Abstract
Uptake of nutrients, such as glucose and amino acids, is critical to support cell growth and is typically mediated by cell surface transporters. An alternative mechanism for the bulk uptake of nutrients from the extracellular space is macropinocytosis, a nonclathrin, and nonreceptor-mediated endocytic process, in which extracellular fluid is taken up into large intracellular vesicles called macropinosomes. Oncogenic transformation leads to the increased metabolic activity of tumor cells, and in the Ras-driven tumor part of this enhanced activity is the stimulation of macropinocytosis. To measure oncogene-dependent macropinocytosis, we used HeLa cells expressing oncogenic HRASG12D driven from a Tet-regulated promoter. Upon oncogenic HRAS expression, the cells undergo metabolic changes that include the elevation of macropinocytosis. We detected macropinocytosis through the uptake of lysine-fixable tetramethyl rhodamine (TMR)-Dextran (70 kDa) from the cell media into nascent intracellular macropinosomes. These macropinosomes were quantified by image-based high-content analysis, with the size, intensity, and position of macropinosomes measured. Using this model system, we ran a full genome-wide siRNA screen (siGenome™; GE) to identify genes involved in controlling oncogenic HRAS-dependent macropinocytosis. Hits from the primary screen were confirmed with siRNA reagents from a different library (GE, OTP), which allowed us to mitigate potential off-target effects. Candidate genes from this screen include known regulators of macropinocytosis as well as novel targets.
Introduction
Activating mutations of RAS gene are found in greater than 90% of pancreatic ductal adenocarcinoma and represent the most frequent and earliest genetic alteration.1,2 It has been shown that RAS-transformed cells internalize extracellular protein, to support central carbon metabolism, through a process termed macropinocytosis.3–5 Furthermore, it has recently been observed that human pancreatic tumors are low in glutamine and display robust levels of macropinocytosis.6
Macropinocytosis is an endocytic process that mediates the uptake of extracellular fluid into large heterogeneous intracellular vesicles, greater than 0.2 μm in size, known as macropinosomes,4 which serve to internalize large volumes of extracellular fluid along with the associated membrane. This endocytic process is initiated by the formation of actin-dependent protrusions of the plasma membrane known as membrane ruffles.4 One of the key mediators of this initial step is the low-molecular-weight G-protein RAC1,7,8 which controls macropinosome formation through membrane fission. A fuller mechanistic understanding of how macropinocytosis is regulated, and how to modulate it, would be of great significance in understanding how oncogenic RAS alters cancer metabolism, ultimately leading to new therapeutic approaches.9,10
To develop a high-throughput full-genome RNAi screen for macropinocytosis, amenable to assay automation and 384-well plates, we adapted a previously developed model.3,10 To undertake our screen, we used the cervical cancer line, HeLa, which expresses wild-type RAS and normally has very low levels of macropinocytosis. These cells were engineered to express tetracycline-regulated oncogenic HRASG12D.5 Upon induction with doxycycline, these cells exhibit an altered phenotype, measured by the elevated uptake of a lysine-fixable high-molecular-weight dextran (70 kDa), labeled with the fluorophore tetramethyl rhodamine (TMR), which is relatively insensitive to pH and therefore ideal for quantitatively labeling endosomal and lysosomal compartments.11 This high-molecular-weight dextran is selectively internalized into macropinosomes, whereas low-molecular-weight dextran may also label other endocytic pathways.3,4 When added to the growth medium for short periods of time, this large lectin molecule does not readily enter cells. However, upon induction with mutant HRAS, dextran enters through macropinocytosis (Fig. 1), which can then be imaged and quantified using multiparametric high-content screening.
Fig. 1.
Dextran uptake in Tet-induced HeLa. Typical phenotype for tetramethyl rhodamine (TMR)-dextran containing macropinosomes in cells (a) following doxycycline withdrawal for 72 h and transfected with negative control RISC-free siRNA. (b) Level of TMR-dextran accumulation in cells maintained on doxycycline. (c) Following doxycycline withdrawal for 72 h and transfected with RAC1 siRNA.
Using this high-throughput assay in 384-well plates, we performed a full-genome siRNA screen, using a siRNA library containing four pooled siRNA oligonucleotides per well (siGenome™ Smartpool™; GE). This pooling is thought to limit off-target effects, driven by single siRNA species. To transfect the HeLa cells with siRNA, we used a reverse transfection approach, which is amenable to high-throughput approaches.12,13
To confirm hits from the primary screen, we performed a confirmation screen using siRNA oligonucleotide pools from the ON-TARGETplus™ Smartpool siRNA library (OTP, GE), to mitigate off-target effects often observed with siRNA reagents. In total, 960 genes were tested in the confirmation screen after first being identified as hits in the primary screen. This rather stringent approach yielded a fairly low hit rate, which did allow us to filter the number of potential candidate genes and molecular pathways, without resorting to extensive and costly characterization of off-target effects,14,15 or gene add-back experiments.16 Examples of hits from the confirmation screen are shown for genes known to regulate macropinocytosis, RAS, and membrane trafficking, demonstrating the robustness and utility of this assay for novel gene target discovery.
The images from the confirmation screen were further characterized to capture data on the intensity and localization of macropinosomes and to cluster hits with known as well as potentially novel mechanisms of action.
Materials and Methods
Cell Culture
HeLa Tet-HRASG12D cells were removed from liquid nitrogen storage and seeded into T175 with Dulbecco's minimal essential medium (DMEM), 10% fetal bovine serum (FBS), and doxycycline. On the day of siRNA transfection, the cells were at 40%–70% confluence. Media were changed on the morning of experiment (DMEM, 10% FBS, no doxycycline).
Reverse Transfection
The siGenome siRNA library (siGenome Smartpools; GE) was stored at 2 μM in 384-well polypropylene storage plates. An intermediate dilution plate, for siRNA library, and controls were prepared in a Corning polypropylene 384-well plate, as follows: 17.5 μL OptiMEM™ was added to a 384-well compound plate using a Thermo Combi™ to columns 3–22. 2.5 μL of siGenome library (2 μM) was transferred with an Agilent Bravo™ liquid handling system, using a 384-well head. Controls were diluted to 10× final concentration in polypropylene tubes and added to dilution plates using a 16-channel pipette (10 μL/well). Doxycyline (25 μM), column 1; RAC1 siRNA Smartpool (20 μM), column 2; RISC-free™ control siRNA17 (20 μM), column 23; and Kif11 siRNA (Silencer Select™; Life Technologies) (5 μM), column 24. OptiMEM (5 μL) was added to each well of assay plate with small-volume cassette on Thermo Combi. Then, 5 μL from the siRNA dilution plate was transferred to the assay plate using the Agilent Bravo. RNAiMax™ transfection reagent was warmed to room temperature immediately before use and mixed using a vortex. RNAiMax was diluted with room temperature OptiMEM at 10× final concentration (0.06 μL/well, 50 μL; 12 μL/mL). Finally, 5 μL of diluted RNAiMax was added to all wells on the assay plate containing siRNA and left at room temperature in tissue culture hood for 25 min. Cells were then added to the assay plate using a Thermo Combi (600 cells/well, 40 μL) in a complete medium with 10% FBS and placed in a tissue culture incubator for 72 h.
Dextran Uptake Assay
On the morning of assay, the cells were starved of FBS for 3 h to increase macropinocytosis.11 Media were changed to 40 μL DMEM, minus FBS, by flicking the media out and adding 40 μL DMEM using Thermo Combi. Cells were then placed back in the incubator for 4 h. TMR-dextran (Life Technologies) was diluted to 10× final concentration (2.5 μg/mL) in DMEM and added to a rectangular reservoir on the Agilent Bravo deck. TMR-dextran (5 μL) was added to the assay plates using the Agilent Bravo 384-well head. Plates were processed in two batches per day with 2.5 min between each plate, which was the amount of time required to wash each plate following incubation. Plates were placed back in the incubator for 30 min following addition of TMR-dextran, before washing six times with 50 μL phosphate-buffered saline (PBS) using a BioTek ELx406™ plate washer. Cells were then fixed and stained, for 20 min, with paraformaldehyde (4%) and Hoechst 33342 (Life Technologies) (4 μg/mL), added using the multichannel peristaltic pump on the BioTek ELx406. Plates were ultimately washed thrice with 50 μL PBS, before being sealed.
Imaging and Analysis
Four fields per well were captured using a 20× objective on a GE INCell 6000™ high-content imager. The Hoechst 33342 image was captured using UV excitation (405 nm) and the TMR-dextran image was captured using the green laser excitation (561 nm). Image sets were analyzed using GE Developer™ software, as follows: The nucleus, stained and identified with Hoechst 33342, and clumped cells segmented. A ring extending 10 μm from the outer edge of the nucleus was used to delineate the cytoplasm. TMR-dextran spots within the cell were identified and segmented using the vesicle segmentation tool in GE Developer. Measurements were made on cell number, nuclear area, and intensity using the Hoechst 33342 image, and on the number, size, intensity, and position of the TMR-dextran spots. The primary endpoint used in the screen was the total intensity of TMR-dextran spots per cell.
All well-level data spreadsheets generated by GE INCell developer were concatenated using KNIME.18 KNIME was then used to merge spreadsheets containing siRNA metadata (gene name, gene ID, gene symbol, well and plate information, etc.). Data were normalized to the positive and negative controls and hit selection criteria set up, again using KNIME (Z-score, and threshold cutoff), with statistical outlier control wells excluded (greater than 3 standard deviations from mean).16,19 Wells with fewer than 100 cells per well were excluded from hit selection.
Results and Discussion
HeLa cells, although being derived from a cervical tumor, have a very low level of dextran uptake through macropinocytosis (Fig. 1). Upon washing of the cells and incubation in doxycycline-free media for 3 days, to induce mutant HRASG12D expression,5 there was a greatly increased level of macropinocytosis, measured using the lysine-fixable 70-kDa dextran labeled with TMR.3 Using high-content analysis, we were able to quantify the uptake of dextran into vesicles in the cytoplasm (Fig. 2a).
Fig. 2.
High-content analysis of macropinosomes. Nuclei were first detected with the DNA intercalating dye, Hoechst 33342. Threshold was applied to identify nuclei (blue), before clump breaking to separate touching and overlapping nuclei. Macropinosomes were identified using a spot detection algorithm within each cell (red), which was delineated by a 10-μm region away from the outer edge of each nuclei (green). Abundant TMR-dextran spots are detectable following growth in doxycycline-free media for 3 days, compared with cells maintained on doxycycline.
Positive and negative controls were used to set up the screen were doxycycline and RISC-free siRNA, respectively. Doxycycline, which will prevent oncogenic HRAS expression, was used as the maximum possible inhibition.20 We also used a siRNA to the low-molecular-weight G-protein, RAC1, which had previously been shown to work well by interfering with initiation of macropinocytosis.7 To determine the best assay endpoint to use, we evaluated the total area and total intensity of TMR-dextran spots per cell. Total intensity per cell had a Z′ of 0.65 when comparing the doxycycline-positive control, the RISC-free negative control, and a Z′ of 0.3 to the RAC1 siRNA-positive control and the RISC-free negative control. The % spot area positive readout had a Z′ of 0.74 comparing doxycycline and the RISC-free negative control, but only a Z′ of −0.14 when comparing the RAC1 siRNA and the RISC-free negative control. We therefore used total spot intensity per cell as the primary readout in the screen, due to the increased sensitivity in detecting weaker hits (Fig. 3).19 The TMR-dextran total spot intensity was normalized to both the negative control (RISC-free siRNA) and positive control (doxycycline) on each plate. All the assay measures used in this screen are based upon well averages and do not take into account the heterogeneity in the amount of TMR-dextran uptake into macropinosomes in individual cells. Since the assay window was large and positive controls and hits have a lower heterogeneity, this did not dramatically affect our hit selection.
Fig. 3.
HTS screen metrics by plate with hit cutoffs. Both spot total intensity per cell and % spot area positive provide excellent assay windows using the doxycycline control (Z′=0.65 and 0.74, respectively). However, the Z′ with the RAC1 siRNA was 0.3 versus −0.14, respectively. Therefore, the spot intensity per cell was used as the primary endpoint.
A siRNA, to kinesin family member 11 (Kif11), induces rapid cell death in dividing cells21,22 and was included on each plate to monitor the performance of the siRNA reverse transfection (Fig. 4). RAC1 siRNA consistently inhibited about 70% of the dextran uptake when compared with doxycycline.
Fig. 4.
HCS data for each plate in primary siRNA screen. Plot of spot total intensity per cell across the entire siRNA genome wide screen. Plate number is plotted on the x-axis; spot total intensity per cell, normalized to doxycycline control (0%), and RISC-free siRNA (100%).
Hit selection in high-throughput screening is ideally based upon a statistical criteria to ensure that the selected hits are not the result of chance or false positives. A typical statistical cutoff is thrice the standard deviation of the negative control data, or a Z-score of 3.16,19 Since this hit selection cutoff would have resulted in a very high hit rate, we used a more strict hit selection criteria of 70% inhibition in the dextran spot total intensity per well (Fig. 4). This was a rather stringent hit selection strategy, since to meet this cutoff, siRNA pools had to be at least as potent as our RAC1 siRNA control. We were also interested in identifying hits that could increase the dextran uptake beyond that normally seen upon doxycycline withdrawal; for this, we set a threshold of double the negative control spot total intensity per cell.
To reduce and coalesce the number of gene targets in our confirmation screen to a manageable number, we used gene ontology clustering methodology (David, NIH) to cluster hits into functional families. The total number of genes selected for rescreen was 960.
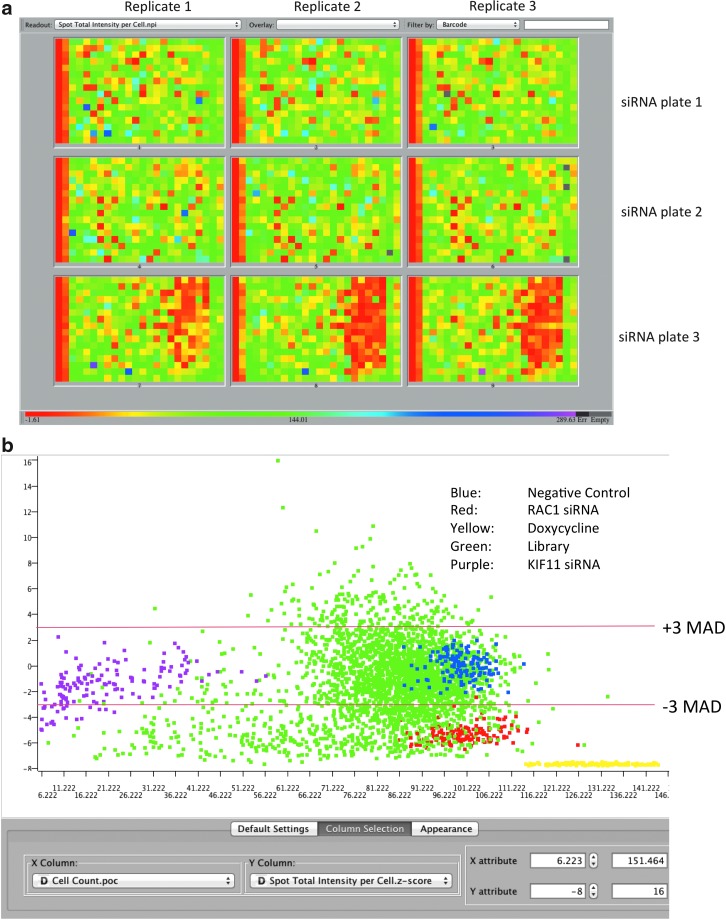
Genes identified as hits in the primary screen, using the siGenome library, were retested in triplicate with the OTP siRNA. This siRNA collection is also pooled with four individual siRNA per gene, and mostly uses different siRNA sequences, compared to siGenome. This approach was taken to rapidly mitigate off-target effects from the primary screen. For 94 of the gene hits from the primary screen, an OTP reagent was not available, in which case, the siGenome siRNA was retested. On the confirmation screen, there was generally very good reproducibility across replicates with a coefficient of variance, between replicate wells, of 0.13 (Fig. 5a). The hit selection criteria used was a robust (median based) Z-score of 3 median absolute deviations (MADs) away from the negative control, for spot total intensity per cell (Fig. 5b). Genes had to have a Z-score greater than 3 MAD in at least two out of the three replicates to be considered a hit. The hit confirmation rate using OTP siRNA was expectedly low at 22%, although the confirmation rate for siGenome siRNA was 84%. Novel genes were identified in this screen as novel regulators of Ras-driven macropinocytosis, the identity of which is not discussed here. However, expected regulators of Ras and vesicle transport mechanisms were identified as hits and examples are shown in Table 1. Examples from the Ras pathway are H-RAS,23,24 FNTA,25 RAN,26 RASSF2,24 and RASGRP2.24 Examples of vesicle-trafficking regulators are ARF1,8,27 RAB13,8 and RAB40B.8
Fig. 5.
Confirmation screen results. (a) Plate hit map of spot total intensity per cell for the hit confirmation screen. The conformation siRNA set was screened in triplicate (technical replicates). OTP siRNA were on plates 1, 2, and 3, while siGenome siRNA are on the right-hand side of plate 3. (b) A hit selection criteria of confirmation screen was based upon plus or minus 3 median absolute deviations (MADs) away from the spot total intensity per plate for the control RISC-free siRNA, for each plate.
Table 1.
Examples of Hits from the Confirmation Screen
| Gene | Pathway | Spot intensity per cell (NPI) | Spot intensity per cell (Z-score) | Cell count (POC) |
|---|---|---|---|---|
| HRAS | Ras | 9.0 | −7.0 | 101.9 |
| FNTA | Ras | 20.9 | −6.1 | 101.2 |
| ARF1 | Vesicle trafficking | 25.2 | −5.8 | 82.4 |
| RAN | Ras | 29.9 | −5.5 | 12.0 |
| RASSF2 | Ras | 29.9 | −5.4 | 66.2 |
| RASGRP2 | Ras | 33.7 | −5.1 | 71.7 |
| RAB13 | Vesicle trafficking | 44.9 | −4.2 | 94.6 |
| RAB40B | Vesicle trafficking | 60.0 | −3.1 | 83.2 |
Examples of hits from the confirmation screen with pathway involvement, normalized spot intensity per cell, Z-score for spot intensity per cell compared with RISC-free negative control siRNA, and cell number expressed as a percentage of the negative control.
As part of a deeper phenotypic analysis of hits from the confirmation screen, we analyzed the position of dextran-containing vesicles within the cell, relative to the nucleus. It was observed that there are very weakly stained vesicles in the doxycycline-positive control, which are perinuclear, compared with the very bright-stained vesicles in the negative control (RISC-free siRNA) cells (Fig. 6). RAC1 siRNA reduces the TMR-dextran uptake as mentioned previously, and the position of these vesicles is in between that seen in doxycycline and the negative control (Fig. 6). The average position of TMR-dextran spots was classified into regions on a scatter plot, for simple classification (Fig. 6). The position of the macropinosomes is of interest, as this might indicate novel mechanisms of action or inhibition at a particular step in the process of macropinocytosis, compared with known modulators such as RAC1, which is thought to be involved in the initiation step of membrane engulfment of macropinosomes.4,8 Several of these hits were identified as shown by examples from regions 3 and 4 in Figure 7. HRAS and other Ras-regulatory genes previously identified as hits (FNTA, RAN, and RAB13) all fall within region 3 along with RAC1 and have TMR-dextran-stained vesicles close to the nucleus. Hits identified in region 4, along with significantly reduced levels of TMR-dextran uptake, have vesicles at a greater distance from the nucleus and several of these have been identified as novel regulators of macropinocytosis, not involved with the regulation of Ras or membrane trafficking.
Fig. 6.
Detailed phenotypic analysis of confirmation screen data. Position of TMR-dextran vesicles relative to the nucleus was measured using high-content analysis. In negative control cells, these are bright and relatively far away from the nucleus, whereas in doxycycline-treated cells, they are very dim and perinuclear.
Fig. 7.
Images of controls and examples from regions 3 and 4. Image intensities are optimized for each image to best show the TMR-dextran staining.
Abbreviations Used
- DMEM
Dulbecco's minimal essential medium
- MAD
median absolute deviation
- PBS
phosphate-buffered saline
- TMR
tetramethyl rhodamine
Acknowledgment
Supported by a grant from The Lustgarten Foundation.
Author Contributions
M.F. and C.C. share the lead authorship on this article.
Disclosure Statement
No competing financial interests exist.
References
- 1.Morris JP, 4th, Wang SC, Hebrok M: KRAS, Hedgehog, Wnt and the twisted developmental biology of pancreatic ductal adenocarcinoma. Nat Rev Cancer 2010;10:683–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kanda M, Matthaei H, Wu J, Hong SM, Yu J, Borges M, Hruban RH, Maitra A, Kinzler K, Vogelstein B, Goggins M: Presence of somatic mutations in most early-stage pancreatic intraepithelial neoplasia. Gastroenterology 2012;142:730–733, e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Commisso C, Davidson SM, Soydaner-Azeloglu RG, Parker SJ, Kamphorst JJ, Hackett S, Grabocka E, Nofal M, Drebin JA, Thompson CB, Rabinowitz JD, Metallo CM, Vander Heiden MG, Bar-Sagi D: Macropinocytosis of protein is an amino acid supply route in Ras-transformed cells. Nature 2013;00:1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kerr MC, Teasdale RD: Defining macropinocytosis. Traffic 2009;10:364–371 [DOI] [PubMed] [Google Scholar]
- 5.Bar-Sagi D, Feramisco JR: Induction of membrane ruffling and fluid-phase pinocytosis in quiescent fibroblasts by ras proteins. Science 1986;233:1061–1068 [DOI] [PubMed] [Google Scholar]
- 6.Kamphorst JJ, Nofal M, Commisso C, Hackett SR, Lu W, Grabocka E, Vander Heiden MG, Miller G, Drebin JA, Bar-Sagi D, Thompson CB, Rabinowitz JD: Human pancreatic cancer tumors are nutrient poor and tumor cells actively scavenge extracellular protein. Cancer Res 2015;75:544–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fujii M, Kawai K, Egami Y, Araki N: Dissecting the roles of Rac1 activation and deactivation in macropinocytosis using microscopic photo-manipulation. Sci Rep 2013;3:2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Egami Y, Taguchi T, Maekawa M, Arai H, Araki N: Small GTPases and phosphoinositides in the regulatory mechanisms of macropinosome formation and maturation. Front Physiol 2014;5:374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kimmelman AC: Metabolic dependencies in RAS-driven cancers. Clin Cancer Res 2015;21:1828–1834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maltese WA, Overmeyer JH: Non-apoptotic cell death associated with perturbations of macropinocytosis. Front Physiol 2015;6:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Commisso C, Flinn RJ, Bar-Sagi D: Determining the macropinocytic index of cells through a quantitative image-based assay. Nat Protoc 2014;9:182–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hasson SA, Kane LA, Yamano K, et al. : High-content genome-wide RNAi screens identify regulators of parkin upstream of mitophagy. Nature 2013;504:291–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sivan G, Martin SE, Myers TG, et al. : Human genome-wide RNAi screen reveals a role for nuclear pore proteins in poxvirus morphogenesis. Proc Natl Acad Sci U S A 2013;110:3519–3524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jackson AL, Burchard J, Schelter J, et al. : Widespread siRNA “off-target” transcript silencing mediated by seed region sequence complementarity. RNA 2006;12:1179–1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brennecke J, Stark A, Russell RB, et al. : Principles of microRNA-target recognition. PLoS Biol 2005;3:404–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mohr SE, Perrimon N: RNAi screening: new approaches, understandings, and organisms. Wiley interdisciplinary reviews. RNA 2012;3:145–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baum P, Fundel-Clemens K, Kreuz S, Kontermann RE, Weith A, Mennerich D, Rippmann JF: Off-target analysis of control siRNA molecules reveals important differences in the cytokine profile and inflammation response of human fibroblasts. Oligonucleotides 2010;20:17–26 [DOI] [PubMed] [Google Scholar]
- 18.Berthold MR, Cebron N, Fabian D, Gabriel TR, Meinl T, Ohl P, Sieb C, Thiel K, Wiswedel B: KNIME: The (K)onstanz (I)nformation (M)iner. Studies in Classification, Data Analysis, and Knowledge Organization. 2007. ISBN: 978-3-540-78239-1
- 19.Birmingham A, Selfors LM, Forster T, et al. : Statistical methods for analysis of high-throughput RNA interference screens. Nat Methods 2009;6:569–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Porat-Shliom N, Kloog Y, Donaldson JG. A unique platform for H-Ras signaling involving clathrin-independent endocytosis. Mol Biol Cell 2008;19:765–775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martens-de Kemp SR, Nagel R, Stigter-van Walsum M, van der Meulen IH, van Beusechem VW, Braakhuis BJ, Brakenhoff RH. Functional genetic screens identify genes essential for tumor cell survival in head and neck and lung cancer. Clin Cancer Res 2013;19:1994–2003 [DOI] [PubMed] [Google Scholar]
- 22.Koller E, Propp S, Murray H, Lima W, Bhat B, Prakash TP, Allerson CR, Swayze EE, Marcusson EG, Dean NM. Competition for RISC binding predicts in vitro potency of siRNA. Nucleic Acids Res 2006;34:4467–4476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakase I, Kobayashi NB, Takatani-Nakase T, et al. : Active macropinocytosis induction by stimulation of epidermal growth factor receptor and oncogenic Ras expression potentiates cellular uptake efficacy of exosomes. Sci Rep 2015;5:10300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.White E. Exploiting the bad eating habits of Ras-driven cancers. Genes Dev 2013;27:2065–2071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang T, Danielson PD, Li BY, et al. : The p21(RAS) farnesyl transferase alpha subunit in TGF-beta and activin signaling. Science 1996;271:1120–1122 [DOI] [PubMed] [Google Scholar]
- 26.Mott HR1, Owen D: Structures of Ras superfamily effector complexes: what have we learnt in two decades? Crit Rev Biochem Mol Biol 2015;50:85–133 [DOI] [PubMed] [Google Scholar]
- 27.Nonnenmacher M, Weber T: Adeno-associated virus 2 infection requires endocytosis through the CLIC/GEEC pathway. Cell Host Microbe 2011;10:563–576 [DOI] [PMC free article] [PubMed] [Google Scholar]