outer space conditions—results of the Lithopanspermia experiments. Icarus 208:735–748 [Google Scholar]
- de la Torre Noetzel R., Sancho L.G., Pintado A., Rettberg P., Rabbow E., Panitz C., Deutschmann U., Reina M., and Horneck G. (2007) BIOPAN experiment LICHENS on the Foton M2 mission: pre-flight verification tests of the Rhizocarpon geographicum-granite ecosystem. Adv Space Res 40:1665–1671 [Google Scholar]
- de Vera J.-P. (2005) Grenzen des Überlebens: Flechten als Modellorganismen für das Potential von Adaptationsmechanismen unter Extrembedingungen. Dissertation at the Heinrich-Heine-University Düsseldorf, ULB Düsseldorf, Germany [Google Scholar]
- de Vera J.-P. and Ott S. (2010) Resistance of symbiotic eukaryotes. Survival to simulated space conditions and asteroid impact cataclysms. In Symbioses and Stress: Joint Ventures in Biology. Cellular Origin, Life in Extreme Habitats and Astrobiology 17, edited by Seckbach J. and Grube M., Springer, Dordrecht, the Netherlands, pp 595–611 [Google Scholar]
- de Vera J.-P., Horneck G., Rettberg P., and Ott S. (2003) The potential of the lichen symbiosis to cope with the extreme conditions of outer space I. Influence of UV radiation and space vacuum on the vitality of lichen symbiosis and germination capacity. International Journal of Astrobiology 1:285–293 [Google Scholar]
- de Vera J.-P., Horneck G., Rettberg P., and Ott S. (2004a) The potential of the lichen symbiosis to cope with the extreme conditions of outer space II: germination capacity of lichen ascospores in response to simulated space conditions. Adv Space Res 33:1236–1243 [DOI] [PubMed] [Google Scholar]
- de Vera J.-P., Horneck G., Rettberg P., and Ott S. (2004b) In the context of panspermia: may lichens serve as shuttles for their bionts in space? In Proceedings of the Third European Workshop on Astrobiology, ESA SP-545, ESA Publications Division, ESTEC, Noordwijk, the Netherlands, pp 197–198 [Google Scholar]
- de Vera J.-P., Rettberg P., and Ott S. (2008) Life at the limits: capacities of isolated and cultured lichen symbionts to resist extreme environmental stresses. Orig Life Evol Biosph 38:457–468 [DOI] [PubMed] [Google Scholar]
- de Vera J.-P., Möhlmann D., Butina F., Lorek A., Wernecke R., and Ott S. (2010) Survival potential and photosynthetic activity of lichens under Mars-like conditions: a laboratory study. Astrobiology 10:215–227 [DOI] [PubMed] [Google Scholar]
- de Vera J.-P., Schulze-Makuch D., Khan A., Lorek A., Koncz A., Möhlmann D., and Spohn T. (2014) Adaptation of an Antarctic lichen to martian niche conditions can occur within 34 days. Planet Space Sci 98:182–190 [Google Scholar]
- Ertl L. (1951) Über die Lichtverhältnisse in Laubflechten. Planta 39:245–270 [Google Scholar]
- Henson J.H., Butler M.J., and Day A.W. (1999) The dark side of the mycelium: melanins of phytopathogenic fungi. Annu Rev Phytopathol 37:447–471 [DOI] [PubMed] [Google Scholar]
- Horneck G., Stöffler D., Ott S., Hornemann U., Cockell C.S., Moeller R., Meyer C., de Vera J.-P., Fritz J., Schade S., and Artemieva N.A. (2008) Microbial rock inhabitants survive hypervelocity impacts on Mars-like host planets: first phase of lithopanspermia experimentally tested. Astrobiology 8:17–44 [DOI] [PubMed] [Google Scholar]
- Horneck G., Klaus D.M., and Mancinelli R.L. (2010) Space microbiology. Microbiol Mol Biol Rev 74:121–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappen L. (1973) Response to extreme environments. In The Lichens, edited by Ahmadjian V. and Hale M.E., Academic Press, London, pp 311–368 [Google Scholar]
- Kappen L. (1985) Lichen habitats as micro-oases in the Antarctic—the role of temperature. Polarforschung 55:49–54 [Google Scholar]
- Kappen L. (1993) Plant activity under snow and ice, with particular reference to lichens. Arctic 46:297–302 [Google Scholar]
- Kappen L. (2000) Some aspects of the great success of lichens in Antarctica. Antarct Sci 12:314–324 [Google Scholar]
- Kappen L. and Lange O.L. (1970) The cold resistance of phycobionts from macrolichens of various habitats. Lichenologist 4:289–293 [Google Scholar]
- Kappen L. and Lange O.L. (1972) Die Kälteresistenz einiger Makrolichenen. Flora 161:1–29 [Google Scholar]
- Kranner I., Cram W.J., Zorn M., Wornik S., Yoshimura I., Stabentheiner E., and Pfeifhofer H.W. (2005) Antioxidants and photoprotection in a lichen as compared with its isolated symbiotic partners. Proc Natl Acad Sci USA 102:3141–3146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranner I., Beckett R., Hochman A., and Nash T.H. (2008) Desiccation-tolerance in lichens: a review. Bryologist 111:576–593 [Google Scholar]
- Mahaffy P.R., Webster C.R., Atreya S.K., Franz H., Wong M., Conrad P.G., Harpold D., Jones J.J., Leshin L.A., Manning H., Owen T., Pepin R.O., Squyres S., Trainer M., and the MSL Science Team. (2013) Abundance and isotopic composition of gases in the martian atmosphere from the Curiosity rover. Science 341:263–266 [DOI] [PubMed] [Google Scholar]
- Meeßen J., Sánchez F.J., Brandt A., Balzer E.M., de la Torre R., Sancho L.G., de Vera J.-P., and Ott S. (2013) Extremotolerance and resistance of lichens: comparative studies on five species used in astrobiological research. I. Morphological and anatomical characteristics. Orig Life Evol Biosph 43:283–303 [DOI] [PubMed] [Google Scholar]
- Meeßen J., Sánchez F.J., Sadowsky A., de Vera J.-P., de la Torre R., and Ott S. (2014a) Extremotolerance and resistance of lichens: comparative studies on five lichen species used in astrobiological research II. Secondary lichen compounds. Orig Life Evol Biosph 43:501–526 [DOI] [PubMed] [Google Scholar]
- Meeßen J., Backhaus T., Sadowsky A., Mrkalj M., Sánchez F.J., de la Torre R., and Ott S. (2014b) Effects of UVC254nm on the photosynthetic activity of photobionts from the astrobiologically relevant lichens Buellia frigida and Circinaria gyrosa. International Journal of Astrobiology 13:340–352 [Google Scholar]
- Meredith P. and Riesz J. (2004) Radiative relaxation quantum yields for synthetic eumelanin. Photochem Photobiol 79:211–216 [DOI] [PubMed] [Google Scholar]
- Millard P.J., Roth B.L., Thi H.P., Yue S.T., and Haugland R.P. (1997) Development of the FUN-1 family of fluorescent probes for vacuole labeling and viability testing of yeasts. Appl Environ Microbiol 63:2897–2905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nier A.O., Hanson W.B., Seiff A., McElroy M.B., Spencer N.W., Duckett R.J., Knight T.C.D., and Cook W.S. (1976) Composition and structure of the martian atmosphere: preliminary results from Viking 1. Science 193:786–788 [DOI] [PubMed] [Google Scholar]
- Noblet A., Stalport F., Guan Y.Y., Poch O., Coll P., Szopa C., Cloix M., Macari F., Raulin F., Chaput D., and Cottin H. (2012) The PROCESS experiment: amino and carboxylic acids under Mars-like surface UV radiation conditions in low-Earth orbit. Astrobiology 12:436–444 [DOI] [PubMed] [Google Scholar]
- Onofri S., de la Torre R., de Vera J.-P., Ott S., Zucconi L., Selbmann L., Scalzi G., Venkateswaran K.J., Rabbow E., Sánchez F.J., and Horneck G. (2012) Survival of rock-colonizing organisms after 1.5 years in outer space. Astrobiology 12:508–516 [DOI] [PubMed] [Google Scholar]
- Øvstedal D.O. and Lewis Smith R.I. (2001) Lichens of Antarctica and South Georgia: A Guide to their Identification and Ecology, Cambridge University Press, Cambridge, UK, pp 66–365 [Google Scholar]
- Owen T., Biemann K., Rushneck D.R., Biller J.E., Howarth D.W., and Lafleur A.L. (1977) The composition of the atmosphere at the surface of Mars. J Geophys Res 82:4635–4639 [Google Scholar]
- Pandey V., Ranjan S., Deeba F., Pandey A.K., Singh R., Shirke P.A., and Pathre U.V. (2010) Desiccation-induced physiological and biochemical changes in resurrection plant, Selaginella bryopteris. Plant Physiol 167:1351–1359 [DOI] [PubMed] [Google Scholar]
- Pannewitz S., Schlensog M., Green T.G.A., Sancho L.G., and Schroeter B. (2003) Are lichens active under snow in continental Antarctica? Oecologia 135:30–38 [DOI] [PubMed] [Google Scholar]
- Poch O., Noblet A., Stalport F., Correia J.J., Grand N., Szopa C., and Coll P. (2013) Chemical evolution of organic molecules under Mars-like UV radiation conditions simulated in the laboratory with the “Mars Organic Molecule Irradiation and Evolution” (MOMIE) setup. Planet Space Sci 85:188–197 [Google Scholar]
- Poch O., Kaci S., Stalport F., Szopa C., and Coll P. (2014) Laboratory insights into the chemical and kinetic evolution of several organic molecules under simulated Mars surface UV radiation conditions. Icarus 242:50–63 [Google Scholar]
- Rabbow E., Rettberg P., Barczyk S., Bohmeier M., Parpart A., Panitz C., Horneck G., von Heise-Rotenburg R., Hoppenbrouwers T., Willnecker R., Baglioni P., Demets R., Dettmann J., and Reitz G. (2012) EXPOSE-E: an ESA astrobiology mission 1.5 years in space. Astrobiology 12:374–386 [DOI] [PubMed] [Google Scholar]
- Rabbow E., Rettberg P., Barczyk S., Bohmeier M., Parpart A., Panitz C., Horneck G., Burfeindt J., Molter F., Jaramillo E., Pereira C., Weiß P., Willnecker R., Demets R., Dettmann J., and Reitz G. (2015) The astrobiological mission EXPOSE-R on board of the International Space Station. International Journal of Astrobiology 14:3–16 [Google Scholar]
- Raggio J., Pintado A., Ascaso C., de la Torre R., de los Ríos A., Wierzchos J., Horneck G., and Sancho L.G. (2011) Whole lichen thalli survive exposure to space conditions: results of Lithopanspermia experiment with Aspicilia fruticulosa. Astrobiology 11:281–292 [DOI] [PubMed] [Google Scholar]
- Rao M.V., Paliyath G., and Ormrod D.P. (1996) Ultraviolet-B- and ozone-induced biochemical changes in antioxidant enzymes of Arabidopsis thaliana. Plant Physiol 110:125–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadowsky A. and Ott S. (2012) Photosynthetic symbionts in Antarctic terrestrial ecosystems: the physiological response of lichen photobionts to drought and cold. Symbiosis 58:81–90 [Google Scholar]
- Sánchez F.J., Mateo-Martí E., Raggio J., Meeßen J., Martínez-Frías J., Sancho L.G., Ott S., and de la Torre R. (2012) The resistance of the lichen Circinaria gyrosa (nom. provis.) towards simulated Mars conditions—a model test for the survival capacity of a eukaryotic extremophile. Planet Space Sci 72:102–110 [Google Scholar]
- Sánchez F.J., Meeßen J., Ruiz M., Sancho L.G., Ott S., Vílchez C., Horneck G., Sadowsky A., and de la Torre R. (2014) UV-C tolerance of symbiotic Trebouxia sp. in the space-tested lichen species Rhizocarpon geographicum and Circinaria gyrosa: role of the hydration state and cortex/screening substances. International Journal of Astrobiology 13:1–18 [Google Scholar]
- Sancho L.G., de la Torre R., Horneck G., Ascaso C., de los Ríos A., Pintado A., Wierzchos J., and Schuster M. (2007) Lichens survive in space: results from 2005 LICHENS experiment. Astrobiology 7:443–454 [DOI] [PubMed] [Google Scholar]
- Schroeter B., Green T.G.A., Kappen L., and Seppelt R.D. (1994) Carbon dioxide exchange at subzero temperatures. Field measurements on Umbilicaria aprina in Antarctica. Cryptogamic Botany 4:233–241 [Google Scholar]
- Shkrob I.A., Chemerisov S.D., and Marin T.W. (2010) Photocatalytic decomposition of carboxylated molecules on light-exposed martian regolith and its relation to methane production on Mars. Astrobiology 10:425–435 [DOI] [PubMed] [Google Scholar]
- Stöffler D., Horneck G., Ott S., Hornemann U., Cockell C.S., Moeller R., Meyer C., de Vera J.-P., Fritz J., and Artemieva N.A. (2007) Experimental evidence for the potential impact ejection of viable microorganisms from Mars and Mars-like planets. Icarus 189:585–588 [Google Scholar]
- Vass I., Szilárd A., and Sicora C. (2005) Adverse effects of UV-B light on the structure and function of the photosynthetic apparatus. In Handbook of Photosynthesis, edited by Pessarakli M., Marcel Dekker, Inc., New York, pp 931–949 [Google Scholar]
- Wang H., Pan Y., Tang X., and Huang Z. (2006) Isolation and characterization of melanin from Osmanthus fragrans seeds. Lebenson Wiss Technol 39:469–502 [Google Scholar]
- Westall F. (2013) Microbial scale habitability on Mars. In Habitability of Other Planets and Satellites. Cellular Origin, Life in Extreme Habitats and Astrobiology 28, edited by de Vera J.-P. and Seckbach J., Springer, Dordrecht, the Netherlands, pp 183–202 [Google Scholar]